- 1Department of Entomology and Nematology, University of Florida, Gainesville, FL, United States
- 2Department of Animals and Plants Sciences, University of Sheffield, Sheffield, United Kingdom
- 3Institute of Evolutionary Biology, University of Edinburgh, Edinburgh, United Kingdom
- 4School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW, Australia
- 5Department of Life Sciences, Imperial College London, London, United Kingdom
In species with biparental care, behavioral coordination in the provisioning of the progeny is hypothesized to increase the number of offspring that survive to independence. Coordination is often quantified by two metrics, alternation and synchrony. Turn-taking (leading to an alternation pattern) can result when one parent's investment strategy is based on the investment of its partner (i.e., conditional cooperation). This should increase the overall provisioning rate and improve offspring body condition. Synchrony might equalize food delivery among offspring and therefore decrease the variance in offspring body condition within the brood. Overall, offspring survival could be increased by parental coordination. Finally, pairs with low coordination, and with potentially lower reproductive success, are expected to be more likely to divorce. In this study, we use a dataset on 473 pairs of house sparrows in a natural insular population to test these hypotheses. We found no effect of the pair's apparent coordination on offspring condition, offspring survival, or divorce rate, questioning the adaptive significance of this behavior. We argue that, in this species, the detection of a higher frequency of alternation and synchrony, when compared to chance expectation, might be induced by the environment, rather than result from an emergent pair behavior selected for fitness benefits.
Introduction
In most animal species, some form of interaction between males and females is necessary to ensure each parent's reproductive success, with examples ranging from courtship display to obligatory biparental care. In this context, the synergy between the two individuals' phenotypes or behavior could be crucial. The emergent property of a pair at the phenotypic level has been shown to be a potential target of mate choice and can lead to large fitness consequences (Ihle et al., 2015). In species with biparental care, the importance of the phenotypic interaction between parents is likely to peak during offspring provisioning, and could take the form of behavioral coordination. Behavioral coordination could potentially improve with familiarity (i.e., pairbond duration; Black, 1996, but see Naves et al., 2007) or be determined by the combination of both partners' personality types (e.g., Both et al., 2005, but see Schielzeth et al., 2011).
Behavioral coordination could take different forms and has sparked significant recent interest (e.g., Mariette and Griffith, 2012, 2015; van Rooij and Griffith, 2013; Johnstone et al., 2014; Bebbington and Hatchwell, 2016; Koenig and Walters, 2016; Iserbyt et al., 2017; Khwaja et al., 2017; Savage et al., 2017; Takahashi et al., 2017; Tyson et al., 2017; Leniowski and Wegrzyn, 2018). First, synchronized feeding (i.e., simultaneous feeding) could potentially ensure that food gets delivered equally to each offspring, limiting sibling competition (Shen et al., 2010). Synchrony could also reduce the conspicuousness of the nest to predators by potentially halving the number of nest visit bouts if parents always visit simultaneously, as opposed to perfectly asynchronously (Mariette and Griffith, 2012, 2015; Bebbington and Hatchwell, 2016). Second, alternated provisioning (or “turn-taking”) could promote greater overall investment by parents, as it has been argued to constitute a simple form of reciprocal cooperation between parents trading-off their current vs. future reproductive effort (Johnstone et al., 2014). Under the conditional cooperation hypothesis, pair members would engage in a tit-for-tat style of provisioning by increasing their return rate to feed the young after their partner has fed, potentially by withholding provisioning until their partner has provisioned (Johnstone et al., 2014). This strategy should lead to alternation (where 100% alternation is when parents take strict turns to provision offspring) and encourage each parent to provide an equal share of care. Theoretically, if pair members follow this rule in real time, an increase in one parent's feeding rate should also lead to an increase in the partner's feeding rate, which would benefit the offspring (Johnstone et al., 2014). Overall, synchrony and alternation, separately or in conjunction, could, at least partly, determine the pair's rearing success. The pair members' behavioral compatibility could also, subsequently, influence the male's or female's decision to retain or divorce their partner.
We aim at testing these hypotheses using the breeding and provisioning data gathered on a wild island population of house sparrows (Passer domesticus) monitored closely for over 12 years where, overall, male and female care are interdependent (Schroeder et al., 2019). In a previous study, we found that alternation and synchrony were higher than expected by chance, but we also demonstrated how this outcome could, in principle, still be explained by passive processes, namely that parents could show correlated behaviors due to their shared environment (Ihle et al., 2019). Therefore, this result was insufficient (albeit necessary) to speculate on the adaptiveness of behavioral coordination.
In the present study, we investigate whether the pair's coordination in provisioning predicts its success in terms of offspring fledging success. In addition, as theory suggests that turn-taking could increase the total number of feeds to the offspring, while synchrony could reduce the effect of sibling competition, we also analyse nestling body condition and within-brood variance in nestling body condition, respectively. Finally, we test whether the degree of pair coordination predicts the likelihood of divorce.
Methods
Parental Rearing Success
We used data collected on a house sparrow population breeding in nestboxes on Lundy, a small island located 19 km off the coast of south-west England (51°10'N, 4°40'W). This population has been closely studied since 2000, and all birds are marked with a unique combination of color rings and a metal ring supplied by the British Trust for Ornithology (Simons et al., 2015).
Each breeding pair was monitored from late April to late August each year. All hatchlings had their blood sampled for later parentage analyses. To construct our population pedigree, we used 13 microsatellite loci to assign genetic parentage (see detailed procedures in Schroeder et al., 2012). In addition, around 50% of the nestlings were cross-fostered 1 or 2 days after they hatched. Nestlings were usually exchanged along with all or some of their siblings among two or three nests, without affecting the original brood size (Winney et al., 2015). We will assume that receiving foreign offspring does not affect parental provisioning (no effect was found in the males of our population: Lattore et al., 2019; no parent-offspring co-adaptation was found in blue tits (Cyanistes caeruleus) despite high power: Lucass et al., 2016; Thomson and Hadfield, 2019). After this cross-fostering event, nestling were measured and weighed when between 4 and 6 days old (hatching = day 0). Finally, nestlings were ringed, weighed, and measured when between 11 and 14 days old. Nestling were weighed with a digital scale with a 0.1 g accuracy and their tarsus length (“minimum tarsus” as defined in the British Trust for Ornithology ringer's manual) were measured with a digital caliper with a 0.01 mm resolution. Offspring body condition was not calculated—its analysis (see below) uses chick mass as the dependent variable and tarsus length as a covariate. Typically, offspring will fledge soon after that date and, therefore, we assumed that each bird that received a ring will have fledged successfully. We chose to analyse offspring survival to fledging (rather than whether offspring bred in the following year, for instance) to maximize our chances of detecting a potential effect of the parents' behavioral compatibility, assuming that fledgling survival after leaving the nest is determined to a much greater degree by factors other than parental care.
In addition to being monitored closely during the breeding season, almost half of the individuals were caught over one or two intense but brief events of mist netting in the winter, allowing relatively accurate information on which individuals were alive during the breeding season (Simons et al., 2015). We considered an individual to have divorced its partner after a given breeding attempt when either or both pair members subsequently engaged in a breeding event with a new partner (in the same or later year), while their former partner was still alive, as judged from sightings, captures, social breeding, and genetic parentage. In 68 broods (from 38 males and 48 females) out of 621 broods that were followed by a subsequent breeding event by the male with an identified female, the male switched to breed with another female before reverting to breed again with their former partner (either fully sequentially or overlapping). Such cases, where the female seemed not to divorce (they did not rear a brood with another male in between), were excluded from the analysis presented below.
Parental Coordination in Provisioning
From 2004 to 2015, nestboxes were videotaped, usually, for two 90-min periods on 2 separate days (typically when nestlings were 6 and 11 days old) during the provisioning phase of each brood (first described in Nakagawa et al., 2007). For each video, we recorded the time (±3 s) at which each sex entered the nest or passed its head through the nest entrance. For all these visits, we assumed that feeding occurred. This seems to be a reasonable assumption given that visit rates predict brood mass changes better than delivery rate based on load size (which cannot always be assessed) in another population of house sparrows (Pelletier et al., 2016; Dave Westneat, personal communication, for the same analysis with a larger sample size). For this study, the duration of time spent in the nestbox (median = 0.3 min, range = 0–47.7 min) was ignored for simplicity and consistency with previous work (Johnstone et al., 2014; Mariette and Griffith, 2015; Bebbington and Hatchwell, 2016).
For each nest watch, we counted the number of alternated visits, A, as the number of times a pair member provisioned after the other; and the number of synchronized visits, S, as the number of times an individual provisioned shortly after its partner (Figure 1). We used an interval of 2 min, as in Bebbington and Hatchwell (2016), to define synchrony (see Ihle et al., 2019 for rationale). Then, we calculated the level of coordination (alternation and synchrony separately) that would be expected by chance. For this, we randomized inter-feeding intervals from each observed bird within each observed nest watch before recounting A and S within each nest watch (in Ihle et al., 2019, we refer to this procedure as “within-individual, within nest watch randomization”). We repeated this procedure 100 times and calculated the median number of alternated and synchronized visits across the 100 randomizations of each observed nest watch. In a previous study, we showed that the most appropriate way to model coordination is to use the deviation of observed alternation and synchrony from random on the log scale to normalize the distribution of counts (Ihle et al., 2019). Therefore, for each observed nest watch, we calculated the deviation of the observed coordination from what would be expected by chance as log(observed)—log(random). We averaged this measure within broods to test whether this affected brood success. We added 0.5 to observed and random values to avoid log transforming zeros that would lead to unrepresentable values (Yamamura, 1999). We use these measures of alternation and synchrony rather than the previously published measures of coordination at the pair level (Bebbington and Hatchwell, 2016), because the latter do not adequately account for the inevitable mathematical relationships between coordination in provisioning and provisioning itself, and therefore between coordination and every fitness proxy that is known to be correlated with provisioning rate (see Ihle et al., 2019 for demonstration).
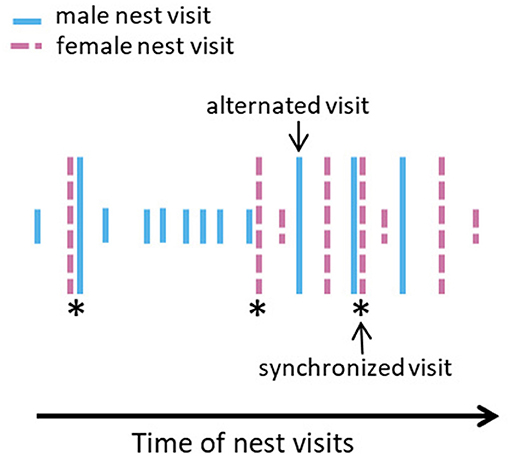
Figure 1. Illustrative timeline of provisioning visits, with each bar representing a visit [female and male visits, in pink (dashed bar) and light blue (solid bar), respectively]. Longer bars represent alternated visits. Asterisks highlight synchronous visits.
Data Selection and Sample Sizes
As we were interested in the effect of parental coordination between social parents when provisioning offspring, we included data on all broods with two known social parents where the nest was video-recorded when nestlings were at least 6 days old. We obtained 1,599 video recordings of ~90 min in length, with, on average, 1.8 video recordings taken per brood, featuring 299 different social mothers and 281 different social fathers, forming 473 different pairs. On average, each parent was observed across 4.7 broods, and each pair was observed across 2.7 broods. This is the same dataset used in a previous study (Ihle et al., 2019).
Statistical Analyses
Offspring survival until fledging was modeled as a binary trait in a generalized linear mixed effect model with binomial error and a logit link function (0: the offspring did not survive, 1: the offspring did survive). Predictors were the deviation in alternation and in synchrony, the mean total provisioning rate for that brood (mean total number of visit per hour across all observations), the mean total provisioning rate squared (added after inspecting the model residuals), brood size [number of offspring surviving to day 5 (see below)], the number of clutches a pair had had together prior to the one being considered (pair clutch number), whether or not one (or both) pair members had nested in that nestbox prior to the breeding attempt (as a binary variable) as a measure of that individual's (and possibly the pair's) familiarity with its environment, and the hatching date as the number of days after 1 April of that year. We also included whether or not the offspring was cross-fostered to account for the cross-fostering design routinely performed on this population. Because, in our population, only chicks that survived until age 2 or 3 days were cross fostered, cross fostering would be expected to artifactually positively predict offspring survival when considering survival from hatching to fledging (Winney et al., 2015). To avoid this artifact, we only analyzed chick survival after cross fostering, i.e., from when they were measured between days 4 and 6 until ringing, and added the number of days between the first measurement and ringing (or the average number of days to ringing when no offspring survived to a later stage within a brood) as a covariable (to account for the number of days between survival checks). Social pair identity, breeding year, and the natal and rearing brood identities were modeled as random effects. This analysis pools all observations (range: 1–3) made on a given brood by averaging provisioning rate and coordination per brood, assuming that parental effort has some level of consistency over the brood stage (from age 5 to 12 days) and that parental coordination at different stages does not have different effects on chick survival. This may not be the case and could be investigated in larger datasets.
Offspring body condition at 11–14 days old (nestling mass with tarsus length as a covariate), was modeled with the same predictors and random effects, with the exception of brood size, which, here, was the number of offspring that were ringed, and the number of days between survival checks, which was replaced here by offspring age at the last measurement. As chicks were cross fostered between nests, the intra-brood variance might have been inflated due to differences in the relatedness of chicks within the same nest. In order to account for this, we used an animal model—a linear mixed effects model that used the relatedness structure among individuals (i.e., the pedigree)—to account for additive genetic effects (Henderson, 1988; Kruuk, 2004). This model was run in a double hierarchical model framework where both the mean and the variance of a trait are modeled simultaneously (Cleasby et al., 2015), so allowing the residual variance in offspring mass to vary across nests. This framework is necessary to test simultaneously the expected positive effect of alternation and synchrony on offspring body condition (in line with the hypothesis of reduction in parental sexual conflict) and the expected negative effect of synchrony on the offspring body condition variance within nest (in line with the hypothesis of increased similarity in food delivery thanks to synchrony). The within-nest standard deviations were assumed to come from a log normal distribution, and were modeled as a function of synchrony, brood size and the interaction between the two. The test of the interaction term was included in order to explore whether the effects of synchrony were more pronounced in nests with more competition (i.e., with larger brood size).
Finally, we modeled whether or not divorce occurred following a specific recorded brood using a generalized mixed effects model with binomial error and a logit link function (0: the pair does not divorce after this brood, 1: the pair does divorce after this brood). Predictors were the deviation in alternation and synchrony, the pair clutch number, male and female ages, the mean total provisioning rate, and the absolute difference in pair members' provisioning rates as a measure of parental investment and asymmetry in parental investment, and the number of ringed nestlings as a measure of reproductive success. We also included whether the brood the pair cared for was mixed (i.e., contained foster offspring). Random effects were the male and female identities, and the breeding year.
Data handling, selection and randomization were performed in R version 3.5.3 (R Development Core Team, 2019) and all codes are available in a permanent repository (http://doi.org/10.5281/zenodo.3459642). The generalized mixed effects models (offspring survival and parental divorce analyses) were performed with the lme4 package (Bates et al., 2015) in R version 3.5.3 (R Development Core Team, 2019), using the bobyqa optimizer. Significance of the predictors was obtained using likelihood ratio tests, comparing nested models with and without the parameter of interest (with the function “drop1”). All predictors, which were all selected a priori, were kept in the model regardless of their significance. The double hierarchical model (offspring mass analysis) was run in Stan (Carpenter et al., 2017) through the RStan package (Stan Development Team, 2019), using 4 chains each with 30,000 iterations (without thinning) and a warmup of 15,000 iterations. Convergence of individual chains was visually assessed, as well as ensuring that the Gelman–Rubin diagnostic (R-hat) across chains was <1.1 (Gelman and Rubin, 1992). Normality of residuals was visually assessed. A p-value for the fixed effects in this double hierarchical model was approximated (pMCMC) as two times the smaller of the number of iterations where (i) a <0 or (ii) a > 0, where a is the parameter value (see e.g., Hadfield et al., 2013). Non-categorical predictors were scaled (i.e., giving them a mean of zero and standard deviation of 1) in all models.
Results
Offspring Survival
The survival of offspring within each brood was negatively affected by the mean deviation in alternation and not affected by the mean deviation in synchrony observed for each brood [effect opposite to expectation for the mean log alternation deviation: odds ratio = 0.86, 95% confidence interval (CI) = 0.74–0.99; mean log deviation in synchrony: odds ratio = 1.02, CI = 0.90–1.17; Table 1, Figure 2]. Offspring survival was also negatively affected by brood size while it was positively affected by hatching date and the total provisioning rate, although it declined (or at least reached a plateau) at high provisioning rates (i.e., the quadratic term was significantly negative, Table 1, Supplementary Figure 1).
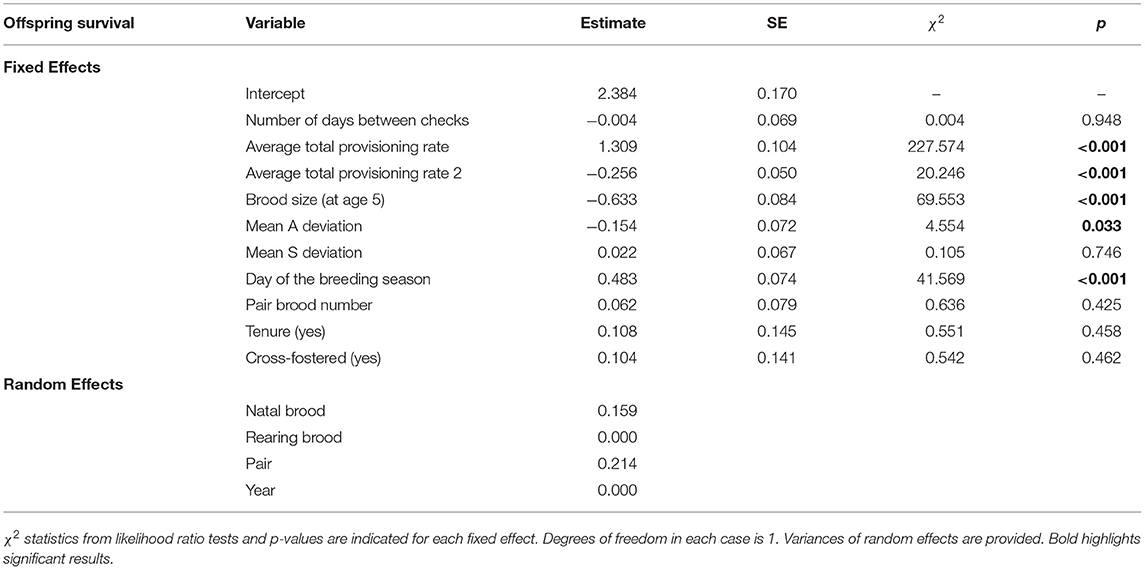
Table 1. Model estimates and standard errors (SE) for each of the predictors of offspring survival to fledging (N = 2,482 offspring alive at age 5, 2,112 survived to ringing).
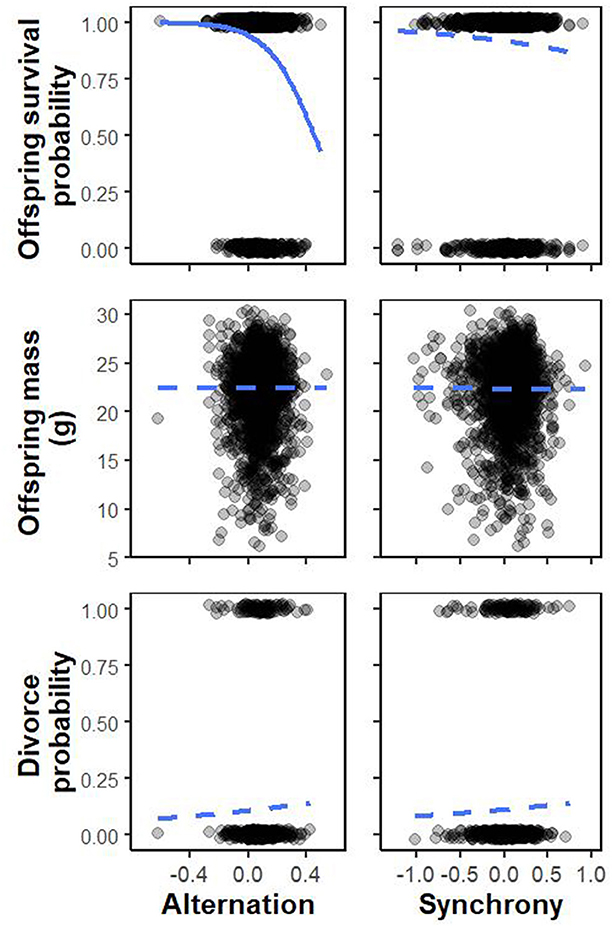
Figure 2. Effect of the pairs' deviation in alternation and synchrony averaged within brood observations and transformed on the log scale on: (top row) the probability of offspring surviving from age 5 days to ringing (middle row), offspring mass at 11 days old (bottom row), the pair's probability of divorcing. Blue curves are dotted when the effects are not significant, and plain when the effect is significant. Note that the only significant effect is opposite expectation. Raw data (top row: offspring survived: 1, offspring did not survive to fledge: 0; bottom row: divorce: 1, did not divorce: 0) are also shown.
Offspring Body Condition
Offspring body condition was not affected by the mean deviation in alternation or synchrony observed for each nestling's rearing brood but was negatively affected by brood size (fixed effects on mean, Table 2, Figure 2). There was no significant interactive effect of synchrony and brood size on within-brood variance in offspring body condition (pMCMC = 0.099; trend opposite to expectation, i.e., the effect of synchrony on within brood variance in body condition is more positive as brood size increases, fixed effect on residual variance, Table 2). There was also no main effect of synchrony on within brood variance in body condition (i.e., at average brood sizes there is no effect of synchrony; fixed effect on residual variance, Table 2, Figure 2).
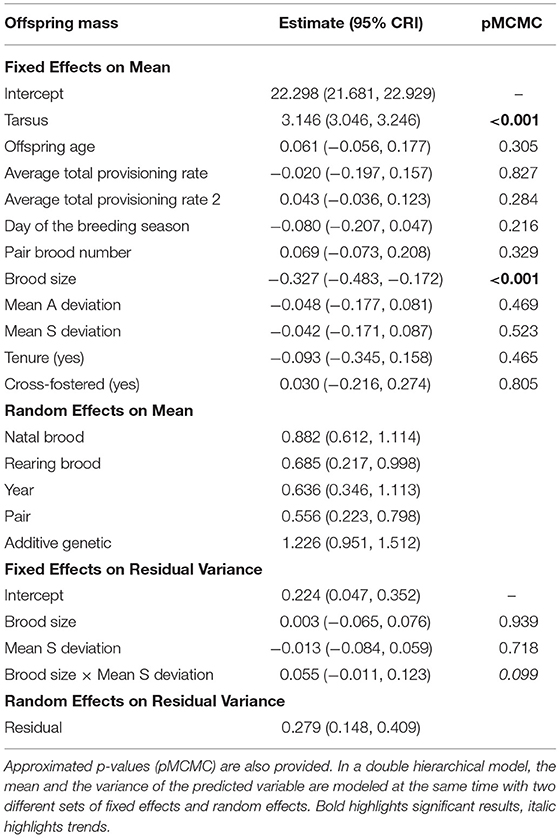
Table 2. Model estimates and 95% credible intervals (CRI) for each of the predictors of offspring body condition (N = 2,098 offspring that were measured around 12 days old).
Divorce
The likelihood of pairs divorcing was not affected by the deviation in alternation or synchrony observed in their previous brood (A: odds ratio = 1.08, CI = 0.82–1.44; S: odds ratio = 0.95, CI = 0.70–1.27; Table 3, Figure 2). Pair divorce was significantly negatively affected by male age (older males were less likely to divorce; Table 3).
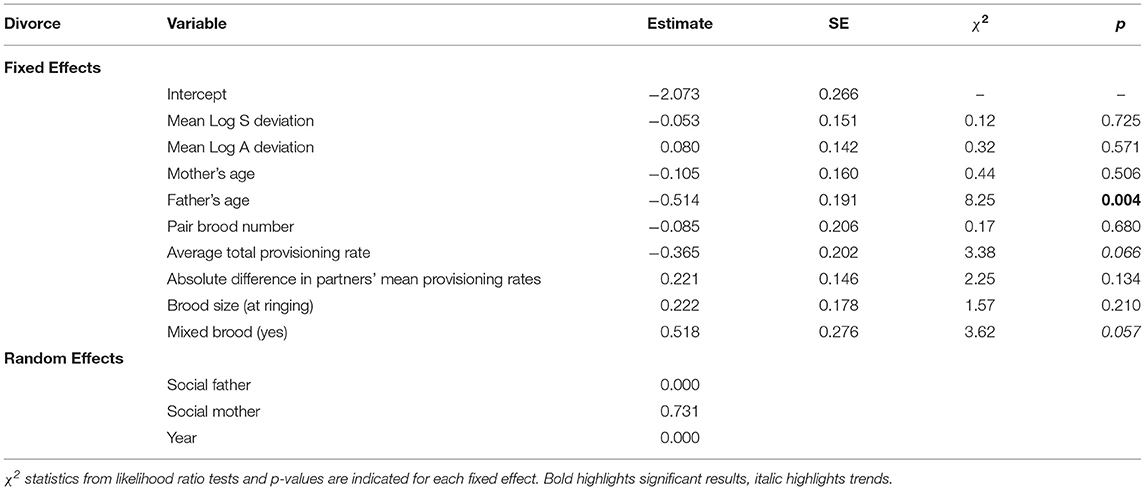
Table 3. Model estimates and standard errors (SE) for each of the predictors of pair divorce (N = 553 broods, subsequent to which 103 divorces occurred).
Discussion
In a previous study, we showed that alternation and synchrony in offspring provisioning in this wild population of house sparrows was higher than expected by chance. This was true when comparing observed data to all of our null models (Ihle et al., 2019). However, such comparisons are not sufficient to allow us to tease apart the patterns due to parental coordination from those induced by the parents each reacting to a shared environment. In this study, we did not find a positive association between offspring survival or body condition and the coordination deviation from randomness, nor did we find that the level of parental coordination predicts the likelihood of divorce. We discuss how these results may fit into, rather than contrast with, the current literature.
Despite increasing interest in behavioral coordination (Mariette and Griffith, 2012, 2015; van Rooij and Griffith, 2013; Johnstone et al., 2014; Bebbington and Hatchwell, 2016; Koenig and Walters, 2016; Iserbyt et al., 2017; Khwaja et al., 2017; Savage et al., 2017; Takahashi et al., 2017; Tyson et al., 2017; Leniowski and Wegrzyn, 2018), the fitness consequences of this emergent property for a pair have been assessed in only a few instances, when coordination was indeed observed to be higher than expected by chance. In an earlier study, a link between coordination in provisioning and provisioning rate itself was presented as evidence for an impact of coordination on fitness (Bebbington and Hatchwell, 2016); however, these variables are mathematically correlated (see Ihle et al., 2019) and, therefore, this evidence should be treated with caution. In Ihle et al. (2019), we showed that even when using a modeling approach intended to account for a mathematical relationship between dependent and independent variables, we could not prevent spurious effects from emerging when correlating coordination deviation and provisioning rate (we could only prevent spurious effects between coordination and variables correlated with provisioning rate). In other passerine species, and in line with our observations, coordination did not affect nestling survival or condition (Mariette and Griffith, 2012, 2015; van Rooij and Griffith, 2013; Bebbington and Hatchwell, 2016; Iserbyt et al., 2017), nor parental survival (Bebbington and Hatchwell, 2016). Nevertheless, evidence suggests that coordination (and especially synchrony of visits at the nest) could reduce the likelihood of depredation (Raihani et al., 2010; Mariette and Griffith, 2012, 2015; van Rooij and Griffith, 2013; Bebbington and Hatchwell, 2016; Leniowski and Wegrzyn, 2018). In contrast, in dual-foraging seabirds (alternating long trips to feed themselves and short trips to provision offspring), coordination in provisioning could prove crucial and strict coordination might be the only way to prevent starvation of the offspring or the partner (Takahashi et al., 2017; Tyson et al., 2017; Wojczulanis-Jakubas et al., 2018). Finally, following the idea that familiarity between partners could positively impact reproductive success through some sort of pair coordination (Black, 1996, but see Naves et al., 2007), one could hypothesize that familiarity between partners specifically increases coordination in provisioning (Westneat and Hatch, 2008). Similarly, pairs with increased coordination, possibly due to an increase in their pairbond duration, might be less likely to divorce. However, we did not find that pair-bond duration was linked to either better coordination in provisioning (Ihle et al., 2019) or improved reproductive success (this study), nor did we find that divorce was predicted by pairbond duration or higher alternation or synchrony (this study). Overall, the fitness consequences of behavioral compatibility in terms of pair coordination in raising offspring have yet to be demonstrated.
While coordination did not impact offspring survival and body condition (apart from an effect opposite expectation), and also did not predict parental divorce, other factors were important. Brood size had a negative effect on offspring mass and on offspring survival, while hatching date within the breeding season of this multi-brooded species positively affected nestling survival. These effects are known and have been discussed in detail elsewhere (e.g., Ringsby et al., 1998; Cleasby et al., 2010; Winney et al., 2015). We also found that the probability of parental divorce declined with male age. This effect has not been shown previously and invites further investigations. Because of the sex-specificity of this age effect, one could explore further the link between mate retention and territoriality, age, and breeding success (e.g., see Bai and Severinghaus, 2012; Culina et al., 2015), as well as with the potentially changing mating strategies with age in males (Hsu et al., 2015; Girndt et al., 2018).
If the adaptive significance of parental coordination in provisioning through conditional cooperation is questioned, how then can we explain that the observed patterns of alternation and synchrony exceed the magnitudes expected by chance alone? We cannot exclude a link between coordination and other aspects of the pairbond (e.g., benefits associated with foraging as a pair), nor can we exclude that taking into account more factors into the randomizations (i.e., subsetting our dataset to nest watches that meet specific criteria) could reveal the effects we expected or, to the contrary, extinguish the difference between the observed and random coordination. However, the positive deviation from randomness might simply be attributable to the coordination of both individuals' behavior to the environment surrounding their nest. As explained extensively in Schlicht et al. (2016), Ihle et al. (2019), and Santema et al. (2019), both pair members might have correlated patterns of inter-feeding intervals due to experiencing the same environmental conditions, which could lead to a pattern of apparently coordinated visits to the nest. Teasing apart environmentally induced patterns of alternation and synchrony from patterns of true coordination emerging from conditional cooperation will not be possible without measuring all environmental parameters (which is in itself probably impossible) or conducting logistically challenging experiments (e.g., Santema et al., 2017). It will therefore be crucial in further studies of pair coordination to explore fitness consequences, instead of solely showing that alternation or synchrony is higher than expected by chance.
Data Availability Statement
All datasets generated for this study are available in a permanent repository (http://doi.org/10.5281/zenodo.3459642).
Author Contributions
MI conceived the study with input from JP, IW, and TB. SN, JS, and TB provided the raw data. MI and JP did the analyses. MI drafted and revised the manuscript with input from all co-authors.
Funding
MI was funded by a grant from the Natural Environment Research Council (NERC, J024597/1) awarded to TB. JP was funded by a Swiss National Science Foundation Early Mobility grant (P2ZHP3 164962). IW was funded by a grant from the Volkswagen Foundation awarded to JS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00405/full#supplementary-material
References
Bai, M.-L., and Severinghaus, L. L. (2012). Disentangling site and mate fidelity in a monogamous population under strong nest site competition. Anim. Behav. 84, 251–259. doi: 10.1016/j.anbehav.2012.05.004
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bebbington, K., and Hatchwell, B. J. (2016). Coordinated parental provisioning is related to feeding rate and reproductive success in a songbird. Behav. Ecol. 27, 652–659. doi: 10.1093/beheco/arv198
Both, C., Dingemanse, N. J., Drent, P. J., and Tinbergen, J. M. (2005). Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 74, 667–674. doi: 10.1111/j.1365-2656.2005.00962.x
Carpenter, B., Gelman, A., Hoffman, M. D., Lee, D., Goodrich, B., Betancourt, M., et al. (2017). Stan: a probabilistic programming language. J. Stat. Softw. 76:1–32. doi: 10.18637/jss.v076.i01
Cleasby, I. R., Nakagawa, S., Gillespie, D. O. S., and Burke, T. (2010). The influence of sex and body size on nestling survival and recruitment in the house sparrow. Biol. J. Linn. Soc. 101, 680–688. doi: 10.1111/j.1095-8312.2010.01515.x
Cleasby, I. R., Nakagawa, S., and Schielzeth, H. (2015). Quantifying the predictability of behaviour: statistical approaches for the study of between-individual variation in the within-individual variance. Methods Ecol. Evol. 6, 27–37. doi: 10.1111/2041-210X.12281
Culina, A., Radersma, R., and Sheldon, B. C. (2015). Trading up: the fitness consequences of divorce in monogamous birds. Biol. Rev. 90, 1015–1034. doi: 10.1111/brv.12143
Gelman, A., and Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472. doi: 10.1214/ss/1177011136
Girndt, A., Chng, C. W. T., Burke, T., and Schroeder, J. (2018). Male age is associated with extra-pair paternity, but not with extra-pair mating behaviour. Sci. Rep. 8:8378. doi: 10.1038/s41598-018-26649-1
Hadfield, J. D., Heap, E. A., Bayer, F., Mittell, E. A., and Crouch, N. M. A. (2013). Intraclutch differences in egg characteristics mitigate the consequences of age-related hierarchies in a wild passerine. Evolution 67, 2688–2700. doi: 10.1111/evo.12143
Henderson, C. R. (1988). Theoretical basis and computational methods for a number of different animal models. J. Dairy Sci. 71, 1–16. doi: 10.1016/S0022-0302(88)79974-9
Hsu, Y.-H., Schroeder, J., Winney, I., Burke, T., and Nakagawa, S. (2015). Are extra-pair males different from cuckolded males? A case study and a meta-analytic examination. Mol. Ecol. 24, 1558–1571. doi: 10.1111/mec.13124
Ihle, M., Kempenaers, B., and Forstmeier, W. (2015). Fitness benefits of mate choice for compatibility in a socially monogamous species. PLoS Biol. 13:e1002248. doi: 10.1371/journal.pbio.1002248
Ihle, M., Pick, J. L., Winney, I. S., Nakagawa, S., and Burke, T. (2019). Measuring up to reality: null models and analysis simulations to study parental coordination in provisioning offspring. Front. Ecol. Evol. 7:142. doi: 10.3389/fevo.2019.00142
Iserbyt, A., Fresneau, N., Kortenhoff, T., Eens, M., and Muller, W. (2017). Decreasing parental task specialization promotes conditional cooperation. Sci. Rep. 7:6565. doi: 10.1038/s41598-017-06667-1
Johnstone, R. A., Manica, A., Fayet, A. L., Stoddard, M. C., Rodriguez-Gironés, M. A., and Hinde, C. A. (2014). Reciprocity and conditional cooperation between great tit parents. Behav. Ecol. 25, 216–222. doi: 10.1093/beheco/art109
Khwaja, N., Hatchwell, B. J., Freckleton, R. P., and Green, J. P. (2017). Sex allocation patterns across cooperatively breeding birds do not support predictions of the repayment hypothesis. Am. Nat. 190, 547–556. doi: 10.1086/693532
Koenig, W. D., and Walters, E. L. (2016). Provisioning patterns in the cooperatively breeding acorn woodpecker: does feeding behaviour serve as a signal? Anim. Behav. 119, 125–134. doi: 10.1016/j.anbehav.2016.06.002
Kruuk, L. E. B. (2004). Estimating genetic parameters in natural populations using the “animal model”. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 873–890. doi: 10.1098/rstb.2003.1437
Lattore, M., Plaza, M., Nakagawa, S., Burke, T., and Schroeder, J. (2019). No evidence for kin recognition in a passerine bird. bioRxiv 560870. doi: 10.1101/560870
Leniowski, K., and Wegrzyn, E. (2018). Synchronisation of parental behaviours reduces the risk of nest predation in a socially monogamous passerine bird. Sci. Rep. 8:7385. doi: 10.1038/s41598-018-25746-5
Lucass, C., Korsten, P., Eens, M., and Müller, W. (2016). Within-family parent–offspring co-adaptation in a wild bird: on static traits, behavioural reaction norms, and sex differences. Funct. Ecol. 30, 274–282. doi: 10.1111/1365-2435.12492
Mariette, M. M., and Griffith, S. C. (2012). Nest visit synchrony is high and correlates with reproductive success in the wild zebra finch Taeniopygia guttata. J. Avian Biol. 43, 131–140. doi: 10.1111/j.1600-048X.2012.05555.x
Mariette, M. M., and Griffith, S. C. (2015). The adaptive significance of provisioning and foraging coordination between breeding partners. Am. Nat. 185, 270–280. doi: 10.1086/679441
Nakagawa, S., Gillespie, D. O. S., Hatchwell, B. J., and Burke, T. (2007). Predictable males and unpredictable females: sex difference in repeatability of parental care in a wild bird population. J. Evol. Biol. 20, 1674–1681. doi: 10.1111/j.1420-9101.2007.01403.x
Naves, L. C., Cam, E., and Monnat, J. Y. (2007). Pair duration, breeding success and divorce in a long-lived seabird: benefits of mate familiarity? Anim. Behav. 73, 433–444. doi: 10.1016/j.anbehav.2006.10.004
Pelletier, K., Oedewaldt, C., and Westneat, D. F. (2016). Surprising flexibility in parental care revealed by experimental changes in offspring demand. Anim. Behav. 122, 207–215. doi: 10.1016/j.anbehav.2016.10.011
R Development Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/
Raihani, N. J., Nelson-Flower, M. J., Moyes, K., Browning, L. E., and Ridley, A. R. (2010). Synchronous provisioning increases brood survival in cooperatively breeding pied babblers. J. Anim. Ecol. 79, 44–52. doi: 10.1111/j.1365-2656.2009.01606.x
Ringsby, T. H., Sæther, B.-E., and Solberg, E. J. (1998). Factors affecting juvenile survival in house sparrow Passer domesticus. J. Avian Biol. 29, 241–247. doi: 10.2307/3677106
Santema, P., Schlicht, E., and Kempenaers, B. (2019). Testing the conditional cooperation model: what can we learn from parents taking turns when feeding offspring? Front. Ecol. Evol. 7:94. doi: 10.3389/fevo.2019.00094
Santema, P., Schlicht, E., Schlicht, L., and Kempenaers, B. (2017). Blue tits do not return faster to the nest in response to either short- or long-term begging playbacks. Anim. Behav. 123, 117–127. doi: 10.1016/j.anbehav.2016.10.016
Savage, J. L., Browning, L. E., Manica, A., Russell, A. F., and Johnstone, R. A. (2017). Turn-taking in cooperative offspring care: by-product of individual provisioning behavior or active response rule? Behav. Ecol. Sociobiol. 71:162. doi: 10.1007/s00265-017-2391-4
Schielzeth, H., Bolund, E., Kempenaers, B., and Forstmeier, W. (2011). Quantitative genetics and fitness consequences of neophilia in zebra finches. Behav. Ecol. 22, 126–134. doi: 10.1093/beheco/arq184
Schlicht, E., Santema, P., Schlicht, R., and Kempenaers, B. (2016). Evidence for conditional cooperation in biparental care systems? A comment on Johnstone et al. Behav. Ecol. 27:e2–e5. doi: 10.1093/beheco/arw036
Schroeder, J., Burke, T., Mannarelli, M. E., Dawson, D. A., and Nakagawa, S. (2012). Maternal effects and heritability of annual productivity. J. Evol. Biol. 25, 149–156. doi: 10.1111/j.1420-9101.2011.02412.x
Schroeder, J., Hannah, L., Dugdale Shinichi, N., Alex, S., and Terry, B. (2019). Social Genetic Effects (IGE) and genetic intra- and intersexual genetic correlation contribute to the total heritable variance in parental care. EcoEvoRxiv. doi: 10.32942/osf.io/nh8m2
Shen, S.-F., Chen, H.-C., Vehrencamp Sandra, L., and Yuan, H.-W. (2010). Group provisioning limits sharing conflict among nestlings in joint-nesting Taiwan yuhinas. Biol. Lett. 6, 318–321. doi: 10.1098/rsbl.2009.0909
Simons, M. J. P., Winney, I., Nakagawa, S., Burke, T., and Schroeder, J. (2015). Limited catching bias in a wild population of birds with near-complete census information. Ecol. Evol. 5, 3500–3506. doi: 10.1002/ece3.1623
Stan Development Team (2019). RStan: The R Interface to Stan. R package version 2.19.2. Available online at: http://mc-stan.org/
Takahashi, L. S., Storey, A. E., Wilhelm, S. I., and Walsh, C. J. (2017). Turn-taking ceremonies in a colonial seabird: does behavioral variation signal individual condition? Auk 134, 530–541. doi: 10.1642/AUK-17-26.1
Thomson, C. E., and Hadfield, J. D. (2019). No evidence for sibling or parent–offspring coadaptation in a wild population of blue tits, despite high power. Evolution 73, 28–41. doi: 10.1111/evo.13642
Tyson, C., Kirk, H., Fayet, A., Van Loon, E. E., Shoji, A., Dean, B., et al. (2017). Coordinated provisioning in a dual-foraging pelagic seabird. Anim. Behav. 132, 73–79. doi: 10.1016/j.anbehav.2017.07.022
van Rooij, E. P., and Griffith, S. C. (2013). Synchronised provisioning at the nest: parental coordination over care in a socially monogamous species. PeerJ 1:e232. doi: 10.7717/peerj.232
Westneat, D. F., and Hatch, M. I. (2008). Familiarity between mates improves few aspects of reproductive performance in house sparrows. Behaviour 145, 365–376. doi: 10.1163/156853908783402867
Winney, I., Nakagawa, S., Hsu, Y. H., Burke, T., and Schroeder, J. (2015). Troubleshooting the potential pitfalls of cross-fostering. Methods Ecol. Evol. 6, 584–592. doi: 10.1111/2041-210X.12341
Wojczulanis-Jakubas, K., Araya-Salas, M., and Jakubas, D. (2018). Seabird parents provision their chick in a coordinated manner. PLoS ONE 13:e0189969. doi: 10.1371/journal.pone.0189969
Keywords: breeding success, house sparrow, divorce, fitness, pairbond, double hierarchical model, brood size, male age
Citation: Ihle M, Pick JL, Winney IS, Nakagawa S, Schroeder J and Burke T (2019) Rearing Success Does Not Improve With Apparent Pair Coordination in Offspring Provisioning. Front. Ecol. Evol. 7:405. doi: 10.3389/fevo.2019.00405
Received: 24 April 2019; Accepted: 09 October 2019;
Published: 30 October 2019.
Edited by:
Camilla Anne Hinde, Wageningen University & Research, NetherlandsReviewed by:
Fumiaki Nomano, Graduate University for Advanced Studies (SOKENDAI), JapanAmanda R. Ridley, University of Western Australia, Australia
Copyright © 2019 Ihle, Pick, Winney, Nakagawa, Schroeder and Burke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malika Ihle, bWFsaWthX2lobGVAaG90bWFpbC5mcg==
 Malika Ihle
Malika Ihle Joel L. Pick2,3,4
Joel L. Pick2,3,4 Shinichi Nakagawa
Shinichi Nakagawa Julia Schroeder
Julia Schroeder Terry Burke
Terry Burke