- 1Animal Evolutionary Ecology, Institute of Evolution and Ecology, University of Tübingen, Tübingen, Germany
- 2Institute for Zoology, Ecological Research Station Rees, University of Cologne, Cologne, Germany
- 3MARE—Marine and Environmental Sciences Centre, ISPA—Instituto Universitário, Lisbon, Portugal
- 4Departamento de Biologia Animal, Faculdade de Ciências, cE3c—Centre for Ecology, Evolution and Environmental Changes, Universidade de Lisboa, Lisbon, Portugal
Selection pressures on signals can be substantially modified by a changing environment, but we know little about how modified selection pressures act on multimodal signals. The currently increasing levels of anthropogenic noise in the ocean may affect the use of acoustic signaling relative to other modalities. In the Painted Goby (Pomatoschistus pictus), visual and acoustic signals are associated during courtship behavior, but females usually rely more heavily on acoustic signals than on visual signals in mate choice. In an aquarium experiment, we compared male courtship behavior and female spawning decisions between silent treatments and treatments with additional noise. We found that the relationships between male characteristics, male visual and acoustic courtship, and spawning success were affected by noise. A path analysis revealed that females pay more attention to visual courtship in noisy circumstances compared to control. We conclude that environmental stressors can cause shifts in the use of different signaling modalities for spawning decisions and discuss how selection pressures on multimodal signals may change with increasing noise-levels.
Introduction
The degree to which a signal is effective in carrying information to a receiver can be substantially modified by a changing environment. Loss of signal efficacy in changing environments may lead to signals being misunderstood or not received at all. To overcome a loss of information transfer, signaling individuals may adjust their signaling behavior by adapting the signal to the environment. For example, Tokay Geckos (Gekko gecko) have been found to increase the duration of their typically brief call notes in a noisy environment, in order to make them more easily heard (Brumm and Zollinger, 2017). Alternatively, signaling individuals may switch to different modalities that are not, or are less affected by environmental changes, such as found in tree frogs that produce more visual signals when background noise is high (Grafe et al., 2012). In addition, in those cases where the receiver benefits from the information contained in the signal (e.g., mate choice), the receiver may also adapt to a loss of signal efficacy by switching its attention to alternative signals or cues, such as stickleback females that pay more attention to chemical than visual signals in a turbid environment (Heuschele et al., 2009).
In fish, acoustic communication is known to play an important role in mating behavior and reproduction (Myrberg and Lugli, 2006). Acoustic signals may be essential for mate attraction and mate selection as seen in the Lusitanian Toadfish (Halobatrachus didactylus), a fish species where mate attraction and reproductive success depends on the male's acoustic courtship performance (Amorim et al., 2016). Sound production is also common in many gadoids and is thought to synchronize gamete release in Haddock, Melanogrammus aeglefinus (Hawkins and Amorim, 2000; Casaretto et al., 2015), and in Cod (Gadus morhua; Rowe and Hutchings, 2006).
Anthropogenic noise is a growing environmental concern, in particular in relation to aquatic life (Slabbekoorn et al., 2010; Popper et al., 2014; Radford et al., 2014). Aquatic animals may be particularly dependent on acoustic communication which overlaps in frequency with anthropogenic noise (Slabbekoorn et al., 2010; van der Sluijs et al., 2011; Radford et al., 2014). Noise can affect the range over which fish can communicate effectively (Vasconcelos et al., 2007; Alves et al., 2016; Stanley et al., 2017), and has been shown to affect acoustic signaling and reproductive behavior in fish (Picciulin et al., 2010, 2012; Bruintjes and Radford, 2013; Holt and Johnston, 2015; Nedelec et al., 2017; de Jong et al., 2018). Because noise can affect both signaling behavior and the propagation of signals, it is likely to affect the way information is transferred to the female during courtship. Therefore, noise could affect sexual selection and, as a result, change the frequency of certain acoustic traits in a population. For example, if male acoustic signals are rendered less effective as a source of information about male quality, females may stop paying attention to acoustic signals, leading to reduced sexual selection for this trait, which could, ultimately, result in trait-loss (Järvenpää and Lindstrom, 2004; Candolin et al., 2007; Tuomainen and Candolin, 2011).
Many animals rely on more than one modality to signal their quality: they may, for example, use sound and visual cues (Rowe, 1999; Hebets and Papaj, 2005). A main hypothesis for the function of multimodal signals is that one modality may be a back-up for a loss of signal efficacy in another modality (Bradbury and Vehrencamp, 1998; Hebets and Papaj, 2005). Such signal redundancy could mitigate effects of noise on acoustic signaling (Brumm and Slabbekoorn, 2005; van der Sluijs et al., 2011; Partan, 2017). However, such an effect would depend on whether and how the receiver uses the information it obtained from these different signals. In Painted Gobies (Pomatoschistus pictus), males lure females to their nests with both visual and acoustic signals, but females have been found to rely more heavily on acoustic than on visual signals for mate choice (Amorim et al., 2013). Therefore, it is an ideal model species to test how noise could affect multimodal communication. In this study, we tested whether this differential use of modalities changed when mating couples were exposed to noise during courtship and spawning. Under the hypothesis that females would pay less attention to signaling in the acoustic modality when this modality is disturbed by noise, we predicted that acoustic signaling would become less important for mating success than visual courtship under noisy conditions.
Materials and Methods
General Design
The experiment was carried out in January and February 2015 at the University of Lisbon. We exposed male gobies to a control (noise-insulated aquaria) or an added noise treatment for 3 days. Females were introduced in a separate compartment within the same aquarium on the evening of day 3 to allow them to habituate to the acoustic environment. On day 4 we removed the partition to release the females into the male compartment and allowed free interaction and spawning. Each male was presented with two free-swimming females and we recorded male courtship behavior and female spawning behavior (added-noise: N = 20, control: N = 16).
Ethics
All experiments were performed in compliance with laws of Portugal. We operated under a permit for catching Painted Gobies from the National Defense Ministry (Autoridade Marítima Nacional-Capitania do Porto de Cascais), permit nr. 550/2013.
Study Species
The Painted Goby, (P. pictus), is a coastal marine species. This small benthic species inhabits shallow gravel and sand substrate areas in the Eastern Atlantic Ocean and in some areas of the Mediterranean Sea (Miller, 1986). The Painted Goby has a polygamous mating system, in which males build nests under bivalve shells, by shoveling sand in a pile over the shell (Bouchereau et al., 2003). Males attract females to spawn and take care of the eggs until they hatch. Males can take care of eggs of several females at the same time and in batches over the season. Males display both visually and acoustically during courtship (Amorim and Neves, 2007). Courtship vocalizations consist of drums and thumps (Amorim and Neves, 2007; Amorim et al., 2013).
Catching and Husbandry
Painted Gobies were caught in January and February 2015 with hand nets in intertidal pools at Parede (38° 41′N, 9° 21′W), Portugal. In the laboratory, males and females were kept separately in recirculated artificial sea water (32–35‰) under a 12 h: 12 h dark/ light regime at 16°C. Fish were fed twice a day ad libitum with a mix of chopped mussel, clams and shrimp. For detailed methods see de Jong et al. (2018).
Experimental Set-Up
Experimental aquaria contained a 3 cm layer of sand and a nest made from a PVC tube with a chimney to accommodate the hydrophone (Figure 1; see Amorim et al., 2013 and de Jong et al., 2018 for further details of the nest and recording setup). The nest was covered inside with a bendable plastic sheet for later removal and photography of the eggs in the nest. The “noise egg,” consisting of an electromotor in a waterproof container, was used to generate a constant low frequency multi-tone with a fundamental frequency around 100 Hz and several strong harmonics (de Jong et al., 2017, 2018) The background noise level in the control treatment was 100 ± 1 dB re 1 μPa (N = 16) compared to 125 ± 6 dB re 1 μPa (N = 20) in the added noise treatments [see (de Jong et al., 2018) for details]. Particle acceleration, measured with an accelerometer (see Klein et al., 2013; de Jong et al., 2018), was elevated on average by 20 dB at 200 Hz (i.e., around the main frequency of courtship drums; Amorim et al., 2013) compared to ambient recordings in the male nest. The harmonic structure of the experimental noise allowed us to unambiguously quantify the number of calls in the added noise treatment as well as in the control treatment. The noise-egg was placed just behind the nest in a cloth bag and weighed down with a stone. In the control treatments, the egg was switched off. Males of both species were allowed to acclimate to the treatment (added noise or control) for 3 days (day 1–3). On day 2 a stimulus female was introduced behind a partition to stimulate nest building, she was removed on day 3. Painted Gobies built nests by shoveling sand in a pile over the plastic tube.
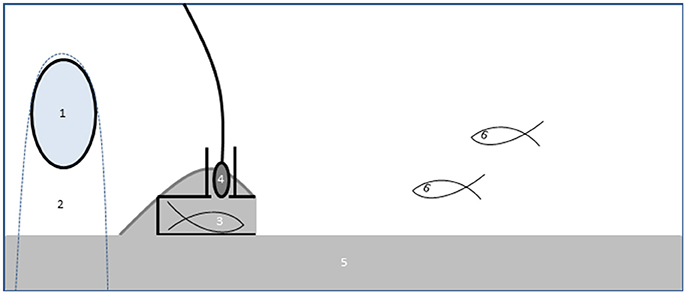
Figure 1. Schematic overview of the experimental set-up in an experiment to test effects of noise on multimodal communication in the Painted Goby. A noise-egg (1) was placed in a cloth with sand as weight (2) behind the male's nest (3). A hydrophone in the nest chimney (4) recorded male sounds. Aquaria contained a layer of sand (5) on the bottom, which males used to build a nest over the provided PVC-tube. The male and the two females (6) could interact freely during the trials.
Test females were measured to the nearest 0.5 mm, weighed to the nearest 0.1 g and introduced to a separate compartment in the experimental aquarium on the evening of day 3. They were allowed to acclimate overnight to the treatment for 12 h. During the acclimation period they could interact with the male behind a transparent partition at 30 cm from the male nest. On day 4, an hour before the start of the trial, we added an opaque divider to obtain a resting period without courtship. We started a trial by lifting the divider to release the females. We recorded acoustic and visual courtship for 30 min and noted whether and when either of the females entered the nest. After the trial, both male and females were left in the aquarium for what was left of day 4 and the morning of day 5, during which we checked the nest for eggs every 3 daylight-hours with a handheld torch. On day 5, we ended the trial and weighed males and females to the nearest 0.1 g, measured them to the nearest 0.5 mm and took a picture of the eggs on the plastic sheet. Male size was on average 41.6 ± 2.99 mm (N = 36) and weight was on average 0.63 ± 0.13 g. We used Fulton's K as a measure of condition, which was calculated by dividing the wet weight in g by the cubic of the length in cm times 100 (Ricker, 1975). Condition was on average 0.86 ± 0.06 (N = 36). There were no significant differences between the treatments in male total length (t-test: t = 0.20, df = 28.7, P = 0.8) or weight (t-test: t = 0.87, df = 31.4, P = 0.4), but there was a trend for males to have a lower condition at the end of the noise treatment compared to the control (t-test: t = 2.04, df = 29.6, P = 0.05).
Analyses
Sound analyses were done using PRAAT version 6.0.19. (Boersma and Weenink, 2017). We counted the number of drums and thumps made during the first 30 min of a trial. Visual courtship was scored from a silent video by an observer that was blind to the treatment (KdJ). Because the minimum time for a female to enter the nest to spawn was as short as 1 min and all visual courtship was completed before spawning, we only report counts for the first minute of visual courtship (as in de Jong et al., 2018). We counted the frequency of hops (the male approaches a female with short hop-like swimming motions), jumps (the male swims over or in front of the female and lands facing the other way, as a component of an eight-display), quivers (shaking the body), leads (the male swims towards the nest waving its tail in a characteristic manner), and fast approaches (including nudges: the male swims quickly toward the female in a straight line, sometimes nudging her in the side) (cf. Amorim and Neves, 2007). We also noted the number of longer swims that were not directed at a female. Female-female interactions (fast approaches) were very scarce and, therefore, left out of the analyses.
Statistical analyses were performed in R 3.4.3 (R Core Team, 2017). We used a simplified form of path analysis, because it is a useful way to visualize changes in relationships within the data. We proposed a single predicted path including all measured male characteristics (Figure 2) and we tested each relationship (arrow) within the path separately for the control and the added noise treatment. We tested each relationship separately, because our sample size did not allow the inclusion of all effects in the same model. We tested the correlation between visual and acoustic courtship with a Spearman's rank correlation test. For all other steps we used generalized linear models with appropriate residual error structures [R packages: lme4 (R Core Team, 2017), MASS (Venables and Ripley, 2002)]. We used a quasibinomial error structure (glm, family = quasibinomial) for the effect of courtship frequency (acoustic and visual) and male characteristics (size and condition) on the likelihood of female spawning. We used Gaussian models (lm) for effects of male characteristics on the log-transformed visual courtship frequency, and we used a quasi-Poisson error structure (glm, family = quasipoisson) for the effects of male characteristics on acoustic courtship frequency. Model fit was verified by visual inspection of the residual plots provided in the plot function in lme4 and we report model results with and without outliers based on Cook's distance. In the model to test the effect of male size on acoustic courtship frequency, we found one data point with a Cook's distance > 1. After removal of this outlier, the estimation of the effect changed from 0.19 (CI: −0.04 to 0.42) to 0.35 (CI: 0.07 to 0.64), and thus from borderline non-significant to borderline significant. However, because we had no a priori reasons to exclude this outlier, and it did not change the results qualitatively, we chose to keep the outlier in the model. We provide estimates and confidence intervals for treatment effects in the figures, for full models see Supplementary Tables 1–8). If the confidence intervals of the estimated effect do not overlap with 0, the effect is significantly different from zero (P < 0.05).
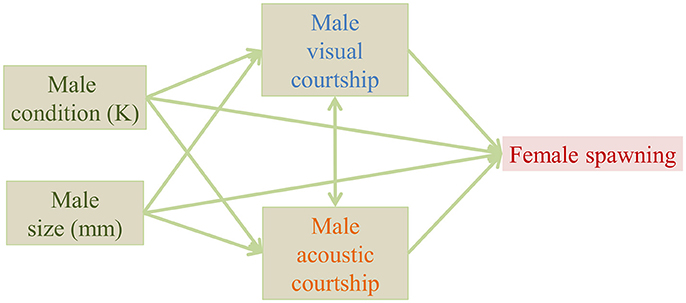
Figure 2. Proposed path of measured variables potentially affecting the likelihood of female spawning in a Painted Goby aquarium experiment.
Results
Painted Gobies produced on average 1.5 ± 2 (N = 36) sounds per minute in a 30 min trial and displayed on average 19 ± 9.2 (mean ± SD, N = 36) visual behaviors in the first minute. Overall differences between the treatments in male visual and male acoustic behavior have been reported in a previous article (de Jong et al., 2018); the frequencies of both behaviors decreased in the added noise treatment.
As in previous studies (Amorim et al., 2013) the likelihood of successful spawning was predominantly correlated with the frequency of male acoustic courtship in the control treatment (Figure 3A). In the treatment with added noise, the frequency of male acoustic courtship still had a significant effect on spawning success, but male visual courtship frequency also had a significant positive effect (Figure 3B). Furthermore, male acoustic courtship frequency was significantly correlated with male visual courtship frequency and male size had a significant effect on male visual courtship frequency (Figure 3B).
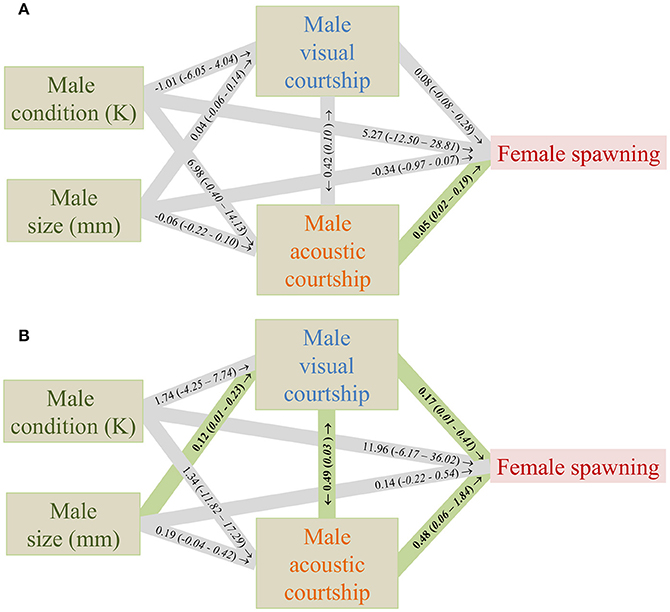
Figure 3. Effects of measured variables on the likelihood of female spawning in an aquarium experiment in the Painted Goby in a control (A) and an added noise (B) treatment (N = 16, 20). Numbers in the lines are effect sizes from generalized linear models with their confidence intervals between brackets. See Supplementary Files for full model reports. The correlation between acoustic and visual courtship was tested with a Spearman's rank test, and therefore the rho is given with the P-value in brackets. Numbers in bold are significant effects (P < 0.05).
The differences between the treatments were most pronounced for average or lower courtship frequencies (Figure 4). Males with high visual or acoustic courtship frequencies were predicted by the model to be successful in both treatments. However, males with lower visual courtship frequencies were less likely to spawn in the added noise treatment than in the control. Males with a lower acoustic courtship frequency, on the other hand, were more likely to spawn in the added noise treatment compared to the control (Figure 4).
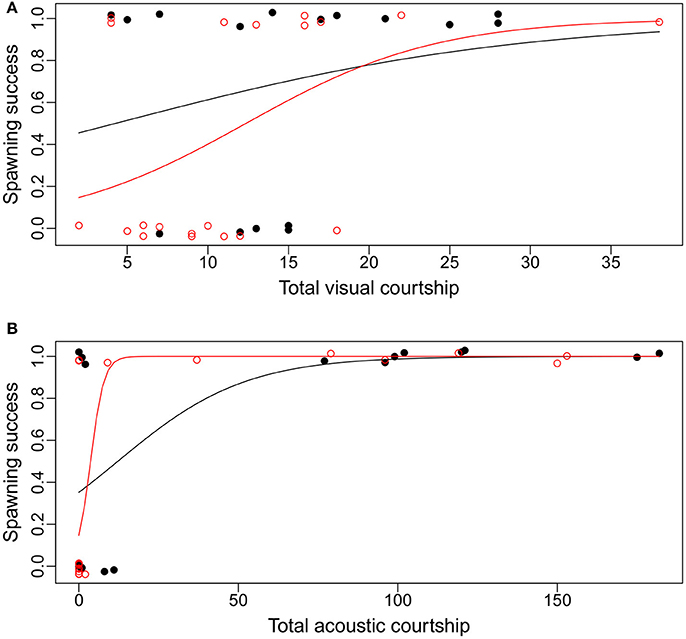
Figure 4. The relationships between visual (A) and acoustic (B) courtship frequency and the probability of spawning success for Painted Goby males in an aquarium experiment to test the effect of additional noise on spawning decisions. Dots are individual males that either did (1) or did not (0) receive eggs from females in the control treatment (black dots) vs. additional noise (red circles). Lines are the model estimates from generalized linear models for the relationship between courtship frequency and spawning success in control (black) vs. additional noise (red). See Supplementary Files for full model reports.
Discussion
We found that noise affects the relationship between male courtship behavior and female spawning decisions in the Painted Goby. While acoustic courtship frequency was the only significant predictor of spawning success in the control treatment, male visual courtship frequency was also a significant predictor of spawning success in the added noise treatment. The model predictions (Figure 4) showed that low acoustic activity is associated with a higher spawning likelihood in the additional noise treatment when compared to the control, while for visual courtship the opposite pattern is seen. Overall this suggests that visual courtship becomes more important in mating communication when noise disturbs acoustic communication in the Painted Goby.
These results could be explained by the sensory compensation hypothesis, which states that multimodal signals may provide a back-up for information loss if the signal components in different modalities are redundant (Hartman and Abrahams, 2000; Hebets and Papaj, 2005; Bro-Jørgensen, 2010; Partan, 2017). If the efficacy of a certain signal component is reduced by noise in one of the modalities, both the signaler and the receiver may shift their communication efforts to another modality, which has been termed a multimodal shift (Partan et al., 2010). Examples of multimodal shifts have been found in all taxa, from invertebrates to mammals (reviewed in Partan, 2017). From the back-up hypothesis, an increase in visual signaling would have been expected under added noise conditions to compensate for the deterioration of acoustic communication, but in the current study male Painted Gobies did not increase visual signaling (de Jong et al., 2018). Nevertheless, females apparently paid more attention to visual signaling in the added noise treatment. A similar mismatch between male and female adjustments was found in the three-spined stickleback (Gasterosteus aculeatus), where males displayed more visual courtship in turbid conditions, while females paid more attention to chemical cues (Candolin et al., 2007; Heuschele et al., 2009). In the three-spined stickleback, this mismatch co-occurred with a weakened sexual selection on visual traits, which could ultimately lead to trait loss in the population (Candolin et al., 2007; Tuomainen and Candolin, 2011).
In addition to the relationship between visual courtship and spawning success, the relationships between visual courtship and acoustic courtship, and the relationship between male size and visual courtship were significant in the additional noise treatment (Figure 3B). The relationship between male size and acoustic courtship was borderline non-significant. One could suspect that this increase in the number of significant relationships in the additional noise treatment compared to the control was caused by an increase in the precision of the model estimates due to the larger sample size in the noise relative to the control treatment (20 vs. 16). Instead, the confidence intervals increased in all cases where we found a significant effect in the noise treatment that was non-significant in the control. This suggests that the increase in the number of significant relationships was caused by an increase in the actual effect sizes, and not by an increase in the precision of the model estimates. Call characteristics, including acoustic courtship frequency of male Painted Gobies have been previously correlated with male quality (Amorim et al., 2013) and may allow females to choose the best mates and also to distinguish between closely-related cryptic species (Pedroso et al., 2012). If such the information in such signals does not reach the female, because the propagation is hampered or because of masking, females may switch to more simple cues of male quality, such as size, which may be assessed directly (if visibility allows) and is most easily advertised in visual courtship. Such a mechanism could potentially explain the stronger relationship between male size, visual courtship frequency and spawning success in the additional noise treatment compared to the control.
Overall, we found a change in the importance of different modalities during mating interactions of the Painted Goby in response to increased noise levels. Although the reliance on acoustic courtship was maintained, visual courtship gained importance in the interactions between males and females under noisy conditions. In addition, male size became significantly associated with visual courtship frequency. Environmental changes have the potential to drastically alter sexual selection on traits (Miller and Svensson, 2014). We show that noise can change relationships between traits, signaling components in different modalities, and spawning success, which is the precursor for such changes in sexual selection. Future studies should focus on whether such changes result in a loss or change of acoustic traits in natural populations.
Data Availability Statement
All data used for this paper is included in the Supplementary Table 9.
Author Contributions
KdJ and KH designed the experiment, with comments from CA and PF. KdJ executed the experiment, analyzed the data and wrote the manuscript with comments and revisions by KH, CA, and PF.
Funding
KdJ was funded by the: Alexander von Humboldt Foundation (NLD /1150888STP). The Volkswagen Foundation funded KH (84 846 / 92 002). The Science and Technology Foundation, Portugal funded CA (strategic projects UID/MAR/04292/2013 to MARE) and PF (UID/BIA/00329/2013 to cE3c).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the University of Lisbon for providing laboratory space. Maria Gouveia, Catarina Rosa, and Joana Vincente for help with catching, husbandry and logistics. We would like to thank Adrian Klein for kindly providing us with particle motion sensors and help with the analyses of the PM-data. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of the University of Tübingen.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00113/full#supplementary-material
References
Alves, D., Amorim, M. C., and Fonseca, P. J. (2016). Assessing acoustic communication active space in the Lusitanian toadfish. J. Exp. Biology 219, 1122–1129. doi: 10.1242/jeb.134981
Amorim, M. C., Conti, C., Sousa-Santos, C., Novais, B., Gouveia, M. D., Vicente, J. R., et al. (2016). Reproductive success in the Lusitanian toadfish: influence of calling activity, male quality and experimental design. Physiol. Behav. 155, 17–24. doi: 10.1016/j.physbeh.2015.11.033
Amorim, M. C. P., Pedroso, S. S., Bolgan, M., Jordão, J. M., Caiano, M., and Fonseca, P. J. (2013). Painted gobies sing their quality out loud: acoustic rather than visual signals advertise male quality and contribute to mating success. Funct. Ecol. 27, 289–298. doi: 10.1111/1365-2435.12032
Amorim, M., and Neves, A. (2007). Acoustic signaling during courtship in the painted goby, Pomatoschistus pictus. J. Marine Biol. Assoc. 87, 1017–1023. doi: 10.1017/S0025315407056822
Boersma, P., and Weenink, D. (2017). PRAAT: Doing Phonetics by Computer. Available online at: www.praat.org
Bouchereau, J.-L., Houder, V., Marques, A., and Rebelo, J. (2003). A new distribution record and the reproductive strategy of Pomatoschistu pictus adriaticus (Pisces: Gobiidae) in the Mediterranean Sea. J. Marine Biol. Assoc. 83, 1157–1161. doi: 10.1017/S0025315403008427h
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles of Animal Communication. Sunderland, MA: Sinauer Associates, Inc.
Bro-Jørgensen, J. (2010). Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol. Evol. 25, 292–300. doi: 10.1016/j.tree.2009.11.003
Bruintjes, R., and Radford, A. N. (2013). Context-dependent impacts of anthropogenic noise on individual and social behaviour in a cooperatively breeding fish. Anim. Behav. 85, 1343–1349. doi: 10.1016/j.anbehav.2013.03.025
Brumm, H., and Slabbekoorn, H. (2005). Acoustic communication in noise. Adv. Study Behav. 35, 151–209. doi: 10.1016/S0065-3454(05)35004-2
Brumm, H., and Zollinger, S. A. (2017). Vocal plasticity in a reptile. Proc. Biol. Sci. B 284:20170451. doi: 10.1098/rspb.2017.0451
Candolin, U., Salesto, T., and Evers, M. (2007). Changed environmental conditions weaken sexual selection in sticklebacks. J. Evol. Biol. 20, 233–239. doi: 10.1111/j.1420-9101.2006.01207.x
Casaretto, L., Picciulin, M., and Hawkins, A. D. (2015). Mating behaviour by the haddock (Melanogrammus aeglefinus). Environ. Biol. Fishes 98, 913–923. doi: 10.1007/s10641-014-0327-7
de Jong, K., Amorim, M. C. P., Fonseca, P. J., Fox, C. J., and Heubel, K. U. (2018). Noise can affect acoustic communication and subsequent spawning success in fish. Environ. Pollut. 237, 814–823. doi: 10.1016/j.envpol.2017.11.003
de Jong, K., Schulte, G., and Heubel, K. U. (2017). The noise egg: a cheap and simple device to produce low-frequency underwater noise for laboratory and field experiments. Methods Ecol. Evol. 8, 268–274. doi: 10.1111/2041-210X.12653
Grafe, T. U., Preininger, D., Sztatecsny, M., Kasah, R., Dehling, J. M., Proksch, S., et al. (2012). Multimodal communication in a noisy environment: a case study of the Bornean rock frog Staurois parvus. PLoS ONE 7:e37965. doi: 10.1371/journal.pone.0037965
Hartman, E. J., and Abrahams, M. V. (2000). Sensory compensation and the detection of predators: the interaction between chemical and visual information. Proc. Biol. Sci. B 267, 571–575. doi: 10.1098/rspb.2000.1039
Hawkins, A., and Amorim, M. (2000). Spawning sounds of the male haddock, Melanogrammus aeglefinus. Environ. Biol. Fishes 59, 29–41. doi: 10.1023/A:1007615517287
Hebets, E. A., and Papaj, D. R. (2005). Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. doi: 10.1007/s00265-004-0865-7
Heuschele, J., Mannerla, M., Gienapp, P., and Candolin, U. (2009). Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 20, 1223–1227. doi: 10.1093/beheco/arp123
Holt, D. E., and Johnston, C. E. (2015). Traffic noise masks acoustic signals of freshwater stream fish. Biol. Conserv. 187, 27–33. doi: 10.1016/j.biocon.2015.04.004
Järvenpää, M., and Lindstrom, K. (2004). Water turbidity by algal blooms causes mating system breakdown in a shallow-water fish, the sand goby Pomatoschistus minutus. Proc. Biol. Sci. B 271, 2361–2365. doi: 10.1098/rspb.2004.2870
Klein, A., Münz, H., and Bleckmann, H. (2013). The functional significance of lateral line canal morphology on the trunk of the marine teleost Xiphister atropurpureus (Stichaeidae). J. Comp. Physiol. A 199, 735–749. doi: 10.1007/s00359-013-0834-6
Miller, C. W., and Svensson, E. I. (2014). Sexual selection in complex environments. Annu. Rev. 59, 427–445. doi: 10.1146/annurev-ento-011613-162044
Miller, P. J. (1986). “Gobiidae,” in Fishes of the North-Eastern Atlantic and the Mediterranean, Vol. 3, ed P. J. P. Whitehead (Paris: UNESCO), 1019–1085.
Myrberg, A. J., and Lugli, M. (2006). “Reproductive behavior and acoustical interactions,” in Communication in Fishes, Vol. 1, ed F. Ladich (Enfield, NH: Science Publishers), 149–176.
Nedelec, S. L., Radford, A. N., Pearl, L., Nedelec, B., McCormick, M. I., Meekan, M. G., et al. (2017). Motorboat noise impacts parental behavior and offspring survival in a reef fish. Proc. Biol. Sci. B 284:0170143. doi: 10.1098/rspb.2017.0143
Partan, S. R. (2017). Multimodal shifts in noise: switching channels to communicate through rapid environmental change. Anim. Behav. 124, 325–337. doi: 10.1016/j.anbehav.2016.08.003
Partan, S. R., Fulmer, A. G., Gounard, M. A., and Redmond, J. E. (2010). Multimodal alarm behavior in urban and rural gray squirrels studied by means of observation and a mechanical robot. Curr. Zool. 56, 313–326.
Pedroso, S. S., Bolgan, M., Jordão, J. M., Fonseca, P. J., and Amorim, M. C. P. (2012). “Acoustic communication in Pomatoschistus spp.: a comparison between closely related species,” in Advances in Experimental Medicine and Biology (New York, NY: Springer), 113–115.
Picciulin, M., Sebastianutto, L., Codarin, A., Calcagno, G., and Ferrero, E. A. (2012). Brown meagre vocalization rate increases during repetitive boat noise exposures: a possible case of vocal compensation. J. Acoust. Soc. Am. 132, 3118–3124. doi: 10.1121/1.4756928
Picciulin, M., Sebastianutto, L., Codarin, A., Farina, A., and Ferrero, E. A. (2010). In situ behavioural responses to boat noise exposure of Gobius cruentatus (Gmelin, 1789, fam. Gobiidae) and Chromis chromis (Linnaeus, 1758, fam. Pomacentridae) living in a marine protected area. J. Exp. Marine Biol. Ecol. 386, 125–132. doi: 10.1016/j.jembe.2010.02.012
Popper, A. N., Hawkins, A. D., Fay, R. R., Mann, D., Bartol, S., Carlson, T., et al. (2014). Sound Exposure Guidelines for Fishes and Sea Turtles: A Technical Report Prepared by ANSI-accredited Standards Committee S3/SC1 and Registered With ANSI. ASA S3/SC1.4 TR-2014, Springerbriefs in Oceanography. Cham: Springer International Publishing; ASA Press.
Radford, A. N., Kerridge, E., and Simpson, S. D. (2014). Acoustic communication in a noisy world: can fish compete with anthropogenic noise? Behav. Ecol. 25, 1022–1030. doi: 10.1093/beheco/aru029
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Ricker, W. E. (1975). Computation and inter-pretation of biological statistics of fish populations. Bull. of the Fish. Res. Board of Canada 191, 1–382.
Rowe, C. (1999). Reciever psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921–931. doi: 10.1006/anbe.1999.1242
Rowe, S., and Hutchings, J. A. (2006). Sound production by atlantic cod during spawning. Trans. Am. Fish. Soc. 135, 529–538. doi: 10.1577/T04-061.1
Slabbekoorn, H., Bouton, N., Van Opzeeland, I., Coers, A., Ten Cate, C., and Popper, A. (2010). A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427. doi: 10.1016/j.tree.2010.04.005
Stanley, J. A., Van Parijs, S. M., and Hatch, L. T. (2017). Underwater sound from vessel traffic reduces the effective communication range in Atlantic cod and haddock. Sci. Rep. 7:14633. doi: 10.1038/s41598-017-14743-9
Tuomainen, U., and Candolin, U. (2011). Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. doi: 10.1111/j.1469-185X.2010.00164.x
van der Sluijs, I., Gray, S. M., Amorim, M. C. P., Barber, I., Candolin, U., Hendry, A. P., et al. (2011). Communication in troubled waters: responses of fish communication systems to changing environments. Evol. Ecol. 25, 623–640. doi: 10.1007/s10682-010-9450-x
Keywords: acoustic communication, aquatic noise pollution, courtship behavior, multimodal shift, sexual selection, mate choice, pomatoschistus pictus, spawning
Citation: de Jong K, Amorim MCP, Fonseca PJ and Heubel KU (2018) Noise Affects Multimodal Communication During Courtship in a Marine Fish. Front. Ecol. Evol. 6:113. doi: 10.3389/fevo.2018.00113
Received: 30 April 2018; Accepted: 10 July 2018;
Published: 31 July 2018.
Edited by:
Varvara Yu. Vedenina, Institute for Information Transmission Problems (RAS), RussiaReviewed by:
Michael J. Pauers, Milwaukee Public Museum, United StatesRyan L. Earley, University of Alabama, United States
Copyright © 2018 de Jong, Amorim, Fonseca and Heubel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen de Jong, S2FyZW4uZGUuam9uZ0BoaS5ubw==; a2FyZW5kZUBhbHVtbmkubnRudS5ubw==
Katja U. Heubel, a2F0amEuaGV1YmVsQHVuaS10dWViaW5nZW4uZGU=
†Present Address: Karen de Jong, Institute of Marine Research, Bergen, Norway
M. Clara P. Amorim, Departamento de Biologia Animal, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal
 Karen de Jong
Karen de Jong M. Clara P. Amorim
M. Clara P. Amorim Paulo J. Fonseca4
Paulo J. Fonseca4 Katja U. Heubel
Katja U. Heubel