
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 12 April 2019
Sec. Behavioral and Evolutionary Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00092
This article is part of the Research Topic Cooperation and Coordination in the Family View all 26 articles
In cooperatively breeding species, the level of investment in young can vary substantially. Despite receiving considerable research attention, how and why investment in young varies with cooperatively breeding group members remains unclear. To investigate the causes of variation in care of young, we assessed patterns of both helper and parental behavior in the cooperatively breeding Western Australian magpie (Cracticus tibicen dorsalis). Observations of 19 helpers and 31 parents provisioning 33 broods raised in 11 different groups over two consecutive breeding seasons revealed substantial variation in offspring care behavior. Our results suggest that the level of investment in young by helpers is strongly influenced by group size, chick age, and individual helper traits (including foraging efficiency, age and sex). Helping behavior was facultative, and individuals from smaller groups were more likely to invest in helping behavior. Overall, the number of broods receiving help was lowest during the nestling phase and highest during the fledgling phase. Female helpers provided more care than both male and juvenile helpers. We found that mothers invest more time in offspring care than do fathers, however fathers increase their effort in the presence of helpers while mothers do not. Overall, helper care was additive to parental care and therefore helping behavior may be beneficial to the brood. Our research reveals that variation in offspring care in magpies is influenced by both social and individual traits.
In cooperatively breeding species, groups are typically comprised of breeders and helpers (group members that help to raise young that they do not have direct parentage of, Cockburn, 1998; Cockburn et al., 2008). Helpers can vary in both the amount and type of helping activities to which they contribute to Ridley and Raihani (2008), Bruintjes and Taborsky (2011), Le Vin et al. (2011), and Green et al. (2016). For example, Clutton-Brock et al. (2001) found substantial variation in the provisioning efforts of meerkat helpers (Suricata suricatta); some helpers fed young only 3% of the food they captured, while others gave away up to 49%. Studies that have identified differences in helper contributions can have important implications for understanding the costs and benefits of helping behavior (Innes and Johnston, 1996; Clutton-Brock et al., 2001; Woxvold et al., 2006). For example, variation in helper contributions explained variation in nesting success of White-throated Magpie-jays (Calocitta formosa) better than helper number alone (Innes and Johnston, 1996). Such findings exemplify how measuring variation in helper effort can allow a more accurate assessment of the benefits of cooperative breeding behavior.
Although several studies have investigated variation in helper contributions, many of these have focused on helper variation in relation to kin selection (Krakauer, 2005; Browning et al., 2012; Green et al., 2016). Helping behavior tends to be costly for the helper, e.g., in terms of loss of time for self-maintenance, predator exposure, and investing in their own reproductive attempts (Cockburn, 1998; Heinsohn and Legge, 1999; Canestrari et al., 2007; Gilchrist, 2007). Hence, kin selection has been hypothesized to explain why individuals invest in costly helping behavior, since they could indirectly benefit from perpetuating genes they share with their relative's offspring (Hamilton, 1963; Krakauer, 2005; Hatchwell, 2009; Bourke, 2011). Thus, the level of relatedness between helper and the young they care for has been proposed to explain differences in helper effort (Emlen and Wrege, 1988; Browning et al., 2012; Green et al., 2016). However, molecular techniques have revealed that group members are often not as closely related as researchers once thought, and unrelated helpers are present in many cooperatively breeding species (Wright et al., 2010; Riehl, 2013; Riehl and Strong, 2015). Consequently, while kin selection can explain variation in helper behavior for some species (see Browning et al., 2012; Green et al., 2016), in other species this explanation does not suffice (see Clutton-Brock et al., 2000; Finn and Hughes, 2001; Gilchrist, 2007; Le Vin et al., 2011). For example, in a review of 44 cooperatively breeding species Kingma (2017) demonstrated that for some species, territory inheritance was able to explain more variation in helping behavior than kin selection. If we consider the direct benefits from cooperative breeding that could motivate helping behavior, contributions may vary according to the individual traits of the carer, or the social traits of the group it is in as these are often linked to potential benefits. For example, in other studies, group size, and position in the social hierarchy appears to be an important predictor of helping behavior, where those that are mostly likely to gain breeding opportunities are more likely to help (Reyer, 1986) while those at the bottom end of the social queue are more likely to disperse (Ekman et al., 2001; Nelson-Flower et al., 2018). It is therefore possible that other social and individual factors could influence a helper's ability and motivation to help (Riehl, 2013; Kingma et al., 2014; Kingma, 2017).
Although there is likely to be a myriad of traits influencing helping behavior, a few key factors have emerged from existing research. For example, helpers have been observed to decrease their individual contributions as group size increases (Anava et al., 2001; Clutton-Brock et al., 2001; Russell et al., 2008; Meade et al., 2010); a behavior also shown by parents, known as load-lightening (Crick, 1992). Another prominent pattern among helpers is for one sex to contribute more to offspring care than the other (Cockburn, 1998; Ridley and Huyvaert, 2007; Koenig et al., 2011). The inequality of contributions between male and female helpers is likely due to sex-biased dispersal patterns, where the philopatric sex is likely to receive more benefits (such as territory inheritance) from helping than the dispersing sex (Greenwood, 1980; Cockburn, 1998). The age of the helper may also affect helper effort, with juveniles often contributing less, probably due to their limited experience and their own costs of continued growth and development (Heinsohn and Cockburn, 1994; Boland et al., 1997; Clutton-Brock et al., 2002). The foraging ability of an individual may also influence their investment rate. When helpers are better able to meet their own energy demands, the costs of provisioning young may be reduced, and therefore helpers with high foraging efficiency may provision young more (Brotherton et al., 2001; Clutton-Brock et al., 2001; Russell et al., 2003). Lastly, the cost-to-benefit ratio of helping has often been linked to body mass, where individuals with a relatively larger body mass than others of similar sizes may contribute more because they may have more resources for self-maintenance, making helping less costly (Gilchrist and Russell, 2007; Le Vin et al., 2011). Therefore, the predominant patterns emerging from previous research into helper contributions suggest that in addition to relatedness, social factors (e.g., group size) and individual characteristics of the helper are potential factors that may influence helper effort.
In this study we assess the causes of individual variation in contributions to the care of young in the cooperatively breeding Western Australian magpie (Cracticus tibicen dorsalis, hereafter referred to as magpie). The magpie is a facultative cooperative breeder with plural breeding (Fulton, 2006), and is an ideal model system for the study of non kin-selected factors influencing variation in the care of young for a number of reasons. Firstly, although helping is associated with kinship in many species, previous research in magpies suggests kin selection may not play a central role in helping behavior (Finn and Hughes, 2001). Genetic analysis on the same magpie population as our study is based on, has revealed extremely high extra-group paternity rates (82%), meaning most offspring are sired by males outside of their territory, thus lowering the level of relatedness within groups (Hughes et al., 2003). Secondly, in magpies some broods receive help while others do not (Kaplan, 2004; Fulton, 2006; Pike, 2016) which allows a comparison of the patterns of offspring care both within and between groups that have helper and non-helper group members. Lastly, our study groups are fully habituated and ringed, enabling us to gather fine-scale foraging, provisioning and body mass data during both the nestling and fledgling phase. This affords us a unique opportunity to quantify the influence of social and individual traits on investment in young and directly compare this between parents and helpers. Here we aim to: (a) measure individual variation in contributions to offspring care from both parents and helpers; and (b) investigate social (i.e., group size and helper presence) and individual (i.e., sex, age, mass, foraging efficiency) causes of variation in investment in group young. We predict that contributions to offspring care will vary between group members and that the level of investment in young will be influenced by the social and individual traits associated with each group member.
The Western Australian magpie is a subspecies of the Australian magpie, found in the south–west of Western Australia (Kaplan, 2004). This medium-sized (250–400 g) passerine bird typically inhabits open grassland and suburban parklands (Rollinson and Jones, 2002; Durrant and Hughes, 2005) where it forages primarily for subterranean invertebrates (Floyd and Woodland, 1981; Kaplan, 2004; Edwards et al., 2015; Mirville et al., 2016). Magpies form social groups typically ranging between 2 to 12 individuals that cooperatively defend a territory year round (Ashton et al., 2018). Magpies are facultative cooperative breeders, whereby most adults within a group attempt to breed, and only some broods receive help from group members other than the breeding pair (Finn and Hughes, 2001; Kaplan, 2004; Fulton, 2006; Pike, 2016). For groups where helping occurs, typically only one brood receives help despite multiple broods simultaneously having nutritionally dependent young (Pike, 2016). Most commonly, the brood receiving help usually only has a single helper (Pike, 2016). Any group member may become a helper and some group members switch to helping only after their brood has failed (26% of adults that don't successfully breed; Pike, 2016). Each group has a roughly equal sex ratio with slightly more adult females than males (mean = 55 ± 10.9% females and 45%± 10.9% males per group). The breeding season typically begins in August for a few months duration and spans austral spring to the beginning of summer (Kaplan, 2004; Edwards et al., 2015). During the breeding season, magpie chicks spend ~4 weeks in the nest before fledging (Pike, 2016). Once they leave the nest, fledglings remain in their natal territory and are dependent on group members for food until they begin to forage independently at ~4–5 weeks post-fledging, and will continue to receive some care from adults until six months of age (Baker et al., 2000; Kaplan, 2004). Magpies can live to ~25 years and typically try to reproduce by their fourth year, once they have acquired their adult plumage (Johnstone and Storr, 2004; Kaplan, 2004). Adults are dichromatic and can easily be sexed by differences in plumage, however these differences are not present in juveniles until they reach sexual maturity around three years of age and they develop adult plumage (see Johnstone and Storr, 2004 for details).
The study population was first established in 1997 by Drs. Ian Rowley and Eleanor Russell. The oldest birds in the current study were at least 23 years old as they were adults at the time of ringing in 1997. The study site was expanded to encompass more magpie groups in 2012. The magpies are habituated to an observer within 2–5 m without disturbing their behaviors (sensu Ridley and Raihani (2007)) and hop on a top balance scale voluntarily (Ohaus Valor 7000 ™) for a small food reward, allowing the collection of regular body mass records (Edwards et al., 2015). At the beginning of each brood observation session, the observer would place c. One gram of shredded cheese atop the top-pan scale (and zeroed) to entice an individual to stand on the scale and once the bird consumed the food reward, their body mass was recorded. This was then repeated with other group members. Individuals are ringed with unique combinations of colored rings for individual identification.
The study population comprised 11 magpie groups, with a group size range of 3–12 members (n = 82 adult and juvenile magpies observed in total). Most group territories in the study population were situated near natural or artificial watercourses, and each was characterized by open grassland with sparse woodlands. Since magpies have high site fidelity and cooperatively defend their territory year round (Kaplan, 2004; Ashton, 2017), individuals could easily be found within a small radius of known foraging sites for each group.
Observations of helping behavior (approved by the Animal Ethics Committee, UWA; Approval number RA 3/100/1272) were carried out over two consecutive breeding seasons (September 2014–March 2016). Data on offspring care behavior was recorded for 33 different broods across 11 magpie groups comprising 82 group members. This resulted in a combined total of 106 brood observation sessions over the nestling to fledgling period (mean = 3.3 ± 1.8, range = 1–6 observations per brood), where a brood observation was defined as an observation session where all visits to the brood by all group members were recorded as they naturally occurred in the field using a pre-defined ethogram (see Supplementary Material S8). All brood observations were performed between 5:30 a.m.−12:30 p.m (when magpies are most active, Edwards et al., 2015) and each brood observation typically lasted 2 h unless it had to be terminated due to unforeseen circumstances such as heavy rain (mean time per observation session = 117 min ± 12 min). Care was taken to ensure an even distribution of brood observations across the 5:30 a.m.−12:30 p.m. sampling time-frame for each brood to avoid a time bias. All observations were collected from wild birds in the field, thus it was not possible to record data blind for this study. Brood observations were conducted over an eight-week period after each brood's estimated hatch date, including the nestling period (4 weeks) and the first four weeks of the post-fledging period, since chicks are still nutritionally dependent during this time. During the fledgling period, generally only one brood survived to fledge in each group; in the one instance where there were two different broods that survived to fledge in the same group, the different broods could be distinguished by age and plumage differences. Newly fledged young aren't very mobile and are easy to observe (Kaplan, 2004): care was taken to record all observations of helping behavior toward all fledglings within a brood observation session. The eggs in a nest were considered to have hatched when group members were first observed feeding chicks, and this was usually detected within 2–3 days of hatching due to intensive fieldwork during the breeding season. In order to ensure that sampling covered contributions over the period of dependency, brood observations were repeated up to six times per brood at 7–9 day intervals. Some broods could not be observed multiple times due to mortality (n = 10).
During each brood observation, the identity of each bird present in the group was noted. For individuals that interacted with the brood, the time and type of helping behavior (i.e., provisioning, guarding, brood defense, or nest sanitation (see Supplementary Material S8 for definitions) and the amount of biomass fed to young was recorded. Food item biomass was determined following the size classification scheme in Edwards et al. (2015) which uses the size of the prey item relative to the birds bill to estimate total wet biomass in grams. All individuals contributing to brood care were categorized as a parent or helper. The female incubating the brood was considered to be the mother, since other group members do not contribute to incubation, and cases of egg-dumping are rare (Durrant and Hughes, 2005). The male who was observed feeding an incubating female and behaving as her social partner was considered the social father. Helpers were all other birds providing any form of care to nestlings or fledglings in the broods observed. Although previous research has identified that social fathers are often not genetic fathers of the offspring (Griffith et al., 2002; Bonderud et al., 2018), there is no evidence to suggest they know they have been cuckolded. We therefore retain their definition as the social father, but investigate sex differences in helping behavior of the parents of each brood to account for the possibility that the high extra-group paternity rate (Hughes et al., 2003) in this species may lead to lower contributions to care by the social father. Helping behaviors were recorded following the ad libitum protocols described in Altmann (1974) and Martin and Bateson (1993), whereby an observer records behaviors in the field as they naturally occur. Using the CyberTracker software (CyberTracker, 2013) the time an individual spent engaged in offspring care behaviors was recorded directly onto an Asus Google Nexus 7 tablet with an error of + 5 s.
A brood was considered “initiated” if a female was observed incubating a nest for at least 30 min (indicating a nest with eggs), and monitored regularly (typically 5 times/week) thereafter to record brood survivorship and hatching date. Upon fledging we also recorded survivorship of individual fledglings until the subsequent March, as fledglings surviving to this date should be no longer dependent on adult care (Carrick, 1972). Magpies typically place their nests high up in trees (>10 m) (Kaplan, 2004), and we therefore could not confidently determine the initial size of the brood. Given that we had limited visibility of nest contents we could not confidently say how many individuals were in a nest. Therefore, nestling survivorship was not comparable between broods, however we were able to compare differences in mortality between helped and unhelped broods, from the time of fledgling until March (see below for details on analysis). To measure body mass change over time, adult and juvenile magpies in each group were weighed at the beginning of each observation session, over the entire chick-rearing period.
To determine helper contributions relative to foraging ability, time-activity focals (sensu Altmann, 1974) were collected from 62 birds over two breeding seasons. The frequency and duration of all foraging activities performed by the focal individual were typically recorded over a 20-min period (mean = 20.18 min ± 2.15 min, range = 15–30 min). Focals were collected between 5:30 a.m.−12:30 p.m. on the same day as the groups brood observation and only included in the analysis if they contained at least 5 min of foraging activity over the focal session as this was considered the minimum amount of time per 20 min focal to get a reliable indicator of foraging behavior (n = 143 focals, mean = 2.3 ± 1.55 focals per individual). To maximize the number of focals which contained a sufficient amount of time foraging per focal, a focal would be abandoned if the bird didn't start foraging within the first 5–10 min of the focal (on average birds foraged for 52 ± 19% of the 20 min focal). While the large number of wild birds we observed limited our ability to do a repeatability analysis of foraging efficiency within individuals, our priority was to gain greater coverage of variation in foraging ability between individuals, as the strength of our data is the ability to relate foraging activity to helping activity during the same observation period. The time spent on all activities by the focal individual was recorded to the nearest five s. Foraging was considered to have begun when an individual was walking slowly while scanning for prey, and a foraging bout was ended when the individual switched to non-foraging behavior such as flying or vocalizing. For each focal, the number and size of all food items captured by the focal individual was recorded following the food item size classification scheme in Edwards et al. (2015) for this study population. This enabled foraging efficiency per focal to be calculated as the total biomass caught (g) /total time spent foraging (min). The proportion of total biomass captured (g) that was fed to young was also recorded, in addition to the identity and age (days post-hatching) of the fed brood.
For both observations of the brood and focal observations of adults and juveniles, we used mixed models to analyze factors affecting helping behavior with group, brood, and individual identity as random terms to account for the potential effect of repeated observation with random intercepts for all mixed models. During brood observations, some group members were observed not interacting with the brood. Consequently, our data set was highly skewed at 0 for the amount of time invested in young. To resolve the difficulty of this zero-inflated distribution whilst still accounting for individual variation in helping activity, we modeled subsets of our data depending on what question was being investigated and thus each subset of data has a different number of observations. For example, we first analyzed which factors affect whether or not an individual becomes a helper (this data subset includes observations of helper and non-helper group members), and then what factors influence how much a helper contributes (this data subset contains only observations of helpers; see below). Because the sex of juveniles is unknown, we used a composite variable incorporating available age and sex information i.e. adults as male/ female and juveniles as unknown. We employed linear (LMM) or generalized linear mixed models (GLMM) using the lme4 package (Bates et al., 2014) with R v3.2.2. (R Core Team, 2018). For both GLMM and LMM's we used model selection based on the Akaike's Information Criterion corrected for small sample size (AICc) using the AICcmodavg package (Mazerolle, 2015), where the model with the smallest AICc value explains the greatest amount of variance in the data (Burnham and Anderson, 2002). We established a “top set” of models containing only those models that were within five AICc units of the top model (Richards, 2005). In the top model set, only models that contained confidence intervals which did not intersect zero were considered significant predictors of data patterns (Symonds and Moussalli, 2011). Classic model averaging was then employed to determine which term/s best explained the distribution of the data (sensu Symonds and Moussalli, 2011).
To determine the factors affecting whether or not an individual provided help to a brood (i.e., became a helper or not), we analyzed the brood observation data using a generalized linear mixed model (GLMM) with a binomial distribution and logit link function. An individual was defined as having the “potential” to help if it didn't breed at all, or if it bred once but failed before the current brood was independent. Breeders were never observed helping at other broods while they had an active brood. Breeders who failed (and didn't re-breed that season) were considered to be available to help only after their own brood failed. Only birds that were not the putative parents of a brood and invested time (i.e., provisioning, guarding, or escorting young) in any brood in their group were considered a helper during a brood observation. The binomial response variable (helper = 1/non-helper = 0 during a brood observation) was tested against; sex [male, female, unknown(juvenile)], group size (juveniles and adults combined) and body mass as well as interactions between terms (taken during the brood observation, n = 152 brood observations for 38 individuals from 10 groups).
Since we did not have foraging efficiency data for all individuals, we used a subset of data for those individuals which we could derive foraging efficiency data. We conducted analyses to determine whether foraging efficiency influenced the likelihood of an individual helping or not. The binomial response variable (helper = 1/non-helper = 0 during a brood observation) was tested against sex, group size and foraging efficiency using a GLMM with a logit link function (n = 66 focal observations for 41 individuals in 11 groups). For both brood observations and focal data chick age was not included in analyses because chick age could not be defined for non-helpers.
In order to assess which factors influence the level of helper investment in a brood, a linear mixed model (LMM) was used. Because each of the different types of helping behavior (i.e., guarding, provision, and escorting) followed a similar pattern of increasing as chicks aged and no other strong pattern was apparent (see Supplementary Materials S9–S11), we combined all helping behaviors to analyse total time invested in young (i.e., the sum of guarding, provisioning, and escorting per observation, per helper). To better satisfy model assumptions our response variable was the proportion of time invested in young per helper, per brood observation, which was log transformed as it produced normally distributed residuals. Factors tested included sex, group size, helper body mass and chick age, and whether the helper was a failed breeder. Not all helper records included a body mass measurement, so a subset of records (n = 66 brood observations) containing body mass were analyzed. However, since body mass was not a significant predictor (see Supplementary Material S4), we analyzed the full dataset without body mass as a predictor, to improve sample size and power (n = 108 brood observations).
Using the focal data set, we investigated the terms influencing how much food individuals fed to young in a LMM. We included observations of both helpers and parents feeding young while foraging (n = 51 focal observations). The amount of biomass (g) fed to young by each adult was the response variable. The factors tested were foraging efficiency, sex, group size, status (parent or helper), and age of the chick fed. A body mass measure was only available for 72% of focals, thus we did not include it for this analysis.
We investigated whether there was a difference in how much time parents invested in young (i.e., total minutes brooding, provisioning, guarding, and shading per observation) between broods with and without helpers (n = 144 brood observations from 33 broods in 11 groups) using a LMM. Because a few brood observations were unequal in duration (i.e., 1, 1.5, or 2 h), a “weights” argument (in the lme4 package; see Bates et al., 2014) was used in the model and “prior statistical weights” were set as the duration of each observation session to account for differences in observation time. This was preferred over using proportion of time as a response variable as it better satisfied normality assumptions. Factors tested were sex, group size, chick age and whether or not the brood had help from other group members during each observation session (coded as 0 for no help, 1 for helped). A subset of brood observations that contained body mass records (n = 82 brood observations) were analyzed, however body mass did not influence adult investment in young (see Supplementary Material S7) and was therefore not included in the final model analyses.
Lastly, we assessed whether the number of fledged chicks surviving to the end of the breeding season was significantly different between broods that received help and those that did not. As helping was relatively rare during the nestling phase, we only assessed the impact of helping on the survival of fledglings. A brood was considered successful if it had at least one fledgling surviving until the end of the breeding season (defined as the beginning of March of the year after hatching). By March, fledglings were on average 20 ± 3 weeks old. Using all observed fledglings (n = 30) over both breeding seasons, a Kaplan-Meier survival analysis using IBM SPSS v22 was used to compare the number of fledglings with and without help that survived to independence.
Although some studies have included territory quality as a potential predictor of helper variation (Koenig et al., 2011; Cusick et al., 2018), we have decided not to include it for this analysis as these magpies live in an urban matrix with many artificial food and water resources easily and equally available (Ishigame et al., 2006). Additionally previous research on these magpie groups found no relationship between group size and territory size (where territory size may be considered a proxy of territory quality, Hidayat, 2018).
Cooperative breeding was observed in nine out of the 11 groups. During the nestling phase, helping behavior was recorded at 12% of all broods observed, and increased to 60% once broods fledged. Helpers consisted of males (42% of helpers and 53% of available males), females (37% of helpers and 50% of available females) and juveniles (21% of helpers and 36% of available juveniles). While slightly more males became helpers than females (42 vs. 37%) the females would help more often (i.e., more helping observations were made by females see Table 1) and their overall contributions were higher than the male helpers (Figure 2). While some individuals that were present during both breeding seasons helped in both years of observation (14% of adults were helpers in both years), many did not. Helpers were observed participating in all offspring care behaviors performed by parents (except incubating) including provisioning, guarding and defending young, escorting and nest sanitation. Overall helpers contributed an average of 10.9 min and 0.25 g of food per individual helper, per observation, compared to 26.93 min, 1.42 g per individual breeder with no help, and parents per individual breeder with help 28.057 min, 1.22 g. The majority of helpers (96%) only helped one brood, even when multiple broods were present. For each brood that received help, usually only one helper contributed, however, 23% of helped broods had more than one helper (mean = 1.4 range 1–3 helpers per brood). A number of helpers only switched to helping after their breeding attempt failed (75% of female helpers and 25% of male helpers).
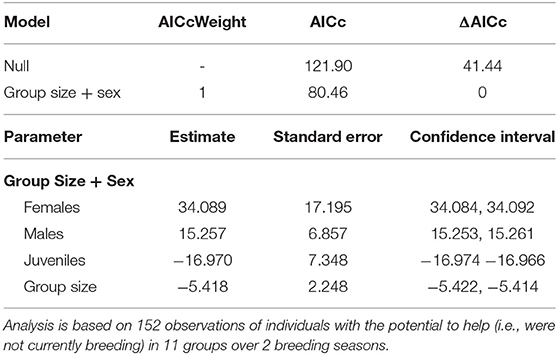
Table 1. Top model set of the factors associated with whether or not an individual displays helping behavior (for a full list of models see Supplementary Material S2).
Overall, 50% of the 38 individuals with the “potential” to help (i.e., those that were not actively breeding), were observed helping. Group size and sex were the strongest predictors affecting whether or not an individual helps (Table 1). In larger groups, a smaller proportion of the individuals “available” to help became helpers and, of those that invested in helping behavior, they were observed helping less often (i.e., helpers in larger groups had more observations sessions where they didn't help at all) compared to helpers in small groups (Figure 1). Helping behavior varied between the sexes, with females helping more often than males or juveniles (Table 1). There was no effect of foraging efficiency on whether an individual invested in helping behavior (Supplementary Material S1).
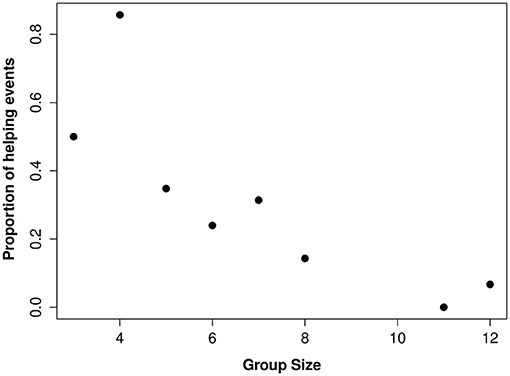
Figure 1. The relationship between group size and proportion of brood observations where a helper contributed to offspring care.
The proportion of time that a helper invested in young varied substantially and ranged between 2–68% of brood observation time. On average, helpers invested ~10% of their time helping young. The strongest predictor of the proportion of time helpers spent with young was chick age (Table 2). Helping was more common during the fledgling phase as only 23% of broods which were raised cooperatively had helpers present during the nestling phase. Most helpers invested a higher proportion of time in fledglings than nestlings (Figure 2). Helpers displayed a continual increase in investment as fledglings aged, peaking at 7–8 weeks post-hatching (when our observations ended). It is possible that helper investment increased even further beyond this age. There was no difference in the proportion of time invested in young between male and juvenile helpers, however females contributed a significantly higher proportion of time to young, equating to approximately twice the amount of average investment for males and juveniles (Table 2, Figure 3). Foraging efficiency was the best determinant of how much biomass individuals fed to chicks (Table 3): more efficient foragers fed young more biomass (Figure 4).
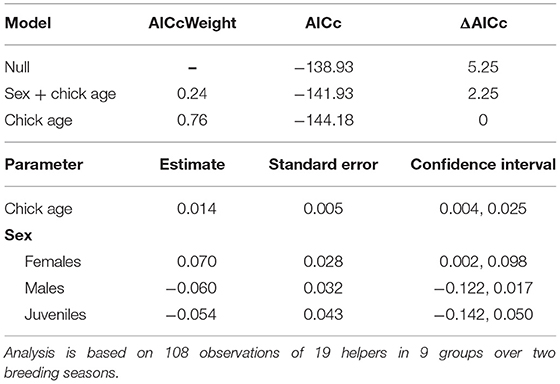
Table 2. Top model set of factors influencing variation in the proportion of time helpers invested in young per brood observation (for full set of models tested see Supplementary Material S3).
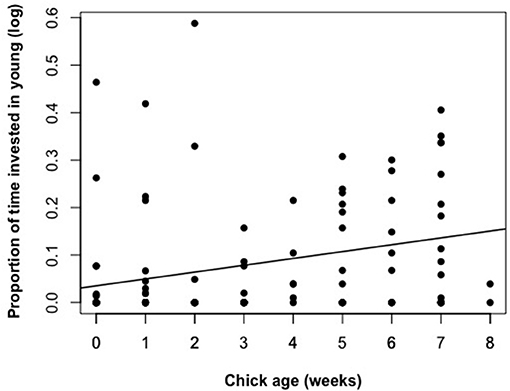
Figure 2. The relationship between chick age (nestling phase 0-4 weeks, fledgling phase 4–8 weeks) and the proportion of time a helper invested in young. Raw data values are displayed against the line of best fit generated from the top model presented in Table 2.
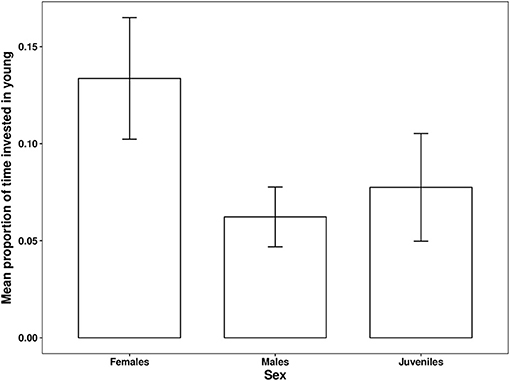
Figure 3. Differences between the average proportions of observation time helpers invested in young according to helper sex per observation session. Error bars generated with ± S.E of the mean.
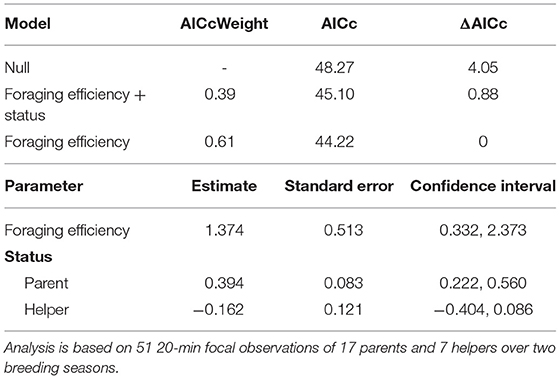
Table 3. Top set of models for the factors influencing the amount of biomass fed to young (for full set of candidate models see Supplementary Material S5).
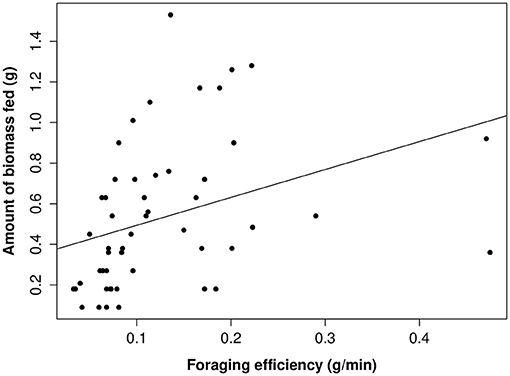
Figure 4. The relationship between the amount of biomass fed to young per 20 min focal by adults and helpers and foraging efficiency. Raw data values are displayed against the line of best fit generated from the top model presented in Table 3.
There was a considerable sex difference in how much time parents invested in parental care. On average mothers invested at least 50% more time in young than did fathers, whether or not the brood had helpers present (Figure 5). When helpers were present, there were sex-specific changes in parental investment (Table 4). Fathers with helpers invested an average of 16% of their time with young, while fathers without help invested 8% of their time. However, for mothers, the opposite trend occurred: on average mothers with helpers spent 27% of their time with young, vs. 39% for mothers with no help (Table 4). On average, parents with helpers investing in their brood spent 44% of observation time with their brood, while parents without help spent 47% of observation time with their brood. Chick age also influenced parental investment (Table 4), with parents spending less time with young as they grew older (Figure 6)—a trend opposite to that found for helpers (Figure 2).
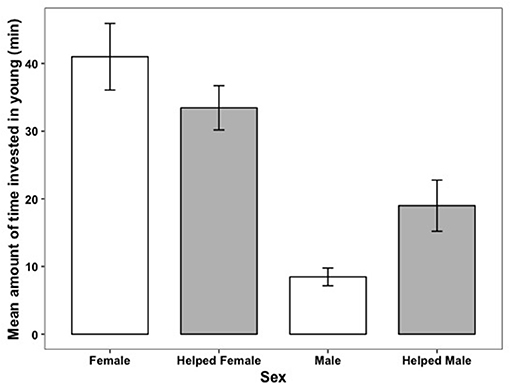
Figure 5. The relationship between how much time parents invested in young and whether they received help (shown in gray) during an observation session. Error bars generated with ± S.E. of the mean.
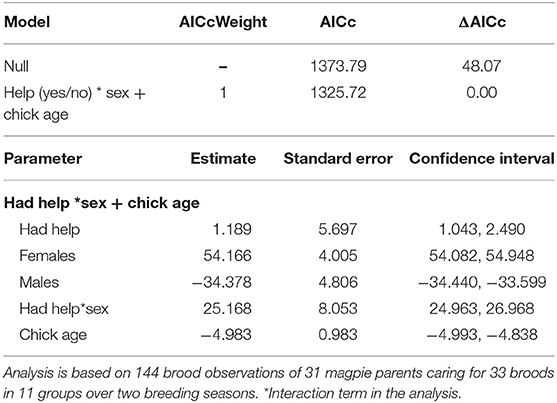
Table 4. The top set of models investigating the factors influencing how much time parents spent with their offspring (for full set of candidate models tested see Supplementary Material S6).
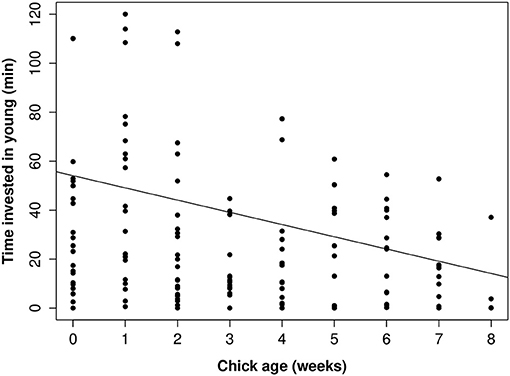
Figure 6. The relationship between how much time parents invested in young according to chick age (nestling phase 0–4 weeks, fledgling phase 4–8 weeks). Raw data values are displayed against the line of best fit generated from the top model presented in Table 4.
Overall brood survivorship was low, with only 22 % of initiated broods having at least one chick surviving until the end of the breeding season over both years. The biggest decline in survivorship was during the nestling stage, with only 47% of hatched broods having at least one chick that survived to fledge. Once chicks fledged, 73% of broods still had at least one chick remaining by the end of the breeding season. There was no significant difference in survival between fledglings with and without help once they had left the nest (Kaplan-Meier survival analysis, log-rank test X2 = 1.044, df = 1, p = 0.307, N = 30 fledglings).
Our main objective was to investigate how individual and social traits influenced contributions to care. Our results reveal that helping behavior in the Western Australian magpie is facultative, and the level of offspring care provided is highly variable. While Finn and Hughes (2001) found no relationship between relatedness and variation in helping behavior for magpies, our study was able to reveal some non-kin selection mechanisms accounting for variation in helping behavior. Our study showed that variation in offspring care is influenced by an individual's age, sex, foraging efficiency, group size, and the presence of helpers.
Although there were many non-breeding group members “available” to help, only some did help, and this propensity to help was affected by group size. Non-breeding individuals in small groups were proportionally more likely to help than those in large groups. The reason for this is unclear. One possibility is the load-lightening effect, where, as the number of individuals contributing to a task increases, the workload per individual decreases, or there is less need for additional individuals to invest in the cooperative breeders (Kokko et al., 2001; Johnstone, 2011; Zöttl et al., 2013b). Magpies are highly territorial and all group members regularly cooperate in territory defense, brood defense and predator detection and mobbing (Farabaugh et al., 1992; Kaplan and Rogers, 2013; Edwards et al., 2015; Mirville et al., 2016). When there are more group members available, groups can become more effective in cooperative tasks (Farabaugh et al., 1992; Ridley et al., 2013; Kingma et al., 2014; Mirville et al., 2016). For example, Farabaugh et al. (1992) found that as magpie group size increased, time needed for defense and individual vigilance decreased, and larger groups were more successful in intergroup “battles” and territory maintenance. This suggests in smaller groups there may be greater need to invest in cooperative breeding, where helping to produce more recruits to maintain a territory and other resources may confer greater benefits in smaller rather than large groups (Wiley and Rabenold, 1984; Kokko et al., 2001; Kingma et al., 2014).
In addition to group size, sex and age influenced the likelihood of an individual to help. Juveniles were less likely to help than both adult males and females. Many other studies have demonstrated that juveniles are less likely to help across a diversity of cooperatively breeding species (Heinsohn and Cockburn, 1994; Clutton-Brock et al., 2001; Clutton-Brock, 2002; Woxvold et al., 2006). In cooperative breeding apostlebirds (Struthidea cinerea) for example, younger individuals were both less likely to help overall, and contributed less when they did help (Woxvold et al., 2006). This may be due in part to the higher energetic costs of growth and development for juveniles (Clutton-Brock et al., 2001). Additionally, juveniles may also have less experience foraging and feeding young, which could limit how much care they can provide (Heinsohn and Cockburn, 1994).
Helping behavior tended to increase after young had fledged, a pattern that was consistent with findings for the south–eastern subspecies of Australian magpie (Cracticus tibicen hypoleuca) (Hughes et al., 1996). While this pattern of investing more in fledglings is not widespread among other cooperative species, this may be in part due to the fact that few studies document helping post-fledgling, as it can be difficult to obtain information on helping behavior once young are out of the nest and highly mobile (Ridley and Raihani, 2007; Tarwater and Brawn, 2010; Covas et al., 2011; Thompson and Ridley, 2013; Van de Loock et al., 2017). The observed increase in helper effort as chicks aged was unlikely to be due to an increase in energy demands per chick because we observed a concurrent reduction in parental effort as chicks aged (Figure 6). Instead, greater helper effort during the fledgling period could possibly be attributed to the fact that fledglings had considerably better survivorship than nestlings. Overall, the number of broods surviving (47%) was lowest during the nestling phase and highest (73% surviving) during the fledgling phase. Differential rates of mortality between early developmental stages have been observed in other avian species (Sankamethawee et al., 2009; Ridley and van den Heuvel, 2012; Van de Loock et al., 2017) and may help explain differential investment by helpers. For example, in long tailed tits, helper investment has no significant effect on nest predation or nestling survival, but in the long-term helper investment does significantly influence fledgling recruitment (Hatchwell et al., 2004). Therefore, for magpies, helping during the nestling phase may have a higher probability of resulting in a cost of care with no benefit (due to high brood mortality).
While both males and females became helpers, we found that female helpers contributed more overall than male helpers. The propensity for one sex to help may be linked to whether or not that sex disperses, since the philopatric sex will receive more of the benefits of helping (Greenwood, 1980; Cockburn, 1998; Clutton-Brock et al., 2002). Veltman and Carrick (1990) found that in eastern Australian magpies, females were philopatric and males were the dispersing sex. However, more recent research has revealed dispersal strategies differ between magpie sub-species (Baker et al., 2000), and full information on dispersal rates for this population is not available. In the six years of close observation on our study population, juveniles have remained with the groups into which they were born and we have not observed a permanent dispersal event between study groups (Ashton et al., 2018). This lack of sex-biased dispersal may help explain our finding that both males and females became helpers but it does not explain why male helpers contributed less. One difference between helping patterns between males and females was that 75% of female helpers switched to helping after their broods failed, something which was less common for male helpers (only 25%). This facultative switch to helping after failed breeding has been observed in a number of species, including long-tailed tits (Aegithalos caudatus), and white-fronted bee-eaters (Merops bullockoides) (Emlen and Wrege, 1988; MacColl and Hatchwell, 2002; McGowan et al., 2003). For females, the costs of egg production and incubation (only females incubate for magpies) are likely to be very high energetically (Visser and Lessells, 2001; Vézina and Williams, 2002; Bowers et al., 2012). Thus, when the success of independent breeding is constrained, it might be more beneficial for females to invest more in helping and abandon any subsequent breeding attempts. Whereas males don't incur this cost and investing more in helping could compete for time and resources to seek out breeding opportunities and may lead to a trade off with time spent helping (Young et al., 2005).
For both parents and helpers, foraging efficiency was the most important parameter influencing the quantity of food provisioned to young. The amount of food that adults provisioned to young was positively correlated with how efficiently they foraged. Similar results have been found for cooperatively breeding meerkats and pied babblers (Turdoides bicolor), where individuals that were better at foraging either fed young more often, or fed young more biomass (Clutton-Brock et al., 2001; Thompson and Ridley, 2013). More efficient foragers will have less difficulty meeting their own energetic demands (Donnelly and Sullivan, 1998), and consequently feeding young may be less costly (Clutton-Brock et al., 2001). This suggests that helping behavior may be dependent on state, whereby helping becomes conditional on the individual's circumstances (such as energy levels, as suggested by Wright et al. (2001b) and others (Wright et al., 2001a; Zöttl et al., 2013a).
When helpers were present, mothers reduced their investment in young, a finding that is in line with previous research demonstrating maternal load-lightening in other cooperatively breeding species (Crick, 1992; Meade et al., 2010; Zöttl et al., 2013b). Studies have demonstrated both theoretically (Johnstone, 2011) and empirically (Blackmore and Heinsohn, 2007; Ridley and Raihani, 2007; Meade et al., 2010) that when helpers reduce parental care load, parents can improve their overall fitness by re-allocating resources to their own survival or future reproduction. However, although helpers elicited a compensatory response in mothers, the same was not found for fathers. Overall, fathers invested considerably less time in young than did mothers. In fact, even when fathers increased their investment in young in the presence of helpers, it was still less than half of the amount contributed by the mothers that had reduced their investment. This relatively lower parental investment by social fathers is likely due to the excessively high extra-group paternity found in this subspecies (Hughes et al., 2003), where 82% of males in a social pair were not the father of the brood they were raising. The net result was that there was little difference in the average parental investment for broods with and without help (47% of time invested by parents without help and 44% by parents with help), indicating that care provided by helpers (on average helpers contributed 10.9 min and 0.25 g to the brood per observation) was additional to parental care. Although we did not detect a significant difference in short-term survival between fledglings with and without help, our analysis may have been limited by the small sample size (N = 30 fledglings) and sampling time frame. When offspring care by helpers is additional to parental care young are often heavier and more likely to fledge than un-helped young, thus the additional offspring care by helpers is potentially beneficial for both mothers and young in some way (including energetic and developmental benefits) (Hatchwell et al., 2004; Ridley and Raihani, 2007; Meade et al., 2010; Cusick et al., 2018).
In summary, our research has revealed social and individual traits that influence the occurrence of, and the level of investment in, the care of young. We demonstrated that for magpies, how much a group member contributes to offspring care is greatly influenced by the individual traits of age, sex, foraging efficiency and the social traits of group size and the presence of others also contributing to the brood. The plasticity of helping behavior and patterns of care seen here highlight the importance of considering the influence of a carer's social and individual traits when evaluating how and why a group member may engage in cooperative breeding.
KP and AR conceived the study. KP conducted the fieldwork and data analysis with guidance and input from AR, KM, and BA. KP wrote the manuscript and AR, KM, and BA reviewed and edited the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Professor Eleanor Russel and Professor Ian Rowley who established this magpie project and allowed research to continue with these enigmatic animals. We thank Alessandro Delli Paoli Carini, Elizabeth Speechly and Claudia Butters for their helpful fieldwork assistance. We also thank Dr. Bruno Buzzato, Dr. Stephanie King and Dr. Michael Renton for valuable advice and discussions about this research. This research was supported by an Australian Research Council Discovery grant awarded to AR (grant number DP140101921) and by the University of Western Australia. Research permits were approved by the Animal Ethics Committee, UWA; Approval number RA 3/100/1272).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00092/full#supplementary-material
Altmann, J. (1974). Observational study of behavior: sampling methods. Behaviour 49, 227–266. doi: 10.1163/156853974X00534
Anava, A., Kam, M., Shkolnik, A., and Degen, A. A. A. (2001). Effect of group size on field metabolic rate of arabian babblers provisioning nestlings. Condor 103, 376–380. doi: 10.1650/0010-5422(2001)103[0376:EOGSOF]2.0.CO;2
Ashton, B. (2017). The Causes and Consequences of Individual Variation in Cognitive Ability in the Cooperatively Breeding Australian magpie (Cracticus tibicen dorsalis). Dissertation, The University of Western Australia (Perth, WA).
Ashton, B. J., Ridley, A. R., Edwards, E. K., and Thornton, A. (2018). Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature 554, 364–367. doi: 10.1038/nature25503
Baker, A. M., Mather, P. B., and Hughes, J. M. (2000). Population genetic structure of Australian magpies : evidence for regional differences in juvenile dispersal behaviour. Heredity 85, 167–176.
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2014). Fitting linear mixed-effects models using lme4. J. stat. soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Blackmore, C. J., and Heinsohn, R. (2007). Reproductive success and helper effects in the cooperatively breeding grey-crowned babbler. J. Zool. 273, 326–332. doi: 10.1111/j.1469-7998.2007.00332.x
Boland, C. R. J., Heinsohn, R., and Cockburn, A. (1997). Experimental manipulation of brood reduction and parental care in cooperatively breeding white-winged choughs. J. Anim. Ecol. 66, 683–691. doi: 10.2307/5921
Bonderud, E. S., Otter, K. A., Burg, T. M., Marini, K. L. D., and Reudink, M. W. (2018). Patterns of extra-pair paternity in mountain chickadees. Ethology 124, 378–386. doi: 10.1111/eth.12747
Bourke, A. F. G. (2011). The validity and value of inclusive fitness theory. Proc. R. Soc. B Biol. Sci. 278, 3313–3320. doi: 10.1098/rspb.2011.1465
Bowers, E. K., Sakaluk, S. K., and Thompson, C. F. (2012). Experimentally increased egg production constrains future reproduction of female house wrens. Anim. Behav. 83, 495–500. doi: 10.1016/j.anbehav.2011.11.026
Brotherton, P. N. M., Clutton-Brock, T. H., O'Riain, M. J., Gaynor, D., Sharpe, L., Kansky, R., et al. (2001). Offspring food allocation by parents and helpers in a cooperative mammal. Behav. Ecol. 12, 590–599. doi: 10.1093/beheco/12.5.590
Browning, L. E., Patrick, S. C., Rollins, L. A., Griffith, S. C., and Russell, A. F. (2012). Kin selection, not group augmentation, predicts helping in an obligate cooperatively breeding bird. Proc. R. Soc. B Biol. Sci. 279, 3861–3869. doi: 10.1098/rspb.2012.1080
Bruintjes, R., and Taborsky, M. (2011). Size-dependent task specialization in a cooperative cichlid in response to experimental variation of demand. Anim. Behav. 81, 387–394. doi: 10.1016/j.anbehav.2010.10.004
Burnham, K., and Anderson, D. (2002). Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. 2nd ed. (Secaucus, NJ: Springer).
Canestrari, D., Marcos, J. M., and Baglione, V. (2007). Costs of chick provisioning in cooperatively breeding crows: an experimental study. Anim. Behav. 73, 349–357. doi: 10.1016/j.anbehav.2006.04.013
Carrick, R. (1972). Population Ecology of the Australian Black-Backed Magpie, Royal Penguin and Silver Gull. United States Department of Interior Wildlife Research Report, Vol. 2. 41–99.
Clutton-Brock, T. (2002). Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72. doi: 10.1126/science.296.5565.69
Clutton-Brock, T. H., Brotherton, P. N., O'Riain, M. J., Griffin, a, S., Gaynor, D., Sharpe, L., et al. (2000). Individual contributions to babysitting in a cooperative mongoose, Suricata suricatta. Proc. Biol. Sci. 267, 301–305. doi: 10.1098/rspb.2000.1000
Clutton-Brock, T. H., Brotherton, P. N. M., O'Riain, M. J., Griffin, a, S., Gaynor, D., Kansky, R., et al. (2001). Contributions to cooperative rearing in meerkats. Anim. Behav. 61, 705–710. doi: 10.1006/anbe.2000.1631
Clutton-Brock, T. H., Russell, A. F., Sharpe, L. L., Young, A. J., Balmforth, Z., and McIlrath, G. M. (2002). Evolution and development of sex differences in cooperative behavior in meerkats. Science 297, 253–256. doi: 10.1126/science.1071412
Cockburn, A. (1998). Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177. doi: 10.1146/annurev.ecolsys.29.1.141
Cockburn, A., Sims, R. A., Osmond, H. L., Green, D. J., Double, M. C., and Mulder, R. A. (2008). Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens. J. Anim. Ecol. 77, 430–438. doi: 10.1111/j.1365-2656.2007.0
Covas, R., Deville, A. S., Doutrelant, C., Spottiswoode, C. N., and Grégoire, A. (2011). The effect of helpers on the postfledging period in a cooperatively breeding bird, the sociable weaver. Anim. Behav. 81, 121–126. doi: 10.1016/j.anbehav.2010.09.022
Crick, H. P. (1992). Load-lightening in cooperatively breeding birds and the cost of reproduction. Ibis 134, 56–61. doi: 10.1111/j.1474-919X.1992.tb07230.x
Cusick, J. A., de Villa, M., DuVal, E. H., and Cox, J. A. (2018). How do helpers help? Helper contributions throughout the nesting cycle in the cooperatively breeding brown-headed nuthatch. Behav. Ecol. Sociobiol. 72:43. doi: 10.1007/s00265-018-2470-1
CyberTracker (2013). CyberTracker software. Available online at: http://www.cybertracker.org/ (Accessed July 27, 2015)
Donnelly, R. E., and Sullivan, K. A. (1998). Foraging proficiency and body condition of juvenile American Dippers. Condor 100, 385–388. doi: 10.2307/1370282
Durrant, K. L., and Hughes, J. M. (2005). Differing rates of extra-group paternity between two populations of the Australian magpie (Gymnorhina tibicen). Behav. Ecol. Sociobiol. 57, 536–545. doi: 10.1007/s00265-004-0883-5
Edwards, E. K., Mitchell, N. J., and Ridley, A. R. (2015). The impact of high temperatures on foraging behaviour and body condition in the Western Australian Magpie Cracticus tibicen dorsalis. Ostrich J. African Ornithol. 86, 37–41. doi: 10.2989/00306525.2015.1034219
Ekman, J., Eggers, S., Griesser, M., and Tegelstrom, H. (2001). Queuing for preferred territories: delayed dispersal of siberian Jays. J. Anim. Ecol. 70, 317–324 doi: 10.1046/j.1365-2656.2001.00490.x
Emlen, S. T., and Wrege, P. H. (1988). The role of kinship in helping decisions among white-fronted bee-eaters. Behav. Ecol. Sociobiol. 23, 305–315. doi: 10.1007/BF00300577
Farabaugh, S. M., Brown, E. D., and Hughes, J. M. (1992). Cooperative territorial defense in the Australian magpie, Gymnorhina tibicen (Passeriformes, Cracticidae), a group-living songbird. Ethology 92, 283–292. doi: 10.1111/j.1439-0310.1992.tb00966.x
Finn, P. G., and Hughes, J. M. (2001). Helping behaviour in Australian magpies, Gymnorhina tibicen. Emu 101, 57–63. doi: 10.1071/MU00066
Floyd, R. B., and Woodland, D. J. (1981). Localization of soil dwelling scarab larvae by the black-backed magpie, Gymnorhina tibicen (Latham). Anim. Behav. 29, 510–517. doi: 10.1016/S0003-3472(81)80112-1
Fulton, G. R. (2006). Plural-breeding Australian Magpies Gymnorhina tibicen dorsalis nesting annually in the same tree. Aust. F. Ornithol. 23, 198–201.
Gilchrist, J. S. (2007). Cooperative behaviour in cooperative breeders: costs, benefits, and communal breeding. Behav. Processes 76, 100–105. doi: 10.1016/j.beproc.2006.12.013
Gilchrist, J. S. and Russell, A. F. (2007). Who cares? Individual contributions to pup care by breeders vs non-breeders in the cooperatively breeding banded mongoose (Mungos mungo). Behav. Ecol. Sociobiol. 61, 1053–1060. doi: 10.1007/s00265-006-0338-2
Green, J. P., Freckleton, R. P., and Hatchwell, B. J. (2016). Variation in helper effort among cooperatively breeding bird species is consistent with Hamilton's rule. Nat. Commun. 7:12663. doi: 10.1038/ncomms12663
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
Griffith, S. C., Owens, I. P. F., and Thuman, K. A. (2002). Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. doi: 10.1046/j.1365-294X.2002.01613.x
Hamilton, W. D. (1963). The evolution of Altruistic behavior. Am. Soc. Nat. 97, 354–356. doi: 10.1086/497114
Hatchwell, B. J. (2009). The evolution of cooperative breeding in birds: kinship, dispersal and life history. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 3217–3227. doi: 10.1098/rstb.2009.0109
Hatchwell, B. J., Russell, A. F., MacColl, A. D. C., Ross, D. J., Fowlie, M. K., and McGowan, A. (2004). Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav. Ecol. 15, 1–10. doi: 10.1093/beheco/arg091
Heinsohn, R., and Cockburn, A. (1994). Helping is costly to young birds in cooperatively breeding white-winged choughs. Proc. R. Soc. B Biol. Sci. 256, 293–298. doi: 10.1098/rspb.1994.0083
Heinsohn, R., and Legge, S. (1999). The cost of helping. Trends Ecol. Evol. 14, 53–57. doi: 10.1016/S0169-5347(98)01545-6
Hidayat, O. (2018). Understanding Relationship Between Group Size and Territory Size by a Social-Living Species, the Western Australian Magpie (Cracticus tibicen dorsalis). Dissertation, The University of Western Australia (Perth, WA).
Hughes, J. M., Hesp, J. D. E., Kallioinen, R., Kempster, M., Lange, C. L., Hedstrom, K. E., et al. (1996). Differences in social behaviour between populations of the australian magpie gymnorhina tibicen. Emu 96, 65–70. doi: 10.1071/MU9960065
Hughes, J. M., Mather, P. B., Toon, A., Ma, J., Rowley, I., and Russell, E. (2003). High levels of extra-group paternity in a population of Australian magpies Gymnorhina tibicen: evidence from microsatellite analysis. Mol. Ecol. 12, 3441–3450. doi: 10.1046/j.1365-294X.2003.01997.x
Innes, K. E., and Johnston, R. E. (1996). Cooperative breeding in the white-throated magpie-jay. How do auxiliaries influence nesting success? Anim. Behav. 51, 519–533. doi: 10.1006/anbe.1996.0057
Ishigame, G., Baxter, G. S., and Lisle, A. T. (2006). Effects of artificial foods on the blood chemistry of the Australian magpie. Austral Ecol. 31, 199–207. doi: 10.1111/j.1442-9993.2006.01580.x
Johnstone, R., and Storr, G. (2004). Handbook of Western Australian Birds. (Perth, WA: Western Australian Museum).
Johnstone, R. A. (2011). Load lightening and negotiation over offspring care in cooperative breeders. Behav. Ecol. 22, 436–444. doi: 10.1093/beheco/arq190.
Kaplan, G. (2004). Australian Magpie: Biology and Behaviour of an Unusual Songbird. (Melbourne: CSIRO Publishing).
Kaplan, G., and Rogers, L. J. (2013). Stability of referential signalling across time and locations: testing alarm calls of Australian magpies (Gymnorhina tibicen) in urban and rural Australia and in Fiji. PeerJ 1:e112. doi: 10.7717/peerj.112
Kingma, S. A. (2017). Direct benefits explain interspecific variation in helping behaviour among cooperatively breeding birds. Nat. Commun. 8, 1–8. doi: 10.1038/s41467-017-01299-5
Kingma, S. A., Santema, P., Taborsky, M., and Komdeur, J. (2014). Group augmentation and the evolution of cooperation. Trends Ecol. Evol. 29, 476–484. doi: 10.1016/j.tree.2014.05.013
Koenig, W. D., Walters, E. L., and Haydock, J. (2011). Variable helper effects, ecological conditions, and the evolution of cooperative breeding in the acorn woodpecker. Am. Nat. 178, 145–158. doi: 10.1086/660832
Kokko, H., Johnstone, R. A., and Clutton-Brock, T. H. (2001). The evolution of cooperative breeding through group augmentation. Proc. Biol. Sci. 268, 187–196. doi: 10.1098/rspb.2000.1349
Krakauer, A. H. (2005). Kin selection and cooperative courtship in wild turkeys. Nature 434, 69–72. doi: 10.1029/2003GC000568
Le Vin, A. L., Mable, B. K., Taborsky, M., Heg, D., and Arnold, K. E. (2011). Individual variation in helping in a cooperative breeder: Relatedness versus behavioural type. Anim. Behav. 82, 467–477. doi: 10.1016/j.anbehav.2011.05.021.
MacColl, A. D. C., and Hatchwell, B. J. (2002). Temporal variation in fitness payoffs promotes cooperative breeding in long-tailed tits Aegithalos caudatus. Am. Nat. 160, 186–194. doi: 10.1086/341013
Martin, P., and Bateson, P. (1993). Measuring Behaviour: An Introductory Guide. 2nd ed. (Cambridge: Cambridge University Press). doi: 10.1017/CBO9781139168342
Mazerolle, M. J. (2015). Model Selection and Multimodel Inference Based on (Q)AIC(c). R package. Available online at: https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf (accessed January, 15, 2019).
McGowan, A., Hatchwell, B. J., and Woodburn, R. J. W. (2003). The effect of helping behaviour on the survival of juvenile and adult long-tailed tits Aegithalos caudatus. J. Anim. Ecol. 72, 491–499. doi: 10.1046/j.1365-2656.2003.00719.x
Meade, J., Nam, K. B., Beckerman, A. P., and Hatchwell, B. J. (2010). Consequences of “load-lightening” for future indirect fitness gains by helpers in a cooperatively breeding bird. J. Anim. Ecol. 79, 529–537. doi: 10.1111/j.1365-2656.2009.01656.x
Mirville, M. O., Kelley, J. L., and Ridley, A. R. (2016). Group size and associative learning in the Australian magpie (Cracticus tibicen dorsalis). Behav. Ecol. Sociobiol. 70, 417–427. doi: 10.1007/s00265-016-2062-x
Nelson-Flower, M. J., Flower, T. P., and Ridley, A. R. (2018). Sex differences in the drivers of reproductive skew in a cooperative breeder. Mol. Ecol. 27, 2435–2446. doi: 10.1111/mec.14587
Pike, K. N. (2016). How Much do Helpers Help? Variation in Helper Behaviour in the Cooperatively Breeding Western Australian magpie. Dissertation, The University of Western Australia (Perth, WA).
R Core Team (2018). R: A Language and Environment for Statistical Computing. Available at: http://www.r-project.org/ (accessed January, 15, 2019).
Reyer, H.-U. (1986). Breeder-helper-interactions in the pied kingfisher reflect the costs and benefits of cooperative breeding. Behaviour 96, 277–303. doi: 10.1163/156853986X00522
Richards, S. A. (2005). Testing ecological theory using the information-theoretic approach : examples and cautionary results. Ecology 86, 2805–2814. doi: 10.1890/05-0074
Ridley, A. R., and Huyvaert, K. P. (2007). Sex-biased preferential care in the cooperatively breeding Arabian babbler. J. Evol. Biol. 20, 1271–1276. doi: 10.1111/j.1420-9101.2007.01356.x
Ridley, A. R., Nelson-Flower, M. J., and Thompson, A. M. (2013). Is sentinel behaviour safe? An experimental investigation. Anim. Behav. 85, 137–142. doi: 10.1016/j.anbehav.2012.10.017
Ridley, A. R., and Raihani, N. J. (2007). Variable postfledging care in a cooperative bird: causes and consequences. Behav. Ecol. 18, 994–1000. doi: 10.1093/beheco/arm074
Ridley, A. R., and Raihani, N. J. (2008). Task partitioning increases reproductive output in a cooperative bird. Behav. Ecol. 19, 1136–1142. doi: 10.1093/beheco/arn097
Ridley, A. R., and van den Heuvel, I. (2012). Is there a difference in reproductive performance between cooperative and non-cooperative species? A southern African comparison. Behaviour 149, 821–848. doi: 10.1163/1568539X-00003005
Riehl, C. (2013). Evolutionary routes to non-kin cooperative breeding in birds. Proc. Biol. Sci. 280:20132245. doi: 10.1098/rspb.2013.2245
Riehl, C., and Strong, M. J. (2015). Social living without kin discrimination: experimental evidence from a communally breeding bird. Behav. Ecol. Sociobiol. 69, 1293–1299. doi: 10.1007/s00265-015-1942-9
Rollinson, D. D. J., and Jones, D. N. (2002). Variation in breeding parameters of the Australian magpie Gymnorhina tibicen in suburban and rural environments. Urban Ecosyst. 6, 257–269. doi: 10.1023/B:UECO.0000004826.52945.ed
Russell, A. F., Langmore, N. E., Gardner, J. L., and Kilner, R. M. (2008). Maternal investment tactics in superb fairy-wrens. Proc. R. Soc. B-Biological Sci. 275, 29–36. doi: 10.1098/rspb.2007.0821
Russell, A. F., Sharpe, L. L., Brotherton, P. N. M., and Clutton-Brock, T. H. (2003). Cost minimization by helpers in cooperative vertebrates. Proc. Natl. Acad. Sci. U. S. A. 100, 3333–3338. doi: 10.1073/pnas.0636503100
Sankamethawee, W., Gale, G. A., and Hardesty, B. D. (2009). Post-fledgling survival of the cooperatively breeding puff-throated bulbul (Alophoixus pallidus). Condor 111, 675–683. doi: 10.1525/cond.2009.090006
Symonds, M. R. E., and Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13–21. doi: 10.1007/s00265-010-1037-6
Tarwater, C. E., and Brawn, J. D. (2010). The post-fledging period in a tropical bird : patterns of parental care and survival. J. Avian Biol. 41, 479–487. doi: 10.1111/j.1600-048X.2010.05006.x
Thompson, A. M., and Ridley, A. R. (2013). Do fledglings choose wisely? An experimental investigation into social foraging behaviour. Behav. Ecol. Sociobiol. 67, 69–78. doi: 10.1007/s00265-012-1426-0
Van de Loock, D., Strubbe, D., De Neve, L., Githiru, M., Matthysen, E., and Lens, L. (2017). Cooperative breeding shapes post-fledging survival in an Afrotropical forest bird. Ecol. Evol. 7, 3489–3493. doi: 10.1002/ece3.2744
Veltman, C. J., and Carrick, R. (1990). Male-biased dispersal in Australian magpies. Anim. Behav. 40, 190–192. doi: 10.1016/S0003-3472(05)80682-7
Vézina, F., and Williams, T. D. (2002). Metabolic costs of egg production in the European starling (Sturnus vulgaris). Physiol. Biochem. Zool. 75, 377–385. doi: 10.1086/343137
Visser, M. E., and Lessells, C. M. (2001). The costs of egg production and incubation in great tits (Parus major). Proc. R. Soc. B Biol. Sci. 268, 1271–1277. doi: 10.1098/rspb.2001.1661
Wiley, H. R., and Rabenold, K. N. (1984). The evolution of cooperative breeding by delayed reciprocity and queuing for favorable social positions. Evolution 38, 609–621. doi: 10.1111/j.1558-5646.1984.tb00326.x
Woxvold, I. A., Mulder, R. A., and Magrath, M. J. L. (2006). Contributions to care vary with age, sex, breeding status and group size in the cooperatively breeding apostlebird. Anim. Behav. 72, 63–73. doi: 10.1016/j.anbehav.2005.08.016
Wright, J., Berg, E., de Kort, S. R., and Khazin, V. (2001a). Safe selfish sentinels in a cooperative bird. J. Anim. Ecol. 70, 1070–1079. doi: 10.1046/j.0021-8790.2001.00565.x
Wright, J., Maklakov, A. A., and Khazin, V. (2001b). State-dependent sentinels: an experimental study in the Arabian babbler. Proc. R. Soc. B Biol. Sci. 268, 821–826. doi: 10.1098/rspb.2000.1574
Wright, J., McDonald, P. G., Te Marvelde, L., Kazem, A. J. N., and Bishop, C. M. (2010). Helping effort increases with relatedness in bell miners, but “unrelated” helpers of both sexes still provide substantial care. Proc. R. Soc. B Biol. Sci. 277, 437–445. doi: 10.1098/rspb.2009.1360
Young, A. J., Carlson, A. A., and Clutton-Brock, T. (2005). Trade-offs between extraterritorial prospecting and helping in a cooperative mammal. Anim. Behav. 70, 829–837. doi: 10.1016/j.anbehav.2005.01.019
Zöttl, M., Chapuis, L., Freiburghaus, M., and Taborsky, M. (2013a). Strategic reduction of help before dispersal in a cooperative breeder. Biol. Lett. 9, 20120878–20120878. doi: 10.1098/rsbl.2012.0878
Keywords: cooperative breeding, helping behavior, social and individual traits, Western Australian magpie, individual variation, contributions to care
Citation: Pike KN, Ashton BJ, Morgan KV and Ridley AR (2019) Social and Individual Factors Influence Variation in Offspring Care in the Cooperatively Breeding Western Australian Magpie. Front. Ecol. Evol. 7:92. doi: 10.3389/fevo.2019.00092
Received: 19 October 2018; Accepted: 11 March 2019;
Published: 12 April 2019.
Edited by:
Camilla Anne Hinde, Wageningen University & Research, NetherlandsReviewed by:
Michelle L. Hall, The University of Melbourne, AustraliaCopyright © 2019 Pike, Ashton, Morgan and Ridley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyana N. Pike, a3lhbmEucGlrZUBteS5qY3UuZWR1LmF1
orcid.org/0000-0001-9259-2899
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.