- 1Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia, Universidade de Coimbra, Coimbra, Portugal
- 2CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus de Vairão, Vairão, Portugal
Cleaning behavior between teleost fish in the marine environment is known to be a classic example of mutualistic cooperation, in which cleaners and their so-called clients exchange benefits. These mutualisms occur globally. However, studies of cleaning interactions in temperate regions are scarce compared with studies in the tropics. Here we focused on the rock cook, Centrolabrus exoletus, a wrasse present in the North-East Atlantic, considered to be the main cleaner inhabiting the coast of Portugal, although little is known about its ecology and behavior. We found that these cleaners attended clients in specific locations while others were roaming freely, leaving open the question of which strategy cleaners preferentially use. Interestingly, interactions were initiated more often by clients and terminated more often by cleaners, suggesting that the intake of parasites are the cleaner's primary interest, which was confirmed by the analysis of their diet, mostly composed of gnathiidae parasites. Moreover, this honesty-based relationship between these cleaners and their clients, calls for a re-interpretation of the very meaning of client-jolts (abrupt movements in response to cleaner mouth touch) since interactions with client jolts lasted longer than interactions with no jolts. This study provides new and important evidence on the mutualistic relationship between C. exoletus and its clientele, thus contributing to a better understanding of the behavioral ecology of this cleaner fish system.
Introduction
Marine cleaning mutualisms are interspecific associations, in which cleaners are recurrently observed to remove parasites and infected tissue from the body surface, mouth and gill chambers of their client fish (Losey, 1987; Côté, 2000). Cleaning interactions occur globally, in almost all known marine environments (Losey et al., 1999) but have mostly been studied in tropical regions, and in two of the most speciose families of teleost fishes: the labridae and gobiidae families. Among these two families, the focus has been on two of the most ubiquitous species of cleaners, in particular: the Indo-Pacific bluestreak cleaner wrasse, Labroides dimidiatus (Randall et al., 1990), and the Caribbean cleaning goby, Elacatinus evelynae (Côté and Soares, 2011). Individuals of both species are classified as obligatory or dedicated cleaners (see Vaughan et al., 2016), as these depend mostly on the ectoparasites ingested during cleaning interactions, throughout their entire life cycle (Limbaugh, 1961). However, most cleaner fish species are described as facultative, as individuals only engage in cleaning interactions during one part of their life cycle, usually when juveniles (Côté, 2000). This is widely reported in regions other than the tropics. For example, Hobson (1971) shows that, in the Californian temperate waters, the sharpnose seaperch Phanerodon atripes cleans largely during the juvenile phase. New Zealand's Coris picta, Coris sandageri, Pseudolabrus luculentus and Pseudolabrus miles are also found to clean facultatively (Ayling and Grace, 1971). More recently, the ornate wrasse (Thalassoma pavo) and the Mediterranean rainbow wrasse (Coris julis), two facultative cleaner species, have been shown to play a significant role in the temperate water reefs of the Azores by selectively inspecting and removing ectoparasites from their client fish (Narvaez et al., 2015). Indeed, while more information is becoming available regarding the important role of facultative cleaners inhabiting temperate and sub-tropical regions (Quimbayo et al., 2018), these reports are usually made of occasional observations on previously unreported cleaning activities in species thought not to clean. Thus, the current knowledge regarding the ecological dynamics of cleaners living in temperate systems is still very limited. Further studies are needed, focusing on cleaner's foraging behavior, life dietary shifts, parasitic preferences and on how these may impact the remaining species (e.g., visiting clientele).
Overall, fish parasite infestations represent costs for the hosts, which can negatively affect their physiology (Shaw et al., 2009), behavior (Garnick and Margolis, 1990) and morphology (Seppälä et al., 2004). Gnathiidae isopods are probably the best studied parasites infesting marine fish (Coile and Sikkel, 2013). Despite their global distribution, gnathiids have been more often found in tropical and temperate waters, being one of the most common ectoparasites of marine reef fish (Grutter, 1994; Grutter and Poulin, 1998) and, therefore, an important food resource for cleaners (Côté, 2000; Grutter, 2002). Gnathiids emerge from the benthos and use their powerful mouthparts to penetrate the fish skin and gills where they can feed on the fish blood, lymph, or mucus (Smit and Davies, 2004). Crustaceans from the Caligidae family are also important in farmed fish parasite infestations. For instance, Lepeophtheirus salmonis is frequently present in the North Atlantic salmon farms (Johnson et al., 2004), while the Caligus spp. are more generalists found in more than 80 species of hosts worldwide (Kabata, 2003). These crustaceans are also commonly found in cleaners' gut contents (Grutter, 1996), which further confirms the significance of being cleaned to the health and dynamics of the client fish community (Grutter, 1999; Grutter et al., 2003; Waldie et al., 2011).
To understand how cleaners depend on parasitic items it is crucial to analyse their diet. Facultative cleaners can also rely on plankton and some benthonic organisms, in addition to the parasitic items (Henriques and Almada, 1997). Obligatory cleaners, on the other hand, are said to depend solely on client-derived parasitic items throughout their life cycle, however these may also vary (Côté, 2000). During cleaning interactions, the cleaner has access not only to ectoparasites but also to scales, mucus and healthy skin from the hosts (Côté and Soares, 2011). The ingestion of these non-parasitic elements, referred to as cheating (Bshary and Grutter, 2002), is harmful to the clients since mucus, for example, protects them against UV damage and other diseases (Ebran et al., 1999). In response, clients usually try to shake cleaners (a behavior known as “body jolt”—a whole body shudder) and to move away (Soares et al., 2008a). In addition, some client species may punish cleaners by chasing them aggressively and ending the interaction. Nevertheless, most cleaner species are thought to prefer to forage on ectoparasites instead of mucus (Bshary and Grutter, 2005), and they may glean on mucus and other tissue when the ectoparasites levels becomes residual (Soares et al., 2008c).
The rock cook wrasse (Labridae), Centrolabrus exoletus, is an important cleaner fish species that inhabits the north eastern Atlantic temperate reefs, from Norway to Portugal and in the south of Spain (Lythgoe and Lythgoe, 1991; Henriques and Almada, 1997; Galeote and Otero, 1998). Potts (1973), was the first to report a cleaning interaction between C. exoletus and a client species, Labrus merula, in the wild. Due to its variable diet, which includes not only parasitic items but also benthonic organisms like crustaceans and molluscs, algae and other fragments (Galeote and Otero, 1998), this species is considered to be a facultative cleaner (Henriques and Almada, 1997). These individuals are thought to search for clients alone or in small groups (of 3 to 8 cleaners), instead of maintaining small territories [usually referred to as cleaning stations, (Potts, 1968)].
The ability of C. exoletus to effectively pick ectoparasites, such as Caligus elongatus (Tully et al., 1995), is evidence of the great potential in using this species in the biological control of parasitic infestations, for commercial fish farms, especially for controlling sea lice infestations in the Northern Atlantic salmon farms (Costello and Bjordal, 1990; Bjordal, 1992; Costello, 1993). However, the increasing interest in C. exoletus and usage contrasts with the limited amount of information available.
This study aims to investigate the cleaning behavior of the facultative cleaner C. exoletus, found in its most southern limit (Algarve, Portugal), strongly contributing to a better understanding of the behavioral ecology of this species, by: (1) analyzing its cleaning behavior, (2) examining gut contents for parasitic items to determine the relevance of these interactions to the overall diets of individuals, (3) determining gnathiid benthic emergence levels in our study sites and, finally, (4) examining the ectoparasite load of selected fish species of the coastal communities in the south of Portugal in order to discuss these cleaners' putative influence on the remaining fish community parasite levels.
Materials and Methods
Study Site and Species
This study was conducted on the rocky reefs between Mato's beach and Grilheira's beach in Lagoa, Algarve, Portugal (37°06′N, 8°30′W) and at Leixão da Gaivota (37°06′N, 8°31′W), located at 200 m from Caneiro's beach, Lagoa (Algarve, Portugal) from July 2015 to March 2016. The marine environment of the south coast of Algarve is quite variable: in the west coast, the type of substrate is predominantly rocky whereas sand is mostly found in the east coast (Gonçalves et al., 2007,2008,2010). The geographic position of the south-Portuguese (Algarve) coast favors its biodiversity due to the confluence of the Mediterranean and tropical and temperate Atlantic waters (Santos et al., 2007). Rocky substratum biodiversity includes brown algae, calcareous red algae, anemones, bryozoans, echinoderms, such as sea urchins and sea cucumbers, gastropods, sponges, as well as benthic fish, such as the rocky goby, and demersal fish, such as the common two-banded seabream (Saldanha, 1995).
Field work was conducted in the morning from 0900 h to 1200 h with SCUBA dive surveys at depths varying between 3 to 10 m. Our focus species, Centrolabrus exoletus, is the main cleaner fish species inhabiting the coastal areas of Portugal, ranging in size from 5 to a maximum of 18 cm (Henriques and Almada, 1997). It has been reported to inspect clients throughout the year but most frequently in the summer months (Galeote and Otero, 1998).
Behavioral Observations
A total of 450 min of behavioral observations were made between July and November 2015 (± SE = 4 ± 2.91 observations per month). Focal observations were made during SCUBA dive surveys to a total of 20 cleaners at depths ranging from 3 to 10 m. Depending on the conditions of the sea (i.e., current, visibility, and water temperature), each observation lasted from a minimum of 20 min to a maximum of 30 min. Observations started after a 2 to 5 min familiarization period to allow the fish to become accustomed to the presence of the diver that remained at a distance of 2 to 5 m from the individuals (Soares et al., 2007).
Each observation period was recorded for each client species seen interacting with the local cleaner (Table 1 includes the description of each behavior). Clients were deemed to start the interaction if posing [species-specific immobile pose signaling the willingness to be cleaned (Côté et al., 1998)] before the onset of cleaning by the cleaner. However, cleaners sometimes approach and start inspecting without any solicitation from clients. The duration of the interaction (in seconds), the number of bites (cleaner mouth contact) taken and the number of jolts by clients were also registered. Jolts are apparently painful reactions to a cleaner's bite and have previously been shown to be dishonest bites by cleaners (Bshary and Grutter, 2002; Soares et al., 2008a); however, the exact meaning of the client's reaction to this C. exoletus interaction is yet to be found. Finally, the interaction location, as well as if it occurred inside or outside of a cleaning station boundary and its depth were recorded.
Centrolabrus exoletus Diet Composition
In total, 20 C. exoletus cleaners, with a total length between 5.9 and 11.3 cm, were haphazardly collected between 0900 and 1200 h, from September 2015 to March 2016. Following their capture, each individual cleaner was immediately placed in a vial containing 70% alcohol to interrupt the digestion process (Soares et al., 2008b). In the lab, each individual was then measured (TL, mm) and weighed (with and without gut content, in grams). All items were separated, identified and counted through a specific identifiable taxonomic part as: non-parasitic content (algae, free copepods, amphipods, gastropods, non-parasitic crustaceans, bivalves and fish scales), parasites (all belonging to the Gnathiidae family) and other fragments like sand grains.
Gnathiid Emergence Levels
To determine gnathiid availability and understand how it may influence the infestation levels of host fishes, gnathiid parasites were sampled using emergence traps—pyramidal shaped traps made with 100 μm plankton mesh attached to a 1 m2 base of PVC tubes. Each trap had a 1 L plastic bottle filled with water linked to a funnel above the base acting as a cod end. To create positive buoyancy, the plastic bottles were also filled with a bubble of air. To prevent gaps between the base and an uneven substratum, PVC tubes were filled with beach sand (Grutter et al., 2000; Sikkel et al., 2004). Samples were collected in 3 different time periods (July, September and December 2015) and in each sampling point 5 traps randomly distributed between 3 to 5 m depth were used. Traps were set between 0900 and 1200 h. Samples were collected after a 24 h period in order to include the night and crepuscular periods (Sikkel et al., 2006). Following retrieval, the contents of each bottle were filtered with a 55 μm plankton mesh and preserved in 70% alcohol (Chambers and Sikkel, 2002). Gnathiids were then counted and identified to the family level using a dissecting microscope.
Client Fish Ectoparasite Infestation Loads
To evaluate the infestation levels of C. exoletus clientele, a total of 50 individuals belonging to 5 different species were captured (client species analyzed: Ctenolabrus rupestris, Coris julis, Diplodus vulgaris, Symphodus bailloni, Symphodus melops). The method followed Sikkel et al. (2004) and Soares et al. (2007) individual fish were steered into a barrier net, caught with a hand net and quickly placed individually into hermetically sealed plastic bags filled with seawater. To limit fish stress and to minimize the time away from the place of capture (max. 1 h), only 5 to 7 fish were collected during each sampling period (Narvaez et al., 2015). On land, each individual was identified and measured (TL, cm) and put in fresh water for 10 min while their entire body was gently brushed with a soft paint brush. The seawater inside the original plastic bag and the fresh water under which the fish was brushed were then sieved through a 55 μm plankton mesh. The content resulting from the filtration of the water belonging to each individual was then preserved in 70% alcohol. Ectoparasites were counted and identified to the family level using a dissecting microscope.
Statistical Analysis
All behavioral observations were estimated per a unit of 20 min. The number of jolts by clients was standardized to 60 s of interaction for better comparison with other studies (see Soares et al., 2008a), and the number of client punishment responses (see Table 1), were counted for each client species. A generalized linear model (GLM) was used to evaluate the influence of client jolts on the duration of cleaner's interaction. Interaction time was used as the dependent variable, assuming a Poisson distribution with logarithmic link function, and the occurrence or absence of jolts as well as the client species (aiming at the five client species that were reported to jolt) were included as fixed factors.
A principal coordinate analysis was used to determine the variation in cleaners' diet composition. This analysis uses the similarity between samples to create a representation in space which enables visualization of the differences found. The matrix used for the multidimensional scale of proximity (PROXCAL) was composed based on food item groups found in the stomach contents of cleaners (gnathiids, scales, algae, free-living copepods, amphipods, gastropods, crustaceans and bivalves) as well as other fragments like sand grains. Measurements were based on counts (chi-square) and the proximities were transformed into intervals. The stress graph showed only two dimensions.
To investigate if there was a relationship between cleaner ontogeny and ectoparasite dependency, the number of ectoparasites found in cleaners' stomachs was regressed against the total length of cleaners with a simple linear regression. The number of gnathiids was used as the dependent variable and the total length was used as the independent variable. In the same way, the number of scales present in the cleaners' stomach contents was related with their total length using the Spearman's correlation to verify if larger and older cleaners (≥7 cm) cheat more than smaller and younger cleaners (< 7 cm).
The variation in the abundance of gnathiid isopods emerging amongst the three different sampling periods was examined by using a generalized linear model (GLM) with a Poisson distribution with logarithmic link function. The number of parasites was used as the dependent variable and the three sampling moments were used as a factor.
Also, the total length of the individuals of each client species and the number of ectoparasites was analyzed with GLM with the Poisson distribution and logarithmic link function. The number of gnathiids was used as the dependent variable, the length of the individuals was used as a covariate and the client species as a factor. Only the client species, in total 4 in this test, observed to be infested with gnathiids isopods were used. In all GLMs Wald X2 statistics were used.
All tests are two-tailed. Analyses were performed with IBM SPSS 22.
Results
Behavioral Observations
Out of a total of 20 focal cleaners, 30% maintained territories located in confined rocky depressions of variable size in which they were clearly seen expecting to receive visits from other fish clients, being thus, identified as cleaning stations. This contrasted with 40% of focal cleaners that were seeking, approaching and cleaning clients while roaming freely in a non-confined territory. The remaining 30% of cleaners engaged in cleaning interactions close to the rocky substratum where they remained for several minutes. However, because they were fast to leave the area during the observation time period, these territories couldn't be considered as cleaning stations.
From a total of 197 cleaner-client events, 89.34% were cleaning interactions (± SE = 8.80 ± 0.76 interactions per observation), whereas in 11.93% of these, cleaners approached the clients without touching them (described as inspection in Table 1). Cleaners were seen rejecting clients more often (total of 50 rejections) than clients rejecting cleaners (total of 10 rejections) (binomial test: p < 0.0001). Cleaners interacted with a total of 8 client species, most frequently with S. bailloni (Table 2).
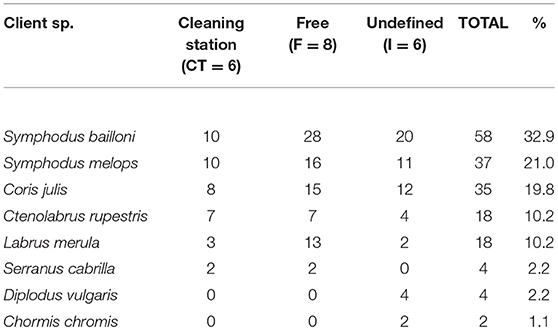
Table 2. Total number of cleaning interactions and respective percentage (%) performed by cleaners within a confined territory (CT), swimming freely (F), or engaging in cleaning interactions both roaming freely or stationary near the rocky substratum (I), per each client species.
Most cleaning interactions were initiated by clients (61.3% of total events) with these adopting a solicitation pose. On the other hand, most interactions were terminated by the cleaner fish (65.9% of total events). S. bailloni was the client species that initiated more interactions (40.7% of total interactions initiated by clients) but also the species with whom cleaners terminated interactions most often (39.6% of total interactions, see Table 3).
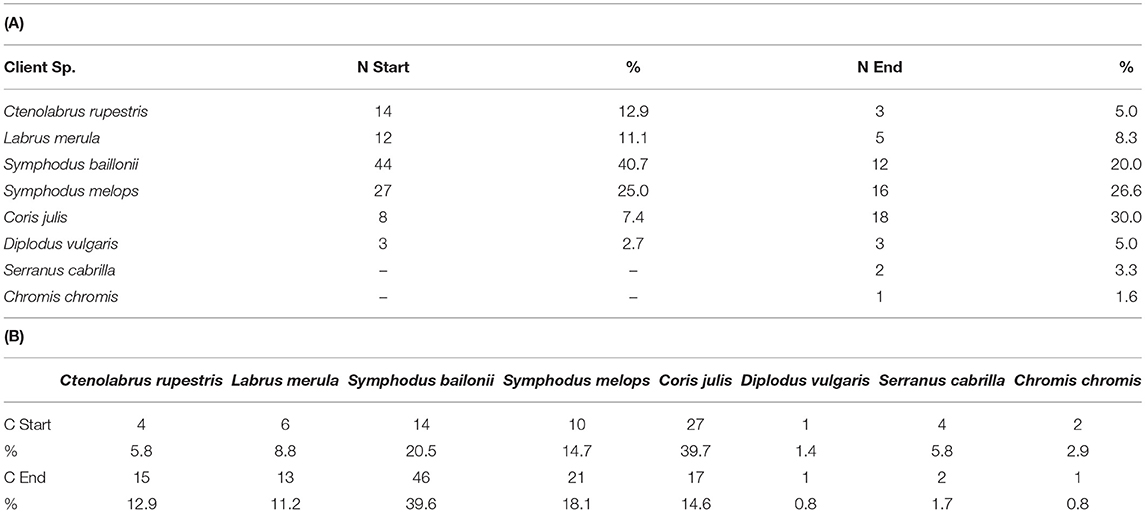
Table 3. (A) Number of interactions started (N Start) and terminated (N End) by each client species with respective percentage (%) and (B) number of interactions started (C start) and terminated (C End) by the cleaner species, Centrolabrus exoletus, with respective percentage (%).
S. melops individuals were observed to jolt more frequently (3.66 jolts.min−1). When comparing the duration of interactions for the five species reported to jolt, these showed a non-significant tendency that differed in relation to the occurrence of jolts [Wald X(1, 1) = 3.82, p = 0.05] and between species [Wald X(1, 4) = 9.29, p = 0.054], with interactions with client jolts lasting longer (Figure 1A) than those without jolts (Figure 1B). Time of interaction did not vary between client species. Punishment only occurred by 3 of the client species observed (Table 4).
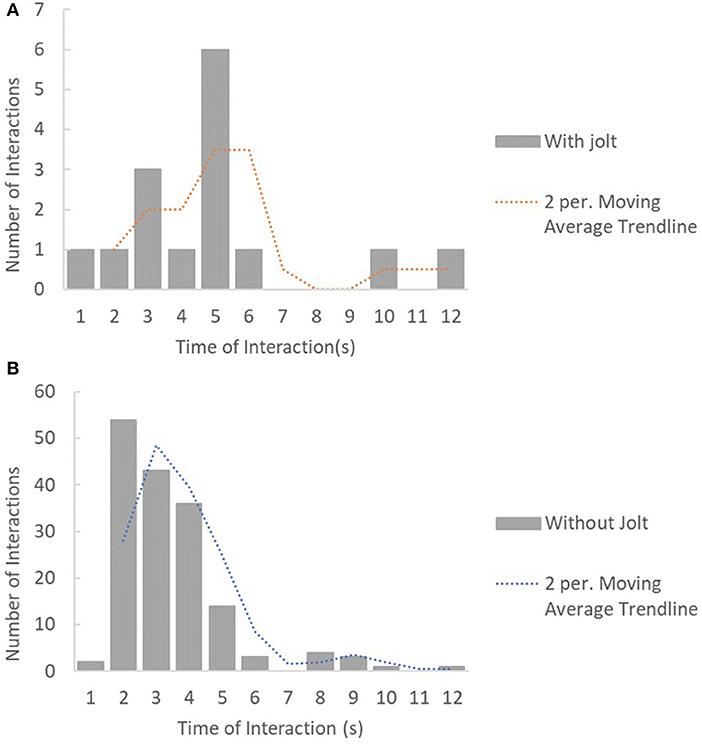
Figure 1. Number of interactions (A) with jolts per time of interaction and (B) without jolts per time of interaction.
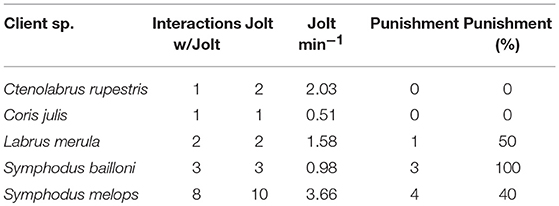
Table 4. Number of interactions with jolts, frequency of jolts, frequency of jolts per minute, total number of punishments and respective percentage.
Diet Composition
The most common item found inside cleaners' stomachs were gnathiid isopods, with a 100% occurrence (Figure 2B). However, the most abundant item was non-parasitic crustaceans with ± SE = 44.98% ± 1.14 (Figure 2A), followed by gnathiid isopods with ± SE = 37.80% ± 0.26 (Figure 2A). The presence of scales occurred in 60% of the stomach contents (Figure 2B) analyzed and had an abundance of 3.92% of total items (Figure 2A).
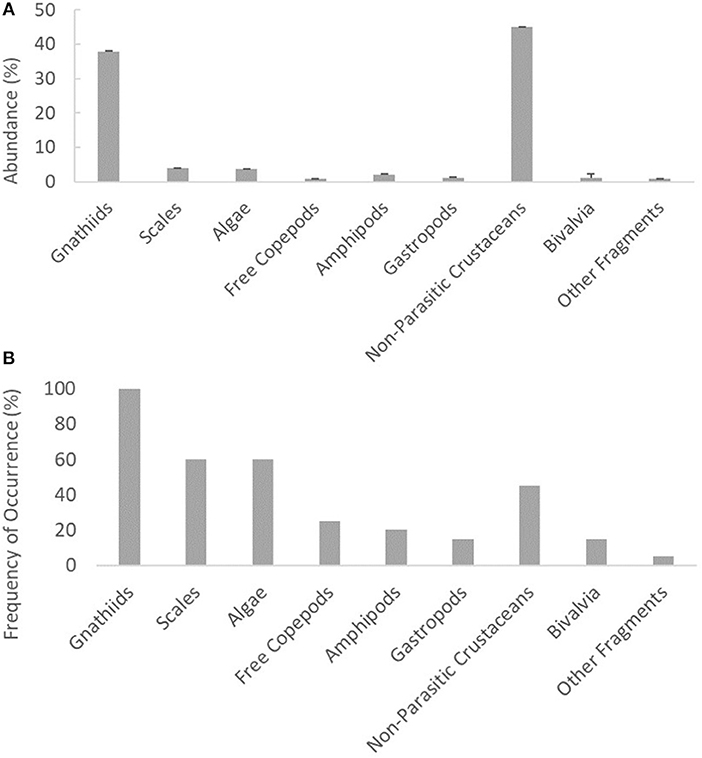
Figure 2. (A) Mean representation (±SE) of the abundance of each item in the cleaner's diet composition. (B) Frequency of occurrence of each item in the cleaner's diet composition.
The principal coordinate analysis (PCoA) (Figure 3) showed that gnathiid isopods and the non-parasitic crustaceans were also the most relevant items, explaining the variation in the cleaner's diet (S-Stress = 0.00702). Moreover, no relation was found between the number of gnathiid parasites and the cleaner's total length [r2 = 0.059, F (0, 1) = 1.138, p = 0.300]. Also, no correlation was found between a cleaner's ingestion of client-gleaned scales and a cleaner's total length (r = 0.346, N = 20, p = 0.135).
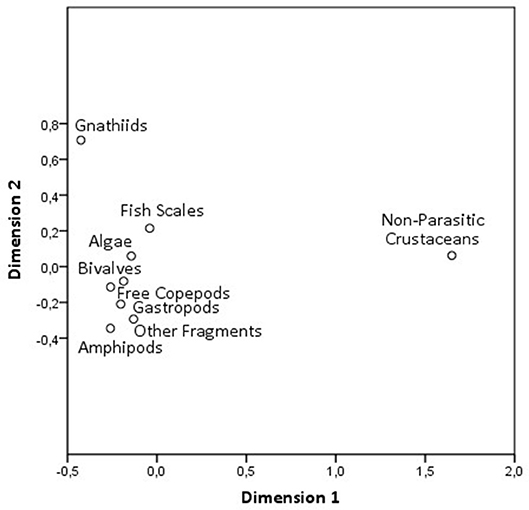
Figure 3. Distribution of all food items and other fragments (such as sand) represented in a two dimensions principal coordination analysis (PCoA), so that species depicted closer tend to co-occur more often.
Gnathiid Emergence Levels
All traps were found to have gnathiid parasites across all the five replicates. The mean number of gnathiid isopods emerging per area was ± SE = 11.53 ± 10.33 larvae.m−2 within a 24 h period. Gnathiid abundance varied significantly among the three sample periods [Wald X2 (1, 2) = 37.3, p < 0.0001, Figure 4]. The predominant classes of non-parasitic organisms found belonged to Malacostraca and Maxillopoda. However, organisms from the class Polychaeta and phylum Chaetognatha and phylum Chordata were also found.
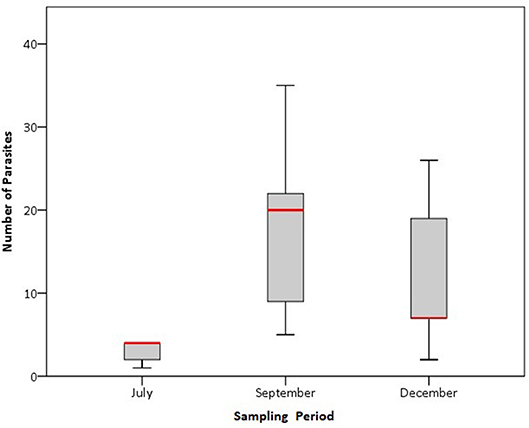
Figure 4. Number (±SE) number of gnathiid isopods found in the emergence traps during the three sampling periods (30 July, 30 September and 2 December). Boxes are medians with 25% and 75% quartiles and wiskers are 5% and 95% quartiles.
Ectoparasite Load
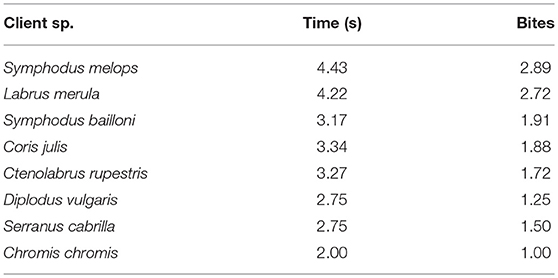
All captured individual clients were infested with gnathiid isopods, except D. vulgaris, which was found with caligidae copepods in just one individual. Significant differences were found between the number of ectoparasites and the client species [Wald X2 (1, 3) = 21.01, p < 0.0001] and between the total number of ectoparasites and client total length [Wald X2 (0, 1) = 4.25, p = 0.039]. Interestingly, the most parasitized client species (Figure 5) was also the one observed interacting with cleaners during longer cleaning bouts (S. melops: ± SE = 5.50 ± 0.93 gnathiids per individual; 4.43 s per interaction; 2.89 bites per interaction; see also Table 5).
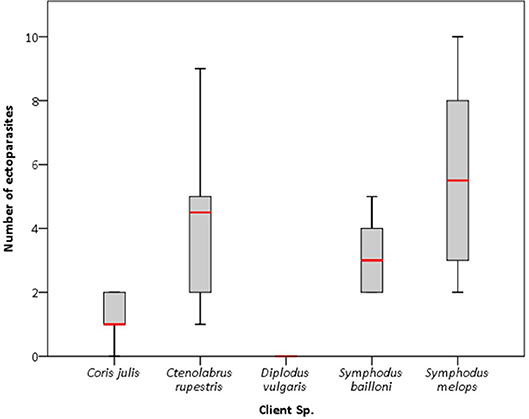
Figure 5. Frequency of ectoparasites found on the client's body by species. Boxes are medians with 25% and 75% quartiles and wiskers are 5% and 95% quartiles.
Discussion
Facultative cleaners are often underestimated when compared to the more notable obligatory tropical cleaners. However, recent studies have shown that these facultative cleaners may indeed be relevant drivers of parasite control while potentially contributing to the health of local fish communities (e.g., Narvaez et al., 2015).
Centrolabrus exoletus individuals were observed attending clients in cleaning stations opportunistically and near the rocky substratum. It remained undefined which strategy cleaners would apply preferentially: wandering around their territories in search of client-patches or having a more stationary cleaning approach. However, the existence of a fixed-territory choice may well be due to client availability and quantity, as well as local parasitic emergence and infestation levels. Overall, this suggests a non-client specificity for the cleaner when engaging in cleaning interactions, which is similar to findings in other facultative species (Narvaez et al., 2015) and to obligatory species (Vaughan et al., 2016). Moreover, the relatively high number of cleaning rejections by C. exoletus compared to those by clients (50 rejections from cleaners vs. 10 rejections from clients) could indicate that these cleaners exhibit preference for some client species. However, to C. exoletus, clients' infestation levels should be the most relevant factor.
Cleaning interactions were initiated more often by clients and terminated more often by cleaners suggesting that: (1) the elimination of parasites and the access to cleaners' physical contact (Soares et al., 2011) should be beneficial for the clients and (2) cleaners' cheating should be residual, with cleaners preferring to end the interaction when ectoparasite availability depletes. In fact, this parasite-based relationship between C. exoletus and clients was further demonstrated in the very meaning of client-jolts: contrary to prior findings (Bshary and Grutter, 2002; Soares et al., 2008a) in other (obligate) cleaner systems, in this study, interactions with client jolts tended to last longer than interactions in the absence of jolts. One possible explanation is that, in the C. exoletus system, client jolts occur in response to the actual parasite removal instead of being a response to cleaners' bites/dishonest behavior (cheating). Moreover, punishment events were rarely observed. All these facts combined point towards a higher value of these cleaners as control vectors of parasitism and potential drivers of community health and welfare.
Centrolabrus exoletus diet analysis confirmed that gnathiidae parasites are the most frequent organisms ingested, similar to several other cleaner fish systems (Vaughan et al., 2016). Gnathiids' occurrence frequency and relative abundance inside cleaners' stomach contents reveal not only a putative preference but a high dependence on these ectoparasites. Nevertheless, other non-parasitic crustaceans were also found along with fish gleaned-scales—the latter indicating that some instances of dishonest behavior could be occurring. The absence of negative responses by the clients (punishment), together with seemingly longer interaction bouts whenever jolts were observed, suggests that the ingestion of these scales could be a bi-product of parasite removal, or most probably, that the ingestion of scales is occurring when gnathiids are no longer available. However, contrary to the cleaning goby system, wherein the ingestion of non-parasitic food items by cleaners work as a signal for clients to leave, eliciting jolts as a way to terminate interactions (see Soares et al., 2008a, 2010) in the C. exoletus case, it is the cleaner that loses interest and terminates the interaction. This is yet another indication of cleaner-client synchrony when it comes to the purpose of these interactions: cleaners really aim to get access to parasites and clients get to really be cleaned.
Interestingly, C. exoletus focused on gnathiids corresponding to what was environmentally available: all the deployed emergency traps used in this study contained gnathiids. However, there was a reasonable level of variation between collections which should have implications to cleaners' food intake. These variations may be crucial for this species ontogenetic dependency on parasites. Surprisingly, cleaners' size (and hence development stage or age—Galeote and Otero, 1998) did not mean a significant dietary shift, as is usually predicted for facultative cleaners. Instead, adult cleaners' diets did not differ from the juveniles; that is, the number of gnathiids and the number of scales ingested showed no relation with the cleaner's total length. Therefore, and in opposition to many other facultative cleaners (Ayling and Grace, 1971; Zander and Sötje, 2002; Narvaez et al., 2015), adult C. exoletus continue to rely on cleaning interactions to obtain food.
Client length was a good predictor of gnathiid infestation on client species as similarly reported in previous studies (Grutter, 1994; Poulin, 2000). Accordingly, it was the most parasitized client species (S. melops) that was observed to spend more time interacting with and receiving more bites from C. exoletus cleaners. On the other hand, caligid copepods were not found in these cleaners' diets and they were hardly accounted for on clients: indeed, out of the 5 client species captured, these copepods were only found on one D. vulgaris individual. While this residual trend could be due to the absence of more pelagic species (in our sample), which could perhaps be more susceptible to being infested by caligids (Costello, 2006), it could simply be a question of lower abundance when compared with gnathiids.
In conclusion, this study provides key evidence on the potential benefits arising from the mutualistic relationship between our focus cleaner species, C. exoletus, and its clientele. We confirm that both cleaner and client exchange benefits during cleaning interactions: the cleaners feed on the items present on the client's body surface and clients enhance their health through the removal of parasites and dead body tissue. The level of synchrony found between cleaners and clients is coherently demonstrated through the cleaners' aim to forage on gnathiids which are environmentally and client-prevalent to the point of requiring a re-interpretation of the meaning of client jolts, which seem to be further evidence of the cleaner's good service quality (Soares et al., 2008a). The potential implications of such a key contributor to the overall fish community are significant and should increase the overall interest regarding the role of facultative cleaners; particularly in locations where these are the sole cleaning-contributors but also in sites where other facultative or obligate cleaners exist (Sazima et al., 2000; Walsh et al., 2017; Quimbayo et al., 2018). The understanding of this cleaner species' behavioral ecology, its dietary coherence (both juveniles and adults engage in cleaning interactions), the lack of client specificity, its parasite-based foraging aims and residual cheating underlie a tremendous potential that is could be used to control parasitic infestations biologically, in fish farms. This study sets the stage for future research aiming, for instance, at cleaners' specific preferences and how these may vary seasonally, but more importantly at finding the health implications (physiology) for clients.
Compliance with Ethical Standards
The study design was approved by (1) the Port Captain of Portimão, Rui Gabriel Martins Santos Pereira, who authorized all behavioral observations, fish collections and the deployment of parasite traps along the Algarve coast, (2) Dr. João Granado Granjo Pires Quintela from University of Algarve, who authorized the use of LEOA—Laboratório Experimental para Organismos Aquáticos facilities and materials to perform the gut content analysis and (3) Maria Margarida Falcão Pinto Almeida from Poeta António Aleixo's High School, who authorized the use of the facilities and materials to identify the ectoparasites found on the clients species and in the parasite emergence traps.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
MCS and NM conceived the work. NM collected and analyzed field data. NM, MCS, and PM wrote the manuscript.
Funding
MCS is currently supported by National Funds through FCT-Foundation for Science and Technlogy. Research on cooperative fish was mostly supported by the Portuguese Foundation for Science and Technology-FCT (grant PTDC/MAR/105276/2008), awarded to MCS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special thanks are due to our scuba dive buddy, Francisco Mesquita, that was always available to accompany and help us during all the field work. Teresa Santos and Pauline Narvaez for providing the emergence traps.
References
Ayling, A. M., and Grace, R. V. (1971). Cleaning symbiosis among New Zealand fishes. N. Z. J. Mar. Freshwater. Res. 5, 205–218. doi: 10.1080/00288330.1971.9515377
Bjordal, A. (1992). “Cleaning symbiosis as an alternative to chemical control of sea lice infestation of Atlantic salmon” in The Importance of Feeding Behaviour for the Efficient Culture of Salmonid Fishes, eds J. E. Thorpe and F. A. Huntingford (Baton Rouge: World Aquaculture Workshops, No. 4, World Aquaculture Society), 53–60.
Bshary, R., and Grutter, A. S. (2002). Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav. 63, 547–555. doi: 10.1006/anbe.2001.1937
Bshary, R., and Grutter, A. S. (2005). Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol. Lett. 1, 396–399. doi: 10.1098/rsbl.2005.0344
Côté, I. M. (2000). Evolution and ecology of cleaning symbioses in the sea. Oceanogr. Mar. Biol. 38, 311–355.
Côté, I. M., Arnal, C., and Reynolds, J. D. (1998). Variation in posing behaviour among fish species visiting cleaning stations. J. Fish Biol. 53, 256–266. doi: 10.1111/j.1095-8649.1998.tb01031.x
Côté, I. M., and Soares, M. C. (2011). “Gobies as cleaners,” in The Biology of Gobies, eds R. Patzner, J. L. Van Tassell, M. Kovacic, B. G. Kapoor (Boca Raton: Science Publishers St. Helier), 525. doi: 10.1201/b11397-28
Chambers, S. D., and Sikkel, P. C. (2002). Diel emergence patterns of ecologically important, fish-parasitic, gnathiid isopod larvae on Caribbean coral reefs. Caribb. J. Sci. 38, 37–43. doi: 10.1007/s12526-017-0756-6
Coile, A. M., and Sikkel, P. C. (2013). An experimental field test of susceptibility to ectoparasitic gnathiid isopods among Caribbean reef fishes. Parasitology 140, 888–896. doi: 10.1017/S0031182013000097
Costello, M. J. (1993). “Review of methods to control sea lice (Caligidae: Crustacea) infestations on salmon (Salmo salar) farms,” in Pathogens of Wild and Farmed, eds G. A. Boxshall and D. Defaye (Chichester: Ellis Horwood Ltd.), 219–252.
Costello, M. J. (2006). Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 22, 475–483. doi: 10.1016/j.pt.2006.08.006
Costello, M. J., and Bjordal, A. (1990). How good is this natural control on sea-lice? Fish Farmer 13, 44–46.
Ebran, N., Julien, S., Orange, N., Saglio, P., Lemaitre, C., and Molle, G. (1999). Pore-forming properties and antibacterial activity of proteins extracted from epidermal mucus of fish. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 122, 181–189. doi: 10.1016/S1095-6433(98)10165-4
Galeote, M. D., and Otero, J. G. (1998). Cleaning behaviour of rock cook, Centrolabrus exoletus (Labridae), in Tarifa (Gibraltar Strait area) Cybium 22, 57–68.
Garnick, E., and Margolis, L. (1990). Influence of four species of helminth parasites on orientation of seaward migrating sockeye salmon (Oncorhynchus nerka) smolts. Can. J. Fish Aquat. Sci. 47, 2380–2389. doi: 10.1139/f90-265
Gonçalves, J. M. S., Monteiro, P., Coelho, R., Afonso, C., Almeida, C., Veiga, P., et al. (2007,2008,2010). Cartografia e caracterização das biocenoses marinhas da Reserva Ecológica Nacional Submarina entre a barra nova do Ancão e a Ponta da Piedade. Faro: Relatórios Finais; CCR Algarve e ARH Algarve; Universidade do Algarve; CCMAR.
Grutter, A. S. (1994). Spatial and temporal variations of the ectoparasites of seven reef fish species from Lizard Island and Heron Island, Australia. Mar. Ecol. Prog. Ser. 115, 21–30. doi: 10.3354/meps115021
Grutter, A. S. (1996). Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Mar. Ecol. Prog. Ser. 130, 61–70. doi: 10.3354/meps130061
Grutter, A. S. (2002). Cleaning symbioses from the parasites' perspective. Parasitology 124, 65–81. doi: 10.1017/S0031182002001488
Grutter, A. S., Lester, R. J., and Greenwood, J. (2000). Emergence rates from the benthos of the parasitic juveniles of gnathiid isopods. Mar. Ecol. Prog. Ser. 207, 123–127. doi: 10.3354/meps207123
Grutter, A. S., Murphy, J. M., and Choat, J. H. (2003). Cleaner fish drives local fish diversity on coral reefs. Curr. Biol. 13, 64–67. doi: 10.1016/S0960-9822(02)01393-3
Grutter, A. S., and Poulin, R. (1998). Intraspecific and interspecific relationships between host size and the abundance of parasitic larval gnathiid isopods on coral reef fishes. Mar. Ecol. Prog. Ser. 164, 263–271. doi: 10.3354/meps164263
Henriques, M., and Almada, V. C. (1997). Relative importance of cleaning behaviour in Centrolabrus exoletus and other wrasse at Arrábida, Portugal. J. Mar. Biol. Assoc. UK 77, 891–898. doi: 10.1017/S0025315400036249
Johnson, S. C., Bravo, S., Nagasawa, K., Kabata, Z., Hwang, J. S., Ho, J. S., et al. (2004). A review of the impact of parasitic copepods on marine aquaculture. Zool. Stud. 43, 229–243. Available online at: http://zoolstud.sinica.edu.tw/Journals/43.2/229.pdf
Kabata, Z. (2003). Copepods Parasitic on Fishes. Key and Notes for the Identification of British Species. Oegstgeest: Synopses of the British fauna (New series), 274.
Limbaugh, C. (1961). Cleaning symbiosis. Sci. Am. 205, 42–49. doi: 10.1038/scientificamerican0861-42
Losey, G. S., Grutter, A. S., Rosenquist, G., Mahon, J. L., and Zamzow, J. (1999). “Cleaning symbiosis: a review” in Behaviour and Conservation of Littoral Fishes, eds V. C.Almada, R. F. Oliveira, and E. J. Goncalves (Lisboa, Portugal: ISPA), 379–395.
Lythgoe, J. N., and Lythgoe, G. I. (1991). Fishes of the sea: the North Atlantic and Mediterranean. Cambridge: Blandford Press, 320.
Narvaez, P., Furtado, M., Neto, A. I., Moniz, I., Azevedo, J. M., and Soares, M. C. (2015). Temperate facultative cleaner wrasses selectively remove ectoparasites from their client-fish in the Azores. Mar. Ecol. Prog. Ser. 540, 217–226. doi: 10.3354/meps11522
Potts, G. W. (1968). The ethology of Crenilabrus melanocercus, with notes on cleaning symbiosis. J. Mar. Biol. Assoc. UK 48, 279–293. doi: 10.1017/S0025315400034482
Potts, G. W. (1973). Cleaning symbiosis among British fish with special reference to Crenilabrus melops (Labridae) J. Mar. Biol. Assoc. UK 53, 1–10. doi: 10.1017/S0025315400056587
Poulin, R. (2000). Variation in the intraspecific relationship between fish length and intensity of parasitic infection: biological and statistical causes. J. Fish Biol. 56,123–137. doi: 10.1111/j.1095-8649.2000.tb02090.x
Quimbayo, J. P., Schlickmann, O. R. C., and Sazima, I. (2018). Cleaning interactions ate the southern limit of tropical reef fishes in the Western Atlantic. Environ. Biol. Fish 101,1195–1204. doi: 10.1007/s10641-018-0768-5
Randall, J. E., Allen, G. R., and Steene, R. C. (1990). Fishes of the Great Barrier Reef and Coral Sea. Honolulu, HI: University of Hawaii Press
Saldanha, L. (1995). Fauna Submarina Atlântica. Portugal Continental, Açores, Madeira. Lisboa: Publicações Europa-América, 364.
Santos, M. N., Erzini, K., Díaz, A., and Manzano, C.(eds.). (2007). “Catálogo de Espécies de Peixes de Interesse Comercial da Costa sul Atlântica da Península Ibérica,” eds I. I. Projecto Getpesca and I. Manual (Seville: Junta de Andalucia).
Sazima, I., Sazima, C., Francini-Filho, R. B., and Moura, R. L. (2000). Daily cleaning activity and diversity of clientes of the barber goby, Elecatinus figaro, on rocky reefs in southeastern Brazil. Environ. Biol. Fish 59, 69–77. doi: 10.1023/A:1007655819374
Seppälä, O., Karvonen, A., and Valtonen, E. T. (2004). Parasite-induced change in host behaviour and susceptibility to predation in an eye fluke–fish interaction. Anim. Behav. 68, 257–263. doi: 10.1016/j.anbehav.2003.10.021
Shaw, J. C., Korzan, W. J., Carpenter, R. E., Kuris, A. M., Lafferty, K. D., Summers, C. H., et al. (2009). Parasite manipulation of brain monoamines in California killifish (Fundulus parvipinnis) by the trematode Euhaplorchis californiensis. Proc. R. Soc. Lond. B. Biol. Sci. 276, 1137–1146. doi: 10.1098/rspb.2008.1597
Sikkel, P. C., Cheney, K. L., and Côté, I. M. (2004). In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Anim. Behav. 68, 241–247. doi: 10.1016/j.anbehav.2003.10.023
Sikkel, P. C., Schaumburg, C. S., and Mathenia, J. K. (2006). Diel infestation dynamics of gnathiid isopod larvae parasitic on Caribbean reef fish. Coral Reefs. 25, 683–689. doi: 10.1007/s00338-006-0154-1
Smit, N. J., and Davies, A. J. (2004). The curious life-style of the parasitic stages of gnathiid isopods. Adv. Parasitol. 58, 289–391. doi: 10.1016/S0065-308X(04)58005-3
Soares, M. C., Bshary, R., Cardoso, S. C., and Côté, I. M. (2008a). The meaning of jolts by fish clients of cleaning gobies. Ethology 114, 209–214. doi: 10.1111/j.1439-0310.2007.01471.x
Soares, M. C., Bshary, R., Cardoso, S. C., and Côté, I. M. (2008b). Does competition for clients increase service quality in cleaning gobies? Etholology 114, 625–632. doi: 10.1111/j.1439-0310.2008.01510.x
Soares, M. C., Côté, I. M., Cardoso, S. C., and Bshary, R. (2008c). The cleaning goby mutualism: a system without punishment, partner switching or tactile stimulation. J. Zool. 276, 306–312. doi: 10.1111/j.1469-7998.2008.00489.x
Soares, M. C., Côté, I. M., Cardoso, S. C., Oliveira, R. F., and Bshary, R. (2010). Caribbean cleaning gobies prefer client ectoparasites over mucus. Ethology 116, 1244–1248. doi: 10.1111/j.1439-0310.2010.01838.x
Soares, M. C., Cardoso, S. C., and Côté, I. M. (2007). Client preferences by Caribbean cleaning gobies: food, safety or something else? Behav. Ecol. Sociobiol. 61, 1015–1022. doi: 10.1007/s00265-006-0334-6
Soares, M. C., Oliveira, R. F., Ros, A. F., Grutter, A. S., and Bshary, R. (2011). Tactile stimulation lowers stress in fish. Nat. Comm. 2:534. doi: 10.1038/ncomms1547
Tully, O., Daly, P., Lysaght, S., Deady, S., and Varian, S. J. A. (1995). Use of cleaner-wrasse (Centrolabrus exoletus (L.). and Ctenolabrus rupestris (L.)). to control infestations of Caligus elongatus Nordmann on farmed Atlantic salmon. Aquaculture 142, 11–24. doi: 10.1016/0044-8486(95)01245-1
Vaughan, D. B., Grutter, A. S., Costello, M. J., and Hutson, K. S. (2016). Cleaner fishes and shrimp diversity and a re-evaluation of cleaning symbioses. Fish Fish. 18, 698–716. doi: 10.1111/faf.12198
Waldie, P. A., Blomberg, S. P., Cheney, K. L., Goldizen, A. W., and Grutter, A. S. (2011). Long-term effects of the cleaner fish Labroides dimidiatus on coral reef fish communities. PLoS One 6:e21201. doi: 10.1371/journal.pone.0021201
Walsh, C. A. J., Pinheiro, H. T., Rocha, L. A., and Goodbody-Gringley, G. (2017). Cleaning service gaps in Bermuda, North Atlantic. Ecology 98, 1973–1974. doi: 10.1002/ecy.1841
Keywords: temperate regions, cleaning mutualisms, facultative cleaner fish, Centrolabrus exoletus, ectoparasites, gnathiidae
Citation: Morado N, Mota PG and Soares MC (2019) The Rock Cook Wrasse Centrolabrus exoletus Aims to Clean. Front. Ecol. Evol. 7:182. doi: 10.3389/fevo.2019.00182
Received: 13 February 2019; Accepted: 06 May 2019;
Published: 31 May 2019.
Edited by:
Kevin R. Theis, Wayne State University, United StatesReviewed by:
Vikram B. Baliga, University of British Columbia, CanadaJuan Pablo Quimbayo, Center for Marine Biology, University of São Paulo, Brazil
Copyright © 2019 Morado, Mota and Soares. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadia Morado, bmFkc2t5LjkmI3gwMDA0MDtnbWFpbC5jb20=; Marta C. Soares, bWFydGEuc29hcmVzJiN4MDAwNDA7Y2liaW8udXAucHQ=
 Nadia Morado
Nadia Morado Paulo G. Mota1,2
Paulo G. Mota1,2 Marta C. Soares
Marta C. Soares