- 1Department of Terrestrial Ecology, The Netherlands Institute of Ecology (NIOO-KNAW), Wageningen, Netherlands
- 2Plant Sciences and Natural Products, Institute of Biology, Leiden University, Leiden, Netherlands
- 3Department of Ecological Sciences - Animal Ecology, VU University, Amsterdam, Netherlands
Soil biota-plant interactions play a dominant role in terrestrial ecosystems. Through nutrient mineralization and mutualistic or antagonistic interactions with plants soil biota can affect plant performance and physiology and via this affect plant-associated aboveground insects. There is a large body of work in this field that has already been synthesized in various review papers. However, most of the studies have been carried out under highly controlled laboratory or greenhouse conditions. Here, we review studies that manipulate soil organisms of four dominant taxa (i.e., bacteria, fungi, nematodes, and soil arthropods) in the field and assess the effects on the growth of plants and interactions with associated aboveground insects. We show that soil organisms play an important role in shaping plant-insect interactions in the field and that general patterns can be found for some taxa. Plant growth-promoting rhizobacteria generally have negative effects on herbivore performance or abundance, most likely through priming of defenses in the host plant. Addition of arbuscular mycorrhizal fungi (AMF) has positive effects on sap sucking herbivores, which is likely due to positive effects of AMF on nutrient levels in the phloem. The majority of AMF effects on chewers were neutral but when present, AMF effects were positive for specialist and negative for generalist chewing herbivores. AMF addition has negative effects on natural enemies in the field, suggesting that AMF may affect plant attractiveness for natural enemies, e.g., through volatile profiles. Alternatively, AMF may affect the quality of prey or host insects mediated by plant quality, which may in turn affect the performance and density of natural enemies. Nematodes negatively affect the performance of sap sucking herbivores (generally through phloem quality) but have no effect on chewing herbivores. For soil arthropods there are no clear patterns yet. We further show that the methodology used plays an important role in influencing the outcomes of field studies. Studies using potted plants in the field and studies that remove target soil taxa by means of pesticides are most likely to detect significant results. Lastly, we discuss suggestions for future research that could increase our understanding of soil biota-plant-insect interactions in the field.
Introduction
Soils are an important source of diversity of microbes worldwide (Ramirez et al., 2018), but soil is also home to various other higher taxa, such as nematodes, root feeding insects or even vertebrates (Bardgett and van der Putten, 2014). The role of soil biota in ecosystem functioning is widely recognized and the study of soil biota-plant interactions has developed into a very active and large field in ecology. Soil organisms fulfill key processes in the soil, such as decomposition and nutrient mineralization. Many microorganisms engage in mutualistic interactions with plant hosts, aiding in the uptake of nutrients and water (e.g., arbuscular mycorrhizal fungi, AMF), in exchange for photosynthates or other plant metabolites. Other groups of soil micro- and macro-organisms have antagonistic effects on plant health, for example via pathogenicity (e.g., pathogenic fungi) or herbivory (e.g., root herbivorous insects). It has been shown previously in studies carried out under artificial/controlled conditions that mutualistic and antagonistic players in the soil not only impact the growth (i.e., biomass production) of plants, but also lead to the alteration of various physiological processes in plant tissues, resulting in changes in tissue quality or palatability of the plant (e.g., Bezemer and van Dam, 2005). Through such mechanisms, soil biota can mediate interactions between the host plant and aboveground organisms, such as insect herbivores and pollinators. Despite all the attention that this subject has received, the majority of published studies have been conducted under more controlled conditions (hereafter “controlled studies”), such as in greenhouses or growth chambers. Hence, an important question is whether the results are a realistic representation of ecological processes that occur in natural systems.
Mechanisms through which soil organisms can affect aboveground insects in the field are mostly plant-mediated (Figure 1). Various organisms, most notably plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF), can boost plant growth (e.g., Saravanakumar et al., 2008; Gadhave et al., 2016), which has been hypothesized to increase plant palatability (i.e., the plant vigor hypothesis; Price, 1991; Cornelissen et al., 2008). On the other hand, plants under biotic or abiotic stress can also be more vulnerable to attack by herbivores (i.e., the plant stress hypothesis; White, 1969). Evidence for the former has been reported from field studies (e.g., for some AMF species in Wolfe et al., 2005; Ueda et al., 2013). Several studies also find support for the plant stress hypothesis (e.g., for nematodes in Alston et al., 1991; Vockenhuber et al., 2013). However, many field studies report plant-mediated effects of soil organisms on aboveground insects, without reporting any effects on plant vigor or stress, which suggests that other factors related to plant performance (see Figure 1) could play an important role in mediating aboveground plant-herbivore interactions.
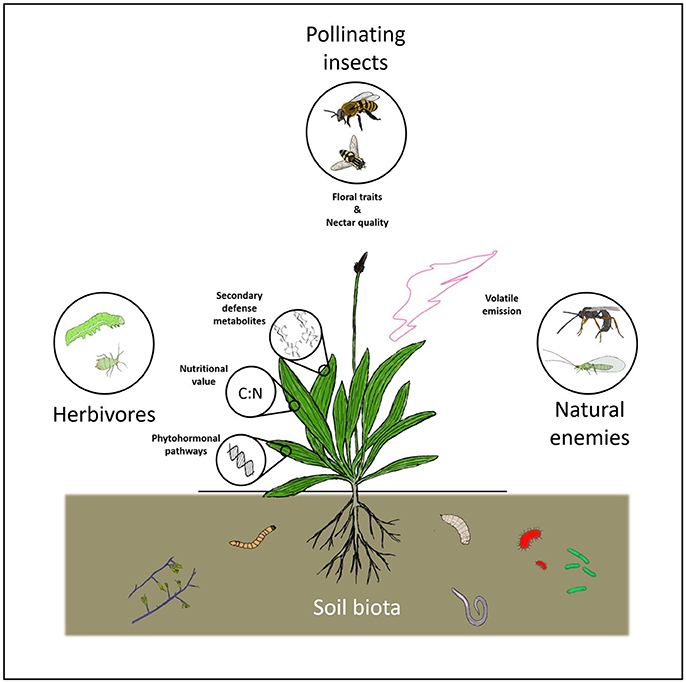
Figure 1. A schematic overview of mechanisms through which soil organisms can affect plant phenotype and associated aboveground insects. Soil organisms can affect a variety of host plant traits, including nutritional quality and palatability, size, morphology and floral traits, as well as the activation of defense pathways and the emission of plant volatile organic compounds. Through these mechanisms they can influence insect herbivores, pollinators and natural enemies.
Plant nutritional value (most importantly, nitrogen and sugar content) in the field can be positively affected by soil organisms (Gange and West, 1994; Gange et al., 2005a,b; Younginger et al., 2009; Moon et al., 2013; Brunner et al., 2015; Godschalx et al., 2015; Ryalls et al., 2016). Moreover, plant secondary defense metabolites, that play a role in the palatability of host plants, can be affected by soil organisms in the field (Wurst et al., 2008; Megías and Müller, 2010). Interactions with soil organisms can also sensitize the immune system of plants so that they can respond faster or more strongly to subsequent attack by antagonists (e.g., Pieterse et al., 2014). This process, better known as induced systemic resistance (ISR), can play an important role in plant-insect interactions in the field (Saravanakumar et al., 2008; Prabhukarthikeyan et al., 2014). Soil organisms can also interfere with plant volatile emissions, which are important cues for herbivores (e.g., for oviposition), as well as for many natural enemies, to detect host plants (Megali et al., 2015). Finally, several studies have shown that, for instance AMF can affect plant functional traits, such as flower size and stamen number (Gange and Smith, 2005; Gange et al., 2005a; Varga and Kytöviita, 2010).
In this review, we aim to answer three main questions. (1) What is the role of whole soil communities and plant-soil feedbacks in mediating aboveground plant-insect interactions in the field? (2) What is the role of the individual taxa of soil organisms in mediating aboveground plant-insect interactions in the field and how do potential patterns compare to those that are observed in controlled studies? (3) How does the experimental methodology used in the field affect the outcome of above-belowground studies? Furthermore, we will discuss potential applications and suggest future directions to advance this scientific field.
Literature Search Methodology
The scientific literature was searched using Web of Science for combinations of “soil ‘faunal group’” AND “insect” AND “field,” in which “faunal group” was replaced by; bacteria, fung*, nematod*, arthropod* or insect*, respectively. Furthermore, the literature was searched for combinations of “plant-soil feedback” AND “insects” AND “field”. Suitable studies were selected first based on title and subsequently on abstract or full manuscript. Additionally, reference lists from suitable papers, as well as from recent reviews (Gehring and Bennett, 2009; Hartley and Gange, 2009; Koricheva et al., 2009; Pineda et al., 2010; Johnson et al., 2012; Soler et al., 2012; Wondafrash et al., 2013) on soil biota-plant-insect interactions were examined to detect additional publications. Lastly, for all suitable publications, the studies that cited these publications were scanned to detect additional studies that were published later.
In total, the literature search yielded 50 field studies, covering a total of 185 individual soil biota-plant-insect interactions (Supplementary Tables 1–4).
Plant-Soil Feedback Effects on Plant-Insect Interactions in the Field
Plants are not only influenced by soil organisms, but they also play an active role in shaping the biome around their roots. Plant species typically manipulate the microbiome around their roots, e.g., via exudation of carbohydrates and other chemical substances (Bais et al., 2006), resulting in specific microbial rhizosphere profiles (Lakshmanan et al., 2014). Such species-specific microbial profiles can influence the performance of other plants that grow later in the same soil (Kostenko et al., 2012; Bezemer et al., 2013; Kos et al., 2015; Heinen et al., 2018). This process is known as plant-soil feedback (Van der Putten et al., 2013) and can be an important driver of plant community dynamics (Kardol et al., 2006). In recent years, it has become evident that such changes in soil microbial communities, via plant-mediated processes, can affect the performance of aboveground organisms that interact with these plants. For example, several greenhouse studies have shown that soil legacy effects, the effects of earlier plant growth on the microbial community in the soil, can have strong effects on aboveground herbivores feeding on later growing conspecific plants in those soils (Kostenko et al., 2012; Kos et al., 2015). A recent study, for example, revealed that soil legacies left by grasses and forbs have contrasting effects on a chewing herbivore that fed on plant communities growing on soils with these legacies (Heinen et al., 2018).
Although most studies on the impact of whole soil microbiomes on plant-insect interactions have been performed in greenhouses and climate chambers, several studies have explored such relationships in the field. For example, in a field experiment, the proportion of ragwort (Jacobaea vulgaris) plants attacked by stem borers, leaf miners and flower feeders was much lower (up to 50%) for plants that were grown in soils with a ragwort legacy compared with plants grown in soils without this legacy, probably because of a soil legacy-induced reduction in plant size (Bezemer et al., 2006). Negative plant-soil feedback is generally seen as a result of the accumulation of pathogenic organisms (Nijjer et al., 2007; Van der Putten et al., 2013), and the effects observed in ragwort and their associated aboveground insects are likely caused by belowground pathogens (e.g., Van de Voorde et al., 2012). Another field study with the same plant species, found a positive correlation between the occurrence of seed feeding insects and colonization of ragwort roots by mycorrhizal arbuscules (Reidinger et al., 2012). These results indicate that soil legacies, most likely driven by soil organisms, can play a role in shaping plant-insect interactions in the field. We have not been able to identify any manipulative studies that have, thus far, investigated plant-insect interactions in a plant-soil feedback framework. However, numerous studies have investigated the effects of the experimental manipulation of various groups of soil organisms on aboveground plant-insect interactions, and this area is discussed in more detail below.
Soil Biota-Plant-Insect Interactions in the Field
Bacteria
Bacteria are a dominant group of organisms in the soil that can have strong effects on plant growth and quality. For example, nitrogen-fixing rhizobia that associate with leguminous plant species fix atmospheric nitrogen and thereby often increase nitrogen content in the plant tissues. On the other hand, plant-growth promoting rhizobacteria (PGPR) are known to have yield enhancing effects on plants, but also are known to induce systemic resistance by priming plants for the activation of defense pathways, which often results in negative effects on insect herbivores in controlled studies (Pineda et al., 2010).
The Effect of Nitrogen-Fixing Rhizobia on Aboveground Herbivores
One would expect that the increased plant quality resulting from plant mutualisms with nitrogen fixing bacteria would benefit aboveground insects. However, this is not necessarily the case, as rhizobia have been shown to also affect plant defense responses directly (e.g., Thamer et al., 2011) and indirectly (Godschalx et al., 2015). The latter is illustrated by a study with potted plants placed in the field that reported positive effects of the addition of Rhizobium sp. on plant protein levels in Lima bean, Phaseolus lunatus, but negative effects on extrafloral sugar content. This, in turn, led to 75% lower visitation numbers of the associated mutualist ant Tetramorium caespitum. Ants can act as natural enemies of herbivores and this study suggests that rhizobia can interfere with this indirect plant defense mechanism. In the presence of rhizobia, cyanogenesis (a chemical defense in legumes) is increased, and this may reduce the need for the plant to produce extrafloral nectar to attract ants (Godschalx et al., 2015).
The Effect of Plant Growth-Promoting Rhizobacteria on Aboveground Herbivores
Plant-mediated effects of the addition of PGPR on aboveground insects in the field are consistently negative in the studied systems. All interactions (n = 17) revealed from the literature search were negative for the aboveground herbivore, regardless of the insect feeding guild (Figure 2A, Supplementary Table 1, Zehnder et al., 1997; Commare et al., 2002; Saravanakumar et al., 2008; Gadhave et al., 2016). For instance, the addition of four different Pseudomonas fluorescens strains (individually, as well as in mixtures) to rice fields in India resulted in a ~3 fold reduction of leaf rolling by the rice leaf roller Cnaphalocrocis medialis (Commare et al., 2002; Saravanakumar et al., 2008). These effects are most likely driven by ISR, as plants generally express higher levels of defense gene transcription after exposure to herbivory in plants that received bacterial treatments (Saravanakumar et al., 2008; Prabhukarthikeyan et al., 2014).
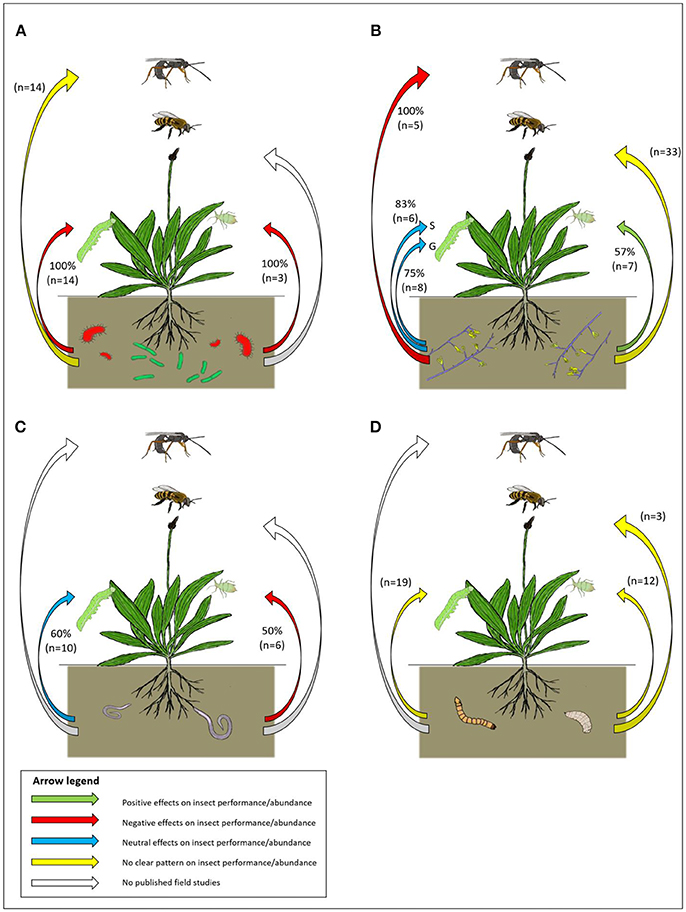
Figure 2. A schematic overview of the effects of (A) plant growth-promoting bacteria, (B) arbuscular mycorrhizal fungi, (C) plant-parasitic nematodes and (D) soil arthropods on the most frequently reported aboveground plant-insect interactions (interactions between plants and chewing and sap sucking herbivores, pollinators and natural enemies, respectively). In (B) S, Specialist; G, Generalist. Arrows indicate plant-mediated effects of soil organisms on aboveground insects. Green arrows represent generally positive indirect effects on aboveground insects, red arrows represent generally negative indirect effects on aboveground insects, blue arrows represent generally neutral effects on aboveground insects. Yellow arrows indicate that effects are observed, but no clear patterns emerged and white arrows indicate that interactions have not been reported in literature. Percentages with the green, red and blue arrows represent the percentage of the total reported interactions that followed the pattern (sample size between brackets).
The Effect of Plant Growth-Promoting Rhizobacteria on Aboveground Natural Enemies
Inoculation with PGPR can also influence the performance or attraction of insects at higher trophic levels, such as predatory insects or parasitoids (Saravanakumar et al., 2008; Gadhave et al., 2016). It is difficult to elucidate clear patterns as from all interactions (n = 18), 50% reported negative effects while 44% of the studies reported positive effects (Figure 2A, Supplementary Table 1). For example, a study investigating the effects of inoculation with Bacillus spp. on field-grown broccoli (B. oleracea) reported consistently reduced numbers of the ladybug (Coccinella septempunctata) and various unidentified syrphid flies on plants that received bacterial inoculations, compared to control plants that did not receive additional bacteria (Gadhave et al., 2016). However, in the same study, the authors found that the percentage of cabbage aphids (B. brassicae) parasitized by the parasitoid wasp Diaraetiella rapae was two to three times higher in plants grown on soils treated with Bacillus cereus and B. subtilis, but not in those treated with B. amyloliquefasciens or a mixture of the species (Gadhave et al., 2016).
Fungi
Soil fungi are a diverse group of organisms and their role in above-belowground interactions has been studied for many years. The most studied taxa are mycorrhizal fungi that associate with the majority of plant species. Ectomycorrhizal fungi (EMF) generally form mutualistic bonds with trees, whereas AMF form mutualisms with plants throughout the plant kingdom. EMF have been poorly studied within the soil biota-plant-insect framework and hence they are only briefly discussed. Relationships between AMF and aboveground insects, mediated by plants, are commonly reported in literature, and these effects have already been summarized in various other reviews (e.g., Pozo and Azcón-Aguilar, 2007; Gehring and Bennett, 2009; Hartley and Gange, 2009; Jung et al., 2012) and a meta-analysis (Koricheva et al., 2009).
The Effect of Ectomycorrhizal Fungi (EMF) on Aboveground Herbivores
Studies on the influence of EMF on plant-insect interactions are limited, but the published reports suggest that they can also affect insects in different directions. One study showed that numbers of the sap sucking poplar aphid Chaitophorus populicola were five times higher on poplar trees (Populus angustifolia x P. fremontii) that were treated with the EMF Pisolithus tinctorius than in controls that did not receive EMF. However, another study showed that various insects, even of the same feeding guild, respond differently to EMF in the same study and more importantly, results differ strongly between the various methodologies used (Gange et al., 2005b), as will be discussed in more detail further onwards in this review.
The Effect of Arbuscular Mycorrhizal Fungi (AMF) on Aboveground Herbivores
A general pattern that has emerged from controlled studies is that AMF negatively influence generalist chewers, while specialist chewers are positively affected by AMF (Hartley and Gange, 2009; Koricheva et al., 2009). From the interactions with generalist chewing herbivores revealed by our literature search (n = 8), 75% reported no effect and 25% reported negative effects of AMF on generalist chewers (Figure 2B, Supplementary Table 2, Gange and West, 1994; Vicari et al., 2002) or herbivore diversity (Guo et al., 2015) in the field. For example, in a field study on ribwort plantain, Plantago lanceolata, caterpillars of the highly polyphagous woolly bear moth, Arctia caja, were 25% smaller in plots with AMF than in plots with AMF removed (Gange and West, 1994). On the other hand, from the interactions with specialist chewers (n = 6) 83% report neutral (Younginger et al., 2009), and 17% reported a positive plant-mediated effect on specialist chewers (Figure 2B, Supplementary Table 2, Barber et al., 2013). Plant-mediated AMF effects on chewing herbivores also differ between different plant functional groups. A recent study showed that AMF presence increased total levels of herbivory in tallgrass prairie plots, but at the plant functional group level herbivory levels only differed between AMF and control plots for C3 grasses, but not for C4 grasses or forbs (Kula and Hartnett, 2015).
In controlled studies, sap sucking insects generally benefit from the presence of AMF and the degree of specialization of the sap sucking insects does not appear to influence the effects of AMF (Hartley and Gange, 2009; Koricheva et al., 2009). From the interactions revealed from our literature search (n = 7), 43% were neutral (Colella et al., 2014) and 57% reported positive plant-mediated effects of AMF on sap suckers (Figure 2B, Supplementary Table 2, Gange and West, 1994; Ueda et al., 2013). For example, a recent field study reports more than tenfold higher numbers of Aulacorthum solani on soybean (G. max) inoculated with Gigaspora margarita, than on untreated control plants (Ueda et al., 2013), which is in line with the commonly observed patterns in controlled studies. Only one study reports that treatment with AMF led to two- to three-fold lower numbers of the poplar aphid Chaitophorus populicola on poplar trees, Populus angustifolia x P. fremontii that were placed in pots in the field (Gehring and Whitham, 2002). Why aphids responded negatively in this study is hard to pinpoint. The authors report no significant effects of AMF on plant performance, but they did not investigate effects on plant chemistry, which may have changed in response to the AMF interaction. AMF effects on plant-insect interactions may also differ among plant functional groups. Most previous studies have been performed with herbaceous species, thus studies on woody shrubs and trees may give contrasting results.
As discussed in Koricheva et al. (2009), patterns in AMF-plant-insect effects on insects belonging to feeding guilds other than leaf chewers and sap suckers, such as cell content feeders and leaf miners, are not straightforward to interpret. However, addition of AMF to plants in the field had neutral (Gange et al., 2003, 2005b; Colella et al., 2014) to positive effects on cell-content feeders, leaf miners and gall makers in several studies (Gange et al., 2003; Younginger et al., 2009; Moon et al., 2013; Ueda et al., 2013). Within the same study system, results may even vary between generations of insects. For instance, when AMF levels were reduced using iprodione, this did not at first affect proportions of leaves mined by the leaf-mining fly Chromoatomyia syngenesiae in ox-eye daisy, Leucanthemum vulgare (Gange et al., 2003). However, in a follow-up study, the authors report AMF species-specific differences in the proportion of Leucanthemum leaves mined by C. syngenesiae, and a 50% increase in pupal biomass of the leafminer in plots with higher levels of AMF. These significant effects were only found for the second generation of flies in the year of study (Gange et al., 2005a).
The Effect of Arbuscular Mycorrhizal Fungi (AMF) on Aboveground Natural Enemies
Several studies have incorporated higher trophic levels in the study of AMF-plant-insect interactions and in all of the studied interactions (n = 5) AMF presence had a negative effect on the performance or density of predatory insects (Ueda et al., 2013) or parasitoids (Gange et al., 2003; Moon et al., 2013). In one study on Sea myrtle, Baccharis halimifolia, parasitism rates of two species of co-occurring leafminers (Amauromyza maculosa and Liriomyza trifolii, respectively) and a gall making fly (Neolasioptera lathami) by parasitoid wasps were all negatively affected by AMF application (Moon et al., 2013). AMF colonization resulted in more leaves per plant, which also had higher nitrogen levels, subsequently leading to healthier and potentially more strongly defended hosts, negatively affecting the respective parasitoids (Moon et al., 2013).
The Effect of Arbuscular Mycorrhizal Fungi (AMF) on Aboveground Pollinators
AMF-plant interactions can have contrasting effects on pollinating insects in the field. From the interactions revealed by our literature search (n = 35), 34% were positive, 17% were negative and 49% reported no effects on pollinators (Figure 2B, Supplementary Table 2). Several studies report higher pollinator visitation or flower probing on plants that received AMF treatment (Gange and Smith, 2005; Wolfe et al., 2005; Cahill et al., 2008; Barber et al., 2013), whereas others report neutral or negative effects on pollinator visitation (Varga and Kytöviita, 2010). It is important to notice that effects of soil organisms on pollinating insects can vary between different levels of measurement (e.g., plot/community/species/pollinator taxa level). For example, in one study, levels of AMF were reduced by application of benomyl and the effects of AMF on six common forb species were investigated (Cahill et al., 2008). At plot level, plots with natural AMF levels showed an overall 67% higher number of pollinator visits per flowering stem, whereas the total number of visits per plot was not affected. AMF associations also led to a three-fold higher visitation by large-bodied bumblebees and a three-fold decrease in visitation by small-bodied pollinators such as bees and flies. At the plant species level, Aster laevis and Solidago missouriensis showed two to four times higher numbers of floral visits by pollinators in plots with higher AMF levels, whereas Cerastium arvensis showed a 80% decrease in total pollinator numbers in plots with higher AMF levels. Pollinator visitation of the herbs Achillea millefolium, Campanula rotundifolia and Erigeron philadelphicus was not affected by soil AMF levels (Cahill et al., 2008). More studies are needed to elucidate patterns for plant-mediated effects of AMF on pollinators in the field.
Nematodes
Nematodes are important soil dwelling organisms that belong to a range of trophic groups in the soil food web, and include bacterial feeders, fungal feeders, root feeders, and predators/carnivores. Their effect on host plants has been studied intensively, although fewer studies have focused on the indirect effects of nematodes on aboveground insects (reviewed in Wondafrash et al., 2013). As the literature search for field studies only revealed studies of plant-parasitic nematodes on aboveground insects, only this group will be discussed here. It should be noted that other nematodes (e.g., fungal feeders, bacterial feeders) may, however, also indirectly affect plant-insect interactions by interacting with other soil organisms. Plant-parasitic nematodes, by feeding on the roots of shared host plants, can influence the defense status and nutritional quality of host plants, potentially leading to effects on herbivores (Bezemer et al., 2003; Bezemer and van Dam, 2005; Wondafrash et al., 2013; Biere and Goverse, 2016). Results from laboratory studies of the effects of plant-parasitic nematodes on aboveground insects are often variable for chewing insects, but generally show negative effects on either the performance or preference of sap sucking insects (Johnson et al., 2012; Wondafrash et al., 2013). As the number of field studies on plant-parasitic nematodes that describe effects on insect herbivores is rather low, we will treat plant-parasitic nematodes (PPNs) with different life styles (free-living, endoparasitic) as one group, and describe their effects on different types of insect herbivores. No studies that incorporated higher trophic levels or pollinating insects have been identified and therefore these are not discussed here.
The Effect of Plant-Parasitic Nematodes on Aboveground Herbivores
From the interactions revealed from our literature search (n = 10), 60% report neutral (e.g., Carter-Wientjes et al., 2004; Kaplan et al., 2009; Guo et al., 2016) and 40% report positive effects of PPNs on aboveground chewing herbivores (Figure 2C, Supplementary Table 3, Alston et al., 1991; Kaplan et al., 2009; Vockenhuber et al., 2013). For example, the addition of the root-knot nematode, Meilodogyne incognita to tobacco (Nicotiana tabacum) in field plots did not affect numbers of the specialist tobacco hornworn, Manduca sexta, or the growth of the generalist beet armyworm, Spodoptera exigua. In contrast, in the same experiment, nematode-treated plants had 30% higher numbers of chewing Epitryx flea beetles than untreated plants (Kaplan et al., 2009). Although correlative data should be interpreted with caution as they do not imply causation, numbers of free-living PPNs were also positively related to the levels of leaf consumption by chewing herbivores, although the observed correlations for PPNs were not significant for the three most abundant nematode genera Tylenchorhynchus, Pratylenchus, and Xiphinema (Kaplan et al., 2009).
From the interactions revealed from our literature search for nematode effects on sap suckers (n = 6), 50% reported no effects (e.g., Vandegehuchte et al., 2010; Heeren et al., 2012) and 50% reported negative effects (Figure 2C, Supplementary Table 3, Kaplan et al., 2009). In soy bean fields, G. max, the presence of the nematode H. glycines did not correlate with total aphid abundance in one study (Heeren et al., 2012), but was negatively correlated with the number of alates of the soy bean aphid Aphis glycines at the onset of the peak season in another study (Hong et al., 2011). It is important to note that in the former study, plant yield was also not affected, whereas yield also negatively correlated with the number of nematode eggs in the latter (Hong et al., 2011; Heeren et al., 2012).
Soil Arthropods
A relatively large number of studies have examined the effect of soil arthropods on aboveground plant-insect interactions. Soil arthropods are an abundant group of macro-invertebrates that can affect plants either directly, via root herbivory or indirectly, via decomposition of organic material. Although an increasing number of studies report on mechanisms through which root herbivory might impact aboveground plant-insect interactions (e.g., reviewed in Soler et al., 2012; Barber and Soper Gorden, 2014), most reviews remain inconclusive about the drivers behind the effects that are often observed. A meta-analysis showed that root herbivory by Diptera generally results in significantly negative effects on aboveground herbivores (Johnson et al., 2012), whereas herbivory by Coleoptera influences only aboveground Homoptera (positively) and herbivorous Hymenoptera (negatively), but has no significant effect on other groups.
The Effect of Root Herbivores on Aboveground Herbivores
From the interactions revealed by our literature search for root herbivore effects (regardless of taxa) on aboveground chewing herbivores (n = 20), 55% reported no effects, 10% reported positive effects and 35% reported negative effects.
Several studies in the 1990's investigated the effects of root herbivores on aboveground insects by means of reducing the total densities of soil arthropods with insecticides. In all of these studies, natural densities of soil arthropods had either no influence (Evans, 1991) or led to an increase (Evans, 1991; Masters et al., 1993, 2001; Masters, 1995) in aboveground herbivory. As there is little specificity in insecticide treatments, it is impossible to disentangle the effects of different soil arthropod taxa on plant-insect interactions from these older studies. Yet, they shed some light on the role of soil arthropods in shaping plant-aboveground insect interactions.
In field studies, plant-mediated effects of coleopteran root herbivores on aboveground chewing herbivores can be neutral (Hunt-Joshi et al., 2004; Barber et al., 2015; Borgström et al., 2017), positive (Wurst et al., 2008), or negative (White and Andow, 2006; Wurst et al., 2008; Megías and Müller, 2010, see Figure 2D, Supplementary Table 4). Interestingly, on ribwort plantain, Plantago lanceolata that were exposed to belowground herbivory by Agriotes spp., aboveground herbivory levels were three times lower on a high-iridoid glycoside (secondary defense metabolites in Plantago) producing lineage, compared to controls without root herbivores. In contrast, herbivory levels were nine times higher in response to the root herbivore on a low iridoid glycoside lineage (Wurst et al., 2008). This study illustrates that the genetic background of a plant can play an important role in determining plant-mediated effects of root insect herbivores on aboveground chewing insect herbivores. Although a meta-analysis (Johnson et al., 2012) concluded that dipteran root herbivores generally have negative plant-mediated effects on aboveground herbivores, there is no consistent support from field studies for this (see Figure 2D, Supplementary Table 4). For example, Cabbage root fly, Delia radicum negatively affected numbers of chewing Phyllotreta sp. leaf beetles (this genus comprises mostly specialists and oligotrophs) in potted black mustard (Brassica nigra) in an experimental garden (Soler et al., 2009), but the addition of root flies had no plant-mediated effect on any lepidopteran chewers (Soler et al., 2009; Pierre et al., 2013).
There seems to be no pattern for the plant-mediated effects of coleopteran root herbivores on sap suckers in the field. From the interactions revealed by our literature search (n = 22), 54% reported no effects, compared to 23% that reported positive effects and 23% that reported negative effects (see Figure 2D, Supplementary Table 4). One study reports positive effects of root herbivory by coleopteran herbivory on aboveground sap suckers (Poveda et al., 2005). However, in other studies, the addition of coleopteran root herbivores had either no effect (Megías and Müller, 2010) or negative effects on sap suckers (Megías and Müller, 2010; Ryalls et al., 2016). For example, addition of larvae of a combination of the two beetle species Morica hybrida and Cebrio gypsicola on Moricandia moricandioides resulted in a more than three times lower number of aphids on the shared host plant, compared to controls. Similarly, in the same study, the addition of soil organisms resulted in a decrease in the total number of unidentified aphids on the plants, compared to controls, whereas the total number of planthoppers was not affected by the treatment with only C. gypsicola, but were 30% lower on plants that received only M. hybrida (Megías and Müller, 2010). This result could be driven by the fact that the latter is largely detritivorous and, thus, these two coleopteran soil arthropods may affect plant physiology in different ways. There is also no consistent effect of dipteran root herbivores on sap sucking herbivores in the field. Plants treated with root herbivores were found to have increased numbers of specialist aphid Brevicoryne brassicae (Pierre et al., 2013) and decreased numbers of the same species in another study (Soler et al., 2009). Numbers of the generalist aphid Myzus persicae were not affected by the presence of root herbivores in either of the two studies (Soler et al., 2009; Pierre et al., 2013).
As we identified only one study that described the effect of root herbivores on other feeding guilds, it is not possible to elucidate patterns. In this study, the abundance of the leafminer Stephensia brunnichella was 30% lower on Wild basil, Clinopodium vulgare plants that were infested with wireworms, Agriotes spp. than on controls without herbivores, whereas the size of the herbivores remained unaffected by the treatments (Staley et al., 2007).
The Effect of Root Herbivores on Aboveground Natural Enemies
The number of studies that have examined the effects of root-feeding insects on aboveground natural enemies in the field is limited. The available reports suggest that the presence of root feeding herbivores may have little effect on aboveground natural enemies in the field (e.g., Soler et al., 2009; Megías and Müller, 2010). Evans (1991) reported that soil arthropod reduction did not affect abundance of unspecified parasitic Hymenoptera, Arachnida and unspecified predatory and entomophagous insects in experimental field plots. In contrast, Megías and Müller (2010) found higher levels of parasitism by the braconid parasitoid Cotesia kazak in larvae of two pierid butterflies, E. crameri and P. daplidice, when soil dwelling larvae of the tenebrionid beetle M. hybrida were present in potted M. moricandioides plants. It is important to note that this beetle species is largely detritivorous and therefore may not directly affect plants, but its presence may influence plant-insect interactions by making nutrients available in the soil that may affect physiological processes in the plant.
The Effect of Root Herbivores on Aboveground Pollinators
The literature is inconclusive on the plant-mediated effects of root herbivores on pollinators. Soil arthropods often cause association-specific effects on their host plants, ranging from changes in flower number to flower size and nectar quality, which all may influence different types of pollinating insects (Barber and Soper Gorden, 2014). Likewise, there is no evident pattern for field studies (Figure 2D, Supplementary Table 4). Three studies investigated the effects of addition of root herbivores on pollinator visits in the field. In all cases, the plants were in pots in the field and the treatment was an addition of coleopteran root herbivores. Addition of wireworms, Agriotes spp. to charlock mustard, Sinapis arvensis consistently resulted in an increase in total pollinator visits (Poveda et al., 2003, 2005). However, in another study using cucumber plants, C. sativus, addition of larvae of the striped cucumber beetle, Acalymma vittatum resulted in half the number of pollinator visits, compared to untreated controls and pollinator visits showed a negative relationship with root herbivore density (Barber et al., 2015).
Methodology Determines the Outcome of Field Experiments
Although similarities between controlled studies and field studies can be found for some soil taxa, the field literature also shows considerable variation in responses and neutral effects are commonly observed for soil biota-plant-insect interactions. This may be at least partly due to the experimental methodologies applied in the field. Three main methodologies are widely applied; (1) Addition of soil organisms to potted plants that are placed in experimental outdoor areas; (2) Addition of soil organisms to plants that are grown in field plots; (3) Removal of specific soil organism taxa by application of pesticides (see Figure 3). Direct comparisons between potted plants and field grown plants were made in two studies. For instance, in Marram grass, presence of a PPN of the genus Heterodera had a negative effect on the aboveground aphid Schizaphis rufula in pots, but in the field this correlation was not significant (Vandegehuchte et al., 2010). In another study, when Eucalyptus trees were grown in pots in the field, addition of EMF had a negative effect on feeding by larvae of the chafer Anomala cupripes, but for trees growing directly in the field, no effect on chafer feeding was observed. Damage by geometrid moths was significantly increased under EMF treatment in the potted plants, whereas it was decreased in the field-grown Eucalyptus. However, the EMF treatment led to a reduction in leaf folding by Strepsicrates sp. in both potted plants in the field and in field-grown plants (Gange et al., 2005b). These two studies clearly illustrate that choice of methodology used in field experiments can strongly influence the outcome, and suggests that studies using potted plants are more likely to show significant effects of belowground organisms on aboveground insects than studies that examine plants grown directly in the soil in the field. This also emphasizes the need for standardized methodologies, in order to make comparisons between different field studies more powerful.
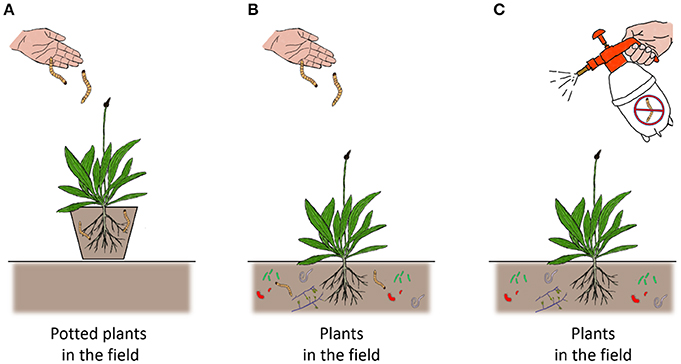
Figure 3. A schematic overview of the three most widely used methodologies to investigate soil biota-plant-insect interactions in the field. In this representation we used additions of wireworms, Agriotes spp. to Ribwort plantain Plantago lanceolata as an example. (A) Potted plants, which are often grown in a greenhouse for a number of weeks, are placed in experimental fields or gardens after being treated with soil organisms. Interactions between the potted plants and natural herbivores or pollinators are then tested in the field. (B) Plants are planted in the field under natural conditions, including a resident soil community. Soil organisms are added to plots and thus in the treated plots the numbers of added soil organisms are augmented, compared to untreated control plots. (C) Plants are planted in the field under natural conditions, including a resident soil community. However, in this method, the soil organisms under investigation are reduced by means of application of a pesticide. Hence, the treated plots have reduced levels of soil organisms, compared to the control plots, which have natural (but higher) levels of the soil organism.
Interestingly, there is a strong difference between effects reported for the different methodologies among the studies compiled in this literature review (see Table 1). In the published literature, only for the taxa soil fungi and soil arthropods were there reports on all three methodologies used in the field (see Figure 3). When we compare methodologies within these two taxa, potted plant studies and field removal studies more often reported significant results (in either direction) than studies where soil organisms were added to field plots. For example, in the studies with fungi, 63% of the interactions studied in pots showed a significant plant-mediated effect (in either direction) on aboveground insects. Field removal studies also showed a significant plant-mediated impact in 73% of the studies, but only 25% of the field addition studies showed significant effects (see Table 1). A similar pattern emerges for the manipulation of soil insects. Here, 64% of the studied interactions resulted in significant plant-mediated effects on insect herbivores in pot experiments. Field removal studies showed significant plant-mediated effects in 70% of the studies, compared to only 33% in the field addition studies (see Table 1). These numbers suggest that there is a strong effect of methodology applied in the field, although it should be noted that publication bias may have also led to a bias toward studies that report significant results and in reality, the fraction of studies that report significant effects may be lower.
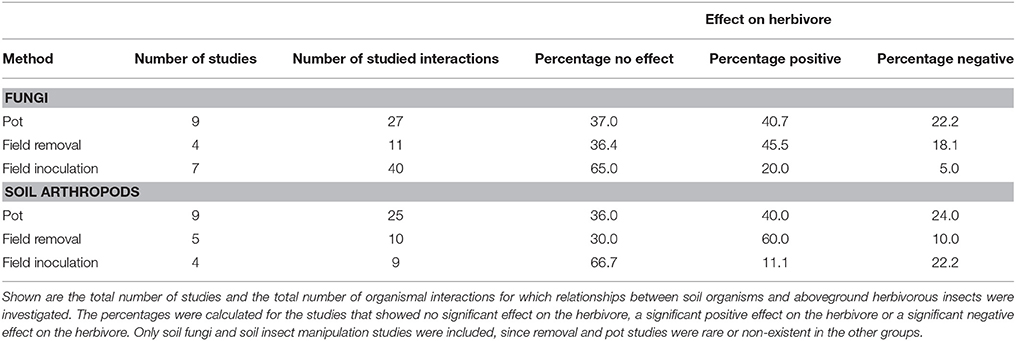
Table 1. Comparison of the three most widely used field methodologies in studies investigating above-belowground interactions (potted plants placed in the field, inoculation of soil organisms in experimental plots, species removal by means of pesticides in experimental plots).
The use of pots comes with a range of disadvantages that may affect the study system, especially so in the field. First of all, studies often use sterilized soil or steamed potting soil, which excludes the interactions with resident soil organisms. Furthermore, pots not only impose a barrier to the root system, but also to the movement of the study organisms. Moreover, it prevents the influx of other soil organisms. Although pots may have the advantage of ensuring that the soil organisms are present at the root system, this methodology may be highly artificial compared to field plots. The barrier also inherently limits plant growth (i.e., pot limitation), leading to changes in plant growth and physiology (Poorter et al., 2012), which may either be beneficial or detrimental to insect performance. Lastly, abiotic conditions in pots can be quite different from conditions in soil. Placing pots (often of dark color, which absorbs more energy) on top of the soil, may increase soil temperature in the pot under warm conditions. Moreover, they may cool down more rapidly under cold conditions. We propose that pots can be extremely useful in studying soil organisms, both in laboratory and field conditions, but that they should be used with caution and that abiotic constraints should be countered as much as possible (for example by burying the pots, using large enough pots and including live soils into the design).
The use of pesticides in field experiments was a common approach in the early years of the development of this niche in ecology. However, this also comes with many obvious disadvantages. Several studies have shown that, although the pesticides are often rather specific and indeed reduce target organisms, there are also undesirable side-effects that influence many other soil processes (e.g., Wang et al., 2004). We propose that addition of soil organisms to field plots may be the best methodology, as this allows for interactions of both the added soil organisms and the plant with resident soil communities. From an applied perspective, results from soil organism addition studies are perhaps also the most useful as these scenarios are most comparable to application of soil organisms (e.g., in Integrated Pest Management). However, it is very hard to standardize both the abiotic and biotic conditions of live field soils, and this can lead to considerable variation between or even within study sites. Introduced soil organisms may encounter antagonists, or effects may be “diluted” as field plots often do not have barriers and organisms may move away.
Discussion and Future Directions
In this review we have explored the scientific literature that discusses the effect of biotic manipulations of the soil on aboveground plant-insect interactions in the field. First, we asked if there is a role for soil organisms in shaping aboveground plant-insect interactions under field conditions. We searched the literature for studies that report on manipulations of the whole soil microbiome and how changes in soil community composition may affect aboveground insects in the field. It appears that there is ample evidence for effects of changes in whole soil communities on insect assemblages, but these findings are all correlative, not causative. This immediately highlights a first gap in the current scientific knowledge; how biotic “soil legacies” or plant-soil feedback (PSF) effects may influence aboveground insect communities in the field. To our knowledge, no studies thus far, have assessed these effects in a field setting. This is an important aspect of above-belowground ecology that deserves more attention in the future. We argue that introducing the PSF concept as a fourth applicable field method to shift soil communities in a certain direction would be less disruptive than the commonly used methodologies and would incorporate more ecological realism.
Our second question was whether the manipulation of specific taxa in the soil has the same effects on aboveground insects in the field as under more controlled conditions in greenhouses or growth chambers. Our survey indicates that this is true for most taxa except for soil arthropods. Bacterial inoculation in the field generally promotes plant growth and depresses abundance and performance of insects in the field, as they do in laboratory studies (e.g., Pineda et al., 2010). For AMF, the effects observed in laboratory settings have been thoroughly reviewed (Gehring and Bennett, 2009; Hartley and Gange, 2009; Koricheva et al., 2009) and the general patterns differ for insects from different feeding guilds and depend on the degree of specialization of the insects. Field studies, we show, report similar patterns; AMF negatively influences generalist chewers, but positively affect specialist chewing insects. AMF also generally benefit sap-sucking insects, regardless of their specialization. Under field conditions, nematodes affect chewing herbivores positively and sap suckers negatively and this is also in line with the general observations in laboratory studies (Wondafrash et al., 2013). Patterns in the effects of soil arthropods are less straightforward. In the current review of field literature, we have not been able to observe a clear pattern. One of the reasons for this could be the variation in abiotic and biotic conditions in the reported study systems. Furthermore, often only very few interactions are studied for each combination of taxa (both below and aboveground). Therefore there is currently a lack of relevant data and this makes it hard to compare the different results more thoroughly, e.g., in a meta-analysis. The same problem arises when we attempt to elucidate patterns for less abundant feeding guilds (such as leaf miners, gall makers or stem borers) or natural enemies and pollinators. Very few studies, so far, have investigated the effects of soil organism manipulations in the field on these less apparent aboveground feeding guilds and this is an area that requires further attention in order to better understand patterns in soil arthropod-plant-insect interactions.
Although we observed similarities between field and laboratory studies, in the field, it is also important to note that a relatively large fraction of the studies that we detected reported neutral effects. We suggest that field methodology can drastically affect the outcome of above-belowground studies and that ecologists should be aware of this when designing experiments. Although there is a current lack of studies that compare the different field methodologies directly, the pattern is rather clear. In the case of pot experiments and removal experiments in the field, the likelihood of observing a statistically significant effect of any kind, are twice as high as those in field addition experiments. However, we argue that the latter is, to date, by far the most realistic and useful methodology to understand ecological processes. Clearly, there are opportunities to explore alternative ways to manipulate soil organisms, or steer soil communities in specific directions. For example through manipulation of soil via plant-soil feedback mechanisms where soils are manipulated in the field by plant species with specific effects on soil communities, or by inoculation of plots with soils that have been conditioned by specific plant species. Moreover, soil organisms can be manipulated via exclusion methods using variable mesh sizes that exclude certain soil taxa based on their sizes (e.g., Johnson et al., 2001, 2002), or via the addition of antagonistic organisms, that can impact specific groups of soil organisms.
Four aspects of the field of above-belowground ecology deserve further development. First, the response of insect species from less apparent feeding guilds (such as gall makers, stem borers, leaf miners and cell content feeders) has often been overlooked so far. In order to further elucidate patterns and more fully understand the ecological role of soil organisms in shaping plant-insect interactions, we need to use a more holistic approach that takes into account players from a broader range of guilds and trophic levels. Responses of natural enemies and pollinators aboveground have been studied infrequently, and are completely missing for certain types of soil manipulations, or soil taxa. The life history of the various natural enemies is quite diverse and their responses to soil biota-plant interactions may vary. Parasitoids and other flying natural enemies may respond more quickly than wingless, cursorial predators like spiders. Furthermore, parasitoids are affected by changes in the quality of their herbivore hosts, as their life cycles intimately depend on host ecophysiology (e.g., MacKauer, 1996; Harvey, 2000; Harvey et al., 2004). Moreover, when we searched for studies in the scientific literature, we could not detect any that focused on the effect of soil organisms, via plants, on interactions between plants and non-arthropod taxa, such as slugs, snails, but also higher vertebrates, such as grazers. As plants are the primary producers that support food chains, it is likely that other organisms will also be affected by belowground organisms.
Second, to increase our ecological understanding, it is important to also include more ecologically realistic model systems, as the current systems are often based on crops, as well as on insect species that are either crop pests or chosen for convenience, rather than based on ecological relevance (Chen et al., 2015). This could be accomplished, for example, by using a range of wild plant species that vary in functional traits, which could give better insight into what traits may predict certain plant responses. Studying their natural associated insect communities may also increase our understanding of which traits are important in mediating soil biota-plant-insect interactions. Future work could fill in these important gaps in our current knowledge.
Third, more emphasis should be placed on the role of time and space in these aboveground-belowground interactions in the field. It is currently unknown whether performing manipulations with the same soil organisms at different locations (e.g., differing in altitude and latitude, as well as abiotic conditions) will lead to differential effects on aboveground insects or not. Future studies should also focus on the temporal aspects of above-belowground interactions in the field. As soil communities are dynamic and species-specific soil communities accumulate over time (Diez et al., 2010; Flory and Clay, 2013; Van der Putten et al., 2013; Heinen et al., 2018), it is likely that these temporal dynamics will strongly influence the performance of aboveground insect communities over time. Various controlled studies have shown that the sequence of arrival of aboveground and belowground herbivores on the plant can greatly alter the outcome of soil biota-plant-insect interactions (e.g., Erb et al., 2011; Wang et al., 2014) and to some extent, this has also been shown in field studies (e.g., Gange et al., 2005a), although the link between temporally changing soil communities and temporal variation in aboveground insect communities has not been made. In the field, insect communities also change throughout the season. How soil treatments affect insects early compared to late in the season, and to what extent this is due to changes in plant-soil interactions or changes in plant-insect interactions is not known.
Fourth, most of the current research is focused on indirect effects that are mediated by shared host plants, but potential direct interactions should not be overlooked. There are various organisms, such as entomopathogens in the soil that can have direct impacts on aboveground insect performance. For instance, infection by entomopathogenic fungi, such as Beauveria bassiana and Metarhizium anisoplae can result in the quick death of many insect species (Meyling and Eilenberg, 2007; Vega et al., 2009, 2012), although its direct effects on aboveground insects in the field has been poorly documented. Interestingly, these fungi can also be endophytic in plants, and can influence both plant and herbivore performance (Meyling and Eilenberg, 2007; Vega et al., 2009, 2012; Senthilraja et al., 2010; Prabhukarthikeyan et al., 2014). Moreover, it has been shown for the fungus Metarhizium that it forms bridges between infected dead insects and plants, through which the fungus can provide the plant with extra nitrogen obtained from the insect bodies, which may also affect plant-insect interactions (Wang and St Leger, 2007; Behie et al., 2012; Sasan and Bidochka, 2012). Little is known about the extent to which aboveground insects pick up soil microorganisms and how this may affect their fitness, either through pathogenicity, or perhaps mutualistic interactions (e.g., in the gut microbiome), leaving an important gap in our current knowledge.
We conclude that there is strong support for a significant role of soil organisms in shaping plant-insect interactions in the field. With the exception of soil arthropods, we find that most field studies report effects that are similar to those of laboratory studies. We argue that future studies should be carefully planned, as the methodology applied in the field strongly affects the chance of finding robust results. Nonetheless, there are ample opportunities to develop this research field further, especially in terms of exploring alternative and more realistic methods to steer soil biomes into a targeted direction. It should be emphasized that there is a large gap in our knowledge when it comes to less apparent insect herbivore taxa such as leaf miners, stem borers and others. There is virtually nothing known about the effects of soil organisms on a broad range of natural enemies (predators and parasitoids). However, as there are consistent reports of effects of soil organism addition in the field on aboveground insects, this opens up opportunities for the exploration of soil organism manipulation in agriculture or ecosystem restoration (e.g., Pineda et al., 2017). Some groups of soil organisms may be promising agents for crop yield enhancement and protection. Other groups of soil organisms may affect aboveground plant diversity at the community level and this gives rise to new opportunities to use soil organisms to “steer” the development of aboveground vegetation (Wubs et al., 2016), which may then subsequently affect aboveground insect communities. A challenge is to disentangle the drivers of soil organism manipulation effects on insects in the field. This will be an important step toward understanding how belowground organisms drive aboveground insect abundance, diversity and impacts in the field.
Author Contributions
TB and RH conceived the idea for the literature review. TB, AB, and RH designed the structural framework of the review. RH performed the literature study. TB and AB provided several additional references. RH wrote the first version of the manuscript. TB, AB, JH, and RH contributed critically to later versions of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was supported by the Netherlands Organization for Scientific Research (NWO VICI grant 865.14.006 to TB). This is publication number 6559 of the Netherlands Institute of Ecology (NIOO-KNAW).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00106/full#supplementary-material
References
Alston, D. G., Bradley, J. R. Jr., Schmitt, D. P., and Coble, H. D. (1991). Response of Helicoverpa zea (Lepidoptera: Noctuidae) populations to canopy development in soybean as influenced by Heterodera glycines (Nematoda: Heteroderidae) and annual weed population densities. J. Econ. Entomol. 84, 267–276. doi: 10.1093/jee/84.1.267
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi: 10.1146/annurev.arplant.57.032905.105159
Barber, N. A., and Soper Gorden, N. L. (2014). How do belowground organisms influence plant–pollinator interactions? J. Plant Ecol. 8, 1–11. doi: 10.1093/jpe/rtu012
Barber, N. A., Kiers, E. T., Hazzard, R. V., and Adler, L. S. (2013). Context-dependency of arbuscular mycorrhizal fungi on plant-insect interactions in an agroecosystem. Front. Plant Sci. 4:338. doi: 10.3389/fpls.2013.00338
Barber, N. A., Milano, N. J., Kiers, E. T., Theis, N., Bartolo, V., Hazzard, R. V., et al. (2015). Root herbivory indirectly affects above-and below-ground community members and directly reduces plant performance. J. Ecol. 103, 1509–1518. doi: 10.1111/1365-2745.12464
Bardgett, R. D., and van der Putten, W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi: 10.1038/nature13855
Behie, S. W., Zelisko, P. M., and Bidochka, M. J. (2012). Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science, 336, 1576–1577. doi: 10.1126/science.1222289
Bezemer, T. M., Wagenaar, R., Van Dam, N. M., and Wäckers, F. L. (2003). Interactions between above-and belowground insect herbivores as mediated by the plant defense system. Oikos 101, 555–562. doi: 10.1034/j.1600-0706.2003.12424.x
Bezemer, T. M., and van Dam, N. M. (2005). Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 20, 617–624. doi: 10.1016/j.tree.2005.08.006
Bezemer, T. M., Harvey, J. A., Kowalchuk, G. A., Korpershoek, H., and van der Putten, W. H. (2006). Interplay between Senecio jacobaea and plant, soil, and aboveground insect community composition. Ecology 87, 2002–2013. doi: 10.1890/0012-9658(2006)87[2002:IBSJAP]2.0.CO;2
Bezemer, T. M., Van der Putten, W. H., Martens, H., Van de Voorde, T. F., Mulder, P. P., and Kostenko, O. (2013). Above-and below-ground herbivory effects on below-ground plant–fungus interactions and plant–soil feedback responses. J. Ecol. 101, 325–333. doi: 10.1111/1365-2745.12045
Biere, A., and Goverse, A. (2016). Plant-mediated systemic interactions between pathogens, parasitic nematodes, and herbivores above-and belowground. Annu. Rev. Phytopathol. 54, 499–527. doi: 10.1146/annurev-phyto-080615-100245
Borgström, P., Strengbom, J., Marini, L., Viketoft, M., and Bommarco, R. (2017). Above-and belowground insect herbivory modifies the response of a grassland plant community to nitrogen eutrophication. Ecology 98, 545–554. doi: 10.1002/ecy.1667
Brunner, S. M., Goos, R. J., Swenson, S. J., Foster, S. P., Schatz, B. G., Lawley, Y. E., et al. (2015). Impact of nitrogen fixing and plant growth-promoting bacteria on a phloem-feeding soybean herbivore. Appl. Soil Ecol. 86, 71–81. doi: 10.1016/j.apsoil.2014.10.007
Carter-Wientjes, C. H., Russin, J. S., Boethel, D. J., Griffin, J. L., and McGawley, E. C. (2004). Feeding and maturation by soybean looper (Lepidoptera: Noctuidae) larvae on soybean affected by weed, fungus, and nematode pests. J. Econ. Entomol. 97, 14–20. doi: 10.1093/jee/97.1.14
Cahill, J. F., Elle, E., Smith, G. R., and Shore, B. H. (2008). Disruption of a belowground mutualism alters interactions between plants and their floral visitors. Ecology 89, 1791–1801. doi: 10.1890/07-0719.1
Chen, Y. H., Gols, R., and Benrey, B. (2015). Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 60, 35–58. doi: 10.1146/annurev-ento-010814-020601
Colella, T., Candido, V., Campanelli, G., Camele, I., and Battaglia, D. (2014). Effect of irrigation regimes and artificial mycorrhization on insect pest infestations and yield in tomato crop. Phytoparasitica 42, 235–246. doi: 10.1007/s12600-013-0356-3
Commare, R. R., Nandakumar, R., Kandan, A., Suresh, S., Bharathi, M., Raguchander, T., et al. (2002). Pseudomonas fluorescens based bio-formulation for the management of sheath blight disease and leaffolder insect in rice. Crop Protection 21, 671–677. doi: 10.1016/S0261-2194(02)00020-0
Cornelissen, T., Wilson Fernandes, G., and Vasconcellos-Neto, J. (2008). Size does matter: variation in herbivory between and within plants and the plant vigor hypothesis. Oikos 117, 1121–1130. doi: 10.1111/j.0030-1299.2008.16588.x
Diez, J. M., Dickie, I., Edwards, G., Hulme, P. E., Sullivan, J. J., and Duncan, R. P. (2010). Negative soil feedbacks accumulate over time for non-native plant species. Ecol. Lett. 13, 803–809. doi: 10.1111/j.1461-0248.2010.01474.x
Erb, M., Robert, C. A., Hibbard, B. E., and Turlings, T. C. (2011). Sequence of arrival determines plant-mediated interactions between herbivores. J. Ecol. 99, 7–15. doi: 10.1111/j.1365-2745.2010.01757.x
Evans, E. W. (1991). Experimental manipulation of herbivores in native tallgrass prairie: responses of aboveground arthropods. Am. Midl. Natur. 125, 37–46. doi: 10.2307/2426367
Flory, S. L., and Clay, K. (2013). Pathogen accumulation and long-term dynamics of plant invasions. J. Ecol. 101, 607–613. doi: 10.1111/1365-2745.12078
Gadhave, K. R., Finch, P., Gibson, T. M., and Gange, A. C. (2016). Plant growth-promoting Bacillus suppress Brevicoryne brassicae field infestation and trigger density-dependent and density-independent natural enemy responses. J. Pest Sci. 89, 985–992. doi: 10.1007/s10340-015-0721-8
Gange, A. C., Brown, V. K., and Aplin, D. M. (2003). Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecol. Lett. 6, 1051–1055. doi: 10.1046/j.1461-0248.2003.00540.x
Gange, A. C., Brown, V. K., and Aplin, D. M. (2005a). Ecological specificity of arbuscular mycorrhizae: evidence from foliar-and seed-feeding insects. Ecology 86, 603–611. doi: 10.1890/04-0967
Gange, A. C., Gane, D. R., Chen, Y., and Gong, M. (2005b). Dual colonization of Eucalyptus urophylla ST Blake by arbuscular and ectomycorrhizal fungi affects levels of insect herbivore attack. Agric. For. Entomol. 7, 253–263. doi: 10.1111/j.1461-9555.2005.00268.x
Gange, A. C., and Smith, A. K. (2005). Arbuscular mycorrhizal fungi influence visitation rates of pollinating insects. Ecol. Entomol. 30, 600–606. doi: 10.1111/j.0307-6946.2005.00732.x
Gange, A. C., and West, H. M. (1994). Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol. 128, 79–87. doi: 10.1111/j.1469-8137.1994.tb03989.x
Gehring, C., and Bennett, A. (2009). Mycorrhizal fungal-plant-insect interactions: the importance of a community approach. Environ. Entomol. 38, 93–102. doi: 10.1603/022.038.0111
Gehring, C. A., and Whitham, T. G. (2002). 12 Mycorrhizae-herbivore interactions: population and community consequences. Mycorrhizal Ecol. 157:295. doi: 10.1007/978-3-540-38364-2_12
Godschalx, A. L., Schädler, M., Trisel, J. A., Balkan, M. A., and Ballhorn, D. J. (2015). Ants are less attracted to the extrafloral nectar of plants with symbiotic, nitrogen-fixing rhizobia. Ecology 96, 348–354. doi: 10.1890/14-1178.1
Guo, X., Petermann, J. S., Schittko, C., and Wurst, S. (2015). Independent role of belowground organisms and plant cultivar diversity in legume-grass communities. Appl. Soil Ecol. 95, 1–8. doi: 10.1016/j.apsoil.2015.05.010
Guo, X., Petermann, J. S., Schittko, C., and Wurst, S. (2016). Root-knot nematodes (Meloidogyne hapla) can modify the positive plant intraspecific diversity-productivity effect on red clover in clover-grass communities. Appl. Soil Ecol. 102, 26–35. doi: 10.1016/j.apsoil.2016.02.007
Hartley, S. E., and Gange, A. C. (2009). Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu. Rev. Entomol. 54, 323–342. doi: 10.1146/annurev.ento.54.110807.090614
Harvey, J. A. (2000). Dynamic effects of parasitism by an endoparasitoid wasp on the development of two host species: implications for host quality and parasitoid fitness. Ecol. Entomol. 25, 267–278. doi: 10.1046/j.1365-2311.2000.00265.x
Harvey, J. A., Bezemer, T. M., Elzinga, J. A., and Strand, M. R. (2004). Development of the solitary endoparasitoid Microplitis demolitor: host quality does not increase with host age and size. Ecol. Entomol. 29, 35–43. doi: 10.1111/j.0307-6946.2004.00568.x
Heeren, J. R., Steffey, K. L., Tinsley, N. A., Estes, R. E., Niblack, T. L., and Gray, M. E. (2012). The interaction of soybean aphids and soybean cyst nematodes on selected resistant and susceptible soybean lines. J. Appl. Entomol. 136, 646–655. doi: 10.1111/j.1439-0418.2011.01701.x
Heinen, R., Sluijs, M., Biere, A., Harvey, J. A., and Bezemer, T. M. (2018). Plant community composition but not plant traits determine the outcome of soil legacy effects on plants and insects. J. Ecol. 106, 1217–1229. doi: 10.1111/1365-2745.12907
Hong, S. C., MacGuidwin, A., and Gratton, C. (2011). Soybean aphid and soybean cyst nematode interactions in the field and effects on soybean yield. J. Econ. Entomol. 104, 1568–1574. doi: 10.1603/EC11084
Hunt-Joshi, T. R., Blossey, B., and Root, R. B. (2004). Root and leaf herbivory on Lythrum salicaria: implications for plant performance and communities. Ecol. Appl. 14, 1574–1589. doi: 10.1890/03-5181
Johnson, D., Leake, J. R., and Read, D. J. (2001). Novel in-growth core system enables functional studies of grassland mycorrhizal mycelial networks. New Phytol. 152, 555–562. doi: 10.1046/j.0028-646X.2001.00273.x
Johnson, D., Leake, J. R., Ostle, N., Ineson, P., and Read, D. J. (2002). In situ13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol. 153, 327–334. doi: 10.1046/j.0028-646X.2001.00316.x
Johnson, S. N., Clark, K. E., Hartley, S. E., Jones, T. H., McKenzie, S. W., and Koricheva, J. (2012). Aboveground–belowground herbivore interactions: a meta-analysis. Ecology 93, 2208–2215. doi: 10.1890/11-2272.1
Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. (2012). Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38, 651–664. doi: 10.1007/s10886-012-0134-6
Kaplan, I., Sardanelli, S., and Denno, R. F. (2009). Field evidence for indirect interactions between foliar-feeding insect and root-feeding nematode communities on Nicotiana tabacum. Ecol. Entomol. 34, 262–270. doi: 10.1111/j.1365-2311.2008.01062.x
Kardol, P., Martijn Bezemer, T., and van der Putten, W. H. (2006). Temporal variation in plant–soil feedback controls succession. Ecol. Lett. 9, 1080–1088. doi: 10.1111/j.1461-0248.2006.00953.x
Koricheva, J., Gange, A. C., and Jones, T. (2009). Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90, 2088–2097. doi: 10.1890/08-1555.1
Kos, M., Tuijl, M. A., Roo, J., Mulder, P. P., and Bezemer, T. M. (2015). Species-specific plant–soil feedback effects on above-ground plant–insect interactions. J. Ecol. 103, 904–914. doi: 10.1111/1365-2745.12402
Kostenko, O., van de Voorde, T. F., Mulder, P. P., Putten, W. H., and Martijn Bezemer, T. (2012). Legacy effects of aboveground–belowground interactions. Ecol. Lett. 15, 813–821. doi: 10.1111/j.1461-0248.2012.01801.x
Kula, A. A. R., and Hartnett, D. C. (2015). Effects of mycorrhizal symbiosis on aboveground arthropod herbivory in tallgrass prairie: an in situ experiment. Plant Ecol. 216, 589–597. doi: 10.1007/s11258-015-0461-0
Lakshmanan, V., Selvaraj, G., and Bais, H. P. (2014). Functional soil microbiome: belowground solutions to an aboveground problem. Plant Physiol. 166, 689–700. doi: 10.1104/pp.114.245811
MacKauer, M. (1996). Sexual size dimorphism in solitary parasitoid wasps: influence of host quality. Oikos 76, 265–272. doi: 10.2307/3546199
Masters, G. J. (1995). The effect of herbivore density on host plant mediated interactions between two insects. Ecol. Res. 10, 125–133. doi: 10.1007/BF02347934
Masters, G. J., Brown, V. K., and Gange, A. C. (1993). Plant mediated interactions between above-and below-ground insect herbivores. Oikos 66, 148–151. doi: 10.2307/3545209
Masters, G. J., Jones, T. H., and Rogers, M. (2001). Host-plant mediated effects of root herbivory on insect seed predators and their parasitoids. Oecologia 127, 246–250. doi: 10.1007/s004420000569
Megali, L., Schlau, B., and Rasmann, S. (2015). Soil microbial inoculation increases corn yield and insect attack. Agron. Sustain. Dev. 35, 1511–1519. doi: 10.1007/s13593-015-0323-0
Megías, A. G., and Müller, C. (2010). Root herbivores and detritivores shape above-ground multitrophic assemblage through plant-mediated effects. J. Anim. Ecol. 79, 923–931. doi: 10.1111/j.1365-2656.2010.01681.x
Meyling, N. V., and Eilenberg, J. (2007). Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol. Control 43, 145–155. doi: 10.1016/j.biocontrol.2007.07.007
Moon, D. C., Barnouti, J., and Younginger, B. (2013). Context-dependent effects of mycorrhizae on herbivore density and parasitism in a tritrophic coastal study system. Ecol. Entomol. 38, 31–39. doi: 10.1111/j.1365-2311.2012.01399.x
Nijjer, S., Rogers, W. E., and Siemann, E. (2007). Negative plant–soil feedbacks may limit persistence of an invasive tree due to rapid accumulation of soil pathogens. Proc. R. Soc. Lond. B Biol. Sci. 274, 2621–2627. doi: 10.1098/rspb.2007.0804
Pierre, S. P., Dugravot, S., Hervé, M. R., Hassan, H. M., van Dam, N. M., and Cortesero, A. M. (2013). Belowground induction by Delia radicum or phytohormones affect aboveground herbivore communities on field-grown broccoli. Front. Plant Sci. 4:305. doi: 10.3389/fpls.2013.00305
Pieterse, C. M., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C., and Bakker, P. A. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. doi: 10.1146/annurev-phyto-082712-102340
Pineda, A., Zheng, S. J., van Loon, J. J., Pieterse, C. M., and Dicke, M. (2010). Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci. 15, 507–514. doi: 10.1016/j.tplants.2010.05.007
Pineda, A., Kaplan, I., and Bezemer, T. M. (2017). Steering soil microbiomes to suppress aboveground insect pests. Trends Plant Sci. 22, 770–778. doi: 10.1016/j.tplants.2017.07.002
Poorter, H., Bühler, J., van Dusschoten, D., Climent, J., and Postma, J. A. (2012). Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 39, 839–850. doi: 10.1071/FP12049
Poveda, K., Steffan-Dewenter, I., Scheu, S., and Tscharntke, T. (2003). Effects of below-and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia 135, 601–605. doi: 10.1007/s00442-003-1228-1
Poveda, K., Steffan-Dewenter, I., Scheu, S., and Tscharntke, T. (2005). Effects of decomposers and herbivores on plant performance and aboveground plant-insect interactions. Oikos 108, 503–510. doi: 10.1111/j.0030-1299.2005.13664.x
Pozo, M. J., and Azcón-Aguilar, C. (2007). Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 10, 393–398. doi: 10.1016/j.pbi.2007.05.004
Prabhukarthikeyan, R., Saravanakumar, D., and Raguchander, T. (2014). Combination of endophytic Bacillus and Beauveria for the management of Fusarium wilt and fruit borer in tomato. Pest Manag. Sci. 70, 1742–1750. doi: 10.1002/ps.3719
Price, P. W. (1991). The plant vigor hypothesis and herbivore attack. Oikos 62, 244–251. doi: 10.2307/3545270
Ramirez, K. S., Knight, C. G., de Hollander, M., Brearley, F. Q., Constantinides, B., Cotton, A., et al. (2018). Detecting macroecological patterns in bacterial communities across independent studies of global soils. Nat. Microbiol. 3:189. doi: 10.1038/s41564-017-0062-x
Reidinger, S., Eschen, R., Gange, A. C., Finch, P., and Bezemer, T. M. (2012). Arbuscular mycorrhizal colonization, plant chemistry, and aboveground herbivory on Senecio jacobaea. Acta Oecol. 38, 8–16. doi: 10.1016/j.actao.2011.08.003
Ryalls, J. M., Moore, B. D., Riegler, M., and Johnson, S. N. (2016). Above–belowground herbivore interactions in mixed plant communities are influenced by altered precipitation patterns. Front. Plant Sci. 7:345. doi: 10.3389/fpls.2016.00345
Saravanakumar, D., Lavanya, N., Muthumeena, B., Raguchander, T., Suresh, S., and Samiyappan, R. (2008). Pseudomonas fluorescens enhances resistance and natural enemy population in rice plants against leaffolder pest. J. Appl. Entomol. 132, 469–479. doi: 10.1111/j.1439-0418.2008.01278.x
Sasan, R. K., and Bidochka, M. J. (2012). The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 99, 101–107. doi: 10.3732/ajb.1100136
Senthilraja, G., Anand, T., Durairaj, C., Kennedy, J. S., Suresh, S., Raguchander, T., et al. (2010). A new microbial consortia containing entomopathogenic fungus, Beauveria bassiana and plant growth promoting rhizobacteria, Pseudomonas fluorescens for simultaneous management of leafminers and collar rot disease in groundnut. Biocontrol Sci. Technol. 20, 449–464. doi: 10.1080/09583150903576949
Soler, R., Schaper, S. V., Bezemer, T., Cortesero, A. M., Hoffmeister, T. S., Van der Putten, W. H., et al. (2009). Influence of presence and spatial arrangement of belowground insects on host-plant selection of aboveground insects: a field study. Ecol. Entomol. 34, 339–345. doi: 10.1111/j.1365-2311.2008.01082.x
Soler, R., Van der Putten, W. H., Harvey, J. A., Vet, L. E., Dicke, M., and Bezemer, T. M. (2012). Root herbivore effects on aboveground multitrophic interactions: patterns, processes and mechanisms. J. Chem. Ecol. 38, 755–767. doi: 10.1007/s10886-012-0104-z
Staley, J. T., Mortimer, S. R., Morecroft, M. D., Brown, V. K., and Masters, G. J. (2007). Summer drought alters plant-mediated competition between foliar-and root-feeding insects. Glob. Chang. Biol. 13, 866–877. doi: 10.1111/j.1365-2486.2007.01338.x
Thamer, S., Schädler, M., Bonte, D., and Ballhorn, D. J. (2011). Dual benefit from a belowground symbiosis: nitrogen fixing rhizobia promote growth and defense against a specialist herbivore in a cyanogenic plant. Plant Soil 341, 209–219. doi: 10.1007/s11104-010-0635-4
Ueda, K., Tawaraya, K., Murayama, H., Sato, S., Nishizawa, T., Toyomasu, T., et al. (2013). Effects of arbuscular mycorrhizal fungi on the abundance of foliar-feeding insects and their natural enemy. Appl. Entomol. Zool. 48, 79–85. doi: 10.1007/s13355-012-0155-1
Vandegehuchte, M. L., De La Peña, E., and Bonte, D. (2010). Interactions between root and shoot herbivores of Ammophila arenaria in the laboratory do not translate into correlated abundances in the field. Oikos 119, 1011–1019. doi: 10.1111/j.1600-0706.2009.18360.x
Van de Voorde, T. F., Ruijten, M., van der Putten, W. H., and Bezemer, T. M. (2012). Can the negative plant–soil feedback of Jacobaea vulgaris be explained by autotoxicity? Basic Appl. Ecol. 13, 533–541. doi: 10.1016/j.baae.2012.08.012
Van der Putten, W. H., Bardgett, R. D., Bever, J. D., Bezemer, T. M., Casper, B. B., Fukami, T., et al. (2013). Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. doi: 10.1111/1365-2745.12054
Varga, S., and Kytöviita, M. M. (2010). Gender dimorphism and mycorrhizal symbiosis affect floral visitors and reproductive output in Geranium sylvaticum. Funct. Ecol. 24, 750–758. doi: 10.1111/j.1365-2435.2010.01708.x
Vega, F. E., Goettel, M. S., Blackwell, M., Chandler, D., Jackson, M. A., Keller, S., et al. (2009). Fungal entomopathogens: new insights on their ecology. Fungal Ecol. 2, 149–159. doi: 10.1016/j.funeco.2009.05.001
Vega, F. E., Meyling, N. V., Luangsa-Ard, J. J., and Blackwell, M. (2012). “Fungal entomopathogens,” in Insect Pathology, 2nd Edn, eds F. E. Vega and H. K. Kaya (Academic Press), 171–220. Available online at: https://www.sciencedirect.com/science/book/9780123849847
Vicari, M., Hatcher, P. E., and Ayres, P. G. (2002). Combined effect of foliar and mycorrhizal endophytes on an insect herbivore. Ecology 83, 2452–2464. doi: 10.1890/0012-9658(2002)083[2452:CEOFAM]2.0.CO;2
Vockenhuber, E., Kabouw, P., Tscharntke, T., and Scherber, C. (2013). Plant–animal interactions in two forest herbs along a tree and herb diversity gradient. Plant Ecol. Divers. 6, 205–216. doi: 10.1080/17550874.2013.782368
Wang, Y. S., Wen, C. Y., Chiu, T. C., and Yen, J. H. (2004). Effect of fungicide iprodione on soil bacterial community. Ecotoxicol. Environ. Saf. 59, 127–132. doi: 10.1016/j.ecoenv.2004.01.008
Wang, C., and St Leger, R. J. (2007). The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryotic Cell 6, 808–816. doi: 10.1128/EC.00409-06
Wang, M., Biere, A., Van der Putten, W. H., and Bezemer, T. M. (2014). Sequential effects of root and foliar herbivory on aboveground and belowground induced plant defense responses and insect performance. Oecologia 175, 187–198. doi: 10.1007/s00442-014-2885-y
White, T. C. R. (1969). An index to measure weather-induced stress of trees associated with outbreaks of psyllids in Australia. Ecology 50, 905–909. doi: 10.2307/1933707
White, J. A., and Andow, D. A. (2006). Habitat modification contributes to associational resistance between herbivores. Oecologia 148, 482–490. doi: 10.1007/s00442-006-0388-1
Wolfe, B. E., Husband, B. C., and Klironomos, J. N. (2005). Effects of a belowground mutualism on an aboveground mutualism. Ecol. Lett. 8, 218–223. doi: 10.1111/j.1461-0248.2004.00716.x
Wondafrash, M., Van Dam, N. M., and Tytgat, T. O. (2013). Plant systemic induced responses mediate interactions between root parasitic nematodes and aboveground herbivorous insects. Front. Plant Sci. 4:87. doi: 10.3389/fpls.2013.00087
Wubs, E. J., van der Putten, W. H., Bosch, M., and Bezemer, T. M. (2016). Soil inoculation steers restoration of terrestrial ecosystems. Nat. Plants 2:16107. doi: 10.1038/nplants.2016.107
Wurst, S., Van Dam, N. M., Monroy, F., Biere, A., and Van der Putten, W. H. (2008). Intraspecific variation in plant defense alters effects of root herbivores on leaf chemistry and aboveground herbivore damage. J. Chem. Ecol. 34, 1360–1367. doi: 10.1007/s10886-008-9537-9
Younginger, B., Barnouti, J., and Moon, D. C. (2009). Interactive effects of mycorrhizal fungi, salt stress, and competition on the herbivores of Baccharis halimifolia. Ecol. Entomol. 34, 580–587. doi: 10.1111/j.1365-2311.2009.01105.x
Keywords: soil, aboveground-belowground interactions, insects, field experiments, fungi, bacteria, nematodes, root herbivores
Citation: Heinen R, Biere A, Harvey JA and Bezemer TM (2018) Effects of Soil Organisms on Aboveground Plant-Insect Interactions in the Field: Patterns, Mechanisms and the Role of Methodology. Front. Ecol. Evol. 6:106. doi: 10.3389/fevo.2018.00106
Received: 13 January 2018; Accepted: 02 July 2018;
Published: 24 July 2018.
Edited by:
Jordi Figuerola, Estación Biológica de Doñana (EBD), SpainReviewed by:
Warwick Allen, Lincoln University, New ZealandPhilip G. Hahn, University of Montana, United States
Copyright © 2018 Heinen, Biere, Harvey and Bezemer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Heinen, ci5oZWluZW5Abmlvby5rbmF3Lm5s
 Robin Heinen
Robin Heinen Arjen Biere
Arjen Biere Jeffrey A. Harvey
Jeffrey A. Harvey T. Martijn Bezemer
T. Martijn Bezemer