- Evolutionary and Organismal Biology Unit, Evolutionary Biology Laboratory, Jawaharlal Nehru Centre for Advanced Scientific Research, Bengaluru, India
Canalization denotes the robustness of a trait against genetic or environmental perturbation. Plasticity, in contrast, indicates the environmental sensitivity of a trait. Stabilizing selection is often thought to increase the canalization of a trait, whereas directional selection is often thought to lead to decanalization. However, the relationship between selection, canalization, and plasticity remains largely unclear. Using experimental evolution, here, we ask whether long-term directional selection for reduced pre-adult development time in Drosophila melanogaster results in the evolution of increased canalization for development time, the trait under primary selection. We additionally investigate whether pre-adult survivorship, a trait only secondarily under selection in this experimental regime, also evolves to become canalized. We examine canalization both in terms of stability of population means and of within population variability across two environmental axes, reflecting macro- and microenvironmental canalization, respectively. We used four large outbred populations of D. melanogaster selected for rapid pre-adult development and early reproduction for 295 generations, and four corresponding ancestral control populations that were not under conscious selection for development time or early reproduction. The selected populations had evolved ~25% reduction in both development time and pre-adult survivorship at the time of this study. We studied development time and pre-adult survivorship of the selected populations and controls across various combinations of rearing temperature and larval density. Development time in the selected populations had become more canalized than controls against both macro- and microenvironmental variation, but only with regard to density, and not temperature. Canalization of development time across density appears to have evolved due to evolutionary changes in the life-history and physiology of the selected populations. Pre-adult survivorship, only a secondary correlate of fitness in the selected populations, did not show any clear trend in terms of canalization with regard to either density or temperature, and overall variation in the trait was greater compared to development time within and across environments. Whether long-term directional selection canalizes or not, therefore, appears to be dependent in a complex way on specific interactions of trait, selection regime, and environmental factor in the context of the ecology and physiology of the populations under study.
Introduction
For organisms developing in the wild, environmental variation at various spatio-temporal scales is ubiquitous; yet, the phenotypic expression of many traits shows a surprising degree of robustness (Félix and Barkoulas, 2015). This is thought to be a result of canalization (Waddington, 1942, 1961), the intrinsic ability of the developmental system to generate a robust phenotype in the face of environmental perturbations or underlying genetic variations (Waddington, 1942, 1961; Dworkin, 2005a). Buffering of the developmental process against environmental perturbation is referred to as environmental canalization (Waddington, 1956) and such perturbations can be macro- or microenvironmental in nature (Falconer and Mackay, 1996; Debat and David, 2001). Macroenvironmental perturbations account for changes in factors like temperature, nutrition, social environment etc. during development. Microenvironmental perturbations, on the other hand, may arise due to small variations in the developmental environment over time and space, and on an even smaller scale because of stochastic fluctuations in the developmental process, also referred to as internal noise or developmental instability (Hall et al., 2007; Morgante et al., 2015). Developmental systems may show micro- or macroenvironmental canalization by maintaining their phenotypic constancy in the face of such perturbations. Genetic variation in development on the other hand, may arise in the form of new mutations, but there could be mechanisms that entrench the developmental process to generate robust phenotypes, masking the underlying genetic variation of a population (Waddington, 1956; Stearns and Kawecki, 1994; Wagner et al., 1997; Rutherford and Lindquist, 1998; Sgrò et al., 2010): this has been termed genetic canalization. By reducing expressed phenotypic variation, genetic canalization may help a population harbor hidden or cryptic genetic variation (Waddington, 1952, 1953; Bateman, 1959; Rendel, 1959; Gibson and Dworkin, 2004). Increase in trait variance of a population in a stressful environment is a common observation. This is thought to be due to breakdown of canalization in extreme environments (Dworkin, 2005a). Failure of the developmental buffering, or decanalization, in extreme environments may reflect breakdown of genetic canalization and/or a lack of environmental canalization in the extreme environment. A trait that has reduced sensitivity to internal or external perturbations during development, and therefore shows a robust phenotypic expression despite perturbations or underlying variation, is said to be canalized.
Canalization or robustness as a phenomenon is intertwined with the related phenomenon of phenotypic plasticity. Phenotypic plasticity reflects the environmental sensitivity of a trait. Traditionally, plasticity referred to the ability of a genotype to give rise to different phenotypes in different environments (Woltereck, 1909). In more contemporary usage, a trait whose phenotypic expression varies in response to environmental variation is called a plastic trait; such traits can range from the simple (e.g., expression level of a protein) to the complex (e.g., a life history trait). Plasticity and canalization of traits have often been thought of as opposite phenomena (Debat and David, 2001), as a trait which is highly canalized is, by definition, less plastic, and vice versa. However, a trait that is plastic with respect to a certain environmental variable could be canalized for another. Moreover, canalization of a trait expressed at one level of biological organization within an organism may require plasticity at an underlying or overlying level of organization (Proulx and Phillips, 2005; Dor and Jablonka, 2010; McDonald et al., 2018). For example, plasticity of a functional trait across environments may entail robustness of a higher-order trait like viability or fitness, leading to the view that both canalization and plasticity are best treated as aspects of the dependence of phenotype on the environment, being two sides of the same coin (de Visser et al., 2003; Flatt, 2005). Studies on phenotypic plasticity have increased in the past couple of decades, and there is greater interest in finding the link between plasticity and evolution (West-Eberhard, 2003; Pigliucci, 2005; Fusco and Minelli, 2010; Moczek et al., 2011; Paaby and Testa, 2018). The relationship between canalization and adaptive evolution, however, has remained largely unclear despite the ability of both processes to shape trait variation and hence influence each other (Fossen et al., 2018; Groth et al., 2018; Jiang et al., 2018; Debat and Le Rouzic, 2019), In this paper we explore the link between adaptive evolution and canalization using a long-running selection experiment involving Drosophila melanogaster.
At a population level, both selection and canalization tend to limit deviations from an optimal phenotype (Eshel and Matessi, 1998; Siegal and Bergman, 2002). Any mechanism that constrains the phenotype to be closer to the optimum is likely to be favored by selection (Rendel, 1967; Stearns and Kawecki, 1994). Therefore, for a trait undergoing selection, the degree of its canalization is predicted to be positively correlated with its impact on fitness (Waddington, 1942; Schmalhausen, 1949). Although theoretical discussions related to this issue are quite old (Waddington, 1942; Schmalhausen, 1949), there is little empirical work done to test these predictions. Although studies of canalization are often focused on either morphological traits or gene expression levels (Dworkin, 2005b,c; Shaw et al., 2014), life history traits are potentially helpful in exploring the evolutionary significance of canalization as they are directly connected to fitness (Prasad and Joshi, 2003; Garland and Rose, 2009). Stearns and Kawecki (1994) and Stearns et al. (1995) studied a suite of life-history traits like development time, body size, lifespan, and early and late fecundity in populations of D. melanogaster and found that the more important a trait is to fitness, the more strongly it is canalized against genetic and environmental perturbations. In our study, we focus on pre-adult development time and pre-adult survivorship in D. melanogaster using the approach of experimental evolution.
Experimental evolution is a powerful approach for addressing questions in evolutionary biology (Garland and Rose, 2009; Kawecki et al., 2012). In this approach, replicate populations are allowed to evolve in the laboratory under specific selection pressures chosen by the experimenter, and the observed changes are studied over a number of generations, and compared to ancestral control populations. It allows the experimenter to observe adaptive evolution in real time, replicate the experimental set up to avoid interpreting stochastic effects as evolutionary outcomes, and draw statistically robust conclusions about evolutionary processes. In view of these, we used a long running experimental evolution study on D. melanogaster to explore the relationship between directional selection and canalization. Our study system involved four large outbred populations of fruitflies subjected to selection for rapid development and early reproduction, relative to ancestral controls, for close to 300 generations in the laboratory [first described in Prasad et al. (2000)], and the four ancestral control populations from which the faster developing populations were selected. The traits that we studied were pre-adult development time and survivorship.
Pre-adult development time had shown a strong response to selection with a 25% reduction in mean development time relative to ancestral controls after 100 generations of selection (Prasad et al., 2001; Prasad and Joshi, 2003). After 245 generations, the magnitude of the selection response remained similar (Ghosh-Modak, 2009). Many other larval and adult traits exhibited correlated responses to selection for rapid development and early reproduction (Joshi et al., 2001; Prasad et al., 2001; Prasad and Joshi, 2003; Shakarad et al., 2005; Ghosh-Modak, 2009; Ghosh-Modak et al., 2009; Ghosh and Joshi, 2012; Dey et al., 2016). In particular, pre-adult survivorship in the faster developing populations had reduced by about 25% compared to controls (Prasad et al., 2000; Ghosh-Modak, 2009).
In view of this, we examined the extent to which direct and correlated responses to directional selection experienced under a stable environment are canalized, by investigating whether the selected populations exhibited canalization for pre-adult development time and/or survivorship across different rearing temperatures and egg densities, compared to their ancestral control populations.
Temperature and pre-adult density are known to affect both development time and survivorship in Drosophila (Prasad and Joshi, 2003). All of the eight (four selected, and four controls) populations used in this study have been maintained at a constant temperature and rearing density throughout the course of selection (Ghosh-Modak, 2009). For this particular study investigating canalization, these populations were reared in nine different environmental conditions, which comprised of three rearing temperatures crossed with three egg densities. One of the treatments corresponded to the standard maintenance conditions of the populations, and the remaining eight were novel environmental conditions to which the flies were not exposed before. We explored canalization and plasticity of the two traits (development time and pre-adult survivorship) in context of the selection pressures experienced by the populations used in the study, and we discuss the possible causes for observed responses of the control and selected lines in specific environmental conditions.
In the faster developing populations, every generation, only the earliest 25% of the eclosing flies were selected and allowed to reproduce, as opposed to the ancestral control populations in which all flies irrespective of their development time were allowed to breed. Development time, therefore, is under strong selection in the faster developing populations in contrast to the controls in which the trait is not under conscious selection. Therefore, we expect development time to be more canalized in the selected populations compared to the controls, and hence show less variation, reflecting microenvironmental canalization. It is important to note, nonetheless, that the nature of selection on development time in our study populations is directional, and not stabilizing. However, in terms of relevance to fitness, the trait is of utmost importance in the faster developing populations unlike that of the controls, and hence we expect the trait to be more canalized in the former than the latter.
We also investigated the plasticity of development time in both sets of populations across different novel environments including conditions that could be moderately stressful (e.g., high rearing densities). The within environment variation for the trait in all assay environments was measured as well. Lower plasticity of development time across environments would indicate greater macroenvironmental canalization in any given population (Nijhout and Davidowitz, 2003; Zelditch et al., 2004; Dworkin, 2005a; Flatt, 2005; Careau et al., 2014), and lower variation within any assay environment would indicate microenvironmental canalization. For development time, if (i) macroenvironmental canalization (low plasticity across environments), and (ii) microenvironmental canalization within different environments, are controlled by shared genetic mechanisms, we expect the faster developing populations to show greater canalization of development time compared to controls for both (i) and (ii).
The other trait we investigated, i.e., pre-adult survivorship, had shown a strong correlated reduction in the faster developing populations compared to the controls in course of selection (Prasad et al., 2000; Ghosh-Modak, 2009). Therefore, pre-adult survivorship for both sets of populations was also tested in all the assay environments. Unlike development time, pre-adult survivorship is relevant to fitness in both sets of our study populations because unless the flies survive to adulthood, they cannot contribute to the gene pool of the next generation, which is true for both the faster developing populations and the controls. However, in the selected populations, development time is the primary trait under selection, as evidenced by a correlated decrease in pre-adult survivorship in these populations as they evolved to become faster developing than controls (Prasad et al., 2000). Thus, we had no a priori expectations for survivorship to be either more canalized or more plastic in the faster developing populations vs. the controls. Nevertheless, we consciously chose the two traits (development time and pre-adult survivorship) to explore canalization in our study system because of the contrast the two traits have with regard to their relationship with fitness in our study populations.
Materials and Methods
Experimental Populations
We used eight laboratory populations of D. melanogaster: four replicate populations selected for rapid development and early reproduction, [FEJ 1−4: Faster development, Early reproduction, derived from JB, first described by Prasad et al. (2000)], and their four matched ancestral control populations [JB 1−4: Joshi Baseline, first described by Sheeba et al. (1998)]. The four JB populations were descendants of a single wild-caught population of D. melanogaster—the IV population described by Ives (1970). They were first maintained in the laboratory for about 110 generations on a 14 day discrete generation cycle. Five populations (B1−5) were then derived from IV populations and reared in the laboratory under similar conditions (Rose and Charlesworth, 1981). After about 360 generations, a set of five populations were derived from B1−5 and christened UU1−5 [Uncrowded as larvae, Uncrowded as adults; described by Joshi and Mueller (1996)]. The UU populations were maintained under similar conditions, but on a 21 day discrete generation cycle. After 170 generations of being maintained as UUs, JB1−4 populations were derived from UU populations (UU 1, 2, 3, 5, respectively) (Sheeba et al., 1998). FEJ1−4 populations were later derived from JB1−4 and subjected to selection for rapid development and early reproduction, relative to their ancestral controls (Prasad et al., 2000).
All populations were maintained on a discrete generation cycle at ~25°C, ~90% relative humidity and constant light, on banana-jaggery food. In both JB and FEJ populations, larvae were reared in 8 dram glass vials (9.5 cm height, 2.4 cm inner diameter) with ~6 mL food at a density of 60–80 larvae per vial, whereas eclosed adults were collected into Plexiglas cages (25 × 20 × 15 cm3) with abundant food, at breeding population sizes of about 1,500 flies. The JBs were maintained on a 3-week discrete generation cycle, and all eclosing adults were part of the breeding population. In JB populations, eggs were collected and dispensed in vials on the 21st day after the egg collection for the previous generation, corresponding to day 11 of adult life. FEJs were maintained under conditions similar to the JBs, except that 120 vials, rather than 40 vials, containing ~60–80 eggs were collected per population, and the vials were monitored for eclosion every 2 h after pupae darkened. As soon as the first 20–25% flies in each vial (12–15 flies) had enclosed, they were transferred into fresh cages containing food plates. These constituted the breeding adults. After 3 days of adult life, eggs were collected from FEJ cages to start the next generation. Thus, the FEJs were under strong primary selection to complete egg-to-adult development fast, and under secondary selection to be relatively fecund on day 3 of adult life.
As each FEJ population was derived from one JB population, selected and control populations bearing identical numerical subscripts were more related to each other than to other populations in the same selection regime. Consequently, control and selected populations with identical subscripts were treated as constituting random blocks in the statistical analyses. At the time of this study, the FEJs had undergone 295 generations of selection, and showed considerable evolutionary reductions in development time (~25%), dry weight (~50%), survivorship (~25%), and general level of activity (Prasad and Joshi, 2003; Ghosh-Modak, 2009; Ghosh and Joshi, 2012).
Collection of Flies for Assays
Prior to assays, all eight populations were reared under a common (control JB type) regime for one complete generation in order to ameliorate non-genetic parental effects. The eggs of these flies, hereafter referred to as standardized flies, were then used for the various assays.
Pre-adult Development Time Assay
A fresh food plate was introduced into the cages and the standardized flies were allowed to lay eggs for 1 h. This plate was then replaced by a second food plate and an egg collection window of 1 h was provided. Eggs used for the assay were collected from the second round of egg lay and the first sets of plates were discarded. This was done to ensure that the eggs used for the assay were developmentally synchronized. Eggs were collected with a moistened paint-brush from the food-surface, counted under the microscope, and exact numbers of eggs for the assay were dispensed into vials with 6 mL of food at a density of 30, 70, or 300 eggs per vial and incubated at three different temperatures namely 18, 25, and 28°C. 25°C and 70 eggs per 6 mL of food represent the normal maintenance conditions for FEJ and JB populations. The egg density of 30 eggs per vial was considered in order to study development at density substantially lower than what the populations normally experienced. Three hundred eggs in 6 mL, on the other hand, represents substantial larval crowding for these Drosophila populations, and the choice of a density that could be somewhat stressful was deliberate as canalization of development (time) or the lack thereof could be explored.
Eight vials were set up for each combination of temperature, density, selection regime and replicate block (total 72 treatments). In all, 576 vials (2 selection regimes × 4 replicate blocks × 3 temperatures × 3 densities × 8 vials) were thus set up for the experiment. The vials were monitored for the first eclosion and, thereafter, checked regularly at 4 h intervals and the number of eclosing flies was recorded. The observations were continued till no new fly eclosed for two consecutive days. The development time in hours was measured by subtracting the time of egg-lay from the time of eclosion. In 4 out of the 72 treatments (2 selection regimes × 4 replicate populations × 3 temperatures × 3 densities), one vial was not viable, possibly due to handling error. For these sets, only data for 7 vials were available. To maintain a balanced design for the statistical analyses, for all 72 treatments the data from one vial, chosen at random, were excluded from the analyses. Hence, data from a total of 504 vials were considered for the final analyses.
Pre-adult Survivorship Assay
From the vials used for the development time assay, the total number of eclosed flies were counted, and the number was divided by the number of eggs (30, 70, 300) for the respective vial to obtain the pre-adult survivorship for any given vial. Similar to development time, survivorship data from a total of 504 vials were considered for analyses.
Outlier Removal
Being large and outbred populations, there is considerable variation for development time, especially within the JB, and once eclosion starts for a given population, it roughly continues for 24–48 h. A temperature of 25°C and density of ~70 eggs per vial represent the standard developmental condition of the JB and FEJ populations. Under such conditions, FEJ flies take 6–7 days to complete their development from egg to eclosion, whereas JB flies take 9–10 days to complete pre-adult development. For the development time data analysis, flies eclosing after 100 h (over 4 days) from the first eclosion for any given set (combination of particular selection regime, temperature, density and replicate population) were not included in the development time data analysis for 30 and 70 egg densities. For 300 egg density, flies eclosing after 200 h (over 8 days) from the first eclosion for any given set were not included in the development time analysis. The criteria for defining outliers, typically few in number and representing potentially pathological individual variants, was decided upon before the experiment, based on past experience of development time distributions in these populations. Ultimately, outlier removal had to be done for only 6 of the 72 treatment combinations and essentially implied the exclusion of one or two extremely late developing flies as outliers in any given vial. For survivorship data, all eclosed flies, including the outliers defined as above, were considered for the analysis as inclusion of these flies did not greatly increase the mean or variance for survivorship.
Statistical Analyses
For both pre-adult development time and survivorship, we examined the mean trait value, and variation in trait values, in each of the eight populations in all nine combinations of rearing density and temperature. We quantified plasticity or macroenvironmental sensitivity of a trait as the relative change in its value from one assay environment to another. For a given selection regime, less plasticity of mean trait value across assay environments would indicate greater macroenvironmental canalization for a trait.
For comparing trait variation across selection regimes and environments, we used the coefficient of variation instead of variance. The coefficient of variation or CV is a standardized, dimensionless quantity measured by dividing the sample standard deviation by the sample mean (often expressed as a percentage and hence, multiplied by 100). Coefficient of variation is used specifically when comparing the variation of two populations independent of the magnitude of their means, or alternatively, while comparing the variations in two traits irrespective of their means (Sokal and Rohlf, 1998). Prior data suggest both development time and survivorship of the faster developing populations used in our study are ~25% less than that of the ancestral controls, and development time is also less variable in selected populations, compared to controls (Prasad et al., 2000, 2001; Ghosh-Modak, 2009). Hence, we used CV as a standardized measure of varaibility for both development time and survivorship in all eight study populations.
Nested mixed-model analyses of variance (ANOVA) were performed on all data, using a completely randomized block design in which selection regime, temperature and density were treated as fixed factors, crossed among themselves, and with random blocks, representing both ancestral lineage and coincident handling of one matched pair of replicate selected and control populations during assays. Vial was treated as a random factor nested within the combinations of block and all three fixed factors. For analyzing across-environment variation in mean trait values, we used vial mean values for development time and vial values for pre-adult survivorship as the input data for the analysis. For comparing development time variation across individuals, the values of CV across individuals within each vial were used as input data for ANOVA. In addition, the across vial variability in mean development time for all combinations of block, selection regime, temperature, and density was also subjected to ANOVA. For survivorship, only vial means could be scored given the nature of the trait, and CV for each vial could not be calculated. Hence, only the across vial variability was used as a measure of survivorship variability for any population and treatment. All analyses were implemented using the software JMP (SAS institute) version 14. For post-hoc comparisons, Tukey's HSD test was performed using JMP. All significant results in the post-hoc comparisons indicate a p-level < 0.05. Since the basic consequential units of analysis in this design are mean values of the replicate populations (e.g., MS selection regime will be tested over MS block × selection regime interaction, etc.), normality can be safely assumed, even though traits like development time are not distributed normally across individuals.
Pre-adult Development Time Data Analysis
Mean Pre-adult Development Time (Trait Mean)
Mean development time was calculated for each vial. A four way mixed model ANOVA was performed on vial means as within-cell replicate values, with selection, temperature, and density being treated as fixed factors, and block as a random factor, crossed with all the other factors. An effect of treatment combination (temperature and density) would then be taken as reflecting macroenvironmental sensitivity of the trait.
Trait Variation Across Individuals (Within Vials)
Trait variation observed in different combinations of temperature and density in the eight populations was also subjected to four-way mixed model ANOVA. Variation among individuals within a vial can reflect (i) genetic variation among individuals, (ii) developmental instability, which is hard to distinguish from (iii) variation due to possible ultra-microenvironmental heterogeneity within a vial, in addition to (iv) stochastic variation in trait measurement. Partitioning the within vial variation in phenotype among individuals into components caused by these four factors cannot be accomplished. However, in the context of our experimental design, if different combinations of the macroenvironmental factors, temperature, and density, affect the extent of within vial variation in phenotype among individuals, it can be cautiously interpreted as a change in developmental instability and/or ultra-microenvironmental sensitivity, given that the macroenvironmental change is unlikely to affect genetic variation or stochastic error. Incidentally, the one time the FEJ and JB populations were examined for developmental instability for any trait, no significant differences were observed (Shakarad et al., 2001). In that study, the trait examined was fluctuating asymmetry of sternopleural bristle number. For the current study, two different measures of trait variation within a population in any specific treatment combination were used. The first one was the standard deviation of development time across individuals, within a vial. The second was CV or coefficient of variation for the trait within each vial. Standard deviations of individual development time were calculated for each vial and divided by the respective vial means for development time to obtain replicate measures of CV, expressed as a percentage, for the trait.
Trait Variation Across Vials
Across vial variation for development time reflects the effects of sampling, since there is within vial variation among individuals, overlaid by effects of microenvironmental heterogeneity, if any. There is evidence for such among vial microenvironmentally induced variation in life-history traits in Drosophila populations such as these in our laboratory (Dey et al., 2006). Again, while it would not be possible to tease apart these two effects within a population, the effects of combinations of the macroenvironmental factors, temperature, and density, on among vial variation can be cautiously assumed to be operating primarily through an effect on the microenvironmental sensitivity, since macroenvironment is unlikely to affect sampling variation. Again, two measures of among vial variation were used, and tested via four-way mixed-model ANOVA: standard deviation of vial means for a particular treatment (selection regimes × replicate populations × temperature × density) and CV (standard deviation of vial means divided by grand mean for the treatment), multiplied by 100.
Pre-adult Survivorship Data Analysis
Data on pre-adult survivorship were treated exactly as described above for development time, except that mean pre-adult survivorship for each population was calculated by averaging the per vial pre-adult survivorship, followed by an arcsin square-root transformation. Since each vial yielded only one pre-adult survivorship value, only among vial variation was examined. The inference of macroenvironmental and microenvironmental sensitivity changes in populations subjected to different combinations of temperature and density was exactly the same as for development time.
Results
Pre-adult Development Time
Mean Pre-adult Development Time Across Macroenvironments
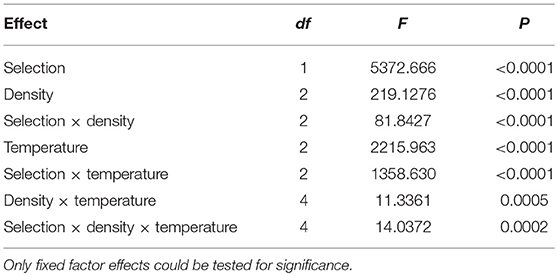
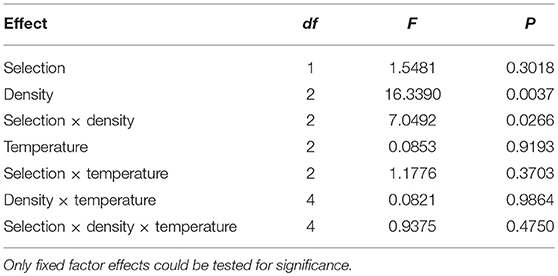
Mean development time of FEJ is significantly less (~75%) than that of JB [F(1, 3) = 5372.666; p < 0.0001] across all combinations of temperature and density (Figure 1; Table 1). Interaction between selection regime and density is significant [F(2, 6) = 81.8427; p < 0.0001] (Table 1). At 30 and 70 eggs per vial, the FEJ flies develop 25% faster than that of JB, across rearing temperature (Figure 1). At a higher rearing density of 300 eggs per vial though, FEJ populations develop 30% faster than JB, due to JB development getting slower at 300. The post-hoc comparisons show that for any given temperature, mean development time in FEJ does not different significantly across density. In contrast, mean development time in JB increases by ~10% from 70 to 300 egg density, averaged across temperatures, and this change is significant (p < 0.05). Mean development time in JB is not significantly different between 30 and 70 egg density. Hence, a culture density of 300 eggs per vial delays JB development significantly but not FEJ. Thus, from a density of 70 to 30 eggs per vial, the selection response is canalized, but not when density increases from 70 to 300 eggs per vial.
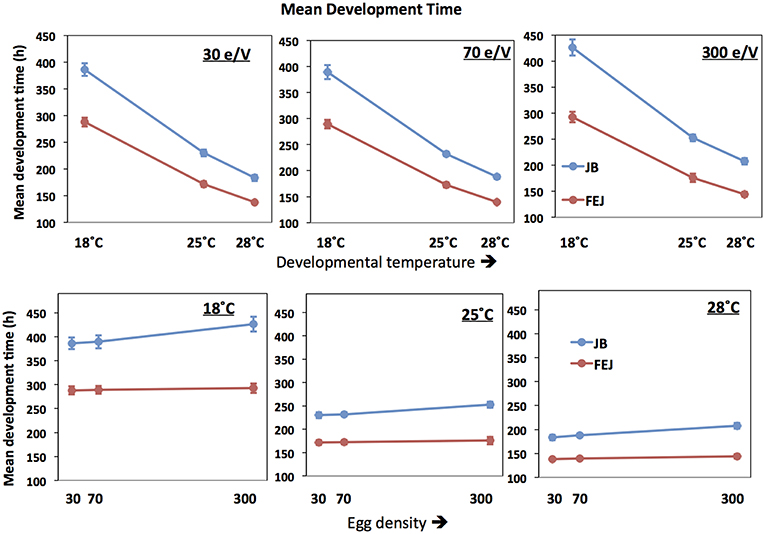
Figure 1. Mean pre-adult development time of FEJ and JB populations. The upper panel shows mean trait value plotted across temperature for each treatment density (e/V = eggs per vial). The lower panel shows mean trait value plotted across egg density for each treatment temperature. The error bars show 95% confidence intervals calculated from the variation among replicate populations in each combination of selection regime, temperature, and density.
In both FEJ and JB, mean development time changes significantly across temperature for any given density but both JB and FEJ show similar changes across temperature. Mean development time in JB and FEJ increases by 68 and 67%, respectively, from 25 to 18°C, averaged across all densities (Figure 1). Mean development time in both JB and FEJ reduces by 19% from 25 to 28°C, averaged across all densities (Figure 1). Thus, the thermal plasticity of mean development time is consistent across densities and selection regimes, resulting in the selection response being canalized across the tested temperature range, from 18 to 28°C. However, there is significant interaction between selection and temperature [F(2, 6) = 1358.630; p < 0.0001], as JB development time shows greater absolute change across temperature (Figure 1; Table 1).
To summarize, overall, mean development time is more plastic along the temperature axis than the density axis (Figure 1). FEJ development is faster than that of the JB, and relatively more canalized (macroenvironmentally) than that of JB across rearing densities. Mean development time, although being highly plastic across temperature, responds similarly to changes in temperature in both the FEJ and JB populations. Thus, though development time as a trait is more plastic with respect to temperature, as compared to density, the extent of plasticity has remained unchanged during the course of evolution in the FEJs, resulting in the greater canalization of the selection response.
Across Vial (Microenvironmental) Variation for Pre-adult Development Time
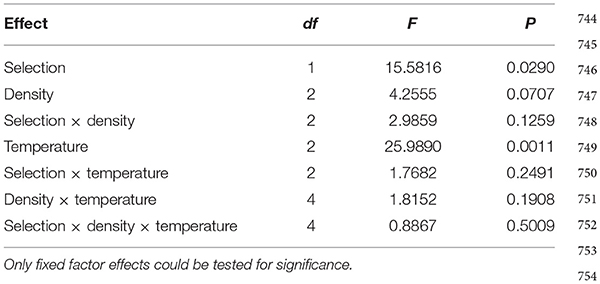
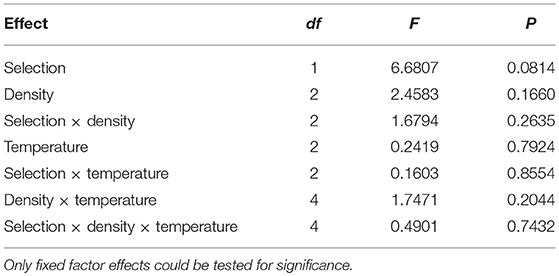
The standard deviation of (vial mean) development time across vials is significantly lower in FEJ than in JB [F(1, 3) = 15.5816; p = 0.029], and is also significantly affected by temperature, being higher at 18°C compared to the higher temperatures in both the JB and FEJ populations [F(2, 6) = 25.98; p = 0.001] (Table 2). However, both these main effects in the ANOVA appear to be due to the standard deviation scaling with the mean. When we examined the CV of development time across vials, there was no significant effect of any factor or interaction in the ANOVA (Table 3), and in both FEJ and JB populations, the CV of development time across vials was about 1, regardless of temperature or rearing density (Figure 2). Thus, the sensitivity of development time to microenvironmental variation does not seem to be affected either by the two macroenvironmental variables (temperature and density), or by selection history.
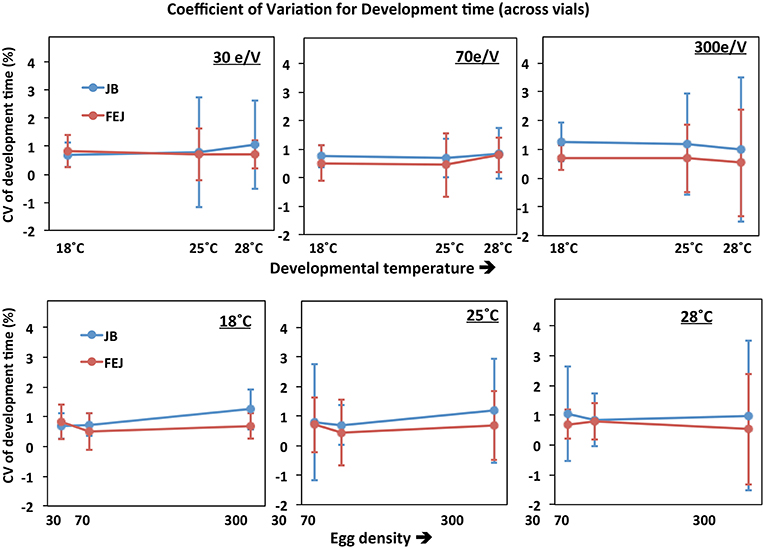
Figure 2. Coefficient of variation (CV) of mean pre-adult development time of FEJ and JB populations across vials. The upper panel shows CV of the trait plotted across temperature for each treatment density (e/V = eggs per vial). The lower panel shows CV of the trait plotted across egg density for each treatment temperature. The error bars show 95% confidence intervals calculated from the variation among replicate populations in each combination of selection regime, temperature, and density.
Across Individual Variation (Within Vial CV, Microenvironmental) for Pre-adult Development Time
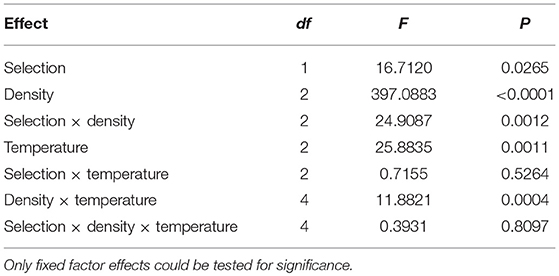
Overall, the among individual within vial CV in development is significantly less in FEJ compared to JB [F(1, 3) = 16.7120; p = 0.0265] (Table 4). The ANOVA also revelaed significant main effects of density [F(2, 6) = 1397.088; p < 0.0001] and temperature [F(2, 6) = 25.883; p = 0.0011], with CV tending to increase with increases in both temperature and density, though the temperature effect was less consistent than that of density and seen prominently only at the highest rearing density of 300 eggs per vial (Figure 3). These patterns are reflected in significant interactions between selection and density [F(2, 6) = 24.9087; p = 0.0012], and density and temperature [F(4, 12) = 11.882; p = 0.0004]. Among individual CV for development time at all three temperatures is significantly lower in FEJ compared to JB only when reared at a density of 300 eggs per vial (p < 0.05), but not at 30 or 70 eggs per vial (Figure 3). CV of JB development time increases by a staggering 138%, on average, from 70 to 300 egg density, and the changes are similar at all temperatures. CV of FEJ development time, on the other hand, increases by only by 22% from 70 to 300 egg density at 18°C, which is not significant. At 25 and 28°C, changes in the CV of FEJ development time are 98 and 78%, respectively, when the density changes from 70 to 300, which are significant (Figure 3). Hence, the CV in FEJ development time is similar to that in JB at lower developmental densities, but at 300 eggs per vial, the CV in FEJ development time is significantly less than that in JBs as the FEJs undergo a much smaller increase in CV between 70 and 30 eggs per vial (Figure 3). CV for development time in both FEJ and JB is significantly greater at 28 than 18°C only when reared at 300 egg density (p < 0.05). Overall, both FEJ and JB populations are similarly affected by temperature (no significant selection by temperature interaction, Table 4), and the temperature effect is greatly enhanced at the highest density of 300 eggs per vial (Figure 3; significant density by temperature interaction, Table 4).
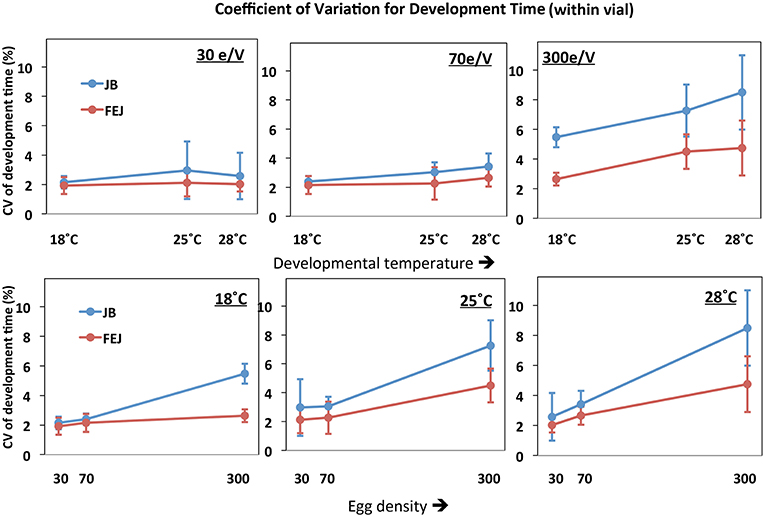
Figure 3. Coefficient of variation (CV) of pre-adult development time of FEJ and JB populations across individuals within a vial. The upper panel shows CV of the trait plotted across temperature for each treatment density (e/V = eggs per vial). The lower panel shows CV of the trait plotted across egg density for each treatment temperature. The error bars show 95% confidence intervals calculated from the variation among replicate populations in each combination of selection regime, temperature, and density.
Pre-adult Survivorship
Mean Pre-adult Survivorship
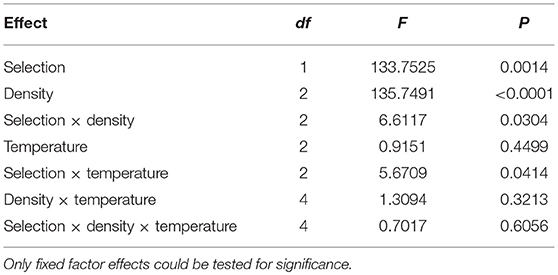
The pattern of macroenvironmental effects on mean pre-adult survivorship in the FEJ and JB is not as consistent as in the case of development time. Overall, mean survivorship in FEJ was less than in JB [F(1, 3) = 133.752; p = 0.0014], as also noted in earlier studies (Prasad et al., 2000; Ghosh and Joshi, 2012). There was also a significant main effect of density [F(2, 6) = 135.749; p < 0.0001], with survivorship dropping markedly at 300 eggs per vial for both selection regimes, compared to that at 30 or 70 eggs per vial (Figure 4). Moreover, selection regime also interacted significantly with both temperature [F(2, 6) = 5.6709; p = 0.0414] and density [F(2, 6) = 6.6117; p = 0.0304], with mean survivorship in FEJ being significantly less than that of JB at 18 and 28°C, but not at 25°C, although the difference is substantial even at 25°C, except at a density of 300 eggs per vial (Figure 4).
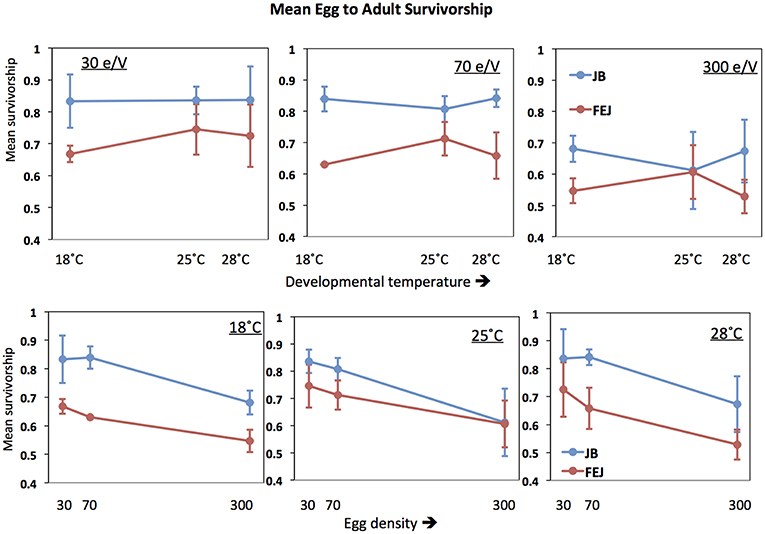
Figure 4. Mean pre-adult survivorship of FEJ and JB populations. The upper panel shows mean trait value plotted across temperature for each treatment density (e/V = eggs per vial). The lower panel shows mean trait value plotted across egg density for each treatment temperature. The error bars show 95% confidence intervals calculated from the variation among replicate populations in each combination of selection regime, temperature, and density.
Mean survivorship in both JB and FEJ is significantly reduced at a density of 300 eggs per vial compared to either of the two lower densities (Figure 4). However, at 25 and 18°C, FEJ undergo a smaller reduction in mean survivorship (14, 13%) compared to JB (24, 19%) when density is increased from 70 to 300 eggs per vial. At 28°C, the reduction in survivorship from 70 to 300 density is similar (20%) in FEJ and JB. Overall, mean survivorship seems to be more affected by changes in density than temperature.
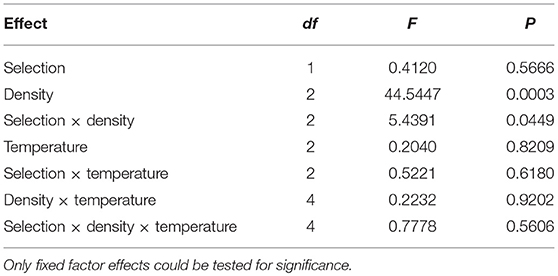
Across Vial Variation (Standard Deviation and CV) for Pre-adult Survivorship
CV of survivorship across vials is consistently and substantially higher than that of development time for both JB and FEJ populations (Figure 5 vs. Figure 2; Figure 6), suggesting that survivorship is more sensitive than development time to microenvironmental variation across vials. For both standard deviation and CV, the only significant ANOVA effects were those of density and the selection by density interaction (Tables 6, 7). Averaged across selection regimes, the CV of survivorship tended to decrease with increasing density, though the bigger drop was from 30 to 70 eggs per vial (Figure 5). However, in the FEJ, CV of survivorship tended to drop substantially from 30 to 70 to 300 eggs per vial, whereas in the JB, CV of survivorship was the least at 70 eggs per vial (Figure 5).
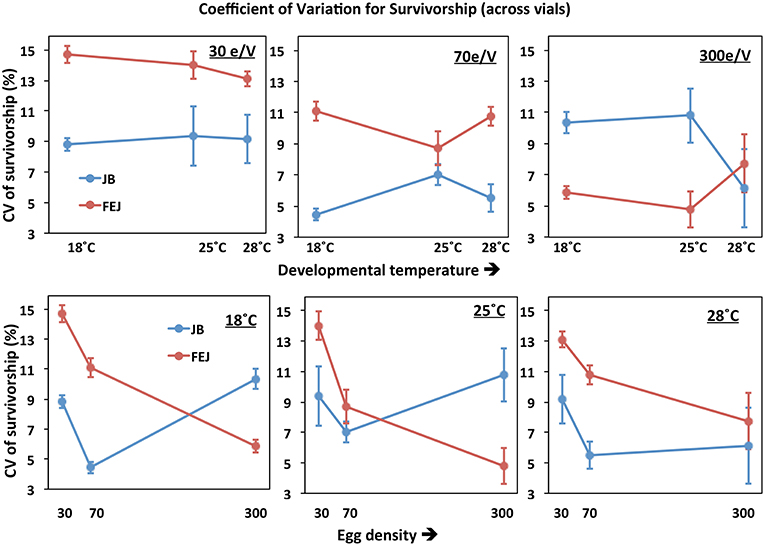
Figure 5. Coefficient of variation (CV) of pre-adult survivorship of FEJ and JB populations across vials. The upper panel shows CV of the trait plotted across temperature for each treatment density (e/V = eggs per vial). The lower panel shows CV of the trait plotted across egg density for each treatment temperature. The error bars show 95% confidence intervals calculated from the variation among replicate populations in each combination of selection regime, temperature, and density.
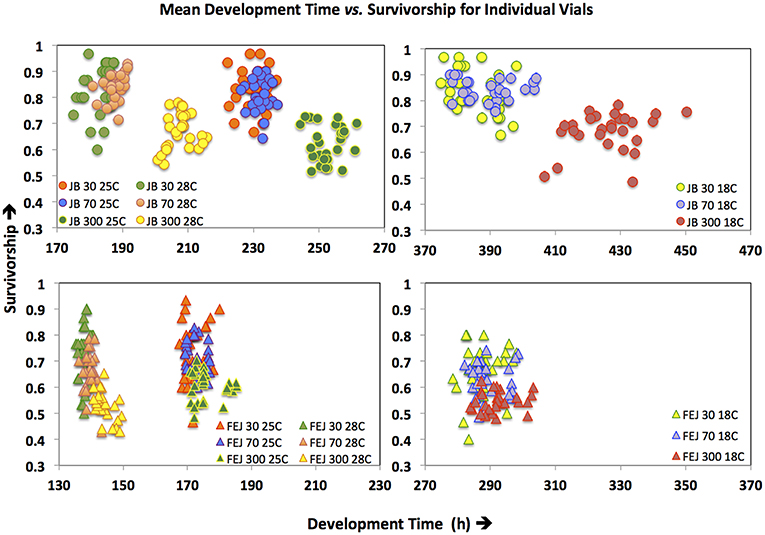
Figure 6. Plot of vial values of survivorship (y-axis) vs. vial mean values of development time (x-axis) for FEJ and JB populations across all treatments. Each circle, or triangle, represents value or mean obtained from one vial.
Thus, CV of pre-adult survivorship across vials is affected more strongly by changes in density than temperature (Figure 6), with different patterns in FEJ and JB. Unlike in the JB, CV of survivorship in FEJ is much higher at the lowest density of 30 eggs per vial (Figures 5, 6). Pre-adult survivorship is known to be reduced at very low densities in Drosophila due to the food medium not getting softened by the feeding activity of enough larvae, and also reduced suppression of fungal growth by larvae (Sang, 1956; Ashburner, 1989). It is possible that pre-adult survivorship in the FEJ is more sensitive to across vial microenvironment variation because the FEJ larvae, being much slower feeders than JB larvae (Prasad et al., 2001), exhibit an Allee effect even at a density of 30 eggs per vial, whereas that density is sufficiently high for JB larvae not to be exhibiting an Allee effect.
Discussion
The structure of our experiment permits us to make several pair-wise comparisons of the effects of long-term directional selection on the sensitivity of fitness-related traits to micro- and macroenvironmental effects. Before drawing these contrasts, and discussing some of the implications for our understanding of the relationship between selection and canalization, we first briefly summarize the patterns seen in our results when we examined pre-adult development time and pre-adult survivorship in the selected populations (FEJ) and their ancestral controls (JB). It should also be noted that though both development time and survival to adulthood are fitness components, it is development time that has the higher correlation with fitness in the FEJ selection regime, with pre-adult survivorship being a secondary fitness correlate.
Macroenvironmental Sensitivity of Pre-adult Development Time: Density vs. Temperature
In terms of the macroenvironmental effects of rearing temperature and density on mean development time in the FEJ and JB, temperature had greater effects than density on mean development time (Figure 1), indicating that the thermal plasticity of this trait is greater than its plasticity to density. Temperature and development time are inversely related in ectotherms (van der Have and de Jong, 1996; Angilletta et al., 2004; Ghosh et al., 2013) and this relationship is well known in Drosophila (Gebhardt and Stearns, 1988; Gibert et al., 2004). Likewise, in our study, mean development time increased at 18°C, and became shorter at 28°C, compared to 25°C, the standard rearing temperature of both FEJ, and JB (Figure 1). Although the JB development time underwent greater absolute change with changing temperature, the relative changes in development time compared to the standard, i.e., 25°C, were same in both JB and FEJ. Thus, despite a large evolutionary reduction of development time in FEJ populations, thermal (macroenvironmental) plasticity of development time has not evolved in FEJ populations.
Like temperature, larval density is a major macroenvironmental variable affecting pre-adult development time in Drosophila, but in a more complex manner. At very low larval densities (10 or so larvae per vial), Drosophila cultures show an Allee effect, which signifies the beneficial effects of larger numbers of larvae to work up and soften the food medium and control the growth of fungi in absence of which development and survival may be affected for the existing larvae (Sang, 1956; Ashburner, 1989; A. Joshi, pers. obs.). Beyond a moderate density (50–100 larvae per vial), increasing larval density tends to increase mean development time, largely by stretching out the right tail of the development time distribution (Borash et al., 1998; Sarangi, 2018) Our results show that mean development time of FEJ populations remains more uniform across rearing densities compared to the JBs, the difference being especially visible at the highest density of 300 eggs per vial, and at the lowest temperature of 18°C (Figure 1). As the egg density rises from 70 to 300 eggs per vial, relative increase in mean development time is 4 times higher in JB than in FEJ, indicating macroenvironmental canalization of development time with regard to larval density in the FEJ, relative to JB controls. We believe the reason for this canalization lies in the reduction of duration of the third instar in FEJ larvae (Prasad et al., 2000). FEJ larvae cease feeding and start wandering in search for pupariation sites soon after reaching their minimum critical size, whereas JB larvae continue to feed for a long time after critical minimum size is attained; this pattern is mirrored in very low levels early in the third instar of FEJ of the transcript of the Dnpf (Drosophila neuropeptide F) gene, reduction in expression of which triggers the shift from feeding to wandering, typically late in the third instar (Satish, 2010). Moreover, as a consequence, FEJ larvae are much smaller than JB larvae at pupariation, and consume less food during the larval stage, resulting in increased carrying capacity (Joshi et al., 2001). Thus, the spreading out of the development time distribution that occurs at high larval density in the JB, and greatly increases mean development time, is likely to occur to a much reduced degree in the FEJ because the transition to wandering stage is time-cued in the selected populations, rather than food/size-cued, as it is in typical D. melanogaster popualtions, including the JB controls. We stress this point because it illustrates how increased canalization against variation in a macroenvironmental variable can result as a by-product of selection in a constant environment, rather than being selected for directly. Here, the increased macroenvironmental canalization of development time with regard to high larval density in FEJ appears to be due to the fact that FEJ larvae stop feeding when still small, rather than being due to any specific developmental buffering mechanism.
It is important to note that the effect of temperature on individual variation for development time, and how the degree of variation changed from one thermal environment to another, varied widely across replicate populations. Thus, neither did the thermal plasticity of development time evolve in FEJs compared to JBs, nor was the extent of trait variation across temperature consistent from one replicate population to another within a selection regime. All of these indicate an overall lack of canalization for development time against macroenvironmental temperature changes in our study populations. This is in contrast with density, where changes in trait variation across treatments were consistent across blocks, and also, FEJ mean development time is more canalized against high density than JBs.
Microenvironmental Sensitivity of Pre-adult Development Time: Density vs. Temperature
Regarding the sensitivity of FEJ and JB development time to microenvironmental variation across vials, we find no evidence that the microenvironmental sensitivity of development time differs between selection regimes or temperature and density levels (Figure 2; Tables 2, 3). However, when we examine the effects of selection regime, temperature and density on among individual within vial variability of development time in this study, the main finding is that the temperature effect on CV, unlike that on the mean development time (Figure 1) is less than that of density, and similar on FEJ and JB (Figure 3). In contrast, the sensitivity of among individual variation to increasing density is substantially greater in JB than in FEJ, indicating that FEJ have evolved increased ultra- microenvironmental canalization of development time with regard to larval density, in tandem with increased macroenvironmental canalization. Again, this is likely a reflection of the much greater effect of high density on JB larvae, due to their larger size and food/size-cued transition from feeding to wandering, as contrasted to the small size of FEJ larvae with a time-cued feeding to wandering transition. Thus, the CV of development time at lower densities remains similar in FEJ and JB populations, but higher densities trigger increases in the development time of several individuals in the JB populations, thereby increasing the CV. In FEJ populations, however, development time is not much affected by high density, indicating microenvironmental canalization of development time in FEJ compared to the JBs.
Pre-adult Survivorship: Environmental Sensitivity
Turning now to sensitivity of the pre-adult survivorship, we observe that macroenvironmental effects of rearing temperature and density on pre-adult survivorship, a correlated response to selection, in the FEJ and JB reveal a pattern different from that seen for pre-adult development time, the trait under direct selection in FEJ populations (Figure 4). Unlike for development time, variation in pre-adult survivorship across densities is greater than that across temperatures (Figure 6). Moreover, there is no evidence for greater canalization of pre-adult survivorship in FEJ or JB with regard to macroenvironmental variation in either temperature or density.
For pre-adult survivorship variation across vials, the extent of variation for the trait is much higher compared to that of development time, in both FEJ and JB populations, and it is also more variable across selection regimes, temperatures and densities (CV 4–15 vs. 1% for development time), although only the effects of selection regime and the selection by density interaction are significant (Figure 5; Tables 6, 7). Mean survivorship tends to reduce at 300 eggs per vial to a similar degree in both the JB and FEJ, compared to the lower densities (Figure 4), and so does the across vial CV in survivorship, but only in the FEJ (Figure 5). Interestingly, the CV of survivorship for FEJ is typically higher than that for JB in all combinations of temperature and density, except at 300 eggs per vial at 18 and 25°C (Figures 5, 6), suggesting that the microenvironmental sensitivity of this trait in FEJ is greater than in the ancestral JB controls, particularly at lower rearing densities. Pre-adult survivorship is known to be reduced at very low densities in Drosophila due to the food medium not getting softened by the feeding activity of enough larvae, and also reduced suppression of fungal growth by larvae (Sang, 1956; Ashburner, 1989). It is possible that pre-adult survivorship in the FEJ is more sensitive to across vial microenvironment variation because the FEJ larvae, being much slower feeders than JB larvae (Prasad et al., 2001), exhibit an Allee effect even at a density of 30 eggs per vial, whereas that density is sufficiently high for JB larvae not to be exhibiting an Allee effect. The cause for across vial CV in survivorship being less in FEJ than JB at 300 eggs per vial could be due to higher density being more stressful for JB than FEJ, owing to the reduced size and enhanced carrying capacity of the latter (see above). Thus, the increased sensitivity of JB survivorship at high density, compared to FEJ, to microenvironmental variation across vials is likely to be specifically due to JB being more susceptible to mortality at 300 eggs per vial than FEJ. The relative degree of microenvironmental sensitivity of pre-adult survivorship in FEJ vs. JB populations thus seems to vary between lower and higher densities, and therefore, no clear trend for microenvironmental canalization of survivorship is apparent. As for temperature, neither the main effect of temperature nor the selection-temperature interaction on across vial CV of pre-adult survivorship was significant, once again indicating no clear trend for microenvironmental canalization of survivorship against temperature in either FEJ or JB.
Selection and Canalization
In the FEJ the nature of selection for development time was directional, at least for the first few hundred generations, although the selection response did eventually plateau (A. Joshi, pers. obs.). While stabilizing selection has been predicted to result in canalization of a trait, directional selection is often thought to lead to decanalization (Schmalhausen, 1949; Rice, 1998; Pélabon et al., 2010; Hayden et al., 2012). Our results, however, suggest that even directional selection can lead to canalization of a trait, as the FEJ appear to have evolved canalization of development time with regard to larval density, both in terms of mean trait value and among individual variability in trait value, reflecting both macro- and microenvironmental canalization of development time against rearing density. More importantly, increased canalization of development time with regard to rearing density in FEJ, relative to the ancestral JB controls, seems to be due to the specific effects of the restructuring of the pre-adult life-stage durations and reduction of body size at pupariation, and the mechanistic causes of macro- and microenvironmental canalization of development time against larval density (or crowding) could be the same in these populations. Interestingly, development time in FEJ populations was not buffered against variation in rearing temperature, either at macro- or microenvironmental levels. Moreover, the magnitude of effects of temperature and density on mean development time and CV of development time across individuals were opposite.
Pre-adult survivorship, a trait that is not under direct selection in our populations but has undergone correlated reduction alongside development time in the faster developing populations, did not show any clear trends for macro- or microenvironmental canalization against either temperature and density.
Overall, then, our results underscore that whether or not canalization results from directional selection may often depend on the trait in question, the environmental factor under study, the definition of canalization, and specific details of the ecology and physiology of the populations studied. Some other recent findings also suggest that directional and disruptive selection may not always lead to decanalization as predicted by theory (Hansen et al., 2006; Hayden et al., 2014). Some caution is, therefore, in order when drawing generalized conclusions about the relationship between different forms of selection and canalization from a hypothetical perspective: such relationships need to be explored on a case-by-case basis, and canalization, like evolution in general, may turn out to be “local” (sensu Rose et al., 2005). Overall the understanding of the mechanistic basis of canalization remains extremely poor, and to add to that, the link between selection and canalization is equally obscure (Stearns, 2002). We need lot more theoretical and empirical work investigating both mechanisms and causes of canalization, and its evolution, to have a clearer understanding of the phenomenon.
Canalization of Morphological vs. Life-History Traits
In our study, we focused on classic life-history traits that are fitness correlates. Historically, though, studies of canalization have typically focused on morphological traits. Waddington (1953, 1956) pioneered the concept of canalization and demonstrated it through his experiments on the morphological phenotypes “crossveinless” and “bithorax” in Drosophila. Thus, the concept of canalization was originally proposed and subsequently studied to describe the robustness of developmental pathways governing morphological characters (Dunn and Fraser, 1958; Rendel, 1959). However, the basic criterion for canalization is robustness of phenotypic expression or maintenance of low phenotypic variation of a trait in the face of mutational perturbation or environmental variation. Given such criteria, canalization, in principle, should be identifiable for any quantitative trait, whether or not morphological. However, studies addressing canalization of morphological traits are more common compared to other traits (Dunn and Fraser, 1958; Rendel, 1959; Gibson and Hogness, 1996; Dworkin, 2005a,b; Hall et al., 2007; Sgrò et al., 2010; Takahashi et al., 2011; Groth et al., 2018). Besides, demonstration of canalizing effect of the chaperone protein Hsp90 in Drosophila (Rutherford and Lindquist, 1998) and Arabidopsis (Sangster et al., 2008) on a wide variety of morphological traits provided the first evidence of canalizing mechanisms at the molecular level. Recent literature shows an upsurge in interest on canalization in molecular biology as canalization is being investigated for suite of traits ranging from transcript abundance to gene regulatory networks to ribozyme diversity (Hayden et al., 2014; Félix and Barkoulas, 2015). However, studies exploring canalization of complex life-history traits are rare. Stearns and Kawecki (1994) and Stearns et al. (1995) investigated the canalization of life-history traits in Drosophila over two decades ago. In the past decade, a few studies addressed canalization of complex traits like reproductive output, body size, developmental period and mate preference (Baer, 2008; Takahashi et al., 2011; Svensson et al., 2014). Canalization, thus, needs to be viewed as a general phenomenon controlling trait variation, connecting genotypes to phenotypes, and limiting studies of canalization to morphological traits alone may hinder achieving a broader understanding of the phenomenon, especially its relationship to different forms of selection.
Conclusion
Our study is exploratory in nature and it sheds light on a number of aspects of the relationship between selection, canalization and plasticity. We demonstrate that (a) a quantitative trait (development time) can evolve changes in its mean value without any change in its plasticity (e.g., thermal plasticity), (b) canalization can evolve for a life-history trait (development time) in laboratory populations within a span of few hundred generations, (c) a trait may show canalization both at macro- and microenvironmetal levels due to same underlying mechanisms, (d) contrary to expectations, directional selection can lead to environmental canalization (against developmental density or crowding) of a life history trait, as a byproduct of the evolved changes in the life history and physiology of the population, and, (e) a trait can be canalized for one environmental factor (density) but may not be canalized for another (temperature). The last two points show that canalization, like many other biological phenomena could be context specific, and one needs to be cautious before drawing generalized conclusions about the causes and manifestations of canalization. Canalization needs to be viewed as a phenomenon controlling trait variation across different hierarchical levels in biology, starting from cellular molecules to complex traits. However, both proximate and ultimate causes of canalization remain poorly understood, and a concerted effort of theoretical and empirical studies are needed to understand this fascinating phenomenon. And last but not the least, this study also demonstrates that experimental evolution can be a powerful tool to understand the intricacies of the evolutionary process, with particular focus on trait variation.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
SG and AJ conceived of the experiments. SG, KS, and MJ carried out the experiments. SG did the data analysis. SG and AJ wrote the manuscript, and both SG and AJ carried out subsequent editing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Archana Mohan for handling data, Ananda T. for help in the experiment and N. Rajanna and M. Manjesh for general help in the laboratory. SG was supported by a DST WOS A fellowship [SR/WOS-A/LS-1179/2015(G)] from the Department of Science & Technology, Government of India, during the preparation of the manuscipt, and by a fellowship from JNCASR during the experimental work. KS thanks Council of Scientific and Industrial Research, Government of India, for a senior research fellowship. The experimental work was supported in parts by funds from the Department of Science and Technology, Government of India, and the preparation of the manuscript by a J.C. Bose National Fellowship of the Science and Engineering Research Board, Government of India, to AJ. We thank the reviewers for comments on earlier drafts of this manuscript.
References
Angilletta, M. J. Jr., Steury, T. D., and Sears, M. W. (2004). Temperature, growth rateand body size in ectotherms: fitting pieces of a life-history puzzle. Integr. Comp. Biol. 44, 498–509. doi: 10.1093/icb/44.6.498
Ashburner, M. (1989). Drosophila: a Laboratory Handbook. New York, NY: Cold Spring Harbour Laboratory Press, Cold Spring Harbour.
Baer, C. F. (2008). Quantifying the decanalizing effects of spontaneous mutations in rhabditid nematodes. Am. Nat. 172, 272–281. doi: 10.1086/589455
Bateman, K. G. (1959). The genetic assimilation of four venation phenocopies. J. Genet. 56, 443–474. doi: 10.1007/BF02984796
Borash, D. J., Gibbs, A. G., Joshi, A., and Mueller, L. D. (1998). A genetic polymorphism maintained by natural selection in a temporally varying environment. Am. Nat. 151, 148–156. doi: 10.1086/286108
Careau, V., Buttemer, W. A., and Buchanan, K. L. (2014). Early-developmental stress, repeatability, and canalization in a suite of physiological and behavioral traits in female zebra finches. Integr. Comp. Biol. 54, 539–554. doi: 10.1093/icb/icu095
de Visser, J. A. G. M., Hermisson, J., Wagner, G. P., Ancel Meyers, L., Bagheri-Chaichian, H., Blanchard, J. L., et al. (2003). Perspective: Evolution and detection of genetic robustness. Evolution 57, 1959–1972. doi: 10.1111/j.0014-3820.2003.tb00377.x
Debat, V., and David, P. (2001). Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 16, 555–561. doi: 10.1016/S0169-5347(01)02266-2
Debat, V., and Le Rouzic, A. (2019). Canalization, a central concept in biology. Semin. Cell. Dev. Biol. 88, 1–3. doi: 10.1016/j.semcdb.2018.05.012
Dey, P., Mendiratta, K., Bose, J., and Joshi, A. (2016). Enhancement of larval immune system traits as a correlated response to selection for rapid development in Drosophila melanogaster. J. Genet. 95, 719–723. doi: 10.1007/s12041-016-0659-5
Dey, S., Dey, S., Mohan, J., and Joshi, A. (2006). Micro-environmental variations in pre-assay rearing conditions can lead to anomalies in the measurement of life-history traits. J. Genet. 85, 53–56. doi: 10.1007/BF02728970
Dor, D., and Jablonka, E. (2010). “Plasticity and canalization in the evolution of linguistic communication: an evolutionary developmental approach,” in The Evolution of Human Language: Biolinguistic Perspectives Approaches to the Evolution of Language, eds R. Larson, V. Déprez, and H. Yamakido (Cambridge: Cambridge University Press, 135–147.
Dunn, R. B., and Fraser, A. S. (1958). Selection for an invariant character - ‘vibrissae number’ - in the house mouse. Nature 181, 1018–1019. doi: 10.1038/1811018a0
Dworkin, I. (2005a). A study of canalization and developmental stability in the sternopleural bristle system of Drosophila melanogaster. Evolution 59, 1500–1509. doi: 10.1111/j.0014-3820.2005.tb01799.x
Dworkin, I. (2005b). Evidence for canalization of distal-less function in the leg of Drosphilia melanogaster. Evol. Dev. 7, 89–100. doi: 10.1111/j.1525-142X.2005.05010.x
Dworkin, I. (2005c). “Canalization, Cryptic Variation and Developmental Buffering: a Critical Examination and Analytical Perspective,” in Variation, eds B. Hallgrimsson and B. K. Hall (Cambridge, MA: Elsevier), 131–158.
Eshel, I., and Matessi, C. (1998). Canalization, genetic assimilation and preadaptation: a quantitative genetic model. Genetics 149, 2119–2133.
Falconer, D. S., and Mackay, T. F. (1996). Introduction to Quantitave Genetics. New York, NY: The Ronald Press Company.
Félix, M., and Barkoulas, M. (2015). Pervasive robustness in biological systems. Nat. Rev. Gen. 16, 483–496. doi: 10.1038/nrg3949
Flatt, T. (2005). The evolutionary genetics of canalization. Q. Rev. Biol. 80, 287–316. doi: 10.1086/432265
Fossen, E. I., Pélabon, C., and Einum, S. (2018). An empirical test for a zone of canalization in thermal reaction norms. J. Evol. Biol., 31, 936–943. doi: 10.1111/jeb.13287
Fusco, G., and Minelli, A. (2010). Phenotypic plasticity in development and evolution: facts and concepts. Phil. Tran. R. Soc. B 365, 547–556. doi: 10.1098/rstb.2009.0267
Garland, T. Jr., and Rose, M. R. (eds.). (2009). Experimental Evolution: Concepts, Methods and Applications of Selection Experiments. Berkeley; Los Angeles, CA: University of California Press.
Gebhardt, M. D., and Stearns, S. C. (1988). Reaction norms for developmental time and weight at eclosion in Drosophila mercatorum. J. Evol. Biol. 1, 335–354. doi: 10.1046/j.1420-9101.1988.1040335.x
Ghosh, S. M., and Joshi, A. (2012) Evolution of reproductive isolation as a by-product of divergent life-history evolution in laboratory populations of Drosophila melanogaster. Ecol. Evol. 2, 3214–26. doi: 10.1002/ece3.413
Ghosh, S. M., Testa, N. D., and Shingleton, A. W. (2013). Temperature-size rule is mediated by thermal plasticity of critical size in Drosophila melanogaster. Proc. Biol. Sci. 280:20130174. doi: 10.1098/rspb.2013.0174
Ghosh-Modak, S. (2009). Evolution of life-history traits, canalization and reproductive isolation in laboratory populations of drosophila melanogaster selected for faster pre-adult development and early reproduction. (Ph.D. Thesis), Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bengaluru.
Ghosh-Modak, S., Satish, K. M., Mohan, J., Dey, S., Raghavendra, N., Shakarad, M., et al. (2009). A possible trade-off between developmental rate and pathogen resistance in Drosophila melanogaster. J. Genet. 88, 253–256. doi: 10.1007/s12041-009-0036-8
Gibert, P., Capy, P., Imasheva, A., Moreteau, B., Morin, J. P., Pétavy, G., et al. (2004). Comparative analysis of morphological traits among Drosophila melanogaster and D. simulans: genetic variability, clines and phenotypic plasticity. Genetica 120, 165–179. doi: 10.1023/B:GENE.0000017639.62427.8b
Gibson, G., and Dworkin, I. (2004). Uncovering cryptic genetic variation. Nat. Rev. Genet. 5, 681–690. doi: 10.1038/nrg1426
Gibson, G., and Hogness, D. S. (1996). Effect of polymorphism in the Drosophila regulatory gene Ultrabithorax on homeotic stability. Science 271, 200–203. doi: 10.1126/science.271.5246.200
Groth, B. R., Huang, Y., Monette, M. J., and Pool, J. E. (2018). Directional selection reduces developmental canalization against genetic and environmental perturbations in Drosophila wings. Evolution 72, 1708–1715. doi: 10.1111/evo.13550
Hall, M. C., Dworkin, I., Ungerer, M. C., and Purugganan, M. (2007). Genetics of microenvironmental canalization in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 104, 13717–13722. doi: 10.1073/pnas.0701936104
Hansen, T. F., Álvarez-Castro, J. M., Carter, A. J. R., Hermisson, J., and Wagner, G. P. (2006). Evolution of genetic architecture under directional selection. Evolution 60, 1523–1536. doi: 10.1111/j.0014-3820.2006.tb00498.x
Hayden, E. J., Bratulic, S., Koenig, I., Ferrada, E., and Wagner, A. (2014). The effects of stabilizing and directional selection on phenotypic and genotypic variation in a population of RNA enzymes. J. Mol. Evol. 78, 101–108. doi: 10.1007/s00239-013-9604-x
Hayden, E. J., Weikert, C., and Wagner, A. (2012). Directional selection causes decanalization in a group I ribozyme. PLoS ONE 7:e45351. doi: 10.1371/journal.pone.0045351
Ives, P. T. (1970). Further studies on the South Amherst population of Drosophila melanogaster. Evolution 24, 507–518. doi: 10.1111/j.1558-5646.1970.tb01785.x
Jiang, P., Kreitman, M., and Reinitz, J. (2018). The Relationship Between Robustness and Evolution. bioRxiv: 268862. doi: 10.1101/268862
Joshi, A., and Mueller, L. D. (1996). Density-dependent natural selection in Drosophila: trade-offs between larval food acquisition and utilization. Evol. Ecol. 10, 463–474. doi: 10.1007/BF01237879
Joshi, A., Prasad, N. G., and Shakarad, M. (2001). K-selection, α-selection, effectiveness, and tolerance in competition: density-dependent selection revisited. J. Genet. 80, 63–75. doi: 10.1007/BF02728332
Kawecki, T. J., Lenski, R. E., Ebert, D., Hollis, B., Olivieri, I., and Whitlock, M. C. (2012). Experimental evolution. Trends Ecol. Evol. 27, 547–560. doi: 10.1016/j.tree.2012.06.001
McDonald, J. M., Ghosh, S. M., Gascoigne, S. J., and Shingleton, A. W. (2018). Plasticity through canalization: the contrasting effect of temperature on trait size and growth in Drosophila. Front. Cell Dev. Biol. 6:156. doi: 10.3389/fcell.2018.00156
Moczek, A. P., Sultan, S., Foster, S., Ledon-Rettig, C., Dworkin, I., Nijhout, H. F., et al. (2011). The role of developmental plasticity in evolutionary innovation. Proc Biol. Sci. 278, 2705–2713. doi: 10.1098/rspb.2011.0971
Morgante, F., Sorensen, P., Sorensen, D. A., Maltecca, C., and Mackay, T. F. (2015). Genetic architecture of micro-environmental plasticity in Drosophila melanogaster. Sci. Rep. 5:09785. doi: 10.1038/srep09785
Nijhout, H. F., and Davidowitz, G. (2003). “Developmental perspectives on phenotypic variation, canalization, and fluctuating asymmetry,” in Developmental Instability: Causes and Consequences, ed M. Polak. (New York, NY: Oxford University Press, 3–13.
Paaby, A. B., and Testa, N. D. (2018). “Developmental plasticity and evolution,” in Evolutionary Developmental Biology, eds. L. Nuno de la Rosa and G. Müller (Cham: Springer, 1–14.
Pélabon, C., Hansen, T. F., Carter, A. J., and Houle, D. (2010). Evolution of variation and variability under fluctuating, stabilizing, and disruptive selection. Evolution 64, 1912–1925. doi: 10.1111/j.1558-5646.2010.00979.x
Pigliucci, M. (2005). Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486. doi: 10.1016/j.tree.2005.06.001
Prasad, N. G., and Joshi, A. (2003). What have two decades of laboratory life-history evolution studies on Drosophila melanogaster taught us? J. Genet. 82, 45–76. doi: 10.1007/BF02715881
Prasad, N. G., Shakarad, M., Anitha, D., Rajamani, M., and Joshi, A. (2001). Correlated responses to selection for faster development and early reproduction in Drosophila: the evolution of larval traits. Evolution 55, 1363–1372. doi: 10.1111/j.0014-3820.2001.tb00658.x
Prasad, N. G., Shakarad, M., Gohil, V. M., Sheeba, V., Rajamani, M., and Joshi, A. (2000). Evolution of reduced pre-adult viability and larval growth rate in laboratory populations of Drosophila melanogaster selected for shorter development time. Genet. Res. 76, 249–259. doi: 10.1017/S0016672300004754
Proulx, S. R., and Phillips, P. C. (2005). The opportunity for canalization and the evolution of genetic networks. Am. Nat. 165, 147–162. doi: 10.1086/426873
Rendel, J. M. (1959). Canalization of the scute phenotype in Drosophila. Evolution 13, 425–439. doi: 10.1111/j.1558-5646.1959.tb03033.x
Rice, S. H. (1998), The evolution of canalization and the breaking of von baer's laws: modeling the evolution of development with epistasis. Evolution 52:647–656. doi: 10.1111/j.1558-5646.1998.tb03690.x
Rose, M. R., and Charlesworth, B. (1981). Genetics of life history in Drosophila melanogaster. II. Exploratory selection experiments. Genetics 97,187–196.
Rose, M. R., Passananti, H. B., Chippindale, A. K., Phelan, J. P., Matos, M., Teotónio, H., et al. (2005). The effects of evolution are local: evidence from experimental evolution in Drosophila. Integr. Comp. Biol. 45, 486–491. doi: 10.1093/icb/45.3.486
Rutherford, S. L., and Lindquist, S. (1998). Hsp90 as a capacitor for morphological evolution. Nature 396, 336–342. doi: 10.1038/24550
Sang, J. H. (1956). The quantitative nutritional requirements of Drosophila melanogaster. J. Expt. Biol. 33, 45–72.
Sangster, T. A., Salathia, N., Lee, H. N., Watanabe, E., Schellenberg, K., Morneau, K., et al. (2008). HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc. Natl. Sci. Acad. U.S.A. 105, 2969–2974. doi: 10.1073/pnas.0712210105
Sarangi, M. (2018). Ecological details mediate different paths to the evolution of larval competitive ability in Drosophila. (Ph.D. thesis), Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bengaluru.
Satish, K. M. (2010). Reverse evolution and gene expression studies on populations of Drosophila melanogaster selected for rapid pre-adult Development and early reproduction. (Ph.D. thesis), Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bengaluru.
Schmalhausen, I. I. (1949). Factors of Evolution: The Theory of Stabilizing Selection. Chicago: University of Chicago Press.
Sgrò, C. M., Wegener, B., and Hoffmann, A. A. (2010). A naturally occurring variant of Hsp90 that is associated with decanalization. Proc. Biol. Sci. 277, 2049–2057. doi: 10.1098/rspb.2010.0008
Shakarad, M., Prasad, N. G., Gokhale, K., Gadagkar, V., Rajamani, M., and Joshi, A. (2005). Faster development does not lead to correlated evolution of greater pre–adult competitive ability in Drosophila melanogaster. Biol. Lett. 1, 91–94. doi: 10.1098/2004.0261
Shakarad, M., Prasad, N. G., Rajamani, M., and Joshi, A. (2001). Evolution of faster development does not lead to greater fluctuating asymmetry of sternopleural bristle number in Drosophila. J. Genet. 80, 1–7. doi: 10.1007/BF02811412
Shaw, J. R., Hampton, T. H., King, B. L., Whitehead, A., Galvez, F., Gross, R. H., et al. (2014). Natural selection canalizes expression variation of environmentally induced plasticity-enabling genes. Mol. Biol. Evol. 31, 3002–3015. doi: 10.1093/molbev/msu241
Sheeba, V., Madhyastha, N. A. A., and Joshi, A. (1998). Oviposition preference for novel versus normal food resources in laboratory populations of Drosophila melanogaster. J. Biosci. 23, 93–100. doi: 10.1007/BF02703000
Siegal, M. L., and Bergman, A. (2002). Waddington's canalization revisited: developmental stability and evolution. Proc. Natl. Acad. Sci. U.S.A. 99, 10528–10532. doi: 10.1073/pnas.102303999
Stearns, S. C. (2002). Progress on canalization. Proc. Natl. Sci. Acad. U.S.A. 99, 10229–10230. doi: 10.1073/pnas.172388999
Stearns, S. C., Kaiser, M., and Kawecki, T. J. (1995). The differential canalization of fitness components against environmental perturbations in Drosophila melanogaster. J. Evol. Biol. 8, 539–557. doi: 10.1046/j.1420-9101.1995.8050539.x
Stearns, S. C., and Kawecki, T. J. (1994). Fitness sensitivity and the canalization of life-history traits. Evolution 48, 1438–1450. doi: 10.1111/j.1558-5646.1994.tb02186.x
Svensson, E. I., Runemark, A., Verzijden, M. N., and Wellenreuther, M. (2014). Sex differences in developmental plasticity and canalization shape population divergence in mate preferences. Proc. Biol. Sci. 281:1636. doi: 10.1098/rspb.2014.1636
Takahashi, K. H., Okada, Y., and Teramura, K. (2011). Genome-wide deficiency mapping of the regions responsible for temporal canalization of the developmental processes of Drosophila melanogaster. J. Hered. 102, 448–457. doi: 10.1093/jhered/esr026
van der Have, T. M., and de Jong, G. (1996). Adult size in ectotherms: temperature effects on growth and differentiation. J. Theo. Biol. 183, 329–340. doi: 10.1006/jtbi.1996.0224
Waddington, C. H. (1942). Canalization of development and the inheritance of acquired characters. Nature 150, 563–565. doi: 10.1038/150563a0
Waddington, C. H. (1952). Selection for the basis of an acquired character. Nature 169:278. doi: 10.1038/169625b0
Waddington, C. H. (1953). Genetic assimilation of an acquired character. Evolution 7, 118–126. doi: 10.1111/j.1558-5646.1953.tb00070.x
Waddington, C. H. (1956). Genetic assimilation of the bithorax phenotype. Evolution 10, 1–13. doi: 10.1111/j.1558-5646.1956.tb02824.x
Waddington, C. H. (1961). Genetic assimilation. Adv. Genet. 10, 257–293. doi: 10.1016/S0065-2660(08)60119-4
Wagner, G. P., Booth, G., and Bagheri-Chaichian, H. (1997). A population genetic theory of canalization. Evolution 51, 329–347. doi: 10.1111/j.1558-5646.1997.tb02420.x
West-Eberhard, M. J. (2003). Developmental Plasticity and Evolution. New York, NY: Oxford University Press.
Woltereck, R. (1909). Weitere experimentelle Untersuchungen uber Artveränderung, speziell uberdas Wesen quantitativer Artunterschiede bei Daphniden. Verh. D. Tsch. Zool. Ges. 19, 110–172.
Keywords: canalization, plasticity, Drosophila, development time, thermal plasticity experimental evolution, directional selection, larval density
Citation: Ghosh SM, Satish KM, Jayaram M and Joshi A (2019) Does Long-Term Selection for Development Time Result in Canalization: A Test Using Drosophila melanogaster. Front. Ecol. Evol. 7:228. doi: 10.3389/fevo.2019.00228
Received: 24 August 2018; Accepted: 31 May 2019;
Published: 18 June 2019.
Edited by:
Jean-Michel Gibert, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Vincent Debat, Muséum National d'Histoire Naturelle, FranceFrançois Mallard, INSERM U1024 Institut de biologie de l'Ecole Normale Supérieure, France
Copyright © 2019 Ghosh, Satish, Jayaram and Joshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shampa M. Ghosh, c2hhbXBhLmdob3NoQGtpaXRiaW90ZWNoLmFjLmlu; Amitabh Joshi, YWpvc2hpQGpuY2Fzci5hYy5pbg==
†Present Address: Shampa M. Ghosh, School of Biotechnology, Kalinga Institute of Industrial Technology (KIIT), Bhubaneswar, India
K. M. Satish, Department of Biotechnology, College of Agriculture, Navile, University of Agricultural and Horticultural Sciences, Shivamogga, India
Mohan Jayaram, Centre of Excellence in Genomics and Translational Medicine, University of Tartu, Tartu, Estonia
 Shampa M. Ghosh
Shampa M. Ghosh K. M. Satish
K. M. Satish Mohan Jayaram
Mohan Jayaram Amitabh Joshi
Amitabh Joshi