- 1Department of Biology, University of North Carolina at Greensboro, Greensboro, NC, United States
- 2Department of Psychology, University of Wisconsin, Madison, WI, United States
An ongoing question related to the evolution of monogamy is how behavioral traits that characterize individuals in monogamous species evolve, and whether monogamy influences the evolution of these traits. One of the most important models for the study of monogamy in mammals is the California mouse (Peromyscus californicus) that uses ultrasonic vocalizations (USVs) in multiple behavioral contexts, including pair-bonding and courtship. Because the genus Peromyscus has many species that both use USVs and express a variety of mating systems, we were able to examine the relationship among USVs, and other ecological (e.g., xeric habitat), physiological (testosterone), and behavioral (e.g., boldness) traits across species. We measured USVs from seven species at the Peromyscus Genetic Stock Center and derived character traits associated with the species' ecology, physiology, and behavior from published studies, including those that had used stocks from the Peromyscus Genetic Stock Center. We determined whether there were USV traits that were particular to monogamous species or whether traits other than mating system best predicted USVs. The trait that best predicted USVs was not related to mating system, but rather, species boldness. Bold species produced few aggressive barks (likely a defensive agonistic USV type) at a higher mean fundamental frequency than less bold species. In relation to mating system, the barks in monogamous species were shorter in duration than the barks in non-monogamous species. Our results suggest that boldness of a species has a higher selection on USVs than the species mating system, ecology, or physiology and that selection has acted on agonistic acoustic signals. Because another type of USV, the sustained vocalization or SV type, did not differ among species in spite of mating system differences, and because all species produced bark types, we suggest that the USVs in rodents evolved as general signals that have generally been co-opted for particular functions within the mating system context that differs across species, as opposed to signals that have been shaped by mating system type.
Introduction
Monogamy is a population or species level characteristic that defines a general trait expressed by individuals within the population. There have been many hypotheses for the evolution of monogamy (review by Klug, 2018). Significant ongoing questions related to the evolution of monogamy are how behavioral and/or physiological traits that characterize individuals in monogamous species evolve, and whether the mating system influences the evolution of these traits (Klug, 2018).
One of the most important models for the study of multiple traits in monogamous mammals is the California mouse (Peromyscus californicus). This species has been used as a model for understanding the ecology of monogamy such as territory and space use (e.g., Ribble and Salvioni, 1990; Ribble, 1991) and habitat selection (e.g., Kalcounis-Rueppell and Millar, 2002; Reid et al., 2013). Research has extended to studies of the behaviors associated with monogamy (e.g., Ribble, 1991; Gubernick and Nordby, 1993; Becker et al., 2012; Gleason et al., 2012; Pultorak et al., 2017, 2018) and associated paternal care (e.g., Gubernick and Teferi, 2000; Kingsbury et al., 2012; Jašarević et al., 2013; Bales and Saltzman, 2016; Stockley and Hobson, 2016; West and Capellini, 2016). Finally, the monogamous behavior of California mice has been explored from the perspective of the physiology, including endocrinology, and neurobiology of monogamy (e.g., Gubernick and Nelson, 1989; Insel et al., 1991; Bester-Meredith et al., 1999; Glasper and DeVries, 2005; Oyegbile and Marler, 2005, 2006; Fuxjager et al., 2010; Gleason and Marler, 2010, 2012; Pultorak et al., 2015; Cushing, 2016).
There are many studies searching for correlations between mating systems and multiple and varied traits. One trait that appears relevant to the monogamous mating system of the California mouse that has not been investigated in this context, is its use of acoustic communication. In the California mouse, ultrasonic vocalizations (USVs) are used for both courtship and pair bonding (Pultorak et al., 2015, 2017, 2018), and USVs are related to transient testosterone increases (T pulses) both through rapid mechanisms (Pultorak et al., 2015) and long-term effects (Timonin et al., 2018). In the presence of an unknown female, a single T pulse increases the proportion of sweeps (defined below; reviewed in Kalcounis-Rueppell et al., 2018a) produced by unpaired males and decreases the proportion of sweeps produced by pair-bonded males (Pultorak et al., 2015). Thus, the behavioral trait of USV production is correlated with both hormonal and mating status of the male producing the USVs, and USV production provides a potential mechanism for pair bond maintenance. The role of pair bond maintenance may be facilitated by the potential for individual recognition of sustained vocalization (SV) calls (defined below; reviewed in Kalcounis-Rueppell et al., 2018a). There is also evidence that females may prefer longer SVs (Pultorak et al., 2017) because shorter SVs are associated with physical aggression (Pultorak et al., 2018; Rieger and Marler, 2018).
USVs in California mice may therefore represent a trait associated with monogamous mating and offer a model for testing the influence of the trait on the mating system and the selective pressures of the mating system on the trait. The North American genus Peromyscus contains over 50 nominal mouse species (Bedford and Hoekstra, 2015) with considerable variation in mating systems and all species tested produce USVs. There are three main types of USVs; sweeps, sustained vocalizations (SVs), and barks also known as “squeaks” (reviewed in Kalcounis-Rueppell et al., 2018a,b). Simple sweeps are one-syllable, typically downward frequency-modulated, short calls with a peak frequency of 40 kHz. Complex sweeps are also frequency-modulated with multiple peaks but exhibit a higher peak frequency, a longer duration, and typically contain multiple inflection points. In Peromyscus, as well as in Rattus and Mus, sweeps are used during courtship interactions (Chabout et al., 2015; Musolf et al., 2015; Neunuebel et al., 2015; Pultorak et al., 2015; Kalcounis-Rueppell et al., 2018a). In Peromyscus, as well as in Rattus and Mus, barks are used during agonistic situations (Grimsley et al., 2013; Hurley and Kalcounis-Rueppell, 2018; Kalcounis-Rueppell et al., 2018a) and were specifically identified as defensive aggressive behaviors in P. californicus by Rieger and Marler (2018). SVs are relatively long in duration and relatively flat with little frequency modulation, have a peak frequency of ~20 kHz, and often occur in bouts that vary in the number of calls (1SV, 2SV, SSV, etc.; Kalcounis-Rueppell et al., 2018a) in a given bout (Briggs and Kalcounis-Rueppell, 2011; Pultorak et al., 2017, 2018). Barks start and end in the audible range (~12 kHz) with a peak around 20 kHz, and are broadband, noisy calls. In Peromyscus, SVs have been implicated in both long range (on the order of meters; Briggs and Kalcounis-Rueppell, 2011; Petric and Kalcounis-Rueppell, 2013; Timonin et al., 2018) and short range (order of centimeters) communication (Pultorak et al., 2015, 2017, 2018; Rieger and Marler, 2018). In two species that regularly use SVs in variable behavioral contexts in the field and differ in mating system (the monogamous California mouse and the polygynous brush mouse, P. boylii), an argument has been made that SVs are general contact calls aimed at conspecifics (Briggs and Kalcounis-Rueppell, 2011, reviewed in Petric and Kalcounis-Rueppell, 2013; Kalcounis-Rueppell et al., 2018a). Rodents other than Peromyscus also produce USVs (see Brudzynski, 2018; Dent et al., 2018). It is interesting to note that rats (Rattus norvegicus) produce long low-modulation USVs between 18 and 24 kHz that are typically used in aversive situations considered to represent negative affect, but that can also be produced by males as appetitive contact calls after copulation (review by Wohr, 2018), suggesting a diversity of functions for SV-type calls.
The diversity of mating systems within the genus Peromyscus allows us to examine how traits define mating systems and whether mating systems appear to have driven the evolution of traits. As described above, there is evidence that links USV traits with monogamy [e.g., T and mate status; (Pultorak et al., 2015)]. There is also evidence that links USV traits in non-monogamous systems, such as female-female interactions (Petric and Kalcounis-Rueppell, 2013). In the case of USVs, there may be general signals that are co-opted for different uses under different contexts that manifest as part of a species mating system. For example, in a monogamous species such as P. californicus, SVs may function for pair bond maintenance (Briggs and Kalcounis-Rueppell, 2011; Pultorak et al., 2017, 2018) whereas in a non-monogamous species SVs might facilitate communication between territorial neighbors (Petric and Kalcounis-Rueppell, 2013). Barks, on the other hand, may be general agonistic signals in multiple contexts and evidence suggests that these are universal signals in muroid rodents that signal aggression [reviewed across rodents in Hurley and Kalcounis-Rueppell, 2018; specifically, as defensive aggression in P. californicus Rieger and Marler (2018)]. If USV traits have evolved with the mating system, we would expect that particular USV parameters would correlate with mating system. On the other hand, if USV signals are general signals that are coopted for different uses under different contexts associated with mating system, we would expect that USV parameters would not correlate with mating system. We can test this hypothesis using recordings of USVs from multiple species of Peromyscus and published data on mating systems from those same species of Peromyscus. Peromyscus is an ideal genus for this hypothesis because it is one of the only rodent lineages for which we have information on mating system and USVs across multiple species.
The objective of our study was to examine whether spectral and temporal characters of SVs and barks (two common USV types; reviewed in Kalcounis-Rueppell et al., 2018a) produced by Peromyscus mice could be predicted by mating system. We also examined whether USV traits were correlated with other aspects of social behavior, reproductive physiology, and ecology. We took advantage of existing characterizations of mating system and personality measures published from mice in the genus Peromyscus (summarized in Wey et al., 2017). Our main reason for examining the influence of mating system on USVs is because of the growing body of literature that the monogamous California mouse (P. californicus) uses USVs, in particular sweeps and SVs, to facilitate pair bond formation (reviewed in Pultorak et al., 2015, 2017, 2018; Kalcounis-Rueppell et al., 2018a). This suggests that, at least in monogamous Peromyscus, USVs function in pair bonding and it remains to be tested whether these USVs have similar functions in other species of monogamous Peromyscus. Our reasoning for examining personality was that we might observe differences in the expression of USVs based on boldness and the potential for encountering individuals in nature. For example, there could a species whose individuals, on average, are bolder than individuals of another species (independent of mating system or level of sociality). For physiological measurements, we obtained measurements of T from published literature using two variables, baseline T and social responsiveness of males to females via testes measurements (Marler et al., 2003; Trainor et al., 2006). Both variables are likely to be important in social behavior (review by Marler et al., 2003; Trainor et al., 2006; Gleason et al., 2012). The measurements of social responsiveness are restricted to testes measurements in the current study that reflect the long-term impact of male exposure to females but that could be related to different reproductive strategies. If USVs mediate reproduction and territoriality, then we expect selection on USVs in relation to baseline T and social responsiveness. Data on ecology were characterized by whether the species was primarily found in tropical or subtropical latitudes or in biomes characterized by hot dry seasons (e.g., xeric). Our reasoning was that in xeric habitat there would be less attenuation of signals due to relative low humidity and vegetation, whereas, in mesic habitats signals would attenuate with increased vegetation and humidity. In general, we did not have specific predictions about how each predictor variable would influence the type and spectral/temporal characteristics of USVs. We were instead interested in whether there was evidence of an association among Peromyscus USVs and the behavioral, physiological, and ecological data available from the literature.
Methods
We collected USVs at the Peromyscus Genetic Stock Center (PGSC) as described in Kalcounis-Rueppell et al. (2010) for P. californicus. During the sampling for Kalcounis-Rueppell et al. (2010) we also recorded USVs from six other species, P. melanophrys, P. aztecus, P. eremicus, P. maniculatus bairdii, P. polionotus, and P. leucopus.
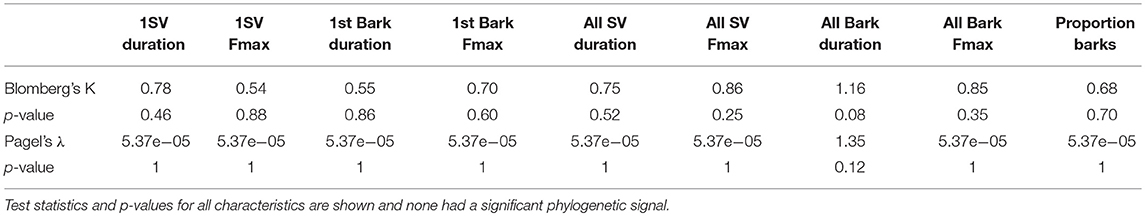
For our dependent variables, we were interested in the USV production and spectral and temporal characteristics of vocalizations. Our independent variables were behavioral, physiological, and ecological characteristics from each species. The seven species of Peromyscus are not phylogenetically independent of one another, therefore, we examined USVs in relation to our behavioral, physiological, and ecological variables while accounting for phylogenetic relationships. The evolutionary relationships of these species are well-resolved, and our tree topology reflects this resolution, with estimated branch lengths, as presented in Bedford and Hoekstra (2015; Figure 1). We used this topology to test for phylogenetic signal and if we did not have data for a particular species, such as no baseline T (as was the case for P. melanophrys), that branch was pruned from the tree for the phylogenetic signal analysis. For all USV characteristics, we tested for phylogenetic signal using Blomberg's K and Pagel's λ (Pagel, 1999; Blomberg et al., 2003).
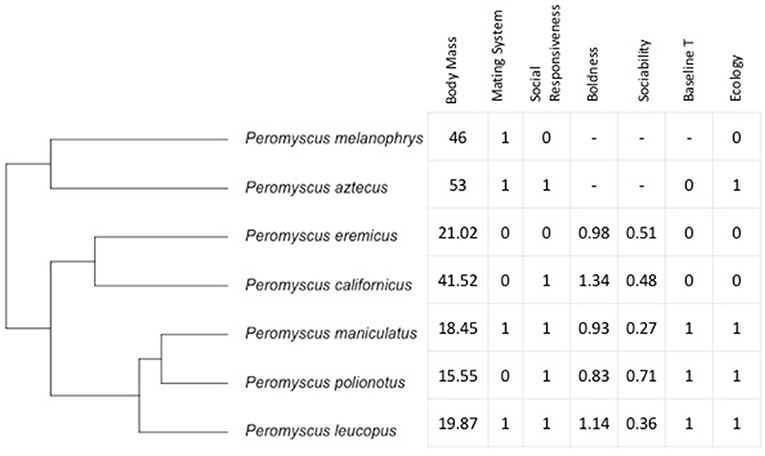
Figure 1. Topology used for phylogenetic analyses (derived from Bedford and Hoekstra, 2015) with predictor variables shown for each species for which there was data available. Body Mass (g); Mating System: 0, predicted monogamous, 1, predicted non-monogamous; Social Responsiveness: 0, testes do not respond to presence of a female, 1, testes do respond to presence of a female; Boldness: unitless measure of latency to exit; Sociability: unitless measure of time spent with same sex stimulus; Baseline T: 0, low, 1, high; Ecology: 0, xeric, 1, mesic. Refer to text for sources of data.
We recorded USVs at the Peromyscus Genetic Stock Center from racks of stock species with a directional microphone (described below) facing the rack of the particular stock. We did not otherwise disturb the cages, beyond limited movement of racks within and between rooms. We recorded USVs opportunistically during a trip to PGSC on March 2nd and 3rd, 2006. As this was opportunistic, we were not able to standardize the length of recording and therefore, we did not calculate rates of USVs and we take averages of all the USVs were able to record from each species. Our recording schedule was primarily driven by our ability to record from isolated racks as opposed to equalizing sampling. In some cases, racks contained adults only in cages and in other cases racks contained adults and their neonate offspring; however, neonate offspring were not isolated from their parents, therefore, recorded calls were more likely produced by adults (reviewed Kalcounis-Rueppell et al., 2018b). Adults were housed following standard protocols for breeding colonies with some species-specific conditions (e.g., adult male female pair of P. californicus were housed together).
USVs from Peromyscus were recorded using a remote bat detector and digital recorder. We recorded with Pettersson D240x ultrasound detectors capable of recording broadband (10–120 kHz) ultrasound (Pettersson Elektronik AB, Uppsala, Sweden). The detector sampled at 307 kHz with 8 bit resolution and was set to continuously record a 1.7 s loop of sound coming through the microphone. Upon detecting any sound in the range of 10–120 kHz the system was triggered and the previous 1.7 s of the sound were slowed down, with time expanded by a factor of ten, and recorded by iRiver digital recorders (iRiver ifp, Reigncom Ltd. Korea) or directly to a laptop. Digital files were downloaded to a computer, converted and saved as.WAV files. Spectrograms of all sound files were played back and visually examined (SonoBat, DNDesign, Arcata, CA) to confirm the file contained Peromyscus USV. We extracted time, amplitude, and frequency characteristics from all spectrograms that contained USVs. The spectrogram rendered by SonoBat used 1,024-point fast Fourier transforms, 192 point windows, and varied window overlap to render the spectrogram with resolution greater than the screen pixel resolution. Our recording system had a frequency response up to 12 kHz and captured ultrasound up to 120 kHz (with the time expansion factor of 10). Maximum frequency resolution of the spectrographic analysis was 154 kHz.
We examined all of the recorded calls. From each unique bout of calls (a series of sounds made from one individual), we analyzed all calls (for a definition of terms see Kalcounis-Rueppell et al., 2018a). For each call analyzed, we manually placed cursors at the start, end, and at the highest and lowest frequency in the call (four cursors total) in SonoBat to determine duration, bandwidth, starting frequency, ending frequency. SonoBat also extracted the frequency at maximum amplitude (frequency at the loudest part of the call). For each species we calculated the average frequency at maximum amplitude (kHz; hereafter frequency) and the duration of two call types, SVs and barks. We further calculated average frequency and duration of only the first SV call in a bout (1SV) and the first call in any bark bout, including bouts with only one call. In addition to calculating the average across all first calls of barks and SVs, we also calculated averages of all bark calls and all SV calls recorded from each species, regardless of where it occurred in a bout. Thus, from our recordings we calculated the following averages for each species: SVDur (duration of 1SV call), SVFmax (frequency at maximum amplitude of 1SV call), barkDur (duration of first call in a bark bout), barkFmax (frequency at maximum amplitude of first call in a bark bout), AllSVDur (duration of all calls in SV bouts), ALLSVFmax, (frequency at maximum amplitude of all calls in SV bouts), ALLbarkdur (duration of all calls in bark bouts), and ALLbarkFmax (frequency at maximum amplitude of all calls in barkbouts). We also calculated the percentage of all recorded bouts that were of the bark type.
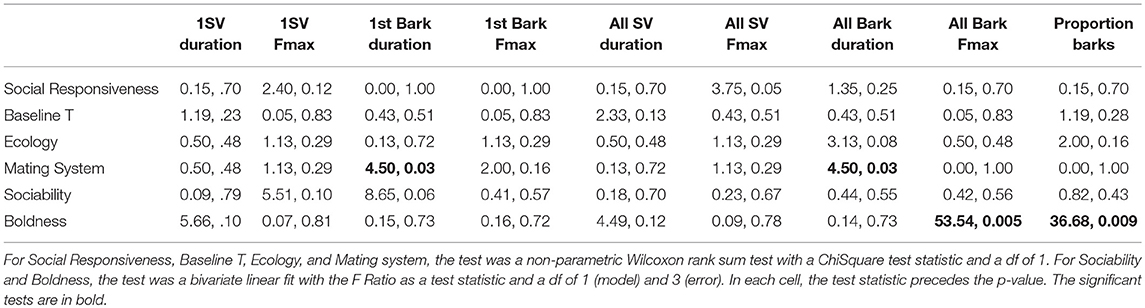
To examine whether social behavior, physiology, and ecology could predict USVs characteristics, we examined available data from the literature as independent variables that characterized each species with determinations as in Figure 1. Note that many measures were obtained from animals housed and bred at PGSC. Specifically, we characterized social behavior based on descriptions of mating system, and measures of social responsiveness, sociality, and boldness. The mating system of these species is described in other comparative studies (Trainor et al., 2006; Wey et al., 2017). Briefly, of the seven species we examined, there is evidence or a prediction of monogamy for P. californicus (Ribble, 1991), P. eremicus (Eisenberg, 1963; Glasper and DeVries, 2005), and P. polionotus (Foltz, 1981). All other species were labeled as not monogamous based on lack of evidence for monogamy, or evidence/prediction of promiscuity or polygyny (e.g., Millar and Xia, 1991; Ribble and Millar, 1996). Measures of social responsiveness were from Trainor et al. (2006) who determined whether males of each species would respond to the presence of a female with changes in reproductive tissue weights and plasma testosterone (T) levels. Specifically, Trainor et al. (2006) determined changes in testes mass (a T sensitive tissue) when housed with an opposite sex conspecific (Trainor et al., 2006). Measures of boldness and sociability were determined from Wey et al. (2017) and include latency to emerge and time spent with a conspecific, same sex stimulus mouse. Notably, measures from Trainor et al. (2006) and Wey et al. (2017) were conducted on mice from the same stocks used in our current study for measuring USVs at the PGSC. We obtained baseline T (no staged social interactions with unfamiliar animals) from Marler et al. (2003). Samples for the baseline T levels were originally obtained from animals from PGSC and Marler laboratory colonies using previously established methods (Trainor and Marler, 2001). P. aztecus, P. californicus and P. leucopus had T levels similar to those found in previous studies (e.g., Klein and Nelson, 1997; Demas and Nelson, 1998; Bester-Meredith and Marler, 2003). Data on ecology were generally characterized as in Trainor et al. (2006) based on whether the distribution of the species was primarily in tropical or subtropical latitudes or in biomes that were characterized by hot dry seasons (e.g., xeric). For a map of the distributions of all seven species see Bedford and Hoekstra (2015). We altered the characterization from Trainor et al. (2006) into a binary categorization by considering the “intermediate” type as mesic such that only “xeric” and “mesic” habitats were used.
We were also interested in whether body size could explain USV characteristics. We therefore used male body mass data as presented in Trainor et al. (2006; Table 1 averaged between “single” and “pair”) and Wey et al. (2017; as presented) as an independent variable. Male body mass was available for all seven species whereas female body mass was only available for five species. For consistency, we used male body mass as the proxy for species body mass. Our results did not change if we used the average between male and female body mass (instead of only male body mass) for the five species where these data were available.
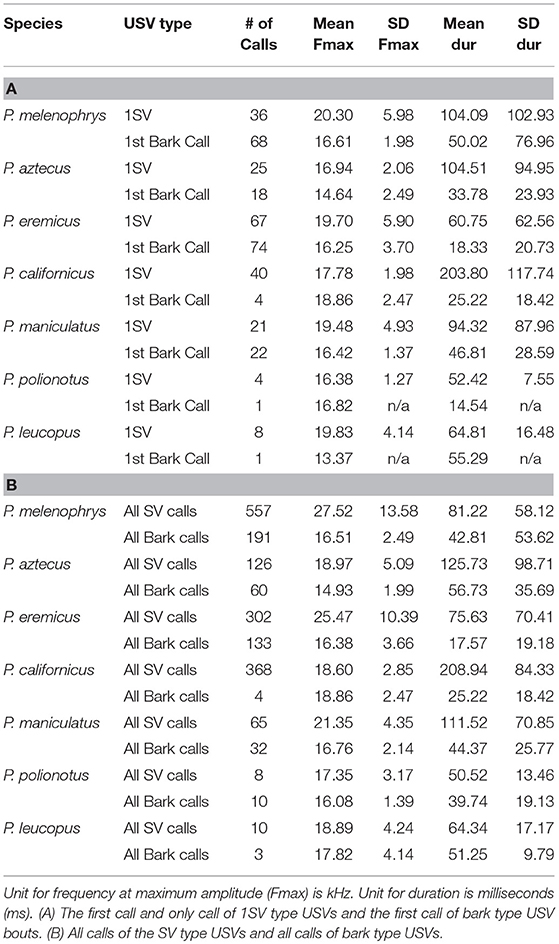
Table 1. Mean (±) SD values for USV calls recorded from seven species at the Peromyscus Genetic Stock Center in 2006.
We used R (R Core Team, 2017) with the packages APE (Paradis et al., 2004) and phytools (Revell, 2012) for all of our phylogenetic analyses. We used JMP 13.1.0 (SAS Institute Inc., Cary, NC) for all other analyses. Our calculations of mean values to assign to each species were performed within Microsoft Excel. We used linear models for our continuous predictor variables (boldness, sociability) and non-parametric Wilcoxon signed-rank tests for our categorical, binary variables (mating system, social responsiveness, baseline T, and ecology). Our rejection criterion was set at p < 0.05.
Results
We recorded 1,882 calls of vocalizations from the seven Peromyscus species of interest. Calls were not recorded evenly among species. The majority of calls were from P. melanophrys (N = 748), P. californicus (N = 385), and P. eremicus (N = 435), a moderate number of sequences of vocalizations were recorded from P. aztecus (N = 186) and P. maniculatus (N = 97), and only a few calls were recorded from P. leucopus (N = 13) and P. polionotus (N = 18). We could not assign calling rates to species because we were recording (1) from racks of different numbers of mice per species, (2) for different time periods based on opportunity, and (3) using a ten times expansion recording system that recorded for 1.7 s on a loop and played back for 17 s once a call was a recorded. Of the 1,882 calls, 188 were from bark bouts and 201 were from 1SV bouts. Thus, our sample size for first call of bark bouts was 188 and our sample size of 1SV calls was 201. The sample size for individual SVs from all SV bouts (such as 1SV, 2SV, 3SV, etc.) from the seven species was 1,436. Our sample size for all individual barks from all bark bouts, was 433. There were additionally 13 frequency modulated (FM) calls recorded from P. californicus that were not included in this analysis because we did not record them from all species, and these very quiet calls are difficult to record without placing the microphone inside the cage (e.g., Pultorak et al., 2015). These are also calls that occur between members of a pair at close proximity (Pultorak et al., 2018) and were more likely produced because P. californicus were housed as pairs. These calls are also produced by other species (reviewed in Kalcounis-Rueppell et al., 2018a) and may occur during courtship (Pultorak et al., 2015). The mean and standard deviation values with sample sizes for all species are presented in Table 1. There was a correlation between the duration of 1SVs and the duration of all SV calls (r = 0.96, P < 0.001) and therefore we only included duration of 1SVs in further analyses.
There was no significant phylogenetic signal in any of our USV characteristics using either Blomberg's K (all values of P > 0.08; Table 2) or Pagel's λ (all values of P > 0.09; Table 2), therefore, for all predictive analyses we did not use phylogenetic corrections or approaches. There was also no effect of body mass on any of the USV characteristics (all values of P > 0.05) and this result was the same whether considering only male body mass or both male and female body mass.
Mating system was a predictor of bark USV duration (Chi Square = 4.5, df = 1, P = 0.03; see below), however, it was not the strongest predictor of USV characteristics. The strongest predictor of USV characteristics was species boldness [F(1, 3) = 53.54, P = 0.005]. When considering all barks recorded, species that tend to be bolder produced barks at higher mean fundamental frequencies (frequency at maximum amplitude) than species with low levels of boldness [F(1, 3) = 53.54, P = 0.005; Table 3, Figure 2A]. In addition, species with high levels of boldness produced proportionally fewer barks compared to species with low levels of boldness [F(1, 3) = 36.68, P = 0.009; Table 3), when considering all barks recorded (Figure 2B). Thus, bold species produced fewer barks in proportion to SVs, but produced barks at higher frequencies than species that are less bold. Species predicted to be monogamous produced bark calls that were shorter in duration than species that were not predicted to be monogamous and this was true for both the first call in a bark bout and all individual barks (Chi Square = 4.5, df = 1, P = 0.03 for both variables; Figure 3). There was no effect of measures of sociality, social responsiveness, baseline T, or ecology on USV characteristics (Table 3). In addition, none of the variables predicted SV characteristics (Table 3).
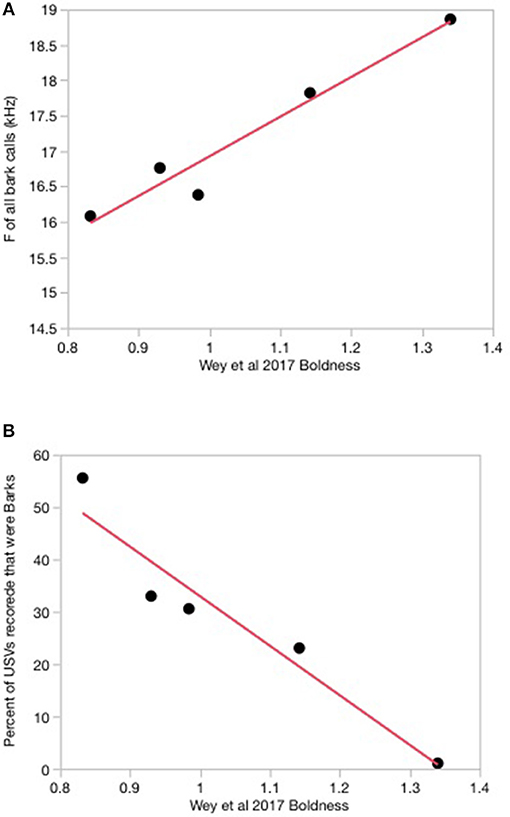
Figure 2. Significant relationships between the trait of boldness from Wey et al. (2017) and bark USVs recorded from 5 species at the Peromyscus Genetic Stock Center in 2006. Species represented, and boldness values are in Figure 1. (A) Linear Fit: F of all bark calls = 11.32 + 5.60* Boldness; F = frequency at maximum amplitude. (B) Linear Fit: Percent of calls that were Barks = 127.60 – 94.63*Boldness.
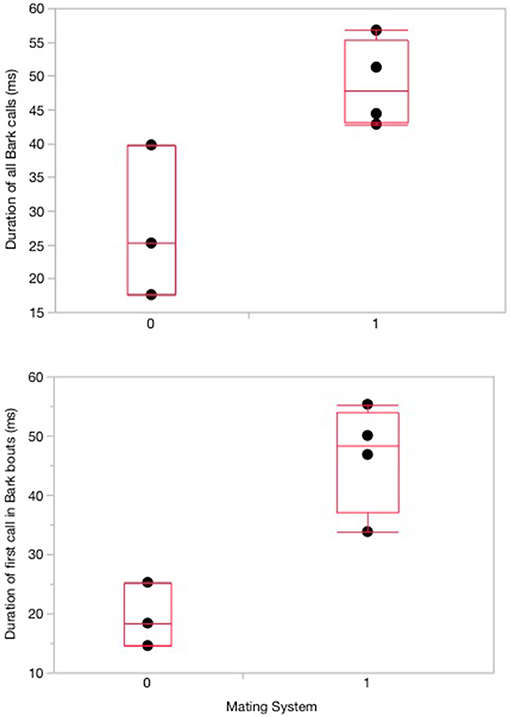
Figure 3. Box and whisker plots with medial values from Wilcoxon signed-ranked test. Significant relationships between predicted mating system and duration of bark USVs recorded from 7 species at the Peromyscus Genetic Stock Center in 2006. Species represented and predicted mating systems for each are in Figure 1.
Discussion
Our analysis was a broad phylogenetic approach to examining whether monogamy was important for shaping the spectral and temporal characteristics of USVs across species of Peromyscus mice. Such a phylogenetic approach across species of Peromyscus has been successful for identifying other traits such as paternal investment as a predictor of monogamy (Jašarević et al., 2013; Wey et al., 2017), but this is the first study examining USVs. As we only calculated a single value for each species, we may not have captured within species variation. It would be very interesting to examine our predictor and response variables within specific Peromyscus species. Regardless, our study serves as a starting point to ask questions about the evolution of ultrasonic vocalizations across rodent species, and the selective pressures that maintain them.
In spite of our broad approach, we found a very compelling result suggesting that personality is more important in defining USV types and parameters than other physiological, behavioral, and ecological species level traits. Specifically, boldness has an impact on the spectral frequency and number of barks produced. Species that are bold produce fewer barks at a higher mean fundamental frequency. Barks are agonistic calls within rodents. Thus, bold species produce agonistic calls at higher frequencies, but produce these calls less often, at least under the conditions under which these calls were collected.
This finding initially is counter intuitive as bold species are typically expected to produce agonistic calls more often. However, our results suggest that at least within Peromyscus mice, the bold species infrequently relies on defensive agonistic calls, and when they do, the effective distance of the calls is lower because higher frequencies attenuate more quickly. The simplest explanation for our result is that bold species are more likely to encounter con- and/or hetero-specifics than non-bold species, and when in close proximity, the calls are not generally broadcasted but instead directed at local individuals. This is a testable hypothesis. Another way of explaining this result is that barks may serve different antagonistic functions depending on the species that is producing them. Bold species may produce high frequency barks because they are more effective for short distance and more directed communications. High frequency barks of bold species would be directed toward a specific individual or group and more likely to occur if individuals are closer together. On the other hand, non-bold species may produce lower frequency barks that travel further and are broadcast as very general signals, that may help with avoidance of close encounters. This also explains the result we observed showing that bold species produce proportionally fewer barks than non-bold species because in this scenario bold species would only produce barks during close encounters, whereas non-bold species would produce barks regularly as advertisement with the function of decreasing interactions. Lastly, if barks represent defensive aggression as suggested by Rieger and Marler (2018), then one could speculate that more bold species would display less appeasement behavior, hence the reduction in number of barks.
We can again only speculate, but it is interesting that in one of the species that is characterized as non-monogamous and bold there appears to be significant variation in how bold individuals are, as defined by how frequently they scent marked an open arena (Fuxjager et al., 2010). Males that were “bolder” and scent marked more were more likely to win a male-male encounter in resource supplemented environments. Peromyscus leucopus expresses significant variation in territorial tendencies in both the laboratory and field (Wolff, 1985; Oyegbile and Marler, 2006). The bolder individuals may be more likely to adopt territorial behavior in which individuals maintain exclusive territories. If the goal is not to establish a dominant-subordinate interaction but rather maintain exclusion, then a call that moderates aggression would be less likely to be used. It might however be a very useful call for moderating aggression between members of a pair bond.
Our results might suggest that agonistic acoustic signaling is used more frequently as a communication modality for less-bold species, however this would need to be tested. This hypothesis could be tested both inter- and intra-specifically. For example, rodent species that are bold would be predicted to use barks less in a natural or experimental context than other species that are not as bold. Similarly, within species, those individuals that were bold should not use as many barks as those individuals that are not bold.
While boldness was most closely associated with the number of barks produced, it is intriguing to note that the duration of barks was of shorter duration in monogamous species. Signal duration increases the effective distance of this signal, and our results suggest that monogamous species use barks with a lower effective distance. The most parsimonious explanation for this result is that for our monogamous species, the barks we recorded were between pairs that were housed together (e.g., they were close together) whereas the barks that we recorded from non-monogamous species were from mice between cages (e.g., they were farther apart) or mice that were not pair-bonded. It is interesting that pair-bonded mice would produce barks with one another but there are behavioral contexts for barks within pairs that are regularly recorded in the wild (Kalcounis-Rueppell, unpublished data). Moreover, while barks decrease over time between an introduced male and female P. californicus as they bond, intersexual barks do occur (Pultorak et al., 2018). Thus, this result suggests that barks are shorter between mates. This hypothesis could be tested intraspecifically because we predict that barks produced in the presence of a mate (for example near or in a nest site) would be shorter than barks produced alone or in the presence of a non-mate (for example at a territorial boundary). We will be able to test this hypothesis with our ongoing field study.
The two variables that related to T were not associated with mating systems. The first is baseline levels of T. Male California mice have surprisingly low levels of baseline T that originally seemed consistent with a framework proposed by Wingfield et al. (1990) suggesting that low baseline levels of testosterone occur because of less male-male-competition (see also Hau, 2007). A previous, non-phylogenetic comparison was made between baseline levels of a smaller subset of monogamous and non-monogamous Peromyscus species (Marler et al., 2003) that did not reveal a pattern related to mating system (Marler et al., 2003). A later comparison was made to investigate whether social stimulation or day length activates testes growth (Trainor et al., 2006) among monogamous and non-monogamous Peromyscus. We used plasticity in size of testes (a testosterone sensitive tissue) as a proxy for T level changes in response to social conditions that could be related to monogamous vs. polygynous mating systems. Testosterone responsiveness to social stimuli as measured in the plasma (review by Gleason et al., 2012) was not tested in the current phylogenetic analysis. Overall there was no evidence that testosterone levels were related to mating systems either in the form of baseline or socially responsive levels.
Whether considering boldness or mating system as a predictor of the USV trait, it is interesting that effects were only seen in bark calls as opposed to SV calls. Peromyscus produce three main calls types, SVs, barks, and sweeps (Kalcounis-Rueppell et al., 2018a). As in other species of mice, including lab mice, barks are normally associated with distress or agonistic interactions (Grimsley et al., 2013; Kalcounis-Rueppell et al., 2018a). In contrast, although there is much to learn about SV calls, they are much more likely to be used as non-aggressive contact calls, especially those with a small number of calls in a bout (e.g., 1SV, 2SV, 3SV; Kalcounis-Rueppell et al., 2018a). Our results suggest that it is the agonistic or distress calls that have more selective pressure to be species specific; in other words, selection might be stronger on the agonistic components of calling as opposed to the affiliative. We cannot draw any conclusions here about sweep calls because they were not recorded as part of this study, likely because sweeps were present but high frequency calls attenuate quickly. Overall, sweeps are difficult to detect unless the microphone is very close to the mouse producing the call (Kalcounis-Rueppell et al., 2018a). We also may have obtained different results if we had considered bouts instead of calls. In our analysis we combined all SV call types independent of whether they were part of a 2SV, 3SV, 4SV etc. Species specific responses may have been found had we examined our USVs in this way, however this was not feasible because we would not have had a balanced data set across species for all the SV types due to sample size. This is something to consider because selection may not be acting on the SV call itself but instead on the way the SV is arranged in bouts.
Our recording paradigm was one of complete eavesdropping. We did not measure age, sex, reproductive condition and we did not stage agnostic or affiliative contexts for any of the mice we were recording. Thus, there was no experimental reason for the mice to be agonistic or affiliative in their cages. Any effects that we observed in barks in this study would likely be enhanced if we had experimentally examined aggression. Similarly, any effects that we observed are most likely muted because we know there is less variation in the spectral and temporal characteristics of USVs recorded in the lab when compared to those in nature (Kalcounis-Rueppell et al., 2010). In addition, we only examined five to seven out of over 50 species of Peromyscus and it could be that our results are unique to this set of species we analyzed. For these reasons, future studies could examine barks in the context of personality in the field under naturally aggressive contexts and include more species of Peromyscus. Another advantage to including more species, would be that the binary scales used herein could be more refined (e.g., distinguishing between obligate and facultative mating patterns and specific ecological associations).
In conclusion, our results suggest that boldness of a species has a higher selection on USVs than the species mating system. Moreover, the effects of boldness and mating system are seen only in defensive barks, as opposed to SV call types. It appears that USVs in rodents evolved as general signals that have been co-opted for particular functions within the mating system context that differs across species.
Ethics Statement
Animals in the Peromyscus Genetic Stock Center are housed and bred under an approved institutional animal care protocol of the University of South Carolina. The Peromyscus Genetic Stock Center is a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International, and in accordance with the Guide for the Care and Use of Laboratory Animals. For our recordings we did not handle animals nor cause any disturbance beyond what they would normally experience under the approved animal care protocol (USC Animal Use Protocol # 1321) that covers their welfare.
Author Contributions
MK-R conceived of the study through discussions with CM and RP. MK-R collected the USV data at the Peromyscus Genetic Stock Center, performed all statistical analyses presented in the study, and wrote the initial draft of the manuscript. RP analyzed all of the Peromyscus USV data that were used as the dependent variables in the study and contributed to the writing of the manuscript. CM contributed to the writing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Janet Crossland at the Peromyscus Genetic Stock Center for facilitating our visit and Tina Wey for discussions. This paper benefitted from 3 careful reviewers and Nancy Solomon. This work was supported by National Science Foundation grants IOS-1132419 and IOS-1355163 to CM and MK-R and IOB-0641530 to MK-R.
References
Bales, K. L., and Saltzman, W. (2016). Fathering in rodents: neurobiological substrates and consequences for offspring. Horm. Behav. 77, 249–259. doi: 10.1016/j.yhbeh.2015.05.021
Becker, E. A., Petruno, S., and Marler, C. A. (2012). A comparison of scent marking between a monogamous and promiscuous species of Peromyscus: pair bonded males do not advertise to novel females. PLoS ONE 7:e32002. doi: 10.1371/journal.pone.0032002
Bedford, N. L., and Hoekstra, H. E. (2015). Peromyscus mice as a model for studying natural variation. eLife 4:e06813. doi: 10.7554/eLife.06813.
Bester-Meredith, J., and Marler, C. A. (2003). Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav. Neurosci. 117, 455–463. doi: 10.1037/0735-7044.117.3.455
Bester-Meredith, J. K., Young, J., and Marler, C. A. (1999). Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm. Behav. 36, 25–38. doi: 10.1006/hbeh.1999.1522
Blomberg, S. P., Garland, T., and Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745.
Briggs, J. R., and Kalcounis-Rueppell, M. C. (2011). Similar acoustic structure and behavioural context of vocalizations produced by male and female California mice in the wild. Anim. Behav. 82, 1263–1273. doi: 10.1016/j.anbehav.2011.09.003
Brudzynski, S. M. (2018) Handbook of Behavioral Neuroscience, Vol. 25. Amsterdam: Elsevier B.V.; Academic Press.
Chabout, J., Sarkar, A., Dunson, D. B., and Jarvis, E. D. (2015). Male mice song syntax depends on social contexts and influences female preferences. Front. Behav. Neurosci. 9:76. doi: 10.3389/fnbeh.2015.00076
Cushing, B. S. (2016). Estrogen receptor alpha distribution and expression in the social neural network of monogamous and polygynous Peromyscus. PLoS ONE 11:e0150373. doi: 10.1371/journal.pone.0150373
Demas, G. E., and Nelson, R. J. (1998). Short-day enhancement of immune function is independent of steroid hormones in deer mice (Peromyscus maniculatus). J. Comp. Physiol. B. 168, 419–426. doi: 10.1007/s003600050161
Dent, M. L., Fay, R. R., and Popper, A. N. (2018) Rodent Bioacoustics Springer Handbook of Auditory Research. Cham: Springer.
Eisenberg, J. F. (1963). The intraspecfic social behavior of some Cricetine rodents of the genus Peromyscus. Am. Midland Nat. 69, 240–246.
Foltz, D. W. (1981). Genetic evidence for long-term monogamy in a small rodent Peromyscus polionotus. Am. Nat. 117, 665–675.
Fuxjager, M. J., Montgomery, J. L., Becker, E. A., and Marler, C. A. (2010). Deciding to win: interactive effects of residency, resources and “boldness” on contest outcome in white-footed mice. Anim. Behav. 80, 921–927. doi: 10.1016/j.anbehav.2010.08.018
Glasper, E. R., and DeVries, A. C. (2005). Social structure influences effects of pair-housing on wound healing. Brain Behav. Immun. 19, 61–68. doi: 10.1016/j.bbi.2004.03.002
Gleason, E. D., Holschbach, M. A., and Marler, C. A. (2012). Compatibility drives female preference and reproductive success in the monogamous California mouse (Peromyscus californicus) more strongly than male testosterone measures. Horm. Behav. 61, 100–107. doi: 10.1016/j.yhbeh.2011.10.009
Gleason, E. D., and Marler, C. A. (2010). Testosterone response to courtship predicts future paternal behavior in the California mouse, Peromyscus californicus. Horm. Behav. 57, 147–154. doi: 10.1016/j.yhbeh.2009.10.006
Gleason, E. D., and Marler, C. A. (2012). A positive link between male testosterone and spacing behavior in pair-bonded California mice. Ethology 118, 1045–1050. doi: 10.1111/eth.12005
Grimsley, J. M., Hazlett, E. G., and Wenstrup, J. J. (2013). Coding the meaning of sounds: contextual modulation of auditory responses in the basolateral amygdala. J. Neurosci. 33, 17538–17548. doi: 10.1523/JNEUROSCI.2205-13.2013
Gubernick, D. J., and Nelson, R. J. (1989). Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm. Behav. 23, 203–210. doi: 10.1016/0018-506X(89)90061-5
Gubernick, D. J., and Nordby, J. C. (1993). Mechanisms of sexual fidelity in the monogamous California mouse, Peromyscus californicus. Behav. Ecol. Sociobiol. 32, 211–219. doi: 10.1007/BF00173779
Gubernick, D. J., and Teferi, T. (2000). Adaptive significance of male parental care in a monogamous mammal. Proc. Biol. Sci. 267, 147–150. doi: 10.1098/rspb.2000.0979
Hau, M. (2007). Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29, 133–144. doi: 10.1002/bies.20524
Hurley, L., and Kalcounis-Rueppell, M. C. (2018). “Ultrasonic vocalizations of mice in the genus Peromyscus,” in Rodent Bioacoustics, Springer Handbook of Auditory Research, eds M. L. Dent, R. R. Fay, and A. N. Popper (Cham: Springer), 202–221.
Insel, T. R., Gelhard, R., and Shapiro, L. E. (1991). The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience 43, 623–630. doi: 10.1016/0306-4522(91)90321-E
Jašarević, E., Bailey, D. H., Crossland, J. P., Dawson, W. D., Szalai, G., and Ellersieck, M. R. (2013). Evolution of monogamy, paternal investment, and female life history in Peromyscus. J. Comp. Psychol. 127, 91–102. doi: 10.1037/a0027936
Kalcounis-Rueppell, M. C., and Millar, J. S. (2002). Partitioning of space, food, and time by syntopic Peromyscus boylii and P. californicus. J. Mammal. 82, 614–625. doi: 10.1644/1545-1542(2002)083<0614:POSFAT>2.0.CO;2
Kalcounis-Rueppell, M. C., Petric, R., Briggs, J. R., Carney, C., Marshall, M. M., and Willse, J. T. (2010). Differences in ultrasonic vocalizations between wild and laboratory California mice (Peromyscus californicus). PLoS ONE 5:e9705. doi: 10.1371/journal.pone.0009705
Kalcounis-Rueppell, M. C., Pultorak, J. D., Blake, B. H., and Marler, C. A. (2018b). “Chapter 14: ultrasonic vocalizations of young mice in the genus Peromyscus” in Handbook of Ultrasonic Vocalizations, ed S. M. Brudzynski (Amsterdam: Academic Press), 149–156.
Kalcounis-Rueppell, M. C., Pultorak, J. D., and Marler, C. A. (2018a). “Chapter 22: ultrasonic vocalizations of mice in the genus Peromyscus,” in Handbook of Ultrasonic Vocalizations, ed S. M. Brudzynski (Amsterdam: Academic Press), 227–235.
Kingsbury, M. A., Gleason, E. D., Ophir, A. G., Phelps, S. M., Young, L. J., and Marler, C. A. (2012). Monogamous and promiscuous rodent species exhibit discrete variation in the size of the medial prefrontal cortex. Brain Behav. Evol. 80, 4–14. doi: 10.1159/000339247
Klein, S. L., and Nelson, R. J. (1997). Sex differences in immunocompetence differ between two Peromyscus species. Amer. J. Physiol. 273, R655–R660. doi: 10.1152/ajpregu.1997.273.2.R655
Klug, H. (2018). Why monogamy? A review of potential ultimate drivers. Front. Ecol. Evol. 6:30. doi: 10.3389/fevo.2018.00030
Marler, C. A., Bester-Meredith, J. K., and Trainor, B. C. (2003). “Paternal behavior and aggression: endocrine mechanisms and nongenomic transmission of behavior,” in Advances in the Study of Behavior, eds P. J. B. Slater, J. S. Rosenblatt, C. T. Snowdon, and T. J. Roper (San Diego, CA: Academic Press), 263–323.
Millar, J. S., and Xia, X. (1991). Genetic evidence of promiscuity in Peromyscus leucopus. Behav. Ecol. Sociobiol. 28, 351–356.
Musolf, K., Meindl, S., Larsen, A. L., Kalcounis-Rueppell, M. C., and Penn, D. J. (2015). Ultrasonic vocalizations of male mice differ among species and females show assortative preferences for male calls. PLoS ONE 10:e0134123. doi: 10.1371/journal.pone.0134123
Neunuebel, J. P., Taylor, A. L., Arthur, B. J., and Egnor, S. E. (2015). Female mice ultrasonically interact with males during courtship displays. Elife 4:e06203. doi: 10.7554/eLife.06203
Oyegbile, T. O., and Marler, C. A. (2005). Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259–267. doi: 10.1016/j.yhbeh.2005.04.007
Oyegbile, T. O., and Marler, C. A. (2006). Weak winner effect in a less aggressive mammal: correlations with corticosterone but not testosterone. Physiol. Behav. 89, 171–179. doi: 10.1016/j.physbeh.2006.05.044
Paradis, E., Claude, J., and Strimmer, K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. doi: 10.1093/bioinformatics/btg412
Petric, R., and Kalcounis-Rueppell, M. C. (2013). Female and male adult brush mice (Peromyscus boylii) use ultrasonic vocalizations in the wild. Behaviour 150, 1747–1766. doi: 10.1163/1568539X-00003118
Pultorak, J. D., Alger, S. J., Loria, S. O., Johnson, A. M., and Marler, C. A. (2018) Changes in behavior ultrasonic vocalizations during pair bonding in response to an infidelity challenge in monogamous California mice. Front. Ecol. Evol. 6:125. doi: 10.3389/fevo.2018.00125
Pultorak, J. D., Fuxjager, M. J., Kalcounis-Rueppell, M. C., and Marler, C. A. (2015). Male fidelity expressed through rapid testosterone suppression of ultrasonic vocalizations to novel females in the monogamous California mouse. Horm.Behav. 70, 47–56. doi: 10.1016/j.yhbeh.2015.02.003
Pultorak, J. D., Matusinec, K. R., Miller, Z. K., and Marler, C. A. (2017). Ultrasonic vocalization production and playback predicts intrapair and extrapair social behaviour in a monogamous mouse. Anim. Behav. 125, 13–23. doi: 10.1016/j.anbehav.2016.12.023
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Reid, R. E. B., Greenwald, E. N., Wang, Y., and Wilmers, C. C. (2013). Dietary niche partitioning by sympatric Peromyscus boylii and P. californicus in a mixed evergreen forest. J. Mammal. 94, 1248–1257. doi: 10.1644/13-MAMM-A-104.1
Revell, L. J. (2012). Phytools: an R package for phylogenetic comparative biology (and other things): phytools: R package. Methods Ecol. Evol. 3, 217–223. doi: 10.1111/j.2041-210X.2011.00169.x
Ribble, D. O. (1991). The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 29, 161–166.
Ribble, D. O., and Millar, J. S. (1996). The mating system of northern populations of Peromyscus maniculatus as revealed by radiotelemetry and DNA fingerprinting. Ecoscience 3, 423–428.
Ribble, D. O., and Salvioni, M. (1990). Social organization and nest co-occupancy in Peromyscus californicus a monogamous rodent. Behav. Ecol. Sociobiol. 26, 9–16.
Rieger, N. S., and Marler, C. A. (2018). The function of ultrasonic vocalizations during territorial defense by pair-bonded male and female California mice. Anim. Behav. 135, 97–108. doi: 10.1016/j.anbehav.2017.11.008
Stockley, P., and Hobson, L. (2016). Paternal care and litter size coevolution in mammals. Proc. Biol. Sci. 283:1829. doi: 10.1098/rspb.2016.0140
Timonin, M., Kalcounis-Rueppell, M., and Marler, C. A. (2018). Male California mice in the field administered testosterone pulses at the nest site increase ultrasonic vocalizations at the nest site. Ethology. 124, 804–815. doi: 10.1111/eth.12812
Trainor, B. C., and Marler, C. A. (2001). Testosterone, paternal behavior, and aggression in the monogamous California mouse, Peromyscus californicus. Horm. Behav. 40, 32–42. doi: 10.1006/hbeh.2001.1652
Trainor, B. C., Martin, I. I., Greiwe, K. M., Kuhlman, J. R., and Nelson, R. J. (2006). Social and photoperiod effects on reproduction in five species of Peromyscus. Gen. Comp. Endocrinol. 148, 252–259. doi: 10.1016/j.ygcen.2006.03.006
West, H. E., and Capellini, I. (2016). Male care and life history traits in mammals. Nat. Commun. 7:11854. doi: 10.1038/ncomms11854
Wey, T. W., Vrana, P. B., and Mabry, K. E. (2017). Mating system as a possible driver of behavioral diversity in Peromyscus. Behav. Ecol. Sociobiol. 71, 1–163. doi: 10.1007/s00265-017-2392-3
Wingfield, J. C., Hegner, R. E., Dufty, A. M. Jr, and Ball, G. F. (1990). The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846.
Wohr, M. (2018). Ultrasonic communication in rats: appetitive 50-kHz ultrasonic vocalizations as social contact calls. Behav. Ecol. Sociobiol. 72:14. doi: 10.1007/s00265-017-2427-9
Keywords: communication, ultrasound, USV, mating system, personality, mouse, boldness
Citation: Kalcounis-Rueppell MC, Petric R and Marler CA (2018) The Bold, Silent Type: Predictors of Ultrasonic Vocalizations in the Genus Peromyscus. Front. Ecol. Evol. 6:198. doi: 10.3389/fevo.2018.00198
Received: 24 June 2018; Accepted: 09 November 2018;
Published: 27 November 2018.
Edited by:
Nancy G. Solomon, Miami University, United StatesReviewed by:
David Ribble, Trinity University, United StatesMeng Zhao, Stanford University, United States
Copyright © 2018 Kalcounis-Rueppell, Petric and Marler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matina C. Kalcounis-Rueppell, bWNrYWxjb3VAdW5jZy5lZHU=
 Matina C. Kalcounis-Rueppell
Matina C. Kalcounis-Rueppell Radmila Petric
Radmila Petric Catherine A. Marler
Catherine A. Marler