- Kharkevich Institute for Information Transmission Problems, Russian Academy of Sciences, Moscow, Russia
Animal females are generally assumed to prefer males that win fights. However, a growing number of studies in numerous animal taxa demonstrate no correlation between male fighting ability and their attractiveness, or even female preferences for fight losers. One of the methods to measure female preferences employs no-choice tests that evaluate a female's latency to mating when placed with a single male. Considering that courtship behavior generally contains multimodal signaling, we analyzed 19 behavioral elements demonstrated by both sexes of the cricket Gryllus bimaculatus during courtship. To estimate male dominance status, males were preliminarily tested in two rounds of fights. Females mounted males with different fighting ability equally often, but the latencies from the start of antennal contact to mount were shorter in fight losers than fight winners. During courtship, males with high fighting ability demonstrated one of the elements of agonistic display, rocking the body, more frequently, and for longer durations than males with low fighting ability. This element was negatively correlated with singing in fight winners but was positively correlated with singing a courtship song in fight losers. Rocking is thereby suggested to have multiple signaling functions in agonistic and courtship behavior. The song parameters were poorly related with male mating success. Fight winners, rather than fight losers, tended to produce a higher number of calling chirps, which could be explained by the inability of males with high fighting ability to quickly shift from aggression to courtship behavior. The results suggest that increased aggression in fight winners is likely to interfere with subsequent courtship.
Introduction
It is generally thought that intrasexual selection (resulting from male-male competition) and intersexual selection (resulting from female choice) are mutually reinforcing processes (Qvarnstrom and Forsgren, 1998; Wong and Candolin, 2005). In the last quarter of a century, however, various studies have suggested a more intricate relationship between these two processes. Female preference for dominant males has been found in many species (Andersson, 1994; Berglund et al., 1996), in particular, in reptiles (Trivers, 1976), birds (Trail, 1985; Kunc et al., 2006), fishes (Far and Travis, 1986; Bisazza and Marin, 1991), crayfish (Aquiloni et al., 2008), flies (Alcock and Pyle, 1979; Borgia, 1981), and crickets (Simmons, 1986). Female preference for dominant males could have direct benefits if these males provide higher quality resources (Andersson, 1994) or better parental care (Hoelzer, 1989). Female preference for dominant males could also provide indirect benefits if these males sire offspring of superior genetic quality (birds: Norris, 1993; Petrie, 1994; Sheldon et al., 1997; crickets: Wedell and Tregenza, 1999; Bretman et al., 2006).
A growing number of studies, however, demonstrate no correlation between male fighting ability and their attractiveness (Qvarnstrom and Forsgren, 1998). This could occur because dominant males provide less parental care (Forsgren, 1997; Wong, 2004), harm their mates (Moore et al., 2001; Ophir and Galef Jr, 2003), have depleted sperm stores (Pitnick and Markow, 1994; Preston et al., 2001), or are more likely to transmit diseases (Folstad and Karter, 1992). Females could also ignore dominant males because these males might not invest as much in mate attraction or courtship as subordinate males. For example, it was shown in crickets Teleogryllus oceanicus that females even preferred subordinate males: rather than investing more in postcopulatory strategies, the subordinate males invested in an alternative precopulatory mating approach (Thomas and Simmons, 2009).
The most common way to investigate female preferences is conducted using simultaneous choice tests. If possible, these tests should exclude male-male interactions; otherwise it would be difficult to evaluate the relationship between intra- and intersexual selection. In crickets, such studies have been conducted using playback experiments (Rantala and Kortet, 2003) or offering filter papers with pheromones of males with different fighting ability (Kortet and Hedrick, 2005). Investigation of long-distance acoustic signals (calling song) could also allow choice tests without male-male interactions (Hedrick and Bunting, 2014).
In choice tests where a female was placed with two male crickets, the winner of the agonistic encounter was more likely to mate (Gryllus bimaculatus: Simmons, 1986; Acheta domesticus: Nelson and Nolen, 1997; Rantala and Kortet, 2004). It was shown that dominant males could prevent subordinate males from courting the females. Because females do not mate with non-courting males (Alexander, 1961), these experiments cannot measure the free choice of females. Moreover, males in the presence of a female were more likely to initiate fights and their fights were more aggressive than in the absence of a female (G. bimaculatus: Simmons, 1986; Tachon et al., 1999; G. veletis: Fitzsimmons and Bertram, 2013; G. assimilis: Montroy et al., 2016). If females prefer to mate with fight winners, thenincreased male aggression might be reinforced by intersexual selection.
A second way to measure female preferences is to conduct no-choice tests. These tests usually measure a female's latency to mating when placed with a single male. In no-choice tests conducted on various species of crickets, the results vary. In A. domesticus (Savage et al., 2005) and G. assimilis (Loranger and Bertram, 2016), males that win fights were shown to be more attractive to females. By contrast, Nelson and Nolen (1997) and Shackleton et al. (2005) showed that females did not prefer males that won fights in A. domesticus and T. commodus. In T. oceanicus, subordinate males upregulated the quantity of a number of cuticular compounds that increase male mating success; at the same time, they produced ejaculates of lower quality and sired fewer offspring than dominant males (Thomas and Simmons, 2009). Thus, the contact pheromones are not always an honest signal of males' quality, and females may not be able to detect this dishonesty.
Many cricket species display a repertoire that includes three structurally distinct signals, termed the calling (a long-range signal), courtship (a close-range signal) and aggression (produced during encounters with other males) songs (Alexander, 1961). In G. bimaculatus, the intensity of calling songs and the repetition rate of chirps and pulses was positively correlated with male size, and larger males gained more matings (Simmons, 1986, 1988). In the field, however, pulse rate was negatively related to male size, while the duration of pulses was positively related to size (Simmons and Zuk, 1992). In G. integer, the percentage of time spent singing calling songs was either negatively correlated with aggressiveness (for males caught in the field) or unrelated to aggressiveness (for males raised in the lab) (Hedrick and Bunting, 2014). The parameters of the courtship song have been more poorly investigated in relation to the cricket dominance status. No effect of body size on the dominant frequency of the courtship song was found in G. bimaculatus (Miyashita et al., 2016). At the same time, higher rates and durations of ticks (the parameters preferred by females) were positively correlated with high immunocompetence, which may indicate that females might benefit by increasing the parasite resistance of their offspring (Rantala and Kortet, 2003).
In no-choice tests, two parameters of female preferences are usually measured: the percentage of females that mated males and the latency to mating. In the current study, we videotaped male-female interactions and measured many different behavioral elements demonstrated by both sexes in no-choice tests. We hypothesized that males that won fights would behave more aggressively toward females than males that lost fights, and this could be a part of reason decrease mating success of fight winners. Taking into account multisensory courtship signals, we tried to evaluate which sensory modalities, chemical or acoustic, could be a better indicator of the male dominance status. We also analyzed different song parameters to determine whether the songs of dominant and subordinate males differed from each other.
Materials and Methods
Crickets
Experimental animals came from a laboratory stock obtained from the Moscow Zoo culture. This culture was originally obtained in 1985–1990 from the cricket farms and pet food stores in Germany and Great Britain. Since this period, the Moscow Zoo culture has been constantly maintained at more than 1,000 individuals. The size of the laboratory stock varied from 30 to 200 individuals at different times; however, this stock was refreshed from the Zoo culture one–two times per year to reduce the potential effects of inbreeding. The crickets were reared in plastic containers (57 × 39 × 42 cm) at 22–27°C under a 12-h:12-h light/dark cycle. Food (dried amphipods and oatflakes) and water were provided ad libitum. The crickets were separated into individual containers (12 × 12 × 7 cm) not later than 24 h after the imaginal molt. Thus, individuals were physically but not acoustically isolated. All behavioral experiments were conducted on virgin individuals of one to 2 weeks old under dim red lights in a temperature controlled room (25°C).
Estimating the Male Social Rank
Individual males were ranked for fighting ability by methods similar to those of Shackleton et al. (2005), Savage et al. (2005), and Thomas and Simmons (2009). We tested males in blocks, with four randomly selected males in each block. The age difference of males within each block varied from 0 to 3 days. For discrimination between opponents during contests, males within each block were marked individually on the pronotum with correction permanent markers.
Males were tested in two rounds of fights (Data Sheet 2). In the first round, pairs of males were randomly assigned and placed in individual open-top plastic container (15 × 15 × 15 cm). Dominance status was usually established within the first few minutes, when a loser (male with low fighting ability) started to avoid all further aggressive encounters with a winner (male with high fighting ability). This generated two males that won and two males that lost their first round of contests. In the second round, the previous winners were paired (Video Clip 1) and the previous losers were paired (Video Clip 2). Only those males that lost (n = 27) or won (n = 29) both contests were used in subsequent experiments with females. The advantage of this method was that every male always competed against another male with the same recent fight history, since the success in previous fights has been shown to increase the likelihood of victory in subsequent fights in crickets (Khazraïe and Campan, 1999; Hofmann and Stevenson, 2000; Savage et al., 2005). By using this method, we also increased the difference between the males with different fighting ability. In all contests, males were left together for a period of 5–7 min. The floor of the arena was covered with a paper towel, which was replaced after each trial to remove any olfactory cues that might be left by the crickets.
Courtship Test Procedure
After the second round of fights (with an interval varied in the range of 1–7 min), we placed a randomly drawn female with the male, one female—with the double winner, and another—with the double loser. The experiments were performed in a cylindrical open-top arena (15 × 15 × 15 cm), in which the floor was covered with a paper towel, and the walls were formed by a metallic grid. We evaluated female preferences based on the readiness of the female to mount the male. During courtship, the male turns away from the female and presents his abdomen, while continuously stridulating. The female reacts by approaching from behind. The male spreads his hind wings and flattens his abdomen, allowing the female to mount him (Video Clip 3). Mounting of the male by the female is a prerequisite for copulation (Alexander, 1961; Adamo and Hoy, 1994). A male was introduced into the arena, and after about 1 min we introduced a female. Each trial lasted for up to 7 min. All trials were video recorded (Sony DCR-TRV 355E), and the video signals were transferred to a PC for analysis of courtship. In male–female interactions, each specimen was used only once.
Courtship behavior was analyzed with the BORIS program (Friard and Gamba, 2016). According to this program, the behavior type can be defined as a “state event” and a “point event” (having and not having duration, correspondingly). Overall, we distinguished 15 state and 4 point events in both male and female behavior (Table 1). All specific elements during male—female interactions were documented as previously described by Adamo and Hoy (1994). We measured the latency from the test start to the onset of the element (for all events), and the duration of the element (for state events only). We also calculated the latencies from the first antennal contact to male rocking, singing, female following and mount (Data Sheet 1).
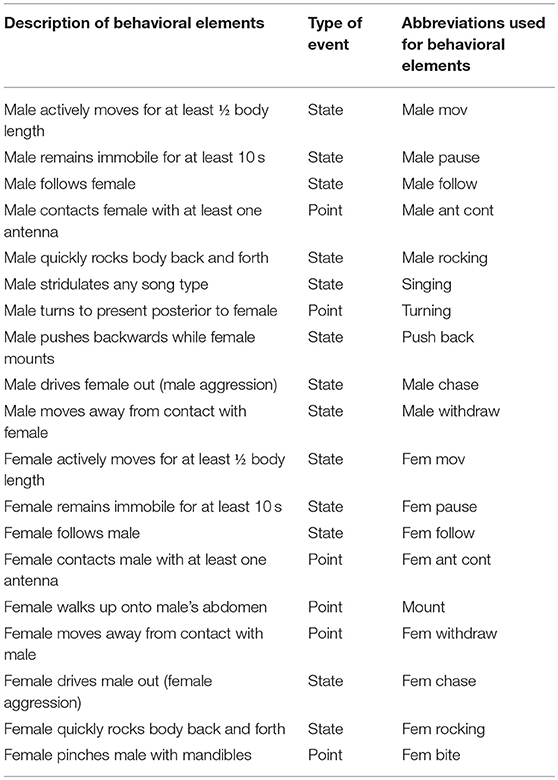
Table 1. Behavioral elements demonstrated by crickets Gryllus bimaculatus during male–female interactions.
Simultaneously with video recording, we conducted song recordings. A microphone (type 4191, 1/2 inch; Bruel & Kjær, Nærum, Denmark) was placed at a height of 8–10 cm from the top of the arena. The output of a conditioning amplifier (Bruel and Kjær 2690) was digitized (100 kHz sampling rate) using a custom-made A/D–D/A interface. The temporal parameters and power spectra of the songs were analyzed with COOLEDIT (Syntrillium, Seattle, WA). During courtship tests, males sometimes produced not only a typical courtship song, but also some elements of calling or rivalry (= aggressive) songs (Figure 1). A courtship song of G. bimaculatus was easy to distinguish from the other two types of the songs: the calling and rivalry songs consisted of chirps containing several pulses; the dominant carrier frequency was ca. 4.5–5.0 kHz (first harmonic). The more variable courtship song was composed of large-amplitude pulses (= ticks) separated by a number of smaller pulses; the energy of ticks was concentrated around 4–5 and 11–16 kHz. The duration of ticks comprised about half of the chirp pulse duration (Rheinlaender et al., 1976; Libersat et al., 1994; Shestakov and Vedenina, 2015). We measured 9 song characters: the ratio of the chirp to tick number, the number of pulses per chirp, the duration and period of the chirp pulses, the dominant frequency of chirps, the duration and period of ticks, the relative amplitude of the courtship pulses and ticks, and the dominant frequency of ticks (Data Sheet 3).
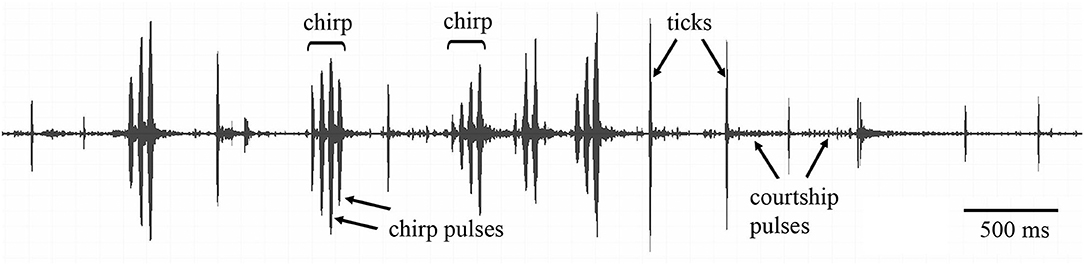
Figure 1. Oscillogram of a song produced during male—female interactions in Gryllus bimaculatus. During courtship, the males could produce not only the elements of courtship song (ticks and courtship pulses), but also the elements of calling song (chirps and chirp pulses).
Results
The Occurrence of Courtship Elements
Most of the specific elements recorded during male–female interactions occurred in more than 50% of trials (Figure 2). Among them, however, only the antennal contact was demonstrated by both sexes in almost all trials. There were no significant differences between males with different fighting ability in the occurrence of any behavioral elements (Fisher's Exact Test, two-tailed; p > 0.05), except for rocking (p = 0.04): fight winners rocked the body (Video Clip 4) more often than fight losers (in 83 vs. 56% of trials). Fight winners also followed females more often (in 69 vs. 44% of trials) and demonstrated pauses less often (in 69 vs. 89% of trials) than fight losers. These differences were, however, not significant (p = 0.1). The elements that usually preceded the mounting response (singing, turn, pushing back) occurred in 58–74% of experiments. Females mounted winners and losers almost equally often (in 59 and 67% of trials, respectively; p = 0.59).
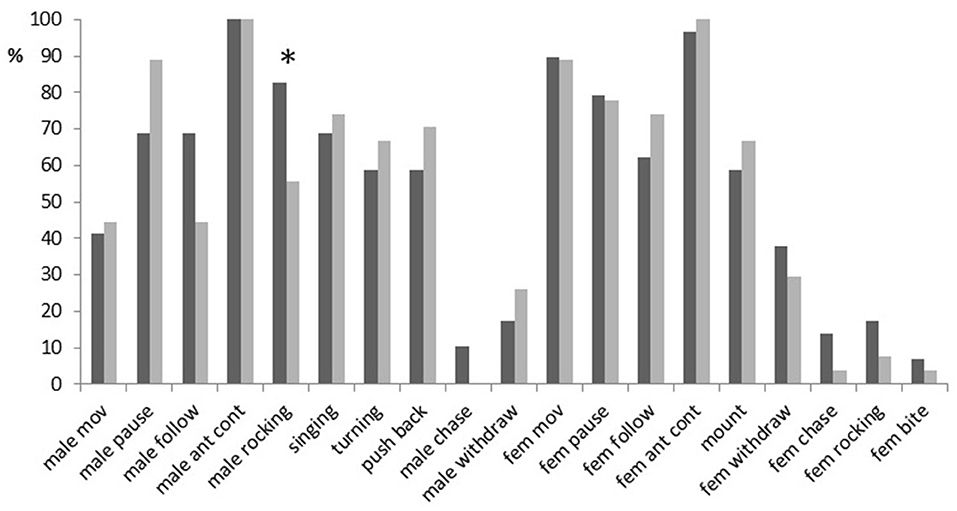
Figure 2. A percentage of trials containing a given behavioral element in courtships of dominant males (black bars) and subordinate males (gray bars) in Gryllus bimaculatus. Abbreviations for behavioral elements are listed in Table 1. Asterisk indicate statistical significance of the differences between dominant and subordinate males (Fisher's Exact Test, two-tailed; *p < 0.05).
Some elements rarely occurred in male–female interactions. Males with high fighting ability moved away (withdrew) from any contacts with females in 17% of the trials, while males with low fighting ability exhibited this behavior in 26% of the trials. Crickets drove conspecifics out (chased) more often in experiments with fight winners (in 10–14% of cases) than in experiments with fight losers (in 0–4% of trials). Females rocked body and bit dominant males more often (in 17 and 7% of trials, respectively) than subordinate males (in 7 and 4%, respectively). None of these differences were statistically significant (0.35 < p < 0.5). We did not analyze latencies or duration of the elements that occurred in < 30% of experiments because of the low sampling number.
The Latencies to Courtship Elements
Analysis of the latencies from the onset of experiment to the start of any element showed many differences between winners and losers. Despite these differences were not significant for all elements (after the correction for the false discovery rate; Benjamini and Hochberg, 1995), the consistent patterns can be found in these differences (Figure 3). For example, winning males started antennal contact (9 s) and rocking (12 s) earlier than losing males (19 and 32 s, respectively). By contrast, fight winners started to sing, follow females, turn and push back later than fight losers (of median values 53 vs. 31 s; 52 vs. 34 s; 61 vs. 42 s; 109 vs. 60 s, respectively). Females started to withdraw earlier from winning than losing males (32 vs. 62 s), and conversely, started to follow winners later than losers (61 vs. 29 s). Females also mount dominant males later than subordinate ones (112 vs. 66 s).
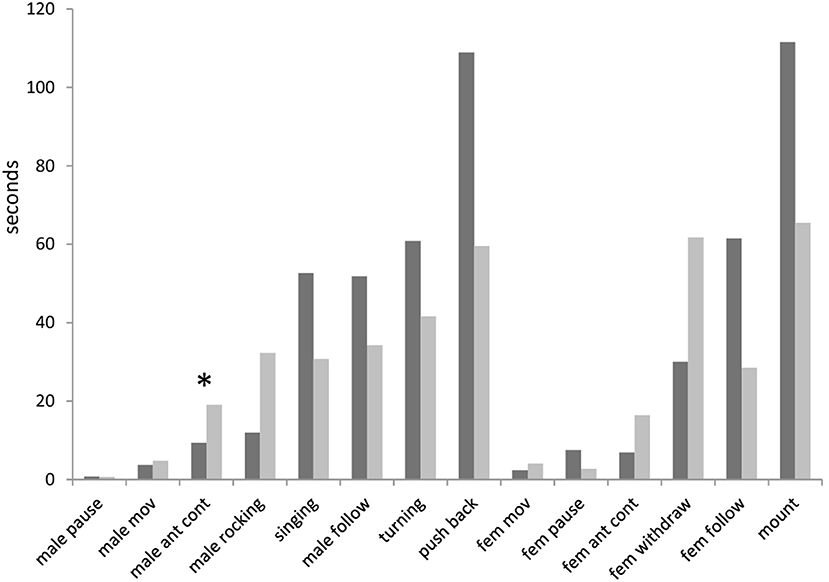
Figure 3. Latencies from the onset of experiment to the start of behavioral elements in courtships of dominant males (black bars) and subordinate males (gray bars) in Gryllus bimaculatus. Experiments with successful courtships are only included (n = 17 for dominant males and n = 18 for subordinate males). Asterisk indicate statistical significance of the differences between dominant and subordinate males (Mann-Whitney U Test; *p < 0.05).
Calculations of the latencies from the onset of antennal contact to the mounting response (Figure 4) revealed significant differences between the males with different fighting ability when conducting Mann–Whitney U-tests (p < 0.03), but not significant differences after the correction for the false discovery rate. The latencies from the onset of male antennal contact to mount were lower in losers (42 s) than winners (89 s). The same was found for the latencies from the onset of female antennal contact to mount (45 s in losers and 89 s in winners).
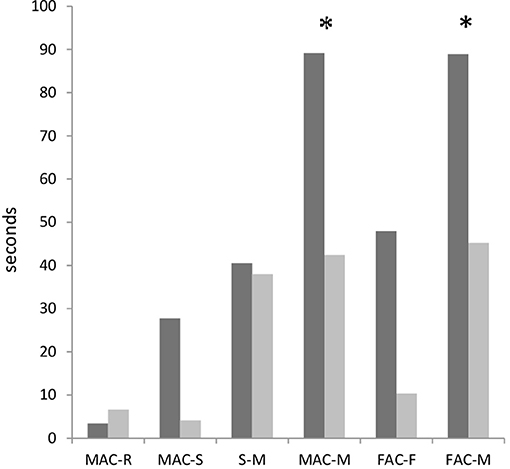
Figure 4. Latencies (median values) from the onset of one to onset of another elements in courtships of dominant males (black bars) and subordinate males (gray bars) in Gryllus bimaculatus. Experiments with successful courtships are only included (n = 17 for dominant males and n = 18 for subordinate males). MAC-R: male antennal contact to rocking; MAC-S: male antennal contact to singing; S-M: singing to mount; FAC-F: female antennal contact to following; FAC-M: female antennal contact to mount. Asterisks indicates statistical significance of the differences between dominant and subordinate males (Mann–Whitney U-Test; *p < 0.05).
We found few correlations between the latencies to different courtship elements in males that won fights. The latencies to male and female antennal contacts highly positively correlated (Spearman rank correlation; r = 0.98, p = 0.0000), and the latency to singing positively correlated with the latencies to pushing back (r = 0.51, p = 0.035) and female movement (r = 0.62, p = 0.01). Notably, we did not find any correlations for the latency to rocking in tests with dominant males. In males that lost fights, more correlations between the latencies to different courtship elements were been found. The latency to rocking positively correlated with latencies to singing and turning (r = 0.86–0.9, p < 0.01). The latency to singing also correlated with latencies to male antennal contact (r = 0.62, p = 0.006), turning (r = 0.93, p = 0.0000), pushing back (r = 0.79, p = 0.0000), following by female (r = 0.66, p = 0.007), and mount (r = 0.79, p = 0.0000). All these correlations were only calculated for successful courtships.
The Duration of Courtship Elements
We found the differences in duration of some courtship elements between males with different fighting ability (Figure 5), but none of these differences was significant after the correction for the false discovery rate. Fight winners demonstrated longer rocking (7% of all courtship duration), singing (23%) and following of females (8%) than fight losers (2, 14 and 4%, respectively). By contrast, fight winners moved less (5%) than fight losers (11%). Also, females withdrew longer from winning males (8%) than from losing males (4.6%).
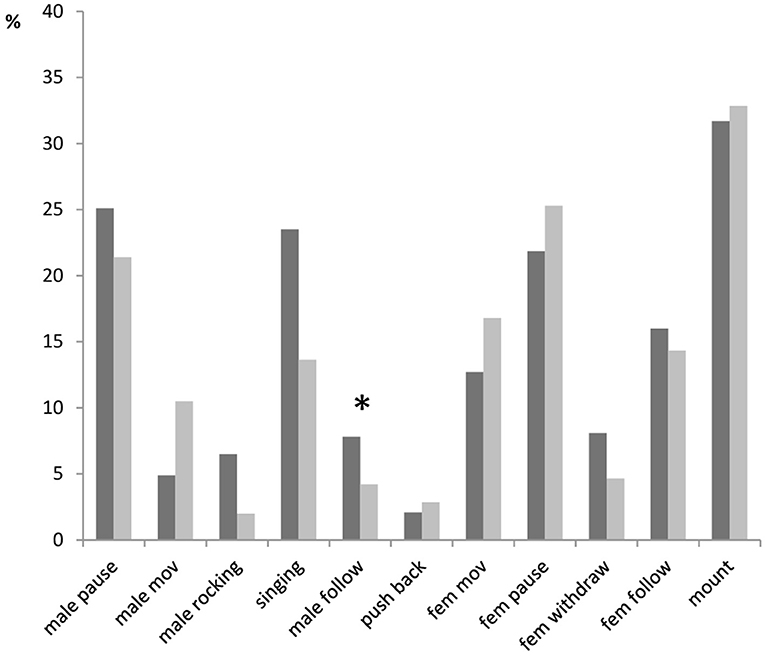
Figure 5. Relative duration (median value) of behavioral elements in courtships of dominant males (black bars) and subordinate males (gray bars) in Gryllus bimaculatus. Experiments with successful courtships are only included (n = 17 for dominant males and n = 18 for subordinate males). Asterisk indicate statistical significance of the differences between dominant and subordinate males (Mann–Whitney U-Test; *p < 0.05).
We found almost no correlation between the durations of different elements for courtships of fight winners. In successful courtships, two elements only, durations of male rocking and singing, were negatively correlated (Spearman rank correlation; r = −0.59, p = 0.045). In courtships of fight losers, we found significant positive correlations between the durations of singing and following of females (r = 0.7, p = 0.035), singing and female movement durations (r = 0.53, p = 0.043), male movement and female following durations (r = 0.9, p = 0.037), male and female pause durations (r = 0.82, p = 0.002). Interestingly, we found a significant negative correlation between the durations of male rocking and mount (r = −0.83, p = 0.01).
The Songs Produced During Courtship Tests
Almost all males singing in our experiments mated: 17 of 20 singing males that won fights and 18 of 20 singing males that lost fights were successful in courtship. The number of chirps emitted by winners was twice as many as by losers; this difference, however, was not significant (Table 2, Figure 1). None of the chirp parameters differed between the songs of winners and losers. Ticks, the main element of the courtship song, appeared to differ significantly between the males in dominant frequency, which was higher in dominant than subordinate males. Other parameters of the courtship song were qualitatively but not significantly different between the males. Dominant males tended to produce longer ticks of a shorter period than subordinate males; low-amplitude pulses that alternated with ticks were more prominent in the songs of subordinate than dominant males.
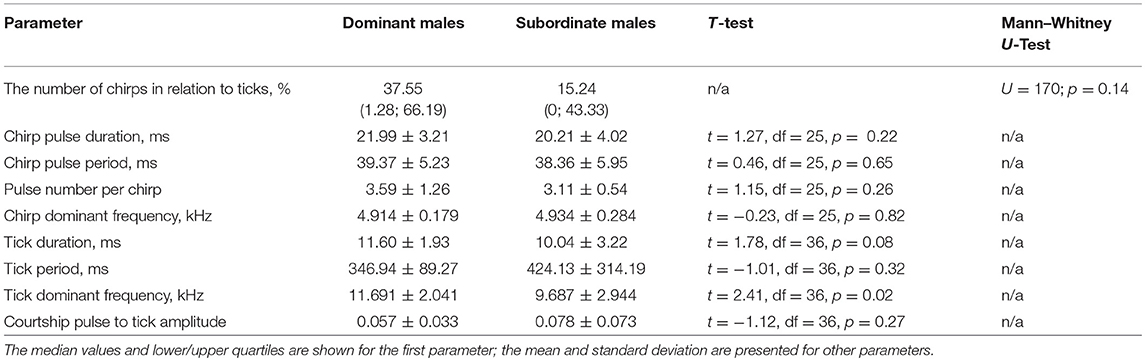
Table 2. The values of the song parameters produced during male-female interactions in Gryllus bimaculatus by dominant and subordinate males, and statistics of comparisons between the two male groups.
Discussion
Females Mate with Fight Losers Faster than with Fight Winners
Our results demonstrated that females of G. bimaculatus mounted equally often fight winners and fight losers. The latencies from the start of the trial to mount also did not differ in males with different fighting ability. However, the latencies from the start of antennal contact to mount were shorter in males that lost fights. We suggest the latter characteristic to be the important one since the antennal sensory cues are crucial for mounting responses (Loher and Rence, 1978; Adamo and Hoy, 1994). Moreover, contact chemoreception, rather than mechanoreception was shown to be the key modality for mate recognition (Balakrishnan and Pollack, 1997; Tyler et al., 2015).
Our results mainly support the data of Nelson and Nolen (1997) and Shackleton et al. (2005) obtained on A. domesticus and T. commodus, who found no difference between winners and losers in mating success in no-choice experiments. The shorter latencies to mating in fight losers demonstrated in our tests may also support the results on pheromone expression in T. oceanicus (Thomas and Simmons, 2009). Subordinate males of T. oceanicus upregulate the quantity of a number of cuticular hydrocarbons (CHC) that increase male mating success. Conversely, dominant males invest less in their pheromone signals but produce ejaculates of higher quality and sire more offspring than subordinate males. Similar results were shown in Drosophila melanogaster: females that became very attractive to males by allocating too many CHC resources produced fewer offspring or offspring of lower quality (Wicker and Jallon, 1995; Howard et al., 2003). In crickets, fighting success was shown to be more strongly linked to an increased investment in overall CHC profile rather than to specific CHC blends (Steiger et al., 2013). At the same time, mating success was tightly linked to both a lower investment in overall CHC expression and the higher relative abundance of specific CHC blends (Simmons et al., 2013; Steiger et al., 2015).
Can High Aggressiveness of Males Interfere with Their Motivation to Court?
Fight winners in our tests started to rock their body earlier and demonstrated more frequent and longer rocking than fight losers. The function of rocking, or juddering, is controversial. Rocking is usually suggested to be a component of agonistic display in crickets (Tachon et al., 1999; Bertram et al., 2010). The energetic expense of this display was shown to be of intermediate level, being, however, much higher than during aggressive stridulation (Hack, 1997). Male crickets also rock as a part of their courtship display (Adamo and Hoy, 1994; Vedenina and Pollack, 2012). In different species of arachnids, juddering was shown to signal male quality (Kotiaho, 2000), stimulate females to mate (Briceño and Bonilla, 2009), or serve multiple signaling functions (Gibson and Uetz, 2008). In our experiments, we found a negative correlation between rocking and singing durations in fight winners, but a positive correlation between rocking latency and latencies to singing and turning (that is usually performed by the male to singing the courtship song) in fight losers. We suggest that fight winners demonstrated rocking as a part of agonistic display, whereas fight losers rocked in the context of courtship display. Thus, rocking in G. bimaculatus can also be regarded as conveying multiple messages.
Fight winners had a tendency to start singing, follow females, turn and push back later than fight losers. Conceivably the high level of aggressiveness that was retained in winners prevented them from starting a “normal” courtship display. Because of the methods used in our tests, the level of the winner aggressiveness was experimentally set too high. Winners used in the courtship tests won fights in two rounds. We allowed a male to compete against another male with the same recent fight history, since success in previous fights increases the likelihood of victory in subsequent fights in crickets (Khazraïe and Campan, 1999; Savage et al., 2005). In our tests, the second round of fight was usually much more severe than the first round of fight (Video Clip 1). To test whether the high level of aggressiveness could interfere with motivation to court, it would be worthwhile to increase the number of fight rounds and study the latencies to singing and other courtship elements after several rounds.
In the wild, males of G. bimaculatus tend to find shelters from which they call to attract receptive females (Alexander, 1961; Simmons, 1986). A calling song, however, attracts not only females but also other males; thus, several successive fights with different males could easily happen in nature. If increased aggressiveness would correlate with increasing latency to court females, as could be expected from our tests, this might be maladaptive. Females, however, were more likely to mate with shelter residents: shelters seemed to benefit males by providing protection since calling may attract both parasitoids and predators (Cade, 1975; Simmons, 1986; Robert et al., 1992; Wagner, 1996). Presumably, such a preference of shelter residents allowed a relaxation of selection pressure for a quick shift from aggression to courtship behavior in dominant males.
In contrast to the high level of winner aggressiveness, the level of the loser submissiveness was not set experimentally too low in our tests. The second round of fight was usually much less fierce than the first one (Video Clip 2). Thus, the level of readiness to court in losers was closer to that of inexperienced male than the level of such readiness in winners. We suggest that this is part of the reason why fight losers tended to demonstrate the shorter latencies to all main courtship elements. In natural habitats, subordinate males have to be capable of quickly shifting from encounters with another male to courting a female. Subordinate males are unlikely to have shelters which could additionally attract females, and selection pressure acting on male behavioral plasticity through female choice could be stronger in subordinate than dominant males.
A Poor Relationship Between Song Parameters and Male Mating Success
The duration of singing was higher in fight winners than in losers. At the same time, the latencies from the start of singing to mount did not differ significantly between the males with different fighting ability. There were no differences in song parameters between winners and losers except for the difference in dominant frequency of ticks. Ticks (high-amplitude pulses) were found to be a crucial component of a successful courtship song (Libersat et al., 1994). Ticks produced by dominant males were of the higher dominant frequency (11.7 kHz) than ticks generated by subordinate males (9.7 kHz). In playback experiments, however, synthesized songs with different carrier frequencies of ticks (varied from 5 to 17 kHz) were as attractive to females as courtship of muted males accompanied by playback of the recorded song (Shestakov and Vedenina, 2015). Thus, the difference in tick dominant frequency between winners and losers was unlikely to influence female preferences but it might reflect differences in body size of males. As it was shown in many animals, both vertebrates and invertebrates, smaller individuals tend to produce higher-frequency calls due to resonant cavities and muscular rate contractions scaling with body size (e.g., Bailey, 1970; Wallschager, 1980; Brown et al., 1996). The lower dominant frequency of ticks shown in fight losers could indicate that they were on average larger than the fight winners. This seems contrary to the results in several studies obtained earlier on different cricket species (Simmons, 1986; Savage et al., 2005; Shackleton et al., 2005), which demonstrated that larger males won more fights. Hofmann and Schildberger (2001), however, found that weight asymmetry was not a very reliable predictor of outcome, duration, or intensity of fights between two males of G. bimaculatus. We measured neither weight nor body length, but noticed that lighter males sometimes won fights even when the weight asymmetry was large.
Female crickets prefer courtship songs with a long duration of ticks (Rantala and Kortet, 2003). In our tests, tick duration tended to be higher in the songs of winners, which were definitely not preferred by females. Our results seem to match the data of our previous study, in which increasing the duration of ticks had a crucial effect on female response rate, decreasing female responsiveness (Shestakov and Vedenina, 2015).
The number of chirps emitted by winners was twice as many as that emitted by losers. Despite this difference was non-significant, we suggest that a tendency to produce a higher number of chirps by dominant males in the presence of a female could be also explained by their inability to quickly shift from aggression to courtship behavior. Because of the relatively low number of pulses per chirp (3-4), we suggest they belong basically to the calling but not to the rivalry song (Zhantiev and Dubrovin, 1974; Rheinlaender et al., 1976). In any case, singing of the calling or rivalry song nearby a female could signal to a female that the male is not ready to copulate. Fight winners thereby appeared to be less ready to mate than fight losers.
Perspectives
Our analysis showed that subordinate males demonstrated shorter latencies from antennal contact to mount as compared with dominant males. This result is not consistent with the traditional view that females should prefer males with increased fighting ability. Using the method of the two-round fights we enhanced the level of aggressiveness in fight winners, thus increasing the difference between winners and losers. As a result, during the male—female interactions, fight winners demonstrated one of the elements of agonistic display, i.e., rocking body, more frequently, and for longer durations than the fight losers, and this behavior seemed to interfere with subsequent courtship. Future research should investigate whether an increase of the fight round number would lead to slower latencies to singing or other courtship elements. It is also possible that the high level of aggressiveness might inhibit the immediate shift to courtship behavior; however, this inhibition may disappear with increasing the delay time from the last fight to courtship. It was shown in G. bimaculatus that the aggressive behavior of males was influenced by prior agonistic experience for 6 h and the effect disappeared entirely after 24 h (Khazraïe and Campan, 1999). The submissive behavior was also shown in subordinate males for at least 3 h (Stevenson and Rillich, 2016). Future studies that estimate the influence of aggressiveness on courtship success depending on the delay time after fight could provide insights into our understanding of the physiological mechanisms underlying such behavior.
Ethics Statement
All animal handling and behavior sampling methods followed the quidelines of the Institutional Animal Ethics Committee and complied with the laws of Russia.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
VV and LS were equally involved in design of the study, conducting the experiments and performing statistical analyses. VV wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor is currently organizing a Research Topic with one of the authors, VV, and confirms the absence of any other collaboration.
Acknowledgments
We are grateful to Varvara Dyakonova for valuable discussion during the manuscript preparation, to Oleg Lazebny for his help with statistic analysis and Irina Vedenina for her help in translating the paper into English. We thank the two reviewers for their helpful comments. The study was supported by Russian Science Foundation (grant 14-50-00150).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00197/full#supplementary-material
Data Sheet 1. Latencies and durations of courtship elements.
Data Sheet 2. Experimental protocol of fights and courtships.
Data Sheet 3. Courtship song measurements.
Video Clip 1. Second round of fight between previous winners.
Video Clip 2. Second round of fight between previous losers.
Video Clip 3. Courtship and copulation.
Video Clip 4. Rocking the body by fight winner.
References
Adamo, S. A., and Hoy, R. R. (1994). Mating behavior of the field cricket Gryllus bimaculatus and its dependence on social and environmental cues. Anim. Behav. 47, 857–868. doi: 10.1006/anbe.1994.1117
Alcock, J., and Pyle, D. W. (1979). The Complex courtship behavior of Physiphora demandata (F.) (Diptera: Otitidae). Ethology 49, 352–362.
Alexander, R. D. (1961). Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17, 130–223. doi: 10.1163/156853961X00042
Aquiloni, A., Burič, M., and Gherardi, F. (2008). Crayfish females eavesdrop on fighting males before choosing the dominant mate. Curr. Biol. 18, R462–R463. doi: 10.1016/j.cub.2008.04.006
Bailey, W. J. (1970). The mechanics of stridulation in bush crickets (Tettigonioidea, Orthoptera) I. Tegminal Generator. J. Exp. Biol. 52, 495–505.
Balakrishnan, R., and Pollack, G. (1997). The role of antennal sensory cues in female responses to courting males in the cricket Teleogryllus oceanicus. J. Exp. Biol. 200, 511–522.
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc. B 57, 289–300.
Berglund, A., Bisazza, A., and Pilastro, A. (1996). Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linnean Soc. 58, 385–399. doi: 10.1111/j.1095-8312.1996.tb01442.x
Bertram, S. M., Rook, V. L. M., and Fitzsimmons, L.P. (2010). Strutting their stuff: victory displays in the spring field cricket, Gryllus veletis. Behaviour 147, 1249–1266. doi: 10.1163/000579510X514535
Bisazza, A., and Marin, G. (1991). Male size and female mate choice in the eastern mosquitofish (Gambusia holbrooki: Poeciliidae). Copeia 3, 730–735. doi: 10.2307/1446400
Borgia, G. (1981). Mate selection in the fly Scatophaga stercoraria: female choice in a male-controlled system. Anim. Behav. 29, 71–80.
Bretman, A., Rodríguez-Muñoz, R., and Tregenza, T. (2006). Male dominance determines female egg laying rate in crickets. Biol. Lett. 2, 409–411. doi: 10.1098/rsbl.2006.0493
Briceño, R. D., and Bonilla, F. (2009). Substrate vibrations in the scorpion Centruroides margaritatus (Scorpiones: Buthidae) during courtship. Rev. Biol. Trop. 57, 267–274.
Brown, W. D., Wideman, J., Andrade, M. C. B., Mason, A. C., and Gwynne, D. T. (1996). Female choice for an indicator of male size in the song of the black-horned tree cricket Oecanthus nigricornis (Orthoptera: Gryllidae: Oecanthinae). Evol. 50, 2400–2411. doi: 10.1111/j.1558-5646.1996.tb03627.x
Cade, W. H. (1975). Acousucally orienting parasitoids: fly phonotaxis to cricket song. Science 190, 1312–1313. doi: 10.1126/science.190.4221.1312
Far, J. A., and Travis, J. (1986). Fertility advertisement by female sailfin mollies, Poecilia latipinna (Pisces: Poeciliidae). Copeia 2, 467–472. doi: 10.2307/1445004
Fitzsimmons, L. P., and Bertram, S. M. (2013). Signaling effort does not predict aggressiveness in male spring field crickets. Behav. Ecol. Sociobiol. 67, 213–220. doi: 10.1007/s00265-012-1441-1
Folstad, I., and Karter, A. J. (1992). Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622.
Forsgren, E. (1997). Female sand gobies prefer good fathers over dominant males. Proc. R. Soc. Lond. B 264, 1283–1286.
Friard, O., and Gamba, M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Gibson, J. S., and Uetz, G. W. (2008). Seismic communication and mate choice in wolf spiders: components of male seismic signals and mating success. Anim. Behav. 75, 1253–1262. doi: 10.1016/j.anbehav.2007.09.026
Hack, M. A. (1997). Assessment strategies in the contests of male crickets, Acheta domesticus (L.). Anim. Behav. 53, 733–747. doi: 10.1006/anbe.1996.0310
Hedrick, A., and Bunting, J. (2014). An attractive male trait and aggressiveness are negatively correlated in wild field crickets, but uncorrelated in lab-reared crickets. Behav. Ecol. Sociobiol. 68, 233–238. doi: 10.1007/s00265-013-1638-y
Hoelzer, G. A. (1989).The good parent process of sexual selection. Anim. Behav. 38, 1067–1078. doi: 10.1016/S0003-3472(89)80146-0
Hofmann, H. A., and Schildberger, K. (2001). Assessment of strength and willingness to fight during aggressive encounters in crickets. Anim. Behav. 62, 337–348. doi: 10.1006/anbe.2001.1746
Hofmann, H. A., and Stevenson, P. A. (2000). Flight restores fight in crickets. Nature 403:613. doi: 10.1038/35001137
Howard, R. W., Jackson, L. L., Banse, H., and Blows, M. W. (2003). Cuticular hydrocarbons of Drosophila birchii and D. serrata: identification and role in mate choice in D. serrata. J. Chem. Ecol. 29, 961–976. doi: 10.1023/A:1022992002239
Khazraïe, K., and Campan, M. (1999). The role of prior agonistic experience in dominance relationships in male crickets Gryllus bimaculatus (Orthoptera: Gryllidae). Behav. Process. 44, 341–348. doi: 10.1016/S0376-6357(98)00058-8
Kortet, R., and Hedrick, A. (2005). The scent of dominance: female field crickets use odour to predict the outcome of male competition. Behav. Ecol. Sociobiol. 59, 77–83. doi: 10.1007/s00265-005-0011-1
Kotiaho, J. S. (2000). Testing the assumptions of conditional handicap theory: costs and condition dependence of a sexually selected trait. Behav. Ecol. Sociobiol. 48, 188–194. doi: 10.1007/s002650000221
Kunc, H. P., Amrhein, V., and Naguib, M. (2006). Vocal interactions in nightingales, Luscinia megarhynchos: more aggressive males have higher pairing success. Animal Behav. 72:25e30. doi: 10.1016/j.anbehav.2005.08.014
Libersat, F., Murray, J. A., and Hoy, R. R. (1994). Frequency as a releaser in the courtship song of two crickets, Gryllus bimaculatus (de Geer) and Teleogryllus oceanicus: a neuroethological analysis. J. Comp. Physiol. A. 174, 485–494. doi: 10.1007/BF00191714
Loher, W., and Rence, B. (1978). The Mating behavior of Teleogryllus commodus (Walker) and its central and peripheral control. Ethology 46, 225–259.
Loranger, M. J., and Bertram, S. M. (2016). The effect of male dominance on female choice in a field cricket (Gryllus assimilis). Anim. Behav. 114, 45–52. doi: 10.1016/j.anbehav.2016.01.020
Miyashita, A., Kizaki, H., Sekimizu, K., and Kaito, C. (2016). No effect of body size on the frequency of calling and courtship song in the two-spotted cricket, Gryllus bimaculatus. PLoS ONE 11:e0146999. doi: 10.1371/journal.pone.0146999
Montroy, K., Loranger, M. J., and Bertram, S. M. (2016). Male crickets adjust their aggressive behavior when a female is present. Behav. Process 124, 108–114. doi: 10.1016/j.beproc.2015
Moore, A. J., Gowaty, P. A., Wallin, W. G., and Moore, P. J. (2001). Sexual conflict and the evolution of female mate choice and male social dominance. Proc. R. Sci. 268, 517–523. doi: 10.1098/rspb.2000.1399
Nelson, C. M., and Nolen, T. G. (1997). Courtship song, male agonistic encounters, and female mate choice in the house cricket, Acheta domesticus (Orthoptera: Gryllidae). J. Insect. Behav. 10, 557–570. doi: 10.1007/BF02765377
Norris, R. (1993). Heritable variation in a plumage indicator of viability in male great tits Parus major. Nature 362, 537–539.
Ophir, A. G., and Galef Jr, B. G. (2003). Female Japanese quail that ‘eavesdrop' on fighting males prefer losers to winners. Anim. Behav. 66, 399–407. doi: 10.1006/anbe.2003.2230
Petrie, M. (1994). Improved growth and survival of offspring of peacocks with more elaborate strains. Nature 371, 598–599. doi: 10.1038/371598a0
Pitnick, S., and Markow, T. A. (1994). Male gametic strategies: sperm size, testes size, and the allocation of ejaculate among successive mates by the sperm-limited fly Drosophila pachea and its relatives. Am. Nat. 143, 785–819.
Preston, B. T., Stevenson, I. R., Pemberton, J. M., and Wilson, K. (2001). Dominant rams lose out by sperm depletion. Nature 409, 681–682. doi: 10.1038/35055617
Qvarnstrom, A., and Forsgren, E. (1998). Should females prefer dominant males? TREE 13, 498–501. doi: 10.1016/S0169-5347(98)01513-4
Rantala, M.J., and Kortet, R. (2003). Courtship song and immune function in the field cricket Gryllus bimaculatus. Biol. J. Linn. Soc. 79, 503–510. doi: 10.1046/j.1095-8312.2003.00202.x
Rantala, M. J., and Kortet, R. (2004). Male dominance and immunocompetence in a field cricket. Behav. Ecol. 15, 187–191. doi: 10.1093/beheco/arg103
Rheinlaender, J., Kalmring, K., Popov, A. V., and Rehbein, H. (1976). Brain projections and information processing of biologically significant sounds by two large ventral-cord neurons of Gryllus bimaculatus DeGeer (Orthoptera, Gryllidae). J. Comp. Physiol. 110, 251–269. doi: 10.1007/BF00659143
Robert, D., Amoroso, J., and Hoy, R. R. (1992). The evolutionary convergence of hearing in a parasitoid fly and its cricket host. Science 258, 1135–1137. doi: 10.1126/science.1439820
Savage, K. E., Hunt, J., Jennions, M. D., and Brooks, R. (2005). Male attractiveness covaries with fighting ability but not with prior fight outcome in house crickets. Behav. Ecol. 16, 196–200. doi: 10.1093/beheco/arh143
Shackleton, M. A., Jennions, M. D., and Hunt, J. (2005). Fighting success and attractiveness as predictors of male mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-choice tests. Behav. Ecol. Sociobiol. 58, 1–8. doi: 10.1007/s00265-004-0907-1
Sheldon, B. C., Merilö, J., Qvarnström, A., Gustafsson, L., and Ellegren, H. (1997). Paternal genetic contribution to offspring condition predicted by size of male secondary sexual character. Proc. R. Soc. Lond. B 264, 297–302.
Shestakov, L. S., and Vedenina, Yu. V. (2015). Broad selectivity for courtship song in the cricket Gryllus bimaculatus. Ethology 121, 966–976. doi: 10.1111/eth.12409
Simmons, L. W. (1986). Inter-male competition and mating success in the field cricket, Gryllus bimaculatus (de Geer). Anim. Behav. 34, 567–579.
Simmons, L. W. (1988). The contribution of multiple mating and spermatophore consumption to the lifetime reproductive success of female field crickets (Gryllus bimaculatus). Ecol. Entomol. 13, 57–69.
Simmons, L. W., Thomas, M. L., Simmons, F. W., and Zuk, M. (2013). Female preferences for acoustic and olfactory signals during courtship: male crickets send multiple messages. Behav. Ecol. 24, 1099–1107. doi: 10.1093/beheco/art036
Simmons, L. W., and Zuk, M. (1992). Variability in call structure and pairing success of male field crickets, Gryllus bimaculatus: the effects of age, size and parasite load. Anim. Behav. 44, 1145–1152.
Steiger, S., Capodeanu-Nägler, A., Gershman, S. N., Weddle, C. B., Rapkin, J., Sakaluk, S. K., et al. (2015). Female choice for male cuticular hydrocarbon profile in decorated crickets is not based on similarity to their own profile. J. Evol. Biol. 28, 2175–2186. doi: 10.1111/jeb.12740
Steiger, S., Ower, G. D., Stökl, J., Mitchell, C., Hunt, J., and Sakaluk, S. K. (2013). Sexual selection on cuticular hydrocarbons of male sagebrush crickets in the wild. Proc. R. Soc. B 280:20132353. doi: 10.1098/rspb.2013.2353
Stevenson, P. A., and Rillich, J. (2016). Controlling the decision to fight or flee: the roles of biogenic amines and nitric oxide in the cricket. Curr. Zool. 62, 265–275. doi: 10.1093/cz/zow028r
Tachon, G., Murray, A. M., Gray, D. A., and Cade, W. H. (1999). Agonistic displays and the benefits of fighting in the field cricket, Gryllus bimaculatus. J. Insect Behav. 12, 533–543.
Thomas, M. L., and Simmons, L. W. (2009). Male dominance influences pheromone expression, ejaculate quality, and fertilization success in the Australian field cricket, Teleogryllus oceanicus. Behav. Ecol. 20, 1118–1124. doi: 10.1093/beheco/arp105
Trail, P. W. (1985). Courtship disruption modifies mate choice in a lek-breeding bird. Science 227, 778–779. doi: 10.1126/science.227.4688.778
Trivers, R. L. (1976). Sexual selection and resource-accruing abilities in Anoils garmani. Evolution 30, 253–269. doi: 10.1111/j.1558-5646.1976.tb00908.x
Tyler, F., Fisher, D., d'Ettorre, P., Rodríguez-Muñoz, R., and Tregenza, T. (2015). Chemical cues mediate species recognition in field crickets. Front. Ecol. Evol. 3:48. doi: 10.3389/fevo.2015.00048
Vedenina, V. Y., and Pollack, G. S. (2012). Recognition of variable courtship song in the field cricket Gryllus assimilis. J. Exp. Biol. 215, 2210–2219. doi: 10.1242/jeb.068429
Wagner, Jr. W. E. (1996). Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav. Ecol. 7, 279–285. doi: 10.1093/beheco/7.3.279
Wallschager, D. (1980). Correlation of sound frequency and body weight in passerine birds. Experientia (Basel) 36:412. doi: 10.1007/BF01975119
Wedell, N., and Tregenza, T. (1999). Ssuccessful fathers sire successful sons. Evolution 53, 620–625. doi: 10.1111/j.1558-5646.1999.tb03797.x
Wicker, C., and Jallon, J. M. (1995). Influence of ovary and ecdysteroids on pheromone biosynthesis in Drosophila melanogaster (Diptera: Drosophilidae). Eur. J. Entomol. 92, 197–202.
Wong, B. B. M. (2004). Superior fighters make mediocre fathers in the Pacific blue-eye fish. Anim. Behav. 67, 583–590. doi: 10.1016/j.anbehav.2003.08.015
Wong, B. B. M., and Candolin, U. (2005). How is female mate choice affected by male competition? Biol. Rev. 80, 559–571. doi: 10.1017/S1464793105006809
Keywords: cricket, Gryllus bimaculatus, multimodal signaling, courtship song, dominance status, aggressiveness, female preference
Citation: Vedenina VY and Shestakov LS (2018) Loser in Fight but Winner in Love: How Does Inter-Male Competition Determine the Pattern and Outcome of Courtship in Cricket Gryllus bimaculatus? Front. Ecol. Evol. 6:197. doi: 10.3389/fevo.2018.00197
Received: 30 April 2018; Accepted: 08 November 2018;
Published: 27 November 2018.
Edited by:
Astrid T. Groot, University of Amsterdam, NetherlandsReviewed by:
Ivar Folstad, UiT The Arctic University of Norway, NorwayRyan L. Earley, University of Alabama, United States
Copyright © 2018 Vedenina and Shestakov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lev S. Shestakov, emljcm9uYUB5YW5kZXgucnU=
 Varvara Yu. Vedenina
Varvara Yu. Vedenina Lev S. Shestakov
Lev S. Shestakov