- 1Department of Entomology, University of Georgia, Athens, GA, United States
- 2Department of Entomology, Rutgers University, New Brunswick, NJ, United States
A diverse diet in polyphagous insects satisfies changing nutritional needs but the choice of host plant may vary throughout the insect and plant life cycle. The behaviors associated with host choice in the immature stages may differ from the egg laying site chosen by the mother. To evaluate this for an important agricultural pest, we looked at host choice over two growing seasons for the invasive Halyomorpha halys. H. halys has a host breadth of over 170 known species in its invaded range and adults can satisfy nutritional needs through a strong dispersal capacity. Nymphs are more limited in their ability to choose host plants and we investigated if they make a choice that differs from the source plant (to simulate maternal choice) and characterized volatile organic compounds that are present during attraction. In a mark-release-recapture experiment we quantified dispersal and host choice by nymphs to four common vegetable hosts throughout the growing season. Applying an attraction index to quantify host choice we identified that nymphs switch host plants depending on host phenology. Plants with maturing fruits were most attractive. Volatile organic compounds were collected from host plants during the same time period. Multivariate and correlation analyses categorized phenol, undecane, decanal, and caryophyllene as compounds associated with host plants during peak attractive periods. Thus, the availability of suitable food and associated olfactory cues appears to be influencing the spatiotemporal distribution of H. halys within the agroecosystem. Exploiting dispersal behavior and olfactory cues may be used to help increase the effectiveness and efficiency of current management practices for this severe and widespread pest.
Introduction
Organisms make many “decisions” during their life to maximize reproductive success, which often are at the cost of compromises. Such decisions may encompass how much to invest in growth relative to defense, mate attraction, spatiotemporal reproduction choices, and whether to disperse in search of resources or to hide from endangerment. Most herbivorous arthropods are considered “specialists” because they specialize in obtaining resources from a narrow range of plant species (Strong et al., 1984). For these specialists, appropriate host selection is essential and chemical cues from host plants may provide the information in order to make such host selection decisions (Dicke, 2000). Herbivore host-choice decisions are largely determined by the mothers, which often show egg laying or ovipositional preference for host plants that may not be the best for their offspring (Mayhew, 1997), essentially making them appear to have less maternal investment (Mayhew, 2001). Scheirs et al. (2000) suggest that insect mothers can select oviposition sites that improve their own fitness at the expense of their offspring. For non-specialist herbivores, polyphagy can provide optimal nutrients required for growth and reproduction as well as predator evasion. These generalist feeders do not have to allocate physiological mechanisms for constitutive host defenses and can invest energy into dispersal mechanisms to meet their physiological requirements (Kant et al., 2015).
Adult stink bugs have been shown to oviposit on unsuitable hosts, seemingly anticipating increased suitability as the host develops (Kiritani et al., 1965). Phenological development stages of hosts can have a significant impact on stink bug nymph survivorship, development time, and subsequent adult survivorship (Panizzi and Alves, 1993). In the lab, when the diet of stink bug nymphs is switched from a poor host to a preferred host, reproductive performance and growth of adults is improved (Panizzi and Saraiva, 1993). Polyphagous behavior in herbivores does not mean that they have no particular preference in host plant selection, but rather that they have complex preferences for certain hosts at ideal growth stages for specific resources, and these preferences can change throughout the life cycle of the insect and the desired hosts (Kiritani et al., 1965) as nutritional needs change (Skillman et al., 2018).
Many species of stink bugs are generalist feeders, most with over 100 host plant species and several prefer to feed on reproductive tissues (Lye and Story, 1988; Siebert et al., 2005; Rice et al., 2014). A changing approach to host plant acceptance throughout a growing season would allow the selection of appropriate dietary needs. The survivorship and developmental duration of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) nymphs are significantly affected by host plant species, phenology, and mixture of diet, with mixed diets having the highest survivorship (Acebes-Doria et al., 2016). Surveys of H. halys on wild and cultivated woody ornamentals and soybean found higher population densities during growth stages with maturing seeds/fruits suggesting that H. halys tracks host plant phenology (Nielsen and Hamilton, 2009; Martinson et al., 2015). Similar behavior occurs in cultivated fruit trees where populations have been observed to follow peach maturity (Hahn et al., 2017).
Halyomorpha halys is an invasive stink bug species from Asia that has caused widespread economic losses in agricultural crops, particularly in the mid-Atlantic region of the U.S.A. This insect has also invaded Canada, Europe, Russia, and Chile, raising its status to that of a global invasive pest (Leskey and Nielsen, 2018). Kuhar et al. (2012) assessed the relative pest risk of H. halys to vegetable crops and determined that sweet corn, beans, bell peppers, tomatoes, eggplant, and okra are highly susceptible to feeding injury by H. halys (Kuhar et al., 2012).
Behavioral and physiological characteristics associated with host plant selection, and subsequent injury to crops, include ability to detoxify plant allelochemicals, ability to penetrate fruiting structures on which they prefer to feed, and ability to move to make that selection. Much of the work on movement of H. halys has naturally focused on the adult stage. In the laboratory, H. halys has demonstrated a strong flight capacity, which can explain its ability for long-distance dispersal in the landscape (Lee and Leskey, 2015; Wiman et al., 2015). Less is known about nymph dispersal via walking or climbing. A nymph's ability to disperse and select a host could potentially counteract a mother's poor host selection (Mayhew, 2001). While they demonstrate a strong capacity for dispersal in response to an olfactory cue, specifically the aggregation pheromone (Lee et al., 2014), the locomotion response of the immature stages to host plant stimuli is unknown.
Understanding how host plant stimuli impact nymph dispersal and colonization of host plants can guide management plans. Our objectives were to (1) identify host plant choice of H. halys nymphs between selected crop species and (2) identify stimuli associated with host plant attractiveness. Through performing the “mother” stink bug duty, we conducted a mark-release-recapture experiment to examine dispersal patterns of H. halys nymphs among four representative host plants; Swiss chard, bell pepper, sweet corn, and soybean. Dispersal was measured at various times throughout the growing season, following progressive phenological stages. In order to investigate the olfactory host cues that may stimulate H. halys nymphs to disperse from one host to find another, we measured the volatile organic compounds from the same four potential host plants: Swiss chard, bell pepper, sweet corn, and soybean.
Materials and Methods
Field Layout and Treatments
Four replicated sets of four experimental 3 × 3 m plots were established within two rows (1.5 m row spacing) of agricultural plastic mulch with four 0.75 × 1 m sub-plots planted with one of four known host plant species (Figure 1). Three host plants bell peppers, sweet corn, and soybean were chosen to represent known, highly attractive host plants with varying phenological stages and the fourth host, Swiss chard, was chosen as a non-fruiting host (Nielsen et al., 2011; Zobel et al., 2016). In 2013, the 3 × 3 m plots were separated by 3 m of bare ground and unplanted plastic mulch which was expanded in 2014 to 5 m. Within the center of each plot, a hole was dug so that a 3.78 l round plastic pot could be placed flush with the surface of the ground, allowing for the deployment of a potted sentinel host plant within each of the 16 plots.
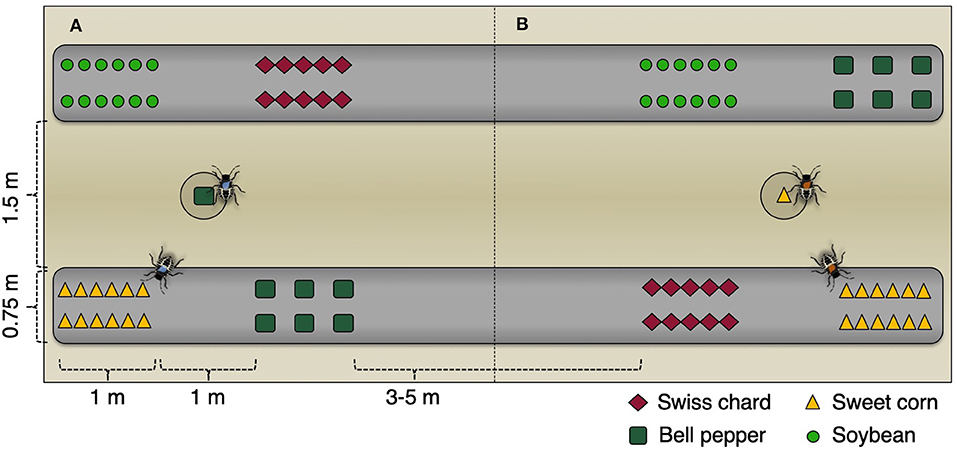
Figure 1. Example field layout of two (A,B) of the 16 experimental plots, illustrating the positioning and spacing of the sub-plots.
The four host plant species were planted directly into the plastic mulch on 23 May 2013 and 6 June 2014 (later due to a hail event on 22 May 2014) in two rows within the 0.75 × 1 m sub-plots and arranged in a Latin square design within each of the 16 main plots. Swiss chard was planted as seedlings at 20 cm spacing, bell peppers were planted as seedlings at 30 cm within row spacing, and sweet corn and soybean were sown as seeds at 15 cm spacing. At the same time of planting, four additional plants from each crop were planted in 3.78 l plastic pots with soil filled to the brim, which were used as portable sentinel host plants. One of each of the sentinel host crops was placed within the center of each of the four replicated sets of experimental plots (Figure 1).
Furthermore, in 2014, following the methods described above, four additional sub-plots were planted at the same time with the four representative host plants. These plants were established in the field to be used for volatile collection to represent the same phenological stage as the host plants in the mark-release-recapture experiment. Each of the four host plant crops were directly planted within the agricultural plastic mulch, 10 m away from the rest of the plants in four replicated sub-plots.
Host Choice
Nymphs were lab reared on a diet of organic carrots, organic green beans, and organic sunflower seeds from adults or nymphs that were originally collected from various host plants in the field. The nymphs released in the field had no prior experience with any of the host plants except for the specific host plant from which they were released. Depending on colony availability, 10–20 H. halys nymphs were marked and prepared for release in each of the 16 plots (Table 1). Initially, only 2nd and 3rd instar nymphs were used, with older instars included as the season progressed to simulate the life stages occurring naturally in the field. Following similar marking methods to Tillman (2008) and Bergh et al. (2017), nymphs were carefully marked with oil-based paint pens (Sharpie, Oak Brook, IL, USA) on the dorsal side of their thorax or abdomen, applying a different color code for each of the 16 plots. Marked nymphs were then placed on their corresponding, separate sentinel host plants in the lab and covered with a 3.78 L fine mesh bag (150 μm; The Cary Company, Addison, IL, USA) to retain the bugs on the plant. After a 48 hr settling period, the sentinel host plants were then placed in the center of each of their corresponding, replicated experimental plots.
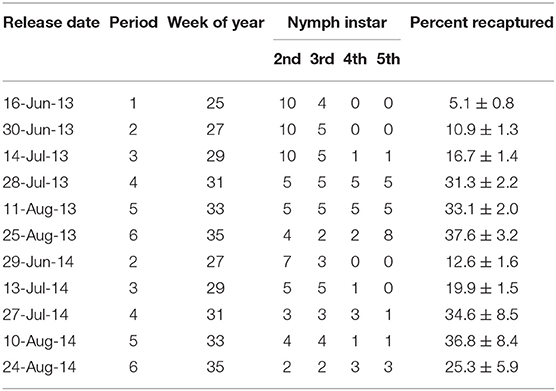
Table 1. Release dates, abundance, and percent recaptured of marked H. halys nymphs and their life stage (instar) released per plot.
Once in the field, the mesh bags were removed from each sentinel plant allowing the nymphs to choose whether or not to disperse from the center sentinel host plant. Prior to the release, any dead nymphs were replaced with newly marked, living nymphs. The location of marked nymphs within each the 16 experimental plots was assessed twice a day (9:00 and 13:00) during days 1, 3, and 5 after each field release. We carefully examined every center sentinel host plant and each of the other surrounding host plants within each subplot, recording the number, age of nymphs, color mark, and on which plants they were recovered. This manner of plant examination created a chance of interference with nymph movement due to human-induced agitation of the plants, but all plants within the study were evaluated and touched in the same manner, so all the nymphs released should have experienced the same type of agitation during their release and dispersal. Unmarked nymphs found attached or near a marked exuvia were recorded as “marked” and included in the subsequent analyses. Observed nymphs were left on the plants in the field until the last examination in the afternoon on the fifth day since release. Then all recovered nymphs were removed from the field and brought back into the laboratory. Releases were made six times over the course of the 2013 growing season, once every 2 weeks starting 14 June, and five times during the 2014 growing season starting 29 June. Consecutive sampling allowed us to assess H. halys nymphal host choice as the plants developed through progressive phenological stages. This field set up was unable to exclude a “group effect” for dispersal, but because nymphs aggregate naturally (Lockwood and Story, 1986; Fucarino et al., 2004), we attempted to mimicked this behavior as much as possible by releasing the H. halys nymphs in groups.
Host attractiveness to H. halys nymphs was quantified by calculating an attraction index. The index is dependent on whether the center sentinel plant is the same crop as the plant on which the marked nymphs are recovered. The attraction index is a proportion that assumes host preference if a nymph does not leave the sentinel host plant. Thus, when the dispersal plant is the same as the sentinel plant, the attraction index = (# nymphs on center plant + # nymphs on dispersal plant)/(# total nymphs recovered in plot). When the dispersal plant is different from the sentinel plant, the attraction index = (# nymphs on dispersal plant)/(# total nymphs recovered in plot). Using the abundance of nymphs observed during our post release plant evaluation, we calculated the attraction index for each host crop for each of the 16 experimental plots for each sampling period, averaging the index from the twice a day and then for the 3-day sampling points.
The attraction indices per plant from each of the 16 plots were averaged for each sample week (6 weeks in 2013 and 5 weeks in 2014). Except for the first sampling period (week 25) in 2013, corresponding sampling periods (2–6) from 2013 to 2014 were combined and the mean attraction indices (arcsine transformed) were compared among the four host plants for each week with a one-way analysis of variance (ANOVA) blocking by year. Week 25 attraction indices were arcsine transformed and compared among host plants using a one-way ANOVA. Additionally, Tukey's HSD was used for pairwise comparisons of attraction indices between host plants for each of the sample weeks. Furthermore, in order to evaluate the correlation between host attraction and plant growth stage, regression analyses with ANOVA were performed for the four host plants. Plant growth stages—vegetative, flowering, fruit set, mature fruit—were determined when more than 50% of the plants within an experimental plot were within that growth stage. Statistical analyses were performed with JMP Pro (Version 14, SAS Institute Inc., Cary, NC, USA).
Headspace Analysis
In order to investigate the olfactory host cues that may stimulate H. halys nymphs to disperse from one host to find another, we measured volatile organic compounds (VOC) from the four host plants: Swiss chard, bell pepper, sweet corn, and soybean. We sampled the most mature sections of the plants (e.g., leaves, flowers, or fruit) of four representatives of each of the four host types (replicated 4 times) and vacuumed off the headspace. Within the field planting, the selected plant parts were enclosed inside a 32 × 20 cm volatile collection bag made of non-absorbent Vac-Pak material (Richmond Aircraft Products, Norwalk, CA, USA). Binder clips were used to close the bag opening around the stem. Following similar methods from Rodriguez-Saona et al. (2009) and Rodriguez-Saona et al. (2011), volatiles from inside the bag were collected in 7.5 cm glass tubes with 30 mg Super-Q adsorbent (Alltech, Deerfield, IL, USA) by pulling air at a rate of 850 ml/min with the use of battery-powered 12 V micro diaphragm vacuum pump (Sensidyne, Clearwater, FL, USA). Volatiles were collected for 3 h (13:00–16:00 h). Measurements were taken at five time points throughout the 2014 growing season that corresponded with each of the five release time points during the 3rd day after H. halys nymphs were released for the mark-release-recapture experiment.
The collected volatiles were eluted from Super-Q trap filters with 150 μl dichloromethane (Sigma-Aldrich, St. Louis, MO, USA) containing 400 ng of n-octane (Sigma-Aldrich) as an internal standard. The solutions were stored at −20°C in 1.5 ml glass vials (Agilent Technologies, Santa Clara, CA, USA). Samples were later analyzed using gas chromatography (GC) (HP 6890 Series, Hewlett Packard, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and an Agilent HP-1 column (10 m × 0.53 mm × 2.65 μm film thickness). The program for separation and quantification followed the methods described in Rodriguez-Saona et al. (2011). Headspace samples (1 μl aliquots) were injected onto the GC-FID helium (He) at a flow rate of 5 ml/min. The temperature program started at 40°C (retained for 1 min), increased at 14°C/min to 180°C (2 min), then at 40°C/min to 200°C, and retained at 200°C for 2 min. The relative amounts of individual compounds (ng per g of dry material per hr) were quantified by comparing peak areas from the GC–FID with that of the internal standard.
Identification of specific compounds was carried out on a Varian 3400 GC coupled to a Finnigan MAT 8230 mass spectrometer (MS), equipped with a Supelco MDN-5S column (30 m × 0.32 mm × 0.25 μm film thickness; Supelco, Bellefonte, PA, USA), and with He as the carrier gas. The program started at 35°C (1 min), increased at 4°C/min to 170°C, then at 15°C/min to 280°C. The MS data were recorded and processed in a Finnigan MAT SS300 data system. Compounds were identified by comparison of spectral data with those from NIST library and by GC retention index (Jennings and Shibamoto, 1980; Adams, 2001).
Multivariate analysis of variance (MANOVA) was performed to identify differences between volatiles (dependent variables) emitted by the host plants across the different phenological stages sampled (independent variables). In order to measure the linear relationship between the relative amounts of the identified volatile organic compounds and the attraction indices we calculated the Pearson product-moment correlation (r) for each of the five sampling dates in 2014 (JMP Pro).
Results
Host Choice
When released from a central host plant, H. halys nymphs readily dispersed to alternative host plants. Overall, recapture of marked bugs was relatively high with an average (±SEM) recovery of 22.4 ± 0.9% in 2013 and 25.8 ± 2.7% in 2014, but there was a considerable range in percentage of bugs recaptured over the five to six sampling periods (Table 1). During the first release period in 2013, Bridgeton, NJ received 2.69 cm of rain on 17 June, and another 4.04 cm on 18 June. These rain events may have resulted in a lower recapture rate during this period. Additionally, interaction with wild, unreleased predators and development into older instars leading to molting and shedding of the “marked” exuviae may have impacted the recapture rate during the release periods.
The host plants in which the H. halys nymphs were recovered from changed throughout the season, indicating that nymphs can and do make host choices independent of their “source plant.” Early in the season when all the plants were in the vegetative stage, during the first sampling period [week 25; F(3, 60) = 4.984, P = 0.004] and the second [week 27; F(3, 123) = 2.849, P = 0.041], Swiss chard had a significantly higher attraction index than any of the other host plants (Figure 2). As the season progressed, attraction for Swiss chard decreased and during week 29 as sweet corn began to tassel—although not significantly higher than that of Swiss chard—was significantly higher than soybean and bell peppers [Figure 2; F(3, 123) = 4.544, P = 0.005]. By week 31, when sweet corn was in the milk stage of its phenology, it had a significantly higher attraction index than any of the other host plants [F(3, 123) = 17.649, P < 0.0001] and by week 33 when the soybean pods began to fill, both corn and soybean were highly attractive to the nymphs (Figure 2; F(3, 123) = 7.442, P = 0.001; Licht, 2014, 2016). During the final sampling period, week 35, as the pods began to mature and the sweet corn dried and hardened, soybean had the highest attraction index of all the host plants [Figure 2; F(3, 123) = 43.265, P < 0.0001]. Although bell peppers flowered and set fruit throughout the season, none of their phenological stages were ever as attractive to H. halys nymphs as the other host plants.
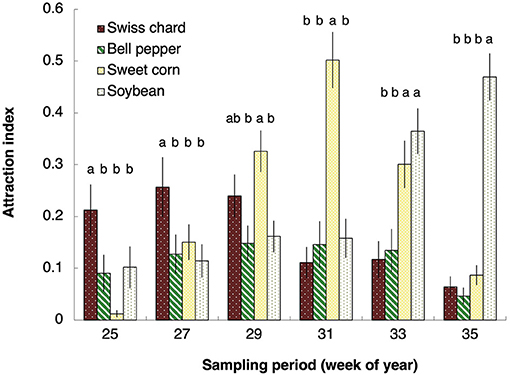
Figure 2. Comparison of Halyomorpha halys nymph attraction to four host plants over a series of six sampling periods (combined 2013 + 2014 data). Bars with the same letter are not significantly different (Tukey's HSD, P < 0.05).
To further assess host plant phenology impact on nymph attraction, we performed regression analysis with ANOVA for the best fit line between the attraction indices and host plant growth stages. Nymph attraction for sweet corn was positively correlated with growth stage progression with a quadratic, bell-shaped relationship where attraction for sweet corn peaked at fruit set (milk and dough stage) and decreased as the kernels matured [Y = 0.05 + 0.19*X−0.12*(X-2.09)2] [Figure 3; R2 = 0.32, F(2, 173) = 36.5, P < 0.001]. Attraction to soybean had a linear relationship with the attraction indices significantly increasing as the plants progressed through subsequent growth stages, with highest attraction with mature seeds [Y = −0.04 + 0.14*X] [Figure 3; R2 = 0.28, F(1, 174) = 64.1, P < 0.001]. Nymph attraction to bell peppers did not significantly change with progression of growth stages [Y = 0.21–0.02*X−0.02*(X-2.79)2] [Figure 3; R2 = 0.005, F(2, 173) = 0.86, P = 0.43]. Swiss chard was maintained in a vegetative state throughout the season, so the regression analysis was not performed for this host plant.
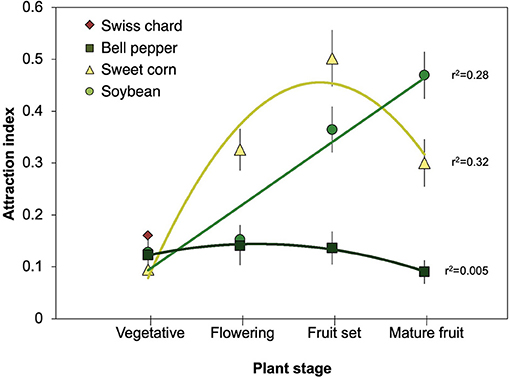
Figure 3. Comparison of mean Halyomorpha halys attraction indices (±standard error) with plant phenological growth stage for host plants. Bell pepper and sweet corn were analyzed with a quadratic regression, soybean with a linear regression, and Swiss chard did not have growth stages beyond “vegetative” so no regression analyses were applied.
Headspace Analysis
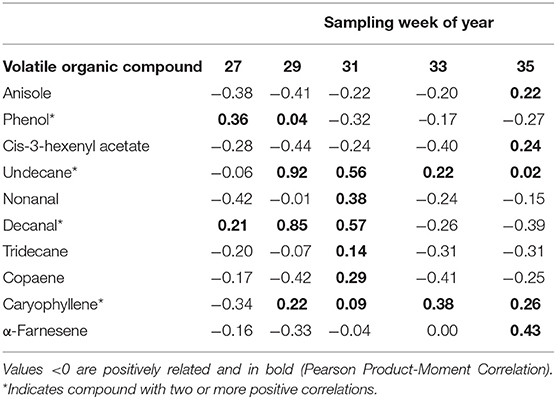
A total of 20 headspace samples were collected for each of the four host crops. Of the dozens of peaks detected with GC-FID, 11 chemicals were identified that were prominent and prevalent across the host plants (Table 2). Total volatile emissions, taken as the sum of the relative amount per host plant (ng) for each compound differed between the host crops across the different phenological growth stages (MANOVA, F = 3.21, P = 0.017). No compound was correlated with the attraction indices during every sampling week, but each of the 10 compounds were positively correlated with attraction during at least one sampling week (Table 3). The number of compounds was further narrowed by categorizing compounds that were positively correlated with attraction two or more times. This was used to categorize phenol, undecane, decanal, and caryophyllene, as compounds associated with host plants when the plants were the most attractive (Table 3).
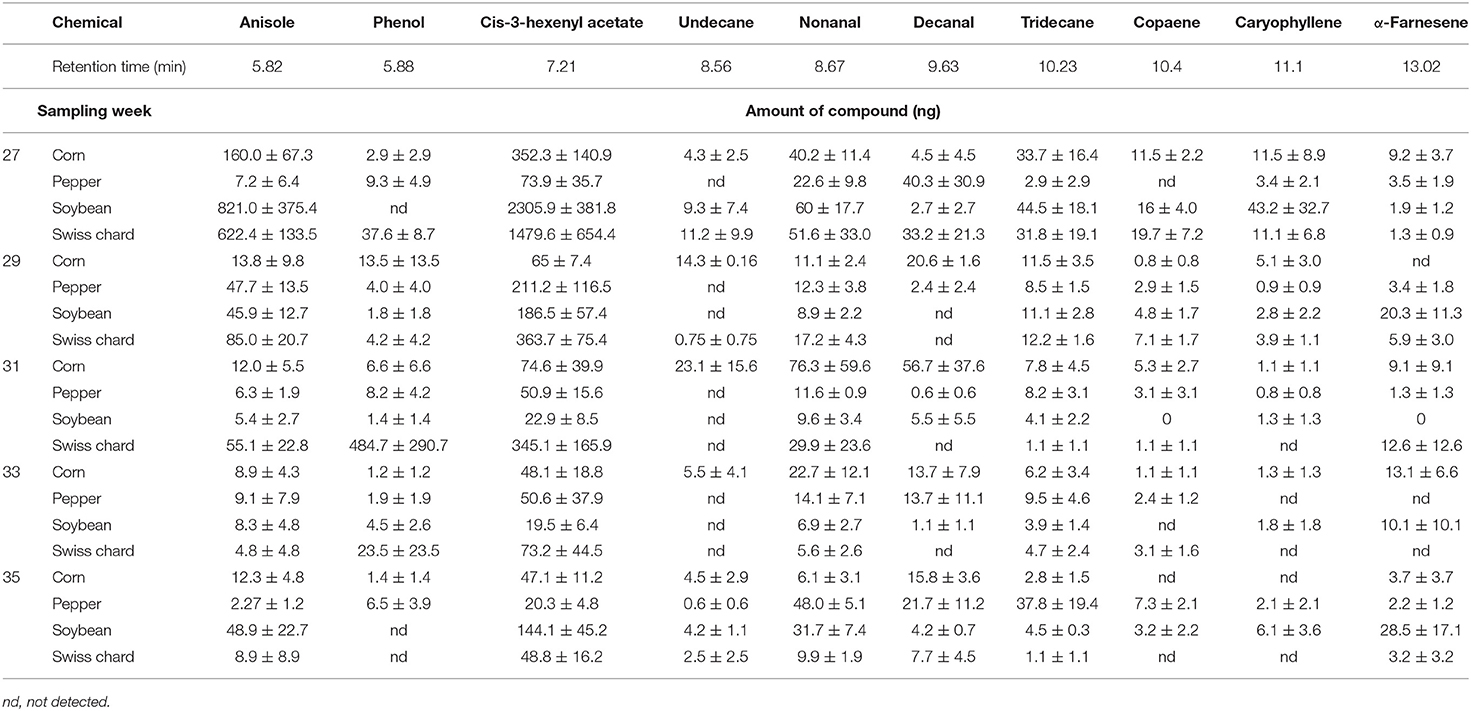
Table 2. List of identified volatile organic compounds detected with GC-FID, corresponding retention times, and average relative amounts (ng) ± standard error of compound per host plant for the five sampling periods (week of year, 2014).
Discussion
Polyphagy with generalist herbivores can provide optimal nutrients required for growth and reproduction, which can be influenced by an organism's behavioral and physiological ability to move in order to make a host selection. While H. halys shows a strong capacity for dispersal in response to an olfactory cue (Lee et al., 2014), the locomotion response of the immature stages to host plant stimuli is largely unknown. We demonstrated that nymphs are capable of moving from one potential host plant to another, which was correlated with host plant phenology (specifically ripening fruiting structures). Assessing movement on a weekly basis throughout the growing seasons of 2013 and 2014, H. halys moved from Swiss chard to sweet corn to soybean, with intermediate attraction to bell peppers, which coincided with host plant development. Nymphs of the southern green stink bug, Nezara viridula L., and the brown stink bug, Euschistus servus (Say), disperse from peanut fields into adjacent cotton fields when cotton bolls develop and are present as a food source (Tillman et al., 2009). What appears to be motivating this spatiotemporal distribution of these stink bug species in the agroecosystem is availability of suitable food in time and associated olfactory cues.
As plants mature, their palatability and thus suitability for insect development changes (e.g., Schumann and Todd, 1982; Awmack and Leather, 2002; Nielsen et al., 2016). We established that H. halys nymph attraction to host plants changes with plant growth stage. When host plants are grown adjacent to one another, even known host plants, such as bell peppers (Kuhar et al., 2012), can appear to have limited attraction due to the attractiveness of other host plant growth stages. Associated with these growth stage changes are likely differences in the volatile organic compounds (VOCs) released. Insects have evolved to utilize VOCs as olfactory stimuli in their host searching behaviors. Here, we demonstrated that H. halys nymphs leave a known host plant in order to locate another more preferred host plant. This choice appears to be based partially on olfactory cues from VOCs released by the host plants during development and fruit ripening. Phenol, undecane, decanal, and caryophyllene were common VOCs detected during the attractive developmental stages of the host plants assessed here. These plant volatiles are commonly found in host plants. For example, Jiménez-Martínez et al. (2004) observed that headspace volatiles from wheat positively influenced attraction of the bird cherry-oat aphid, Rhopalosiphum padi (Homoptera: Aphididae), which included three of the attractive compounds collected in this study: undecane, decanal, and caryophyllene. Similarly, phenol, undecane, decanal, and caryophyllene were all found in the headspace measured in apples throughout the season (Vallat et al., 2005). Apples are a highly attractive fruit crop for H. halys adults and nymphs (Morrison et al., 2019), and the use of plant volatiles has the potential to enhance current monitoring and/or management tools for H. halys (Morrison et al., 2018a). As generalists with a wide host range, perception of crop development and associated VOCs may influence the spatiotemporal distribution of H. halys within an agroecosystem.
The immature stages of many herbivorous insects have few opportunities to change the host or location on which they develop. Thus, parents, or more specifically, the mother determines available nutrition and exposure to the environment and natural enemies. Adult stink bugs have been shown to oviposit on unsuitable hosts, seemingly anticipating increased suitability as the host develops (Kiritani et al., 1965). While we were unable to replicate mother's “choice” by utilizing nymphs transferred to the release plants rather than egg masses oviposited directly to the plants, the directed movement made by the nymphs post-release establishes an apparent host choice by immature H. halys. Additionally, applying an attraction index allowed correction of unidirectional movement toward the same host plant as released from, accounting for natural dispersal behaviors. Adult stink bugs readily move between host plants (Jones and Sullivan, 1982; Tillman et al., 2009; Aigner et al., 2017; Blaauw et al., 2017), and the results from this work demonstrate that H. halys nymphs can also move amongst host plants. While nymphs are generally the more vulnerable stage of pentatomids, finding an adequate food resource may outweigh the risk of dispersing from their oviposited location.
The results of this study indicate that not only do H. halys adults, but also the nymphs, possess the capacity to disperse and select more favorable plants, which is an interesting ecological behavior that has the potential to be useful in agricultural pest management. We need to better understand the farmscape ecology and spatiotemporal distribution of H. halys to strategically manage stink bug populations. Understanding dispersal behavior may help with management of stink bugs by focusing management in time and space, such as through border-targeted insecticide applications (Blaauw et al., 2015, 2016), to more effectively and efficiently control such pests. Host attractiveness changes depending on host species and plant phenology, which is likely influenced by visual and olfactory cues. Thus, crop layout and trap crop utilization (Blaauw et al., 2017), in addition to chemical attractants (Weber et al., 2017; Morrison et al., 2018b), may also be used in pest management strategies for H. halys. Furthermore, four VOCs were identified here that were associated with highly attractive host plants, which is encouraging for the future understanding of dispersal cues and attractants. Previous research looked at the behavioral response of H. halys to host plant stimuli augmented with semiochemicals (Morrison et al., 2018a). While plant volatiles increased the retention time of H. halys adults and nymphs, the same volatiles did not increase attraction to pheromone-baited traps. Thus, continued research on dispersal behavior and olfactory cues of H. halys nymphs and adults is needed to incorporate such strategies into field-applicable and effectivere management practices.
Conclusions
We demonstrated that H. halys nymphs are capable of moving from one potential host plant to another, where attraction was correlated with volatile organic compounds produced by ripening of host plant fruiting structures. Thus, the availability of suitable food and associated olfactory cues appears to be influencing the spatiotemporal distribution of H. halys nymphs within the agroecosystem. Exploiting dispersal behavior and olfactory cues may be used to help increase the effectiveness and efficiency of current management practices for this severe and widespread pest.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
BB designed and implemented experiments, analyzed data, and co-wrote the paper. GH designed experiments and co-wrote the paper. CR-S designed experiments, analyzed data, and co-wrote the paper. AN designed and implemented experiments, and co-wrote the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Ann Rucker, Dr. Elvira de Lange, Rob Holdcraft, and the Nielsen lab team for their technical support throughout this project. Additionally, we would like to thank Dr. Thomas Hartman (Rutgers University, Mass Spectrometry Support Facility, New Brunswick, NJ) for identification of volatiles. This work was supported by USDA—NIFA Organic Agriculture Research and Extension Initiative program # 2012-51300-20097.
References
Acebes-Doria, A. L., Leskey, T. C., and Bergh, J. C. (2016). Host plant effects on Halyomorpha halys (Hemiptera: Pentatomidae) nymphal development and survivorship. Environ. Entomol. 45, 663–670. doi: 10.1093/ee/nvw018
Adams, R. P. (2001). Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J. Am. Soc. Mass Spectrom. 16, 1902–1903.
Aigner, B. L., Kuhar, T. P., Herbert, D. A., Brewster, C. C., Hogue, J. W., and Aigner, J. D. (2017). Brown marmorated stink bug (Hemiptera: Pentatomidae) infestations in tree borders and subsequent patterns of abundance in soybean fields. J. Econ. Entomol. 110, 487–490. doi: 10.1093/jee/tox047
Awmack, C. S., and Leather, S. R. (2002). Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844. doi: 10.1146/annurev.ento.47.091201.145300
Bergh, J. C., Morrison, W. R., Joseph, S. V., and Leskey, T. C. (2017). Characterizing spring emergence of adult Halyomorpha halys using experimental overwintering shelters and commercial pheromone traps. Entomol. Exp. Appl. 162, 336–345. doi: 10.1111/eea.12539
Blaauw, B. R., Jones, V. P., and Nielsen, A. L. (2016). Utilizing immunomarking techniques to track Halyomorpha halys (Hemiptera: Pentatomidae) movement and distribution within a peach orchard. PeerJ 4:e1997. doi: 10.7717/peerj.1997
Blaauw, B. R., Morrison, W. R. III., Mathews, C. R., Leskey, T. C., and Nielsen, A. L. (2017). Measuring host plant selection and retention of Halyomorpha halys by a trap crop. Entomol. Exp. Appl. 163, 197–208. doi: 10.1111/eea.12571
Blaauw, B. R., Polk, D., and Nielsen, A. L. (2015). IPM-CPR for peaches: incorporating behaviorally-based methods to manage Halyomorpha halys and key pests in peach. Pest Manag. Sci. 71, 1513–1522. doi: 10.1002/ps.3955
Dicke, M. (2000). Chemical ecology of host-plant selection by herbivorous arthropods: a multitrophic perspective. Biochem. Syst. Ecol. 28, 601–617. doi: 10.1016/S0305-1978(99)00106-4
Fucarino, A., Millar, J. G., McElfresh, J. S., and Colazza, S. (2004). Chemical and physical signals mediating conspecific and heterospecific aggregation behavior of first instar stink bugs. J. Chem. Ecol. 30, 1257–1269. doi: 10.1023/B:JOEC.0000030276.32665.cb
Hahn, N. G., Rodriguez-Saona, C., and Hamilton, G. C. (2017). Characterizing the spatial distribution of brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera: Pentatomidae), populations in peach orchards. PLoS ONE 12:e0170889. doi: 10.1371/journal.pone.0170889
Jennings, W., and Shibamoto, T. (1980). Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography. New York, NY: Academic Press.
Jiménez-Martínez, E. S., Bosque-Pérez, N. A., Berger, P. H., Zemetra, R. S., Ding, H., and Eigenbrode, S. (2004). Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus–infected transgenic and untransformed wheat. Environ. Entomol. 33, 1207–1216. doi: 10.1603/0046-225X-33.5.1207
Jones, W. A., and Sullivan, M. J. (1982). Role of host plants in population dynamics of stink bug pests of soybean in South Carolina. Environ. Entomol. 11, 867–875. doi: 10.1093/ee/11.4.867
Kant, M. R., Jonckheere, W., Knegt, B., Lemos, F., Liu, J., Schimmel, B. C. J., et al. (2015). Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 115, 1015–1051. doi: 10.1093/aob/mcv054
Kiritani, K., Hokyo, N., Kimura, K., and Nakasuji, F. (1965). Imaginal dispersal of the southern green stink bug, Nezara viridula L., in relation to feeding and oviposition. Jpn. J. Appl. Entomol. Zool. 9, 291–297. doi: 10.1303/jjaez.9.291
Kuhar, T. P., Kamminga, K. L., Whalen, J., Dively, G. P., Brust, G., Hooks, C. R. R., et al. (2012). The pest potential of brown marmorated stink bug on vegetable crops. Plant Health Prog. doi: 10.1094/PHP-2012-0523-01-BR
Lee, D. H., and Leskey, T. C. (2015). Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Bull. Entomol. Res. 105, 566–573. doi: 10.1017/S0007485315000462
Lee, D. H., Nielsen, A. L., and Leskey, T. C. (2014). Dispersal capacity and behavior of nymphal stages of Halyomorpha halys (Hemiptera: Pentatomidae) evaluated under laboratory and field conditions. J. Insect Behav. 27, 639–651. doi: 10.1007/s10905-014-9456-2
Leskey, T. C., and Nielsen, A. L. (2018). Impact of the invasive brown marmorated stink bug in North America and Europe: history, biology, ecology, and management. Annu. Rev. Entomol. 63, 599–618. doi: 10.1146/annurev-ento-020117-043226
Licht, M. (2014). PM 1945: Soybean Growth and Development. Iowa State University, Extension and Outreach, 28.
Licht, M. (2016). CROP 3094A: Corn Growth and Development - Corn Staging. Iowa State University, Extension and Outreach, 1.
Lockwood, J. A., and Story, R. N. (1986). Adaptive functions of nymphal aggregation in the southern green stink bug, Nezara viridula (L.) (Hemiptera: Pentatomidae). Environ. Entomol. 15, 739–749. doi: 10.1093/ee/15.3.739
Lye, B. H., and Story, R. N. (1988). Feeding preference of the southern green stink bug (Hemiptera: Pentatomidae) on tomato fruit. J. Econ. Entomol. 81, 522–526. doi: 10.1093/jee/81.2.522
Martinson, H. M., Venugopal, P. D., Bergmann, E. J., Shrewsbury, P. M., and Raupp, M. J. (2015). Fruit availability influences the seasonal abundance of invasive stink bugs in ornamental tree nurseries. J. Pest Sci. 88, 461–468. doi: 10.1007/s10340-015-0677-8
Mayhew, P. J. (1997). Adaptive patterns of host-plant selection by phytophagous insects. Oikos 79, 417–428. doi: 10.2307/3546884
Mayhew, P. J. (2001). Herbivore host choice and optimal bad motherhood. Trends Ecol. Evol. 16, 165–167. doi: 10.1016/S0169-5347(00)02099-1
Morrison, W. R. III., Allen, M., and Leskey, T. C. (2018a). Behavioural response of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to host plant stimuli augmented with semiochemicals in the field. Agric. For. Entomol. 20, 62–72. doi: 10.1111/afe.12229
Morrison, W. R. III., Blaauw, B. R., Nielsen, A. L., Talamas, E., and Leskey, T. C. (2018b). Predation and parasitism by native and exotic natural enemies of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) eggs augmented with semiochemicals and differing host stimuli. Biol. Control 121, 140–150. doi: 10.1016/j.biocontrol.2018.02.016
Morrison, W. R. III., Blaauw, B. R., Short, B. D., Nielsen, A. L., Bergh, J. C., Krawczyk, G., et al. (2019). Successful management of Halyomorpha halys (Hemiptera: Pentatomidae) in commercial apple orchards with an attract-and-kill strategy. Pest Manag. Sci. 75, 104–114. doi: 10.1002/ps.5156
Nielsen, A. L., Dively, G. P., Pote, J. M., Zinati, G., and Mathews, C. (2016). Identifying a potential trap crop for a novel insect pest, Halyomorpha halys (Hemiptera: Pentatomidae), in organic farms. Environ. Entomol. 45, 472–478. doi: 10.1093/ee/nvw006
Nielsen, A. L., and Hamilton, G. (2009). Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in Northeastern United States. Ann. Entomol. Soc. Am. 102, 608–616. doi: 10.1603/008.102.0405
Nielsen, A. L., Hamilton, G. C., and Shearer, P. W. (2011). Seasonal phenology and monitoring of the non-native Halyomorpha halys (Hemiptera: Pentatomidae) in soybean. Environ. Entomol. 40, 231–238. doi: 10.1603/EN10187
Panizzi, A. R., and Alves, R. M. L. (1993). Performance of nymphs and adults of the southern green stink bug (Heteroptera: Pentatomidae) exposed to soybean pods at different phenological stages of development. J. Econ. Entomol. 86, 1088–1093. doi: 10.1093/jee/86.4.1088
Panizzi, A. R., and Saraiva, S. I. (1993). Performance of nymphal and adult southern green stink bug on an overwintering host plant and impact of nymph to adult food-switch. Entomol. Exp. Appl. 68, 109–115. doi: 10.1111/j.1570-7458.1993.tb01694.x
Rice, K. B., Bergh, C. J., Bergmann, E. J., Biddinger, D. J., Dieckhoff, C., Dively, G., et al. (2014). Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 5, A1–A13. doi: 10.1603/IPM14002
Rodriguez-Saona, C., Vorsa, N., Singh, A. P., Johnson-Cicalese, J., Szendrei, Z., Mescher, M. C., et al. (2011). Tracing the history of plant traits under domestication in cranberries: potential consequences on anti-herbivore defences. J. Exp. Bot. 62, 2633–2644. doi: 10.1093/jxb/erq466
Rodriguez-Saona, C. R., Rodriguez-Saona, L. E., and Frost, C. J. (2009). Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. J. Chem. Ecol. 35, 163–175. doi: 10.1007/s10886-008-9579-z
Scheirs, J., De Bruyn, L., and Verhagen, R. (2000). Optimization of adult performance determines host choice in a grass miner. Proc. R. Soc. B 267, 2065–2069. doi: 10.1098/rspb.2000.1250
Schumann, F. W., and Todd, J. W. (1982). Population dynamics of the southern green stink bug (Heteroptera: Pentatomidae) In relation to soybean phenology. J. Econ. Entomol. 75, 748–753. doi: 10.1093/jee/75.4.748
Siebert, M. W., Leonard, B. R., Gable, R. H., and LaMotte, L. R. (2005). Cotton boll age influences feeding preference by brown stink bug (Heteroptera: Pentatomidae). J. Econ. Entomol. 98, 82–87. doi: 10.1093/jee/98.1.82
Skillman, V. P., Wiman, N. G., and Lee, J. C. (2018). Monitoring nutrient status of brown marmorated stink bug adults and nymphs on summer holly. Insects 9:E120. doi: 10.3390/insects9030120
Strong, D. R., Lawton, J. H., and Southwood, S. (1984). Insects on Plants: Community Patterns and Mechanisms. Cambridge: Harvard University Press.
Tillman, P. G. (2008). Populations of stink bugs (Heteroptera: Pentatomidae) and their natural enemies in peanuts. J. Entomol. Sci. 43, 191–207. doi: 10.18474/0749-8004-43.2.191
Tillman, P. G., Northfield, T. D., Mizell, R. F., and Riddle, T. C. (2009). Spatiotemporal patterns and dispersal of stink bugs (Heteroptera: Pentatomidae) in peanut-cotton farmscapes. Environ. Entomol. 38, 1038–1052. doi: 10.1603/022.038.0411
Vallat, A., Gu, H., and Dorn, S. (2005). How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 66, 1540–1550. doi: 10.1016/j.phytochem.2005.04.038
Weber, D. C., Morrison, W. R., Khrimian, A., Rice, K. B., Leskey, T. C., Rodriguez-Saona, C., et al. (2017). Chemical ecology of Halyomorpha halys: discoveries and applications. J. Pest Sci. 90, 989–1008. doi: 10.1007/s10340-017-0876-6
Wiman, N. G., Walton, V. M., Shearer, P. W., Rondon, S. I., and Lee, J. C. (2015). Factors affecting flight capacity of brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). J. Pest Sci. 88, 37–47. doi: 10.1007/s10340-014-0582-6
Keywords: phenology, volatile, attraction, mark-release-recapture, nymph
Citation: Blaauw BR, Hamilton G, Rodriguez-Saona C and Nielsen AL (2019) Plant Stimuli and Their Impact on Brown Marmorated Stink Bug Dispersal and Host Selection. Front. Ecol. Evol. 7:414. doi: 10.3389/fevo.2019.00414
Received: 08 July 2019; Accepted: 17 October 2019;
Published: 12 November 2019.
Edited by:
Juergen Gross, Julius Kühn-Institut, GermanyReviewed by:
Lara Maistrello, University of Modena and Reggio Emilia, ItalyAntonino Cusumano, Wageningen University & Research, Netherlands
Copyright © 2019 Blaauw, Hamilton, Rodriguez-Saona and Nielsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brett R. Blaauw, YmJsYWF1dyYjeDAwMDQwO3VnYS5lZHU=
 Brett R. Blaauw
Brett R. Blaauw George Hamilton2
George Hamilton2