
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 18 July 2018
Sec. Behavioral and Evolutionary Ecology
Volume 6 - 2018 | https://doi.org/10.3389/fevo.2018.00101
Predators that depend on patchily distributed prey face the problem of finding food patches where they can successfully compete for prey. While the competitive exclusion principle suggests that species can only coexist if their ecological niches show considerable differences, newer theory proposes that local coexistence can be facilitated by so-called stabilizing and equalizing mechanisms. A prerequisite to identify such mechanisms is the understanding of the strength and the nature of competition (i.e., interference or exploitation). We studied the interaction between two open-space foraging bats by testing if common noctule bats Nyctalus noctula shift their space use in response to simulated aggregations of conspecifics or heterospecific Pipistrellus nathusii. When confronted with playbacks of heterospecifics, N. noctula increased their activity in early summer, but decreased activity in late summer. This pattern was accompanied by a decrease in the proportion of large insects in late summer, suggesting a more intense competition for food in late compared to early summer. When confronted with playbacks of conspecifics, N. noctula did not change their activity, irrespective of season. Our results indicate that in early summer, intraspecific competition is more severe than interspecific competition for insectivorous bats. Likely, conspecifics engage in interference competition for flight space, and may suffer from reduced prey detectability as echolocation calls of conspecifics interfere with each other. During insect rich times, interspecific competition on the other hand may be mediated by fine scale vertical partitioning and the use non-interfering echolocation frequencies. In contrast, when food is scarce in late summer, bats may engage in exploitation competition. Our data suggests that N. noctula avoid aggregations of more agile bats like P. nathusii, probably due to impeded hunting success. Yet, as fast and efficient fliers, N. noctula may be able to escape this disadvantage by exploiting more distant foraging patches.
All predators face the same problem of finding and catching prey. In large carnivores, the capture rate is commonly limited by the high failure rates (e.g., Eaton, 1970; Holekamp et al., 1997) during energetically demanding capture attempts (e.g., Heglund et al., 1974; Gorman et al., 1998). In contrast, predators feeding on relatively small prey items like invertebrates, insects, or Krill and Zooplankton depend more strongly on the detection of prey aggregations and the abundance or energetic value of single prey items (Morse, 1971; Lubin et al., 1977; Nowacek et al., 2011). Especially aerial hunting insectivores such as bats and birds often hunt on patchily distributed insects swarms which they may locate only over short distances. However, individuals may improve their search efficiency by using public information that is inadvertently provided by conspecifics or heterospecifics with similar food requirements (Danchin et al., 2004). While group foraging birds can increase their hunting success by visual observations of other birds (Greene, 1987), aerial-hawking bats may do so by eavesdropping on the echolocation calls of other foraging bats (Balcombe and Fenton, 1988; Gillam, 2007; Dechmann et al., 2009; Dorado-Correa et al., 2013). Since bats use specialized calls, so called feeding buzzes (Kalko, 1995), to capture their prey, conspecifics and heterospecifics can use such acoustic information to locate promising prey patches. Indeed, there is evidence that foraging bats of some species stay in an optimal eavesdropping distance to each other when they hunt in large groups, thus forming a sensory network that allows them to scan an area much larger than their individual detection range for insect prey (Cvikel et al., 2015). While two bats are flying within hearing range of each other during prey search, they may both profit from an increase in effective prey detection range, yet they would still compete when both are reaching the respective prey patch. Moreover, most insectivorous bat species hunt mainly during the first few hours after sunset (Kunz, 1973), probably because the activity of airborne insects usually declines substantially afterwards (Taylor and O'Neill, 1988; Meyer et al., 2004; Milne et al., 2005). This short period of prey availability limits the temporal partitioning of resources by competing species and thus increases interspecific competition for taxa that hunt on the same prey. Since competing bat species often also overlap in other aspects of their biology, e.g., roost and habitat preferences, competition may become even more exacerbated. The competitive exclusion principle suggests that species with an overly high niche overlap cannot coexist (Gause, 1934; Hardin, 1960; see also e.g., Levine and HilleRisLambers, 2009). However, recent developments in coexistence theory suggest that equalizing or stabilizing mechanisms could promote the coexistence of ecologically similar taxa, next to those mechanisms purely driven by environmental niche differences (Chesson, 2000). Within this framework, stabilizing mechanisms are a condition for coexistence; given that intraspecific competition is stronger than interspecific competition, a population's growth rate will increase at low abundances of that species. Equalizing mechanisms on the other hand support coexistence by reducing fitness disadvantages of the inferior of competing species. Movement behavior may act as such a mechanism, e.g., when competing species alter their movements and thus their space use in such a way that they avoid aggregations of strong competitors (Jeltsch et al., 2013; Schlaegel in review).
To explore the presence and extent of such mechanisms, one ideally should evaluate the nature and the strength of intra- and interspecific competition within the investigated species ensemble (sensu Fauth et al., 1996). For bats that hunt on ephemeral insects, it is often assumed that food resources within a patch of swarming insects are virtually unlimited (cf Bell, 1980; Anthony et al., 1981; Arlettaz, 1999, see also Kalko, 1995: maximum capture rate of swarming insects by medium sized pipistrelle bats is roughly 7 insects/min). Exploitation competition among insectivorous bats is thus unlikely during insect rich times. Yet, large groups of hunting bats may still engage in interference competition since they need a certain amount of flight space during aerial foraging. Large open-space foraging bats like Nyctalus noctula usually use an area of at least 1 ha during spatially concentrated hunting bouts over preferred foraging patches (Roeleke et al., 2016; Roeleke et al. in preparation; Voigt et al. in preparation). Indeed, Amichai et al. (2015) recently showed that large aggregations of bats are foraging less effective, since the respective individuals have to direct their attention more often toward conspecifics, and are thus not able to detect prey items at the same time. This is in concordance with some early studies showing that bats that are on collision course may use special calls described as honk calls when approaching conspecifics too closely (Suthers, 1965; Fenton and Bell, 1979). To date, it remains unclear whether these calls are just a warning to avoid collision, or could also be interpreted as aggressive vocalizations (Voigt-Heucke et al., 2010). Moreover, vocalizations emitted by several bats at the same time may also interfere with the detection of each other's specialized hunting calls. Indeed, Corcoran and Conner (2014) showed that Tadarida brasiliensis, a species that forms roosting communities of millions of individuals, uses specialized aggressive vocalizations during competition for prey. Through broadcasting of ultrasounds that jam the sound detection of their competitors, they make them unable to detect a prey item that was recognized before. Under such a framework of interference competition, we would assume that intraspecific competition within limited flight space is higher than interspecific competition, given that heterospecifics, but not necessarily conspecific bats might still be able to show fine-scale spatial segregation (Salsamendi et al., 2012, own observations at study site), due to their respective wing morphologies and resulting flight and foraging modes (Norberg and Rayner, 1987; Arlettaz, 1999; Schnitzler and Kalko, 2001; Voigt et al., 2010; Voigt and Holderied, 2012).
However, seasonal as well as possibly anthropogenically driven changes in insect availability might violate our assumption of constant, unlimited food resources for aerial hawking bats. Recent studies show that in Central Europe insect abundance is decreasing toward late summer (Anthony et al., 1981; Hallmann et al., 2017; Heim et al., 2017), which coincides with the time when several bat species face a trade-off between spending their time for feeding, mating, and either finding a winter roost or migrating southwards. Given that foraging time as well as prey availability can be limited in late summer, competition might then change toward the exploitation of resources, which will bring an advantage to the smaller and more maneuverable fliers (Norberg and Rayner, 1987) that might be more successful in catching a limited number of prey items within short time. Yet, the question remains whether larger and faster species can mitigate this increase in interspecific competition by exploiting more distant but possibly less rich and yet unoccupied prey patches to equalize this potential disadvantage.
Here we used playback experiments to examine the nature of competition between two co-occurring and potentially competing bat species by recording their reactions toward simulated aggregations of con- and heterospecifics during different life-history stages (i.e., early season during which breeding and molting occurs and late season during which mating, search for winter roost, and potentially migration occurs). Our playback approach makes use of the vocalization and hearing ability of aerial hawking insectivorous bats, which allows to measure spatial changes in activity in response to experimental acoustic treatments by quantifying bat activity through the number of ultrasonic calls that can be recorded within the experimental area. Our focal species was the common noctule bat N. noctula (Schreber, 1774), a fast flying and partially migratory bat. At our study site in Germany, we exposed locally foraging N. noctula to playbacks of either hunting conspecifics, or hunting Pipistrellus nathusii. These two species have similar activity patterns (Heim et al., 2016) and a high niche overlap in terms of diet, habitat use, and roost preference (Eichstädt, 1997; Vaughan, 1997). Based on the above speculations on the nature of intra- and interspecific competition in aerial hawking bat ensembles, we hypothesized that the reaction of N. noctula toward the different playback types depends on the overall density of competitors within the area and the season, and that this reaction will be linked to different prey availability within the different seasons. In particular, we predicted that N. noctula will increase foraging activity during conspecific playbacks in the early season, when prey is plentiful, and that N. noctula will abandon hunting grounds during con- or heterospecifics playbacks in the late season, when prey is scarce.
In 2016, we conducted playback experiments directed toward N. noctula during the non-migratory breeding season (mid-June to mid-August, hereafter referred to as early season) and during its mating and potentially migratory season (beginning of September until beginning of October, hereafter referred to as late season). Playback experiments took place in Northeastern Germany, i.e., in northern parts of the federal country Brandenburg, called Uckermark. The Uckermark is dominated by agricultural fields, but includes many waterbodies, ranging from small kettle holes to relatively large lakes. We aimed at conducting playback experiments at the shores of 23 of these limnic habitats. Although there are only few forest remnants, and roosting opportunities in the area are thus expected to be scarce, we knew from previous GPS studies (Roeleke et al., 2016; Roeleke et al. unpublished data) that N. noctula colonies in the Uckermark preferentially forage above waterbodies within distances of at least 7 km from their roost. The 23 playback locations were distributed over an area of ~60,000 ha. During each experimental night, we conducted playback experiments at two sites simultaneously. Distance between the paired playback locations ranged from 1 to 5 km. Since we conducted the experiments roughly at the same time, it is unlikely that we broadcasted playbacks to the same individuals at the different sites during a given night. We further aimed at conducting playbacks twice at each site—once during the early and once during the late season (see section Playback Experiments). However, due to spatial and temporal variation in bat activity, we could not always achieve this for all sites. To avoid pseudo-replication, we only visited each site once per season.
At each site, we broadcasted three different playback types toward foraging N. noctula; feeding buzzes of N. noctula, feeding buzzes of P. nathusii, and a sine tone between 20 and 40 kHz as a control (Supplement Material 1). Feeding buzzes are specialized bat calls that are designed for the terminal phase of prey capture, and which are unambiguously identifiable. The single playback trials were 3 min long and consisted of three phases; (i) 1 min of silence (baseline), in order to record the acoustic baseline activity of N. noctula at the respective site, (ii) 1 min of broadcasting the respective playback (playback), and (iii) 1 min of silence again (post), in order to see potential post- playback effects (cf. Übernickel et al., 2013; Voigt-Heucke et al., 2016).
We started the playback trials as soon as we observed foraging activity of one or more N. noctula via the recording setup. After each trial, we waited at least 3 min and checked again for acoustic foraging activity before we broadcasted the next playback. In most nights, we conducted the experiments shortly after sunset when the first N. noctula arrived. However, in case all bats left the area during the playbacks, we tried to conduct a second round of playback experiments later on when N. noctula activity over the area was more stable. At around midnight, N. noctula activity always declined to low levels. If we did not manage to conduct our experiment until midnight, we stopped the experiments to ensure that all bats within our study were confronted with playbacks in a similar situation, i.e., during their first foraging bout of the night. During some experimental trials, we also noticed P. nathusii foraging close to the shoreline and thus close to our experimental setup. However, since the natural P. nathusii activity was low compared to the broadcasted stimuli, we are certain that their potential effect was negligible. We did not evaluate potential effects of the playbacks on P. nathusii since the playbacks were not directed toward them, and we thus could not assure consistent baseline activity of P. nathusii before broadcasting.
We only performed experiments at wind speeds ≤ 3 m/s during nights without rainfall. Please see Supplement Material 1 for a detailed description of playback preparation and the experimental setup.
We analyzed the acoustic records with SasLabPro (Avisoft Bioacoustics), using a hamming window spectrogram, with fast Fourier transformation of 1024, and 87.5% time overlap. We identified and counted calls of N. noctula which had signal to noise ratio higher than 30 dB for each of the three phases of the respective playback trials, thereby accounting for the difference of ~30 dB between our playbacks and the assumed sound pressure levels foraging bats produce.
At each playback site, we trapped flying insects with a custom built ultra-violet (UV) light trap (light source of about 365 nm wavelength). When insects were approaching the light, they collided with a smooth plastic surface in front of the lamp and subsequently slipped into a bottle filled with 95% ethanol. We placed the traps at the shore of the respective waterbodies, ~5 m from the playback setup, at 3 m height. As soon as we noticed the first N. noctula with our recording setup, we switched on the UV light of the trap and attached the bottle with the ethanol for 1 h. Thus, we ensured that insects were not attracted to the UV light before the onset of the playback experiment.
To derive the most important prey measurements from a bats point of view, we sorted and counted insects by para-taxonomic groups reflecting a combination of order and size (Table 1). We dried the sorted samples for 72 h at 50°C and measured dry mass with an electronic balance (ME5, Sartorius, Germany, 0.001 mg resolution).
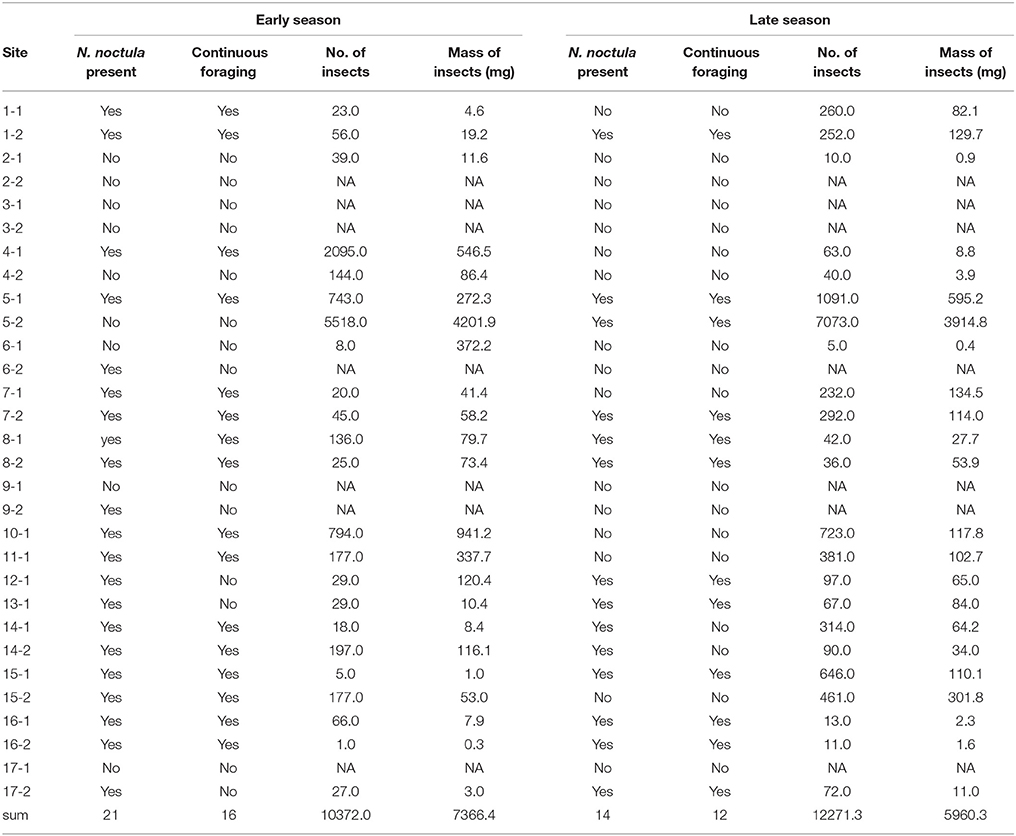
Table 1. Presence and continuous foraging activity of N. noctula and total number and mass of insects at sampled sites.
Our acoustic analysis showed that sometimes bat activity stopped for a longer time during any of the three phases of our experiment (i.e., baseline, playback, post). We excluded these trials from further analysis since we could not be sure if the focus animals were really foraging in such cases. Please see Supplement Material 2 for a detailed description of the estimation of the experimental area and the subsequent data cleaning.
To evaluate the relative difference of N. noctula activity between the pre phase and the playback phase, we calculated the relative difference between pre and playback phase as:
We then modeled the relative difference with a linear mixed effect model (R package lme4, Bates et al., 2015). As predictor variables we used the three-fold interaction of playback type (i.e., conspecifics, heterospecifics, control), baseline activity (number of N. noctula calls during the pre-phase), and season. As random effect we included experimental trial nested within site. To test whether the effects of the playback would last longer than the broadcasting of the playback itself, we ran a similar model with the relative difference between pre- and post- phase as dependent variable. We ensured normal distribution of modeled residuals by visually checking quantile plots of the models. We calculated pseudo-R-squared values with the R package MuMIn (Barton, 2014) and effect sizes of the predictor variables with the R package effects (Fox, 2003). We assumed statistical significant effects of predictor variables when the 95% confidence intervals did not span 0. After confirming with Kolmogorov-Smirnov-Tests that numbers and masses from the different insect groups were not normally distributed, we used paired Mann-Whitney-U-Tests to test whether there was an effect of season on mass or number of caught insects, or on the relative number of the different size classes within each sample. We used all samples for this test, including those from location and season combinations were we did not obtain data from the playback experiments. All data handling and analyses were done with R 3.3.2 (R Core Team, 2016). Review and approval of the experiments was not required by national guidelines, since no animals were caught, handled, or physically manipulated.
We sampled 30 different waterbodies in the study area for N. noctula activity. More waterbodies were used by foraging N. noctula in the early than in the late season (Chi2-Test, Chi2 = 4.65, N = 30, p = 0.03). At sites where N. noctula was present in both seasons, the level of N. noctula activity did not differ between seasons (Mann-Whitney-U-Test, W = 124, N = 28, p = 0.21) (Table 1).
Most caught insects were of rather small size. Number and dry mass of caught insects varied largely between the sampled sites (Table 1). Although there seemed to be a slight shift from large (body length >9 mm) to small insects (body length < 6 mm) from the early to the late season, we did not detect any significant differences for number and mass of the different insect size classes (paired Mann-Whitney-U-tests, N = 46, Figure 1). Yet, paired Mann-Whitney-U-tests (N = 46) showed that the relative number of large insects was significantly higher in the early season (V = 132, p = 0.045), while the relative number of small insects was higher in the late season (V = 57, p = 0.025). Please see Supplement Material 3 for a site specific presentation of abundances and masses of the different insect classes.
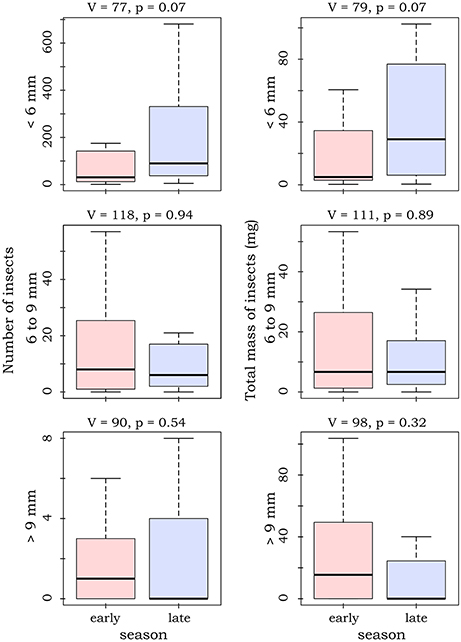
Figure 1. Boxplots and test statistics for number of insects and dry mass for the different seasons, sorted by different size classes of insects. Whiskers depict at maximum 1.5 times the inter-quartile range. For graphical reasons outliers are not shown. Please see Table 1 for total numbers at the sampled sites.
N. noctula did not change their activity when confronted with our control treatment, a sine tone between 20 and 40 kHz. In general, N. noctula showed less shifts in activity when their initial density (i.e., number of calls recorded during the pre-phase) was comparably high. However, when including activity during the pre-phase not as an interaction term but only as a main effect in the model, it turned out that N. noctula generally responded negatively toward playbacks when the initial density of conspecifics was high (i.e., around 500 calls per minute during the pre-phase, see Supplement Material 3 for the effect plot). Our full model with the three-fold interaction between initial density, season, and playback type had a pseudo R2-value of 0.42. This model revealed that N. noctula did not react toward the hunting calls of heterospecific P. nathusii. Further, there was only a slight positive response toward hunting calls of conspecifics at rather low initial densities (Figure 2A). However, this turned into a clear avoidance of conspecifics in the late season when the initial density was low to medium. At the same time, at least at low initial densities, N. noctula activity increased when we broadcasted heterospecific playbacks in the late season. Only at high initial densities, N. noctula started to avoid the experimental area during the P. nathusii playbacks (Figure 2B).
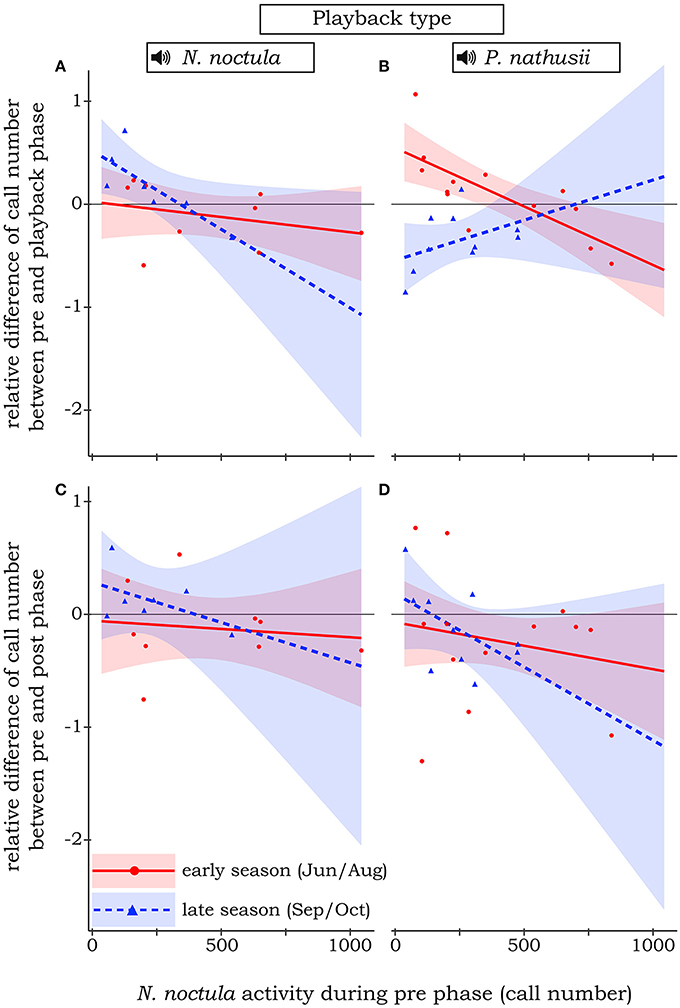
Figure 2. Relative difference of N. noctula activity between pre-phase and playback phase (A,B) or the pre-phase and post phase (C,D) of the experiment, depending on N. noctula activity during the pre -phase, the different playback types, and the season. Raw data is depicted by circles and triangles. Lines show the estimated effect, shaded areas show the corresponding 95% confidence intervals. Solid red line and red circles: early season. Dashed blue line and blue triangles: late season. For simplicity, insignificant effects toward the control treatment (sine tone) are not shown.
Irrespective of playback type and season, the number of calls during the pre-phase and the post-phase of the playback did not differ significantly, i.e., the relative difference was fluctuating around 0 (Figures 2C,D).
Insectivorous bat species can co-occur despite high overlaps in their ecological niches (e.g., Bell, 1980; Fenton, 1990; Salsamendi et al., 2012). Yet, the mechanisms that prevent ensembles of competing bats from competitive exclusion are not fully resolved. The aim of this study was to better understand the nature and relative strength of intraspecific and interspecific competition, and to reveal seasonal changes in competition. Therefore, we directed playbacks of foraging conspecifics and heterospecifics toward foraging N. noctula during two different seasons, i.e., early and late summer. N. noctula responded only marginally toward playbacks of conspecifics. The response of N. noctula toward playbacks of competing heterospecifics, on the other hand, turned from an increase of activity in the early season to a decrease of activity in the late season. We conclude that the studied insectivorous bats experienced stronger intraspecific than interspecific competition during the early season, whereas the opposite was true during the late season.
During late summer, foraging N. noctula used fewer waterbodies in our study area than during the early summer. This seems to be counter-intuitive at first glance, since one would expect higher abundances of foraging bats during the late season, due to the by then weaned offspring. Further, migrating bats from northern and north-eastern countries are arriving around late August to late October in Central Europe, including our study area (Ahlén et al., 2009; Furmankiewicz and Kucharska, 2009; Ciechanowski et al., 2010). The influx of migrating N. noctula is probably the reasons why Heim et al. (2016) found an increase of N. noctula activity above agricultural fields in the study area in late summer. We suggest that the observed decrease in use of our sampled waterbodies was not due to an overall reduced activity in the area, but rather due to a shift of habitat use from limnic to terrestrial foraging grounds. This is in concordance with isotopic analyses by Voigt et al. (2016) who found that N. noctula feeds less on aquatic insect during late summer than during early summer. The shift in habitat use may partially result from the need to mate in the late season. Male bats have to establish and defend solitary roosts, while females search for these so-called mating roost. Thus, males may have to feed nearby their roost, and females may save time when feeding opportunistically during their search for mating roosts rather than at designated foraging areas such as waterbodies. A recent tracking study suggests such a strategy, at least for females, by showing that female N. noctula cover large areas and focus less on single waterbodies for foraging in late summer (Roeleke et al., 2016).
Contrary to our expectations and past studies (Black, 1974; Janzen and Pond, 2009; Hallmann et al., 2017; Heim et al., 2017, but see Hails, 1982), we could not detect differences in number or biomass of flying nocturnal insects between the early and the late season. Yet, in the late season, there seemed to be a tendency that fewer big insects (i.e., body length > 9 mm) were present at the sampled waterbodies (cf. Gloor et al., 1995), and we detected a significant decrease of the proportion of large insects compared to the early season. While there are many dietary studies that show that N. noctula is an opportunistic feeder, most studies agree that relatively large insects are important components of its diet (reviewed in Vaughan, 1997). A decrease of relatively large insects at the sampled waterbodies may thus have increased competition for prey items. This provides a further explanation why fewer waterbodies were used by foraging N. noctula, since a decrease in feasible prey items may have forced N. noctula to forage in habitats with less competitors. Such a temporarily insect rich surrogate foraging habitat could have been agricultural land. Heim et al. (2016) speculate that harvesting activity during September could temporarily increase insect availability in the area (cf Plucinski et al., 2015). Voigt et al. (2015) found that Eptesicus serotinus, an open space foraging bat with a similar wing morphology as N. noctula (Norberg and Rayner, 1987), feeds on terrestrial and aquatic insects alike, which suggests flexibility in the habitat use of feeding open space foragers.
However, we must acknowledge that by using UV light traps, our sampling method was selective toward light sensitive insects. Further, we were limited to place the traps at the shores of the waterbodies at about 4 m height, whereas N. noctula were mostly foraging at altitudes of about 8 to 12 m above the water surfaces. Therefore, our insect sampling provides most likely only a proxy for general insect activity, but does not necessarily reflect actual prey availability for N. noctula.
We did not detect any significant effects of the experimental treatment in the post playback phase, i.e., the bat activity almost instantly went back to the baseline activity level after the broadcasting of playbacks. This shows that N. noctula conceives new competitive situations very quickly, and adjusts its space use likewise quickly and dynamically.
N. noctula only reacted toward our playbacks when the baseline activity was low to medium (i.e., less than 500 calls per minute). Possibly, N. noctula perceived acoustic information from actual present conspecifics more reliable than our playback. However, feeding buzzes are naturally fainter than search calls (Holderied et al., 2005), and high acoustic search call activity may hinder the acoustic detection of feeding con- or heterospecifics in experimental as well as natural situations. However, it may also be that the space that could be efficiently used for foraging was already saturated with individuals. Using densely occupied foraging patches can be ineffective (Amichai et al., 2015), which may result in an individual partitioning of foraging space (cf Beauchamp and Fernández-Juricic, 2005).
It was only during late summer that N. noctula showed a moderate positive response toward the playbacks of conspecifics. On the other hand, N. noctula was clearly attracted toward the playbacks of foraging P. nathusii in early summer, yet this turned into a clear avoidance during late summer. As mentioned above, all these responses held true for low to medium baseline activity of N. noctula (i.e., < 500 calls per minute), but vanished or even reversed when large aggregations of individuals were present. We suggest that the seasonally different responses toward our playbacks were driven by changes in the strength of intra- and interspecific competition.
In particular, the increased activity during playbacks of heterospecifics during early summer indicates that eavesdropping on foraging heterospecifics is an advantageous strategy for bats that hunt for patchily distributed prey in this season. Yet, it appears surprising that N. noctula did not show such a positive response toward playbacks of foraging conspecifics, since conspecifics theoretically should have the highest overlap of dietary requirements, and should thus be the most reliable indicator for availability of preferred insect prey. The observed lack of response toward foraging conspecifics, coupled with the positive response toward foraging heterospecifics, suggests a strong intraspecific competition, and at the same time, a negligible interspecific competition during early summer. As a consequence, we propose that bats are not competing for prey items, but rather for flight space and “soundspace” in early summer. By soundspace, we mean a multidimensional entity that is defined by a 3-dimensional spatial component, time, and the range of ultrasonic frequencies that bats use to echolocate. Echolocating bats need this space to broadcast their ultrasonic calls, and to receive the reflected echoes of their calls, in order to locate prey and obstacles. Nearby conspecifics use the same flight space and soundspace, and may thus interfere with each other during flight and during acoustic detection of prey, respectively. In contrast to that, heterospecifics individuals may partition foraging space vertically and overlap less in their soundspace due to the use of different echolocation frequencies. Fine scale vertical segregation has been shown for a variety of competing taxa that make excessive use of 3-dimensional foraging space (e.g., Saiful et al., 2001; Kiszka et al., 2011; Navarro et al., 2013; Humphries et al., 2016; Mansor and Ramli, 2017). Although investigating fine scale vertical segregation of aerial hawkers is technically challenging, recent recordings of flight altitude of open space bats suggest vertical segregation, yet without clear evidence for foraging activity (Roemer et al., 2017). At our sample sites, we frequently observed that P. nathusii generally foraged at altitudes of approx. 4 to 8 m, while N. noctula often used altitudes of 8 to 15 m.
As mentioned above, heterospecific bats also show partitioning of their echolocation frequencies (approximately peak frequencies for N. noctula 20 kHz and for P. nathusii 40 kHz, Skiba, 2003). Since the auditory system of bats is finely tuned to their own frequency range (reviewed by Hiryu et al., 2016; Pollak, 2016), acoustic interference across these two species should be negligible. Given that prey is not limited, vertical partitioning of foraging space and call frequency partitioning should thus allow an ensemble of these two species to efficiently forage at higher densities than it would be possible for an aggregation of any of these two species alone.
Contrary to the pattern observed in early summer, N. noctula showed decreased activity when exposed to playbacks of foraging heterospecifics during late summer. At the same time, there was an, admittedly very moderate, positive response toward playbacks of foraging conspecifics during low baseline activities (i.e., < 250 calls per minute). We conclude that the strength of interspecific competition must have drastically changed from early to late summer. In particular, N. noctula seemed to expect strong interspecific competition when we broadcasted feeding buzzes of P. nathusii, which forced them to abandon the respective foraging areas during the playback. Given that interference of heterospecifics is probably negligible, we conclude that the observed negative response was driven by increased exploitation competition, due to low prey availability. Since large insects were relatively scarce during late summer, open space foragers like N. noctula might not have been able to forage efficiently at patches already occupied by P. nathusii. Probably P. nathusii can catch prey quicker than N. noctula in such a situation, due to its higher maneuverability (Norberg and Rayner, 1987). Further, its lower flight altitude suggests that P. nathusii may catch ascending insects before these reach the spheres of higher foraging bats like N. noctula. Marggraf et al. (in review) found that P. nathusii decreased activity in response to playbacks of foraging conspecifics, but did not react toward playbacks of foraging N. noctula, which indicates that interspecific competition is not symmetric in these two species. Thus, especially during times of prey scarcity, it would be crucial for N. noctula to locate patches of prey that are not exploited by superior foragers like P. nathusii. Therefore, we suggest that eavesdropping on hunting conspecifics is the most promising strategy when prey is limited, as long as density of conspecifics is not too high for efficient foraging.
We found that the aerial hawking open space foraging bat N. noctula actively seeks heterospecific P. nathusii during foraging bouts in early summer, but avoids patches occupied by foraging heterospecifics in late summer. N. noctula did not respond to foraging conspecifics in early summer, but showed a slight positive response to conspecifics in late summer. We conclude that the number of aerial hawking open space foragers at a food patch is limited by intraspecific interference competition for flight space and soundspace in early summer, but that interspecific exploitation competition for insect prey is limiting the number of bats in late summer. High intraspecific competition may thus act stabilizing on insectivorous bat ensembles when food resources are plentiful. During probably lower prey availability in late summer, aerial hawkers that are specialized for fast flight in uncluttered habitats may suffer from inferior capture rate compared to more maneuverable bats like P. nathusii. Water bodies were used less during this time. We speculate that fast flying aerial hawkers can use farther away or less rich hunting grounds, since their specialized wing morphology allows them to fly large distances at low energetic costs (Winter and von Helversen, 1998). This adaption to fast yet cheap flight may equalize fitness disadvantages toward superior foragers. One could even speculate that habitats which are suboptimal from a foraging perspective may support the diversity of bats by offering refuges from interspecific competition.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
CV and MR conceptualized the study. CV supervised the students. MR and LJ performed the experiments. LJ analyzed the acoustic recordings. MR did the statistical analysis. MR and CV wrote the manuscript.
MR was funded by the Deutsche Forschungsgemeinschaft (DFG-GRK 2118/1 - BioMove). The publication of this article was funded by the Deutsche Forschungsgemeinschaft (DFG-GRK 2118/1 - BioMove) and the Open Access Fund of the Leibniz Association.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JM and handling Editor declared their shared affiliation.
We are grateful to Maris Pärn for help with sorting insect samples. We thank Olga Heim, Silke Voigt-Heucke, and Lara Marggraf for providing bat call records and help with preparing the playback files. Viktoriia Radchuk for statistical advice. The Leibniz Centre for Agricultural Landscape Research and Gernot Verch for providing facilities during the fieldwork. Niels Blaum, Florian Jeltsch, and members from the RTG BioMove for discussion of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00101/full#supplementary-material
Ahlén, I., Baagøe, H. J., and Bach, L. (2009). Behavior of Scandinavian bats during migration and foraging at sea. J. Mammal. 90, 1318–1323. doi: 10.1644/09-MAMM-S-223R.1
Amichai, E., Blumrosen, G., and Yovel, Y. (2015). Calling louder and longer: how bats use biosonar under severe acoustic interference from other bats. Proc. Biol. Sci. 282:20152064. doi: 10.1098/rspb.2015.2064
Anthony, E. L. P., Stack, M. H., and Kunz, T. H. (1981). Night roosting and the nocturnal time budget of the little brown bat, Myotis lucifugus: effects of reproductive status, prey density, and environmental conditions. Oecologia 51, 151–156. doi: 10.1007/BF00540593
Arlettaz, R. (1999). Habitat selection as a major resource partitioning mechanism between the two sympatric sibling bat species Myotis myotis and Myotis blythii. J. Anim. Ecol. 68, 460–471. doi: 10.1046/j.1365-2656.1999.00293.x
Balcombe, J. P., and Fenton, M. B. (1988). Eavesdropping by bats: the influence of echolocation call design and foraging strategy. Ethology 79, 158–166. doi: 10.1111/j.1439-0310.1988.tb00708.x
Barton, K. (2014). MuMIn: Multi-Model Inference. R package version 1.12.11. Available online at: https://CRAN.R-project.org/package=MuMIn
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Beauchamp, G., and Fernández-Juricic, E. (2005). The group-size paradox: effects of learning and patch departure rules. Behav. Ecol. 16, 352–357. doi: 10.1093/beheco/arh169
Bell, G. P. (1980). Habitat use and response to patches of prey by desert insectivorous bats. Can. J. Zool. 58, 1876–1883. doi: 10.1139/z80-256
Black, H. L. (1974). A north temperate bat community: structure and prey populations. J. Mammal. 55, 138–157. doi: 10.2307/1379263
Chesson, P. (2000). Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. doi: 10.1146/annurev.ecolsys.31.1.343
Ciechanowski, M., Zajac, T., Zielinska, A., and Dunajski, R. (2010). Seasonal activity patterns of seven vespertilionid bat species in Polish lowlands. Acta Theriol. 55, 301–314. doi: 10.1007/BF03193234
Corcoran, A. J., and Conner, W. E. (2014). Bats jamming bats: food competition through sonar interference. Science 346, 745–747. doi: 10.1126/science.1259512
Cvikel, N., Egert Berg, K., Levin, E., Hurme, E., Borissov, I., Boonman, A., et al. (2015). Bats aggregate to improve prey search but might be impaired when their density becomes too high. Curr. Biol. 25, 206–211. doi: 10.1016/j.cub.2014.11.010
Danchin, E., Giraldeau, L. A., Valone, T. J., and Wagner, R. H. (2004). Public information: from nosy neighbors to cultural evolution. Science 305, 487–491. doi: 10.1126/science.1098254
Dechmann, D. K. N., Heucke, S. L., iuggioli, L., Safi, K., Voigt, C. C., and Wikelski, M. (2009). Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. Biol. Sci. 276, 2721–2728. doi: 10.1098/rspb.2009.0473
Dorado-Correa, A. M., Goerlitz, H. R., and Siemers, B. M. (2013). Interspecific acoustic recognition in two European bat communities. Front. Physiol. 4:192. doi: 10.3389/fphys.2013.00192
Eaton, R. L. (1970). Hunting behavior of the cheetah. J. Wildl. Manage. 34, 56. doi: 10.2307/3799492
Eichstädt, H. (1997). Ressourcennutzung und nischengestaltung einer fledermausgemeinschaft im nordosten brandenburgs. Säugetierkundliche Mitteilungen 40, 3–171.
Fauth, J. E., Bernardo, J., Camara, M., Resetarits, W. J., Van Buskirk, J. Jr., and McCollum, S. A. (1996). Simplifying the jargon of community ecology: a conceptual approach. Am. Nat. 147, 282–286. doi: 10.1086/285850
Fenton, M. B. (1990). The foraging behaviour and ecology of animal-eating bats. Can. J. Zool. 68, 411–422. doi: 10.1139/z90-061
Fenton, M. B., and Bell, G. P. (1979). Echolocation and feeding behaviour in four species of Myotis (Chiroptera). Can. J. Zool. 57, 1271–1277. doi: 10.1139/z79-163
Fox, J. (2003). Effect displays in R for generalised linear models. J. Stat. Softw. 8, 1–27. doi: 10.2307/271037
Furmankiewicz, J., and Kucharska, M. (2009). Migration of bats along a large river alley in Southwestern Poland. J. Mammal. 90, 1310–1317. doi: 10.1644/09-MAMM-S-099R1.1
Gillam, E. H. (2007). Eavesdropping by bats on the feeding buzzes of conspecifics. Can. J. Zool. 85, 795–801. doi: 10.1139/Z07-060
Gloor, S., Stutz, H. B., and Ziswiler, V. (1995). Nutritional habits of the noctule bat Nyctalus noctula (Shreber, 1774) in Switzerland. Myotis 32–33, 231–242.
Gorman, M. L., Mills, M. G., Raath, J. P., and Speakman, J. R. (1998). High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 391, 479–481. doi: 10.1038/35131
Greene, E. (1987). Individuals in an osprey colony discriminate between high and low quality information. Nature 329, 239–241. doi: 10.1038/329239a0
Hails, C. J. (1982). A comparison of tropical and temperate aerial insect abundance. Biotropica 14, 310. doi: 10.2307/2388092
Hallmann, C. A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. doi: 10.1371/journal.pone.0185809
Hardin, G. (1960). The competitive exclusion principle. Science 131, 1292–1297. doi: 10.1126/science.131.3409.1292
Heglund, N. C., Taylor, C. R., and McMahon, T. A. (1974). Scaling stride frequency and gait to animal size: mice to horses. Science 186, 1112–1113. doi: 10.1126/science.186.4169.1112
Heim, O., Lorenz, L., Kramer-Schadt, S., Jung, K., Voigt, C. C., and Eccard, J. A. (2017). Landscape and scale-dependent spatial niches of bats foraging above intensively used arable fields. Ecol Process. 6:24. doi: 10.1186/s13717-017-0091-7
Heim, O., Schröder, A., Eccard, J., Jung, K., and Voigt, C. C. (2016). Seasonal activity patterns of European bats above intensively used farmland. Agric. Ecosyst. Environ. 233, 130–139. doi: 10.1016/j.agee.2016.09.002
Hiryu, S., Mora, E. C., and Riquimaroux, H. (2016). “Behavioral and physiological bases for doppler shift compensation by echolocating bats,” in Bat Bioacoustics, eds M. B. Fenton, A. D. Grinnell, A. N. Popper, and R. R. Fay (New York, NY: Springer), 239–263.
Holderied, M. W., Korine, C., Fenton, M. B., Parsons, S., Robson, S., and Jones, G. (2005). Echolocation call intensity in the aerial hawking bat Eptesicus bottae (Vespertilionidae) studied using stereo videogrammetry. J. Exp. Biol. 208, 1321–1327. doi: 10.1242/jeb.01528
Holekamp, K. E., Smale, L., Berg, R., and Cooper, S. M. (1997). Hunting rates and hunting success in the spotted hyena (Crocuta crocuta). J. Zool. 242, 1–15. doi: 10.1111/j.1469-7998.1997.tb02925.x
Humphries, N., Simpson, S., Wearmouth, V., and Sims, D. (2016). Two's company, three's a crowd: fine-scale habitat partitioning by depth among sympatric species of marine mesopredator. Mar. Ecol. Prog. Ser. 561, 173–187. doi: 10.3354/meps11937
Janzen, D. H., and Pond, D. M. (2009). A comparison, by sweep sampling, of the arthropod fauna of secondary vegetation in Michigan, England and Costa Rica. Trans. R. Entomol. Soc. Lond. 127, 33–50. doi: 10.1111/j.1365-2311.1975.tb00551.x
Jeltsch, F., Bonte, D., Pe'er, G., Reineking, B., Leimgruber, P., and Balkenhol, N. (2013). Integrating movement ecology with biodiversity research - exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 1:6. doi: 10.1186/2051-3933-1-6
Kalko, E. K. V. (1995). Insect pursuit, prey capture and echolocation in pipestirelle bats (Microchiroptera). Anim. Behav. 50, 861–880. doi: 10.1016/0003-3472(95)80090-5
Kiszka, J., Simon-Bouhet, B., Martinez, L., Pusineri, C., Richard, P., and Ridoux, V (2011). Ecological niche segregation within a community of sympatric dolphins around a tropical island. Mar. Ecol. Prog. Ser. 433, 273–288. doi: 10.3354/meps09165
Kunz, T. H. (1973). Resource utilization: temporal and spatial components of bat activity in Central Iowa. J. Mammal. 54, 14–32. doi: 10.2307/1378869
Levine, J. M., and HilleRisLambers, J. (2009). The importance of niches for the maintenance of species diversity. Nature 461, 254–257. doi: 10.1038/nature08251
Lubin, Y. D., Montgomery, G. G., and Young, O. P. (1977). Food resources of anteaters (Edentata: Myrmecophagidae) I. A year's census of arboreal nests of ants and termites on Barro Colorado Island, Panama canal zone. Biotropica 9, 26–34. doi: 10.2307/2387856
Mansor, M. S., and Ramli, R. (2017). Foraging niche segregation in Malaysian babblers (Family: Timaliidae). PLoS ONE 12:e0172836. doi: 10.1371/journal.pone.0172836
Meyer, C. F. J., Schwarz, C. J., and Fahr, J. (2004). Activity patterns and habitat preferences of insectivorous bats in a West African forest–savanna mosaic. J. Trop. Ecol. 20, 397–407. doi: 10.1017/S0266467404001373
Milne, D. J., Fisher, A., Rainey, I., and Pavey, C. R. (2005). Temporal patterns of bats in the top end of the Northern Territory, Australia. J. Mammal. 86, 909–920. doi: 10.1644/1545-1542(2005)86[909:TPOBIT]2.0.CO;2
Morse, D. H. (1971). The insectivorous bird as an adaptive strategy. Annu. Rev. Ecol. Syst. 2, 177–200. doi: 10.1146/annurev.es.02.110171.001141
Navarro, J., Votier, S. C., Aguzzi, J., Chiesa, J. J., Forero, M. G., and Phillips, R. A. (2013). Ecological segregation in space, time and trophic niche of sympatric planktivorous petrels. PLoS ONE 8:e62897. doi: 10.1371/journal.pone.0062897
Norberg, U. M., and Rayner, J. M. V. (1987). Ecological morphology and flight in bats (Mammalia, Chiroptera) - Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. Lond. 316, 337–419. doi: 10.1098/rstb.1987.0030
Nowacek, D. P., Friedlaender, A. S., Halpin, P. N., Hazen, E. L., Johnston, D. W., Read, A. J., et al. (2011). Super-aggregations of krill and humpback whales in Wilhelmina Bay, Antarctic Peninsula. PLoS ONE 6:e19173. doi: 10.1371/journal.pone.0019173
Plucinski, T., Zmihorski, M., and Plucinski, P. (2015). Impact of night-time crop harvesting on bat activity in agricultural landscape. Zool. Ecol. 25, 1–7. doi: 10.1080/21658005.2014.999501
Pollak, G. D. (2016). “The neural processing of frequency modulations in the auditory system of bats.” in Bat Bioacoustics, eds M. B. Fenton, A. D. Grinnell, A. N. Popper, and R. R. Fay (New York, NY: Springer), 239–263.
Roeleke, M., Blohm, T., Kramer-Schadt, S., Yovel, Y., and Voigt, C. C. (2016). Habitat use of bats in relation to wind turbines revealed by GPS tracking. Sci. Rep. 6:28961. doi: 10.1038/srep28961
Roemer, C., Disca, T., Coulon, A., and Bas, Y. (2017). Bat flight height monitored from wind masts predicts mortality risk at wind farms. Biol. Conserv. 215, 116–122. doi: 10.1016/j.biocon.2017.09.002
Saiful, A. A., Norma, Y. R., and Azarae, H. I. (2001). Niche segregation among three sympatric species of squirrels inhabiting a lowland dipterocarp forest, Peninsular Malaysia. Mamm. Stud. 26, 133–144. doi: 10.3106/mammalstudy.26.133
Salsamendi, E., Garin, I., Arostegui, I., Goiti, U., and Aihartza, J. (2012). What mechanism of niche segregation allows the coexistence of sympatric sibling rhinolophid bats? Front. Zool. 9:30. doi: 10.1186/1742-9994-9-30
Schnitzler, H. U., and Kalko, E. K. V. (2001). Echolocation by insect-eating bats. Bioscience 51, 557–569. doi: 10.1641/0006-3568(2001)051[0557:ebieb]2.0.co;2
Skiba, R. (2003). Europäische Fledermäuse : Kennzeichen, Echoortung und Detektoranwendung, 1st Edn. Hohenwarsleben: Westarp-Wiss.
Suthers, R. A. (1965). Acoustic orientation by fish-catching bats. J. Exp. Zool. 158, 319–347. doi: 10.1002/jez.1401580307
Taylor, R. J., and O'Neill, M. G. (1988). Summer activity patterns of insectivourous bats and their prey in Tasmania. Aust. Wilflife Res. 55, 533–539.
Übernickel, K., Tschapka, M., and Kalko, E. K. V. (2013). Selective eavesdropping behaviour in three neotropical bat species. Ethology 119, 66–76. doi: 10.1111/eth.12038
Vaughan, N. (1997). The diets of British bats (Chiroptera). Mamm. Rev. 27, 77–94. doi: 10.1111/j.1365-2907.1997.tb00373.x
Voigt, C. C., and Holderied, M. W. (2012). High manoeuvring costs force narrow-winged molossid bats to forage in open space. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 182, 415–424. doi: 10.1007/s00360-011-0627-6
Voigt, C. C., Lehmann, D., and Greif, S. (2015). Stable isotope ratios of hydrogen separate mammals of aquatic and terrestrial food webs. Methods Ecol. Evol. 6, 1332–1340. doi: 10.1111/2041-210X.12414
Voigt, C. C., Lindecke, O., Schönborn, S., Kramer-Schadt, S., and Lehmann, D. (2016). Habitat use of migratory bats killed during autumn at wind turbines. Ecol. Appl. 26:771–783. doi: 10.1890/15-0671
Voigt, C. C., Schuller, B.-M., Greif, S., and Siemers, B. M. (2010). Perch-hunting in insectivorous Rhinolophus bats is related to the high energy costs of manoeuvring in flight. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 180, 1079–1088. doi: 10.1007/s00360-010-0466-x
Voigt-Heucke, S. L., Taborsky, M., and Dechmann, D. K. N. N. (2010). A dual function of echolocation: bats use echolocation calls to identify familiar and unfamiliar individuals. Anim. Behav. 80, 59–67. doi: 10.1016/j.anbehav.2010.03.025
Voigt-Heucke, S. L., Zimmer, S., and Kipper, S. (2016). Does interspecific eavesdropping promote aerial aggregations in European pipistrelle bats during autumn? Ethology 122, 745–757. doi: 10.1111/eth.12519
Keywords: aerial, biodiversity, coexistence, flight, insectivore, movement, Nyctalus noctula, playback
Citation: Roeleke M, Johannsen L and Voigt CC (2018) How Bats Escape the Competitive Exclusion Principle—Seasonal Shift From Intraspecific to Interspecific Competition Drives Space Use in a Bat Ensemble. Front. Ecol. Evol. 6:101. doi: 10.3389/fevo.2018.00101
Received: 10 April 2018; Accepted: 27 June 2018;
Published: 18 July 2018.
Edited by:
Deseada Parejo, Universidad de Extremadura, SpainReviewed by:
Darryl Jones, Griffith University, AustraliaCopyright © 2018 Roeleke, Johannsen and Voigt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel Roeleke, cm9lbGVrZUBpenctYmVybGluLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.