- 1Laboratorio de Conservación Biológica, Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Santiago, Chile
- 2Laboratorio de Estudios del Antropoceno, Departamento de Manejo de Bosques y Medio Ambiente, Facultad de Ciencias Forestales, Universidad de Concepción, Concepción, Chile
- 3Asociación Kauyeken, Santiago, Chile
Mitigation of carnivore-human conflict due to domestic animal predation represents an imperative challenge. Although livestock management strategies aimed at reducing predation have recently received attention by wildlife managers and producers, the information regarding ecological attributes of studied predators, and environmental characteristics of the areas where conflicts occur is largely missing. We conducted a global review to characterize the literature of carnivore-livestock conflict, identifying the set of reported predators, and assessing the ecological attributes of these species and areas where predation has occurred. A total of 391 published peer-reviewed research papers on carnivore-livestock conflict containing 783 predation study cases were evaluated. Carnivore-livestock conflict research was largely conducted in Asian and African countries (80% of published studies). Fifty-two carnivores were reported in conflict-related studies being Felidae and Canidae the most frequently studied groups (80% of study cases). Carnivores more often reported to prey on domestic animals exhibit larger home ranges and body masses, and are also subject to larger reductions in their distribution ranges. They also show a generalist habitat behavior, a strictly carnivore diet, and cathemeral activity. Predation of domestic animals consistently increased with vegetation cover, decreased with distance from human settlement and was higher in young animals. The analysis conducted separately for large and meso carnivores showed that predation on domestic animals by large carnivores (>21.5 kg) increased near protected areas and far from human settlements. Current information regarding conflicts exhibits a notable variation in research effort toward some regions and large-bodied and broadly distributed species. This asymmetry could reflect the role of human perspectives in research based on species-level traits, research facilities and funding opportunities, though also underlies ecological processes induced by land transformation occurring in some regions across the globe. As encroached habitat increases, species with restricted distributions and behaviors, or smaller home ranges such as meso carnivores, will roam into human-dominated landscapes, increasing their probability of interacting with livestock activity. Identifying ecological attributes that distinguish carnivores and areas as “conflict-prone” may contribute to set evidence-based management approaches in frameworks ready to anticipate, reduce, or prevent human-carnivore conflict, complementing the use of other strategies.
Introduction
Predation upon livestock is the triggering factor of human–carnivore conflicts in production-oriented landscapes (Loveridge et al., 2010). Livestock predation can impose important economic costs to local communities (Treves and Karanth, 2003; Woodroffe and Frank, 2005) and the subsequent elimination of “problematic” individuals as a retaliatory action is one of the most ubiquitous and difficult problems faced by carnivore conservation today. A wide range of species are involved in predation on domestic animals, including wolf (Canis lupus), bear (Ursus spp.), and lynx (Lynx spp.) in North America and Europe (Thorn et al., 2013; Smith et al., 2014); tigers (Panthra tigris), snow leopards (Panthera uncia), and leopards (Panthera pardus) in Asia (Miller, 2015); hyenas (Hyaena spp.), wild dogs (Lycaon pictus), jackals (Canis mesomelas and Canis auereus), lions (Panthera leo), and cheetahs (Acinonyx jubatus) in Africa (Thorn et al., 2013); and jaguars (Panthera onca), pumas (Puma concolor), and foxes (Lycalopex spp.) in Central and South America (Palmeira et al., 2008; Gonzalez et al., 2012; Soto-Shoender and Main, 2013). On the other hand, these carnivores also prey on a wide array of domestic animals, including poultry, sheep (Ovis spp.), goats (Capra spp.), and cattle (Bos spp.) (Graham et al., 2005).
Carnivore-livestock conflict poses an urgent challenge in heavily-cleared landscapes where the requirements of carnivore populations are often at odds with those of human activities (Dickman, 2010). Whereas, livestock husbandry practices have recently received attention by conservationist and wildlife managers to mitigate the conflict in these landscapes (Miller et al., 2016; Eklund et al., 2017; Van Eeden et al., 2017; Moreira-Arce et al., 2018), ecological characteristics of carnivores that prey on domestic animals have rarely been considered (Graham et al., 2005; Miller, 2015). For instance, in mosaic landscapes containing natural and anthropogenic lands, carnivores displaying large home-ranges and wide habitat requirements are expected to wander frequently in areas associated with livestock managed under extensive grazing systems (Balme et al., 2010). Similarly, diet-generalist species and nocturnal and pack hunters may have increased predation rates on livestock (Kruuk, 1972; Kleiman and Eisenberg, 1973; Gittleman, 1989; Cozzi et al., 2012), creating a potential conflict with livestock owners.
Carnivores occurring in human-dominated landscapes usually respond to habitat attributes depending on how they prey on wild species, use remnant habitats as refuges and avoid human presence as expected from habitat selection, optimal foraging, and landscape of fear theories (Brown et al., 1999; Boyce, 2006; Schooley and Branch, 2007). Understanding the relations among key socio-ecological factors such as landscape and habitat configurations, and management practices can offer data regarding how these variables affect predation on domestic animals and thus aid in identifying “conflictive hotspots” in livestock-raising landscapes (e.g., Baker et al., 2008; Treves et al., 2011; Abade et al., 2014; Miller, 2015). For instance, increases in livestock predation may emerge from changes in the relative abundances of native to domestic prey as well as the presence of landscape elements that might favor encounters between carnivores and domestic animals (Baker et al., 2008; Miller, 2015).
Unraveling the ecological characteristics of species reported in the carnivore-conflict literature and under what ecological conditions specific areas may be susceptible to livestock predation are need steps to setting evidence-based management approaches to prioritize and co-ordinate future research effort and to anticipate or reduce human-carnivore conflict (Inskip and Zimmermann, 2009; Miller, 2015; Lozano et al., 2019). Within this context, the aim of the work was to provide a global perspective of carnivore-livestock conflict research to determine to what extent different carnivore species are reported in the conflict-related literature. Specifically, the present study: (i) evaluated the conflict in taxonomic terms; and (ii) assessed the ecological traits of the reported carnivores as well as the environmental, ecological conditions, and management practices of areas where predation of livestock occurs.
Methods
A search was performed on the Web of Science (Science Citation Index Expanded) for papers about every terrestrial carnivore using the following search terms: carnivore-livestock conflict* OR human-carnivore interaction* OR predation risk*. Our peer-reviewed literature included studies dealing with direct predation events, as well as studies where carnivores were perceived as livestock predators but not necessarily confirmed (mostly based on surveys; e.g., Minnie et al., 2015). Studies that presented only reviews, opinions, or meta-analyses were excluded. The diversity of carnivores and domestic prey involved in carnivore-livestock conflicts was assessed and information detailing general information of the published studies that included geographic location was extracted. Likewise, the season and moment of the day when the predation event occurred was also assessed.
The frequency of each carnivore in the carnivore-livestock conflict literature was assessed as the number of times each species was reported across selected studies. This frequency was contrasted against the general published literature of each species to explore the frequency distribution of research effort (Brooke et al., 2014). Subsequently, the frequency of large and medium-size carnivores was also assessed separately in order to explore whether research effort may be biased by carnivore body size. Although meso carnivores are best identified on the basis of characteristics of a given food web (Prugh et al., 2009), to separate these two groups of species (large and medium size) we used a mass of 21.5 kg based on mass-related energetic requirements of carnivores (Carbone et al., 1999). More specifically, a set of ecological attributes of reported species was evaluated based on previous studies dealing with descriptive bibliometric analyses and species traits (Brooke et al., 2014). These attributes included body size (kg), home range sizes (km2), social structure (solitary/group), and activity cycle (nocturnal/diurnal/crepuscular or cathemeral), habitat (number of habitats used), and diet breadth (number of dietary items consumed). Ecological data from reported carnivores were obtained from the PanTHERIA database (Jones et al., 2009) and The Handbook of the Mammals of the World (Wilson and Mittermeier, 2009). Finally, we assessed the habitat shrinkage for a subset of reported carnivores for which current and historic distribution ranges were available in the IUCN database. Then, a Decline Distribution Index for each species was estimated by calculating 1—the ratio between both current and historic ranges. Values ranged between 0 (no reduction in distribution range) and 1 (maximum reduction in distribution range). Associations between the frequencies of each species reported in conflict-related studies and the different ecological attributes above mentioned were tested by using Spearman correlations implemented in R package software. Spatial analyses were conducted using QGIS 2.16.
To test whether different characteristics of killing sites effectively influence predation on domestic animals, a subset of 94 studies that presented quantitative information regarding predation on domestic animals was used. Then, the predation ratio on domestic animals (obtained as the number of animals lost, percentage of the stock preyed or predation rate) was calculated under different ecological/environmental conditions: native prey density (high/low), vegetation cover (dense/open), season (dry/wet), and distances from forest, protected areas, and human settlements (far/near). Due to the fact that vulnerability to predation may vary according to the body size of the prey (Knarrum et al., 2006) and light availability (Kavanau and Ramos, 1975), and both conditions can be managed by livestock producers, the ratio of predation on domestic animals of different age (young/adult) and the time of day when a predation event occurred (day/night) were also calculated. Other variables that may affect predation such as predator abundance, elevation, distance to roads, distance to water courses and slope (e.g., Miller, 2015) could not be assessed due to small sample sizes. The analyses were performed in two steps. First, the effect of above conditions on the variation of livestock predation was assessed by accounting for the entire suite of carnivores reported in the selected studies. Second, the effect on large and meso carnivores was assessed separately by following the body-size criteria described above. For those conditions in which it was not possible to separate the effect on predation by large or meso carnivores, we only reported the effect using the complete diversity of carnivores. For all ratio analyses, 0.1 was added to every value and l was applied to standardized ratios. A one sample t-test (Zar, 1974) was performed, implemented in R package to check if the average of the predation ratio under a particular ecological factor was different from 0 (i.e., no change in predation).
Results
After reviewing 868 scientific publications from 1992 to 2019 that met the inclusion criteria, 391 publications dealing with carnivore-livestock conflict were considered (information available upon request). Because some studies involved more than one species, the total number of carnivore-livestock conflicts reached 783 study cases. Publications involving a single carnivore were more frequent (n = 211) than those containing multiple carnivores (2–10 species, n = 171). Nine publications could not identify the predator species (e.g., Marker et al., 2005). Geographically, the research was conducted in Asia (30.6%), Africa (30.1 %), Europe (18.2%), North America (12.2%), South America (6.3%), Central America (1.8%), and Oceania (0.8%).
A total of 23 species of domestic animals were reported to have been preyed upon by carnivores. Considering that publications also reported more than one domestic prey, cattle was reported in 59.8%, sheep (Ovis spp.) and domestic goat (Capra spp.) in 54.5 and 46.3%, respectively. A smaller proportion of publications reported horses (Equus caballus) (21.2%), donkey (Equus africanus) (15.3%), poultry (11.3%), domestic dog (Canis lupus familiaris) (10.5%), pork (Sus scrofa) (8.7%), and yaks (Bos grunniens) (6.9%).
The number of carnivore species reported to prey on domestic animals (N = 52; 22 and 30 large and meso carnivores, respectively; mean body size = 11.5 kg) has increased over time, particularly after 2013 (Figure 1). The distribution of carnivore-livestock research per species differed from that expected according to their general occurrence in the research literature (G–test of goodness-of-fit with Bonferroni corrections, d.f. = 51, P < 0.001), whereas mesocarnivore species were reported less often than expected according to species richness (d.f. = 1, P < 0.001; Figure 2). Felidae and Canidae were the most frequently reported groups (51.3 and 28.2%, respectively), followed by Hyaenidae (9.2%), Ursidae (8.2%), Musteliade (1.5%), Viverridae (0.9%), Eupleridae (0.4%), Mephitidae (0.1%), and Procyonidae (0.1%). Species more frequently covered by scientific literature focusing on livestock-carnivore conflicts were wolf (13.4%), leopard (12.1%), lion (8.7%), spotted hyenna (Crocuta crocuta) (6.6%), tiger (5.7%), brown bear (4.9%), cheetah (4.7%), and Eurasian lynx (Lynx lynx) (4.1%), which were reported in 64.8% of study cases.
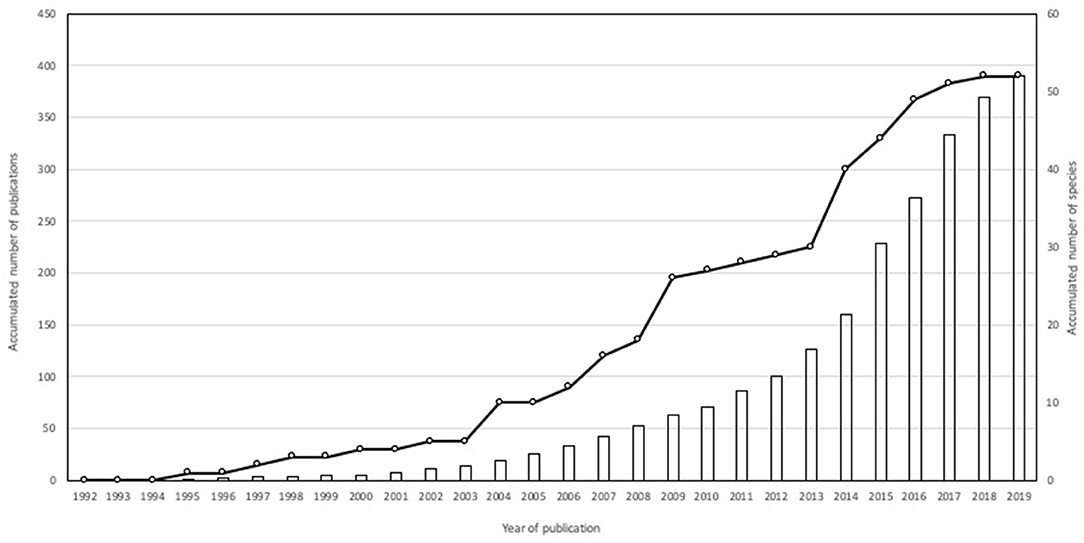
Figure 1. Accumulated number of publications (bar) and carnivores reported (circle) in the reviewed sample between 1992 and 2019 (N = 391).
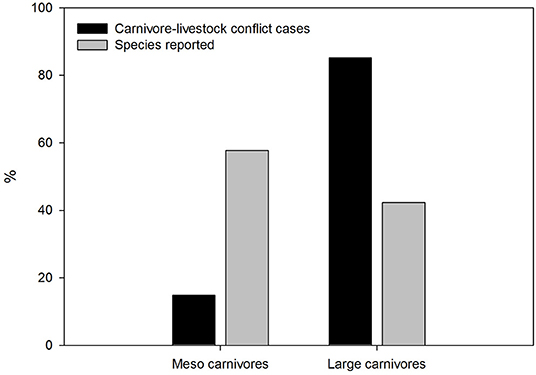
Figure 2. Percentage of large and meso carnivore species reported in the carnivore-livestock conflict literature (gray bar; N = 393), and reported frequency in canivore-livestock conflict cases (black bar; N = 783).
Carnivores reported in conflict-related studies covered a wide array of body sizes and ranges of movement (N = 52): body mass ranged from 2.7 to 196.5 kg (median = 20.9 kg) and home range varied between 0.2 and 395.9 km2 (median: 11.4 km2) (Figure 3). Carnivores more frequently reported occupy a wide variety of habitats (min = 1, max = 9, median = 5) and consume few food items (min = 1, max = 6, median = 1) (Figure 3). They also exhibited larger home ranges (Spearman's rank correlation coefficient, rho = 0.6, p < 0.01) and body masses (rho = 0.8, p < 0.01), and showed a larger reduction in their geographic range of distribution (rho = 0.5, p < 0.01). Data on the carnivores reported in the conflict-related literature also showed a positive association between their body masses and their geographical range decline (Pearson correlation coefficient, rs = 0.75, p << 0.01). Crepuscular or cathemeral carnivores were mostly reported in carnivore-livestock literature (61.5% of study cases), followed by nocturnal (26.9%) and diurnal (11.6%) species, whereas more than half of study cases (60.1%) involved solitary carnivores.
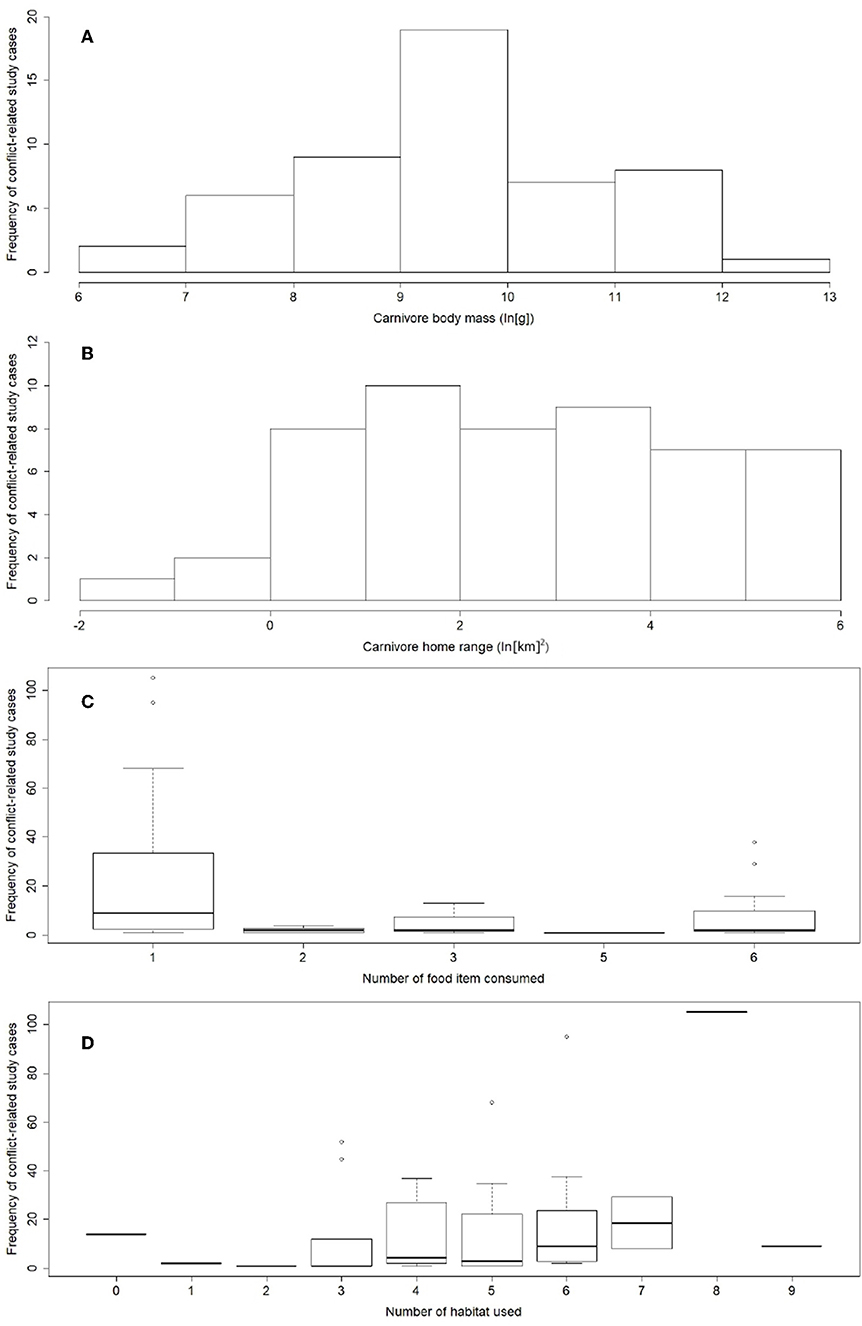
Figure 3. Ecological diversity of carnivores reported in the reviewed carnivore-livestock conflict publications. (A) Predators spanning a wide range of body sizes were reported. (B) Home range sizes of species studied were diverse. (C) Conflict-related research effort focused on carnivores using a diverse range of habitats and (D) showing a narrow dietary breadth. Trait data were taken from a sample of 52 species, and reported frequency from 783 study cases (information available upon request).
A total of 94 papers included quantitative information of predation ratio under different conditions (ecological/environmental conditions and management practices), completing 221 study cases of predation (i.e., an event of predation on individuals of domestic animal species by a particular carnivore). Predation on domestic animals increased with vegetation cover (t = 2.31, p < 0.03) and decreased with distance from human settlement (t = 4.13, p < 0.01). Predation was not related to distance to forests (t = −0.1, p > 0.05), distance to protected areas (t = −0.45, p > 0.05) or density of native prey (p = 0.29, p > 0.05). Predation on domestic animals occurred similarly in wet and dry seasons (t = 0.06, p > 0.05) and during day or night (t = 1.23, p > 0.05). Finally, young animals were preyed upon more often than adults (t = −2.38, p = 0.02) (Figure 4). The disaggregated analysis by carnivore groups showed the predation on domestic animals by large carnivores increased near protected areas (t = −2.54, p < 0.03) and away from human settlements (t = 4.0, p < 0.05), and was higher on young animals (t = −2.65, p < 0.02) (Figure 4). No effects were found for landscape attributes, management practices or environmental conditions on predation by meso carnivores (all p > 0.05).
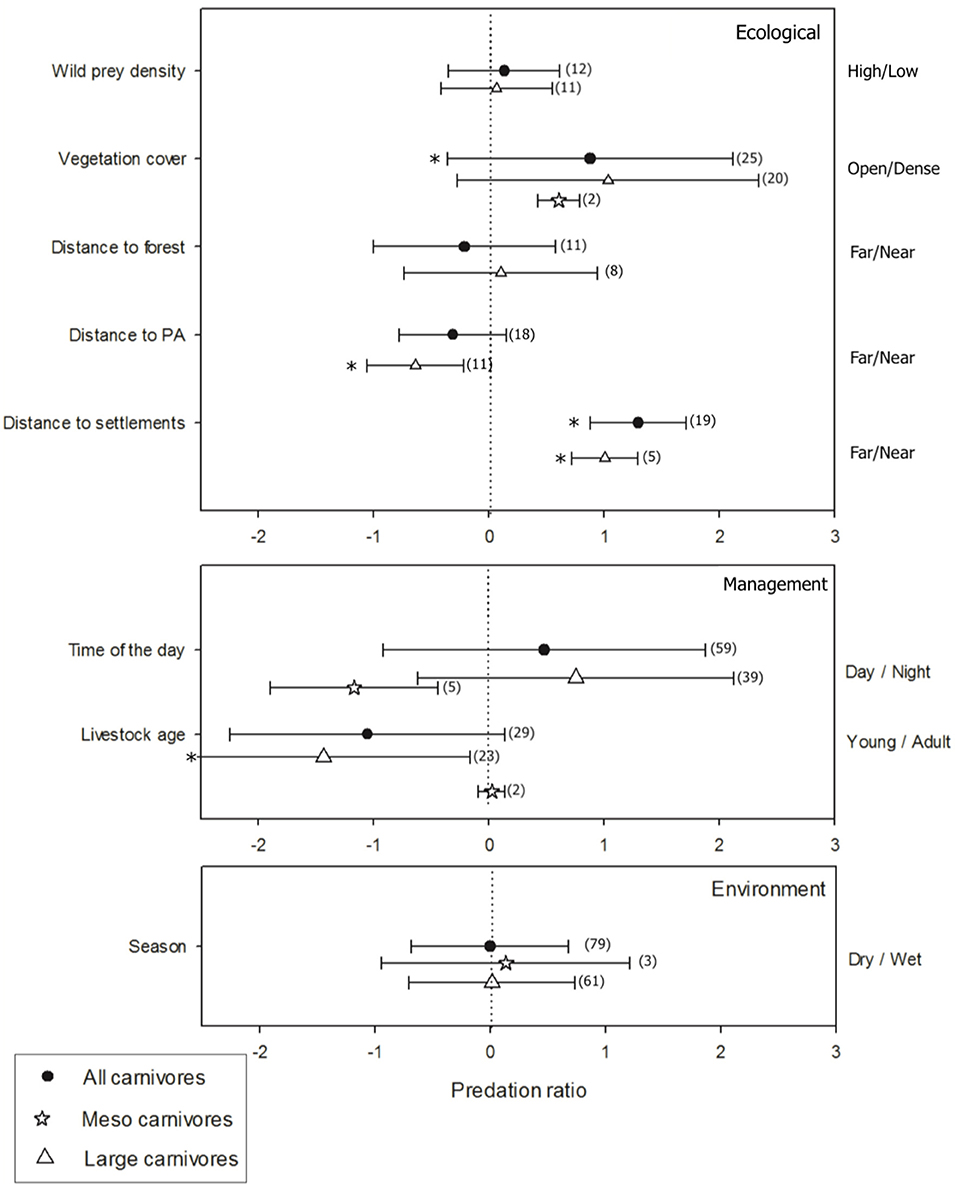
Figure 4. Predation ratio (ln) as a response to ecological conditions: wild prey abundance (high/low), vegetation cover (dense/open), and distance to forest patch, protected area and human settlement (far/near); environmental conditions: season (dry/wet); and management practices: livestock age (young/adults) and moment of the day (day/ night). The number of study cases considered for each ecological/environmental condition and management practice is shown in parentheses. *Denotes significant effect at p < 0.05.
Discussion
Carnivores-livestock conflict is a worldwide and increasing phenomenon that needs to be tackled, considering that 30% of terrestrial carnivores are threatened by retaliation (IUCN, 2016). Although the conflict is a by-product of the socio-economic and political landscapes upon which livestock is raised, ecological information is also required to undertake evidence-based management (e.g., Graham et al., 2005).
Our findings show that research effort on carnivore-livestock conflict exhibits a wide geographic, taxonomic and ecological variation. Near 60% percent of conflict-related studies were conducted in Asian and African countries (see also Van Eeden et al., 2017). Furthermore, ca. 80% of conflict-related cases were focused on large carnivores such as wolves and brown bears (Ursus arctos; widespread in Europe, North America, and Asia), leopards, lions and spotted hyenas (widespread Africa and Asia), followed by tigers, cheetahs (Asia and Africa) and Eurasian lynx (Europe and Asia). In contrast, meso carnivores (<21.5 kg) were largely under-represented, yet they accounted for 67% of species richness reported in the carnivore-livestock conflict. Ecological attributes of terrestrial carnivore reported in the conflict literature showed that research effort focused on habitat generalists, solitary hunters, strictly dietary, and cathemeral species. The limited sample size of carnivores reported in the conflict literature along with the low variation of study cases among species prevented the use of predictive approaches to test these associations. The relationship of some ecological and life-history traits with well-studied carnivores have been previously documented and reflect the human perspectives in research attention toward charismatic and abundant species, accessibility to research locations where these species occur, and species with funding opportunities (Brooke et al., 2014). Accordingly, the descriptive approach used in this study provides valuable insights on the potential effect of species trait to explain the differential research effort in the carnivore-livestock literature.
The over-representation of some species and regions in carnivore-livestock conflict studies may not only prevent drawing general conclusions regarding how widespread current conflict is according to across ecosystems, but also might conceal the relevance of ecological attributes in determining whether some species are “conflict-prone.” As more habitat is encroached, more likely large predators will be extirpated (Crooks et al., 2011; Winterbach et al., 2013; Ripple et al., 2014) and new species with restricted distributions, smaller home ranges or ecological opportunism will roam into human-dominated landscapes (Prugh et al., 2009), increasing the probability to encounter with, and prey upon domestic animals. Since the effectiveness of management techniques aimed to reduce predation on domestic animals vary according to the predator body size (Moreira-Arce et al., 2018), policies, regulations, and evidence-based management strategies based on large carnivores only will be ineffective in production-oriented landscapes where species involved are small-bodied predators.
Besides the bias toward large-bodied and conspicuous species, it should be noted that large carnivores are a experiencing significant replacement of their native habitats for agricultural and livestock raising, particularly in sub-Saharan Africa, and southeastern and northern Asia (e.g., Ellis et al., 2010; Crooks et al., 2011; this study). The size of the geographical range is a predictor of extinction risk in large mammal species (Cardillo et al., 2005) and larger body sized species demand larger home ranges that frequently extend beyond natural habitat borders into livestock-raising areas, where mortality increases due to retaliation (Woodroffe and Ginsberg, 1998; Inskip and Zimmermann, 2009). On the other hand, the ecological traits of species such as hunting habits, habitat, and feeding behaviors might predispose carnivores to use novel habitats such as livestock-raising lands when food become scarce in wild habitats, increasing their probability and success of preying on domestic animals (Gittleman, 1989; Inskip and Zimmermann, 2009; Cozzi et al., 2012; Sol et al., 2013). For instance, solitary and elusive species such as jaguar, puma and tiger mostly retreat to natural areas and away from human activity for hunting (Kissling et al., 2009; Zarco-González et al., 2013; Miller et al., 2015). On the contrary, social and active roaming hunters such as wolves are effective predators in flat and open areas (e.g., Behdarvand et al., 2014). Although current evidence is still insufficient and biased toward some species to assess what carnivores' attributes (or combination of them) would determine whether species are “conflict-prone,” the role of ecological traits in the carnivore-livestock conflict should not be underestimated.
Some ecological conditions and management practices do consistently affect the likelihood of predation in grazing areas. Thus, the analyses of the present study revealed that this may have important consequences when managing the conflict with large or meso carnivores. Dense vegetation coverage steadily incremented predation upon domestic animals. High rates of domestic animal predation in places containing dense vegetation have been previously reported with felids such as jaguars (Panthera onca), pumas (Puma puma) (Sunquist and Sunquist, 1989), Eurasian lynx (Stahl et al., 2002), leopard, jackal (C. mesomelas), Caracal (Caracal caracal) (Thorn et al., 2013) tiger (Miller et al., 2015). Dense vegetation provides stalking cover for these ambush predators (Sunquist and Sunquist, 1989). On the other hand, consistent evidence was found for the effects of distance to protected areas and human settlements on livestock predation by large carnivores only. The proximity to a protected area has been associated with an increased predation of livestock by leopard and spotted hyena (Gusset et al., 2009), tiger (Karanth et al., 2013), and lion (Van Bommel et al., 2007), however, with a decrease of predation by wolf (Behdarvand et al., 2014). Similarly, carnivores such as tigers are more likely to kill livestock farther from roads and villages in China (Soh et al., 2014) and India (Miller et al., 2015), but the proximity to towns and villages was an important factor that shaped the predation risk by wolves in Iran (Behdarvand et al., 2014; see for more details in Miller, 2015). Contrary to previous considerations, an effect of wild prey density on predation was not empirically supported. Although density of carnivores in most natural areas is strongly correlated to prey biomass (Carbone and Gittleman, 2002), limited available literature in production-oriented lands show that prey can be positively or negatively correlated to predation rates (Miller, 2015 and this study). The definition of prey availability (prey density x prey accessibility) and the spatial extent at which the availability is quantified may hide the effect of this variable on livestock predation (Fuller et al., 2007; Keim et al., 2011; Gorini et al., 2012). Our findings also suggest that livestock age needs to be considered when preventive measures are employed to reduce animal predation. For instance, the use of measures such as enclosures to protect animals after calving season may be a feasible solution to reduce susceptibility to predation on young animals by large carnivores (Moreira-Arce et al., 2018).
Although with the available information no differences in the predation ratio were found between dry and wet seasons, weather plays a significant role on structuring predator-prey system throughout primary productivity, particularly in subtropical dry ecosystem (Hatton et al., 2015), and may have consequences on domestic animal predation. In these biomes wet-season migration of herbivorous such as ungulates triggers movements of their predators from protected areas onto community village lands leading to an increment of domestic animal predation (Kissui, 2008). Wet season also matches with calving season when cattle calves are more vulnerable to predation, as shown by studies conducted on jaguar and puma in Sonora region (Rosas-Rosas et al., 2008). However, during dry season, riparian habitats adjacent to water sources can also concentrate higher density of livestock increasing the vulnerability to predation (Rosas-Rosas et al., 2008). Additional research is clearly needed to determine whether predators show consistent seasonal preferences for wildlife prey vs. livestock. Although based on a small quantitative sample of conflict-related studies, these findings suggest that landscape attributes and environmental information can be used to reduce livestock predation complementing non-lethal techniques used at finer scale (Moreira-Arce et al., 2018).
Management Implications
Carnivore-livestock conflict resolution needs to move toward evidence-based policy and practice. The evidence should rely on studies of predation based on evaluations of effect and causality of ecological attributes and containing relevant databases. This evaluation has to be founded in unbiased research efforts in order to deal with knowledge gaps on species and ecosystems. We encourage wildlife managers to partner with livestock producers to take advantage of landscape heterogeneity of production-oriented lands to assess the effect of landscape and habitat configuration on herds vulnerability. Expanding the knowledge toward less-studied predators including meso carnivores and recently altered ecosystems will strengthen public policies and practices to better manage the diversity of context where carnivore-livestock conflict occurs.
Author Contributions
DM-A and JS conceived and designed the review. CU and DM-A performed the data collection and analyzed the data. DM-A, CU, and JS wrote the paper.
Funding
This work was supported by CONICYT FONDECYT/Postdoctoral Grant No. 3160056 and FONDECYT grant 11181180, and Asociación Kauyeken.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful for the comments by the editor V. Penteriani and the two reviewers who helped to improve this manuscript. We also thank Silvio Crespin for English proofreading.
References
Abade, L., MacDonald, D. W., and Dickman, A. J. (2014). Assessing the relative importance of landscape and husbandry factors in determining large carnivore depredation risk in Tanzania's Ruaha landscape. Biol. Conserv. 180, 241–248. doi: 10.1016/j.biocon.2014.10.005
Baker, P. J., Boitani, L., Harris, S., Saunders, G. R., and White, P. C. L. (2008). Terrestrial carnivores and human food production. Mamm. Rev. 38, 123–166. doi: 10.1111/j.1365-2907.2008.00122.x
Balme, G. A., Slotow, R., and Hunter, L. T. B. (2010). Edge effects and the impact of non-protected areas in carnivore conservation: leopards in the Phinda-Mkhuze Complex, South Africa. Anim. Conserv. 13, 315–323. doi: 10.1111/j.1469-1795.2009.00342.x
Behdarvand, N., Kaboli, M., Ahmadi, M., Nourani, E., Mahini, A. S., and Aghbolaghi, M. A. (2014). Spatial risk model and mitigation implications for wolf–human conflict in a highly modified agroecosystem in western Iran. Biol. Conserv. 177, 156–164. doi: 10.1016/j.biocon.2014.06.024
Boyce, M. S. (2006). Scale for resource selection functions. Divers. Distrib. 12, 269–276. doi: 10.1111/j.1366-9516.2006.00243.x
Brooke, Z. M., Bielby, J., Nambiar, K., and Carbone, C. (2014). Correlates of research effort in carnivores: body size, range size and diet matter. PLoS ONE 9:e93195. doi: 10.1371/journal.pone.0093195
Brown, J. S., Laundre, J. W., and Gurung, M. (1999). The ecology of fear: optimal foraging, game theory, and trophic interactions. J. Mammal. 80, 385–399. doi: 10.2307/1383287
Carbone, C., and Gittleman, J. L. (2002). A common rule for the scaling of carnivore density. Science 295, 2273–2276. doi: 10.1126/science.1067994
Carbone, C., Mace, G. M., Roberts, S. C., and Macdonald, D. W. (1999). Energetic constraints on the diet of terrestrial carnivores. Nature 402:286. doi: 10.1038/46266
Cardillo, M., Mace, G. M., Jones, K. E., Bielby, J., Bininda-Emonds, O. R., Sechrest, W., et al. (2005). Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241. doi: 10.1126/science.1116030
Cozzi, G., Broekhuis, F., Mcnutt, J. W., Turnbull, L. A., Cozzi, G., Broekhuis, F., et al. (2012). Fear of the dark or dinner by moonlight? Reduced temporal partitioning among Africa' s large carnivores. Ecology 93, 2590–2599. doi: 10.1890/12-0017.1
Crooks, K. R., Burdett, C. L., Theobald, D. M., Rondinini, C., and Boitani, L. (2011). Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 2642–2651. doi: 10.1098/rstb.2011.0120
Dickman, A. J. (2010). Complexities of conflict: the importance of considering social factors for effectively resolving human-wildlife conflict. Anim. Conserv. 13, 458–466. doi: 10.1111/j.1469-1795.2010.00368.x
Eklund, A., López-Bao, J. V., Tourani, M., Chapron, G., and Frank, J. (2017). Limited evidence on the effectiveness of interventions to reduce livestock predation by large carnivores. Sci. Rep. 7:2097. doi: 10.1038/s41598-017-02323-w
Ellis, E. C., Klein Goldewijk, K., Siebert, S., Lightman, D., and Ramankutty, N. (2010). Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 19, 589–606. doi: 10.1111/j.1466-8238.2010.00540.x
Fuller, A. K., Harrison, D. J., and Vashon, J. H. (2007). Winter habitat selection by Canada lynx in Maine: prey abundance or accessibility? J. Wildl. Manage. 71, 1980–1986. doi: 10.2193/2006-288
Gittleman, J. L. (1989). Carnivore Behavior, Ecology, and Evolution. London: Cornell University Press. doi: 10.1007/978-1-4613-0855-3
Gonzalez, A., Novaro, A., Funes, M., Pailacura, O., Bolgeri, M. J., and Walker, S. (2012). Mixed-breed guarding dogs reduce conflict between goat herders and native carnivores in Patagonia. Hum. Wildl. Interact. 6, 327–334. doi: 10.26077/2agv-bw33
Gorini, L., Linnell, J. D. C., May, R., Panzacchi, M., Boitani, L., Odden, M., et al. (2012). Habitat heterogeneity and mammalian predator-prey interactions. Mamm. Rev. 42, 55–77. doi: 10.1111/j.1365-2907.2011.00189.x
Graham, K., Beckerman, A. P., and Thirgood, S. (2005). Human-predator-prey conflicts: ecological correlates, prey losses and patterns of management. Biol. Conserv. 122, 159–171. doi: 10.1016/j.biocon.2004.06.006
Gusset, M., Swarner, M. J., Mponwane, L., Keletile, K., and McNutt, J. W. (2009). Human–wildlife conflict in northern Botswana: livestock predation by endangered African wild dog Lycaon pictus and other carnivores. Oryx 43, 67–72. doi: 10.1017/S0030605308990475
Hatton, I. A., McCann, K. S., Fryxell, J. M., Davies, T. J., Smerlak, M., Sinclair, A. R., et al. (2015). The predator-prey power law: Biomass scaling across terrestrial and aquatic biomes. Science 349:aac6284. doi: 10.1126/science.aac6284
Inskip, C., and Zimmermann, A. (2009). Human-felid conflict: a review of patterns and priorities worldwide. Oryx 43, 18–34. doi: 10.1017/S003060530899030X
IUCN (2016). The IUCN Red List of Threatened Species. Version 2016.4. Available online at: http://www.iucnredlist.org. Downloaded on October 26, 2016
Jones, K. E., Bielby, J., Cardillo, M., Fritz, S. A., O'Dell, J., Orme, C. D. L., et al. (2009). PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648–2648. doi: 10.1890/08-1494.1
Karanth, K. K., Naughton-Treves, L., DeFries, R., and Gopalaswamy, A. M. (2013). Living with wildlife and mitigating conflicts around three Indian protected areas. Environ. Manage. 52, 1320–1332. doi: 10.1007/s00267-013-0162-1
Kavanau, J. L., and Ramos, J. (1975). Influences of light on activity and phasing of carnivores. Am. Nat. 109, 391–418. doi: 10.1086/283009
Keim, J. L., Dewitt, P. D., and Lele, S. R. (2011). Predators choose prey over prey habitats: evidence from a lynx - Hare system. Ecol. Appl. 21, 1011–1016. doi: 10.1890/10-0949.1
Kissling, W. D., Fernández, N., and Paruelo, J. M. (2009). Spatial risk assessment of livestock exposure to pumas in Patagonia, Argentina. Ecography 32, 807–817. doi: 10.1111/j.1600-0587.2009.05781.x
Kissui, B. M. (2008). Livestock predation by lions, leopards, spotted hyenas, and their vulnerability to retaliatory killing in the Maasai steppe, Tanzania. Anim. Conserv. 11, 422–432. doi: 10.1111/j.1469-1795.2008.00199.x
Kleiman, D. G., and Eisenberg, J. F. (1973). Comparisons of canid and felid social systems from an evolutionary perspective. Anim. Behav. 21, 637–659. doi: 10.1016/S0003-3472(73)80088-0
Knarrum, V., Sørensen, O. J., Eggen, T., Kvam, T., Opseth, O., Overskaug, K., et al. (2006). Brown bear predation on domestic sheep in central Norway. Ursus 17, 67–75. doi: 10.2192/1537-6176(2006)17[67:BBPODS]2.0.CO;2
Kruuk, H. (1972). The spotted hyena; a study of predation and social behavior. Wildl. Behav. Ecol. 42:335. doi: 10.2307/3145
Loveridge, A., Wang, S. W., Frank, L., and Seidensticker, J. (2010). “People and wild felids: conservation of cats and management of conflicts,” in Biology and Conservation of Wild Felids, eds D. W. Macdonald and J. A. Loveridge (New York, NY: Oxford University Press, 161–195.
Lozano, J., Olszanska, A., Morales-Reyes, Z., Castro, A. A., Malo, A. F., Moleón, M., et al. (2019). Human-carnivore relations: a systematic review. Biol. Conserv. 237, 480–492. doi: 10.1016/j.biocon.2019.07.002
Marker, L. L., Dickman, A. J., and Macdonald, D. W. (2005). Perceived effectiveness of livestock-guarding dogs placed on namibian farms. Rangeland Ecol. Manage. 58, 329–336. doi: 10.2111/1551-5028(2005)058[0329:PEOLDP]2.0.CO;2
Miller, J. R., Jhala, Y. V., Jena, J., and Schmitz, O. J. (2015). Landscape-scale accessibility of livestock to tigers: implications of spatial grain for modeling predation risk to mitigate human–carnivore conflict. Ecol. Evol. 5, 1354–1367. doi: 10.1002/ece3.1440
Miller, J. R. B. (2015). Mapping attack hotspots to mitigate human–carnivore conflict: approaches and applications of spatial predation risk modeling. Biodivers. Conserv. 24, 2887–2911. doi: 10.1007/s10531-015-0993-6
Miller, J. R. B., Stoner, K. J., Cejtin, M. R., Meyer, T. K., Middleton, A. D., and Schmitz, O. J. (2016). Effectiveness of contemporary techniques for reducing livestock depredations by large carnivores. Wildl. Soc. Bull. 40, 806–815. doi: 10.1002/wsb.720
Minnie, L., Boshoff, A. F., and Herley, G. I. H. (2015). Vegetation type influences livestock predation by leopards: implications for conservation in agro-ecosystems. Afr. J. Wildl. Res. 45, 204–214. doi: 10.3957/056.045.0204
Moreira-Arce, D., Ugarte, C. S., Zorondo-Rodríguez, F., and Simonetti, J. A. (2018). Management tools to reduce carnivore-livestock conflicts: current gap and future challenges. Rangeland Ecol. Manage. 71, 389–394. doi: 10.1016/j.rama.2018.02.005
Palmeira, F. B. L., Crawshaw, P. G., Haddad, C. M., Ferraz, K. M. P. M. B., and Verdade, L. M. (2008). Cattle depredation by puma (Puma concolor) and jaguar (Panthera onca) in central-western Brazil. Biol. Conserv. 141, 118–125. doi: 10.1016/j.biocon.2007.09.015
Prugh, L. R., Stoner, C. J., Epps, C. W., Bean, W. T., Ripple, W. J., Laliberte, A. S., et al. (2009). The rise of the mesopredator. Bioscience 59, 779–791. doi: 10.1525/bio.2009.59.9.9
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world's largest carnivores. Science 343:1241484. doi: 10.1126/science.1241484
Rosas-Rosas, O. C., Bender, L. C., and Valdez, R. (2008). Jaguar and puma predation on cattle calves in northeastern Sonora, Mexico. Rangeland Ecol. Manage. 61, 554–560. doi: 10.2111/08-038.1
Schooley, R. L., and Branch, L. C. (2007). Spatial heterogeneity in habitat quality and cross-scale interactions in metapopulations. Ecosystems 10, 846–853. doi: 10.1007/s10021-007-9062-7
Smith, J. B., Nielsen, C. K., and Hellgren, E. C. (2014). Illinois resident attitudes toward recolonizing large carnivores. J. Wildl. Manage. 78, 930–943. doi: 10.1002/jwmg.718
Soh, Y. H., Carrasco, L. R., Miquelle, D. G, Jiang, J., Yang, J., Stokes, E. J., et al. (2014). Spatial correlates of livestock depredation by Amur tigers in Hunchun, China: relevance of prey density and implications for protected area management. Biol. Conserv. 169, 117–127. doi: 10.1016/j.biocon.2013.10.011
Sol, D., Lapiedra, O., and González-Lagos, C. (2013). Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Soto-Shoender, J. R., and Main, M. B. (2013). Differences in stakeholder perceptions of the jaguar Panthera onca and puma Puma concolor in the tropical lowlands of Guatemala. Oryx 47, 109–112. doi: 10.1017/S003060531200107X
Stahl, P., Vandel, J. M., Ruette, S., Coat, L., Coat, Y., and Balestra, L. (2002). Factors affecting lynx predation on sheep in the French Jura. J. Appl. Ecol. 39, 204–216. doi: 10.1046/j.1365-2664.2002.00709.x
Sunquist, N., and Sunquist, F. (1989). “Ecological constraints on predation by large felids,” in Carnivore Behavior, Ecology and Evolution, ed J. L. Gittleman (Boston, MA: Cornell University Press), 283–301. doi: 10.1007/978-1-4757-4716-4_11
Thorn, M., Green, M., Scott, D., and Marnewick, K. (2013). Characteristics and determinants of human-carnivore conflict in South African farmland. Biodivers. Conserv. 22, 1715–1730. doi: 10.1007/s10531-013-0508-2
Treves, A., and Karanth, K. U. (2003). Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv. Biol. 17, 1491–1499. doi: 10.1111/j.1523-1739.2003.00059.x
Treves, A., Martin, K. A., Wydeven, A. P., and Wiedenhoeft, J. E. (2011). Forecasting environmental hazards and the application of risk maps to predator attacks on livestock. Bioscience 61, 451–458. doi: 10.1525/bio.2011.61.6.7
Van Bommel, L. B. D. V., Bij de Vaate, M. D., De Boer, W. F., and De Iongh, H. H. (2007). Abstract. Afr. J. Ecol.45, 490–498. doi: 10.1111/j.1365-2028.2007.00759.x
Van Eeden, L. M., Crowther, M. S., Dickman, C. R., MacDonald, D. W., Ripple, W. J., Ritchie, E. G., et al. (2017). Managing conflict between large carnivores and livestock. Conserv. Biol. 32, 26–34. doi: 10.1111/cobi.12959
Wilson, D. E., and Mittermeier, R. A. (2009). Handbook of the Mammals of the World, Vol. 1. Carnivores. Barcelona: Lynx Edicions.
Winterbach, H. E. K., Winterbach, C. W., Somers, M. J., and Hayward, M. W. (2013). Key factors and related principles in the conservation of large African carnivores. Mamm. Rev. 43, 89–110. doi: 10.1111/j.1365-2907.2011.00209.x
Woodroffe, R., and Frank, L. G. (2005). Lethal control of African lions (Panthera leo): local and regional population impacts. Anim. Conserv. 8, 91–98. doi: 10.1017/S1367943004001829
Woodroffe, R., and Ginsberg, J. R. (1998). Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128. doi: 10.1126/science.280.5372.2126
Keywords: carnivore management, human–wildlife conflicts, landscapes attributes, livestock predation, production-oriented lands
Citation: Ugarte CS, Moreira-Arce D and Simonetti JA (2019) Ecological Attributes of Carnivore-Livestock Conflict. Front. Ecol. Evol. 7:433. doi: 10.3389/fevo.2019.00433
Received: 26 April 2019; Accepted: 23 October 2019;
Published: 14 November 2019.
Edited by:
Vincenzo Penteriani, Spanish National Research Council (CSIC), SpainReviewed by:
Fredrik Dalerum, University of Oviedo, SpainMaria Delgado, University of Oviedo, Spain
Giulia Bombieri, University of Oviedo, Spain, in collaboration with reviewer MD
Copyright © 2019 Ugarte, Moreira-Arce and Simonetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darío Moreira-Arce, bW9yZWlyYS5kYXJpb0BnbWFpbC5jb20=
 Carolina S. Ugarte
Carolina S. Ugarte Darío Moreira-Arce
Darío Moreira-Arce Javier A. Simonetti
Javier A. Simonetti