- 1Monarch Joint Venture, Department of Fisheries, Wildlife, and Conservation Biology, University of Minnesota, St. Paul, MN, United States
- 2U.S. Fish and Wildlife Service, Inventory and Monitoring, National Wildlife Refuge System, Fort Collins, CO, United States
- 3Department Integrative Biology, Oklahoma State University, Stillwater, OK, United States
- 4U.S. Fish and Wildlife Service, Neal Smith National Wildlife Refuge, Prairie City, IA, United States
- 5U.S. Fish and Wildlife Service, Bloomington, MN, United States
- 6U.S. Geological Survey, Great Lakes Science Center, Chesterton, IN, United States
- 7Iowa Department of Natural Resources, Des Moines, IA, United States
- 8U.S. Fish and Wildlife Service, Ecological Services, Tucson, AZ, United States
- 9U.S. Geological Survey, Upper Midwest Environmental Sciences Center, La Crosse, WI, United States
- 10University of Wisconsin-Madison Arboretum, University of Wisconsin, Madison, WI, United States
Steep declines in North American monarch butterfly (Danaus plexippus) populations have prompted continent-wide conservation efforts. While monarch monitoring efforts have existed for years, we lack a comprehensive approach to monitoring population vital rates integrated with habitat quality to inform adaptive management and effective conservation strategies. Building a geographically and ecologically representative dataset of monarchs and their habitat will improve these efforts. These data will help track long-term changes in the distribution and abundance of monarchs and their habitats, refine population and habitat models, and illuminate how conservation activities affect monarchs and their habitats. The Monarch Conservation Science Partnership developed the Integrated Monarch Monitoring Program (IMMP) to profile breeding habitats and their use by monarchs in North America. A spatially balanced random sampling framework guides site selection, while also allowing opportunistic inclusion of sites chosen by participants, such as conservation areas. The IMMP weaves new protocols together with those from existing monitoring programs to improve data compatibility for assessing milkweed (Asclepias spp.) density, nectar resources, monarch reproduction and survival, and adult monarch habitat use. Participants may select a protocol subset according to interests or local monitoring objectives, thereby maximizing contributions. Conservation partners, including public and private land managers, academic researchers, and citizen scientists contribute data to a national dataset available for analyses at multiple scales. We describe the program and its development, implementation elements that make the program robust and feasible, participation to date, and how IMMP data can advance research and conservation for monarchs, pollinators, and their habitats.
Purpose and Rationale
Monarch butterflies (Danaus plexippus) exhibit one of the most spectacular animal migrations (Urquhart, 1976; Brower, 1977). East of the Rocky Mountains in North America, monarchs migrate up to 4,500 km each fall to overwinter in high-altitude fir forests in central Mexico; west of the Rockies, monarchs overwinter in groves along the California coast. In spring, monarchs return to their breeding grounds; several generations move and breed across most of North America throughout the summer. Migrating and breeding butterflies rely on nectar sources for food; to reproduce monarchs depend solely on larval host plants in the milkweed subfamily (primarily Asclepias spp.).
Like many pollinator species (Biesmeijer et al., 2006; Potts et al., 2010; Powney et al., 2019), North American monarch populations have declined over the past two decades (Brower et al., 2012; Vidal and Rendón-Salinas, 2014; Semmens et al., 2016; Schultz et al., 2017), motivating range-wide conservation efforts. Breeding range conservation has focused on enhancing milkweed and nectar availability, as reduction of these resources is implicated in monarch population declines (Pleasants and Oberhauser, 2013; Pleasants, 2017; Thogmartin et al., 2017a; Zaya et al., 2017; Malcolm, 2018; Stenoien et al., 2018). Conservation efforts are driven by population targets, e.g., those in the national pollinator strategy (Pollinator Health Task Force, 2015) and related national habitat goals (Thogmartin et al., 2017b).
Monarch conservation goals are generally based on models of monarch population viability (Semmens et al., 2016; Schultz et al., 2017), geographic prioritization (Flockhart et al., 2015; Oberhauser et al., 2017), threats (Saunders et al., 2017; Thogmartin et al., 2017a), and habitat (Thogmartin et al., 2017b) developed using limited datasets and expert opinion. While some studies have examined breeding habitat use (e.g., Stenoien et al., 2015; Kasten et al., 2016; Pitman et al., 2018; Kantola et al., 2019), they are limited in scope and geography. Citizen science program (e.g., Journey North1, Monarch Watch2, Monarch Larva Monitoring Project (MLMP)3) data have been instrumental to modeling efforts and expanding general knowledge of monarchs (Oberhauser et al., 2015; Ries and Oberhauser, 2015; Tracy et al., 2019), but are often concentrated near population centers and lack geographical balance (Bird et al., 2014; Nail et al., 2015). Furthermore, use of largely self-selected monitoring locations that often contain high-quality habitat (e.g., butterfly gardens or butterfly monitoring sites) prevents robust statistical inference about average conditions or extrapolation to other land-use types (Bird et al., 2014). Lastly, many programs record monarch locations opportunistically, without measured and repeated effort, making it difficult to identify long-term trends. A monitoring scheme that overcomes these limitations is needed (National Research Council, 2007) to accurately track progress toward habitat and population goals, identify habitat deficiencies, and assess the success of conservation actions.
The Monarch Conservation Science Partnership (MCSP), a collaborative group of scientists addressing information gaps in monarch conservation and ecology, developed an integrated strategy for monitoring conservation progress, starting with the end goal and working backward to determine the details (Thogmartin et al., 2015; Reynolds et al., 2016). Through review of existing programs (Oberhauser et al., 2009), 3 years of design meetings, and pilot testing, the strategy became the Integrated Monarch Monitoring Program (IMMP). The IMMP collects geographically and ecologically representative data using a stratified randomized sampling framework. Data from conservation sites, such as private lands enrolled in Farm Bill conservation programs, are included to provide insight into the effectiveness of management actions. The sampling framework optimizes statistical robustness while minimizing the number of samples needed by prioritizing sites where collecting information will be most valuable.
The IMMP has three primary objectives: to (1) track long-term changes in the distribution and abundance of monarchs and their habitats (2) provide geographically and ecologically representative information to fill data gaps and update current population and habitat models, and (3) acquire information about how habitat conservation actions affect monarchs and their habitat. Metrics include milkweed density, indices of blooming plant abundance, adult monarch abundance, egg and larval abundance, egg and larval survival estimates, and fire ant occurrence.
Below, we highlight design elements of the newly implemented IMMP that make it robust, efficient, and feasible for large-scale, multi-partner data collection and use. We discuss the benefits of the program to researchers, land managers, and citizen scientists, as well as the benefit of compatible and representative long-term data generated over a broad geography.
Spatially Balanced Random Sampling
A key IMMP element is its proactive sampling design that obtains geographically and ecologically representative data throughout the monarch's breeding range. Geographically distributed data allow evaluation of how monarch habitat and its use vary across ecoregions, latitude, elevation, and climatic conditions. Ecologically representative sampling emphasizes all habitats that may be suitable for monarchs rather than just easily accessible sites or known habitat locations (Bird et al., 2014).
To establish representative sampling locations for the IMMP, we used a Generalized Random Tessellation Stratified (GRTS) sampling design (Stevens and Olsen, 1999, 2003, 2004). GRTS provides a spatially balanced set of sample units with a randomized component for unbiased representation and can represent multiple strata to reduce variability of parameter estimates. GRTS produces a hierarchical sample list such that for any sample size or geographic subset, the sample will be spatially balanced if the sample list is followed in order (Loeb et al., 2015).
We applied GRTS to rank, and thereby prioritize for sampling, each 10 × 10-km cell within a grid of cells (“blocks” hereafter) superimposed over the contiguous United States (Figure 1). Within each block, a second-stage GRTS draw ranked points for unbiased plot location within each sampling stratum in each block. For the eastern population, the strata comprise five land types associated with milkweed: agriculture, protected grassland-shrubland, unclassified grassland-shrubland, rights-of-way, and developed areas (Thogmartin et al., 2017b). In the west, a model of habitat suitability for milkweed was built from environmental variables (Dilts and Forister, 2017), so western strata are high, medium, and low expected suitability for milkweed.
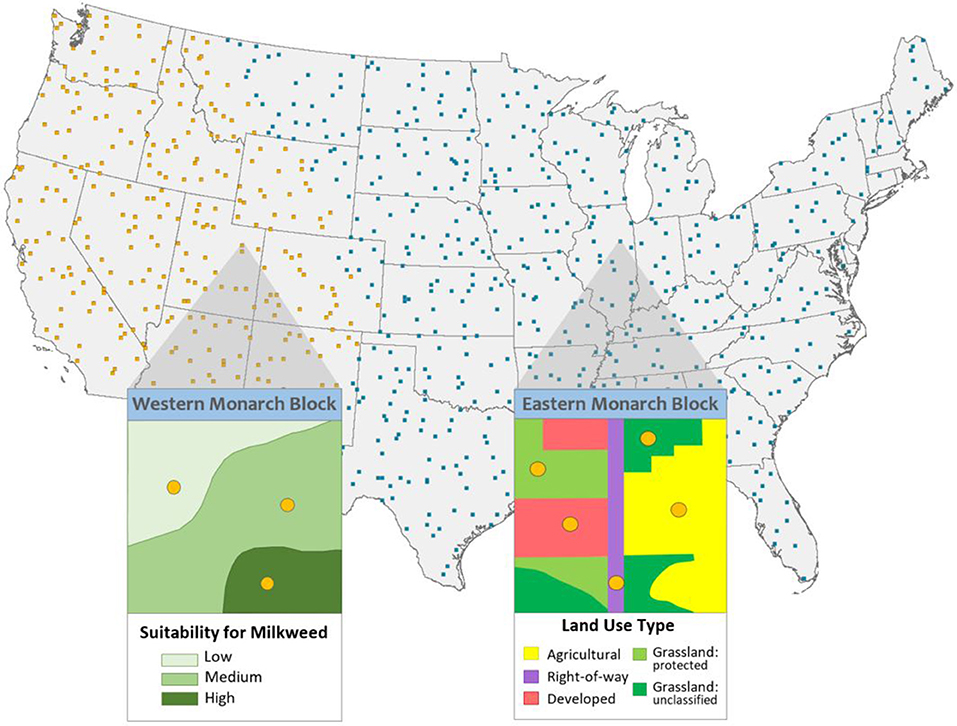
Figure 1. Top 500 randomized [Generalized Random Tessellation Stratified (GRTS)] 10-km by 10-km sampling cells (‘monarch blocks') for the western North American population of monarch butterflies (in yellow) and the eastern population (in blue). Inset 1: an example monarch block in the range of the western population, showing three strata, areas modeled as low-, medium-, and high-suitability for milkweed, per the western milkweed habitat suitability project (Dilts and Forister, 2017). Inset 2: an example monarch block from the eastern population depicting top random site locations for each of the five major land-use strata for sampling (Thogmartin et al., 2017b).
GRTS can also incorporate data from non-random locations, such as legacy or volunteer-selected sites (Overton and Stehman, 1993; Olsen et al., 1999). Non-random sites may not represent the full landscape (Williams et al., 2001; Kinkead et al., in review), but can provide data from spatially rare land-use types that are poorly represented in the random draw but might be of particular interest (e.g., state parks, Conservation Reserve Program). During analysis, data from non-random sites can be down-weighted to reduce bias while improving statistical power for the entire dataset (Austin, 2011).
Data Collection
Integrated Monarch Monitoring Program (IMMP) surveys are modular; data are valuable regardless of whether all protocols are completed at each site. Participants collect data relevant to their interests using Survey123 (data survey application, Esri, Redlands, California) on a mobile device or paper with online data entry. Below, we give a brief overview of the primary field surveys; a complete guide (Monarch Joint Venture, 2019) is posted on the IMMP webpage4. A U.S. Fish and Wildlife Service (USFWS) protocol will provide full documentation of the purpose, rationale, and monitoring procedures from design to reporting, to be posted in ServCat5 (USFWS information repository).
Plot Description
A site is sampled by a 1-hectare (ha) rectangle (200 × 50 m) or square (100 × 100 m) monitoring plot originating from a random starting point (Figure 2A). Longer, narrower plots, (400–500 m long) are used in linear areas (e.g., rights-of-way); alternative configurations fit irregularly shaped sites. Consistency in monitoring plot size reduces variation from area effects and differential effort. Participants collect data regarding site characteristics and management practices in consultation with project managers and/or landowners. Continuity in monitoring sites across years is preferred for trend detection, but shorter-term inventories can inform regional and sector comparisons.
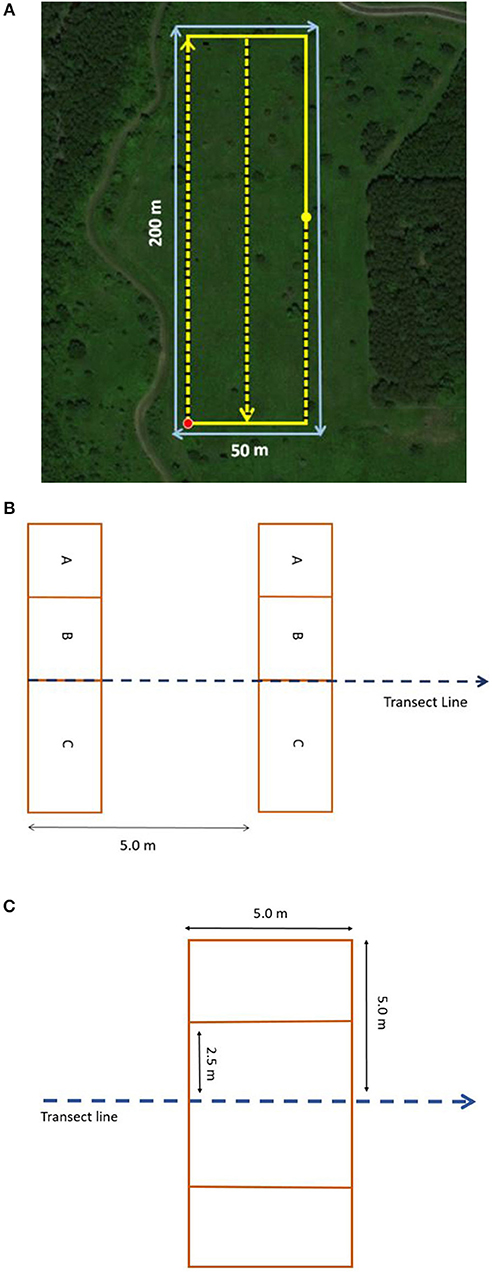
Figure 2. (A) Standard 1-ha sampling plot layout, anchored at random sampling point (red dot), 200 m by 50 m. Plots are defined with an ArcGIS Online mapping tool to minimize bias derived from habitat conditions encountered in the field. From a random point, we anchor a rectangle within patch of particular land-use type (must include <10% non-target land-use type). If this does not fit, we rotate the plot clockwise, shift the rectangle while still encompassing the point, use a square, or delineate an irregular shape to fit within the patch (in decreasing order of preference). For non-random sites, plots are centered within the field or management unit of interest, following the same guidelines. Within plots, nectar plants and milkweed are surveyed along two 200-m transects and one 100-m transect (indicated by yellow lines with black hash marks), and adult monarch surveys are conducted around the plot perimeter (blue solid line). (B) Rectangular quadrats are placed first to the left and then to the right of transects, every 5 m, for a total of 100 quadrats per plot. (C) To count adult monarchs, surveyors move along the perimeter of the rectangle, using a modified Pollard walk with a moving data recording window of 5 m on both sides.
Milkweed and Blooming Plant Survey
Participants survey milkweed and blooming plants in 100 quadrats placed every 5 m along transects (0.5 × 1-m frames are placed to each side of transects equaling 1-m2 area per quadrat). Transects run the length of the plot, 25-m apart (Figure 2B), with variation for small, linear, and irregularly shaped sites. Three nested sections within quadrats aid in frequency sampling (Elzinga et al., 1998). To estimate milkweed density, milkweed plants and stems are counted by species within quadrats. To generate an index of nectar availability, all blooming plants are either identified or their presence simply noted and assigned to the smallest quadrat section in which they occur to generate frequency scores (when species are identified, richness and diversity are also calculated). Additional blooming species are recorded during a meandering walk through the plot (following Szigeti et al., 2016a). Surveys average 2.5 h; the recommended interval is monthly during the season of monarch use.
Egg and Larva Survey
Egg and larva data are used to examine how immature monarch densities (monarchs per plant) vary spatially, within seasons, and among years. Surveyors examine up to 100 milkweed plants within the monitoring plot, recording the number of monarch eggs and larvae per milkweed plant observed, and identifying larvae to stadium (instar) using visual cues. To representatively sample (and account for aggregations; Zalucki and Kitching, 1982; Pitman et al., 2018), surveyors search milkweed within quadrats. If milkweed is sparse, surveyors also search within 1 m of transects. If milkweed is abundant, only every second, third or fifth plant is searched. This protocol was adapted from the MLMP, allowing data to be combined for analysis. Survey time averages 1 h, and weekly surveys are recommended.
Adult Monarch Survey
Adult monarch surveys provide data on the abundance and phenology of monarchs throughout breeding and migration periods. Participants conduct a modified Pollard walk (Pollard, 1977), counting adult monarchs within 5 m on each side of a 500-m transect (Figure 2C) and documenting monarch behaviors (e.g., nectaring and associated plant species). Surveys produce a time-specific index of adult monarch abundance (number/ha) compatible with existing butterfly monitoring programs (e.g., North American Butterfly Monitoring Network6). Surveys average 25 min to complete; bi-weekly surveys are recommended during the season of monarch use. Nectar plant selection can be quantified by combining nectaring adult data and the relative abundance of nectar plant species at the same site and date (Manly et al., 2002).
Survival and Parasitism
To estimate larval survival and measure spatiotemporal variation in mortality, participants collect fourth or fifth instar larvae from just outside the monitoring plots and rear them indoors to track outcomes (e.g., adult monarch, parasitism by tachinid fly, mortality due to other causes). Before releasing the newly emerged monarchs, participants use a sticker to screen for a protozoan parasite, Ophryocystis elektroscirrha; stickers are sent to Project Monarch Health (PMH)7. While daily monitoring of known cohorts would provide more complete survival data, rearing late instars with ample exposure to disease, parasites, and parasitoids provides a broad-scale relative index of larval outcomes across time, regions, dates, and land use types. Rearing and parasite testing protocols were adapted from the MLMP and PMH, yielding compatible data sets.
Pilot Testing and Protocol Refinement
Field testing and protocol refinement spanned 2016–2018, on 97 sites surveyed by USFWS technicians, 82 by Monarch Joint Venture (MJV) staff, 60 by University of Wisconsin technicians, and 127 by MJV-trained volunteers. During 2017–2018, the MJV trained 171 citizen scientists, biologists, and conservation staff representing 25 organizations.
A power analysis was conducted on 2016 and 2017 data to estimate the sampling effort needed to detect trends in densities of milkweed, eggs, and adult monarchs and to compare densities across strata or regions (insufficient pilot nectar data were available; Weiser et al, in revision). The consequences of survey frequency (surveys per year), numbers of quadrats for milkweed, and the number of sites and years were examined. Based on limited pilot data, the numbers of sites and years contributed more than the number of quadrats or visits per year to the statistical power to detect trends or differences, indicating the importance of repeatedly sampling large numbers of sites through time.
The power analysis and feedback from participants led to protocol revisions to improve ease of data collection, including simplification of the transect placement process, reductions in the number of quadrats, and capping the number of milkweed plants examined for eggs and larvae. These changes reduced the time required to collect data and improved participant experiences. In 2018, 86% of participant survey respondents (n = 43) reported positive program experiences and intent to participate in the next season.
Data Management
A centralized database and GIS platform readily available to participants and partners is hosted by the MJV8; USFWS maintains a database for their staff. Data are documented according to the Darwin core standards as described in (Wieczorek et al., 2012) and are shareable with efforts such as the Trinational Monarch Knowledge Network9. Data sharing agreements enable land owners to specify the level of geographic precision for data sharing (e.g., at the scale of the monarch block rather than at a specific point location). Data are available to participants and researchers upon request. Web-hosted data summaries and visualization tools (e.g., graphs and maps) for milkweed densities, monarch distributions, and nectar plant species composition are in development.
Participation
Involvement from a broad array of partners is essential for implementing a successful monitoring program for such a widely distributed species. Integration with existing naturalist networks has been a successful strategy for spurring participation in the IMMP. Collaboratives (e.g., Monarchs Across Georgia10), nature centers, government agencies, or volunteer groups serve as IMMP “hubs.” These entities connect IMMP methods with local conservation goals and expand implementation by recruiting and training local participants. Outreach and training workshops held with these groups have amplified data collection in new localities and mobilized larger audiences.
Participation is fostered by a number of tools hosted by MJV on the IMMP webpage, including activity instructions, training resources (including video), mapping tools, and data entry portal. USFWS hosts guidance documents, site-screening and mapping tools, data collected by their staff, and associated products on ServCat11.
By participating in the IMMP, citizen scientists and private landowners deepen their connection with monarchs and appreciation for their conservation challenges. Their participation can broaden civic engagement within local communities (Lewandowski and Oberhauser, 2017), and contribute more representative data than is possible by agencies working alone.
Relevance
Monitoring is a key element of adaptive management and strategic habitat conservation (National Ecological Assessment Team, 2006). The IMMP will provide conservation professionals with an enhanced understanding of the dynamics of monarch populations, their habitats, and their response to conservation efforts. IMMP results can readily be entered to the USFWS's Monarch Conservation Database12, which tracks monarch conservation efforts and informs decisions about the butterfly's status. The IMMP may be used to achieve research and monitoring objectives within monarch conservation plans (e.g., Midwest Association of Fish and Wildlife Agencies, 2018), including monitoring monarch habitat, estimating milkweed distribution across different land-use sectors, monarch distribution, vital rates, nectar resource selection, and understanding effects of disease and pathogens.
The IMMP is already instrumental to local, regional, and national conservation assessment and research programs. Several county conservation boards in Iowa (K. Kinkead personal communication) and dozens of private landowners in the eastern U.S. are using it to evaluate the quality of monarch habitat on their conservation lands. Statewide collaboratives such as Missourians for Monarchs13 employ it to track progress toward achieving their statewide milkweed stem goals. IMMP data were used to parameterize a milkweed density index in a model of monarch reproductive use on an Iowa landscape (Grant et al., 2018; Grant and Bradbury, 2019). Regionally, IMMP protocols were used by (Lukens et al., in review) to evaluate conservation projects in the Upper Midwest and in landscape-scale studies of habitat quality and monarch survivorship at University of Wisconsin. Federal programs also use the IMMP, for example, to assess monarch habitat and use on USFWS refuges, and to compare with the U.S. Department of Agriculture's Natural Resources Conservation Service assessment of monarch habitat (i.e., Wildlife Habitat Evaluation Guide).
Impact
The scale and potential impact of the IMMP compare to other large-scale programs such as the United Kingdom Butterfly Monitoring Scheme (Roy et al., 2001; Brereton et al., 2006), North American Bat Monitoring Program (Loeb et al., 2015), and the Breeding Bird Survey, which have influenced conservation policy (Hudson et al., 2017). In three pilot years, the IMMP has already been implemented at hundreds of sites across Georgia, Iowa, Illinois, Kentucky, Michigan, Minnesota, Missouri, North Dakota, Ohio, Oklahoma, Texas, and Wisconsin, showing strong potential to reach the scale and impact of other successful large-scale monitoring programs.
The IMMP will greatly improve our knowledge of monarch biology, particularly in historically under-surveyed geographies and land-use types. The multi-dimensionality of IMMP data, which pairs quantitative habitat data with monarch use, provides an opportunity to assess how monarchs in several life stages interact with a variety of spatially and temporally explicit habitat characteristics. IMMP protocols can also be used to address priority research questions such as the location of gaps in nectar resources along migration routes, or how proximity to fields routinely treated with pesticides affects monarch recruitment and survival (Midwest Association of Fish and Wildlife Agencies, 2018).
IMMP nectar plant information can benefit broader pollinator conservation efforts and efforts for other declining species that rely on flowering plants (e.g., Rusty-patched Bumblebee). Data on nectar plant species richness and frequency can help land managers gauge progress toward habitat goals, such as establishing plants with staggered bloom times recommended by many pollinator plans. While more frequent visits may better characterize nectar availability for pollinators (Szigeti et al., 2016b, 2018), IMMP data can contribute to larger phenology databases (e.g., USA National Phenology Network14) and ultimately contribute to our understanding of habitat availability in a changing climate.
Broad and diverse participation is necessary to achieve the desired breadth and depth of sampling and to ensure the IMMP's long-term sustainability. Success will depend on mobilizing partners across government, academia, and NGOs, alongside a cadre of citizen scientists. These efforts are only just beginning, and the potential for long-term scientific payoff is enormous. Ultimately, monarch conservation relies on the cooperation of all stakeholders not only in protecting and restoring habitat, but also in understanding and evaluating this species and the habitats on which it relies.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
AC led refinements to the protocol and writing the manuscript. HH, JW, and KO led initial stages of design. JW is the USFWS lead in documenting the full protocol. LL and JT worked on revisions and lead current implementation. KT developed tools for data entry, GIS, and data management. WT and EW provided the sampling framework and statistical analyses. KB, WC, PD, RD, RG, KH, CH, KyK, KaK, JM, and TT contributed to program development, documentation, and implementation.
Funding
Funding support has been provided by U.S. Fish and Wildlife Service, the U.S. Forest Service, and the National Fish and Wildlife Foundation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Monarch Conservation Science Partnership developed the IMMP over the course of several years, and we acknowledge (in addition to the authors) participants in several meetings through which the strategy was developed [including but not limited to Greg Butcher (USFS), Melissa Martin (NRCS), Richard Easterbrook, Allen Gilbert, Dan Konzek, Jana Newman, (USFWS), Diane Larson (USGS), and John Pleasants (Iowa State University)]. We also thank the multitude of participants of the IMMP, including private land owners who provided access to their land and volunteers and technicians who collected data. Funding support has been provided by USFWS, the U.S. Forest Service, and the National Fish and Wildlife Foundation. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service or other coordinating NGOs, local or state agencies. Any use of trade, product, or firm names are for descriptive purposes only and do not imply endorsement by the U.S. Government.
Footnotes
2. ^https://www.monarchwatch.org/
4. ^https://www.monarchjointventure.org/IMMP/
5. ^https://ecos.fws.gov/ServCat/Reference/Profile/109175
6. ^https://www.thebutterflynetwork.org/
8. ^https://monarchjointventure.org/IMMP/
9. ^https://birdscanada.org/birdmon/tmkn/
10. ^https://www.eealliance.org/monarchs-across-ga
11. ^https://ecos.fws.gov/ServCat/Reference/Profile/109175
12. ^https://www.fws.gov/savethemonarch/mcd.html
References
Austin, P. C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multiv. Behav. Res. 46, 399–424. doi: 10.1080/00273171.2011.568786
Biesmeijer, J. C., Roberts, S. P. M, Reemer, M., Ohlemüller, R., Edwards, M., et al. (2006). Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. doi: 10.1126/science.1127863
Bird, T. J., Bates, A. E, Lefcheck, J. S, Hill, N., et al. (2014). Statistical solutions for error and bias in global citizen science datasets. Biol. Conserv. 173, 144–154. doi: 10.1016/j.biocon.2013.07.037
Brereton, T., Roy, D., and Greatorex-Davies, N. (2006). Thirty years and counting: the contribution to conservation and ecology of butterfly-monitoring in the UK. British Wildlife 17, 162–170. Available online at: http://nora.nerc.ac.uk/id/eprint/4024.
Brower, L. P., Taylor, O. R, Williams, E. H, Slayback, D. A., et al. (2012). Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv. Divers. 5, 95–100. doi: 10.1111/j.1752-4598.2011.00142.x
Dilts, T., and Forister, M. (2017). Western Monarch and Milkweed Habitat Suitability Model: Final Maps. Unpublished report prepared in collaboration with the Xerces Society and the Fish, US, and Wildlife Service. University of Nevada, Reno, NV.
Elzinga, C. L., Salzer, D. W., and Willoughby, J. W. (1998). Measuring and Monitoring Plant Populations. Denver, CO, Bureau of Land Management.
Flockhart, D. T., Pichancourt, P., Norris, D. R., and Martin, T. G. (2015). Unravelling the annual cycle in a migratory animal: breeding-season habitat loss drives population declines of monarch butterflies. J. Anim. Ecol. 84, 155–165. doi: 10.1111/1365-2656.12253
Grant, T. J., and Bradbury, S. P. (2019). The role of modeling in monarch butterfly research and conservation: a review and a challenge. Front. Ecol. Evol.
Grant, T. J., Parry, H. R., Zalucki, M. P., and Bradbury, S. P. (2018). Predicting monarch butterfly (Danaus plexippus) movement and egg-laying with a spatially-explicit agent-based model: the role of monarch perceptual range and spatial memory. Ecol. Modell. 374, 37–50. doi: 10.1016/j.ecolmodel.2018.02.011
Hudson, M. A. R., Francis, C. M., Campbell, K. J., Downes, C. M., Smith, A. C., Pardieck, K. L., et al. (2017). The role of the North American Breeding Bird Survey in conservation. Condor 119, 526–545. doi: 10.1650/CONDOR-17-62.1
Kantola, T., Tracy, J. L., Baum, K. A., Quinn, M. A., and Coulson, R. N. (2019). Spatial risk assessment of eastern monarch butterfly road mortality during autumn migration within the southern corridor. Biol. Conserv. 231, 150–160. doi: 10.1016/j.biocon.2019.01.008
Kasten, K., Stenoien, C., Caldwell, W., and Oberhauser, K. S. (2016). Can roadside habitat lead monarchs on a route to recovery? J. Insect Conserv. 20, 1047–1057. doi: 10.1007/s10841-016-9938-y
Kinkead, K. E., Harms, T. M., Dinsmore, S. J., Frese, P. W., and Murphy, K. T. (in review). Design implications for surveys to monitor monarch butterfly population trends. Front. Ecol. Evol.
Lewandowski, E. J., and Oberhauser, K. S. (2017). Butterfly citizen scientists in the United States increase their engagement in conservation. Biol. Conserv. 208, 106–112. doi: 10.1016/j.biocon.2015.07.029
Loeb, S. C., Rodhouse, T. J., Ellison, L. E., Lausen, C. L., Reichard, J. D., Irvine, K. M., et al. (2015). A plan for the North American Bat Monitoring Program (NABat). U.S. Department of Agriculture, Forest Service, General Technical Report SRS-208.
Malcolm, S. (2018). Anthropogenic impacts on mortality and population viability of the monarch butterfly. Annu. Rev. Entomol. 63, 277–302 doi: 10.1146/annurev-ento-020117-043241
Manly, B. F. J., McDonald, L. L, Thomas, D. L, McDonald, T., et al. (2002). Resource Selection by Animals, Statistical Design and Analysis for Field Studies, 2nd Edn. Kluwer Academic Publishers, Norwell, MA.
Midwest Association of Fish Wildlife Agencies (2018). Mid-America Monarch Conservation Strategy, 2018-2038, Version 1.0. Available online at: http://www.mafwa.org/wp-content/uploads/2018/07/MAMCS_June2018_Final.pdf.
Nail, K. R., Stenoien, C., and Oberhauser, K. S. (2015). Immature monarch survival: effects of site characteristics, density, and time. Ann. Entomol. Soc. Am. 108, 680–690. doi: 10.1093/aesa/sav047
National Ecological Assessment Team, NEAT. (2006). Strategic Habitat Conservation: a Report From the National Ecological Assessment Team. Fish, U. S., and Wildlife Service, Washington, DC., and Geological Survey, U. S., Reston, Virginia, USA. Available online at: http://training.fws.gov/EC/resources/shc/shc_finalrpt.pdf.
National Research Council, Division on Earth and Life Studies, Board on Life Sciences, Board on Agriculture and Natural Resources, and Committee on the Status of Pollinators in North America. (2007). Status of Pollinators in North America. Washington, DC: National Academies Press.
Oberhauser, K., Batalden, R., and Howard, E. (2009). Monarch Butterfly Monitoring in North America: Overview of Initiatives and Protocols. Commission for Environmental Cooperation, Montreal, QC.
Oberhauser, K., Wiedenholt, R., Diffendorfer, J., Semmens, D., Ries, L., Thogmartin, W., et al. (2017). A trans-national monarch butterfly population model and implications for regional conservation priorities. Ecol. Entomol. 42, 51–60 doi: 10.1111/een.12351
Oberhauser, K. S., Ries, L., Altizer, S., Batalden, R. V., Kudell-Ekstrum, J., Garland, M., et al. (2015). “Chapter 2: contributions to monarch biology and conservation through citizen science: 70 years and counting,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds K. S. Oberhauser, K.R. Nail, and S. M. Altizer (Ithaca, NY: Cornell University Press), 13–30.
Olsen, A. R., Sedransk, J., Edwards, D., Gotway, C., Liggett, W., Rathbun, S., et al. (1999). Statistical issues for monitoring ecological and natural resources in the United States. Environ. Monit. Assess. 54, 1–45. doi: 10.1023/A:1005823911258
Overton, W. S., and Stehman, S. V. (1993). Properties of designs for sampling continuous spatial resources from a triangular grid. Commun. Statist. Part A-Theory Methods. 22, 2641–2660. doi: 10.1080/03610928308831175
Pitman, G. M., Flockhart, D. T. T., and Norris, D. R. (2018). Patterns and causes of oviposition in monarch butterflies: implications for milkweed restoration. Biol. Conserv. 217, 54–65. doi: 10.1016/j.biocon.2017.10.019
Pleasants, J. (2017). Milkweed restoration in the Midwest for monarch butterfly recovery: estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the monarch population. Insect Conserv. Divers. 10, 42–53. doi: 10.1111/icad.12198
Pleasants, J. M., and Oberhauser, K. S. (2013). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Divers. 6, 135–144. doi: 10.1111/j.1752-4598.2012.00196.x
Pollard, E. (1977). A method for assessing changes in the abundance of butterflies. Biol. Conserv. 12, 115–134. doi: 10.1016/0006-3207(77)90065-9
Pollinator Health Task Force (2015). National Strategy to Promote the Health of Honey Bees and other Pollinators. Available online at: https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/Pollinator%20Health%20Strategy%202015.pdf (accessed December17, 2018).
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., and Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol. 6, 345–353. doi: 10.1016/j.tree.2010.01.007
Powney, G. D., Carvell, C., Edwards, M., Morris, R. K. A., Roy, H. E., Woodcock, B. A., et al. (2019). Widespread losses of pollinating insects in Britain. Nat. Commun. 10:1018. doi: 10.1038/s41467-019-08974-9
Reynolds, J. H., Knutson, M. G, Newman, K. B, Silverman, E., et al. (2016). A road map for designing and implementing a biological monitoring program. Environ. Monit. Assess. 188:399. doi: 10.1007/s10661-016-5397-x
Ries, L., and Oberhauser, K. S. (2015). A citizen-army for science: quantifying the contributions of citizen scientists to our understanding of monarch butterfly biology. Bioscience 65, 419–420. doi: 10.1093/biosci/biv011
Roy, D. B., Rothery, P., Moss, D., Pollard, E., and Thomas, J. A. (2001). Butterfly numbers and weather: predicting historical trends in abundance and the future effects of climate change. J. Anim. Ecol. 70, 201–217. doi: 10.1111/j.1365-2656.2001.00480.x
Saunders, S. P., Ries, L., Oberhauser, K. S., Thogmartin, W. E., and Zipkin, E. F. (2017). Local and cross-seasonal associations of climate and land use with abundance of monarch butterflies Danaus plexippus. Ecography 40, 1–12. doi: 10.1111/ecog.02719
Schultz, C. B., Brown, L. M., Pelton, E., and Crone, E. E. (2017). Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol. Conserv. 214, 343–346. doi: 10.1016/j.biocon.2017.08.019
Semmens, B. X., Semmens, D. J., Thogmartin, W. E., Wiederholt, R., López-Hoffman, L., Diffendorfer, J. E., et al. (2016). Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6:23265. doi: 10.1038/srep23265
Stenoien, C., Nail, K., and Oberhauser, K. S. (2015). Habitat productivity and temporal patterns of monarch butterfly egg densities in the eastern United States. Ann. Entomol. Soc. Am. 108, 670–679. doi: 10.1093/aesa/sav054
Stenoien, C., Nail, K. R., Zalucki, J. M., Parry, H., Oberhauser, K. S., and Zalucki, M. P. (2018). Monarchs in decline: a collateral landscape-level effect of modern agriculture. Insect Sci. 25, 528–541. doi: 10.1111/1744-7917.12404
Stevens, D. L., and Olsen, A. R. (1999). Spatially restricted surveys over time for aquatic resources. J. Agric. Biol.Environ. Stat. 4, 415–428. doi: 10.2307/1400499
Stevens, D. L., and Olsen, A. R. (2003). Variance estimation for spatially balanced samples of environmental resources. Environmetrics 14, 593–610. doi: 10.1002/env.606
Stevens, D. L., and Olsen, A. R. (2004). Spatially balanced sampling of natural resources. J. Am. Stat. Assoc. 99, 262–278. doi: 10.1198/016214504000000250
Szigeti, V., Korösi, Á., Harnos, A., and Kis, J. (2018). Temporal changes in floral resource availability and flower visitation in a butterfly. Arthropod Plant Interact. 12, 177–198. doi: 10.1007/s11829-017-9585-6
Szigeti, V., Korösi, Á., Harnos, A., Nagy, J., and Kis, J. (2016a). Comparing two methods for estimating floral resource availability for insect pollinators in semi-natural grasslands. Ann. Soc. Entomol. France 52, 289–299. doi: 10.1080/00379271.2016.1261003
Szigeti, V., Korösi, Á., Harnos, A., Nagy, J., and Kis, J. (2016b). Measuring floral resource availability for insect pollinators in temperate grasslands - a review. Ecol. Entomol. 41, 231–240. doi: 10.1111/een.12298
Thogmartin, W. E., López-Hoffman, L., Rohweder, J., Diffendorfer, J., Drum, R., Semmens, D., et al. (2017b). Restoring monarch butterfly habitat in the Midwestern US: all hands on deck. Environ. Res. Lett. 12:101003. doi: 10.1088/1748-9326/aa7637
Thogmartin, W. E., Ward, J. P, Grundel, R., Oberhauser, K., Newman, J., et al. (2015). Integrated Strategy for Understanding Effectiveness of Monarch Conservation. Unpublished document. Available online at: https://ecos.fws.gov/ServCat/DownloadFile/161949.
Thogmartin, W. E., Wiederholt, R., Oberhauser, K., Drum, R. G., Diffendorfer, J. E., Altizer, S., et al. (2017a). Monarch butterfly population decline in North America: identifying the threatening processes. R. Soc. Open Sci. 4:170760. doi: 10.1098/rsos.170760
Tracy, J. L., Kantola, T., Baum, K. A., and Coulson, R. N. (2019). Modeling fall migration pathways and spatially identifying potential migratory hazards for the eastern monarch butterfly. Landsc. Ecol. 34, 443–458. doi: 10.1007/s10980-019-00776-0
Vidal, O., and Rendón-Salinas, E. (2014). Dynamics and trends of overwintering colonies of the monarch butterfly in Mexico. Biol. Conserv. 180, 165–175. doi: 10.1016/j.biocon.2014.09.041
Wieczorek, J. D., Bloom, R., Guralnick, S., Blum, M., Döring, R., De Giovanni, T., et al. (2012). Darwin core: an evolving community-developed biodiversity data standard. PLoS ONE 7:e29715. doi: 10.1371/journal.pone.0029715
Williams, B. K., Nichols, J. D., and Conroy, M. J. (2001). Analysis and Management of Animal Populations. San Diego, CA: Academic Press.
Zalucki, M. P., and Kitching, R. L. (1982). Dynamics of oviposition in Danaus plexippus (Insecta: Lepidoptera) on milkweed, Asclepias spp. J. Zool. Lond. 198, 103–116. doi: 10.1111/j.1469-7998.1982.tb02063.x
Keywords: butterfly counts, citizen science, conservation effectiveness, habitat assessment, monarch butterflies (Danaus plexippus), cooperative monitoring, milkweed, nectar plants
Citation: Cariveau AB, Holt HL, Ward JP, Lukens L, Kasten K, Thieme J, Caldwell W, Tuerk K, Baum KA, Drobney P, Drum RG, Grundel R, Hamilton K, Hoang C, Kinkead K, McIntyre J, Thogmartin WE, Turner T, Weiser EL and Oberhauser K (2019) The Integrated Monarch Monitoring Program: From Design to Implementation. Front. Ecol. Evol. 7:167. doi: 10.3389/fevo.2019.00167
Received: 28 February 2019; Accepted: 25 April 2019;
Published: 29 May 2019.
Edited by:
Laurentiu Rozylowicz, University of Bucharest, RomaniaReviewed by:
Adam Korösi, MTA-ELTE-MTM Ecology Research Group, HungaryArthur M. Shapiro, University of California, Davis, United States
Copyright © 2019 Cariveau, Holt, Ward, Lukens, Kasten, Thieme, Caldwell, Tuerk, Baum, Drobney, Drum, Grundel, Hamilton, Hoang, Kinkead, McIntyre, Thogmartin, Turner, Weiser and Oberhauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison B. Cariveau, YWxpc29uLmNhcml2ZWF1QGdtYWlsLmNvbQ==
 Alison B. Cariveau
Alison B. Cariveau Holly L. Holt1
Holly L. Holt1 James P. Ward
James P. Ward Laura Lukens
Laura Lukens Kyle Kasten
Kyle Kasten Wendy Caldwell
Wendy Caldwell Karen Tuerk
Karen Tuerk Kristen A. Baum
Kristen A. Baum Pauline Drobney
Pauline Drobney Ryan G. Drum
Ryan G. Drum Ralph Grundel
Ralph Grundel Karen Kinkead
Karen Kinkead Wayne E. Thogmartin
Wayne E. Thogmartin Emily L. Weiser
Emily L. Weiser