- 1Psychology Program, SUNY Polytechnic Institute, Utica, NY, United States
- 2Department of Biological Sciences, Nova Southeastern University, Ft. Lauderdale, FL, United States
- 3Department of Biology and Chemistry, SUNY Polytechnic Institute, Utica, NY, United States
In polygynandrous species, males face the trade-off between the pursuit of increased mating opportunities and securing paternity. Within such systems, males need to accurately assess the social composition of the local environment to maximize fitness. Here, we investigated this capability in the water strider (Aquarius remigis), a semi-aquatic insect known to exhibit a broad spectrum of mating behaviors and inhabit a socially diverse and changing environment. Using a combination of methodological designs to track both within- and between-subject effects, individuals remained in same-sex housing prior to being exposed to slightly biased operational sex ratios (2:1 vs. 1:2) with or without prior cohabitation to determine the effects on mating duration. Results show that males were sensitive to these subtle differences in social conditions, mating for longer periods within male-biased environments, but this was true only under conditions with prior cohabitation. In particular, when individuals could acclimate to the testing environment, mating duration dropped precipitously in female-biased conditions. These findings do not support the view that male water striders have consistent behavioral syndromes, and instead show that individuals are able to differentiate between, and adaptively respond to, small changes in the local sex ratio. In addition to improving our understanding of the plasticity of male mating behavior in this species, this study offers new insights for future laboratory research studying reproductive competition across a diverse range of polygynandrous animals.
Introduction
Male fitness is typically associated with the number of reproductive partners acquired (Parker, 1979; Gavrilets et al., 2001; Chapman et al., 2003). However, in scenarios where both sexes mate with multiple partners (polygynandry), individual matings are not a guarantee of success because of sperm competition (Rubenstein, 1989; Kvarnemo and Ahnesjo, 1996). Therefore, although prolonged mating periods and mate guarding might seem counterintuitive as other potential mating opportunities are lost, extended mating duration and shielding of females can increase the chances of paternity from any given mating (Parker, 1970; Alcock, 1994; Kvarnemo and Ahnesjo, 1996; Danielsson, 1998).
The common water strider (Aquarius remigis) provides a model system for the study of mate competition and plasticity as it displays a spectrum of reproductive behaviors (Rubenstein, 1989; Jabłonski and Kaczanowski, 1994; Rowe et al., 1994; Jablonski and Vepsäläinen, 1995; Vepsäläinen and Savolainen, 1995; Vermette and Fairbairn, 2002; Arnqvist and Rowe, 2005). Males are known to mate with females from as short as 15 min to over 10 h (Sih et al., 2002), and freely migrate across social environments that vary considerably in population density and sex ratio (Krupa and Sih, 1993). Mating behaviors have shown to vary based on food availability (Clark, 1988), predation risk (Sih et al., 1990), pool/group size and composition (Montiglio et al., 2017; Sih et al., 2017), as well as access to other mating opportunities (Krupa and Sih, 1993). In particular, mate duration tends to be longer in male-biased environments, whereby males benefit by maximizing the paternity success of rare mating opportunities (Clark, 1988; Krupa and Sih, 1993; Han et al., 2012; Wey et al., 2015; Montiglio et al., 2017). To date, research examining the role of sex ratio on A. remigis mate competition has used between-subjects comparisons across exaggerated sex ratios (of at least 3:1, and vice versa) or only compared male-biased conditions to equal sex ratios.
Here, we employed both within- and between-subjects design comparisons, taking an individual-level approach to investigate the plasticity of male mating behavior when exposed to slightly male- and female-biased sex ratios (2:1 vs. 1:2) with or without a period of cohabitation. Considering that individuals freely disperse among social groups in natural populations (Eldakar et al., 2010a), we predicted that males would accurately assess differences in social composition and demonstrate flexibility in their reproductive behavior. Specifically, we hypothesized males would mate for longer periods within male-biased conditions, and that, consistent with observations in a similar species (G. lacustris; Jablonski and Vepsäläinen, 1995; Vepsäläinen and Savolainen, 1995), this difference would be more pronounced following a period of cohabitation in the testing environment.
Methods
Experiment 1
A total of 101 (63 male, 38 female) water striders were collected at the end of May 2018 from a stream that runs through Marcy, New York. Striders were individually marked with a colored oil-based paint on their dorsum, and separated by sex into housing pools (63.7 × 56.5 × 13.1 cm; 5 cm water depth) that were aerated to maintain the surface tension. Females were housed in one pool, whereas males were evenly distributed across two separate pools. A foam raft was provided for resting and striders were fed crickets ad libitum.
Behavioral testing occurred 2 days later and continued for 2 weeks. Using three additional pools with the same dimensions, one male-biased pool (6:3) and two female-biased pools (3:6) were created each testing day. Starting at 1055 h, selected males were placed into the testing pools for 5 min, at which point females were introduced. Using a within-subjects design, males were first tested in one sex-biased condition and a second time 4–6 days later in the opposite condition (order counterbalanced). Initial selection of males for testing was performed randomly, with an equal number of individuals being exposed to both conditions each day. Due to reduced mating opportunities in the male-biased pools, males failing to mate during initial testing were tested up to two times in this condition. Females were distributed randomly across trials, but were not tested on 2 consecutive days.
During testing, pools were continuously scanned to identify the start and ending of all matings. Mating duration for each male was measured in minutes, and this was averaged in the case of multiple matings within the same condition. Observations concluded at 1500 h, and striders were returned to the sex-specific housing pools. The final sample included 36 males.
Experiment 2
The day after Experiment 1 ended, 45 of the same striders (21 males; 24 females) were distributed randomly across five testing pools of the same dimensions to form two male-biased and three female-biased conditions with the same operational sex ratios (6:3; 3:6), where they remained for the entirety of this experiment. After 3 days of continuous cohabitation, pools were observed for 2 days between 1000 h and 1600 h every 15 min to identify mating pairs and record mating duration. Thus, unlike Experiment 1, striders could acclimate to the social conditions prior to testing. Outside observation periods, striders were provided rafts for resting and fed large crickets ad libitum. Two males in the male-biased pools failed to mate during testing, and thus the final sample included 19 males.
Analyses
Mating duration was compared between sex ratio conditions separately in Experiment 1 and Experiment 2 using Generalized Linear Mixed Models (GLMM), with condition as the fixed factor and individual ID, testing group, and days contained within the same-sex housing prior to testing (Experiment 1) entered as random factors. To assess the effects of cohabitation on mating duration, a separate GLMM was run including condition and experiment as fixed factors and individual ID and testing group as random factors. Correlations were performed to assess the degree of within-subject plasticity in mating duration between conditions and experiments. Total mating duration (in min) was constrained by the respective observation windows (240 and 360 min), and matings that lasted <15 min (Experiments 1 and 2) were considered unsuccessful and not included in the analysis since they rarely result in ejaculation or sperm transfer (Rubenstein, 1989; Campbell and Fairbairn, 2001). For Experiment 1, exclusion criteria also included any matings that were forcibly separated, or disrupted, by rivals (Eldakar and Gallup, 2013).
Results
Experiment 1
Ninety-six matings (55 female-biased; 41 male-biased) occurred during testing. Although males mated for slightly longer in the male-biased pools compared to the female-biased pools (median: 227.50 vs. 216.50 min), this difference was not significant [F(1, 70) = 1.682, p = 0.199]. In addition, there was no correlation between the mating duration observed in one pool and the other (r36 = −0.209, p = 0.222).
Experiment 2
Fifty-one matings (27 female-biased; 24 male-biased) occurred during testing. Unlike experiment 1, there was a significant difference in mating duration between these conditions [F(1, 18) = 14.444, p = 0.001]. In particular, median mating duration within the male-biased pools was over 2.5 times longer compared to the female-biased pools (77.50 vs. 30.00 min).
Comparison Between Experiments
Within-subjects data across both experiments revealed significant main effects for both sex ratio [F(1, 34) = 12.663; p < 0.001] and cohabitation [F(1, 34) = 27.503; p < 0.001], with longer mating durations in male-biased conditions and with no cohabitation. There was also a significant interaction between these two factors [F(1, 34) = 9.640; p = 0.004; Figure 1]. Pair-wise comparisons revealed that following a period of cohabitation, mating duration was significantly shorter in the female-biased pools compared to all other conditions (p < 0.01). No other comparisons were significant. In addition, there was no correlation between the mating duration observed in Experiment 1 and Experiment 2 across the same sex ratio conditions (r19 = −0.126, p = 0.609).
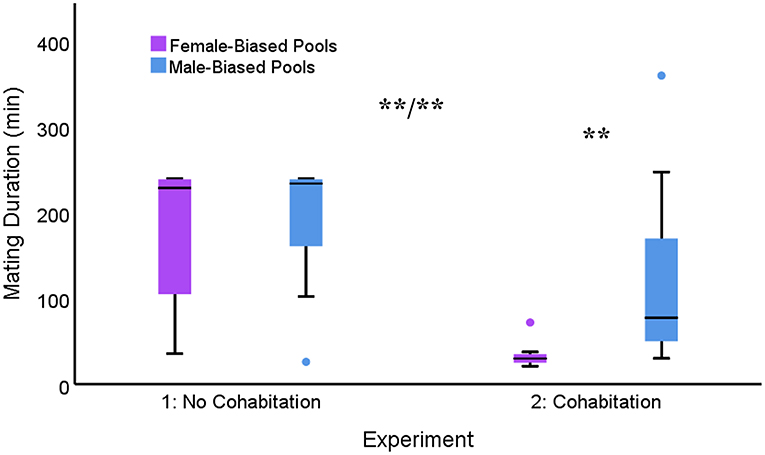
Figure 1. Box plots depicting mating duration across different sex-ratio conditions for males tested in both Experiment 1 (max 240 min) and Experiment 2 (max 360 min). There were significant main effects for sex-ratio condition and experiment (i.e., cohabitation), as well as an interaction between these factors. Median and quartiles presented (**p < 0.01).
Discussion
As predicted from the polygynandrous mating system, and past research in this area (e.g., Clark, 1988; Arnqvist, 1992; Jablonski and Vepsäläinen, 1995; Vepsäläinen and Savolainen, 1995), male water striders were capable of detecting slight differences in the operational sex ratio and adjusted their reproductive behaviors accordingly following a period of cohabitation. Here, we show that previous cohabitation magnified observed differences in mating duration as a function of the operational sex ratio. We speculate that male isolation from females via sex-specific housing, which is typical within comparable laboratory studies, is likely to influence subsequent male mating behaviors by temporarily increasing mating duration over baseline (1:1 sex ratio) conditions. As a result, there was no difference in mating duration across the two conditions in Experiment 1. In contrast, prior cohabitation with females in Experiment 2 allowed males to assess the social surroundings. This modification resulted in a robust difference in mating duration between conditions, with males mating for significantly less time within the female-biased environments. Although these manipulations are not entirely reflective of natural settings, which permit free-movement between groups, our findings suggest that behavioral adjustments in response to slight changes to the social environment are more likely to be identified with extended cohabitation.
Although the focus of this report was on male behavior, females have been shown to influence the duration of mating pairs in related species. In members of a similar genus, Gerris, for example, mating ends once the male withdraws his genitalia (Rowe, 1992; Vepsäläinen and Savolainen, 1995; Rowe and Arnqvist, 1996; Arnqvist and Danielsson, 1999; Danielsson, 2001), but the post-copulatory guarding phase terminates with a struggle initiated by the female (Rowe, 1992; Vepsäläinen and Savolainen, 1995). In contrast, A. remigis water striders have excessively large male genitalia along with reduced counter-adaptation by females (Andersen, 1997; Fairbairn et al., 2003). Males inflate their phallus inside of the female for the entire duration of the pairing, and once sperm transfer is completed the male releases and the pairing ends without any post-copulatory mate guarding (Campbell and Fairbairn, 2001). These effects, coupled with the fact that we tracked individual males and randomly assigned females across treatments, suggest that differences reported here are due to male behavior. Nonetheless, it remains possible that A. remigis females do alter mating duration (Weigensberg and Faribairn, 1994, 1996; Sih et al., 2002), and thus future research could examine how the sexes influence this outcome under differing operational sex ratios.
The revelation that ejaculation occurs at the end of matings in A. remigis, as opposed to near the beginning, calls into question the purpose of extended mating if not for mate guarding. Nonetheless, mating females are still effectively removed from access to rival males regardless of when sperm is transferred, which should benefit mating males in highly competitive environments under natural conditions with dispersal. In addition, female water striders can store sperm for up to 24 days post-mating (Rubenstein, 1989), supporting the observation that mating duration does not reliably predict paternity (Vermette and Fairbairn, 2002). These findings suggest that sperm competition could play a greater role in this species than previously suggested.
The sequential testing of experimental conditions is a limitation to this study. For example, it remains possible that slight aging or prior mating experience influenced subsequent behavior. While previous research has shown that individual differences in male mating behavior remain consistent across the mating season (Eldakar et al., 2009, 2010b), the current findings demonstrate substantial variability in mating duration. Future research could investigate how varying periods of cohabitation alter mating duration and whether individual differences influence this response (Wey et al., 2015; Montiglio et al., 2017). In addition, while this is the first study to our knowledge to compare differences in mating behavior between 2:1 and 1:2 sex ratios in this species, further research could investigate the point at which males can reliably discriminate between sex ratios departing from 1:1 (e.g., 2:3 vs. 3:2, or 4:3 vs. 3:4).
In sum, we used varied methodological approaches to study the plasticity of mating strategies in male water striders based on slight differences in operational sex ratio. Building upon previous research in this area (e.g., Clark, 1988; Arnqvist, 1992; Jablonski and Vepsäläinen, 1995; Vepsäläinen and Savolainen, 1995), these findings reveal that water striders possess both the ability to accurately assess the local competitive landscape, and the behavioral flexibility expected for a species that inhabits heterogeneous stream environments with dynamically changing social conditions.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the Nova Southeastern University repository (https://nsuworks.nova.edu/cnso_bio_datasets/1/).
Ethics Statement
This research was performed using invertebrate animals.
Author Contributions
AG and OE contributed to the conception and design of the research. KP collected and organized the dataset. AG performed the statistical analyses. AG, KP, and OE wrote the paper.
Funding
This study was funded by the College of Arts and Sciences (to AG) and the Student Undergraduate Research Program (to KP) at SUNY Polytechnic Institute.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alcock, J. (1994). Postinsemination associations between males and females in insects: the mate guarding hypothesis. Ann. Rev. Entomol. 39, 1–21. doi: 10.1146/annurev.en.39.010194.000245
Andersen, N. M. (1997). A phylogenetic analysis of the evolution of sexual dimorphism and mating systems in water striders (Hemiptera: Gerridae). Biol. J. Linn. Soc. 61, 345–368. doi: 10.1111/j.1095-8312.1997.tb01796.x
Arnqvist, G. (1992). Pre-copulatory fighting in a water strider: inter-sexual conflict or mate assessment? Anim. Behav. 43, 559–567. doi: 10.1016/S0003-3472(05)81016-4
Arnqvist, G., and Danielsson, I. (1999). Postmating sexual selection: the effects of male body size and recovery period on paternity and egg production rate in a water strider. Behav. Ecol. 10, 358–365. doi: 10.1093/beheco/10.4.358
Campbell, V., and Fairbairn, D. J. (2001). Prolonged copulation and the internal dynamics of sperm transfer in the water strider Aquarius remigis. Can. J. Zool. 79, 1801–1812. doi: 10.1139/z01-148
Chapman, T., Arnqvist, G., Bangham, J., and Rowe, L. (2003). Sexual conflict. Trends Ecol. Evol. 18, 41–47. doi: 10.1016/S0169-5347(02)00004-6
Clark, S. J. (1988). The effects of operational sex ratio and food deprivation on copulation duration in the water strider (Gerris remigis Say). Behav. Ecol. Sociobiol. 23, 317–322. doi: 10.1007/BF00300578
Danielsson, I. (2001). Antagonistic pre–and post–copulatory sexual selection on male body size in a water strider (Gerris lacustris). Proc. R. Soc. London B: Biol. Sci. 268, 77–81. doi: 10.1098/rspb.2000.1332
Eldakar, O. T., Dlugos, M. J., Holt, G. P., Wilson, D. S., and Pepper, J. W. (2010a). Population structure influences sexual conflict in wild populations of water striders. Behaviour 147, 1615–1631. doi: 10.1163/000579510X510520
Eldakar, O. T., Dlugos, M. J., Pepper, J. W., and Wilson, D. S. (2009). Population structure mediates sexual conflict in water striders. Science 326, 816–816. doi: 10.1126/science.1180183
Eldakar, O. T., and Gallup, A. C. (2013). Mate disruption in the water strider Aquaris remigis Say, 1832 (Hemiptera: Gerridae). Aquat. Insect. 35, 89–97. doi: 10.1080/01650424.2014.948457
Eldakar, O. T., Wilson, D. S., Dlugos, M. J., and Pepper, J. W. (2010b). The role of multilevel selection in the evolution of sexual conflict in the water strider Aquarius remigis. Evolution 64, 3183–3189. doi: 10.1111/j.1558-5646.2010.01087.x
Fairbairn, D. J., Vermette, R., Kapoor, N. N., and Zahiri, N. (2003). Functional morphology of sexually selected gentalia in the water strider Aquarius remigis. Can. J. Zool. 81, 400–413. doi: 10.1139/z03-021
Gavrilets, S., Arnqvist, G., and Friberg, U. (2001). The evolution of female mate choice by sexual conflict. Proc. R. Soc. London B: Bio. Sci. 268:531539. doi: 10.1098/rspb.2000.1382
Han, C. S., Kang, C. K., Shin, H. S., Lee, J. H., Bae, M. R., Lee, S. I., and Jablonski, P. G. (2012). Insects perceive local sex ratio in the absence of tactile or visual sex-specific cues. Behav. Ecol. Sociobiol. 66, 1285–1290. doi: 10.1007/s00265-012-1382-8
Jabłonski, P., and Kaczanowski, S. (1994). Influence of mate-guarding duration on male reproductive success: an experiment with irradiated water strider (Gerris lacustris) males. Ethology 98, 312–320. doi: 10.1111/j.1439-0310.1994.tb01079.x
Jablonski, P., and Vepsäläinen, K. (1995). Conflict between sexes in the water strider, Gerris lacustris: a test of two hypotheses for male guarding behavior. Behav. Ecol. 6, 388–392.
Krupa, J. J., and Sih, A. (1993). Experimental studies on water strider mating dynamics: spatial variation in density and sex ratio. Behav. Ecol. Sociobiol. 33, 107–120. doi: 10.1007/BF00171662
Kvarnemo, C., and Ahnesjo, I. (1996). The dynamics of operational sex ratios and competition for mates. Trends Ecol. Evol. 11, 404–408. doi: 10.1016/0169-5347(96)10056-2
Montiglio, P. O., Wey, T. W., Chang, A. T., Fogarty, S., and Sih, A. (2017). Correlational selection on personality and social plasticity: morphology and social context determine behavioural effects on mating success. J. Anim. Ecol. 86, 213–226. doi: 10.1111/1365-2656.12610
Parker, G. A. (1970). Sperm competition and its evolutionary consequences in the insects. Biolo. Rev. 45, 525–567. doi: 10.1111/j.1469-185X.1970.tb01176.x
Parker, G. A. (1979). “Sexual selection and sexual conflict” in Sexual Selection and Reproductive Competition in Insects. Eds M. S. Blum and N. A. Blum (New York, NY: Academic Press), 123–166.
Rowe, L. (1992). Convenience polyandry in a water strider: foraging conflicts and female control of copulation frequency and guarding duration. Anim. Behav. 44, 189–202. doi: 10.1016/0003-3472(92)90025-5
Rowe, L., and Arnqvist, G. (1996). Analysis of the causal components of assortative mating in water striders. Behav. Ecol. Sociobiol. 38, 279–286. doi: 10.1007/s002650050243
Rowe, L., Arnqvist, G., Sih, A., and Krupa, J. J. (1994). Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol. 9, 289–293. doi: 10.1016/0169-5347(94)90032-9
Rubenstein, D. I. (1989). Sperm competition in the water strider, Gerris remigis. Anim. Behav. 38, 631–636. doi: 10.1016/S0003-3472(89)80008-9
Sih, A., Krupa, J., and Travers, S. (1990). An experimental study on the effects of predation risk and feeding regime on the mating behavior of the water strider. Am. Nat. 135, 284–290. doi: 10.1086/285044
Sih, A., Lauer, M., and Krupa, J. J. (2002). Path analysis and the relative importance of male– female conflict, female choice and male–male competition in water striders. Anim. Behav. 63, 1079–1089. doi: 10.1006/anbe.2002.2002
Sih, A., Montiglio, P.-O., Wey, T. W., and Fogarty, S. (2017). Altered physical and social conditions produce rapidly reversible mating systems in water striders. Behav. Ecol. 28, 632–639 doi: 10.1093/beheco/arx021
Vepsäläinen, K., and Savolainen, R. (1995). Operational sex ratios and mating conflict between the sexes in the water strider Gerris lacustris. Am. Nat. 146, 869–880.
Vermette, R., and Fairbairn, D. J. (2002). How well do mating frequency and duration predict paternity success in the polygynandrous water strider Aquarius remigis?. Evolution 56, 1808–1820. doi: 10.1111/j.0014-3820.2002.tb00195.x
Weigensberg, I., and Faribairn, D. J. (1994). Conficts of interest between the sexes: a study of mating durations in a semiaquatic bug. Anim. Behav. 48, 893–901. doi: 10.1006/anbe.1994.1314
Weigensberg, I., and Faribairn, D. J. (1996). The sexual arms race and phenotypic correlates of mating success in the waterstrider Aquarius remigis (Hemitpera: Gerridae). J. Insect Behav. 9:307–319. doi: 10.1007/BF02213873
Keywords: sperm competition, operational sex ratio, male-male competition, conditional strategies, sexual selection
Citation: Gallup AC, Pietruch K and Eldakar OT (2019) Plasticity of Mating Duration in Response to Slightly Biased Operational Sex Ratios in the Water Strider (Aquarius remigis): The Effect of Cohabitation Under Standard Laboratory Conditions. Front. Ecol. Evol. 7:75. doi: 10.3389/fevo.2019.00075
Received: 17 September 2018; Accepted: 27 February 2019;
Published: 21 March 2019.
Edited by:
Carlos Alonso Alvarez, Spanish National Research Council (CSIC), SpainReviewed by:
Inger Suzanne Prange, Appalachian Wildlife Research Institute, United StatesPiotr Jablonski, Seoul National University, South Korea
Copyright © 2019 Gallup, Pietruch and Eldakar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew C. Gallup, YS5jLmdhbGx1cEBnbWFpbC5jb20=
 Andrew C. Gallup
Andrew C. Gallup Krista Pietruch
Krista Pietruch Omar Tonsi Eldakar
Omar Tonsi Eldakar