- 1General and Systematic Zoology, Zoological Institute and Museum, University of Greifswald, Greifswald, Germany
- 2Department of Bioscience, Aarhus University, Aarhus, Denmark
- 3Department of Biology, Lund University, Lund, Sweden
Some semelparous species show terminal investment by suicidal offspring provisioning. This requires internal cellular disintegration for the production of regurgitated food and in preparation for the sacrifice of the female body to the offspring, however, we have limited insights into the extent and costs of such physiological modifications. Extreme provisioning is hypothesized to be limited to reproducing individuals because it requires physiological alterations triggered by reproduction. However, non-reproducing helpers-at-the-nest have been shown to engage in suicidal provisioning, prompting us to ask whether helpers undergo similar physiological alterations to brood provisioning as mothers, which would represent an adaptation to cooperative breeding. Using an experimental approach, we investigated the physiological consequences of extended maternal care in the solitary spider Stegodyphus lineatus and the cooperative breeder S. dumicola, and whether non-reproducing helpers (virgin allomothers) in S. dumicola show physiological adaptations to brood provisioning. To identify costs of offspring provisioning, we determined the energy expenditure (standard metabolic rate; SMR) and tissue disintegration over the course of brood care. In both species, brood care is associated with elevated SMR, which was highest in allomothers. Brood care results in progressive disintegration of midgut tissue, which also occurred in allomothers. On experimental offspring removal, these responses are reversible but only until the onset of regurgitation feeding, marking a physiological “point-of-no-return.” The mechanism underlying the onset of physiological responses is unknown, but based on our finding of mature eggs in mothers and allomothers, as opposed to the undeveloped eggs in virgins of the solitary species, we propose that oocyte maturation is a central adaptation in non-reproducing helpers to provide terminal allomaternal care.
Introduction
Parental care involves a wide range of behavioral and physiological adaptations that increase the fitness of a parent's offspring (Trivers, 1972; Clutton-Brock, 1991; Royle et al., 2012). Parental care is most commonly performed by females, and in the most extreme cases it involves regurgitation feeding of the offspring for a prolonged period and matriphagy, in which the female sacrifices her body through cellular disintegration as a terminal investment in her brood (Smiseth et al., 2012). The investment in parental care relative to somatic maintenance or growth is strongly influenced by life history and ecology (Stearns, 1992). In iteroparous species, which reproduce more than once in their lifetime, trade-offs between current and future reproduction are expected to lead to progressive increase in reproductive effort with age as residual reproductive value declines (Pianka, 1976; Clutton-Brock, 1991). Semelparous species that reproduce only once are under selection to allocate all available energy into their single brood (Stearns, 1992; Roff, 2002; Alonso-Alvarez and Velando, 2012), and this terminal investment may favor major and potentially irreversible physiological adaptions to increase the efficiency of maternal care. Our knowledge of the physiology and plasticity of responses associated with extreme brood provisioning is, however, limited. This applies both to solitarily reproducing species, and perhaps even more so for cooperative breeders, where non-reproducing helpers also engage in extreme offspring care.
Cooperative breeders show reproductive division of labor, where a few individuals produce the offspring and closely related helpers take over some or all aspects of parental care. The provision of extended brood care by non-reproducing helpers is known from cooperatively breeding insects, spiders, birds, and mammals (Wilson, 1971; Choe and Crespi, 1997; Lubin and Bilde, 2007; Cant, 2012). The inclusive fitness benefits obtained by helpers through their investment in brood care may favor traits that increase the effectiveness of alloparental care (Wilson, 1971; Creel et al., 1991; Adkins-Regan, 2005; Montgomery et al., 2018). Such traits could be physiological adaptations as for example thermoregulation or regurgitation of nectar to produce honey by the worker bee (Wilson, 1971; Choe and Crespi, 1997; Cant, 2012). A particularly interesting question in the context of parental care is whether direct offspring provisioning requires physiological adaptations in non-reproducing helpers. For example, the ability to lactate is expected to be triggered by hormones or development of organs associated with reproduction (Patton and Neville, 1997), and may therefore be limited to reproducing individuals within the group. Interestingly, the ability to perform spontaneous lactation in mongoose helpers is coupled with pseudopregnancy, which indicates an adaptation to cooperative breeding (Creel et al., 1991). Regurgitation feeding of the offspring with previously digested food is also expected to require special adaptations, and may depend on cellular degradation of the gut tissue (Nawabi, 1974; Salomon et al., 2015). The exhibition of physiological traits in non-reproducing helpers that enable offspring provisioning by regurgitation feeding therefore represents an adaptation to cooperative breeding, a hypothesis that has not yet been investigated.
Spiders exhibit maternal care by wrapping their eggs in silk cases and guarding the offspring (Foelix, 2011), or provisioning the offspring with captured prey (Avilés, 1997; Lubin and Bilde, 2007). Some species show extended care by performing regurgitation feeding, i.e., females provide a nourishing fluid for the offspring by regurgitation. This process is thought to be an energetically demanding task that is accompanied by physiological changes involving degradation of the midgut (Nawabi, 1974; Salomon et al., 2015), which functions as a storage organ for fat and glycogen (Alberti and Storch, 1983). Several genera also show matriphagy, as females are consumed by their offspring following the provisioning period (Kullmann, 1968; Toyama, 1999; Kim et al., 2000; Viera et al., 2007; Foelix, 2011). Here, we investigated the metabolic cost and physiological consequences of reproduction and offspring provisioning in two species of semelparous spiders of the genus Stegodyphus, specifically in one solitary and one cooperatively breeding species. In both species, mothers provide extended maternal care including regurgitation feeding and matriphagy, and in the social species also the helpers (allomothers) engage in regurgitation feeding and are consumed by the offspring (Kraus and Kraus, 1988; Lubin and Bilde, 2007). We hypothesized that in both species, reproduction and regurgitation provisioning are associated with an up-regulation of cost intensive processes in relation to egg production and organ restructuring for offspring care until matriphagy (Speakman and McQueenie, 1996; Vanfleteren and DeVreese, 1996; Ruhland et al., 2016; Fowler and Williams, 2017), which can result in elevated standard metabolic rate (SMR) (Barnes and Partridge, 2003; Metcalfe and Alonso-Alvarez, 2010). We tested this prediction experimentally by determining changes in SMR in response to oviposition and regurgitation provisioning. In parallel, we investigated the dynamics of internal morphological changes in response to brood care, with focus on the midgut tissue as the primary storage organ in spiders. We determined when structural changes of the midgut occur during the reproductive cycle of both species. Experimentally, we also examined whether physiological changes to the midgut are permanent once the process has been initiated, or reversible upon experimental removal of the eggs or offspring. A female can produce a replacement clutch if she loses her brood (Schneider and Lubin, 1997a; Futami and Akimoto, 2005; Viera et al., 2007), but depending on when the brood is lost, there might be a point of no return in the physiological dynamics of offspring provisioning.
The cooperatively breeding Stegodyphus species show reproductive skew in which up to 80 percent of females in a nest are unmated (Salomon et al., 2008). Mothers as well as female helpers provide extended maternal and allomaternal care (Lubin and Bilde, 2007; Salomon and Lubin, 2007; Junghanns et al., 2017). Since allomaternal care is provided by mated, reproducing females as well as by unmated, non-reproducing females (Junghanns et al., 2017), this warrants the question of whether the evolution of allomaternal care by non-reproducing helpers is associated with physiological adaptations that trigger the ability to provide regurgitation feeding. Using the cooperative S. dumicola, in which both mothers and allomothers perform similar tasks and share the workload (Junghanns et al., 2017), we examined if non-reproducing helpers exhibit adaptations to offspring provisioning, and whether potential changes in energy allocation patterns (SMR) and dynamics of changes in the midgut tissue (histological examinations) in response to offspring provisioning are permanent or reversible.
The mechanisms that trigger the onset of physiological preparations for regurgitation provisioning are not well understood. If reproduction activates the ability to provide for the offspring, this would support the hypothesis that mating or oviposition initiates an internal maturation process that physiologically enables mothers to provide regurgitation feeding (Krafft and Horel, 1980; Feneron et al., 1996; Schal et al., 1997; Schneider, 2002; Mas and Kolliker, 2008; Pinilla et al., 2012). We investigated whether oocyte maturation is a proxy for reproductive maturation and a prerequisite for the ability to provide regurgitation feeding. In the solitarily breeding S. lineatus, experimental cross-fostering previously revealed that non-reproducing (virgin) females do not adopt and care for cross-fostered brood (Schneider, 2002). In contrast to the solitary species, however, non-reproducing helpers in the cooperatively breeding Stegodyphus engage in all aspects of allomaternal care (Junghanns et al., 2017), suggesting the evolution of adaptations to offspring provisioning in non-reproducing helpers. This ability may be triggered by oocyte development, as unmated S. dumicola can produce unfertilized egg sacs (A. Junghanns and C. Holm, pers. obs.), in contrast to unmated solitary S. lineatus (Y. Lubin, J Schneider, and T. Bilde, pers. obs.). We propose that reproductive maturation is a prerequisite for triggering extended brood care in prospective allomothers, and predict that unmated females undergo development of their reproductive organs in preparation for brood provisioning as helpers. We investigated this prediction by comparing oocyte maturation as a proxy for brood provision ability between mothers and non-reproducers of the solitary S. lineatus and mothers, non-reproducing helpers and non-helpers of the cooperative S. dumicola.
Materials and Methods
Study Species
The spider genus Stegodyphus (Eresidae) contains 20+ species (Kraus and Kraus, 1988; World Spider Catalog, 2018), most of which are solitarily breeding, subsocial species that show extended offspring care. Cooperative breeding has evolved independently three times, suggesting that subsocial behavior is the ancestral state (Johannesen et al., 2007; Settepani et al., 2016). Females are semelparous, and mothers and helpers of the social species provide extensive maternal care, in which offspring are provisioned by regurgitation feeding and female self-sacrifice (Lubin and Bilde, 2007). The solitary S. lineatus oviposits March-June, and tends the egg sac for 30 days (Millot and Bourgin, 1942). Females provision the offspring with regurgitated fluids and are consumed by their offspring about 2 weeks after hatching (Schneider, 1995). The social spider S. dumicola lives in communal nests, which arise from a single mated female and her offspring (Lubin and Bilde, 2007; Settepani et al., 2017). Females oviposit December-February, and mothers and allomothers care cooperatively for the offspring for several months until they are consumed by the offspring (Seibt and Wickler, 1987; Salomon and Lubin, 2007).
Collection Sites and Animal Maintenance
Stegodyphus lineatus was collected in Israel in April 2012, from dry water courses at two sites, Mt. Amasa (31.31N, 35.12E) and Lehavim (31.36N, 34.83E), with a total number of 215 individuals. Females were collected before they matured to adulthood and therefore prior to oviposition, to follow them through their entire reproductive and maternal care period (mothers), and to assure that we had virgin females available (virgin controls). Mothers and virgin controls were kept within their natural nest in individual plastic containers (90 × 70 mm), at a constant temperature of 25°C and a 12:12 h light:dark period. Until oviposition, mothers were provided with a diet of houseflies or crickets two-three times/week, after which feeding was stopped as they do not forage during brood care (Schneider et al., 2003). Virgin controls followed the same feeding scheme and were not fed after mothers had oviposited.
The cooperative S. dumicola was collected in South Africa during two consecutive summers before females matured. The first collection took place in November 2013 at three sites, Shingwedzi (−22.98S, 31.30E), Middelfontein (−24.68S, 28.55E), and Mokopane (−24.40S, 28.78E), where a total number of 24 nests was collected. The second collection took place in November 2014 from two sites, Shingwedzi (−22.98S, 31.30E) and Skukuza (−24.9S3, 31.69E), with four nests used for histological analysis. To ensure virginity, subadult females were separated from males and raised to adulthood. Some of the mature females were paired overnight with a male from the same nest. If traces of secretion were found on the females' genital openings the next day, she was considered mated (Junghanns pers. obs.) and was then used as a mother in small experimental colonies created for studying brood care. Unmated, adult females were used as allomothers (helpers) and were grouped with a mother from the same nest. In both seasons, virgin females from laboratory colonies that contained unmated, non-helping females (kept without males and reproducing females) were used to assess potential internal changes in the absence of brood care (virgin control).
In the first season, the experimental colonies of S. dumicola contained 1-2 mated females and three allomothers with a total number of 334 groups. All spiders were kept in a climate chamber at 25°C with a 13:11 h light:dark period. In the second season, 21 colonies consisting of one mated female and three allomothers were used for histological examinations. Experimental and control colonies experienced a 12:12 light:dark period and temperatures of 19°C at night and 27°C during the day with a peak temperature of 30°C for 2 h at noon. Experimental colonies were kept in transparent plastic containers (122 × 82 × 52 mm) with a plastic ring (diameter 53 mm) for silk attachment. Control virgins were kept in hexagonal plastic boxes (180 × 180 × 60 mm). All colonies were fed two to three times per week during the entire experiment with a diet of houseflies and crickets.
Experimental Design
The experimental design is outlined in Figure 1. First, we assessed changes in SMR and morphology (midgut and ovaries) in females at different stages during the natural brood care period in both S. lineatus and S. dumicola (natural group). Second, we determined the reversibility of physiological changes by experimentally removing eggs or offspring from mothers at different stages during the brood care period (removal groups). Since in S. dumicola, all colonies contained mothers and allomothers (non-reproducing helpers), we were at the same time able to investigate the effect of egg sacs and offspring removal on allomothers at different stages. To assess physiological changes that are not due to brood care, we established virgin controls, i.e., virgin females that we followed over time. The natural and removal groups in combination with virgin controls enabled us to address the following questions: (1) Does extreme brood care involve physiological changes in mothers? (2) Are physiological changes during brood care reversible, and if so, until which stage(s) during the brood care period? (3) Do allomothers experience similar physiological changes as mothers? (4) Is the ability to provide extreme brood care associated with oocyte maturation?
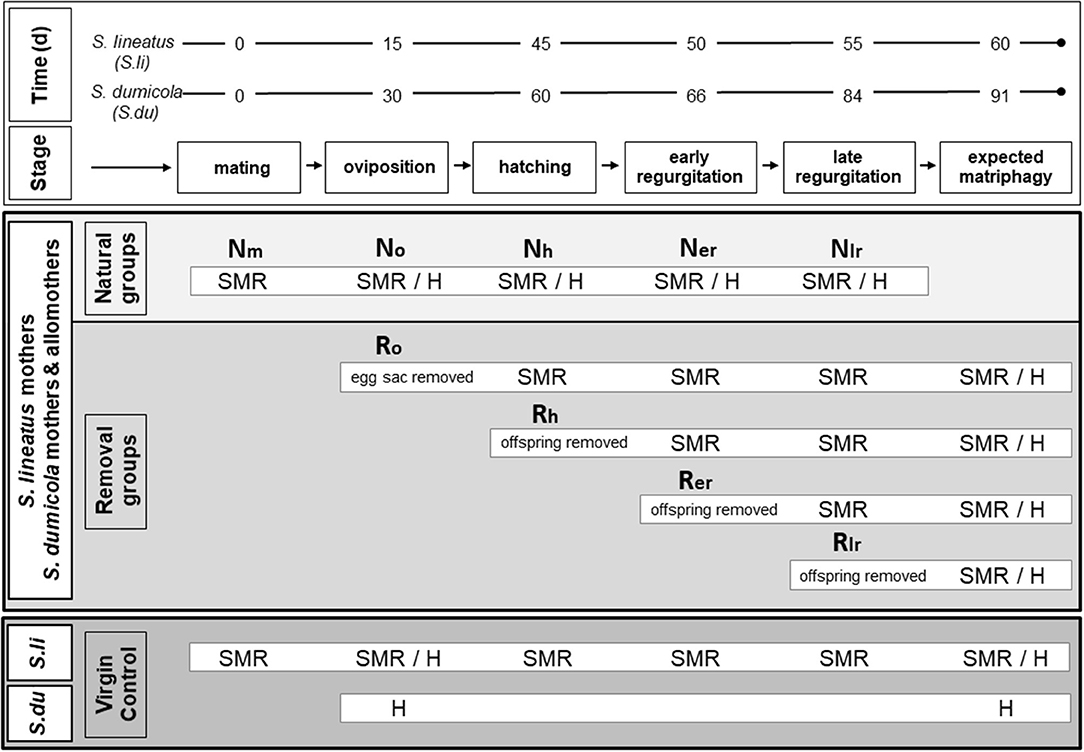
Figure 1. Experimental design for examining physiological effects of brood care in females of the subsocial S. lineatus and cooperative S. dumicola. Upper box (white): representative time frames in days (d) for the reproductive cycle in the solitary breeding S. lineatus (S. li) and the cooperative breeding S. dumicola (S. du). Middle box (light/medium gray): treatment of the experimental females (S. li: mothers; S. du: mothers and non-reproducing helpers/allomothers). Natural groups (light gray): SMR measurements or chemical fixation for histological analyses (H) took place after mating (Nm), oviposition (No), hatching of offspring (Nh), early in the regurgitation period (Ner; S. li = 5 days after hatch; S. du = 6 days after hatch) and late in the regurgitation period (Nlr; S. li = 10 days after hatch; S. du = 24 days after hatch). Removal groups (medium gray): egg sacs or offspring were removed at different stages (Ro/oviposition, Rh/hatching, Rer/early and Rlr/late regurgitation), and females were kept alive until the time of expected matriphagy (S. li = 15 days after hatching; S. du = 31 days after hatching) and then chemically fixed for histological analyses. SMR was measured at all following stages after experimental removal. Lower box (dark gray): virgin controls. In S. lineatus SMR of virgin controls was measured whenever a mother from the natural group was measured and at expected matripaghy. Virgin controls of both species (virgin S. li and virgin, non-helping S. du) were examined histologically in the beginning and the end of the experimental period.
All S. lineatus mothers and the experimental colonies of S. dumicola were checked every day for oviposition. After oviposition, individuals/colonies were randomly assigned either to the natural group or to a removal group (Figure 1). The natural group followed an undisturbed course of brood care and standard metabolic rate (SMR) was measured at the following stages of the females' reproductive cycle: “Nm” mating, “No” oviposition, “Nh” the day when offspring hatched (hatching), “Ner” midway in the phase of regurgitation feeding (early), and “Nlr” end of regurgitation feeding (late) (Figure 1, natural group). For histology, some spiders from all groups (except for Nm and at the time of matriphagy) were chemically fixed (Figure 1). SMR measurements and chemical fixation was done 1 day after the respective stage was reached.
The colonies in the four removal groups (Figure 1) were manipulated by removing either the eggs or offspring at different stages corresponding to those of the natural groups as explained above (termed Ro, Rh, Rer, Rlr), to examine the effect of removal on SMR (measured the day after removal), midgut morphology, and oocyte stage. In all removal groups, females were maintained until their expected death by matriphagy, at day 15 after hatching of the spiderlings for S. lineatus (Schneider, 1995) and at day 31 for S. dumicola (Henschel et al., 1995; Reut Berger-Tal pers. comm.). If a replacement clutch was laid, it was removed. At the time of expected matriphagy the females were chemically fixed to investigate midgut integrity and oocyte stage histologically.
Whenever SMR was measured in a S. lineatus mother of the natural group, a virgin control was measured in parallel. Mother and virgin control were matched as to the amount of time that had passed from the beginning of the experiment (Figure 1). In SMR measurements of S. dumicola, mothers and allomothers from the same experimental colony were measured simultaneously. For histology, virgin non-caring controls of both species were sampled and chemically fixed to assess the state of their midgut and ovaries in the beginning and at the end of the experimental period. In S. lineatus, these virgin controls were approximately between 60 and 70 days old (since maturation to adulthood) when used for histology. This corresponds to the age of senescence of reproducing females, destined to be consumed by their offspring. Based on their age and on multiple experiments raising females both under laboratory and semi-natural conditions, we are quite confident that virgin S. lineatus do not produce egg sacs. As virgin controls in S. dumicola matured within colonies it was impossible to determine the exact age of a female. However, S. dumicola virgins were on average younger than S. lineatus virgins. This suggests that the pattern of egg maturation (mature eggs in virgin S. dumicola, immature eggs in virgin S. lineatus) is likely to be robust: despite the older age of virgin S. lineatus they still had less developed ovaries.
Measuring Standard Metabolic Rate
Standard metabolic rate (SMR) was estimated from the rate of CO2 production (VCO2) by repeated measurements using stop-flow respirometry (Lighton and Halsey, 2011) in a setup as described by Jensen et al. (2014). Individual spiders were randomly assigned to a measuring chamber (glass cylinder L: 9 × D: 2 cm), which was held at a constant temperature of 25°C with a 12:12 h light:dark period (S. lineatus) or 13:11 h light:dark period (S. dumicola). To avoid desiccation, a piece of filter paper (15 × 15 mm) with 0.25 ml solution of 2% agar was added to each chamber. The system used two parallel 8 channel multiplexers (RM Gas Flow Multiplexer, Sable Systems, Las Vegas, Nevada, USA) allowing for measurements of 16 parallel respirometry chambers that were measured sequentially by opening and closing the chambers. These measurements were repeated over a period between 18 and 24 h. Measurements were obtained by flushing the chambers with CO2 free air (washed in a soda lime column, MERCK Millipore, Darmstadt, Germany) at a fixed rate of 250 mL min−1. The flow was controlled by a flow meter (Side-Trak®, Sierra Instruments, Monterey, California, USA) and a flow controller (MFC 2-channel v. 1.0, Sable Systems, Las Vegas, Nevada, USA). Each chamber was flushed every 30 min (S. lineatus) or 40 min (S. dumicola) resulting in ~30–48 or 35 independent measurements of metabolic rate during the entire measurement period. The first three measurements were excluded to eliminate effects of stress from handling, and as these spiders are very sedentary, we estimated SMR from the average of the three lowest values obtained during the day of measurement. This was done to gain the intrinsic metabolic rate and not the total energy budget that would include phases of activity and handling stress.
The rate of CO2 production was calculated from the raw data, with a script in Mathematica (version 7.0, Wolfram Research, Champaign, Illinois, USA) by assessing a baseline for each CO2 peak and integrating the area below the curve. Any abnormalities in the plot were discarded by manual checks. See further details in Jensen et al. (2014). Data are reported as mass specific metabolic rate (μL/min/g).
Statistical Analysis of SMR
SMR measurements were analyzed using general linear mixed models (glmm) with Gaussian errors in the R package “lme4” v. 1.1–15 (Bates et al., 2015). As the different removal groups were initiated at different stages of the reproductive cycle, each differed in the number of stages that followed removal (Figure 1). For example, females that had their eggs removed at oviposition (Ro) were measured four times from the stage of hatching to matriphagy, while females that had their offspring removed at the time of hatching (Rh) were measured three times from time of early regurgitation to the time of expected matriphagy. For this reason, we constructed separate models for each removal group. In each model, the measurement from the removal group was compared to the measurements from the respective stage of the natural group (e.g., Ro compared to No). Due to limited data, the late regurgitation stages were not statistically analyzed (Rlr vs. Nlr, see Tables S2–S4 for details).
For S. lineatus, SMR comparisons were performed between (1) the sequence of stages of the natural group and the virgin controls, and (2) in separate comparisons of each removal group stage with the corresponding natural group (oviposition: No vs. Ro; hatching: Nh vs. Rh; early regurgitation: Ner vs. Rer; Table S2 for sample sizes). Each full model consisted of the fixed effect natural group, removal group, or virgin control, stage across the reproductive period (continuous variable, with the first stage present in a model being 0 and subsequent stages 1, 2, 3…), and the interaction between group and stage. Hence, in case of a significant interaction, model coefficients for the main fixed effect of group refer to the difference between groups in the first stage of the model. As spiders were measured multiple times across and within stages, spider ID was included as a random effect. If we identified a significant difference in SMR between a natural and a removal groups, we subsequently compared this removal group to the virgin controls. Accounting for multiple measures by the inclusion of spider ID as a random effect caused some structure in the residual plots. We identified this structure as an overfitting of the individuals only measured once. To ensure that this did not produce spurious significance, we validated all significant p-values using subsets of the data. We did not find any deviation between the original results and the results in the validation (Table S1).
In S. dumicola, mothers and allomothers of the natural groups (Tables S3, S4 for sample sizes) were compared by constructing a glmm including stage of reproductive cycle (continuous as for S. lineatus) and reproductive role (mother, allomothers) as fixed effects as well as their interaction. The colonies were included as random effects in all models of S. dumicola (see Text S1 for details). To investigate whether mothers and allomothers were affected differently across the different removal groups, we compared each removal group with the corresponding stage of the natural group as was done for S. lineatus, however, with the inclusion of an additional fixed effect differentiating mothers from allomothers. The group comparisons performed were No vs. Ro, Nh vs. Rh, and Ner vs. Rer. The full models for each pairwise group comparison contained the fixed effects stage (continuous as for S. lineatus), group and female role, and all possible interactions.
For all models, significance of the highest order interaction term was evaluated by comparing the full model with a reduced model in which the highest order interaction term had been omitted. If the interaction term was non-significant it was omitted from the full model, which was then further reduced to evaluate the significance of each of the lower order terms and so forth. In case of a significant interaction, further model reductions and significance testing of involved main effects were halted. Models fulfilled assumptions of parametric analysis unless noted and all model comparisons were performed with likelihood ratio tests. All statistical analyses were performed in R (R Development Core Team, 2018).
Histology
Natural groups: To assess morphological changes in the midgut tissue during the natural course of brood care, brood caring females were chemically fixed at four stages as in the SMR analyses: No) at oviposition by the mothers, Nh) after hatching of the offspring, Ner) in an early regurgitation phase and Nlr) in a late regurgitation phase (Figure 1). To this aim, the opisthosomata of 9 S. lineatus mothers, 14 S. dumicola mothers and 35 S. dumicola allomothers (from 10 nests) were fixed on the day or 1 day after the respective stages were reached.
Removal group: To investigate whether potential changes are reversible, we investigated 11 S. lineatus mothers, 19 S. dumicola mothers and 49 S. dumicola allomothers (from 10 nests) from which eggs or offspring had been removed at the same life stages as given above (Ro, Rh, Rer, Rlr). The females were maintained until the expected date of matriphagy (see Figure 1 “removal groups”) and then chemically fixed.
We additionally examined five virgin females of S. lineatus and 14 unmated non-helping females (virgin control) from seven nests of S. dumicola, the latter having been kept in colonies consisting of unmated females only. All females were anesthetized with CO2 before their opisthosomata were separated from the prosoma and the region around the spinnerets was cut off to enable sufficient penetration of the tissue by the fixative. The opisthosomata were chemically fixed in Duboscq-Brasil after (Bouin, 1887) for at least 1 week. The samples were dehydrated in an alcohol series, transferred to Tetrahydrofuran (THF) and embedded in paraffin (Rotiplast). Five micrometers sections were produced with a rotation microtome HM 360 and then stained with AZAN (Geidies, 1954). AZAN stains basophilic structures in red while acidophilic structures are stained blue. As a result, the nuclei are stained red, connective tissue light blue, secretion blue, and granules of the cells blue, red, or yellow (Burck, 1988). Staining does not stain regurgitate only, but also other material of similar biochemical properties. Thus, we focus on liquefied (blue) material in the gut region. More coarse material was not considered regurgitate but food remnants. The samples were analyzed and photographed using an Olympus BX60 System Microscope and Zeiss Axio Vision 4.8. To avoid interpretation bias, the histological sections were analyzed blind with regard to the identity of the samples. We categorized morphological traits of the midgut tissue as correlates for changes during brood care: the abundance of secretion granules (blue stained granules) and the abundance of extracellular fluids, both of which are considered to accumulate for regurgitation purposes (Nawabi, 1974; Salomon et al., 2015). Sample sizes are reported in Table S5.
Results
Variation in SMR Over the Maternal Care Period in S. lineatus
We found a significant and increasing difference in SMR between mothers in the natural group and virgin controls over time, with mothers showing higher SMR than virgins, illustrated by a significant interaction term between stage and group (Table 1; Figure 2A). This effect was mainly driven by a decrease in SMR over time in virgin controls, while mothers in the natural group had a stable SMR across reproductive stages (Table 1; Figure 2A). We compared mothers of the four removal groups with the mothers of the corresponding natural groups. In response to egg removal, Ro mothers had a significantly and consistently lower SMR than No mothers (Table 1, Figure 3). SMR of mothers in the removal groups at the time of hatching (Rh) and of early regurgitation (Rer) did not differ significantly from that of Nh and Ner mothers (Table 1; Figure 3). These results suggest that (1) mothers in the period where they provide offspring care maintain a higher SMR than virgin females; and (2) the elevated SMR during brood care is reversible: the SMR of mothers that have their egg sac removed before hatching of the eggs steadily returns to a state similar to that of a virgin female, while removal of offspring after hatching does not cause a reduction in SMR (compare Figure 2A and Figure 3).
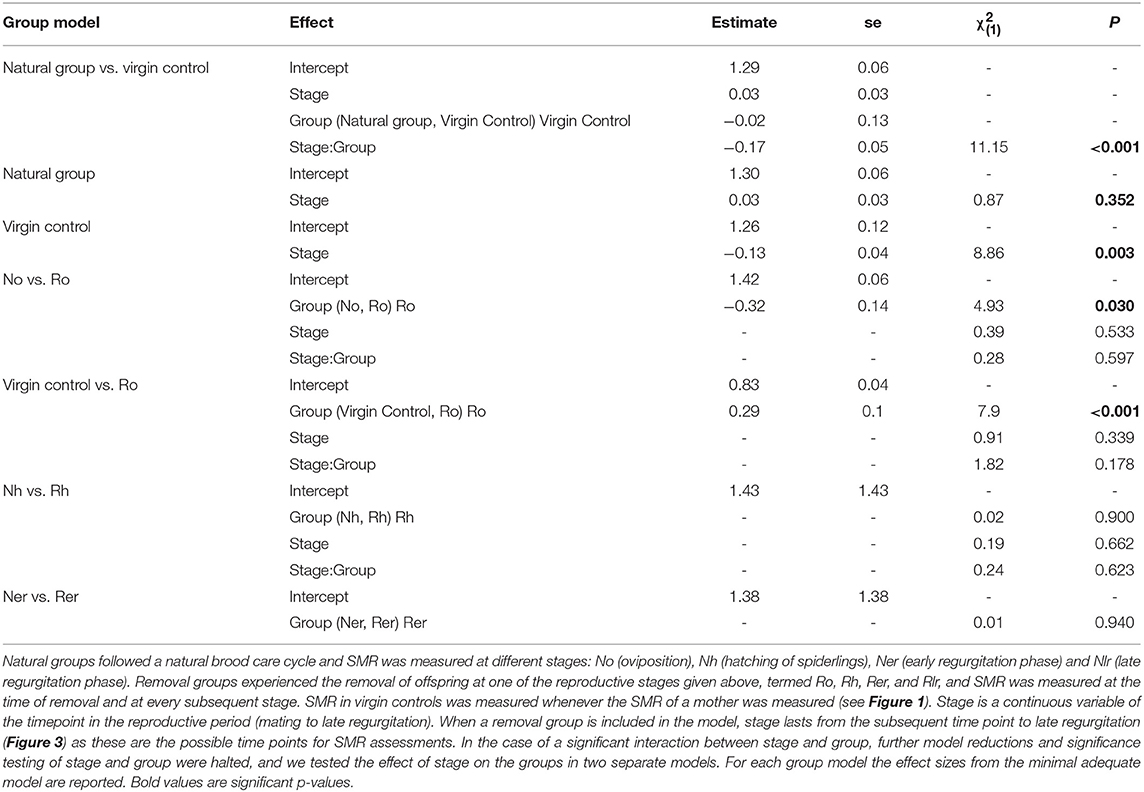
Table 1. Statistical analyses of SMR in mothers of the natural and removal groups and virgin control females in the subsocial S. lineatus.
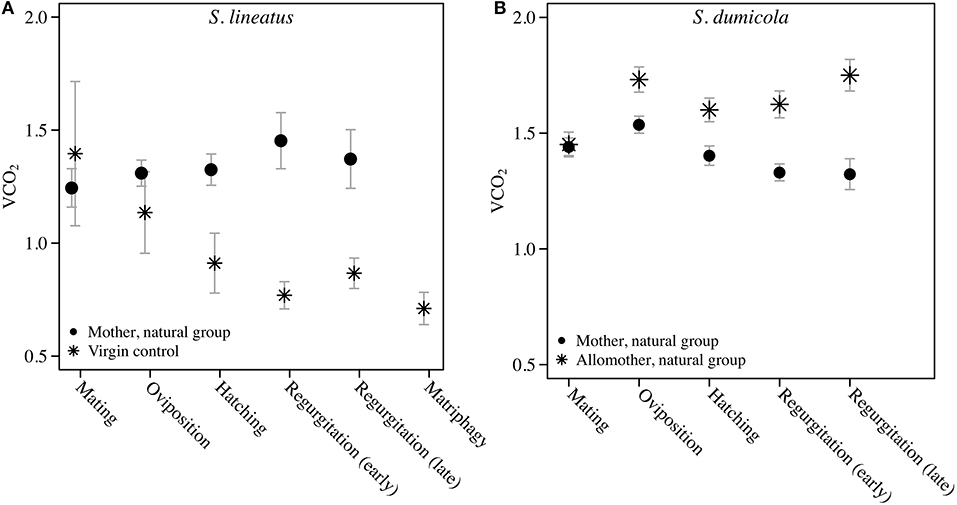
Figure 2. Data on Standard Metabolic Rate measured as VCO2 (μL CO2/minute/gram) during the natural brood care period (natural group) in mothers and virgin control females of the solitary spider S. lineatus (A), and in mothers and allomothers (non-reproducing helpers) of the cooperative spider S. dumicola (B). For each measurement of mothers, simultaneous measurements were performed on virgin controls (S. lineatus) or allomother females (S. dumicola). The mothers succumbed to matriphagy and were thus not measured at this stage. The VCO2 measure provided is the average of the 3 lowest measurements out of 40. Error bars show the standard error of the mean.
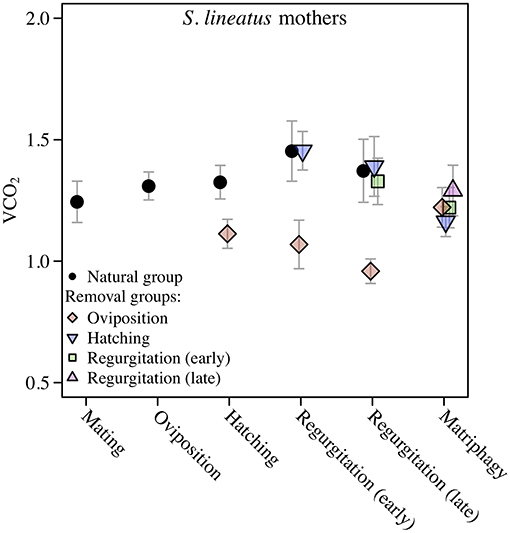
Figure 3. Data on Standard Metabolic Rate (SMR) measured as VCO2 (μL CO2/minute/gram) during the maternal care period in mothers of the solitary spider S. lineatus. SMR of natural mothers (reproducing females that followed a natural brood care cycle) was measured at mating, oviposition, hatching of offspring, early regurgitation and late regurgitation. Natural mothers succumbed to matriphagy and were thus not measured at this stage. In the removal groups, mothers were measured after removal of the egg sac or offspring at the same stages as well as at expected time of matriphagy. The VCO2 measure provided is the average of the 3 lowest measurements out of 40. Error bars show the standard error of the mean. The natural group data presented here is the same data as presented in Figure 2A.
Changes in SMR Over the Maternal Care Period in S. dumicola
Comparing SMR between allomothers and mothers in the natural groups revealed consistently higher SMR in allomothers from the time of oviposition and onwards (Figure 2B). A significant interaction term between stage and reproductive role (allomother/mother) indicates that the disparity in SMR of allomothers and mothers increased over time (Table 2; Figure 2B). SMR in S. dumicola mothers showed a decreasing trend over the brood care period while allomothers did not (Table 1, Figure 2B). As in S. lineatus mothers, both mothers and allomothers in S. dumicola showed a sharp decrease in SMR after egg sac removal (compare Figure 3 and Figures 4A,B), suggesting that experimental removal of the egg sac resulted in a significant reduction in SMR in both mothers and allomothers compared to females in the natural groups (Table 2). After hatchlings were removed, S. dumicola allomothers showed higher SMR than mothers (Table 2, compare Figure 4A and Figure 4B). There was no reduction in SMR compared to the females from the natural group (Nh) (Table 2; Figures 4A,B). When regurgitation feeding had begun, removal of offspring likewise did not cause a significant reduction in SMR in mothers and allomothers compared to females from the natural groups, but SMR of allomothers was again higher compared to mothers (Table 2; Figures 4A,B). Overall, this suggests for the social S. dumicola that (1) allomothers exhibit a higher SMR than mothers over the entire period of offspring care; and (2) the elevated SMR during brood care is reversible both for mothers and allomothers if the egg sac is removed.
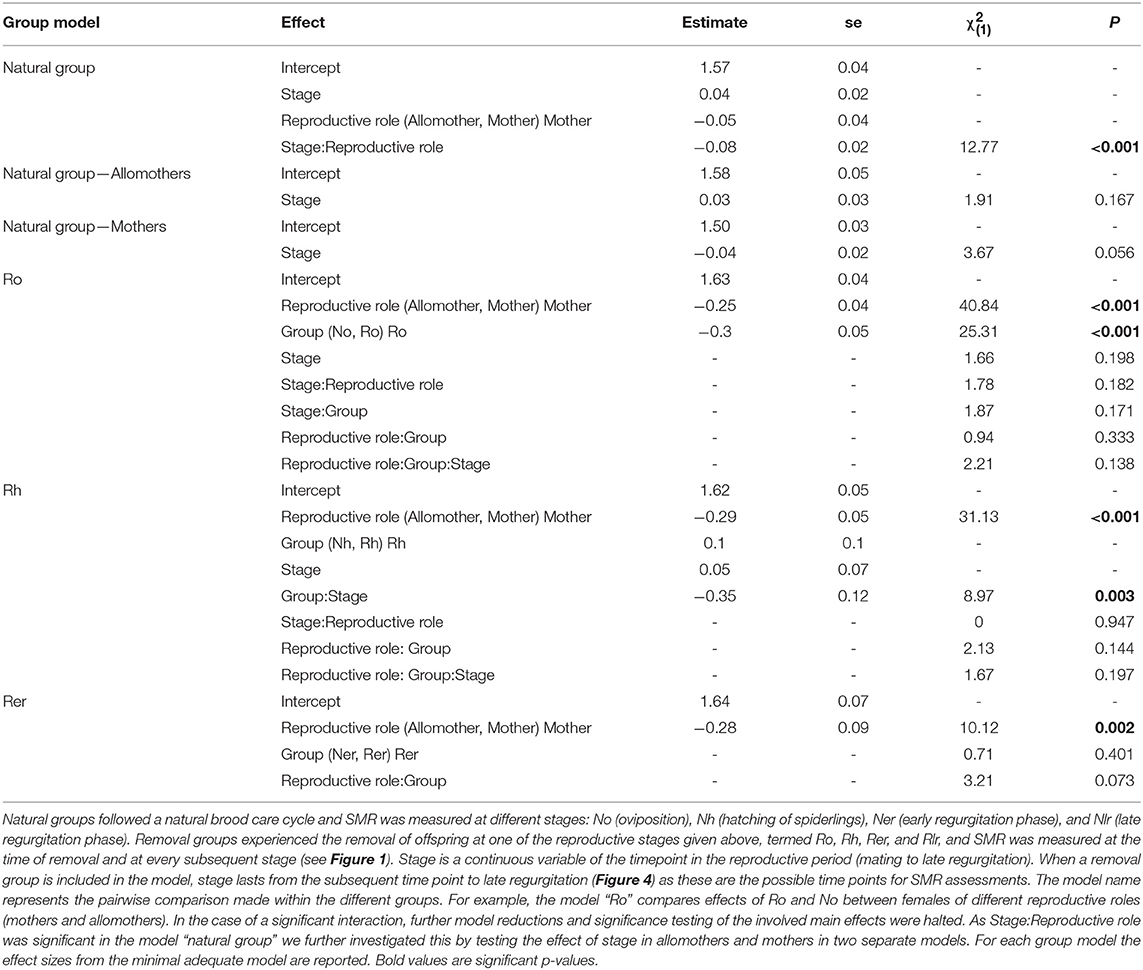
Table 2. Statistical analyses of SMR in mothers and allomothers of the natural and removal groups in the social S. dumicola.
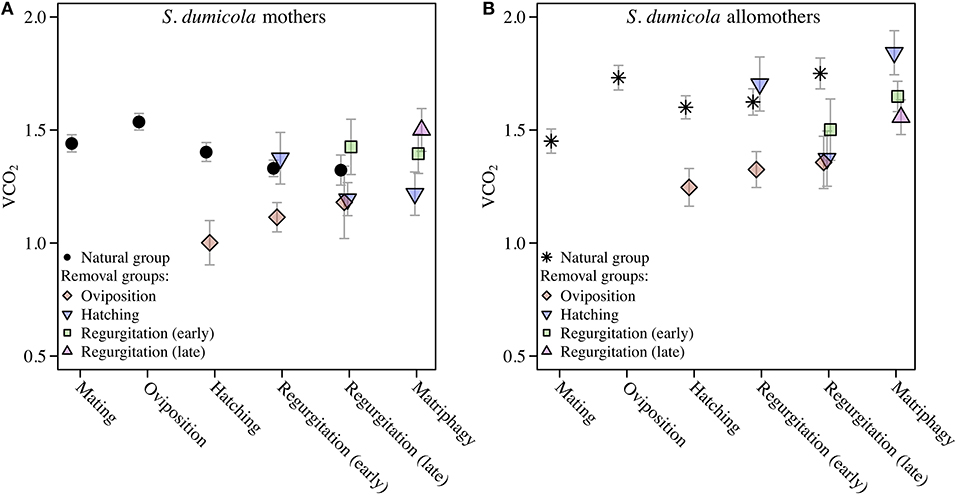
Figure 4. Standard Metabolic Rate given as VCO2 (μL CO2/minute/gram) in cooperative S. dumicola mothers (A) and allomothers (non-reproducing, virgin females) (B). Natural groups followed a natural brood care cycle. SMR of mothers and allomothers was measured at mating, oviposition, hatching of offspring, early regurgitation and late regurgitation (same data as in Figure 2B). Females in the natural group succumbed to matriphagy and were thus not measured at this stage. In the removal groups, SMR was measured after removal of the eggs (oviposition) or offspring at the same stages as given above and at expected time of matriphagy. The VCO2 measure provided is the average of the 3 lowest measurements out of 40. Error bars show the standard error of the mean.
Histology: General Results for Both Species (see Also Tables S6–S9)
In the midgut, extracellular fluids that accumulate in lumina and other extracellular spaces in preparation of regurgitation feeding can be distinguished from food remnants by their structure: while food remnants have a coarse and flaky structure and are stained pinkish or gray (see for example Figure 8B), regurgitate appears finely structured and stains appear blue after AZAN staining (see for example Figure 8D).
We examined the ovaries of all females to determine the developmental stages of the oocytes. The undeveloped ovaries contain homogeneously structured pre-vitellogenic oocytes (Trabalon et al., 1992), which appear pink in AZAN staining and do not show larger granules (e.g., Figure 5A). Pre-vitellogenic oocytes were smaller than 150 μm in S. lineatus and smaller than 100 μm in S. dumicola. Maturing early (S. lineatus: up to 300 μm, S. dumicola: up to 170; e.g., Figures 8D,E) and late vitellogenic oocytes (S. lineatus: up to 370 μm, S. dumicola: up to 270; e.g., Figure 5B) are considerably larger and exhibit a grained structure. As females with early or late vitellogenic oocytes usually also contained less matured oocyte stages, we classified them by the most mature oocyte stage found in the ovaries (see also Tables S6–S9).
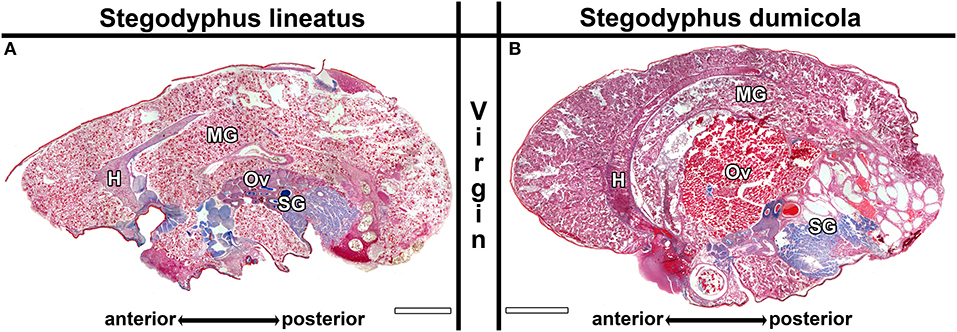
Figure 5. Midgut sections of virgin control females of Stegodyphus lineatus (A) and S. dumicola (B) sampled at the beginning of the experimental period. The midgut tissue (MG) consists of diverticula embedded in storage tissue and surrounds the heart (H), reproductive organs (Ov) and the silk glands (SG). No blue stained secretion granules or extracellular materials are visible. The ovary of S. lineatus contains exclusively pre-vitellogenic oocytes while the ovary from S. dumicola shows far matured oocytes. Virgin Controls sampled at the end of the experimental period did not differ. Scale bars are 2,000 μm.
Morphological Changes in S. lineatus Natural Groups
Virgin controls from the beginning of the experimental period and the end of the experimental period did not differ in the traits investigated. In virgin controls and natural mothers at the stage of oviposition (No), blue stained secretion granules were either absent, or only present in clusters and in low amounts (Figures 5A, 6A), with the exception of one female that was chemically fixed one day after oviposition and showed massive amounts of secretion granules. In contrast, in Nh mothers (hatching; Figure 6C) and Ner mothers (early regurgitation; Figure 6E) secretion granules were common or abundant. In mothers at the late regurgitation stage (Nlr), the secretion granules were less frequent (Figure 6G) or absent. In many females at Nh and onwards, large lacunae were present, but mainly the middle parts of the midgut region. These lacunae were filled with large amounts of dense and finely structured extracellular fluids that often stained blue (Figures 6C,E). The abundance of these fluids and their tendency to stain blue was decreased at Nlr (compare Figures 6E,G). At this time the size of the female's opisthosomata was smaller compared to earlier stages (compare scale bars Figures 6C,E to Figure 6G). Interestingly, even in the late brood care stages part of the midgut tissue stayed intact (Figure 6G). Lacunae were never observed in virgin controls. These histological investigations show that brood care is associated with progressive tissue disintegration.
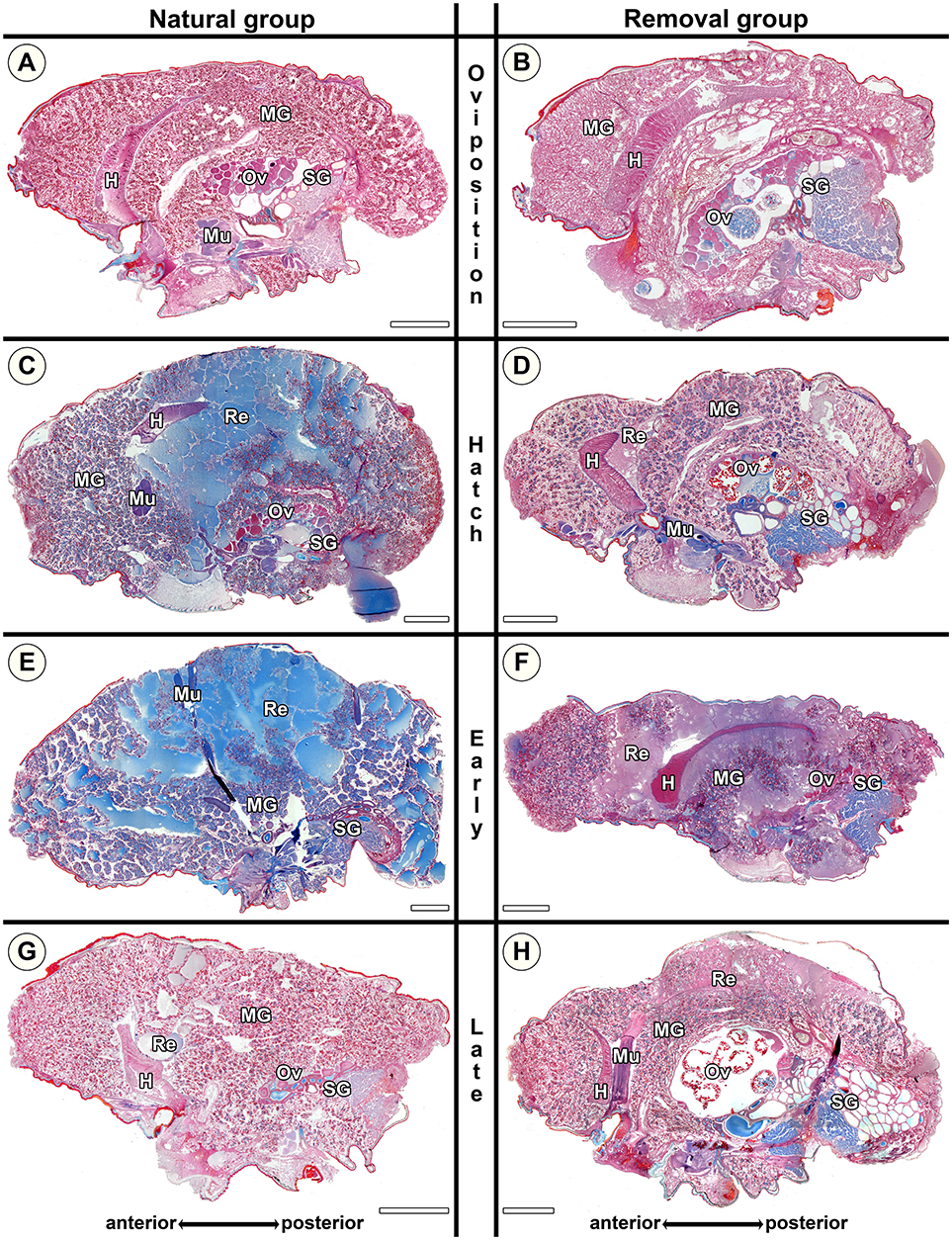
Figure 6. Histological sections of opisthosomata of Stegodyphus lineatus mothers of the natural group (left column) and mothers of the removal group (right column). Females were either chemically fixed (natural group) or the eggs sac or offspring was removed (removal group) at (A,B) oviposition (No, Ro); (C,D) hatching of spiderlings (Nh, Rh); (E,F) early regurgitation phase (Ner, Re; 5 days after hatching); (G,H) late regurgitation phase (Nlr, Rlr; 10 days after hatching). Females in the removal groups were chemically fixed 15 days after hatching of the offspring when matriphagy would occur under natural conditions. In the natural group by the time offspring hatches (C), massive changes have occurred compared to virgin females or females at oviposition (A). Blue stained secretion granules are abundant, and parts of the midgut tissue are dissolved with stained extracellular material (Re) accumulating especially in the mid-region of the midgut tissue while anterior parts stay intact for the longest time. At the late hatching phase almost no extracellular material (interpreted as regurgitant fluid) is left and the opisthosoma has shriveled, but parts of the midgut tissue are still intact. When mothers were separated from offspring directly after hatching (D) they were able to terminate and reverse processes, almost no extracellular material is visible. During regurgitation the ability to reverse the cellular disintegration diminishes as extracellular material remains. Oocytes might mature in the removal group even until the late regurgitation phase. H, heart; MG, midgut; Mu, muscle; Ov, ovary; Re, extracellular material (regurgitant); SG, silk gland. All scale bars are 2,000 μm.
Morphological Changes in S. lineatus Removal Groups
Stegodyphus lineatus mothers from which the egg sac had been removed at oviposition (Ro) did not show secretion granules in the midgut tissue (Figure 6B). When the offspring had been removed at hatching (Rh) or early or late regurgitation (Rer, Rlr), the abundance of secretion granules varied strongly. In these stages, extracellular fluids were visible (Figures 6D,F,H), similar to mothers from the corresponding natural groups. However, in contrast to natural mothers, fluids often appeared less dense and often did not stain blue. At Rh, the amount of extracellular fluid found in the midgut of the mother was low or very low (Figure 6D). The lacunae were small and often diverticula did not show marked natural lumina. In Rer mothers, the amount of extracellular fluids varied from high amounts of extracellular fluids (Figure 6F) to none. Rlr mothers showed comparable amounts of extracellular fluids as natural mothers at Nlr (compare Figure 6G and Figure 6H). The comparison of mothers from the natural groups and removal groups suggests that disintegration of the midgut tissue is reversible until the hatching stage and that the ability of mothers to reverse these processes diminishes once regurgitation feeding has begun.
Morphological Changes in S. dumicola Natural Groups
In the natural groups, we found similar progressive tissue disintegration of the midgut along the brood care period in both mothers and allomothers. Control virgins exanimated at the beginning and the end of the experimental period did not differ in the investigated traits (Figure 5B), as mothers and allomothers at No (Figures 7A,B) never showed blue stained secretion granules or accumulation of extracellular fluids (Figure 5B). In contrast, the blue stained granules were abundant in mothers and allomothers at Nh (Figures 7C,D). During the regurgitation phase (Ner, Nlr), less secretion granules were found in mothers and allomothers (Figures 7E–H). Mothers of the natural group showed small to middle sized lacunae with extracellular fluids in the anterior parts of the midgut starting at Nh (Figures 7C,E,G), and in allomothers from Ner onwards (Re in Figures 7F,H). The lacunae never occurred in the posterior parts of the midgut (Figure 7F), but sometimes lumina filled with extracellular fluids were found in the mid-regions of the midgut (Figure 7H). In Nlr females, only small amounts of extracellular fluids were observed (Figure 7G and Re in Figure 7H).
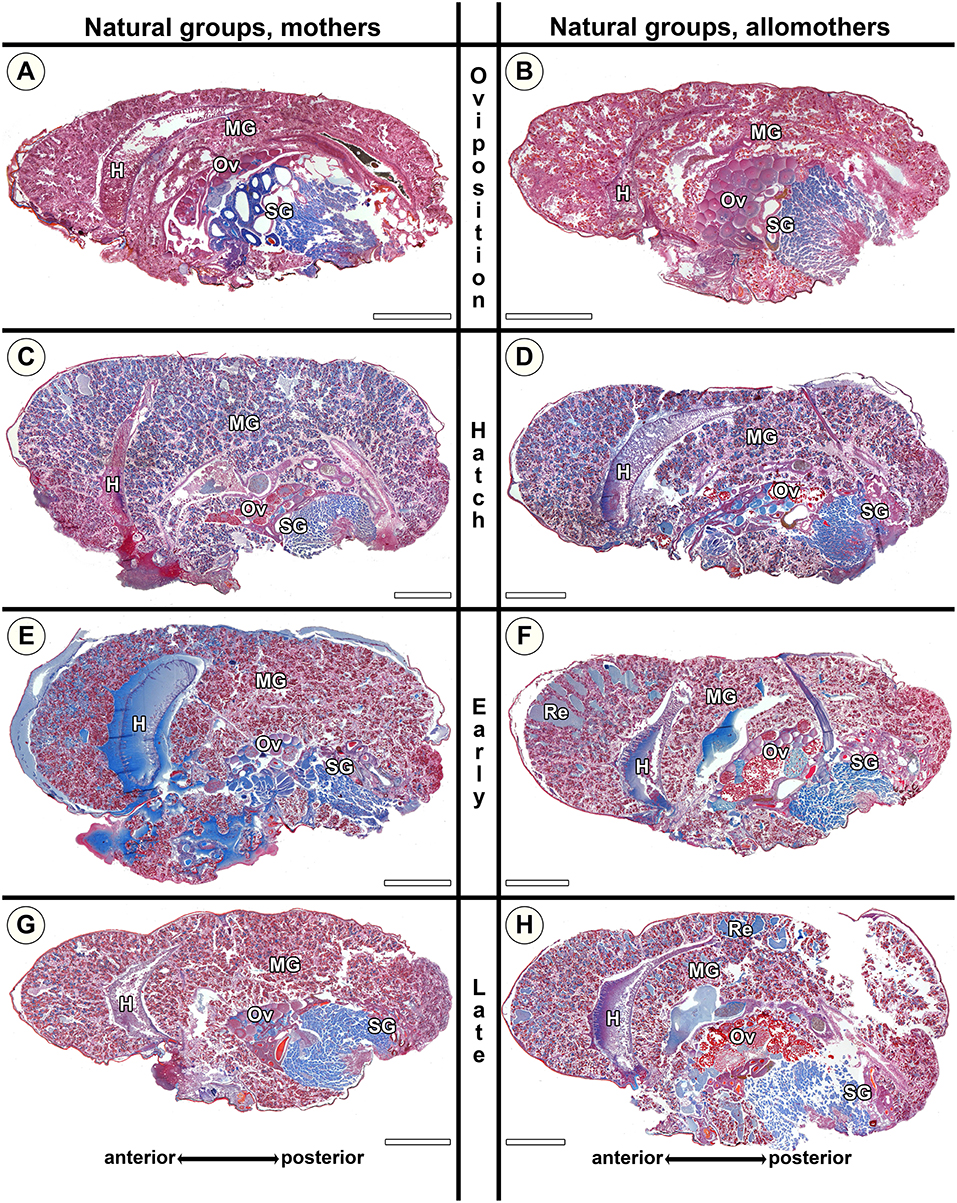
Figure 7. Histological sections of opisthosomata of Stegodyphus dumicola mothers (left column) and allomothers (right column) of the natural group that followed a natural brood care cycle. Females were chemically fixed at (A,B) oviposition (No); (C,D) hatching of spiderlings (Nh); (E,F) early regurgitation phase (Ner; 6 days after hatching); (G,H) late regurgitation phase (Nlr; 24 days after hatching). Mothers as well as allomothers show similar changes with blue stained granules accumulating in the cells at the time of hatching (C,D). Extracellular material is only visible in small amounts often in anterior parts of the midgut tissue (e.g., E,F). Oocytes may undergo maturation in mothers (C) and allomothers (F,H). H, heart; MG, midgut; Ov, ovary; Re, extracellular material (regurgitant); SG, silk gland. All scale bars are 2,000 μm.
The morphological changes in natural mothers and allomothers of S. dumicola were similar to those in natural S. lineatus mothers. However, as the size of the lacunae in S. dumicola midgut tissue never reached the same extent as in S. lineatus (compare Figures 6C,E with Figures 7C–F), changes in S. dumicola appeared less pronounced. At Ner, the amount of secretion granules was lower in S. dumicola than in S. lineatus (compare Figure 6E with Figures 7E,F). However, at Nlr the midgut of S. dumicola mothers and allomothers contained more secretion granules compared to S. lineatus mothers at the same stage (compare Figure 6G with Figures 7G,H).
Our histological investigations of S. dumicola showed that the provisioning of extreme maternal care is associated with progressive changes of the midgut tissue in both mothers and allomothers, but changes in the midgut were less pronounced than those of S. lineatus.
Morphological Changes in S. dumicola Removal Groups
Rh mothers showed low amounts of secretion granules and almost no extracellular fluids (Figure 8C). In contrast, allomothers at the same stage showed middle-sized lacunae and had accumulated extracellular fluids when they were scrutinized (Figure 8D). At Rer and Rlr, both mothers and allomothers showed high levels of secretion granules and medium to large amounts of extracellular fluids which were present in natural lumina of the diverticula, and middle-sized lacunae in the anterior to the middle parts of the midgut (Figures 8E–H). The comparison of natural S. dumicola females to the females of the removal group shows that the amount of secretion granules was lower in Rh compared to Nh (compare Figures 7C,D to Figures 8C,D). However, compared to the natural groups Ner and Nlr, the amount of extracellular fluids was higher in Rer and Rlr mothers (compare Figures 8G,E to Figures 7G,E) and in allomothers from the hatching stage (Rh) onwards (compare Figures 8D,F,H to Figures 7D,F,H). These data suggest that in mothers of S. dumicola, disintegration of the midgut tissue is reversible until the hatching stage as in S. lineatus mothers. In contrast, allomothers of S. dumicola were seemingly not able to terminate and reverse processes when offspring were removed at hatching.
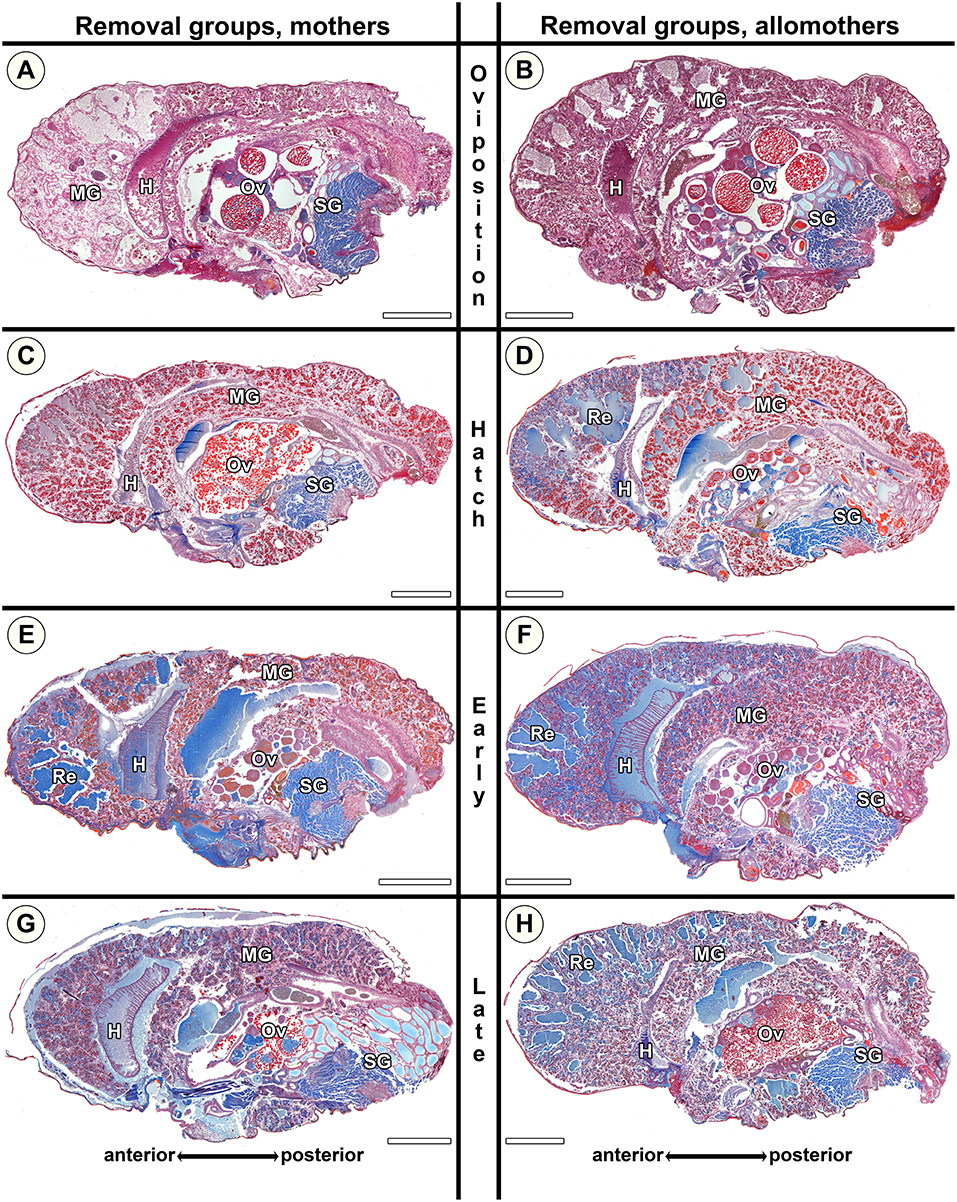
Figure 8. Histological sections of opisthosomata of Stegodyphus dumicola mothers (left column) and allomothers (right column) of the removal group of which egg sacs or offspring had been removed. Offspring was removed at (A,B) oviposition (Ro); (C,D) hatching of spiderlings (Rh); (E,F) early regurgitation phase (Rer; 6 days after hatching); (G,H) late regurgitation phase (Rlr; 24 days after hatching). Females were chemically fixed 31 days after hatching at the time matriphagy would occur under natural conditions. Mothers and allomothers show almost no blue stained secretion granules when offspring were removed at time of hatching (C,D) compared to females of the same stage in the natural group. Extracellular material (regurgitant) is accumulating more in mothers from early regurgitation and in allomothers from hatching onwards than in control groups, suggesting the inability to terminate and reverse production of material for regurgitation feeding when offspring were removed at hatching. Ovaries of mothers and allomothers frequently show late-vitellogenic oocytes (A–C,G,H). H, heart; MG, midgut; Ov, ovary; Re, extracellular material (regurgitant); SG, silk gland. All scale bars are 2,000 μm.
Ovaries of S. lineatus and S. dumicola
The ovaries of virgin S. lineatus females did not differ between individuals examined at the beginning and the end of the experimental period and showed exclusively pre-vitellogenic oocytes (Ov in Figure 5A and Table S6). Similarly, the ovaries of natural mothers from all stages of brood care often contained pre-vitellogenic oocytes (Ov in Figures 6A,C,G). In the removal groups, most mothers exhibited late vitellogenic oocytes in their ovaries at least in one stage (e.g., Ov in Figure 6B,H, see detailed results in Table S6). These results suggest that mothers keep the ability to mature oocytes if they lose their brood, even if this happens late in the brood care period.
In S. dumicola, our data shows that allomothers as well as virgin non-helping females are able to mature oocytes in their ovaries. Virgin controls exhibited pre-vitellogenic, early vitellogenic, or late vitellogenic oocytes (Figure 5B and Table S7) in their ovaries. There was no significant difference between virgin controls sampled at the beginning and those sampled at the end of the experimental period. The ovaries of natural mothers contained pre-vitellogenic (Figure 7A) or early vitellogenic oocytes (Figure 7C), and some exhibited late vitellogenic oocytes in their ovaries. In contrast, natural allomothers of all stages had early vitellogenic (Figure 7B) or late vitellogenic oocytes (Figure 7F) in their ovaries. In the removal groups, late vitellogenic (Figures 8A,G) and early vitellogenic oocytes (Figure 8E) was present in most mothers. Allomothers from removal groups showed early or late vitellogenic oocytes in most cases (Figure 8D).
Discussion
Does Extreme Brood Care Lead to Physiological Changes in Females?
We investigated the physiological response to maternal care in spiders with a semelparous life history. We found an energetic cost of brood care in the solitary S. lineatus, with the highest SMR exhibited by mothers during the regurgitation-feeding period. This is consistent with an up-regulation of energy demanding processes associated with offspring care resulting in elevated SMR (Barnes and Partridge, 2003; Metcalfe and Alonso-Alvarez, 2010). Interestingly, in the cooperative S. dumicola we found a consistently higher SMR of unmated helpers than that of mothers. This can be interpreted as allomothers investing relatively more in parental care than mothers do. A possible explanation for this difference in investment is that allomothers do not allocate resources to reproduction, but instead invest all of their resources into the care of the brood, which represents their entire reproductive fitness. Furthermore, S. dumicola mothers are sometimes observed to produce an additional brood (Junghanns, unpublished), for which resources may be preserved (discussed further below).
The removal experiment strongly suggests that brood care is correlated with an elevated SMR, as mothers of S. lineatus as well as mothers and allomothers of S. dumicola experienced a significant reduction in SMR after removal of the eggs. Although regurgitation feeding has not yet begun at this stage, histological data suggests that the transformation of midgut tissue in preparation for provisioning of young is already initiated when an egg sac is present. The ability to reduce SMR in the removal experiment could indicate metabolic and morphological plasticity as a strategy to save energy for a second reproductive event in case the first brood is lost (Ricklefs and Wikelski, 2002). We hypothesized that an increase in SMR reflects investment in maternal care, but a decrease in SMR could instead reflect energy allocated to egg production and a down regulation of energy allocation to self-maintenance (Naya et al., 2007). However, oviposition and brood provisioning in S. lineatus mothers coincided with elevated SMR relative to virgin females, consistent with our primary hypothesis.
We note that SMR of virgin S. lineatus females declined over time. We do not have an explicit explanations for this pattern, but to provide the most realistic comparison between virgin females and mothers, who do not feed after oviposition, the virgin females were also not fed after this point. This could explain the decline in SMR compared to mothers. This is also consistent with the observation that virgins do not mature eggs and never produce an egg sac unless they are mated, and therefore do not need to allocate energy to these processes. This result emphasizes that reproducing females upregulate SMR in response to brood care.
In the solitary S. lineatus, the histological data support the observed differences in metabolic rate between reproducing and virgin females, with disintegration of the midgut tissue and accumulation of extracellular fluids occurring only in mothers. This suggests that reproducing females undergo morphological changes to meet the demands of regurgitation feeding with liquefied body tissue (Kullmann, 1968; Nawabi, 1974; Salomon et al., 2015). Accumulation of material for secretion, perhaps of alkaline content (Burck, 1988), in preparation for regurgitation feeding started at oviposition. By offspring hatching, the midgut tissue of mothers formed large extracellular lacunae that indicate ongoing disintegration of midgut tissue, and lacunae filled with fine-grained fluids post-hatching most likely contain the fluids that females will regurgitate to the young. During regurgitation, females lose weight (Salomon et al., 2005), which is reflected by reduced amounts of fluids in the late regurgitation stage of S. lineatus and a smaller opisthosoma compared to earlier stages. Morphological changes similar to those in S. lineatus but not as comprehensive, were observed in mothers and allomothers of the social S. dumicola. In S. dumicola, extracellular blue stained fluids did not accumulate in high amounts in lacunae but were often limited to natural lumina of the diverticula in the anterior part of the midgut. The less dramatic changes of the midgut tissue in the social S. dumicola are likely to reflect adjustment of resource allocation to a longer maternal provisioning period, as reproduction is not entirely synchronized within a nest, and the provisioning period in social Stegodyphus is longer than that of solitary congeners (Seibt and Wickler, 1988). The ability to perform continuous allomaternal provisioning may also provide an insurance against high female mortality (Jones and Riechert, 2008). Collectively, the data show that mothers and allomothers undergo physiological changes in preparation for regurgitation provisioning, which is initiated at oviposition.
Are Physiological Adaptations to Brood Provisioning Reversible?
We examined whether physiological alterations are irreversible once reproduction is initiated as an adaptation to a semelparous life history, or whether some modulation is possible in case of brood mortality. Our data indicate that physiological adaptations to maternal care are reversible if the offspring are removed early in the maternal care period. SMR decreased significantly in mothers following egg removal, whereas removal of the offspring at different times during the regurgitation period did not result in a marked decrease in SMR. Histological examination showed that mothers of both species, after removal of hatched offspring, showed a reduced amount of secretion granules, and in S. lineatus mothers extracellular fluids also diminished. This suggests that mothers were able to terminate the production of secretion for regurgitation and to reabsorb existing extracellular material using the remaining intact diverticula. The implication could be that mothers retain sufficient resources to produce a second clutch if the first brood is lost early in the provisioning period (Schneider and Lubin, 1997a), this is a common scenario as the risk of brood loss to infanticidal males, predation, or parasitism in nature is high (Schneider and Lubin, 1997a,b; Bilde et al., 2007). The ability to produce a replacement clutch was confirmed by the presence of late vitellogenic oocytes in the ovaries of some females in late stages of regurgitation feeding. Indications of reversal processes were less pronounced when removal of offspring occurred in the regurgitation feeding period, at which point S. lineatus mothers appeared unable to reabsorb regurgitation fluids. Similarly, in S. dumicola, extracellular fluids accumulated when the offspring had been removed during regurgitation, indicating that these females were incapable of reabsorbing extracellular fluids, and unable to stop the process of producing additional fluids. This suggests that physiological plasticity in mothers of both species diminishes from the onset of regurgitation, marking the time of regurgitation feeding a physiological ‘point of no return'. The life history of Stegodyphus, in which high mortality may have favored semelparity, appears to be aligned with physiological adaptations to extreme maternal care that are irreversible once regurgitation feeding has begun. At this point, females may have invested an amount of energy that reduces the likelihood that their energy budget would meet the threshold for yet another successful reproductive bout (Drent and Daan, 1980; Stearns, 1992). Intriguingly, as mentioned above, we have observed that S. dumicola females can produce a second brood, but we need a better understanding of the circumstances under which this happens. The solitary S.lineatus, only produces a replacement brood if they lose their first egg sac (Schneider and Lubin, 1997a), and it is possible that the ability of S. dumicola females to produce a second brood depends on the timing and relative investment in the first brood.
Do Allomothers Experience Similar Physiological Changes as Mothers?
In the solitary S. lineatus, maternal care behavior occurs exclusively in reproducing females (Schneider, 2002), while unmated helpers of the cooperative species engage in maternal care (Salomon and Lubin, 2007; Junghanns et al., 2017), suggesting that the physiological ability of unmated helpers to provision the offspring is an adaptation to cooperative breeding. Allomothers consistently experienced higher maintenance cost measured as SMR compared to mothers, indicating that helpers may even experience a higher cost of brood care than mothers. This elevated cost could be explained by allomothers engaging more frequently in prey capture, web building and other tasks of colony maintenance in addition to food provisioning (Junghanns et al., 2017). The histological examination confirmed that allomothers undergo similar changes in the midgut as mothers. Already at the stage of hatching, allomothers appeared unable to terminate and reverse these internal processes, instead, they continuously accumulated extracellular fluids. This may reflect that allomothers care for all offspring produced by several mothers in the family group, i.e. in the event of loss of one brood, there is still demand for allomaternal provisioning. Since the unmated allomothers cannot invest their resources in their own offspring, their reproductive fitness is determined by inclusive fitness benefits from raising their sisters' brood (Hamilton, 1964a,b; Smith and Wynneedwards, 1964). The ability of unmated females to engage in suicidal brood provisioning is therefore key for acquiring indirect benefits of helping by kin selection, and likely represents an adaptation to cooperative breeding.
Are Morphological Adaptations to Extreme Brood Care Associated With Egg Maturation?
The physiological capacity to engage in regurgitation feeding may rely on an internal maturation process triggered by mating or oviposition (Feneron et al., 1996; Mas and Kolliker, 2008; Pinilla et al., 2012). Interestingly, allomothers and virgin non-helping females of the cooperative S. dumicola showed early and late vitellogenic oocytes in their ovaries—a mating event does therefore not seem required for egg maturation. The presence of late stage oocytes in their ovaries likely indicates the physiological maturation process that precedes and triggers regurgitation feeding. In ants, ovarian maturation of workers is linked with the performance of certain tasks in the nest, with nursing workers showing the most developed ovaries (Feneron et al., 1996). The link between ovarian maturation and brood care may have played a role in the evolution of cooperative breeding within the genus Stegodyphus, since in the solitary species S. lineatus, virgin females only contained pre-vitellogenic oocytes, and did not oviposit or provide care when in contact with spiderlings. We therefore suggest that maturation of ovaries in allomothers of cooperative Stegodyphus is associated with the onset of physiological preparations for brood provisioning and represents an adaptation to engage in cooperative breeding.
In conclusion, the onset of reproduction triggers an increase in standard metabolic rate (SMR) and causes structural changes in the abdominal midgut tissue, indicating physiological responses to regurgitation feeding with liquefied body tissue. Females are able to terminate and partly reverse these internal morphological changes until the start of regurgitation feeding, which marks a physiological “point of no return.” Remarkably, allomothers show similar or even stronger physiological response to brood care than mothers. This could be an adaptation to continued allomaternal care over prolonged periods in social nests, or it could facilitate the production of a second brood by mothers, who retain the ability to mature oocytes upon the loss of offspring. In contrast to virgin females of the subsocial S. lineatus, unmated S. dumicola allomothers often contained early and late vitellogenic oocytes in their ovaries. This suggests that oocyte maturation is a prerequisite for the onset of extreme brood care in unmated helpers, and that oocyte maturation has shifted to an earlier stage in ontogeny as an adaptation to cooperative breeding.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
GU, TB, AJ, CH, and JO contributed to conceive the ideas and designed the experiments. AJ and CH collected the data. JO and HM supervised SMR data collection and analyses. GU supervised histology. MS performed statistical analyses. TB, GU, AJ, and CH wrote the manuscript.
Funding
The study was supported by the European Research Council with ERC StG-2011-282163 to TB. CH was supported by Weis-Fogh Foundation. JO was funded by The Danish Council of Independent Research. AJ was supported by a stipend from the federal state of Mecklenburg-Vorpommern, Germany and received funding from the young academics and mobility program of the International Office, University of Greifswald.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Yael Lubin for hosting CH in Sede Boger, Israel, and Michelle Greve for hosting CH and AJ in Pretoria, South Africa. We thank Lena Grinsted for a steady hand in preparing S. lineatus, and Bram Vanthournout for help with measuring S. lineatus. We are indebted to everyone in The Spider Lab Aarhus University and the Uhl lab at the University of Greifswald for stimulating work environments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00305/full#supplementary-material
References
Adkins-Regan, E. (2005). Hormones and Animal Social Behaviour. Princeton, NJ: Princeton University Press.
Alberti, G., and Storch, V. (1983). Zur Ultrastruktur der Mitteldarmdrüse von Spinnentieren (Scorpiones, Araneae, Acari) unter verschiedenen Ernährungsbedingungen. Zool. Anz. 211, 145–160.
Alonso-Alvarez, C., and Velando, A. (2012). “Benefits and costs of parental care,” in The Evolution of Parental Care Royle, eds N. J., Smiseth, P. T., and M. Kölliker (Oxford,: Oxford University Press), 40–61.
Avilés, L. (1997). “Causes and consequences of cooperation and permanent-sociality in spiders,” in The Evolution of Social Behavior in Insects and Arachnids, eds, J. C. Choe and B. J. Crespi (Cambridge, UK: Cambridge University Press), 476–498. doi: 10.1017/CBO9780511721953.024
Barnes, A. I., and Partridge, L. (2003). Costing reproduction. Anim. Behav. 66, 199–204. doi: 10.1006/anbe.2003.2122
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67:1–48. doi: 10.18637/jss.v067.i01
Bilde, T., Coates, K. S., Birkhofer, K., Bird, T., Maklakov, A. A., Lubin, Y., et al. (2007). Survival benefits select for group living in a social spider despite reproductive costs. J. Evol. Biol. 20, 2412–2426. doi: 10.1111/j.1420-9101.2007.01407.x
Bouin, P. (1887). Phénoménes cytologiques anormaux dans l'histogenése et l'atrophie expérimentale du tube séminifére. Nancy: Université de Nancy.
Burck, H. C. (1988). Histologische Technik. Leitfaden für die Herstellung mikroskopischer Präparate in Unterricht und Praxis. Stuttgart: Georg Thieme Verlag.
Cant, M. A. (2012). “Cooperative breeding systems,” in The Evolution of Parental Care Royle, N. J., Smiseth, P. T., and M. Kölliker (Oxford: Oxford University Press, 206–225.
Choe, J. C., and Crespi, B. J. (eds.) (1997). The Evolution of Social Behavior in Insects and Arachnids. Cambridge, UK: Cambridge University Press. doi: 10.1017/CBO9780511721953
Clutton-Brock, T. H. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton University Press.
Creel, S. R., Monfort, S. L., Wildt, D. E., and Waser, P. M. (1991). Spontaneous lactation is an adaptive result of pseudopregnancy. Nature 351, 660–662. doi: 10.1038/351660a0
Drent, R. H., and Daan, S. (1980). The prudent parent - energetic adjustments in avian breeding. Ardea 68, 225–252. doi: 10.5253/arde.v68.p225
Feneron, R., Durand, J. L., and Jaisson, P. (1996). Relation between behaviour and physiological maturation in a ponerine ant. Behaviour 133, 791–806. doi: 10.1163/156853996X00477
Foelix, R. F. (2011). Biology of Spiders. New York, NY; Oxford: Oxford University Press; Georg Thieme Verlag.
Fowler, M. A., and Williams, T. D. (2017). A physiological signature of the cost of reproduction associated with parental care. Am Natural. 190, 762–773. doi: 10.1086/694123
Futami, K., and Akimoto, S. (2005). Facultative second oviposition as an adaptation to egg loss in a semelparous crab spider. Ethology 111, 1126–1138. doi: 10.1111/j.1439-0310.2005.01126.x
Hamilton, W. D. (1964a). Genetical evolution of social behaviour 1. J. Theor. Biol. 7, 1–16. doi: 10.1016/0022-5193(64)90038-4
Hamilton, W. D. (1964b). Genetical evolution of social behaviour 2. J. Theor. Biol. 7, 17–52. doi: 10.1016/0022-5193(64)90039-6
Henschel, J. R., Lubin, Y. D., and Schneider, J. (1995). Sexual Competition in an inbreeding social spider, Stegodyphus dumicola (Araneae, Eresidae). Insect. Soc. 42, 419-426. doi: 10.1007/BF01242170
Jensen, P., Overgaard, J., Loeschcke, V., Schou, M. F., Malte, H., and Kristensen, T. N. (2014). Inbreeding effects on standard metabolic rate investigated at cold, benign and hot temperatures in Drosophila melanogaster. J. Insect Physiol. 62, 11–20. doi: 10.1016/j.jinsphys.2014.01.003
Johannesen, J., Lubin, Y., Smith, D. R., Bilde, T., and Schneider, J. M. (2007). The age and evolution of sociality in Stegodyphus spiders: a molecular phylogenetic perspective. Proc. R. Soc. B Biol. Sci. 274, 231–237. doi: 10.1098/rspb.2006.3699
Jones, T. C., and Riechert, S. E. (2008). Patterns of reproductive success associated with social structure and microclimate in a spider system. Anim. Behav. 76, 2011–2019. doi: 10.1016/j.anbehav.2008.07.033
Junghanns, A., Holm, C., Schou, M. F., Sorensen, A. B., Uhl, G., and Bilde, T. (2017). Extreme allomaternal care and unequal task participation by unmated females in a cooperatively breeding spider. Anim. Behav. 132, 101–107. doi: 10.1016/j.anbehav.2017.08.006
Kim, K. W., Roland, C., and Horel, A. (2000). Functional value of matriphagy in the spider Amaurobius ferox. Ethology 106, 729–742. doi: 10.1046/j.1439-0310.2000.00585.x
Krafft, B., and Horel, A. (1980). Comportement maternel et relations mères-jeunes chez les araignées. Reprod. Nutr. Dév. 20, 747–758. doi: 10.1051/rnd:19800501
Kraus, O., and Kraus, M. (1988). “The genus Stegodyphus (Arachnida, Araneae). Sibling species, species groups, and parallel origin of social living,” in Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg (NF) 30. ed O. Kraus (Hamburg; Berlin: Verlag Paul Parey, 151–254.
Lighton, J. R. B., and Halsey, L. G. (2011). Flow-through respirometry applied to chamber systems: pros and cons, hints and tips. Comp. Biochem. Physiol. Mol. Integr. Physiol. 158, 265–275. doi: 10.1016/j.cbpa.2010.11.026
Lubin, Y., and Bilde, T. (2007). The evolution of sociality in spiders. Adv. Study Behav. 37, 83–145. doi: 10.1016/S0065-3454(07)37003-4
Mas, F., and Kolliker, M. (2008). Maternal care and offspring begging in social insects: chemical signalling, hormonal regulation and evolution. Anim. Behav. 76, 1121–1131. doi: 10.1016/j.anbehav.2008.06.011
Metcalfe, N. B., and Alonso-Alvarez, C. (2010). Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996. doi: 10.1111/j.1365-2435.2010.01750.x
Millot, J., and Bourgin, P. (1942). Sur la biologie des Stegodyphus solitaires (Aranéides Érésides). Bull. Biol. France Belg. 76, 298–313.
Montgomery, T. M., Pendleton, E. L., and Smith, J. E. (2018). Physiological mechanisms mediating patterns of reproductive suppression and alloparental care in cooperatively breeding carnivores. Physiol. Behav. 193, 167–178. doi: 10.1016/j.physbeh.2017.11.006
Nawabi, S. (1974). Histologische untersuchungen an der mitteldarmrüse von stegodyphus pacificus (Pocock 1900) (Araneae: Salticidae) (Doctoral thesis). Universität Bonn, Bonn, Germany.
Naya, D. E., Lardies, M. A., and Bozinovic, F. (2007). The effect of diet quality on physiological and life-history traits in the harvestman Pachylus paesslerik. J. Insect Physiol. 53, 132–138. doi: 10.1016/j.jinsphys.2006.11.004
Patton, S., and Neville, M. C. (1997). Maternal adaptation to lactation. J. Mammary Gland Biol. Neoplasia 2, 201–203. doi: 10.1023/a:1026376119456
Pianka, E. R. (1976). Natural selection of optimal reproductive tactics. Am. Zool. 16, 775–784. doi: 10.1093/icb/16.4.775
Pinilla, L., Aguilar, E., Dieguez, C., Millar, R. P., and Tena-Sempere, M. (2012). Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev. 92, 1235–1316. doi: 10.1152/physrev.00037.2010
R Development Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ricklefs, R. E., and Wikelski, M. (2002). The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. doi: 10.1016/S0169-5347(02)02578-8
Royle, N. J., Smiseth, P. T., and Kolliker, M. (2012). The Evolution of Parental Care. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780199692576.001.0001
Ruhland, F., Petillon, J., and Trabalon, M. (2016). Physiological costs during the first maternal care in the wolf spider Pardosa saltans (Araneae, Lycosidae). J. Insect Physiol. 95, 42–50. doi: 10.1016/j.jinsphys.2016.09.007
Salomon, M., Aflalo, E. D., Coll, M., and Lubin, Y. (2015). Dramatic histological changes preceding suicidal maternal care in the subsocial spider Stegodyphus lineatus (Araneae: Eresidae). J. Arachnol. 43, 77–85. doi: 10.1636/B14-15.1
Salomon, M., and Lubin, Y. (2007). Cooperative breeding increases reproductive success in the social spider Stegodyphus dumicola (Araneae, Eresidae). Behav. Ecol. Sociobiol. 61, 1743–1750. doi: 10.1007/s00265-007-0406-2
Salomon, M., Mayntz, D., and Lubin, Y. (2008). Colony nutrition skews reproduction in a social spider. Behav. Ecol. 19, 605–611. doi: 10.1093/beheco/arn008
Salomon, M., Schneider, J., and Lubin, Y. (2005). Maternal investment in a spider with suicidal maternal care, Stegodyphus lineatus (Araneae, Eresidae). Oikos 109, 614–622. doi: 10.1111/j.0030-1299.2005.13004.x
Schal, C., Holbrook, G. L., Bachmann, J. A. S., and Sevala, V. L. (1997). Reproductive biology of the German cockroach, Blattella germanica: juvenile hormone as a pleiotropic master regulator. Arch. Insect Biochem. Physiol. 35, 405–426.
Schneider, J. M. (1995). Survival and growth in groups of a subsocial spider (Stegodyphus lineatus). Insect. Soc. 42, 237–248. doi: 10.1007/BF01240418
Schneider, J. M. (2002). Reproductive state and care giving in Stegodyphus (Araneae: Eresidae) and the implications for the evolution of sociality. Anim. Behav. 63, 649–658. doi: 10.1006/anbe.2001.1961
Schneider, J. M., and Lubin, Y. (1997a). Does high adult mortality explain semelparity in the spider Stegodyphus lineatus (Eresidae)? Oikos 79, 92–100. doi: 10.2307/3546094
Schneider, J. M., and Lubin, Y. (1997b). Infanticide by males in a spider with suicidal maternal care, Stegodyphus lineatus (Eresidae). Anim. Behav. 54, 305–312. doi: 10.1006/anbe.1996.0454
Schneider, J. M., Salomon, M., and Lubin, Y. (2003). Limited adaptive life-history plasticity in a semelparous spider, Stegodyphus lineatus (Eresidae). Evolut. Ecol. Res. 5, 731–738.
Seibt, U., and Wickler, W. (1987). Gerontophagy versus cannibalism in the social spiders Stegodyphus mimosarum (Pavesi) and Stegodyphus dumicola (Pocock). Anim. Behav. 35, 1903–1905. doi: 10.1016/S0003-3472(87)80087-8
Seibt, U., and Wickler, W. (1988). Why do family spiders, Stegodyphus (Eresidae), Live in Colonies. J. Arachnol. 16, 193–198.
Settepani, V., Bechsgaard, J., and Bilde, T. (2016). Phylogenetic analysis suggests that sociality is associated with reduced effectiveness of selection. Ecol. Evol. 6, 469–477. doi: 10.1002/ece3.1886
Settepani, V., Schou, M. F., Greve, M., Grinsted, L., Bechsgaard, J., and Bilde, T. (2017). Evolution of sociality in spiders leads to depleted genomic diversity at both population and species levels. Mol. Ecol. 26, 4197–4210. doi: 10.1111/mec.14196
Smiseth, P. T., Kölliker, M., and Royle, N. J. (eds.). (2012). “What is parental care?,” in The Evolution of Parental Care Royle (Oxford: Oxford University Press), 1–17. doi: 10.1093/acprof:oso/9780199692576.003.0001
Smith, J. M., and Wynneedwards, V. C. (1964). Group selection and Kin selection. Nature 201, 1145–1147. doi: 10.1038/2011145a0
Speakman, J. R., and McQueenie, J. (1996). Limits to sustained metabolic rate: the link between food intake, basal metabolic rate, and morphology in reproducing mice, Mus musculus. Physiol. Zool. 69, 746–769. doi: 10.1086/physzool.69.4.30164228
Toyama, M. (1999). Adaptive advantages of maternal care and matriphagy in a foliage spider, Chiracanthium japonicum (Araneae : Clubionidae). J. Ethol. 17, 33–39. doi: 10.1007/BF02769295
Trabalon, M. A., Bautz, M., Moriniere, M., and Porcheron, P. (1992). Ovarian development and correlated changes in hemolymphatic ecdysteroid levels in two spiders, Coelotes terrestris and Tegenaria domestica (Araneae, Agelenidae). Gen. Comp. Endocrinol. 88, 128–136. doi: 10.1016/0016-6480(92)90201-T
Trivers, R. L. (1972). “Parental investment and sexual selection,” in Sexual Selection and the Descent of Man: The Darwinian Pivot. eds B. Campbell (Chicago, IL: Aldine-Atherton), 136–179. doi: 10.4324/9781315129266-7
Vanfleteren, J. R., and DeVreese, A. (1996). Rate of aerobic metabolism and superoxide production rate potential in the nematode Caenorhabditis elegans. J. Exp. Zool. 274, 93–100.
Viera, C., Costa, F. G., Ghione, S., and Benamu-Pino, M. A. (2007). Progeny, development and phenology of the sub-social spider Anelosimus cf. studiosus (Araneae, Theridiidae) from Uruguay. Stud. Neotrop. Fauna Environ. 42, 145–153. doi: 10.1080/01650520600884553
Keywords: brood-provisioning, allomaternal care, histology, physiology, semelparity, metabolic-rate, midgut
Citation: Junghanns A, Holm C, Schou MF, Overgaard J, Malte H, Uhl G and Bilde T (2019) Physiological Adaptations to Extreme Maternal and Allomaternal Care in Spiders. Front. Ecol. Evol. 7:305. doi: 10.3389/fevo.2019.00305
Received: 04 May 2019; Accepted: 30 July 2019;
Published: 13 September 2019.
Edited by:
James Luke Savage, University of Sheffield, United KingdomReviewed by:
Per T. Smiseth, University of Edinburgh, United KingdomAnne Danielson-Francois, University of Michigan–Dearborn, United States
Copyright © 2019 Junghanns, Holm, Schou, Overgaard, Malte, Uhl and Bilde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trine Bilde, dHJpbmUuYmlsZGVAYmlvcy5hdS5kaw==; Gabriele Uhl, Z2FicmllbGUudWhsQHVuaS1ncmVpZnN3YWxkLmRl
†These authors share first authorship
‡These authors share senior authorship
 Anja Junghanns
Anja Junghanns Christina Holm2†
Christina Holm2† Hans Malte
Hans Malte Gabriele Uhl
Gabriele Uhl Trine Bilde
Trine Bilde