
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 22 August 2018
Sec. Behavioral and Evolutionary Ecology
Volume 6 - 2018 | https://doi.org/10.3389/fevo.2018.00126
This article is part of the Research Topic As Above so Below? Below-ground Interactions in Ecological Processes View all 19 articles
 Nikolaos Garantonakis1
Nikolaos Garantonakis1 Maria L. Pappas2†
Maria L. Pappas2† Kyriaki Varikou1†
Kyriaki Varikou1† Vasiliki Skiada3
Vasiliki Skiada3 George D. Broufas2
George D. Broufas2 Nektarios Kavroulakis1*
Nektarios Kavroulakis1* Kalliope K. Papadopoulou3*
Kalliope K. Papadopoulou3*Belowground symbiosis of plants with beneficial microbes is known to confer resistance to aboveground pests such as herbivorous arthropods and pathogens. Similarly, microbe-induced plant responses may also impact natural enemies of pests via the elicitation of plant defense responses and/or alteration of plant quality and growth. Nesidiocoris tenuis is a zoophytophagous predator and an efficient biological control agent of greenhouse pests. Its usefulness in plant protection is often hindered by its ability to damage plants at high predator population densities or when prey is scarce. In this study, we investigated the effect of Fusarium solani strain K (FsK), an endophytic fungal isolate that colonizes tomato root tissues, on the capability of N. tenuis to cause necrotic rings, an easily discernible symptom, on tomato stems and leaves. We found significantly less necrotic rings formed on FsK-inoculated plants for all tomato cultivars tested. FsK has been previously shown to confer ethylene-mediated tomato resistance to both foliar and root fungal pathogens; thus, the ethylene-insensitive Never ripe (Nr) and epinastic (epi) tomato plant mutant lines were included in our study to assess the role of ethylene in the recorded FsK-mediated plant damage reduction. The jasmonic acid (JA)-biosynthesis tomato mutant def-1 was also used since JA is known to mediate major anti-herbivore plant responses. We show that ethylene and JA are required for FsK to efficiently protect tomato plants from N. tenuis feeding. No necrotic rings were recorded on FsK-inoculated epi plants suggesting that ethylene overproduction may be key to tomato resistance to N. tenuis feeding.
Plants are associated with a vast diversity of microbes that exert beneficial effects on their performance. Soil-borne microbes in particular, such as endophytic fungi, plant growth promoting fungi and rhizobacteria as well as arbuscular mycorrhizae have long been recognized for their benefits to plant growth and nutrition (Smith and Smith, 2011; Hadar and Papadopoulou, 2012; Finkel et al., 2017). In addition, certain root colonizing microbes are known to antagonize soil-borne pathogens and/or prime plant defense against future attackers (Pineda et al., 2010; Pieterse et al., 2014).
Soil-borne beneficial microbes can affect aboveground herbivores both positively and negatively (Hartley and Gange, 2009; Shikano et al., 2017). Improved plant growth and/or nutrition by plant-growth promoting fungi and rhizobacteria have been shown to result in positive effects on herbivore performance (Pineda et al., 2010; Ahemad and Kibret, 2014). On the other hand, defense priming triggered by beneficial microbes, often referred to as Induced Systemic Resistance (ISR), can impact herbivores via direct or indirect defense elicitation (Pineda et al., 2010; Pieterse et al., 2014).
Microbe-ISR is mediated by phytohormones that also control plant defense against herbivores. In particular, ISR is mediated by priming of defense-related genes upon attack and involves an increased sensitivity to jasmonic acid (JA) and ethylene (ET) (Rosenblueth and Martínez-Romero, 2006; Van Wees et al., 2008). JA-mediated plant responses can be directly effective against chewing herbivores but also against phloem feeders, such as aphids and whiteflies, which normally activate the SA-signaling pathway to counteract JA-defenses via crosstalk (Walling, 2000; Schaller, 2008). Ethylene on the other hand is a modulator of the JA and SA signaling pathways in plant defense against pathogens and acts either by synergizing JA or by enabling SA antagonism with JA (Pieterse et al., 2012). To date, very little is known on the role of ET in plant responses against herbivores (Stahl et al., 2018).
Beneficial microbes can, also, impact the so-called plant's indirect defense. Upon herbivore attack plants normally emit a blend of volatiles that attract its natural enemies (Karban and Baldwin, 1997; Dicke and Baldwin, 2010). The JA signaling pathway is the key regulator of this process, suggesting that ISR in microbe-inoculated plants could modify the volatiles emitted in response to herbivory (Pineda et al., 2010). Indeed, selected soil beneficial microbes are capable of altering the composition or the emission rate of this blend and thus the attractiveness of the infested plant to certain predators and parasitoids (e.g., Fontana et al., 2009; Schausberger et al., 2012; Pineda et al., 2013). Nevertheless, besides behavior, plant-mediated effects of beneficial microbes on the performance of natural enemies have only been scarcely addressed so far (e.g., Battaglia et al., 2013; Prieto et al., 2017).
In this regard, zoophytophagous predators are of particular interest as they feed on both plant and prey. Nesidiocoris tenuis is one such predator and an efficient biocontrol agent of several plant pests. Nevertheless, it may also cause significant plant damage at high predator population densities or when prey is scarce (Sánchez and Lacasa, 2008; Sanchez, 2009; Arnó et al., 2010; Castañé Cristina et al., 2011) as it can feed on the plant i.e. shoots and petioles, specifically the phloem and neighboring parenchyma cells (Raman and Sanjayan, 1984). Necrotic rings are the externally visible symptoms around the stems and leaf petioles caused by the frequent stylet penetration and tissue sap feeding by N. tenuis along the stylet track, which result in wound response, cell necrosis and increased protein content at the feeding site. Besides necrotic rings on stems and leaves, flower abortion and punctures on fruits are the main symptoms related to N. tenuis feeding on tomato (Calvo et al., 2009; Arnó et al., 2010; Castañé Cristina et al., 2011).
In this study, we assessed the effects of Fusarium solani strain K (FsK) on N. tenuis, specifically its ability to cause necrotic rings on tomato plants. FsK is an endophytic fungus isolated from the roots of tomato plants grown on suppressive compost. It colonizes the roots, including vascular tissues but ingress ceases at the root crown area and fungal growth is not detected in aboveground tomato tissues (Kavroulakis et al., 2007, Skiada, unpublished data). FsK has been previously shown to confer resistance not only against root but also foliar plant pathogens in tomato. In addition, it was shown that an intact ethylene signaling pathway was necessary to confer resistance to foliar pathogens by FsK (Kavroulakis et al., 2007), indicating that FsK can induce systemic responses to the plant. We, thus, hypothesized that FsK mediates effectual tomato responses against arthropods that attack aboveground tissues of the plant, too. To explore putative defense mechanisms mediating the effects of FsK on the formation of necrotic rings by N. tenuis, ethylene and jasmonate plant mutant lines were used in parallel with their wild type progenitors.
A F. solani strain FsK (Kavroulakis et al., 2007) routinely cultured on potato dextrose broth (PDB) at 25°C for 5 days in the dark was used in the experiments. Following removal of mycelium fragments by sieving, conidia were recovered by centrifugation at 4000 g, counted using a haemocytometer and suspended in an appropriate volume of 0.85% NaCl in order to achieve the desired inoculum concentration. Application of the inoculum of strain FsK with 104 conidia cm−3 of potting mix was performed as water drench 1 week after seed sowing.
Wild-type tomato (Solanum lycopersicum) cultivars Pearson, VFN8 and Castlemart and their mutant lines Nr, epi, and def-1, respectively as well as the commercial cultivar ACE55 were used in this study. The wild type cultivars are the progenitors of the mutant plant lines. Nr plants block ethylene perception (Lanahan et al., 1994) whereas epi is an ethylene overproducing tomato line (Fujino et al., 1988). Def-1 plants are deficient in JA accumulation in response to wounding and systemin (Howe et al., 1996). Pearson, VFN8, Nr and epi seeds were obtained from the Tomato Genetics Resource Center (University of California, Davis). Castlemart and def-1 seeds were kindly provided by Greg Howe (Michigan State University).
Seeds were surface-sterilized in 2.5% NaOCl and sown directly into 10 cm diameter pots, each containing approximately 300 cm3of peat blended with an NPK fertilizer (20-20-20) to a total concentration of 0.8 g l−1 of potting mix. The pots were placed in a climate room with a temperature of 25 ± 1°C, 65 ± 5% relative humidity (RH) and a 16L:8D photoperiod. Plants were regularly watered and once a week fertilized with a balanced nutrient solution which consisted of the following macronutrients (mM): Ca(NO3)2.4H2O (11.1); NaH2PO4.2H2O (0.0094); Na2HPO4.12H2O (0.006); K2SO4 (6.410); MgSO4.7H2O (3,840); CaCl2.2H2O (2); and micronutrients (μM): H3BO3 (69); MnSO4.4H2O(10.4); ZnSO4.7H2O (1.2); CuSO4.5H2O (1.7); NaMoO4.2H2O (0.13); and FeEDDHA (0.3).
Nesidiocoris tenuis was reared on Nicotiana tabacum plants, which can support N. tenuis feeding (Calvo et al., 2012; Bueno et al., 2013; Sukhoruchenko et al., 2015). The rearing was initiated with nymphs and adults collected from Solanum nigrum plants in the area of Ierapetra, eastern Crete in the summer of 2012, and kept in wooden-framed muslin cages (100 cm length × 50 cm width × 70 cm height) in a climate room with 25 ± 1°C, 65 ± 5% RH and 16L:8D. Eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) were provided ad libitum with a thin brush on the leaves of N. tabacum plants as supplemental food for the predator.
Three to four weeks-old tomato plants of all plant cultivars were inoculated with FsK as described above and individually transferred in cylindrical net cages (30 cm length × 10 cm diameter). Control (uninoculated) plants received water only. A pair of N. tenuis adults (male and female, < 1 week old) was introduced in each cage without food or prey so as to be forced to feed on the plant. Total number of necrotic rings on shoot and leaves as well as the number of live predators on each plant were recorded after 1 week. At this time period, no predator nymphs had hatched as anticipated (Martínez-García et al., 2016). All cages were kept in a climate room (25 ± 0.5°C, 65 ± 5% RH, and 16L:8D). Experiments with wild-type (WT) (n = 10–18) and mutant lines (n = 14–17) were carried out in parallel in two blocks in time.
FsK colonization of root tissues was verified for all tomato genotypes both in control and N. tenuis-exposed plants. Root tissues were collected from four replicates of each treatment 1 week after exposure to N. tenuis. Samples were used for whole genomic DNA extraction using the “NuncleoSpin® Plant II genomic DNA extraction” kit (MACHEREY-NAGEL GmbH &Co.KG, Duren, Germany). FsK colonization of root tissues was assessed via qPCR, by using primers pair FFsITS (5′-TGGTCATTTAGAGGAAGTAA-3′) and RFsITS (5′-GGTATGTTCACAGGGTTGATG−3′), specific for a ca 100 bp fragment of F. solani ITS region. An external standard curve was generated in order to quantify the copy number of ITS gene in total DNA extracted from root tissues of FsK-inoculated plants. The standard curve was generated as follows: ITS gene was amplified using FsK genomic DNA as template, the PCR product was purified and ligated into pGEM-T Easy vector (Promega, Madison, USA) and transformed to competent Escherichia coli DH5a cells. The recombinant plasmid was extracted again (NucleoSpin Plasmid, Macherey Nagel) and its concentration was determined via Qubit 3.0 Fluorometer. The copy numbers of the targeted gene were calculated from the concentration of the extracted plasmid DNA.
Serial 10-fold dilutions of the recombinant plasmid ranging from 5.9 × 100 to 5.9 × 108 copies/μl were subjected in triplicate to qPCR to construct the standard curve. qPCR amplification efficiencies for the under-study gene were 99.77%, with r2 value of 0.998 and a slope of −3.327. Amplification occurred in a 10 μl reaction mixture containing Kapa SYBR FAST qPCR Master Mix (1x) Universal, 200 nM of each primer, and 1 μl of DNA, using the following thermocycling protocol: 3 min at 95°C; 45 cycles of 15 s at 95°C, 20 s at 58°C followed by a melting curve to check the specificity of the products. PCR products were furthermore analyzed on a 1.5% agarose gel in order to check for potential non-targeted amplifications.
Two-way analysis of variance (ANOVA) was used to evaluate the effect of tomato cultivar and plant inoculation status (FsK inoculated/non-inoculated) and their interaction on the number of necrotic rings recorded on tomato plants when exposed to N. tenuis. Data were log(x+1) transformed to meet the criteria for parametric analysis. Pairwise comparisons by Student's t-test were used to compare the number of necrotic rings on wild-type tomato cultivars (ACE55, Castlemart, Pearson, and VFN8) and FsK inoculated or non-inoculated mutants (def-1, Nr, and epi) when exposed to N. tenuis as well as to compare FsK colonization levels between N. tenuis exposed wild-type plants and their mutants. In the cases homoscedasticity's assumption was not met, the non-parametric Mann-Whitney U-test was used. All statistics were performed in SPSS (SPSS, 2011).
Different tomato cultivars were used to assess putative cultivar-dependent effects of FsK on plant damage by N. tenuis. Plant feeding by the zoophytophagous predator for 1 week produced similar numbers of necrotic rings in all tomato cultivars [F(3, 105) = 0.839, P = 0.475] used in this study (Figure 1). Inoculating plants with FsK resulted in a significant reduction in the number of necrotic rings [F(1, 105) = 82.128, P = 7.75E−15] in all cultivars compared to control (non-inoculated) plants (Figure 1). The interaction between cultivar and inoculation status (FsK-inoculated/non-inoculated) was not significant [F(3, 105) = 0.013, P = 0.806]. No effect was observed on the survival of the predators, which all remained alive at the end of the experiment (100% survival rate).
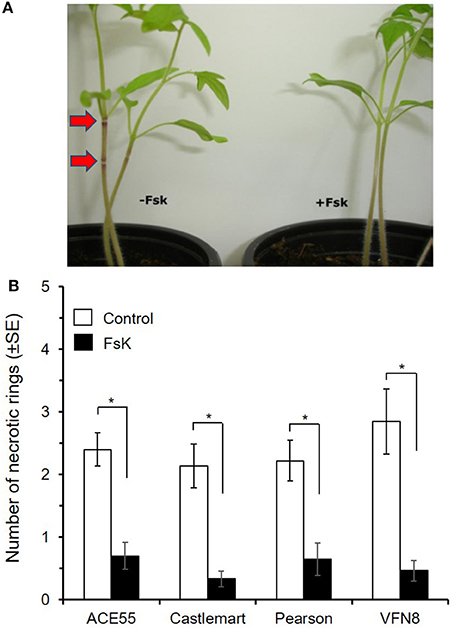
Figure 1. Plant damage by Nesidiocoris tenuis is reduced on tomato plants inoculated with Fusarium solani strain K (FsK). Tomato FsK-inoculated (black bars, N = 10–17) or control (white bars, N = 10–18) plants were exposed to N. tenuis (one male, one female) for 1 week. (A) Necrotic rings (red arrows, left) caused by N. tenuis feeding on ACE55 control (-FsK) plants compared to FsK-inoculated (+FsK, right) plants where no symptoms are depicted (B) Mean (±SE) total number of necrotic rings on stems and leaves recorded for each tomato cultivar (ACE55, Castlemart, Pearson, VFN8) on day 7. Asterisks indicate significant differences within each cultivar after Student t-test (P < 0.001).
We hypothesized that FsK-mediated tomato resistance to N. tenuis feeding may be linked to tomato JA-defenses since these constitute a major anti-herbivore defense (Howe et al., 1996; Karban and Baldwin, 1997; Walling, 2000). In addition, because FsK was previously shown to mediate tomato resistance against pathogens via the ethylene signaling pathway (Kavroulakis et al., 2007), we assumed ethylene might also be essential for FsK-mediated tomato resistance against N. tenuis. To test these, we investigated the effect of inoculating tomato mutant plant lines with FsK on the ability of N. tenuis to cause necrotic rings, to determine the involvement of the ethylene and jasmonic acid defense pathways in the FsK mode of action.
In the absence of FsK, we found that the numbers of necrotic rings recorded on both Nr and def-1 plants were not significantly different compared to those on their wild-type relatives (Castlemart and Pearson, respectively) (Figures 2A,B), while significantly reduced number of rings were observed on epi mutant plants (Figure 2C). This indicates that although basal levels of ethylene or JA cannot protect tomato plants from the phytophagy, elevated ethylene levels may have a protective role against N. tenuis in tomato. In the presence of FsK, inoculated Nr and def-1 plants displayed similar numbers of necrotic rings to non-inoculated mutants (Figures 2A,B). The plants of both mutant lines were not affected by the endophyte and the necrotic rings measured were significantly higher than those recorded on FsK-inoculated Castlemart (t = −3.862; df = 28; P = 0.0006) and Pearson (t = 2.102; df = 32; P = 0.043) wild-type plants, respectively (Figures 2A,B). In contrast, the presence of the endophyte further increased the response against N. tenuis feeding observed in epi mutant plants, resulting in significantly less necrotic rings on epi compared to wild-type VFN8 plants (t = −2.744; df = 26; P = 0.011). These results suggest that ethylene and jasmonate biosynthesis and signaling are essential for the expression of the FsK-mediated reduction of plant damage caused by N. tenuis.
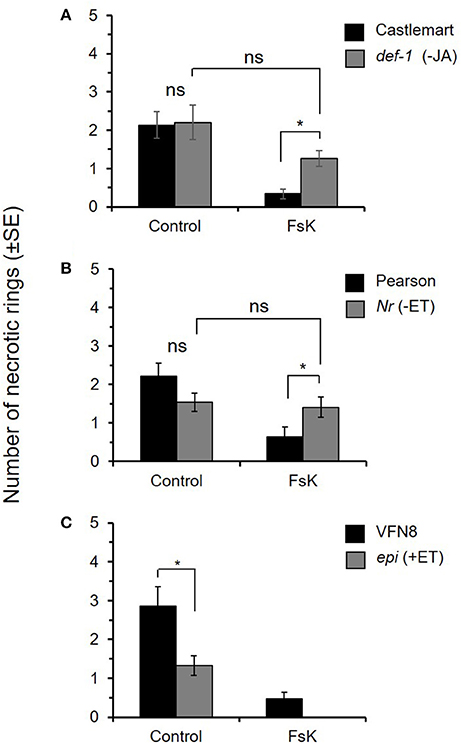
Figure 2. Ethylene and jasmonate signaling is essential for the expression of Fusarium solani strain K (FsK) systemic effects on plant damage by Nesidiocoris tenuis. FsK-inoculated (FsK, N = 14–17) or non-inoculated (Control, N = 13–18) tomato plants were exposed to N. tenuis (one male, one female) for 1 week. Mean (±SE) total number of necrotic rings on stems and leaves on (A) Castlemart wild-type and JA-biosynthesis def-1 mutant, (B) Pearson wild-type and ethylene-insensitive Nr mutant and (C) VFN8 wild-type and ethylene overproducing epi mutant. Bars depicting numbers of necrotic rings on WT plants (except ACE55) are the same as those shown in Figure 1. Asterisks indicate significant differences (P < 0.05); ns, not significant.
To investigate the possibility that the differences observed in the activity of FsK in the various mutant plant lines could be attributed to a colonization efficiency of the FsK in these genotypes, we estimated by quantitative PCR the colonization levels of FsK in all tomato cultivars at the time of sampling. No significant differences were recorded in FsK colonization levels of tomato cultivars in all combinations [Pearson vs. Nr: U = 7, P = 0.773; VNF8 vs. epi: t(5.96) = −0.78; P = 0.465; Castlemart vs. def-1: t(3.74) = 1.09; P = 0.341]. Thus, the recorded reduction in the N. tenuis-caused plant damage could not be related to the colonization efficiency of FsK.
Microbes are considered capable of affecting plant-arthropod interactions (Hartley and Gange, 2009; Shikano et al., 2017). Induced plant responses by multiple biocontrol agents, such as zoophytophagous predators and soil-borne beneficial microbes may be mediated by interacting plant signaling pathways (Pappas et al., 2017). In this study, we report a mutualistic relationship between tomato and the fungal endophyte F. solani strain K (FsK), shown herein to mediate resistance to plant damage caused by the zoophytophagous predator N. tenuis. In addition, our data show that ethylene and jasmonic acid are required for the endophyte to effectively protect tomato, whereas ethylene overproduction results in null damage by N. tenuis.
Feeding intensity by N. tenuis is known to be affected by abiotic conditions (e.g., temperature) but also prey availability, with necrotic ring number increasing when prey is scarce, and vice versa (Arnó et al., 2006, 2010; Sanchez, 2008; Calvo et al., 2009). In addition, specific tomato cultivars suffer more damage by N. tenuis than others (Pérez-Hedo and Urbaneja, 2016), suggesting that symptom intensity may also be related to plant traits. In our study, no cultivar-dependent difference on the symptoms developed was found and intensity of plant damage caused by N. tenuis was similar on all wild-type tomato cultivars tested. Moreover, FsK inoculation resulted in similar reduction in the number of necrotic rings across all wild-type tomato cultivars. In addition, no prey was available for the predators and experiments were conducted under controlled environmental conditions, suggesting that mainly plant-related factors should have affected N. tenuis ability to cause less necrotic rings. The fact that no differences were recorded between cultivars when FsK was present suggests the involvement of similar mechanisms mediating tomato resistance to N. tenuis feeding across all cultivars.
Reduction of feeding damage by N. tenuis on FsK-inoculated plants may be related with tomato resistance mechanisms. For example, antixenosis and/or antibiosis could be involved when N. tenuis is reluctant to feed on the plant due to the induction of plant defense-related responses or changes in plant nutritional quality by FsK. On the other hand, FsK-inoculated plants may display increased tolerance via accelerated healing of symptoms caused by N. tenuis feeding. The latter was shown for necrotic rings that completely disappeared after exposing tomato plants to N. tenuis only temporarily, for a few days (Arnó et al., 2006, 2010). Thus, antixenosis, antibiosis and/or tolerance may be involved in FsK-mediated tomato resistance to N. tenuis feeding but this needs to be further explored. In our study, all predators introduced to the control and the FsK-inoculated plants survived at the end of the experimental period, indicating that either the changes in plant response conferred by FsK have no direct impact on the predator or N. tenuis was not affected for the experimental period of this work. A more detailed investigation into the performance and feeding preferences of the predator will be needed to address the effects on the predator.
The prominent role of ethylene-mediated tomato responses in its interaction with N. tenuis, is clearly depicted by the significant reduction of necrotic rings in the ethylene overproducing epi mutant plants. This effect was evidently amplified by the presence of the endophyte and resulted in augmented tomato resistance against N. tenuis, since no rings were detected on FsK-inoculated epi plants. It is not known whether the endophyte is capable of inducing ethylene production in the plant and, thereby, further enhancing the positive impact of elevated levels of ethylene against N. tenuis. This putative mode of action resembles the reported induction of ethylene biosynthesis as a mechanism of plant protection against root-knot nematodes by Trichoderma harzianum (Leonetti et al., 2017). A focused study on the effect of FsK colonization on ethylene biosynthesis and signaling pathway, which would involve measurements of hormonal levels in plant tissues, will provide further insight on this mode of action.
On the other hand, both an intact ethylene and jasmonic acid pathway is shown to be essential for the expression of FsK-mediated resistance to N. tenuis feeding in this study. Our previous results show that FsK is able to colonize the root of tomato plants (Kavroulakis et al., 2007) and we have not been able to detect fungal ingress in the stems and leaves of the plant under our experimental conditions. Thus, a systemic effect of FsK on hormonal balance is anticipated. Hormone crosstalk is a well-established mechanism of plant resistance against pathogens and herbivores. Although for arthropods there is no general model that can describe the type of regulation exerted by the hormonal pathways and there is a strong influence of the feeding guild, JA signaling appears to be central to plant resistance against arthropods (Stahl et al., 2018). Ethylene, as a modulator of JA and SA signaling pathways has been shown to act by synergizing JA or enabling SA antagonism with JA and, thus, to variably impact arthropods studied so far (Pieterse et al., 2012; Stahl et al., 2018). FsK inoculation did not increase resistance against N. tenuis feeding neither in the ethylene perception-deficient Nr nor the jasmonic-deficient def-1 mutant plants. This indicates towards a synergistic role between ethylene and JA in this case. In this regard, ethylene involvement in SA antagonism to JA cannot be concluded by the present study; further studies including the SA-deficient transgenic nahG tomato line are needed to study the putative involvement of SA in this tripartite interaction. Finally, we have not observed any differences in the capability of FsK to colonize the various mutant genotypes when compared with the progenitor plant lines. This suggests that the endophyte triggers a systemic response in the plant, which is not related to its colonization level or to its physical presence and interaction with N. tenuis.
Zoophytophagous predators such as N. tenuis and Macrolophus pygmaeus are known to induce JA defenses in response to their phytophagy on tomato (Pappas et al., 2015, 2016; Pérez-Hedo et al., 2015a,b). Nevertheless, relatively little is known about the effects of JA- or SA-mediated plant responses on their performance and behavior. The expression of proteinase inhibitors, known to be induced by wounding, was recently shown not to affect the development and survival of N. tenuis in barley (Hamza et al., 2018). On the other hand, Podisus maculiventris preferred jasmonate-insensitive plants and their survival was higher on these compared to jasmonate-overexpressing plants (Thaler et al., 2015). Finally, M. pygmaeus development was shown to be positively affected by tomato inoculation with Trichoderma longibrachiatum strain MK1, which also increased plants attractiveness to this predator possibly via the involvement of both the SA and JA signaling pathways (Battaglia et al., 2013). To date, no study has ever explored JA/SA-mediated responses on N. tenuis feeding behavior nor the underlying mechanisms involved in zoophytophagous predator-plant-microbe interactions.
We conclude that inoculating tomato plants with FsK results in significant reduction in plant damage caused by N. tenuis feeding. In addition, we show evidence for the involvement of the ethylene and JA signaling pathways in FsK-mediated tomato resistance to N. tenuis. The ecological implications of these results are highly relevant to biological control because tomato association with FsK is shown to provide substantial benefits to the plant by conferring resistance not only to pathogens but also against arthropods. Plant damage caused by N. tenuis feeding poses an important limitation in the use of an otherwise highly efficient biocontrol agent, when prey is scarce or at high predator populations. The fact that FsK negatively affects N. tenuis feeding damage on plants is promising but needs to be further explored by considering effects on performance and predation efficiency, also in the presence of prey. In this regard, it is important to understand the regulatory mechanisms involved in FsK-mediated resistance to N. tenuis in tomato.
NK, KV, and KP designed the study. NK, KV, NG, and VS performed experiments. NK, KV, MP, GB, and KP analyzed data. NK, MP, KV, and KP wrote the paper, with contribution from all authors.
Fusarium solani FsK is patented (20070100563/1006119, issued by the Industrial Property Organization to NK, KP).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was partially supported by the Postgraduate Programs 3817 and 3439 of the Department of Biochemistry and Biotechnology, University of Thessaly. MP was supported by the Onassis Foundation (grant number R-ZJ 003).
Ahemad, M., and Kibret, M. (2014). Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J. King Saud Univ. Sci. 26, 1–20. doi: 10.1016/j.jksus.2013.05.001
Arnó, J., Castañé, C., Riudavets, J., and Gabarra, R. (2010). Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bull. Entomol. Res. 100, 105–115. doi: 10.1017/S0007485309006841
Arnó, J., Castañé, C., Riudavets, J., Roig, J., and Gabarra, R. (2006). Characterization of damage to tomato plants produced by the zoophytophagous predator Nesidiocoris tenuis. IOBC/WPRS Bull. 29, 249–254. Available online at: http://www.iobc-wprs.org/pub/bulletins/iobc-wprs_bulletin_2006_29_04.pdf#page=261
Battaglia, D., Bossi, S., Cascone, P., Digilio, M. C., Prieto, J. D., Fanti, P., et al. (2013). Tomato below ground–above ground interactions: Trichoderma longibrachiatum affects the performance of Macrosiphum euphorbiae and its natural antagonists. Mol. Plant Microbe Interact. 26, 1249–1256. doi: 10.1094/MPMI-02-13-0059-R
Bueno, V. H. P., Van Lenteren, J. C., Lins, J. R. J. C., Calixto, A. M., Montes, F. C., Silva, D. B., et al. (2013) New records of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) predation by Brazilian Hemipteran predatory bugs. J. Appl. Entomol. 137, 29–34. doi: 10.1111/jen.12017
Calvo, F. J., Bolckmans, K., and Belda, J. E. (2012). Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl 57, 809–817. doi: 10.1007/s10526-012-9455-1
Calvo, J., Bolckmans, K., Stansly, P. A., and Urbaneja, A. (2009). Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 54, 237–246. doi: 10.1007/s10526-008-9164-y
Castañé Cristina, C., Arnó, J., Gabarra, R., and Alomar, O. (2011). Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 59, 22–29. doi: 10.1016/j.biocontrol.2011.03.007
Dicke, M., and Baldwin, I. T. (2010). The evolutionary context for herbivore-induced plant volatiles: beyond the 'cry for help'. Trends Plant Sci. 15, 167–175. doi: 10.1016/j.tplants.2009.12.002
Finkel, O. M., Castrillo, G., Herrera Paredes, S., Salas González, I., and Dangl, J. L. (2017). Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 38, 155–163. doi: 10.1016/j.pbi.2017.04.018
Fontana, A., Reichelt, M., Hempel, S., Gershenzon, J., and Unsicker, S. B. (2009). The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. J. Chem. Ecol. 35, 833–843. doi: 10.1007/s10886-009-9654-0
Fujino, D. W., Burger, D. W., Yang, S. F., and Bradford, K. J. (1988). Characterization of an ethylene overproducing mutant of tomato Lycopersicon esculentum Mill. Cultivar VFN8. Plant Physiol. 88, 774–779. doi: 10.1104/pp.88.3.774
Hadar, Y., and Papadopoulou, K. K. (2012). Suppressive composts: microbial ecology links between abiotic environments and healthy plants. Annu. Rev. Phytopathol. 50, 133–153. doi: 10.1146/annurev-phyto-081211-172914
Hamza, R., Pérez-Hedo, M., Urbaneja, A., Rambla, J. L., Granell, A., Gaddour, K., et al. (2018). Expression of two barley proteinase inhibitors in tomato promotes endogenous defensive response and enhances resistance to Tuta absoluta. BMC Plant Biol. 18:24. doi: 10.1186/s12870-018-1240-6
Hartley, S. E., and Gange, A. C. (2009). Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu. Rev. Entomol. 54, 323–342. doi: 10.1146/annurev.ento.54.110807.090614
Howe, G. A., Lightner, J., Browse, J., and Ryan, C. A. (1996). An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8, 2067–2077. doi: 10.1105/tpc.8.11.2067
Karban, R., and Baldwin, I. T. (1997). “Induced responses to herbivory,” in Induced Responses to Herbivory, eds R. Karban and I. T.Baldwin (Chicago, IL: The University of Chicago Press), 330.
Kavroulakis, N., Ntougias, S., Zervakis, G. I., Ehaliotis, C., Haralampidis, K., and Papadopoulou, K. K. (2007). Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. J. Exp. Bot. 58, 3853–3864. doi: 10.1093/jxb/erm230
Lanahan, M. B., Yen, H. C., Giovannoni, J. J., and Klee, H. J. (1994). The never ripe mutation blocks ethylene perception in tomato. Plant Cell 6, 521–530. doi: 10.1105/tpc.6.4.521
Leonetti, P., Zonno, M. C., Molinari, S., and Altomare, C. (2017). Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. Plant Cell Rep. 36, 621–631. doi: 10.1007/s00299-017-2109-0
Martínez-García, H., Román-Fernández, L. R., Sáenz-Romo, M. G., Pérez-Moreno, I., and Marco-Mancebón, V. S. (2016). Optimizing Nesidiocoris tenuis (Hemiptera: Miridae) as a biological control agent: mathematical models for predicting its development as a function of temperature. Bull. Entomol. Res. 106, 215–224. doi: 10.1017/S0007485315000978
Pappas, M. L., Broekgaarden, C., Broufas, G. D., Kant, M. R., Messelink, G. J., Steppuhn, A., et al. (2017). Induced plant defences in biological control of arthropod pests: a double-edged sword. Pest Manag. Sci. 73, 1780–1788. doi: 10.1002/ps.4587
Pappas, M. L., Steppuhn, A., and Broufas, G. D. (2016). The role of phytophagy by predators in shaping plant interactions with their pests. Commun. Integr. Biol. 9, 1–4. doi: 10.1080/19420889.2016.1145320
Pappas, M. L., Steppuhn, A., Geuss, D., Topalidou, N., Zografou, A., Sabelis, M. W., et al. (2015). Beyond predation: the zoophytophagous predator Macrolophus pygmaeus induces tomato resistance against spider mites. PLoS ONE 10:e0127251d doi: 10.1371/journal.pone.0127251
Pérez-Hedo, M., Bouagga, S., Jaques, J. A., Flors, V., and Urbaneja, A. (2015a). Tomato plant responses to feeding behavior of three zoophytophagous predators (Hemiptera: Miridae). Biol. Control 86, 46–51. doi: 10.1016/j.biocontrol.2015.04.006
Pérez-Hedo, M., and Urbaneja, A. (2016). “The zoophytophagous predator Nesidiocoris tenuis: a successful but controversial biocontrol agent in tomato crops,” in Advances in Insect Control and Resistance Management, eds A. R. Horowitz and I. Ishaaya (Cham: Springer International Publishing), 121–138.
Pérez-Hedo, M., Urbaneja-Bernat, P., Jaques, J. A., Flors, V., and Urbaneja, A. (2015b). Defensive plant responses induced by Nesidiocoris tenuis (Hemiptera: Miridae) on tomato plants. J. Pest Sci. 88, 543–554. doi: 10.1007/s10340-014-0640-0
Pieterse, C. M., Van Der Does, D., Zamioudis, C., Leon-Reyes, A., and Van Wees, S. C. M. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. doi: 10.1146/annurev-cellbio-092910-154055
Pieterse, C. M., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C., and Bakker, P. A. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. doi: 10.1146/annurev-phyto-082712-102340
Pineda, A., Soler, R., Weldegergis, B. T., Shimwela, M. M., Van Loon, J. J., and Dicke, M. (2013). Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signalling. Plant Cell Environ. 36, 393–404. doi: 10.1111/j.1365-3040.2012.02581.x
Pineda, A., Zheng, S. J., van Loon, J. J., Pieterse, C. M., and Dicke, M. (2010). Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci. 15, 507–514. doi: 10.1016/j.tplants.2010.05.007
Prieto, J. D., Castañé, C., Calvet, C., Camprubi, A., Battaglia, D., Trotta, V., et al. (2017). Tomato belowground–aboveground interactions: Rhizophagus irregularis affects foraging behavior and life history traits of the predator Macrolophus pygmaeus (Hemiptera: Miridae). Arthropod Plant Interact. 11, 15–22. doi: 10.1007/s11829-016-9465-5
Raman, K., and Sanjayan, K. P. (1984). Histology and histopathology of the feeding lesions by Cyrtopeltis tenuis Rent. (Hemiptera: Miridae) on Lycopersicon esculentum Mill. (Solanaceae). Proc. Anim. Sci. 93, 543–547. doi: 10.1007/BF03186303
Rosenblueth, M., and Martínez-Romero, E. (2006). Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 19, 827–837. doi: 10.1094/MPMI-19-0827
Sanchez, J. A. (2008). Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric. For. Entomol. 10, 75–80. doi: 10.1111/j.1461-9563.2007.00357.x
Sanchez, J. A. (2009). Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biol. Control 51, 493–498. doi: 10.1016/j.biocontrol.2009.09.006
Sánchez, J. A., and Lacasa, A. (2008). Impact of the zoophytophagous plant bug ?esidiocoris tenuis (Heteroptera: Miridae) on tomato yield. J. Econ. Entomol. 101, 1864–1870. doi: 10.1603/0022-0493-101.6.1864
Schausberger, P., Peneder, S., Jürschik, S., and Hoffmann, D. (2012). Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct. Ecol. 26, 441–449. doi: 10.1111/j.1365-2435.2011.01947.x
Shikano, I., Rosa, C., Tan, C. W., and Felton, G. W. (2017). Tritrophic interactions: microbe-mediated plant effects on insect herbivores. Annu. Rev. Phytopathol. 55, 313–331. doi: 10.1146/annurev-phyto-080516-035319
Smith, S. E., and Smith, F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62, 227–250. doi: 10.1146/annurev-arplant-042110-103846
Stahl, E., Hilfiker, O., and Reymond, P. (2018). Plant–arthropod interactions: who is the winner? Plant J. 93, 703–728. doi: 10.1111/tpj.13773
Sukhoruchenko, G. I., Belyakova, N. A., Pazyuk, I. M., and Ivanova, G. P. (2015). The toxic effect of greenhouse insecticides on the predatory bugs Nesidiocoris tenuis Reuter and Macrolophus pygmaeus H.-S.(Heteroptera, Miridae). Entomol. Rev. 95, 1166–1173. doi: 10.1134/S0013873815090031
Thaler, J. S., Olsen, E. L., and Kaplan, I. (2015). Jasmonate-induced plant defenses and prey availability impact the preference and performance of an omnivorous stink bug, Podisus maculiventris. Arthropod Plant Interact. 9, 141–148. doi: 10.1007/s11829-015-9357-0
Van Wees, S. C., Van der Ent, S., and Pieterse, C. M. (2008). Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 11, 443–448. doi: 10.1016/j.pbi.2008.05.005
Keywords: biological control, endophyte, ethylene, jasmonic acid, plant damage, tomato, zoophytophagous predator
Citation: Garantonakis N, Pappas ML, Varikou K, Skiada V, Broufas GD, Kavroulakis N and Papadopoulou KK (2018) Tomato Inoculation With the Endophytic Strain Fusarium solani K Results in Reduced Feeding Damage by the Zoophytophagous Predator Nesidiocoris tenuis. Front. Ecol. Evol. 6:126. doi: 10.3389/fevo.2018.00126
Received: 24 March 2018; Accepted: 02 August 2018;
Published: 22 August 2018.
Edited by:
Ainhoa Martinez Medina, German Center for Integrative Biodiversity Research, GermanyReviewed by:
Eunice Jingmei Tan, Yale-NUS College, SingaporeCopyright © 2018 Garantonakis, Pappas, Varikou, Skiada, Broufas, Kavroulakis and Papadopoulou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nektarios Kavroulakis, bmthdnJvdWxha2lzQG5hZ3JlZi1jaGEuZ3I=
Kalliope K. Papadopoulou, a2FscGFwYWRAYmlvLnV0aC5ncg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.