- 1Biology Department, Concordia University, Montreal, QC, Canada
- 2Zoology Museum, Pontificia Universidad Católica del Ecuador, Quito, Ecuador
One common advantage proposed for group-living in animals is social facilitation of feeding, by which food acquisition by an individual is made easier by feeding neighbors. The present paper provides an explanation of social facilitation of feeding in a gregarious leaf-chewing insect, demonstrating how caterpillars cooperate to overwhelm plant trichome defenses and pierce a hole in the leaf on which they feed collectively. Specifically, it tests the hypotheses that Mechanitis menapis caterpillars feed collectively, that the glandular trichomes of Solanum acerifolium are effective defenses against this specialist herbivore, and that feeding by early-instar M. menapis is socially facilitated in the presence of glandular trichomes. A field survey showed that Mechanitis menapis on trichome–bearing plants feed collectively on the abaxial surface of leaves during the first larval instars. In a lab experiment comparing feeding on control and ethanol-washed leaves, caterpillars on the glandular-trichome-free washed sides of leaves initiated feeding sooner and had higher survival rates, suggesting that glandular trichomes are effective defenses. Behavioral observations showed that feeding is socially facilitated in response to glandular trichomes: caterpillars on the washed sides of leaves were more likely to begin feeding alone and initiated more separate feeding sites, suggesting that caterpillars are less able to initiate feeding independently in the presence of glandular trichomes. These results demonstrate a novel mechanism of cooperation among folivores, showing how they can benefit from grouping to tackle food sources that would be unavailable to isolated individuals. This study thus demonstrates that social facilitation of feeding extends to insect folivores, a hyper-diverse and abundant feeding guild.
Introduction
Some resources are better exploited by groups, providing one of the main arguments for gregariousness in animals (Prokopy and Roitberg, 2001) as individuals aggregate to benefit from feeding together. Social facilitation of feeding can be defined in a variety of ways, but the simplest and broadest definition, which makes no assumptions about an animal's motivational state, states that facilitation occurs when food acquisition by an individual is made easier by the feeding action of neighbors (Costa and Pierce, 1997; Weed, 2010; Ward and Webster, 2016). Social facilitation of feeding can be considered a form of cooperation—defined as an interaction in which individuals confer mutual fitness benefits onto each other (Barker et al., 2017). This cooperation can occur via various mechanisms (Ward and Webster, 2016; Barker et al., 2017)—collective feeding either buffers variability in resource acquisition (e.g., foraging seabirds share information about location of fish schools), reduces time and energy required to acquire resources, or makes accessible resources unavailable to solitary individuals (e.g., a group of predators can take down larger prey).
A classic example of social facilitation of feeding involves cooperation between mammalian predators to overwhelm prey defenses (Ward and Webster, 2016), but herbivorous insects could also provide fertile ground for exploring how acquisition of defended resources by an individual is made easier by the feeding action of neighbors. Indeed, the food sources of herbivorous insects are often plentiful but bear mechanical and chemical defenses that constitute a considerable obstacle to feeding (Schoonhoven et al., 2005), and collective feeding could be a means to overcome those defenses.
Group living is widespread among herbivorous insects and takes many diverse forms (Costa and Pierce, 1997; Fitzgerald and Costa, 1999; Costa, 2006). Cooperation via social facilitation of feeding is one of the several advantages put forward for gregariousness, and has been well-documented in a few classic systems: for example, bark beetle mass attacks can exhaust tree defenses and kill them (Raffa et al., 2008), and aphids both create nutrient sinks that redirect plant resources to the colony and collectively secrete enzymes to neutralize plant defenses (Will et al., 2013). Many leaf-chewing larvae also live in groups and have been proposed to benefit from social facilitation of feeding via trail-based recruitment to feeding sites, collective building of feeding shelters and overcoming of plant defenses (Costa and Pierce, 1997). Studies designed to exclude anti-predator or thermoregulatory effects of group-living have documented group-enhanced growth rates that appear linked to collective overwhelming of plant defenses in many herbivorous caterpillars (Tsubaki and Shiotsu, 1982; Cornell et al., 1987; Lawrence, 1990; Stamp and Bowers, 1990; Clark and Faeth, 1997; Denno and Benrey, 1997; Reader and Hochuli, 2003; Inouye and Johnson, 2005; Pescador-Rubio, 2009; Allen, 2010; Fiorentino et al., 2014) as well as sawfly (McMillen and Wagner, 1998) and beetle (Nahrung et al., 2001; Weed, 2010) larvae. However, the plant defenses that leaf-chewing herbivores could overcome by feeding collectively are not understood as clearly as they are for xylophages and phloem-feeders.
One potential mechanism for social facilitation of feeding in folivores involves overcoming trichome defenses by collective production of silk (Rathcke and Poole, 1975; Fordyce and Agrawal, 2001). Trichomes are hair-like appendages extending from aerial surfaces of plants and constitute important defenses that can significantly reduce herbivore survival and damage by physically impeding or even impaling small herbivores, or by exuding toxic or sticky compounds to deter, kill or entrap them (Levin, 1973). Plants in the genus Solanum exhibit several different types of trichomes: non-glandular trichomes are purely mechanical defenses and can take several shapes from simple hairs to stellate rosettes, whereas glandular trichomes contain toxic or sticky secretions (Tian et al., 2012).
Solanum trichomes, especially glandular trichomes (Tian et al., 2012), have been shown to be important defenses against multiple herbivores (Simmonds and Gurr, 2005). For instance, Helicoverpa armigera (Lepidoptera: Noctuidae) neonates (Simmonds et al., 2004) and Phthorimaea operculella (Lepidoptera: Gelechiinae) larvae (Gurr and McGrath, 2001) are entrapped on glandular trichome exudates of pubescent tomato varieties, and washing leaves with ethanol to remove these secretions decreases P. operculella mortality (Gurr and McGrath, 2002). Similarly, both Helicoverpa zea and Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) feed more and perform better on tomato varieties with fewer glandular trichomes (Tian et al., 2012), and early-instar Manduca sexta neonates initiate feeding faster on trichome-free plants (Kariyat et al., 2018).
Several studies suggest mechanisms by which insects counter plant trichome defenses, including specialized morphology, like elongated tarsi to avoid contact with trichome exudates or tarsal hooks to anchor onto trichomes (Boligon and Medeiros, 2007), and behavioral adaptations (Voigt et al., 2007). Pardasena diversipennis (Lepidoptera: Nolidae) feeding on pubescent Solanum coccineum mow the abundant stellate trichomes prior to initiating feeding on the underlying tissue (Hulley, 1988). Similarly, H. armigera remove trichomes on pubescent Solanums, leading to a delay in feeding when compared to individuals on glabrous plants (Shelomi et al., 2010). Mechanitis polymnia isthmia (Lepidoptera: Nymphalidae) feeding on pubescent Solanums use silk mats and trails to move around and feed out of reach of trichomes (Rathcke and Poole, 1975).
Collective feeding could significantly enhance these behavioral trichome countermeasures. For instance, in Battus philenor (Lepidoptera: Papilionidae) larvae feeding on Aristolochia californica plants (Aristolochiaceae), not only did caterpillars show preference for portions of leaves with trichomes removed, individuals feeding on trichome-rich plant parts aggregated more than those on leaves with fewer trichomes (Fordyce and Agrawal, 2001). Detailed studies of M. polymnia isthmia feeding on pubescent Solanum hispidum and S. jamaicense show that early-instar caterpillars collectively build a silk network over the trichomes to move around on the leaf and engage in lengthy probing among trichomes before initiating feeding. Once an individual has begun feeding, others join it and expand the feeding hole, mowing the abundant stellate trichomes and leaving them to accumulate at the edge of the feeding hole (Young and Moffett, 1979b). Individual consumption and growth rate of M. polymnia isthmia are higher in large groups than in small groups (Young and Moffett, 1979a), suggesting social facilitation of feeding.
The present study uses the closely related Mechanitis menapis to examine the role of collective feeding in enabling Mechanitis caterpillars to colonize glandular-trichome-defended Solanums. Specifically, it tests the hypothesis that social facilitation of feeding enables Mechanitis menapis caterpillars to initiate a feeding edge on pubescent Solanum acerifolium. First, a field survey and a lab experiment test for collective feeding was conducted. Next, the lab experiment compares behavior and survival of Mechanitis menapis on control and ethanol-washed leaf surfaces to further investigate whether glandular trichomes impede feeding and whether feeding is socially facilitated in response to glandular trichomes.
Methods
Study Species
Mechanitis menapis mantineus (Nymphalidae: Ithomiini) larvae are gregarious and use silk trails to move around on host plants (Santacruz-Endara, 2012; Despland and Santacruz-Endara, 2016). Known host plants are within the Solanum subgenus Leptostemonum (Robinson et al., 2010), the “spiny Solanums,” characterized by sharp epidermal prickles and stellate trichomes (Levin et al., 2006).
The present study was conducted on Solanum acerifolium (Leptostemonum: Acanthophora), the most common host plant on which M. menapis is found in the study region (personal observation). S. acerifolium is a 1–3 m tall herb and bears prickles on veins of both adaxial and abaxial leaf-surfaces, as well as simple hyaline hairs and shorter simple glandular trichomes on both surfaces, and 4-5 rayed stellate hairs on the abaxial surface (http://eol.org/pages/5695079/details).
Study Design
The study was designed as follows to test hypotheses: first about collective feeding, second about the defensive role of glandular trichomes, and third about social facilitation of feeding in response to glandular trichomes. To test whether caterpillars feed collectively, a field survey recorded aggregation by caterpillars throughout development and a lab experiment tested whether caterpillars move toward each other to feed. The lab experiment tested whether glandular trichomes impede feeding by comparing the time budget, latency to begin feeding (Kariyat et al., 2018) and survival of caterpillars on washed and control leaves. The lab experiment also tests whether feeding in the presence of glandular trichomes is socially facilitated by comparing, between leaf treatments, the proportion of feeding events that occurred alone vs. with a neighbor and by counting the number of feeding holes.
Field Survey
Solanum acerifolium plants (N = 1,200) were surveyed from April to October 2017 to record whether Mechanitis menapis feed collectively. Surveys were conducted in the Mindo valley (00°03′44.1′′S 78°45′41.7′′W), located in cloud forest at 1,250 m a.s.l. on the Western slope of the Andes in the province of Pichincha, Ecuador. Plants were found in shaded to partially shaded ecotone locations including old overgrown fields and roadside edges in secondary forest.
We recorded all developmental stages of Mechanitis menapis observed (eggs, larvae, and pupae), as well as their position on the plant and whether they were aggregated. Insects were considered to be aggregated if they were <5 mm away from their nearest neighbor (Santacruz-Endara, 2012; Despland and Santacruz-Endara, 2016). Each plant was surveyed only once; eggs were collected and hatchlings were used in behavioral observations. Surviving individuals were released after use.
Laboratory Experiment
Mid-level mature leaves (leaf number 3–6 starting from the apical bud) were cut from S. acerifolium plants and placed immediately in water. These leaves were selected because they are the ones on which M menapis eggs and larvae were generally found during the field survey.
Experiments were conducted in a field laboratory under natural conditions, with an LD 12: 12 h photocycle at 25°C (day) and 16°C (night), and 75% relative humidity.
One side (left or right) of each leaf was washed with 70% ethanol by stroking with a paintbrush for 60 s on both surfaces (adaxial and abaxial) then rinsing with abundant water (Gurr and McGrath, 2002). Control sides were brushed with water. Leaves were observed under a stereomicroscope (20x magnification) to compare density of stellate, simple and glandular hairs with exudate-filled heads. The number of each type of hair was counted on a 4 mm2 disc; 3 discs were counted on each leaf used in the experiments and pooled to create an average value per leaf.
Three to six (depending on availability) hatchlings from field-collected eggs were placed 2 cm apart from each other on the adaxial surface of washed and control sides of a leaf 1 h following the washing treatment. Sixteen replicate trials were conducted and a total of 62 control- and 63 washed-treatment insects were used.
Caterpillar behavior was recorded individually using 5-min interval sampling for 2 h 30 min following placement using Noldus Observer XT 12 (i.e., 30 intervals per individual caterpillar; of the expected 3,750 intervals, 3,529 intervals were retained for analysis since some were lost to disturbances). The ethogram contained the following behaviors: resting (the caterpillar is motionless), feeding (the head moves up and down in a characteristic motion and a hole appears in the leaf), grazing trichomes [the head moves up and down and from side to side as the caterpillar bites trichomes to remove them (Shelomi et al., 2010)], spinning (the head moves rhythmically from side to side as the caterpillar lays down a silk mat on the leaf surface), walking (the legs and prolegs move alternately as the body moves forward), or grooming [the head waves around irregularly as the forelegs contact the mouthparts; this behavior is often seen after feeding or trichome grazing and is thought to involve cleaning plant compounds from the mouthparts (Gurr and McGrath, 2001)]. The experiment also recorded the leaf surface on which the caterpillar was located (adaxial, abaxial or edge), aggregation (whether the caterpillar was within 5 mm of nearest neighbor) and how far it had moved since the previous observation.
At the end of the observation, survival was evaluated by leaving caterpillars for 24 h on the leaf on which they had been observed in mesh cages that exclude predators. Short-term hatchling survival in the absence of predation provides an index of success in overcoming plant defenses and initiating feeding (Despland, 2018). The number of holes in the leaf (feeding initiation sites) was also recorded to test whether caterpillars on washed leaves were more likely to have initiated feeding independently rather than use a feeding edge created by a conspecific.
All statistical analyses were done with the R 3.5.3 package. In all cases, data distributions were checked prior to assigning the appropriate error structure. Analyses include GLMs with the error structure indicated, Cox proportional hazard models for survival analysis (survival 2.44-1 package) and binomial tests to compare proportions.
Results
Collective Feeding: Field Survey
The field survey shows that M. menapis eggs are grouped in small clusters on the adaxial surface of the leaf (see Figure 1): 1,077 groups of eggs were observed, ranging in size from 1 to 15 eggs (median = 4). Non-aggregated eggs were usually seen several to a leaf, and it is impossible to distinguish whether such eggs represent intermittent laying by the same female or separate oviposition events from different females. Early instar larvae were always recorded on leaf abaxial surfaces or on stems (never on adaxial surfaces), and were also generally in groups. Presence on leaf undersides, combined with the pale color of the larvae and their tropical habitat, suggests that thermoregulation is not involved in driving aggregation.
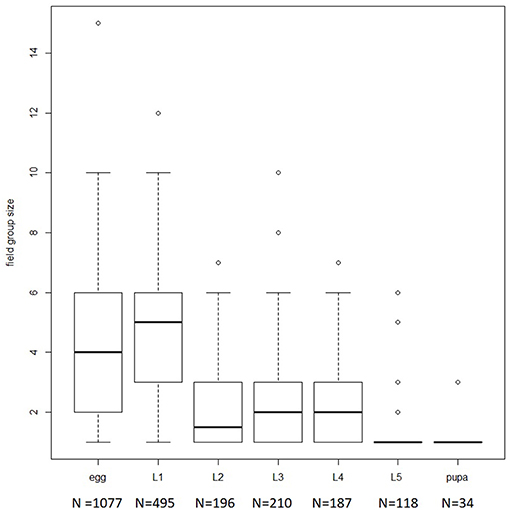
Figure 1. Boxplot representing distribution of group sizes observed in the field by developmental stadium. Number in parentheses below x-axis represents the number of individuals observed per stadium.
First instar larvae appeared to actively aggregate—group size for first instar larvae was larger than for eggs (GLM with Poisson error structure df = 1,570, p = 0.0004). When several larvae were present on the same leaf, they were almost always aggregated. Group size declined during development, such that most pupae were seen alone (see Figure 1).
Effectiveness of Leaf Washing Treatment
The ethanol washing treatment significantly decreased the average density of glandular trichomes on both adaxial and abaxial surfaces (Figure 2; GLM with quasi-Poisson error structure: p = 7.51e-08 with 60 d.f.; effects of leaf surface and interaction not significant). However, there was no significant evidence that washing influenced average density of stellate or simple trichomes (Figure 2; GLM with Poisson error structure for stellate: p = 0.074 and for simple trichomes: p = 0.71 on 60 d.f).
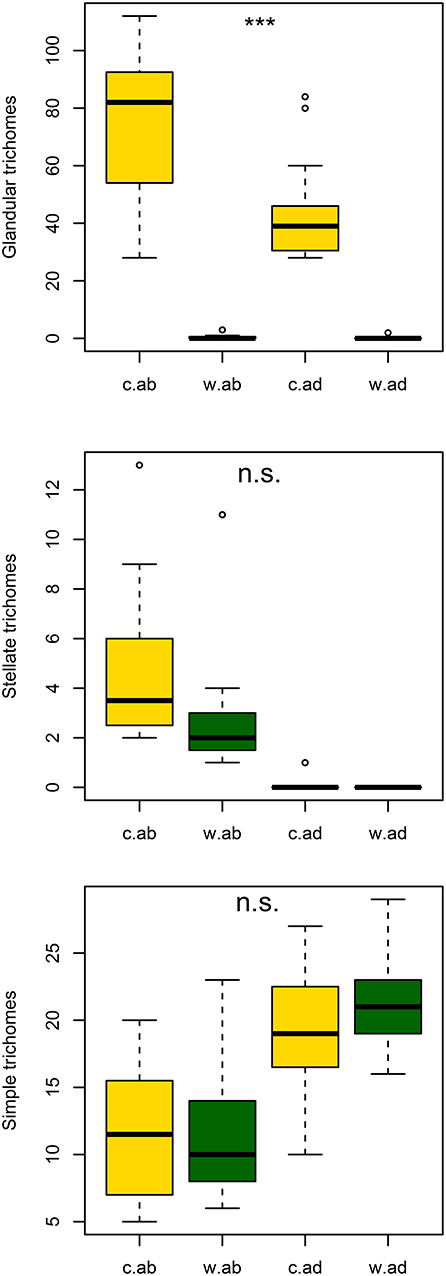
Figure 2. Number of stellate, simple and glandular trichomes with a visible glandular head containing exudate on abaxial and adaxial surfaces of washed and control leaves. ***Implies significance at p < 0.001.
Collective Feeding: Lab Experiment
On both treatments, caterpillars moved to the abaxial surface of the leaf, either by chewing a hole through the lamina, or by walking over the edge (median latency: control, 38 min.; washed, 36 min; Cox proportional hazards analysis p = 0.54 from the survival 2.44–1 package in R). However, movement was generally limited (see Figure 3) for total distances traveled) and no individual crossed over to the other treatment (washed or control) on its leaf.
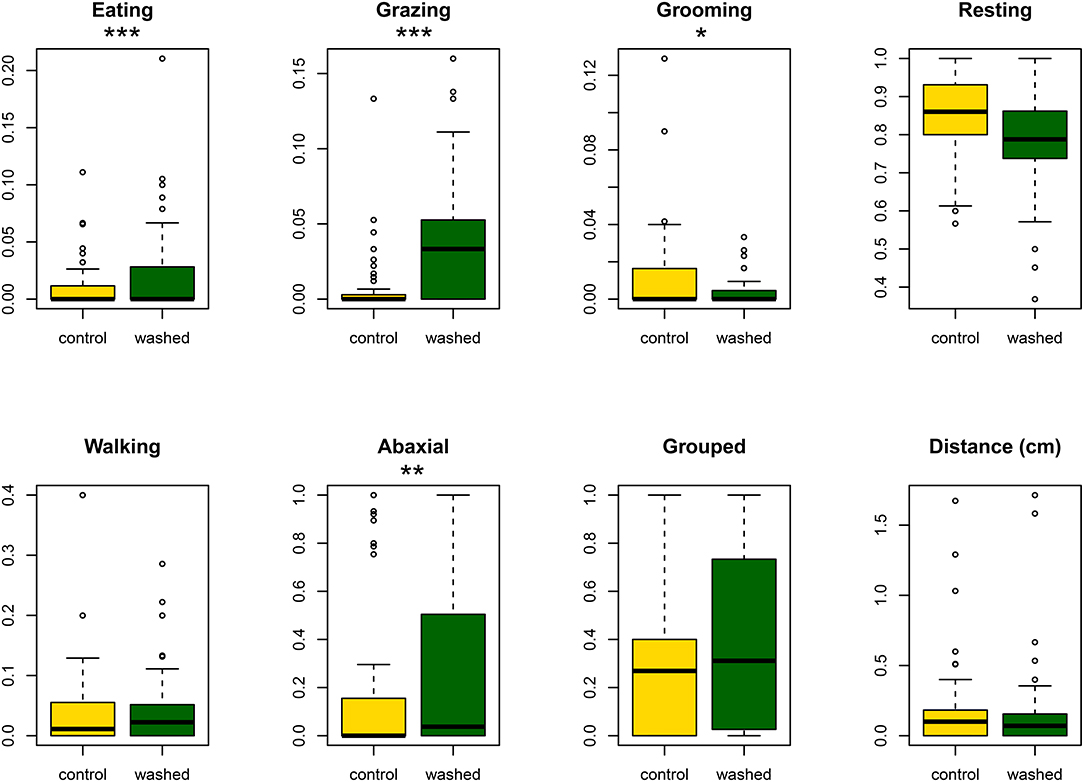
Figure 3. Boxplots showing, for control and washed sides of leaves, the proportion of observations spent eating, trichome grazing, grooming, walking and resting. The proportion of observations recorded on the abaxial surface of the leaf, and the total distance traveled during the assay are also given. Asterisks indicate significance: *p < 0.05; **p < 0.01; ***p < 0.001.
All individuals moved to join at least one neighbor during the assay period and the latency to aggregate did not differ between treatments (median latency: control, 32 min.; washed, 28 min; Cox proportional hazards analysis p = 0.64).
Trichome Effects on Feeding and Survival
Larvae began feeding sooner on the washed than on the control side of leaves: the median latency to feeding was 55 min on the washed side (1 censored individual never fed within the duration of the assay) and 87 min (3 censored individuals) on the control side. Cox proportional hazards survival analysis shows this difference to be significant (coefficient for the washed treatment: 2.32; Wald statistic: 7.37, p = 0.0065). Latency to initiate feeding can reflect food palatability (Clissold, 2008), and hence this result suggests that the washing treatment did not significantly decrease leaf palatability.
Analysis of the time budget of those individuals that did feed during the assay showed that larvae spent more time eating and grazing trichomes on washed leaves and less time grooming, but showed no difference in time spent walking or spinning or in total distance traveled during the assay (see Figure 3).
In total, feeding was observed 97 times on the washed side of leaves, compared with 65 times on controls. The caterpillar groomed its mouthparts after 86% of these feeding events on controls but only after 40% of feeding events on the washed side of leaves (proportions test chi-square = 22.9, p < 0.0001).
Finally, survival over the first 24 h period was higher on washed (70%; N = 63 caterpillars) than on control (40%; N = 62 caterpillars) sides of leaves (χ2 = 10.9, df = 1, p < 0.001). The absent caterpillars were retrieved dead: no individuals had disappeared, confirming that mortality was linked to plant defenses or other mortality agents (e.g., pathogens) associated with the plant surface that had been removed by the washing treatment. All individuals were recovered alive at the end of the behavioral observations.
Trichome Effects on Social Facilitation of Feeding
Initiation of feeding was facilitated by a feeding neighbor, especially on the control side of leaves: of the 65 individual feeding events that were observed on controls, only 5 occurred when the caterpillar was not in an aggregation (7.69 %), compared to 45 of the 97 observations of feeding (46.4%) on the washed sides of leaves (proportions test chi-square = 24.8, p < 0.0001). Aggregated caterpillars always shared a feeding edge as soon as it had been initiated.
Furthermore, observation of leaves after 24 h showed that the number of feeding initiation sites was higher in the washed treatment: 0.81 ± 0.072 S.E. holes per surviving individual on washed, 0.46 ± 0.038 S.E. on control sides of leaves (GLM with binomial error structure p = 0.006 on 15 d.f.).
Discussion
In response to the questions posed in the introduction, both the field survey and lab experiment showed that caterpillars aggregated and fed collectively. In the lab experiment, leaf washing destroyed gladular trichomes and could also have affected other aspects of leaf surface chemistry, any effects of which are likely to be minor relative to those of trichome head removal (Gurr and McGrath, 2001). The lab experiment further showed that glandular trichomes are effective defenses for the plant: they delay caterpillar feeding initiation and decrease survival. The increased time spent grooming on control leaves further suggests that trichomes contain noxious substances that caterpillars on washed leaves do not need to contend with (Gurr and McGrath, 2001). Finally, the experiment demonstrated social facilitation of feeding in response to these trichomes, as solitary feeding was observed more frequently on washed than on control leaves, leading to a greater number of feeding initiation sites on washed leaves. By contrast, caterpillars on control leaves generally fed at a site where another individual had already pierced the cuticle and created a feeding edge (Clissold, 2008). Together, these results suggest that glandular trichomes inhibit a caterpillar's ability to initiate solitary feeding, and that caterpillars' adaptive response to trichomes is to aggregate, using social facilitation to overwhelm these defenses.
In general, the leaf surface influences the ability of insect herbivores to establish a feeding site, especially in the early larval instars when mandibles are small (Despland, 2018) as newly emerged larvae must pierce the leaf cuticle to access the nutritious tissues underneath (Clissold, 2008). Tough leaves, or additional surface features such as waxes or trichomes, can increase the food processing time required by caterpillars to remove or circumvent these obstructions in order to access the nutritious leaf parenchyma (Voigt et al., 2007; Shelomi et al., 2010). Glandular trichome exudates can entrap or poison small larvae before they even initiate feeding (Gurr and McGrath, 2001). Our results show that caterpillars cooperate to collectively overcome the barrier posed by glandular trichomes and open up a feeding edge, which is then exploited by all the individuals. This form of cooperation would fall under the category of “collaborative production of shared group benefits” (Barker et al., 2017).
Two potentially complementary mechanisms exist by which grouping can facilitate feeding initiation on trichome-bearing plants: first, previous work suggests that caterpillars collectively lay down silk to avoid trichome exudates (Rathcke and Poole, 1975), and second, the present study shows that individuals share the feeding edge created by one of their neighbors. M. menapis collectively lay down a network of silk threads to move around suspended above the trichomes (Despland and Santacruz-Endara, 2016), and this behavior was observed on control leaves in our experiment (Supplementary Image 1). However, larvae on washed leaves were observed walking directly on the leaf surface, around the base of non-glandular trichomes (Supplementary Videos 1, 2), suggesting that the silk bridge is used to avoid glandular trichomes and their exudates. As soon as a control-side larva established a feeding edge, it fed on the leaf lamina, cutting trichomes, including glandular trichomes, at the base, presumably avoiding exudates contained in the trichome head. Feeding was often followed by grooming behavior, presumably to remove secretions the larva had been unable to avoid (Gurr and McGrath, 2001). Similarly, cooperation by sharing the task of producing a feeding edge has been proposed to explain social facilitation of feeding in folivores on particularly tough leaves. Experiments suggest that small larvae need to work together to cut tough leaves: young larvae grow better in larger groups but the difference disappears when they are reared on pre-damaged leaves [Pryeria sinica Lepidoptera: Zygaenidae (Tsubaki and Shiotsu, 1982); Chlosyne lacinia Lepidoptera: Nymphalidae (Clark and Faeth, 1997); Chrysophtharta agricola Coleoptera: Chrysomelidae (Nahrung et al., 2001)].
Scaling relationships imply that trichomes are especially dangerous for small larvae (Zalucki et al., 2001), and indeed, in M. menapis as in other species, groups tend to break up as caterpillars grow, such that in the field, late instar larvae are often observed alone (see Figure 1). Laboratory observations confirm that late-instar M. menapis eat glandular and non-glandular trichomes apparently effortlessly (Supplementary Video 3).
The genus Mechanitis is specialized on the trichome-bearing Leptostemonum clade within the mega-genus Solanum, and many species are gregarious (Giraldo and Uribe, 2012). Mechanitis menapis actually exhibits relatively small group-sizes and flexible behavior (Despland and Santacruz-Endara, 2016) compared to some congenerics (Young and Moffett, 1979b; Young, 1983; Brown and Freitas, 1994; Arantes, 2001; Giraldo and Uribe, 2010). Previous work has suggested that other Mechanitis larvae cut trichomes prior to feeding, leading to the accumulation of piles of uneaten trichomes near the feeding hole (Young and Moffett, 1979b), and that they use silk to cover plant trichomes and to facilitate movement and feeding (Rathcke and Poole, 1975), suggesting that adaptations to overcoming trichomes are widespread in this genus that specializes on trichome-defended plants. The phylogeny of Mechanitis is still unresolved and data are insufficient to form hypotheses about the evolution of gregariousness and host plant use in this genus.
The present study provides the first evidence, to my knowledge, demonstrating a mechanism of cooperation between conspecific folivores, and explains the mechanism via overcoming a plant's trichome-based defenses and facilitating entry to the leaf. There are many known examples whereby insect feeding decreases plant quality for subsequent, con- or hetero-specific, herbivores by stimulating induced plant defenses (Schoonhoven et al., 2005; Johnson et al., 2012; Stam et al., 2014). Instances where attack by an herbivore facilitates feeding by others are less well-documented: a review suggests that 20–40% of interactions between different herbivore species on the same plant are facilitative (Stam et al., 2014), and that these primarily occur between species, even between feeding guilds [e.g., chewing and sucking guilds (Stam et al., 2014), above- and belowground feeders (Johnson et al., 2012)]. For instance, leaf-rollers create novel habitats on a plant and thus facilitate colonization by many other herbivores (Vieira and Romero, 2013). The present study shows that feeding activity can facilitate further feeding by conspecifics by overcoming trichome defenses and opening up a feeding edge.
The ways in which foragers interact and shape one another's behavior have been widely studied within the framework of social foraging (Giraldeau and Caraco, 2000), and perhaps the best-known advantage to foraging in a group is access to information (Ward and Webster, 2016). The generation of public goods that are qualitatively different from those a single individual could produce has received less attention. The present study shows that insect folivores can benefit from grouping to tackle food sources that would be unavailable to isolated individuals. This type of cooperation implies direct fitness benefits to group members irrespective of the identity of other group members, and therefore can occur between unrelated individuals (Barker et al., 2017), demonstrating an alternate pathway to group living in insects independent of cooperative breeding (Costa, 2006).
Author Contributions
ED conceived and conducted the study, analyzed the data, and wrote the text.
Funding
This research was funded by a Fonds de recherche du Québec–Nature et Technologies (FRQNT) échanges hors Québec de professeurs award (grant # FRQ-NT 211156).
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Paola Santacruz and Tania Iza for help collecting eggs, and to Hacienda San Vicente for access to their property.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00232/full#supplementary-material
Supplementary Image 1. Second instar M. menapis caterpillar suspended on a silk mat above the surface of a S. acerifolium leaf to avoid glandular trichomes. Photo taken with Aven Mighty Scope 1.3 M Digital Microscope.
Supplementary Video 1. Short video sequence of second instar M. menapis caterpillars walking on the surface of control S. acerifolium leaves. Video shot with Aven Mighty Scope 1.3 M Digital Microscope.
Supplementary Video 2. Short video sequence of second instar M. menapis caterpillars walking on the surface of ethanol-washed S. acerifolium leaves. Video shot with Aven Mighty Scope 1.3 M Digital Microscope.
Supplementary Video 3. Short video sequence showing a fifth instar M. menapis caterpillar feeding on a S. acerifolium leaf and eating trichomes. Video shot with Aven Mighty Scope 1.3 M Digital Microscope.
References
Allen, P. E. (2010). Group size effects on survivorship and adult development in the gregarious larvae of Euselasia chrysippe (Lepidoptera, Riodinidae). Insec. Soc. 57, 199–204. doi: 10.1007/s00040-010-0068-3
Arantes, A. H. (2001). Defensa química em larvas da borboleta Mechanitis polymnia (Nymphalidae: Ithomiinae). Ph.D. thesis, Instituto de Biologia, Universidade Estadual de Campinas, Brazil.
Barker, J. L., Bronstein, J. L., Friesen, M. L., Jones, E. I., Reeve, H. K., Zink, A. G., et al. (2017). Synthesizing perspectives on the evolution of cooperation within and between species. Evolution 71, 814–825. doi: 10.1111/evo.13174
Boligon, D., and Medeiros, L. (2007). Adaptations of two specialist herbivores to movement on the hairy leaf surface of their host, Solanum guaraniticum hassl (Solanaceae). Rev. Brasil. Entomol. 51, 210–216. doi: 10.1590/S0085-56262007000200011
Brown, K. S., and Freitas, A. V. L. (1994). Juvenile stages of Ithomiinae: Overview and systematics (Lepidoptera: Nymphalidae). Trop. Lepidopter. 5, 9–20.
Clark, B. R., and Faeth, S. H. (1997). The consequences of larval aggregation in the butterfly Chlosyne lacinia. Ecol. Entomol. 22, 408–415. doi: 10.1046/j.1365-2311.1997.00091.x
Clissold, F. (2008). “The biomechanics of chewing and plant fracture: mechanisms and implications,” in Advances in Insect Physiology: Insect Mechanics and Control, eds J. Casas and S. J. Simpson (London, Academic Press), 317–372.
Cornell, J. C., Stamp, N. E., and Bowers, D. (1987). Developmental change in aggregation, defense and escape behavior of buckmoth caterpillars, Hemileuca lucina (Saturniidae). Behav. Ecol. Sociobiol. 20, 383–388. doi: 10.1007/BF00302980
Costa, J. T., and Pierce, N. E. (1997). “Social evolution in the Lepidoptera: ecological context and communication in larval societies,” in The Evolution of Social Behaviour in Insects and Arachnids, eds J. C. Choe and B. J. Crespi (Cambridge: Cambridge University Press), 407–442. doi: 10.1017/CBO9780511721953.021
Denno, R. F., and Benrey, B. (1997). Aggregation facilitates larval growth in the neotropical nymphalid butterfly Chlosyne janais. Ecol. Entomol. 22, 133–141. doi: 10.1046/j.1365-2311.1997.t01-1-00063.x
Despland, E. (2018). Effects of phenological synchronization on caterpillar early-instar survival under a changing climate. Can. J. Forest Res. 48, 247–254. doi: 10.1139/cjfr-2016-0537
Despland, E., and Santacruz-Endara, P. (2016). Silk drives aggregation and following in the neotropical Ithomiine caterpillar Mechanitis menapis. Physiol. Entomol. 41, 274–280. doi: 10.1111/phen.12153
Fiorentino, V. L., Murphy, S. M., Stoepler, T. M., and Lill, J. T. (2014). Facilitative effects of group feeding on performance of the saddleback caterpillar (Lepidoptera: Limacodidae). Environ. Entomol. 43, 131–138. doi: 10.1603/EN13144
Fitzgerald, T. D., and Costa, J. T. (1999). “Collective behavior in social caterpillars,” in Information Processing in Social Insects, eds C. Detrain, J. L. Deneubourg, and J. M. Pasteels (Basel: Birkhaüser Verlag, 379–400.
Fordyce, J. A., and Agrawal, A. A. (2001). The role of plant trichomes and caterpillar group size on growth and defence of the pipevine swallowtail Battus philenor. J. Anim. Ecol. 70, 997–1005. doi: 10.1046/j.0021-8790.2001.00568.x
Giraldeau, L. A., and Caraco, T. (2000). Social Foraging Theory. Princeton: Princeton University Press. doi: 10.1515/9780691188348
Giraldo, C. E., and Uribe, S. I. (2010). Registro de Mechanitis polymnia (Lepidoptera: Ithomiinae) en Solanum jamaicense y ciclo de vida en laboratorio. Revis. Colomb. Entomol. 36, 165–168. Available online at: http://www.scielo.org.co/scielo.php?pid=S0120-04882010000100025&script=sci_arttext&tlng=en
Giraldo, C. E., and Uribe, S. I. (2012). Taxonomy of Mechanitis (F.) (Lepidoptera: Nymphalidae) from the west Colombian Andes: an integrative approach. Neotr. Entomol. 41, 472–484. doi: 10.1007/s13744-012-0071-7
Gurr, G. M., and McGrath, D. (2001). Effect of plant variety, plant age and photoperiod on glandular pubescence and host-plant resistance to potato moth (Phthorimaea operculella) in Lycopersicon spp. Ann. Appl. Biol. 138, 221–230. doi: 10.1111/j.1744-7348.2001.tb00106.x
Gurr, G. M., and McGrath, D. (2002). Foliar pubescence and resistance to potato moth, Phthorimaea operculella, in Lycopersicon hirsutum. Entomol. Exp. Appl. 103, 35–41. doi: 10.1046/j.1570-7458.2002.00960.x
Hulley, P. E. (1988). Caterpillar attacks plant mechanical defence by mowing trichomes before feeding. Ecol. Entomol. 13, 239–241. doi: 10.1111/j.1365-2311.1988.tb00351.x
Inouye, B. D., and Johnson, D. M. (2005). Larval aggregation affects feeding rate in Chlosyne poecile (Lepidoptera: Nymphalidae). Florida Entomol. 88, 247–252. doi: 10.1653/0015-4040(2005)088[0247:LAAFRI]2.0.CO;2
Johnson, S. N., Clark, K. E., Hartley, S. E., Jones, T. H., McKenzie, S. W., and Koricheva, J. (2012). Aboveground–belowground herbivore interactions: a meta-analysis. Ecology 93, 2208–2215. doi: 10.1890/11-2272.1
Kariyat, R. R., Hardison, S. B., Ryan, A. B., Stephenson, A. G., De Moraes, C. M., and Mescher, M. C. (2018). Leaf trichomes affect caterpillar feeding in an instar-specific manner. Commun. Integr. Biol. 11, 1–6. doi: 10.1080/19420889.2018.1486653
Lawrence, W. S. (1990). The effects of group size and host species on development and survivorship of a gregarious caterpillar, Halisidota carye (lepidoptera: Arctiidae). Ecol. Entomol. 15, 53–62. doi: 10.1111/j.1365-2311.1990.tb00783.x
Levin, D. A. (1973). The role of trichomes in plant defense. Q. Rev. Biol. 48, 3–15. doi: 10.1086/407484
Levin, R. A., Myers, N. R., and Bohs, L. (2006). Phylogenetic relationships among the “spiny solanums” (Solanum subgenus Leptostemonum, Solanaceae). Am. J. Botany 93, 157–169. doi: 10.3732/ajb.93.1.157
McMillen, J. D., and Wagner, M. R. (1998). Influence of host plant vs. natural enemies on the spatial distribution of a pine sawfly, Neodiprion autumnalis. Ecol. Entomol. 23, 397–383. doi: 10.1046/j.1365-2311.1998.00146.x
Nahrung, H. F., Dunstan, P. K., and Allen, G. R. (2001). Larval gregariousness and neonate establishment of the eucalypt-feeding beetle Chrysophtharta agricola (Coleoptera: Chrysomelidae: Paropsini). Oikos 98, 358–364. doi: 10.1034/j.1600-0706.2001.940217.x
Pescador-Rubio, A. (2009). Growth and survival of a tropical polyphagous caterpillar: effects of host and group size. Southwestern Entomol. 34, 75–84. doi: 10.3958/059.034.0107
Prokopy, R. J., and Roitberg, B. D. (2001). Joining and avoidance behavior in nonsocial insects. Annu. Rev. Entomol. 46, 631–365. doi: 10.1146/annurev.ento.46.1.631
Raffa, K., Aukema, B., Bentz, B., Carroll, A., Hicke, J., Turner, M., et al. (2008). Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58, 501–517. doi: 10.1641/B580607
Rathcke, B. J., and Poole, R. W. (1975). Coevolutionary race continues: butterfly larval adaptation to plant trichomes. Science 187, 175–176. doi: 10.1126/science.187.4172.175
Reader, T., and Hochuli, D. (2003). Understanding gregariousness in a larval Lepidopteran: the roles of host plant, predation, and microclimate. Ecol. Entomol. 28, 729–737. doi: 10.1111/j.1365-2311.2003.00560.x
Robinson, G. S., Ackery, P. R., Kitching, I. J., Beccaloni, G. W., and Hernández, L. M. (2010). HOSTS - A Database of the World's Lepidopteran Hostplants. London: Natural History Museum. Available online at: http://Www.nhm.ac.uk/hosts.~2018
Santacruz-Endara, P. (2012). Historia Natural, Ciclo de Vida y Parasitismo de la Mariposa Mechanitis Menapis Mantineus. Licencia Biologia, Pontificia Universidad Católica del Ecuador.
Schoonhoven, L. M., van Loon, J. J. A., and Dicke, M. (2005). Insect-Plant Biology. Oxford: Oxford University Press.
Shelomi, M., Perkins, L. E., Cribb, B. W., and Zalucki, M. P. (2010). Effects of leaf surfaces on first-instar Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) behaviour. Austr. J. Entomol. 49, 289–295. doi: 10.1111/j.1440-6055.2010.00766.x
Simmonds, A. T., and Gurr, G. M. (2005). Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agr. Forest Entomol. 8, 1–11. doi: 10.1111/j.1461-9563.2006.00271.x
Simmonds, A. T., Gurr, G. M., McGrath, D., Martin, P. M., and Nicol, H. I. (2004). Entrapment of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on glandular trichomes of Lycopersicon species. Austr. J. Entomol. 43, 196–200. doi: 10.1111/j.1440-6055.2004.00414.x
Stam, J. M., Kroes, A., Li, Y., Gols, R., van Loon, J. J., Poelman, E. H., et al. (2014). Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 65, 689–713. doi: 10.1146/annurev-arplant-050213-035937
Stamp, N. E., and Bowers, M. D. (1990). Body temperature, behavior, and growth of early-spring caterpillars (Hemileuca lucina: Saturniidae). J. Lepidopter. Soc. 44, 143–155.
Tian, D., Peiffer, M., Chung, S. H., and Felton, G. W. (2012). Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236, 1053–1066. doi: 10.1007/s00425-012-1651-9
Tsubaki, Y., and Shiotsu, Y. (1982). Group feeding as a strategy for exploiting food resources in the burnet moth Pryeria sinica. Oecologia 55, 12–20. doi: 10.1007/BF00386712
Vieira, C., and Romero, G. Q. (2013). Ecosystem engineers on plants: indirect facilitation of arthropod communities by leaf-rollers at different scales. Ecology 94, 1510–1518. doi: 10.1890/12-1151.1
Voigt, D., Gorb, E., and Gorb, S. (2007). Plant surface–bug interactions: Dicyphus errans stalking along trichomes. Arthropod. Plant Inter. 1:221. doi: 10.1007/s11829-007-9021-4
Ward, A., and Webster, M. (2016). Sociality: The Behaviour of Group-Living Animals. Springer. doi: 10.1007/978-3-319-28585-6
Weed, A. S. (2010). Benefits of larval group feeding by Chrysolina aurichalcea asclepiadis on vincetoxicum: improved host location or feeding facilitation? Entomol. Exp. Et Appl. 137, 220–338. doi: 10.1111/j.1570-7458.2010.01057.x
Will, T., Furch, A. C., and Zimmerman, M. R. (2013). How phloem-feeding insects face the challenge of phloem-located defenses. Front. Plant Sci. 4:336. doi: 10.3389/fpls.2013.00336
Young, A. (1983). On the evolution of egg placement and gregariousness of caterpillars in the Lepidoptera. Acta Biotheor. 32, 43–60. doi: 10.1007/BF00047974
Young, A. M., and Moffett, M. W. (1979a). Behavioral regulatory mechanisms in populations of the butterfly Mechanitis isthmia in Costa Rica: adaptations to host plants in secondary and agricultural habitats (Lepidoptera: Nymphalidae: Ithomiinae). Deutsc. Entomol. Zeitschr. 26, 21–38. doi: 10.1002/mmnd.4800260104
Young, A. M., and Moffett, M. W. (1979b). Studies on the population biology of the tropical butterfly Mechanitis isthmia in Costa Rica. Am. Midl. Natur. 101, 309–319. doi: 10.2307/2424596
Keywords: plant-insect interactions, group-living, lepidoptera, aggregation, social facilitation, cooperation, solanaceae, collective foraging
Citation: Despland E (2019) Caterpillars Cooperate to Overcome Plant Glandular Trichome Defenses. Front. Ecol. Evol. 7:232. doi: 10.3389/fevo.2019.00232
Received: 14 November 2018; Accepted: 04 June 2019;
Published: 02 July 2019.
Edited by:
Jacob D. Davidson, Max Planck Institute of Ornithology, GermanyReviewed by:
Arthur de Fouchier, EA4443 Laboratoire d'Éthologie Expérimentale et Comparée (LEEC), FranceKonrad Fiedler, University of Vienna, Austria
Copyright © 2019 Despland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emma Despland, ZW1tYS5kZXNwbGFuZEBjb25jb3JkaWEuY2E=
 Emma Despland
Emma Despland