
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Ecol. Evol. , 25 June 2019
Sec. Behavioral and Evolutionary Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00234
This article is part of the Research Topic Contributions of Behavior and Physiology to Conservation Biology View all 13 articles
The amphibian decline crisis has been challenging to address because of the complexity of factors—and their multitude of interactive effects—that drive this global issue. Dissecting such complexity could benefit from strategies that integrate multiple disciplines and address the mechanistic underpinnings of population declines and extirpations. We examine how the disciplines of behavior and physiology could be used to develop conservation strategies for amphibians and identify eight research gaps that provide future directions for the emerging fields of conservation behavior and conservation physiology. We present two case studies on imperiled salamanders that show how studies of behavior and physiology may support amphibian conservation efforts. We found several applications of stress physiology to amphibian conservation, but long-term studies are needed to understand how stress ultimately affects individual fitness and population resilience. Additionally, multiple measures of physiological health are needed to provide a more holistic assessment of an individual's overall condition. Previous behavioral and physiological studies have been instrumental for understanding how amphibians respond to habitat modification, pathogens and parasites, contaminants, and invasive species. Some behavior-based approaches to mitigating invasive species issues have been successful in short-term studies with individual species. However, widespread application of these tactics has not yet been integrated into conservation and management strategies for ecologically similar species. A diversity of modeling approaches has enhanced understanding of how climate variability may impact amphibian populations, but model predictions need empirical tests to provide conservation managers with workable approaches to multiple perturbations associated with global environmental change. We illustrate that behavior and physiology can have broad utility for amphibian conservation, but evidence is scant that such studies have actually been used to inform strategies for amphibian conservation and management.
A substantial challenge in addressing the biodiversity crisis is the inherent complexity of environmental problems that drive population declines (Blaustein et al., 2011). Because of this complexity, there is a need to incorporate interdisciplinary approaches in conservation planning. This need has given rise to such fields as conservation genetics (Hedrick, 2001; Shaffer et al., 2015), conservation physiology (Wikelski and Cooke, 2006; Cooke et al., 2013, 2014), conservation behavior (Sutherland, 1998; Blumstein and Fernández-Juricic, 2004; Buchholz, 2007), and integrative behavioral ecotoxicology (Clotfelter et al., 2004; Peterson et al., 2017). Interdisciplinary approaches can directly address the mechanistic underpinnings of population processes (e.g., extinction risk and metapopulation connectivity), thus providing insight into the causes of biodiversity loss. The merger of physiology, behavior, and genetics with conservation, for example, has the potential to generate “cause-and-effect” relationships that can reveal how various stressors may contribute to population declines (Cooke et al., 2013; Birnie-Gauvin et al., 2017). Despite the promise that these emerging disciplines hold, a disconnect continues between basic research and applied wildlife conservation and management, particularly with behavior (Merrick and Koprowski, 2017; but see Fortin et al., 2005 for an illustrative counterexample). Moreover, the knowledge and tools that interdisciplinary approaches produce need to not only be used to document problems, but to also develop and test management strategies to solve complex conservation problems.
Integrative approaches could be particularly relevant in the case of amphibians which, among the vertebrate classes, have experienced the highest rates of diversity loss with an estimated 43% of all known species declining globally (Wake and Vredenberg, 2008). Amphibian population declines have been well-documented and are attributed to various stressors—and their synergistic interactions—such as habitat modification, disease, contaminants, competition and predation from both non-indigenous and native species, climate change, and overexploitation (Egea-Serrano et al., 2012; Grant et al., 2016; Blackburn et al., 2019). Because of the compounding effects of interacting stressors, the causes for amphibian declines are complex (Hayes et al., 2010; Blaustein et al., 2011). Studies of amphibian population declines traditionally have not addressed this complexity but, rather, have often focused on single factors (e.g., disease) affecting particular amphibian species (Blaustein et al., 2011). Because of their interactive nature, multidisciplinary approaches are well-suited to disentangle this complexity, uncover the behavioral, physiological, and other mechanisms that drive population declines and extirpations, and reveal pathways forward to developing conservation solutions.
Stressors are biotic or abiotic factors that can challenge individual homeostasis, resulting in an acute glucocorticoid (GC) stress response. During such reactions, energy stores are mobilized and rapid behavioral and physiological changes typically occur (Greenberg and Wingfield, 1987). However, severe or prolonged exposure to stressors can negatively influence reproduction, immune function, and growth, and can impair subsequent responses to additional stressors because the hypothalamic–pituitary–adrenal/interrenal axis (HPA/HPI) becomes non-responsive (Sapolsky et al., 2000; McEwen and Wingfield, 2003). As the magnitude of a perceived stressor increases, GC levels may be upregulated or downregulated (Gendron et al., 1997; Gabor et al., 2018a), indicating that GCs may correlate with physiological health, population declines, and loss of genetic variation (Dantzer et al., 2014).
In addition to physiological indicators of environmental stress, behavioral “proxies” of population viability can also provide early indications of population decline, allowing proactive management and conservation before extinction risk escalates (Gerber, 2006; Janin et al., 2011; Gabor et al., 2018a; Madliger et al., 2018). Thus, given the unprecedented population declines and extirpations that amphibians are experiencing, we believe that the integration of behavior and physiology with conservation is both timely and likely urgent for this taxonomic group. We address the question: how can these disciplines be integrated and used to develop conservation strategies for amphibians? We review key studies in behavior and physiology to examine their potential to inform amphibian conservation. We then present two case studies with imperiled salamanders—one of a long-term program of behavioral research and the other from Gabor's research on physiology and behavior—that show how such studies may support conservation efforts. Finally, we identify research gaps and potential directions that could enable future behavioral and physiological studies to help develop conservation approaches for declining amphibian populations.
We compiled studies of conservation behavior/physiology with amphibians, using Google Scholar and our knowledge of the literature (Table 1). We performed a search for publications on “conservation behavior and amphibians” and “conservation physiology and amphibians” in Google Scholar, primarily from 2000 to present (unless otherwise indicated). We additionally searched the Thomson Reuters Web of Science™ database across all years for literature on contaminants, behavior, and physiology, using the search terms “amphibian behavior and contaminants” and “amphibian physiology and contaminants.” We compiled selected studies that are relevant to five known stressors that impact amphibian populations [climate change; pathogens and parasites; invasive species; habitat modification (including urbanization); and contaminants: (Hayes et al., 2010)] and one tactic that is widely used as part of recovery strategies for threatened species (ex situ conservation/captive breeding). Although we include captive breeding here, we caution that captive breeding is a measure of last resort for critically endangered species that should not usurp protection of animals in the wild. This compilation, though not exhaustive, provides insight into ways behavioral and physiological studies can reveal amphibian responses to various factors, thus helping to design and improve approaches to their conservation and management.
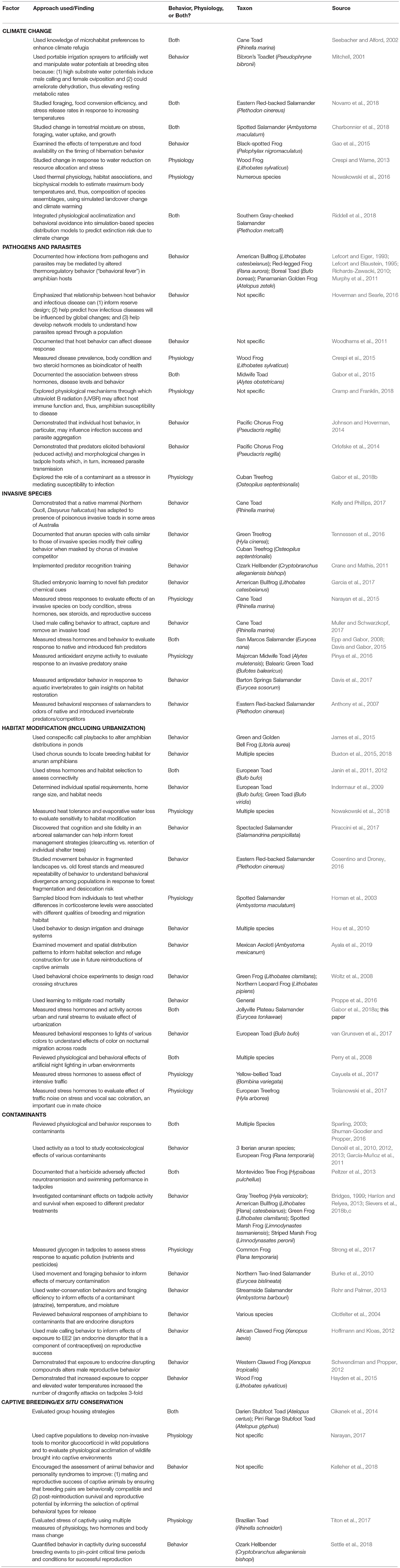
Table 1. Selected examples of studies in behavior and physiology that could help inform amphibian conservation.
Understanding how amphibians may cope with climatic variability has involved examining microhabitat preferences, as well as species' behavioral and physiological responses to increasing temperatures and decreasing water availability (Griffis-Kyle, 2016; Table 1). Such studies have helped develop a climate adaptation plan for at least one declining species (Mitchell, 2001). Understanding host behavior has been instrumental in revealing how amphibians can sometimes mediate (through altered thermoregulatory behavior) infections from parasites and pathogens, and physiological studies have helped elucidate how abiotic factors may affect host immune function and susceptibility to disease (Table 1). Vocalization behavior has been used to attract individuals to newly created habitats to ameliorate the effects of habitat loss and fragmentation and several physiological metrics—e.g., stress responses, heat tolerance, and evaporative water loss—have helped evaluate amphibian responses to habitat modification (including urbanization). Both behavioral and physiological studies have revealed how species respond to invasive predators and findings have generated novel ways to reduce predation risk (Table 1).
In addition to direct lethal impacts, contaminants and disease can have numerous sublethal effects—including behavioral and physiological responses—that, while not overtly causing mortality, may weaken an animal and make it more vulnerable to other stressors (Sparling, 2003; Shuman-Goodier and Propper, 2016; Rollins-Smith, 2017). For example, behavior and physiology can be sensitive indicators of endocrine disruption (Schwendiman and Propper, 2012). Behavioral studies have provided insight into strategies for reducing the impact of emerging infectious diseases (Hoverman and Searle, 2016). Experimental studies have illustrated the role that contaminants could play in compromising reproductive success, therefore potentially contributing to the global problem of amphibian decline (Table 1). In the context of ex situ conservation, physiological assays have helped evaluate acclimation of animals brought into captive environments (Narayan, 2017). Likewise, an understanding of animal behavior and personality syndromes can inform the selection of optimal behavioral types for release and ensure that captive breeding pairs are behaviorally compatible, thus improving mating success, post-reintroduction survival and reproductive potential (Kelleher et al., 2018). Evidence is growing that cognitive behavior, decision making, and personality syndromes occur in amphibians (Jaeger et al., 2016; Kelleher et al., 2018) and could have many conservation and management applications (Blumstein and Berger-Tal, 2015; Owen et al., 2016; Table 1).
Despite the large body of evidence that these various stressors unequivocally affect amphibian populations, the application of this information to conservation issues—i.e., to the development of specific conservation strategies—remains uncommon. Similarly, Mahoney et al. (2018) found that an increase in conservation physiology research has not led to an increased application of physiological tools in recovery planning for threatened and endangered species in the United States. For example, for recovery plans developed over an 11-year period, only 17% incorporated current physiological methodologies as recovery or monitoring activities. Thus, the need to integrate such knowledge into concrete conservation actions remains a critical need in recovery planning for imperiled species.
The Eastern Red-backed Salamander (Plethodon cinereus: hereafter, Red-backed Salamander) is widespread on forest floors of northeastern North America. By comparison, the Shenandoah Salamander (P. shenandoah) is restricted to only three populations, isolated on three mountain tops in Shenandoah National Park, Virginia, United States (Jaeger, 1980). These three populations inhabit Pleistocene-age talus slopes that contain sparse shade and are drier than the surrounding, deeper soil in well-shaded forests (Jaeger et al., 2016). Red-backed Salamanders are abundant in these forests and completely surround each Shenandoah Salamander population. Also, the talus slopes are slowly eroding, with Red-backed Salamanders found in these intruding soils. Jaeger (1970, 1971a,b); Jaeger (1972) hypothesized that the Shenandoah Salamander is an extinction-prone species based on these observations. He then plotted the distributions of both species and found that Red-backed Salamanders rarely entered the talus whereas Shenandoah Salamanders moved into the surrounding deeper, moister soil, but seldom are able to progress more than 3 m from the talus' edge into the forest on Hawksbill Mountain. Laboratory experiments indicated that both species prefer moist soil vs. drier rocky areas and that the Shenandoah Salamander is more tolerant of drier habitats than is the Red-backed Salamander.
Experiments in outdoor enclosures indicated that the Red-backed Salamander competitively excludes Shenandoah Salamanders from the deep-soil forest and, thus, essentially confines it to talus-refugia. Additional research indicated that the two species compete for prey on the forest floor (Jaeger et al., 2016). On Hawksbill Mountain, several small areas of talus occur below the large talus on top of the mountain. Jaeger (1980) conducted a 14-year census in these isolated talus patches and found that in one of them, the Shenandoah Salamander became extinct during a long drought. In general, amphibians have numerous adaptations that minimize water loss but, regardless, dehydration ultimately occurs when water loss exceeds an individual's physiological tolerance and ability to store water internally (Griffis-Kyle, 2016). To prevent mortality from dehydration during droughts, one management tactic could be to water talus patches during extended dry periods, as has been suggested to extend pond hydroperiods for aquatic-breeding amphibians (Griffis-Kyle, 2016).
Behavioral experiments in the laboratory showed that both species avoided pheromones of each other and that individuals preferred to reside on their own substrate pheromones (Jaeger and Gergits, 1979). This suggested that both species behaved as if territorial. Wrobel et al. (1980) also conducted behavioral experiments in the laboratory; the two species aggressively displayed and often bit each other, again suggesting interspecific competition as a mechanism driving the spatial distributions of the two species. Later research (Griffis and Jaeger, 1998) on Hawksbill Mountain found that Red-backed Salamanders successfully defend territories (under rocks and logs) against the Shenandoah Salamander. This inhibits the latter species' movements from the source talus on top of the mountain to the sink populations below: a rare example of how interspecific competition—mediated by aggressive interference and territoriality—can lead to the endangerment of one of the competing species. Ultimately, this research contributed to listing the Shenandoah Salamander as a federally endangered species in 1989 [U.S. Fish and Wildlife Service USFWS (1989)]. Therefore, the use of observations and experiments in both laboratory and natural habitats provided the necessary information to protect and manage this vulnerable species but, to our knowledge, no specific management actions (related to interspecific competition as a factor in declines) have yet been implemented (USFWS, 2013).
Land-use modification (e.g., urbanization and agriculture) is often linked to higher environmental temperatures, pollution, and eutrophication in water bodies (Smith et al., 2006). Changes in water quality can affect the behavior and endocrine systems of aquatic organisms, and nitrogen and phosphorous are among the top contributors to waterway degradation (Cook et al., 2018). Nitrogen release—from fertilizers in agriculture and suburban settings, animal waste, and wastewater effluents—results in elevated levels of active forms of nitrogen (e.g., nitrate, nitrite, ammonia, and ammonium). Nitrate is a major contaminant in freshwater aquatic environments and is especially toxic to amphibians (Rouse et al., 1999; Kellock et al., 2018). Thus, understanding risks, susceptibility, and behavioral and physiological responses of aquatic species to changes in land use is necessary to mitigate potential stressors, especially as habitat conversion continues (Pauchard et al., 2006).
Gabor et al. (2018a) examined the relation between corticosterone (the main amphibian GC) and urbanization in a stream dwelling amphibian, the Jollyville Plateau Salamander (Eurycea tonkawae). This plethodontid is a neotenic (completely aquatic) species found in karst-associated groundwater in the Edwards-Trinity aquifer system of central Texas, United States. This spring water is oligotrophic, has constant temperatures, and supports a unique assemblage of aquatic species adapted to these conditions. A recent phylogenomic analysis revealed extensive cryptic species diversity in the salamander assemblage endemic to this aquifer system (Devitt et al., 2019). However, because of overexploitation of this groundwater resource, regional climate and hydrologic models project that these salamanders are at high risk of extinction within the next century (Devitt et al., 2019). Indeed, the Jollyville Plateau Salamander has already been listed as threatened under the Endangered Species Act of 1973 (USFWS, 2019) due to threats from urbanization. Counts of this species have declined in areas that had the largest increases in urbanization (measured by residential development) and salamander densities were negatively correlated with residential development throughout the species' range (Bendik et al., 2014).
Gabor et al. (2018a) examined baseline corticosterone and stress response (as an indication of chronic stress) for 3 years in urban sites (≥25% impervious cover) vs. rural sites. They obtained corticosterone release rates using a non-invasive water-borne hormone assay and found that corticosterone was higher in urban sites than in rural ones in 2 of the 3 years of the study. Salamanders showed general stress responsiveness (to agitation) across all years and populations, indicating that even if salamanders were physiologically stressed they were not necessarily chronically stressed. They also found that “background corticosterone,” measured directly from the stream in which the salamanders were sampled, was higher in urban than rural streams and was positively correlated with baseline corticosterone across populations and years. Background corticosterone provides an efficient, indirect method of evaluating stress levels and physiological health in aquatic vertebrates, allowing for more expeditious management decisions and evaluation of their effectiveness for imperiled species.
Mondelli (2016) used a behavioral assay (activity) in combination with a corticosterone assay to further examine the effects of urbanization on the Jollyville Plateau Salamander. Corticosterone and activity were positively related in two urban sites yet negatively related in two rural ones (Figure 1). The relation between activity and corticosterone release rates in urban and rural sites (albeit in opposite directions) demonstrates that measures of activity provide an additional indication of salamander responsiveness to their environment; for this species, salamanders in more urban habitats that release higher levels of corticosterone will be more active. Yet, higher activity can make individuals more vulnerable to visual predators (Epp and Gabor, 2008). This suggests a possible trade-off in consequences of behavioral activity, particularly in urban environments, for some aquatic amphibians.
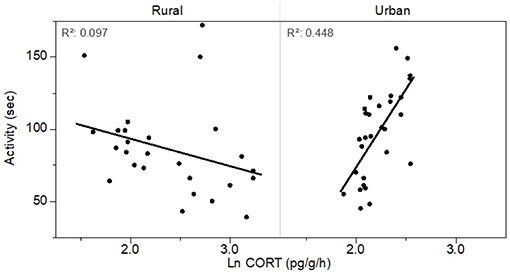
Figure 1. Relationship between total movement activity (in seconds) of Jollyville Plateau Salamanders (Eurycea tonkawae) and natural log of corticosterone release rates for salamanders collected in more rural vs. more urban streams. Modified from Mondelli (2016).
These results have not yet been used directly in conservation and management strategies for amphibians. Moreover, we are unaware of any other studies in which behavioral and/or physiological findings with respect to urbanization, in general, have been used in this manner. However, because aquatic urban habitats often accumulate toxic contaminants that can affect amphibians at sublethal levels (Sievers et al., 2018a,b), behavioral and physiological assays can be additional tools in the conservation toolbox with which to assess effects of urban-associated contaminants and may serve as early warning signals of potential population declines. Although the specific contaminants that may have affected corticosterone in Mondelli (2016) are not known, exposure to nitrate and nitrite affects behavior, physiology, and survival in some aquatic species (Hecnar, 1995; Jannat et al., 2014; Pottinger, 2017). An important next step in this research will be to examine potential links among specific contaminants, physiology and behavior. A combination of laboratory and field studies that examine the relationship between corticosterone and behavior across a range of environmentally-relevant levels of active nitrogen could aid in understanding the consequences of urbanization on population health. If indeed nitrate affects behavior and/or physiology, then a management plan could include the use of vegetated buffer zones around water to reduce the amount of nitrate entering the water through runoff, as suggested by Rouse et al. (1999).
Herein we have highlighted representative examples of how studies of behavior and physiology could help inform amphibian conservation efforts, but substantial deficiencies remain. Studies with amphibians are generally under-represented in the conservation behavior and physiology literature, indicating a clear need for greater focus on amphibians in future research (Madliger et al., 2018). Long-term studies could help elucidate how behavior and physiological stress ultimately affect individual fitness and population resilience. Considerable advancements have been made in conservation behavior: for example, the scientific community now has a greater understanding of how native amphibian species respond to invasive predators, and novel behavior-based approaches have been designed to minimize the impact of invasive species (Table 1). However, widespread application of actions has not yet been integrated into conservation and management strategies for a broad array of ecologically-similar species. Other than the species-specific climate adaptation plan implemented by Mitchell (2001), it is unclear what targeted conservation strategies have been developed as a consequence of many physiological and behavioral studies. Thus, fully integrating the information gained from research in behavior and physiology into concrete conservation actions is the most critical need at the interface of these three disciplines.
In addition, there are several areas of research in amphibian behavior and physiology that could be strengthened to better integrate these disciplines with conservation. For example:
1. The terms “stress” and “stressor” are often used without a clear physiological basis in the conservation literature. Species' threats are often identified as “stressors,” even though the physiological effects of such factors on individuals and populations may not have been empirically established (Mahoney et al., 2018) or, if examined, may have been found to not occur (Gunderson et al., 2017). Contrary to the expectation of stressor effects on amphibians, there are several examples of how pathogens (Kiesecker and Blaustein, 1999), contaminants (Boone et al., 2004; Boone, 2018), and certain predators (Werner and Anholt, 1996; Davis and Gabor, 2015) can have positive (indirect) or otherwise unanticipated effects on populations. Thus, the effects of stressors can vary within and among populations (e.g., Hopkins et al., 2016) and species, and across scales (Grant et al., 2016; Muths et al., 2017). This variation underscores the need to test assumptions about the physiological basis of stress, especially when used in the context of species' threats.
2. Anthropogenic change can often occur over short time periods, and physiological indicators of how well a species may be able to respond to environmental disruptions could help reveal the potential for populations to persist. Steroid levels can fluctuate rapidly with environmental modifications and variation in individual responses can be high. Repeatability of these variable responses (multiple phenotypes) could indicate that endocrine traits exhibit heritable variation and, thus, the ability to evolve in response to environmental change (Hau et al., 2016). Miles et al. (2018) documented support for this hypothesis with respect to corticosterone but more work is needed. Thus, measuring repeatability in stress hormone activity is an important area of future research because it could provide an indication of how well a given species may be able to respond to environmental change.
3. Multiple measures of physiological health (e.g., glucose and lactate concentrations, metabolic rate or telomere length as a measure of metabolic cost) would provide a more holistic assessment of an individual's condition and overall resilience of a population than would individual measures.
4. Physiological and behavioral attributes of relatively healthy populations—both of endangered and common species—need to be documented. Doing so could provide a comparative basis for evaluating responses to environmentally-induced stress. Non-threatened species can also undergo localized population declines and losses, yet typically are not the beneficiaries of conservation interventions, as are imperiled species. Proactively implementing conservation of common species could lead to early detection of conservation problems before endangerment occurs.
5. Behavior-based approaches to climate change adaptation are still in their infancy (Caro, 2016). Phenological shifts are key responses of species to recent climate change, yet the use of experimental approaches to gain insights into phenology are still uncommon (Gao et al., 2015). Both behavioral and physiological responses to climate change have been simulated with biophysical and species distribution models (Table 1), but these predictions need empirical tests to provide conservation managers with workable solutions to climate change.
6. The potential for behavior to mitigate disease outbreaks also warrants further study, especially as it relates to informing the design of refugia from pathogens and parasites (Hoverman and Searle, 2016).
7. Nitrate has recently been considered an endocrine disruptor that has many negative effects on amphibians and other aquatic organisms (Poulsen et al., 2018). Indeed, cortisol release rates in fish were elevated below wastewater plants where nitrate levels were high compared to upstream sites (Pottinger, 2017). More studies are needed with amphibians to examine the relationship between corticosterone and active nitrogen. If there is a link, then just measuring active nitrogen in an aquatic system could provide a first approximation of population health.
8. Last, the fundamental questions in any mechanistic study are, first, how do behavioral and/or physiological phenomena “scale up” from the individual to that of the population and community levels (e.g., Jaeger et al., 2016; Saaristo et al., 2018)? Secondly, do responses observed under controlled, experimental conditions translate into measurable effects on population demography in nature (Saaristo et al., 2018)? Answers to these questions will ultimately determine the extent to which behavioral and physiological studies may influence conservation strategies for amphibians.
Our review and highlighted case studies illustrate the potential utility of studies in behavior and physiology for informing strategies for amphibian conservation and management. Caro (2016) stated that examples of useful behavioral applications for conservation practitioners are “principally restricted to ex situ conservation.” However, we found numerous examples of behavioral and physiological studies that address key stressors known to impact amphibian populations—climate change, pathogens and parasites, invasive species, habitat change, and contaminants—in addition to ex situ conservation.
Our first case study highlighting Jaeger's research (Jaeger et al., 2016) illustrates the value of long-term investigations and, together with the studies by Gabor (Mondelli, 2016; Gabor et al., 2018a), shows the dynamic nature of relationships among environmental factors, physiology, and behavioral attributes as climate change and habitat alteration escalates. These highlighted research programs illustrate how examining a species' extinction risk through the lens of behavior and physiology can help elucidate why an extinction-prone species is currently declining, as well as provide early indicators of future population declines in at-risk species. In the future, longer-term studies, use of multiple measures of physiological health, and a focus on scaling-up from individual behavior and physiology to the population and community levels could help pinpoint how stressor impacts compound to affect population persistence and community organization. Understanding mechanisms by which various stressors may be contributing to population declines is a fundamental first step in fostering solutions to the amphibian decline crisis. However, in many cases, specific conservation strategies based on such knowledge have yet to be developed or implemented and are priorities for future conservation and management of this imperiled group of vertebrates.
Both authors contributed to the review through development of ideas, along with drafting, and revision of text. CG prepared the figure.
This work was funded, in part, by the U.S. Geological Survey's Amphibian Research and Monitoring Initiative (ARMI) to SW and a Texas State Wildlife Grant (#T-99-1) in cooperation with the U.S. Fish and Wildlife Service, Wildlife and Sport Fish Restoration Program to CG.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to C. D. Anthony, R. G. Jaeger, and J. C. Mitchell for comments on earlier drafts of this manuscript and J. T. Thibodeaux for administrative assistance. R. G. Jaeger generously summarized his work for inclusion in this paper. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This work is ARMI contribution number 687.
Anthony, C. D., Hickerson, C. M., and Venesky, M. D. (2007). Responses of juvenile terrestrial salamanders to introduced (Lithobius forficatus) and native centipedes (Scolopocryptops sexspinosus). J. Zool. 271, 54–62. doi: 10.1111/j.1469-7998.2006.00202.x
Ayala, C., Ramos, A. G., Merlo, A., and Zambrano, L. (2019). Microhabitat selection of axolotls, Ambystoma mexicanum, in artificial and natural aquatic systems. Hydrobiologia 828, 11–20. doi: 10.1007/s10750-018-3792-8
Bendik, N. F., Sissel, B. N., Fields, J. R., O'Donnell, L. J., and Sanders, M. S. (2014). Effect of urbanization on abundance of Jollyville plateau salamanders (Eurycea tonkawae). Herpetol. Conserv. Biol. 9, 206–222.
Birnie-Gauvin, K., Walton, S., Delle Palme, C. A., Manouchehri, B. A., Venne, S., Lennox, R. J., et al. (2017). Conservation physiology can inform threat assessment and recovery planning processes for threatened species. Endang. Species Res. 32, 507–513. doi: 10.3354/esr00831
Blackburn, T. M., Bellard, C., and Ricciardi, A. (2019). Alien versus native species as drivers of recent extinctions. Front. Ecol. Environ. doi: 10.1002/fee.2020
Blaustein, A. R., Han, B., Relyea, R. A., Johnson, P. T. J., Buck, J. C., Gervasi, S. S., et al. (2011). The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann. NY Acad. Sci. 1223, 108–119. doi: 10.1111/j.1749-6632.2010.05909.x
Blumstein, D. T., and Berger-Tal, O. (2015). Understanding sensory mechanisms to develop effective conservation and management tools. Curr. Opin. Behav. Sci. 6, 13–18. doi: 10.1016/j.cobeha.2015.06.008
Blumstein, D. T., and Fernández-Juricic, E. (2004). The emergence of conservation behavior. Conserv. Biol. 18, 1175–1177. doi: 10.1111/j.1523-1739.2004.00587.x
Boone, M. D. (2018). An amphibian with a contracting range is not more vulnerable to pesticides in outdoor experimental communities than common species. Environ. Toxicol. 37, 2699–2704. doi: 10.1002/etc.4236
Boone, M. D., Semlitsch, R. D., Fairchild, J. F., and Rothermel, B. B. (2004). Effects of an insecticide on amphibians in large-scale experimental ponds. Ecol. Appl. 14, 685–691. doi: 10.1890/02-5308
Bridges, C. M. (1999). Effects of a pesticide on tadpole activity and predator avoidance behavior. J. Herpetol. 33, 303–306. doi: 10.2307/1565728
Buchholz, R. (2007). Behavioural biology: an effective and relevant conservation tool. Trends Ecol. Evol. 22, 401–407. doi: 10.1016/j.tree.2007.06.002
Burke, J. N., Bergeron, C. M., Todd, B. D., and Hopkins, W. A. (2010). Effects of mercury on behavior and performance of northern two-lined salamanders (Eurycea bislineata). Environ. Pollut. 158, 3546–3551. doi: 10.1016/j.envpol.2010.08.017
Buxton, V. L., Ward, M. P., and Sperry, J. H. (2015). Use of chorus sounds for location of breeding habitat in 2 species of anuran amphibians. Behav. Ecol. 26, 1011–1018. doi: 10.1093/beheco/arv059
Buxton, V. L., Ward, M. P., and Sperry, J. H. (2018). Evaluation of conspecific attraction as a management tool across several species of anurans. Diversity 10:6. doi: 10.3390/d10010006
Caro, T. (2016). Behavior and conservation, conservation and behavior. Curr. Opin. Behav. Sci. 12, 97–102. doi: 10.1016/j.cobeha.2016.09.008
Cayuela, H., Quay, L., Dumet, A., Léna, J. P., Miaud, C., and Rivière, V. (2017). Intensive vehicle traffic impacts morphology and endocrine stress response in a threatened amphibian. Oryx 51, 182–188. doi: 10.1017/S0030605315000812
Charbonnier, J., Pearlmutter, J., Vonesh, J., Gabor, C., Forsburg, Z., and Grayson, K. (2018). Cross-life stage effects of aquatic larval density and terrestrial moisture on growth and corticosterone in the spotted salamander. Diversity 10:68. doi: 10.3390/d10030068
Cikanek, S. J., Nockold, S., Brown, J. L., Carpenter, J. W., Estrada, A., Guerrel, J., et al. (2014). Evaluating group housing strategies for the ex-situ conservation of harlequin frogs (Atelopus spp.) using behavioral and physiological indicators. PLoS ONE 9:e90218. doi: 10.1371/journal.pone.0090218
Clotfelter, E. D., Bell, A. M., and Levering, K. R. (2004). The role of animal behaviour in the study of endocrine-disrupting chemicals. Anim. Behav. 68, 665–676. doi: 10.1016/j.anbehav.2004.05.004
Cook, S. C., Housley, L., Back, J. A., and King, R. S. (2018). Freshwater eutrophication drives sharp reductions in temporal beta diversity. Ecology 99, 47–56. doi: 10.1002/ecy.2069
Cooke, S. J., Blumstein, D. T., Buchholz, R., Caro, T., Fernández-Juricic, E., Franklin, C. E., et al. (2014). Physiology, behavior, and conservation. Physiol. Biochem. Zool. 87, 1–14. doi: 10.1086/671165
Cooke, S. J., Sack, L., Franklin, C. E., Farrell, A. P., Beardall, J., Wikelski, M., et al. (2013). What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv. Physiol. 1:cot001. doi: 10.1093/conphys/cot001
Cosentino, B. J., and Droney, D. C. (2016). Movement behaviour of woodland salamanders is repeatable and varies with forest age in a fragmented landscape. Anim. Behav. 121, 137–146. doi: 10.1016/j.anbehav.2016.08.013
Cramp, R. L., and Franklin, C. E. (2018). Exploring the link between ultraviolet B radiation and immune function in amphibians: implications for emerging infectious diseases. Conserv. Physiol. 6:coy035. doi: 10.1093/conphys/coy035
Crane, A. L., and Mathis, A. (2011). Predator-recognition training: a conservation strategy to increase post release survival of hellbenders in head-starting programs. Zoo Biol. 30, 611–622. doi: 10.1002/zoo.20358
Crespi, E. J., Rissler, L. J., Mattheus, N. M., Engbrecht, K., Duncan, S. I., Seaborn, T., et al. (2015). Geophysiology of wood frogs: landscape patterns of prevalence of disease and circulating hormone concentrations across the eastern range. Integr. Comp. Biol. 55, 602–617. doi: 10.1093/icb/icv096
Crespi, E. J., and Warne, R. W. (2013). Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integr. Comp. Biol. 53, 989–1001. doi: 10.1093/icb/ict087
Dantzer, B., Fletcher, Q. E., Boonstra, R., and Sheriff, M. J. (2014). Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv. Physiol. 2:cou023. doi: 10.1093/conphys/cou023
Davis, D. R., DeSantis, D. L., and Gabor, C. R. (2017). Antipredator behavior of the Barton Springs salamander (Eurycea sosorum) in response to aquatic invertebrates: potential consequences of habitat restoration. Hydrobiologia 795, 129–137. doi: 10.1007/s10750-017-3124-4
Davis, D. R., and Gabor, C. R. (2015). Behavioral and physiological antipredator responses of the San Marcos salamander, Eurycea nana. Physiol. Behav. 139, 145–149. doi: 10.1016/j.physbeh.2014.11.013
Denoël, M., Bichot, M., Ficetola, G. F., Delcourt, J., Ylieff, M., Kestemont, P., et al. (2010). Cumulative effects of road de-icing salt on amphibian behavior. Aquat. Toxicol. 99, 275–280. doi: 10.1016/j.aquatox.2010.05.007
Denoël, M., D'Hooghe, B., Ficetola, G. F., Brasseur, C., De Pauw, E., Thomé, J. P., et al. (2012). Using sets of behavioral biomarkers to assess short-term effects of pesticide: a study case with endosulfan on frog tadpoles. Ecotoxicology 21, 1240–1250. doi: 10.1007/s10646-012-0878-3
Denoël, M., Libon, S., Kestemont, P., Brasseur, C., Focant, J. F., and De Pauw, E. (2013). Effects of a sublethal pesticide exposure on locomotor behavior: a video-tracking analysis in larval amphibians. Chemosphere 90, 945–951. doi: 10.1016/j.chemosphere.2012.06.037
Devitt, T. J., Wright, A. M., Cannatella, D. C., and Hillis, D. M. (2019). Species delimitation in endangered groundwater salamanders: implications for aquifer management and biodiversity conservation. Proc. Natl. Acad. Sci. U.S.A. 116, 2624–2633. doi: 10.1073/pnas.1815014116
Egea-Serrano, A., Relyea, R. A., Tejedo, M., and Torralva, M. (2012). Understanding of the impact of chemicals on amphibians: a meta-analytic review. Ecol. Evol. 2, 1382–1397. doi: 10.1002/ece3.249
Epp, K. J., and Gabor, C. R. (2008). Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology 114, 607–615. doi: 10.1111/j.1439-0310.2008.01494.x
Fortin, D., Beyer, H. L., Boyce, M. S., Smith, D. W., Duchesne, T., and Mao, J. S. (2005). Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320–1330. doi: 10.1890/04-0953
Gabor, C. R., Davis, D. R., Kim, D. S., Zabierek, K. C., and Bendik, N. F. (2018a). Urbanization is associated with elevated corticosterone in Jollyville Plateau salamanders. Ecol. Indic. 85, 229–235. doi: 10.1016/j.ecolind.2017.10.047
Gabor, C. R., Fisher, M. C., and Bosch, J. (2015). Elevated corticosterone levels and changes in amphibian behavior are associated with Batrachochytrium dendrobatidis (Bd) infection and Bd lineage. PLoS ONE 10:e0122685. doi: 10.1371/journal.pone.0122685
Gabor, C. R., Knutie, S. A., Roznik, E. A., and Rohr, J. R. (2018b). Are the adverse effects of stressors on amphibians mediated by their effects on stress hormones? Oecologia 186, 393–404. doi: 10.1007/s00442-017-4020-3
Gao, X., Jin, C., Llusia, D., and Li, Y. (2015). Temperature-induced shifts in hibernation behavior in experimental amphibian populations. Sci. Rep. 5:11580. doi: 10.1038/srep11580
Garcia, T. S., Urbina, J. C., Bredeweg, E. M., and Ferrari, M. C. O. (2017). Embryonic learning and developmental carry-over effects in an invasive anuran. Oecologia 184, 623–631. doi: 10.1007/s00442-017-3905-5
García-Muñoz, E., Guerrero, F., and Parra, G. (2011). Larval escape behavior in anuran amphibians as a wetland rapid pollution biomarker. Mar. Freshw. Behav. Physiol. 44, 109–123. doi: 10.1080/10236244.2011.557855
Gendron, A. D., Bishop, C. A., Fortin, R., and Hontela, A. (1997). In vivo testing of the functional integrity of the corticosterone-producing axis in mudpuppy (Amphibia) exposed to chlorinated hydrocarbons in the wild. Environ. Toxicol. Chem. 16, 1694–1706. doi: 10.1002/etc.5620160818
Gerber, L. R. (2006). Including behavioral data in demographic models improves estimates of population viability. Front. Ecol. Environ. 4, 419–427. doi: 10.1890/1540-9295(2006)4[419:IBDIDM]2.0.CO;2
Grant, E. H., Miller, D. A. W., Schmidt, B. R., Adams, M. J., Amburgey, S. M., Chambert, T., et al. (2016). Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci. Rep. 6:25625. doi: 10.1038/srep25625
Greenberg, N., and Wingfield, J. C. (1987). “Stress and reproduction: reciprocal relationships,” in Hormones and Reproduction in Fishes, Amphibians and Reptiles, eds D. O. Norris and R. E. Jones (New York, NY: Plenum, 461–489.
Griffis, M. R., and Jaeger, R. G. (1998). Competition leads to an extinction-prone species of salamander: interspecific territoriality in a metapopulation. Ecology 79, 2494–2502. doi: 10.1890/0012-9658(1998)079[2494:CLTAEP]2.0.CO;2
Griffis-Kyle, K. L. (2016). Physiology and ecology to inform climate adaptation strategies for desert amphibians. Herpetol. Conserv. Biol. 11, 563–582.
Gunderson, A. R., King, E. E., Boyer, K., Tsukimura, B., and Stillman, J. H. (2017). Species as stressors: heterospecific interactions and the cellular stress response under global change. Integr. Comp. Biol. 57, 90–102. doi: 10.1093/icb/icx019
Hanlon, S. M., and Relyea, R. (2013). Sublethal effects of pesticides on predator–prey interactions in amphibians. Copeia 2013, 691–698. doi: 10.1643/CE-13-019
Hau, M., Casagrande, S., Ouyang, J. Q., and Baugh, A. T. (2016). Chapter two - Glucocorticoid-Mediated phenotypes in vertebrates: multilevel variation and evolution. Adv. Study Behav. 48, 41–115. doi: 10.1016/bs.asb.2016.01.002
Hayden, M. T., Reeves, M. K., Holyoak, M., Perdue, M., King, A. L., and Tobin, S. C. (2015). Thrice as easy to catch! Copper and temperature modulate predator-prey interactions in larval dragonflies and anurans. Ecosphere 6:art56. doi: 10.1890/ES14-00461.1
Hayes, T. B., Falso, P., Gallipeau, S., and Stice, M. (2010). The cause of global amphibian declines: a developmental endocrinologist's perspective. J. Exp. Biol. 213, 921–933. doi: 10.1242/jeb.040865
Hecnar, S. J. (1995). Acute and chronic toxicity of ammonium nitrate fertilizer to amphibians from southern Ontario. Environ. Toxicol. Chem. 14, 2131–2137. doi: 10.1002/etc.5620141217
Hedrick, P. W. (2001). Conservation genetics: where are we now? Trends Ecol. Evol. 16, 629–636. doi: 10.1016/S0169-5347(01)02282-0
Hoffmann, F., and Kloas, W. (2012). Estrogens can disrupt amphibian mating behavior. PLoS ONE 7:e32097. doi: 10.1371/journal.pone.0032097
Homan, R. N., Regosin, J. V., Rodrigues, D. M., Reed, J. M., Windmiller, B. S., and Romero, L. M. (2003). Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim. Conserv. 6, 11–18. doi: 10.1017/S1367943003003032
Hopkins, G. R., Brodie, E. D. Jr., Neuman-Lee, L. A., Mohammadi, S., Brusch, G. A. IV., Hopkins, Z. M., et al. (2016). Physiological responses to salinity vary with proximity to the ocean in a coastal amphibian. Physiol. Biochem. Zool. 89, 322–330. doi: 10.1086/687292
Hou, W. S., Chang, Y. H., Wang, H. W., and Tan, Y. C. (2010). Using the behavior of seven amphibian species for the design of banks of irrigation and drainage systems in Taiwan. Irrig. Drain. 59, 493–505. doi: 10.1002/ird.515
Hoverman, J. T., and Searle, C. L. (2016). Behavioural influences on disease risk: implications for conservation and management. Anim. Behav. 120, 263–271. doi: 10.1016/j.anbehav.2016.05.013
Indermaur, L., Gehring, M., Wehrle, W., Tockner, K., and Naef-Daenzer, B. (2009). Behavior-based scale definitions for determining individual space use: requirements of two amphibians. Am. Nat. 173, 60–71. doi: 10.1086/593355
Jaeger, R. G. (1970). Potential extinction through competition between two species of terrestrial salamanders. Evolution 24, 632–642. doi: 10.1111/j.1558-5646.1970.tb01797.x
Jaeger, R. G. (1971a). Competitive exclusion as a factor influencing the distributions of two species of terrestrial salamanders. Ecology 52, 632–637. doi: 10.2307/1934151
Jaeger, R. G. (1971b). Moisture as a factor influencing the distributions of two species of terrestrial salamanders. Oecologia 6, 191–207. doi: 10.1007/BF00344914
Jaeger, R. G. (1972). Food as a limited resource in competition between two species of terrestrial salamanders. Ecology 53, 535–546. doi: 10.2307/1934249
Jaeger, R. G. (1980). Density-dependent and density-independent causes of extinction of a salamander population. Evolution 34, 617–621. doi: 10.1111/j.1558-5646.1980.tb04001.x
Jaeger, R. G., and Gergits, W. F. (1979). Intra- and interspecific communication in salamanders through chemical signals on the substrate. Anim. Behav. 27, 150–156. doi: 10.1016/0003-3472(79)90134-9
Jaeger, R. G., Gollmann, B., Anthony, C. D., Gabor, C. R., and Kohn, N. R. (2016). Behavioral Ecology of the Eastern Red-Backed Salamander: 50 Years of Research. New York, NY: Oxford University Press.
James, M. S., Stockwell, M. P., Clulow, J., Clulow, S., and Mahony, M. J. (2015). Investigating behaviour for conservation goals: conspecific call playback can be used to alter amphibian distributions within ponds. Biol. Conserv. 192, 287–293. doi: 10.1016/j.biocon.2015.10.001
Janin, A., Léna, J. P., Deblois, S., and Joly, P. (2012). Use of stress-hormone levels and habitat selection to assess functional connectivity of a landscape for an amphibian. Conserv. Biol. 26, 923–931. doi: 10.1111/j.1523-1739.2012.01910.x
Janin, A., Léna, J. P., and Joly, P. (2011). Beyond occurrence: body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad. Biol. Conserv. 144, 1008–1016. doi: 10.1016/j.biocon.2010.12.009
Jannat, M., Fatimah, R., and Kishida, M. (2014). Nitrate (NO3(-)) and nitrite (NO2(-)) are endocrine disruptors to downregulate expression of tyrosine hydroxylase and motor behavior through conversion to nitric oxide in early development of zebrafish. Biochem. Biophys. Res. Commun. 452, 608–613. doi: 10.1016/j.bbrc.2014.08.114
Johnson, P. T., and Hoverman, J. T. (2014). Heterogeneous hosts: how variation in host size, behaviour and immunity affects parasite aggregation. J. Anim. Ecol. 83, 1103–1112. doi: 10.1111/1365-2656.12215
Kelleher, S. R., Silla, A. J., and Byrne, P. G. (2018). Animal personality and behavioral syndromes in amphibians: a review of the evidence, experimental approaches, and implications for conservation. Behav. Ecol. Sociobiol. 72:79. doi: 10.1007/s00265-018-2493-7
Kellock, K. A., Moore, A. P., and Bringolf, R. B. (2018). Chronic nitrate exposure alters reproductive physiology in fathead minnows. Environ. Pollut. 232, 322–328. doi: 10.1016/j.envpol.2017.08.004
Kelly, E., and Phillips, B. L. (2017). Get smart: native mammal develops toad-smart behavior in response to a toxic invader. Behav. Ecol. 28, 854–858. doi: 10.1093/beheco/arx045
Kiesecker, J. M., and Blaustein, A. R. (1999). Pathogen reverses competition between larval amphibians. Ecology 80, 2442–2448. doi: 10.1890/0012-9658(1999)080[2442:PRCBLA]2.0.CO;2
Lefcort, H., and Blaustein, A. R. (1995). Disease, predator avoidance, and vulnerability to predation in tadpoles. Oikos 74, 469–474. doi: 10.2307/3545992
Lefcort, H., and Eiger, S. M. (1993). Antipredatory behaviour of feverish tadpoles: implications for pathogen transmission. Behaviour 126, 13–27. doi: 10.1163/156853993X00317
Madliger, C. L., Love, O. P., Hultine, K. R., and Cooke, S. J. (2018). The conservation physiology toolbox: status and opportunities. Conserv. Physiol. 6:coy029. doi: 10.1093/conphys/coy029
Mahoney, J. L., Klug, P. E., and Reed, W. L. (2018). An assessment of the US endangered species act recovery plans: using physiology to support conservation. Conserv. Physiol. 6:coy036. doi: 10.1093/conphys/coy036
McEwen, B. S., and Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. doi: 10.1016/S0018-506X(02)00024-7
Merrick, M. J., and Koprowski, J. L. (2017). Should we consider individual behavior differences in applied wildlife conservation studies? Biol. Conserv. 209, 34–44. doi: 10.1016/j.biocon.2017.01.021
Miles, M. C., Vitousek, M. N., Husak, J. F., Johnson, M. A., Martin, L. B., Taff, C. C., et al. (2018). Standing variation and the capacity for change: are endocrine phenotypes more variable than other traits? Integr. Comp. Biol. 58, 751–762. doi: 10.1093/icb/icy062
Mitchell, N. J. (2001). Males call more from wetter nests: effects of substrate water potential on reproductive behaviours of terrestrial toadlets. Proc. R. Soc. B 268, 87–93. doi: 10.1098/rspb.2000.1334
Mondelli, M. J. (2016). Effects of Urbanization on Stress Response of Texas Eurycea salamanders (Masters Degree). Texas State University.
Muller, B. J., and Schwarzkopf, L. (2017). Success of capture of toads improved by manipulating acoustic characteristics of lures. Pest Manage. Sci. 73, 2372–2378. doi: 10.1002/ps.4629.
Murphy, P. J., St.-Hilaire, S., and Corn, P. S. (2011). Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis. Aquat. Organ. 95, 31–42. doi: 10.3354/dao02336
Muths, E., Chambert, T., Schmidt, B. R., Miller, D. A. W., Hossack, B. R., Joly, P., et al. (2017). Heterogeneous responses of temperate-zone amphibian populations to climate change complicates conservation planning. Sci. Rep. 7:17102. doi: 10.1038/s41598-017-17105-7
Narayan, E. (2017). Evaluation of physiological stress in Australian wildlife: embracing pioneering and current knowledge as a guide to future research directions. Gen. Comp. Endocrinol. 244, 30–39. doi: 10.1016/j.ygcen.2015.12.008
Narayan, E. J., Jessop, T. S., and Hero, J. M. (2015). Invasive cane toad triggers chronic physiological stress and decreased reproductive success in an island endemic. Funct. Ecol. 29, 1435–1444. doi: 10.1111/1365-2435.12446
Novarro, A. J., Gabor, C. R., Goff, C. B., Mezebish, T. D., Thompson, L. M., and Grayson, K. L. (2018). Physiological responses to elevated temperature across the geographic range of a terrestrial salamander. J. Exp. Biol. 221:jeb178236. doi: 10.1242/jeb.178236
Nowakowski, A. J., Watling, J. I., Thompson, M. E., Brusch, G. A. IV., Catenazzi, A., Whitfield, S. M., et al. (2018). Thermal biology mediates responses of amphibians and reptiles to habitat modification. Ecol. Lett. 21, 345–355. doi: 10.1111/ele.12901
Nowakowski, A. J., Watling, J. I., Whitfield, S. M., Todd, B. D., Kurz, D. J., and Donnelly, M. A. (2016). Tropical amphibians in shifting thermal landscapes under land-use and climate change. Conserv. Biol. 31, 96–105. doi: 10.1111/cobi.12769
Orlofske, S. A., Jadin, R. C., Hoverman, J. T., and Johnson, P. T. (2014). Predation and disease: understanding the effects of predators at several trophic levels on pathogen transmission. Freshw. Biol. 59, 1064–1075. doi: 10.1111/fwb.12329
Owen, M. A., Swaisgood, R. R., and Blumstein, D. T. (2016). Contextual influences on animal decision-making: significance for behavior-based wildlife conservation and management. Integr. Zool. 12, 32–48. doi: 10.1111/1749-4877.12235
Pauchard, A., Aguayo, M., Peña, E., and Urrutia, R. (2006). Multiple effects of urbanization on the biodiversity of developing countries: the case of a fast-growing metropolitan area (Concepción, Chile). Biol. Conserv. 127, 272–281. doi: 10.1016/j.biocon.2005.05.015
Peltzer, P. M., Junges, C. M., Attademo, A. M., Bassó, A., Grenón, P., and Lajmanovich, R. C. (2013). Cholinesterase activities and behavioral changes in Hypsiboas pulchellus (Anura: Hylidae) tadpoles exposed to glufosinate ammonium herbicide. Ecotoxicology 22, 1165–1173. doi: 10.1007/s10646-013-1103-8
Perry, G., Buchanan, B. W., Fisher, R. N., Salmon, M., and Wise, S. E. (2008). “Effects of artificial night lighting on amphibians and reptiles in urban environments,” in Urban Herpetology, eds J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew (Salt Lake City, UT: Society for the Study of Amphibians and Reptiles, 239–256.
Peterson, E. K., Buchwalter, D. B., Kerby, J. L., Lefauve, M. K., Varian-Ramos, C. W., and Swaddle, J. P. (2017). Integrative behavioral ecotoxicology: bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Curr. Zool. 63, 185–119. doi: 10.1093/cz/zox010
Pinya, S., Tejada, S., Capó, X., and Sureda, A. (2016). Invasive predator snake induces oxidative stress response in insular amphibian species. Sci. Total Environ. 566–567, 57–62. doi: 10.1016/j.scitotenv.2016.05.035
Piraccini, R., Cammarano, M., Costa, A., Basile, M., Posillico, M., Boitani, L., et al. (2017). Habitat trees and salamanders: conservation and management implications in temperate forests. For. Ecol. Manag. 384, 17–25. doi: 10.1016/j.foreco.2016.10.048
Pottinger, T. G. (2017). Modulation of the stress response in wild fish is associated with variation in dissolved nitrate and nitrite. Environ. Pollut. 225, 550–558. doi: 10.1016/j.envpol.2017.03.021
Poulsen, R., Cedergreen, N., Hayes, T., and Hansen, M. (2018). Nitrate: an environmental endocrine disruptor? A review of evidence and research needs. Environ. Sci. Technol. 52, 3869–3887. doi: 10.1021/acs.est.7b06419
Proppe, D. S., McMillan, N., Congdon, J. V., and Sturdy, C. B. (2016). Mitigating road impacts on animals through learning principles. Anim. Cogn. 20, 19–31. doi: 10.1007/s10071-016-0989-y
Richards-Zawacki, C. L. (2010). Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc. R. Soc. Lond. B 277, 519–528. doi: 10.1098/rspb.2009.1656
Riddell, E. A., Odom, J. P., Damm, J. D., and Sears, M. W. (2018). Plasticity reveals hidden resistance to extinction under climate change in the global hotspot of salamander diversity. Sci. Adv. 4:eaar5471. doi: 10.1126/sciadv.aar5471
Rohr, J. R., and Palmer, B. D. (2013). Climate change, multiple stressors, and the decline of ectotherms. Conserv. Biol. 27, 741–751. doi: 10.1111/cobi.12086
Rollins-Smith, L. A. (2017). Amphibian immunity-stress, disease, and climate change. Dev. Comp. Immunol. 66, 111–119. doi: 10.1016/j.dci.2016.07.002
Rouse, J. D., Bishop, C. A., and Struger, J. (1999). Nitrogen pollution: an assessment of its threat to amphibian survival. Environ. Health Perspect. 107, 799–803. doi: 10.1289/ehp.99107799
Saaristo, M., Brodin, T., Balshine, S., Bertram, M. G., Brooks, B. W., Ehlman, S. M., et al. (2018). Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. R. Soc. B 285:20181297. doi: 10.1098/rspb.2018.1297
Sapolsky, R. M., Romero, L. M., and Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. doi: 10.1210/er.21.1.55
Schwendiman, A. L., and Propper, C. R. (2012). A common environmental contaminant affects sexual behavior in the clawed frog, Xenopus tropicalis. Physiol. Behav. 106, 520–526. doi: 10.1016/j.physbeh.2012.03.035
Seebacher, F., and Alford, R. A. (2002). Shelter microhabitats determine body temperature and dehydration rates of a terrestrial amphibian (Bufo marinus). J. Herpetol. 36, 69–75. doi: 10.1670/0022-1511(2002)036[0069:SMDBTA]2.0.CO;2
Settle, R. A., Ettling, J. A., Wanner, M. D., Schuette, C. D., Briggler, J. T., and Mathis, A. (2018). Quantitative behavioral analysis of first successful captive breeding of endangered Ozark Hellbenders. Front. Ecol. Evolut. 6:205. doi: 10.3389/fevo.2018.00205
Shaffer, H. B., Gidiş, M., McCartney-Melstad, E., Neal, K. M., Oyamaguchi, H. M., Tellez, M., et al. (2015). Conservation genetics and genomics of amphibians and reptiles. Annu. Rev. Anim. Biosci. 3, 113–138. doi: 10.1146/annurev-animal-022114-110920
Shuman-Goodier, M. E., and Propper, C. R. (2016). A meta-analysis synthesizing the effects of pesticides on swim speed and activity of aquatic vertebrates. Sci. Total Environ. 565, 758–766. doi: 10.1016/j.scitotenv.2016.04.205
Sievers, M., Hale, R., Swearer, S. E., and Parris, K. M. (2018a). Frog occupancy of polluted wetlands in urban land scapes. Conserv. Biol. 33, 389–402. doi: 10.1111/cobi.13210
Sievers, M., Hale, R., Swearer, S. E., and Parris, K. M. (2018c). Contaminant mixtures interact to impair predator-avoidance behaviours and survival in a larval amphibian. Ecotoxicol. Environ. Saf. 161, 482–488. doi: 10.1016/j.ecoenv.2018.06.028
Sievers, M., Parris, K. M., Swearer, S. E., and Hale, R. (2018b). Stormwater wetlands can function as ecological traps for urban frogs. Ecol. Appl. 28, 1106–1115. doi: 10.1002/eap.1714
Smith, V. H., Joye, S. B., and Howarth, R. W. (2006). Eutrophication of freshwater and marine ecosystems. Limnol. Oceanogr. 51, 351–355. doi: 10.4319/lo.2006.51.1_part_2.0351
Sparling, D. W. (2003). “A review of the role of contaminants in amphibian declines,” in Handbook of Ecotoxicology, 2nd Edn. eds D. J. Hoffman, B. A. Rattner, G. A. Burton, Jr., and J. Cairns, Jr. (Boca Raton, FL: Lewis Publishers), 1099–1128.
Strong, R., Martin, F. L., Jones, K. C., Shore, R. F., and Halsall, C. J. (2017). Subtle effects of environmental stress observed in the early life stages of the common frog, Rana temporaria. Sci. Rep. 7:44438. doi: 10.1038/srep44438
Sutherland, W. J. (1998). The importance of behavioural studies in conservation biology. Anim. Behav. 56, 801–809. doi: 10.1006/anbe.1998.0896
Tennessen, J. B., Parks, S. E., Tennessen, T. P., and Langkilde, T. (2016). Raising a racket: invasive species compete acoustically with native treefrogs. Anim. Behav. 114, 53–61. doi: 10.1016/j.anbehav.2016.01.021
Titon, S. C. M., Assis, V. R., Titon Junior, B., Cassettari, B. D. O., Fernandes, P. A. C. M., and Gomes, F. R. (2017). Captivity effects on immune response and steroid plasma levels of a Brazilian toad (Rhinella schneideri). J. Exp. Zool. Part A Ecol. Integr. Physiol. 327, 127–138. doi: 10.1002/jez.2078
Troïanowski, M., Mondy, N., Dumet, A., Arcanjo, C., and Lengagne, T. (2017). Effects of traffic noise on tree frog stress levels, immunity, and color signaling. Conserv. Biol. 31, 1132–1140. doi: 10.1111/cobi.12893
USFWS (U. S. Fish and Wildlife Service) (1989). Endangered and threatened wildlife and plants; determination of threatened status for the Cheat Mountain Salamander and endangered status for the Shenandoah Salamander. Fed. Regist. 54, 34464–34468.
USFWS (U. S. Fish and Wildlife Service) (2013). Endangered and threatened wildlife and plants; determination of endangered species status for the Austin Blind Salamander and threatened species status for the Jollyville Plateau Salamander throughout their ranges. Fed. Regist. 78, 51278–51326.
USFWS (U. S. Fish Wildlife Service) (2019). Shenandoah Salamander recovery plan Implementation Progress. Available online at: https://ecos.fws.gov/ecp0/reports/implementation-activity-status-ore-report?documentId=600307&entityId=200 (accessed March 10, 2019).
van Grunsven, R. H. A., Creemers, R., Joosten, K., Donners, M., and Veenendaal, E. M. (2017). Behaviour of migrating toads under artificial lights differs from other phases of their life cycle. Amphib. Reptil. 38, 49–55. doi: 10.1163/15685381-00003081
Wake, D. B., and Vredenberg, V. T. (2008). Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. U.S.A. 105, 11466–11473. doi: 10.1073/pnas.0801921105
Werner, E. E., and Anholt, B. R. (1996). Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology 77, 157–169. doi: 10.2307/2265664
Wikelski, M., and Cooke, S. J. (2006). Conservation physiology. Trends Ecol. Evol. 21, 38–46. doi: 10.1016/j.tree.2005.10.018
Woltz, H. W., Gibbs, J. P., and Ducey, P. K. (2008). Road crossing structures for amphibians and reptiles: informing design through behavioral analysis. Biol. Conserv. 141, 2745–2750. doi: 10.1016/j.biocon.2008.08.010
Woodhams, D. C., Bosch, J., Briggs, C. J., Cashins, S., Davis, L. R., Lauer, A., et al. (2011). Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front. Zool. 8:8. doi: 10.1186/1742-9994-8-8
Keywords: amphibians, climate change, contaminants, disease, ex situ conservation, habitat change, invasive species, urbanization
Citation: Walls SC and Gabor CR (2019) Integrating Behavior and Physiology Into Strategies for Amphibian Conservation. Front. Ecol. Evol. 7:234. doi: 10.3389/fevo.2019.00234
Received: 24 December 2018; Accepted: 05 June 2019;
Published: 25 June 2019.
Edited by:
Elise Huchard, UMR5554 Institut des Sciences de l'Evolution de Montpellier (ISEM), FranceReviewed by:
Laura Gangoso, Spanish National Research Council (CSIC), SpainCopyright © 2019 Walls and Gabor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susan C. Walls, c3dhbGxzQHVzZ3MuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.