- 1School of Biological Sciences, Faculty of Science, The University of Western Australia, Perth, WA, Australia
- 2The Oceans Graduate School, Faculty of Engineering and Mathematical Sciences, The University of Western Australia, Perth, WA, Australia
- 3The Oceans Institute, Faculty of Engineering and Mathematical Sciences, The University of Western Australia, Perth, WA, Australia
- 4Lions Eye Institute, Faculty of Health and Medical Sciences, The University of Western Australia, Perth, WA, Australia
Sensory input to the central nervous system is the primary means by which animals respond to variation in their physical and biological environments. It is well established that key threats such as habitat destruction, the introduction of non-native species, and climate change are imposing significant pressures on natural ecosystems, yet surprisingly few studies have examined how these threats impact the senses or determine species' responses to environmental change. This review focuses on how anthropogenic impacts on aquatic ecosystems can have a detrimental effect on the sensory systems of aquatic organisms and how these modalities can act to influence genetic and non-genetic (e.g., developmental) responses to environmental change, which in turn can cause knock-on effects in a range of other biological systems. Species often exhibit unique sensory specializations that are suited to their behavioral requirements; at present it is unclear whether and how sensory systems have the capacity to respond to environmental change through genetic adaptation and/or sensory plasticity, and on what timescale this might occur. Sensory systems lie at the forefront of how various species respond to environmental perturbation. As such, determining the important role they play in determining fitness is critical for understanding the effects of external processes such as habitat degradation and climate change. Given the current consensus that human impacts and environmental changes are potentially highly detrimental to the delicate balance of the biome, knowing how organisms respond, and to what degree adaptation is physiologically and behaviorally limited, warrants urgent attention.
Introduction
The most important challenge facing biologists today is understanding how animal populations respond to human impacts such as climate change, overexploitation, the introduction of non-native species, and habitat degradation (Sutherland et al., 2013). Environmental disruption is known to cause changes in the abundance and distribution of species (Moritz et al., 2008; Smale and Wernberg, 2013), which often leads to a loss of biodiversity, but populations also express phenotypic responses to environmental change (Hoffmann and Sgrò, 2011; Merilä and Hendry, 2014). Monitoring these phenotypic responses, and determining whether trait changes are based on genetic or environmental factors (or both), can reveal how altered patterns of selection affect individual fitness and population survival (Charmantier et al., 2008; Gienapp et al., 2008). However, when the relationships between the environment, phenotypic change, and the fitness of an individual organism are considered, a crucial step is missing—the response of sensory systems to environmental disruption (Figure 1). Sensory systems provide the fundamental link between the physical and biological environments of an organism and both its physiology and behavior. Sensory systems are, therefore, not only directly (and indirectly) affected by environmental change, but they mediate species-specific responses that determine individual fitness.
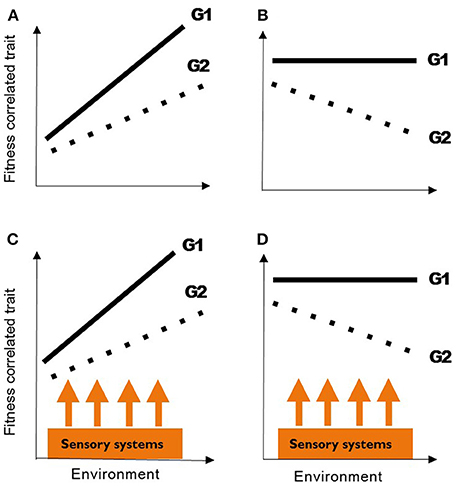
Figure 1. Phenotypic plasticity can result in different phenotypes produced by the same genotype (A G1 and G2). If a genotype maintains function under environmental stress, plasticity may allow the same phenotype to be produced [i.e., flat reaction norm; (B) G1]. The latter form of phenotypic plasticity is termed “phenotypic buffering” (Reusch, 2014). G1 shows greater phenotypic plasticity and thus has a steeper reaction norm, and higher fitness than G2 (A). Species that respond to environmental change with a decrease in fitness will undergo population decline (B: G2), while phenotypic buffering may allow others to persist (B: G1). Understanding natural variation in phenotypic traits is therefore important for predicting how populations will respond to human-impacts, such as temperature increases under climate change. Here, we suggest that the key role of sensory systems, which underpin both phenotypic plasticity (C) and phenotypic buffering (D), have been overlooked. Figure re-drawn and modified from Reusch (2014).
Sensory systems are used by organisms to perceive the physical structure and temporal dynamics of the environment, including its chemical and spectral composition, and biophysical information relating to ambient thermal, electrical and magnetic fields. Sensory systems also provide biologically important information, such as the suitability of potential habitats, the identity of conspecifics, and the risk(s) posed by predators (Collin and Marshall, 2003). Sensory systems determine the behavior of an individual, because senses such as vision, olfaction, and audition are essential for acquiring and defending resources, recognizing conspecifics, selecting mates, and avoiding predators. For example, there is a vast amount of literature on the role of chemical cues in affecting the behavior of freshwater fishes, including species recognition (Wong et al., 2005), courtship decisions (Fisher and Rosenthal, 2006), shoaling behavior (Brown and Smith, 1994), and predator avoidance (Brown, 2003). While it is well established that these behaviors are impacted by environmental change, there has been a focus on shifts in behavior rather than the responses of the underlying sensory systems that underpin these behavioral changes.
Altered environmental conditions in aquatic environments might impact sensory systems either directly or indirectly. Pollutants may directly affect the senses; for example, by directly blocking, masking or disrupting sensory reception (such as olfaction), thus leading to a shift in behaviors related to the affected sense. Indeed, sensory structures are, by necessity, directly exposed to the aquatic environment and thus materials in the water column, such as contaminants, may easily interfere with their function (Hara and Thompson, 1978). Altered environmental conditions, such as poor visibility, noise pollution and chemical contaminants, also affect the transmission, detection and reception of sensory information. Sensory systems may be impaired or less reliable if the propagation of sensory signals is impeded; for example, visual signals can be readily diminished or altered when water becomes turbid as a result of human activities (e.g., eutrophication, dredging, terrestrial run-off). Both direct and indirect affects of changed environmental conditions on sensory systems can cause changes in animal behavior. Behavioral changes can occur at different levels, and can be used as indicators of environmental disturbance (Kelley et al., 2018). Sensory systems may respond to environmental disruption via evolutionary genetic change (i.e., sensory adaptation) over a number of generations and/or via behavioral or sensory plasticity. For example, individuals may also display changes in behavior according to the perceived quality of information obtained, switching to information obtained from other senses or sensory cues that may be more reliable (Partan, 2017). Furthermore, since the senses do not act independently, many forms of environmental disturbance will have multisensory affects or disrupt a range of neurological processes, leading to changes in cognition and behavior.
In this review, we examine how environmental change can directly or indirectly (via changes in sensory signal transmission, detection, and reception) disrupt the function of sensory systems. We examine how different types of environmental disturbance are likely to have direct physiological affects on the senses, and whether these impacts result in short or long term sensory disruption. We also consider how environmental change affects signaling environments (e.g., changes in the spectral, chemical, and acoustic environments) and whether there are concomitant changes in behavior, and we ask whether we can predict the outcomes of behavioral change on individual fitness. An understanding of the link between natural environmental variation and within-species sensory system diversity (i.e., current observed environmental tolerance) is an important prerequisite for predicting the outcomes of human-induced environmental change on species' survival. We, therefore, provide examples of evolutionary adaptation and sensory plasticity in response to natural environmental variation, such as habitat diversity. Our review primarily focuses on fishes, because they are among the best-studied aquatic vertebrates, but we include other examples, where appropriate. Finally, we determine whether there is any evidence that human activities are causing evolutionary changes in sensory systems and whether sensory systems can exhibit phenotypic plasticity. We conclude by suggesting target areas for future research, particularly for sensory modalities that have been less well studied with respect to anthropogenic impacts.
Degradation of Aquatic Ecosystems and Sensory Disruption
Coastal aquatic ecosystems are vulnerable to localized activities that cause deterioration in water quality due to eutrophication, sedimentation, and contamination by metals and chemical pollutants from agriculture, pharmaceutical, and manufacturing industries. Coastal habitats are also vulnerable to environmental disruption at a global scale, such as the effects of climate change, including ocean and freshwater acidification and rising water temperatures. Ecotoxicological studies have revealed that contaminants can disrupt olfactory processes and inhibit fish behavior (Atchison et al., 1987; Scott and Sloman, 2004; Tierney et al., 2010). Common pollutants, such as surfactants that are found in household detergents (e.g., linear alkylbenzene sulphonate or LAS) are known to physically damage the gustatory receptors of yellow bullhead catfish (Ictalurus natalis), causing a diminished action potential following a few hours of exposure, before there are visible signs of histological tissue damage (Bardach et al., 1965). In whitefish (Coregonus clupeaformis), the electrical response of the olfactory bulb is diminished on exposure to the surfactant sodium lauryl sulphonate (SLS) (Hara and Thompson, 1978). The observation that whitefish were attracted to SLS at sublethal doses, but showed no preference at high concentrations, provides further evidence that SLS reduces chemoreceptor function at sublethal doses but largely blocks the response at high doses (Hara and Thompson, 1978).
Subsequent studies with Arctic charr (Salvelinus alpinus) have demonstrated the behavioral outcomes of chemosensory disruption; charr that were previously attracted to conspecific odor showed reduced or diminished preferences, depending on the concentration and duration of exposure to LAS (Olsen and Hoglund, 1985). Surfactants can affect shoaling behavior even at very low doses; for example exposure of rainbow trout (Oncorhynchus mykiss) to 0.5 μgl−1 of 4-nonylphenol (4-NP) for 1 h caused a change in association preference, while higher doses (1–2 μgl−1) caused non-dosed fish to show strong avoidance of treated conspecifics (Ward et al., 2008). In this case, short-term exposure to a chemical pollutant has not inhibited the olfactory sensitivity of this species, but exposure has still resulted in a change in social behavior with important consequences for behaviors such as foraging efficiency and predator defense.
Storm water run-off often contains metal contaminants such as copper and cadmium, and synthetic compounds such as bisphenol A (found in plastics) and polychlorinated biphenyls or PCBs, which are used in the electrical industry. These common contaminants are known to have toxicological effects on the mechanosensory lateral line system of fishes, a sensitive sensory modality that is externally located, and hence directly exposed to compounds in the surrounding environment. The mechanosensory lateral line plays an important role in behaviors such rheotaxis (body orientation relative to water flow), prey capture and shoaling (Montgomery et al., 2014). In zebrafish (Danio rerio), for example, the level of damage to the lateral line hair cells (neuromasts) depends on the concentration of dissolved copper, with some loss of hair cells at doses above 20 μg/L and almost complete cell death after 3 h of exposure at 50 μg/L (Linbo et al., 2006). Larvae that were exposed to the lower dose and transferred to uncontaminated water began to regenerate the hair calls within 24 h, while those exposed to the highest concentrations (50 μg/L) displayed permanent lateral line damage (Linbo et al., 2006). Subsequent studies with larval zebra fish have shown that exposure to both copper (CuSO4) and silver (AgNO3) metal salts is associated with a reduction in the number of neuromasts and a failure to orientate into a water current (McNeil et al., 2014). Storm water run-off is often filtered to remove chemical contaminants; this can restore lateral line development in zebrafish, but not in coho salmon (Oncorhynchus kisutch), suggesting that species likely differ in sensitivity to contaminants (Young et al., 2018).
An important source of contamination in marine environments is petroleum products such as polycyclic aromatic hydrocarbons (PAHs). A recent comprehensive study examined the effect of PAH concentration on settlement, anti-predator behavior and survival rates of larval coral reef fishes found that all of these traits were altered as a result of PAH exposure (Johansen et al., 2017). The changes in anti-predator behavior included a reduction in shoal size in some species, as well as increased movement between habitats and increased time spent in open areas (Johansen et al., 2017). Since PAHs can affect the development of the peripheral nervous system in teleosts (Irie et al., 2011), it is possible that the central nervous system may also be affected, leading to a change in behaviors associated with higher order cognition (Johansen et al., 2017).
Freshwater Acidification and Chemosensory Impairment
The processes underlying the acidification of freshwater ecosystems is relatively well known and occurs because the combustion of fossil fuels releases carbon dioxide, sulfur dioxide, and nitrogen oxides that combine with water to produce highly acidic precipitation (acid rain). A large number of studies have shown that acidification can affect the ability of freshwater fishes to respond to alarm cues, which are an important cue for predation risk in aquatic environments. Alarm cues are chemicals present in the epidermis of the skin that are released when the skin is damaged (e.g., during a predator attack) and elicit an unlearned anti-predator response in conspecifics (reviewed by Brown, 2003; Wisenden, 2003). For example, the ability of fathead minnows (Pimephales promelas), finescale dace (Phoxinus neogaeus), pumpkinseed fish (Lepomis gibbosus), rainbow trout (O. mykiss), and brook charr (Salvelinus fontinalis) to detect and respond to olfactory cues from conspecifics (alarm cues) is reduced under low pH conditions (pH 6.0–6.1) (Brown et al., 2002; Leduc et al., 2003, 2004a).
Experimental manipulation of the pH of the skin extract of minnows also results in the loss of an alarm response, suggesting that even weakly acidic conditions result in degradation of the alarm molecule, rather than the loss of olfactory sensitivity of the fish (Brown et al., 2002). A reciprocal transplant experiment, in which juvenile Atlantic salmon (Salmo salar) were reared in neutral or acidic conditions and tested under the opposite pH conditions, revealed no long-term effects of acidic conditions on the production or detection of alarm cues (Leduc et al., 2010). Thus, acidic conditions cause a reversible and short-term reduction in olfactory sensitivity, a chemical disruption of the alarm cue under acidic conditions, or a combination of both these effects suggesting that these effects are environmental, rather than physiological or behavioral (Leduc et al., 2010). The chemical composition of alarm cues includes nitrogen oxides such as hypoxanthine-3-N-oxide (Brown et al., 2000), which is converted to 6,8-dioxypurine with a loss of the 3-N-oxide group when treated with acid (Kawashima and Kumashiro, 1969), and may explain the temporary loss of alarm function (Leduc et al., 2013). Irrespective of the mechanisms involved, the loss of alarm functions has significant implications for predator-prey interactions, including the response of populations to introduced novel predators.
The olfactory systems of fishes can be highly sensitive to the chemical signals of conspecifics, and production and detection of these cues plays an important role in reproductive physiology, shoaling behavior, migration, individual recognition, and antipredator responses (Liley, 1982). Nonetheless, there is evidence that acidic conditions can diminish olfactory sensitivity in pink salmon (Oncorhynchus gorbuscha) exposed to constant [450 (control), 1,000 or 2,000 μatm] or fluctuating CO2 conditions (450–200 μatm). Salmon showed diminished growth in both freshwater and seawater, but also showed impaired olfactory responses to alarm cues, common odorants (amino acids), and a reduced response to predation risk (Ou et al., 2015). In particular, fish exposed to elevated CO2 levels showed corresponding reductions in the electro-olfactogram responses recorded at the olfactory epithelium, suggesting that CO2 acts to impair olfactory sensitivity (Ou et al., 2015). Indeed, in Atlantic salmon (S. salar) exposed to water with a reduced pH, higher concentrations of olfactory cues are required to produce a behavioral response (toward urine and testosterone, which are important mediators of social behavior) that matches the response of non-treated fish (Moore, 1994).
Ocean Acidification and Chemosensory Impairment
Recent studies examining sensory system responses to environmental change have focused on the projected impacts of climate change in marine environments, and particularly the effects of ocean acidification. Ocean acidification occurs when carbon dioxide, which is emitted from the burning of fossil fuels, enters the ocean, and combines with water to form carbonic acid. This results in the production of hydrogen ions, which react with carbonate ions and aragonite to form bicarbonate (Figure 2). This process leads to a decrease in the pH of ocean water, and a reduction in the availability of carbonate, which is required for the survival of coral reefs and invertebrates (Hoegh-Guldberg et al., 2007).
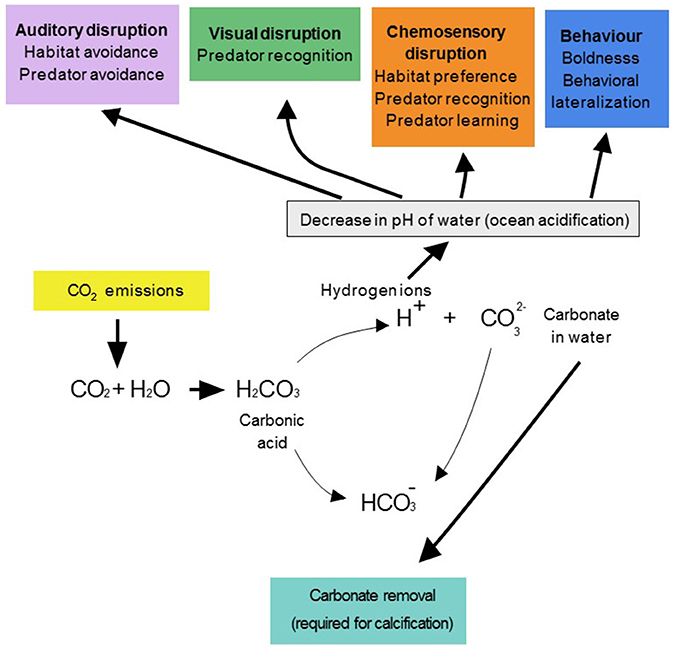
Figure 2. Excess levels of CO2 from burning fossil fuels enter the ocean and react with water to form carbonic acid. Carbonic acid disassociates into bicarbonate and hydrogen ions that decrease the pH of the water, increasing ocean acidity. Hydrogen ions also combine with carbonate to produce more carbonate, thereby reducing the availability of carbonate required for the production of calcium carbonate, a critical process for shell formation, and coral growth. Ocean acidification has been shown to disrupt behaviors mediated by auditory (purple), visual (green), and chemosensory (orange) modalities.
Munday et al. (2009) were the first to demonstrate that ocean acidification can result in loss of olfactory discrimination for preferred settlement sites. Under control conditions (current seawater pH of 8.15), orange clownfish (Amphiprion percula) preferred vegetation cues that were consistent with their settlement on tropical islands rather than vegetation cues that contained aversive oils (Munday et al., 2009). When reared under projected acidification conditions (pH 7.8), the preference for tropical vegetation settlement cues was reduced and larvae showed a strong response to vegetation that was previously aversive (Munday et al., 2009). Scanning electron microscopy revealed no difference in the surface structure of the olfactory epithelium in fish reared in low pH conditions, suggesting that the behavioral changes observed were mediated via induced changes in the transmission of chemosensory signals (via the olfactory receptor cells) rather than changes in the gross morphology of the olfactory system (Munday et al., 2009).
Importantly, a subsequent study by Munday et al. (2010) illustrated the fitness consequences of impaired olfactory discrimination, showing that damselfish larvae (Pomacentrus wardi) that had been exposed to elevated CO2 conditions (850 ppm) for 4 days had 5–9 times higher mortality rates than those exposed to current (control) CO2 conditions (390 ppm). Experiments simulating ocean acidification have also revealed changes in the ability of orange clownfish larvae to recognize and respond to olfactory cues from predators. Both newly hatched and settlement stage clownfish larvae can discriminate between the odors of predators and non-predators, but this ability is diminished in settlement stage fish exposed to CO2 acidified water (Dixson et al., 2010).
Disruption of Learning Processes: Olfactory and Visual Cues
Experience with predator olfactory cues is one of the most fundamental ways in which fishes can learn the identity of novel predators, allowing them to develop an anti-predator response toward cues that were previously unfamiliar or associated with low levels of risk (Brown, 2003; Kelley and Magurran, 2003). Studies with both marine and freshwater fishes have revealed that individuals that have been conditioned by exposing them to chemical cues (e.g., the odor from a novel predator), in conjunction with alarm cues, fail to learn a response to predators under acidic conditions (Leduc et al., 2004b; Smith et al., 2008; Ferrari et al., 2012a). In juvenile rainbow trout (O. mykiss), for example, fish learned to recognize a novel predator odor irrespective of the pH of the water, but the learned response was only retained when the pH was unchanged between the learning experience and subsequent chemical cue encounters (Smith et al., 2008). Staged encounters between rainbow trout and largemouth bass (Micropterus salmoides) have demonstrated that this disrupted response to alarm cues imposes significant survival costs, even following brief (24 h) rainfall events, which cause a rapid drop in stream pH (0.68 pH units) (Leduc et al., 2009).
Ocean acidification conditions also affect assessment of risk using visual cues. In a similar experiment, juvenile damselfish (Pomacentrus amboinensis) were exposed to visual cues from a predator of coral reef fishes i.e., adult spiny damselfish (Acanthochromis polycanthus), after being maintained at different CO2 concentrations (Ferrari et al., 2012b). Pomacentrus amboinensis displayed appropriate anti-predator behaviors toward A. polycanthus at all treatment concentrations except the highest concentration (850 μatm), where they failed to display the reduction in foraging behavior, activity levels and area use that is typically observed in fishes (Ferrari et al., 2012b). The observation that acidification affects the behavioral assessment of predation risk via both visual and olfactory cues suggests that neural (afferent) pathways are affected, rather than the peripheral sensory organs (Ferrari et al., 2012b). A subsequent study revealed that elevated CO2 levels reduces the critical flicker fusion threshold of the retina of spiny damselfish, a visual capability that allows animals to track moving stimuli that is likely important for escaping predators (Chung et al., 2014). Treatment with gabazine restored retinal function, again highlighting the importance of the GABAA receptors (see below) in mediating behavioral functions (Chung et al., 2014).
In the above mentioned studies, it is unclear how the acidification conditions (lowered pH or elevated CO2) result in the observed olfactory and visual responses. If cognitive function is affected, causing fish to engage in risky behaviors, predation rates may become high (Munday et al., 2010). The only mechanism proposed to date is based on the altered function of gamma-aminobutyric acid type A (GABA-A) receptors found in the vertebrate brain (Tresguerres and Hamilton, 2017). A study by Nilsson et al. (2012) found that exposure to gabazine (a GABA-A receptor antagonist) restored the loss of olfactory discrimination in orange clownfish (A. percula), and reversed the loss of lateralisation behavior in damselfish (Neopomacentrus azysron), that were reared in high CO2 conditions.
Elevated CO2, Aquatic Contaminants, and GABAA Receptor Function
Under conditions of high CO2, marine fishes regulate their acid balance by accumulating bicarbonate ions () and releasing chloride (Cl−) ions; regulatory changes that lead to a reversal in GABAA receptor function (inhibition to excitation) and impairment of behavioral processes (Nilsson et al., 2012; Tresguerres and Hamilton, 2017). Altered function of GABAA receptors, with corresponding effects on fish behavior, has since been reported in a number of studies on both marine and freshwater organisms (reviewed by Tresguerres and Hamilton, 2017) and is caused by a change in intracellular and extracellular levels in the brain and blood plasma (Heuer et al., 2016). However, beyond this, little is known about distribution and subunit composition of GABAA receptors in fish, or the function and regulation of other neural pathways involved in and Cl− transport (Heuer et al., 2016). For example, glycine receptors, which are responsible for generating motor patterns and spinal reflexes, are also permeable to . Therefore, these neurotransmitter pathways would most likely be affected by elevated levels of CO2, with corresponding, and potentially additive, effects on behavioral impairment (Tresguerres and Hamilton, 2017).
Studies addressing other types of human impacts, such as chemical contaminants in aquatic environments (Brodin et al., 2014), have also suggested a role for GABAA receptors. In Atlantic salmon (S. salar), smolt that were exposed to low concentrations of oxazepam (a common anxiolytic pharmaceutical agent and modulator of GABAA receptors) migrated faster than smolt that were not exposed to this agent (Hellström et al., 2016). Thus, although changes to GABAA receptor function have helped explain many of the studies reporting an effect of acidification on behavior, there is an urgent need to examine whether other neural mechanisms (including neurotransmitter pathways) are affected, what interspecific differences are present, and whether the effects vary over different stages of development (Tresguerres and Hamilton, 2017).
Ocean Acidification and Auditory Impairment
Most marine organisms are known to respond to auditory cues underwater and the acidification of aquatic environments has the ability to disrupt physiological and behavioral traits pertaining to the auditory system. Although there is no information on the impact of acidification on the auditory abilities of marine mammals, the auditory ability of bony fishes is known to be altered from elevated levels of CO2 (Ashur et al., 2017).
The mechanism of sound perception in bony fishes is mediated within the inner ear, which contains dense carbonate earbones, the otoliths. These CaCO3 concretions have an essential function in sound detection (particle acceleration) and as orientation sense organs (Tohse and Mugiya, 2001; Tohse et al., 2004). Given their composition, otoliths are susceptible to either the reduced availability of carbonate ions in seawater at low pH, or to changes in the concentrations of bicarbonate and carbonate ions caused by acid-base regulation in fish exposed to high CO2 levels (Munday et al., 2011b; Heuer and Grosell, 2014). An increase in otolith size was revealed in a range of species following exposure to as little as 64 μatm of additional CO2 compared to control levels of CO2, in species such as sea bass larvae (Atractoscion nobilis) (Checkley et al., 2009), clownfish (A. percula) larvae (Munday et al., 2011b), juvenile walleye Pollock (Theragra chalcogramma) (Hurst et al., 2012), cobia (Rachycentron canadum) larvae (Bignami et al., 2013a,b), cod (Gadus morhua) larvae (Frommel et al., 2012; Maneja et al., 2013), juvenile sticklebacks (Gasterosteus aculeatus) (Schade et al., 2014), mahi-mahi (Coryphaena hippurus) larvae (Bignami et al., 2014), juvenile sea bream (Sparus aurata) (Réveillac et al., 2015), and mulloway (Argyrosomus japonicus) larvae (Rossi et al., 2016b). However, the otoliths of juvenile spiny damselfish (Acanthochromis polyacanthus) (Munday et al., 2011a), juvenile clownfish (A. percula) (Simpson et al., 2011), Atlantic herring (Clupea harengus) larvae (Franke and Clemmesen, 2011), and juvenile scup (Stenotomus chrysops, (Perry et al., 2015) showed no size differences at increased levels of CO2, whereas the size of the otoliths in marine medaka larvae, Oryzias melastigma, were even observed to be reduced (Mu et al., 2015). This reveals that the deposition and chemical composition of fish otoliths is dependent on CO2 levels, and that the effects may be variable (depending on ocean acidification conditions), species-specific, and sensitive to the duration of the study. Although the increase in otolith size in species exposed to high CO2 levels has not yet been directly linked to behavioral endpoints, variations in the size, shape and symmetry of the otoliths will have a major bearing on the mechanotransduction process and each individual's ability to effectively detect sound (Popper and Lu, 2000; Gagliano et al., 2008). Bignami et al. (2013a) used a computer model to demonstrate that enlarged otoliths of larval cobia (R. canadum) in high CO2 conditions could affect auditory sensitivity, including a 50% increase in hearing range, which may be beneficial for perceiving biologically-relevant cues but detrimental by increasing sensitivity to background noise, hence masking ecologically vital information.
Several behavioral studies have demonstrated impaired acoustic behaviors of reef fish species in low pH conditions. Reef fish larvae use a suite of sensory cues to orient toward and discriminate between potential settlement sites, and ambient reef sounds have been shown to be an important cue for settlement (Montgomery et al., 2001; Leis et al., 2011). Ocean acidification has been shown to alter the ability of larval fishes to use these acoustic habitat cues. For example, clownfish larvae showed a change in their directional response to a predator-rich, daytime reef recording when reared in elevated CO2 conditions (Simpson et al., 2011), suggesting an impairment of auditory behavior critical for survival. Similarly, mulloway (A. japonicus) and barramundi (Lates calcarifer) larvae exposed to high CO2 levels avoided playback recordings of ambient reef sounds, while individuals reared in normal pH conditions were attracted to these habitat cues (Rossi et al., 2015, 2016b). The perception of soundscapes of reef systems was also shown to be degraded in these fish species as a result of the reduced intensity and frequency of snapping shrimp (Alpheus novaezelandiae) sounds (Rossi et al., 2016a,b), thereby, indirectly, influencing reef larval settlement.
Marine invertebrates, like cephalopods, cnidarians, and arthropods, can sense vibrational stimuli through external sensory hairs and/or statocysts. Statocysts generally include gravity and particle acceleration (sound) receptors (Maturana and Sperling, 1963; Budelmann and Williamson, 1994) and a statolith organ, which is analogous in function and structure to a fish otolith. The composition of these statoliths varies among taxa, but many are composed of calcium carbonate (Fabry et al., 2008) and therefore may be affected by ocean acidification, as is the case for the otoliths of bony fishes (see above). However, contrary to fish otoliths, which show increased growth rates under low pH/high CO2 conditions, at least in some species (Ashur et al., 2017), statolith size is reported to decrease in a range of invertebrate species including squid larvae Doryteuthis pealeii (Kaplan et al., 2013; Navarro et al., 2016), Chilean abalone larvae Concholepas concholepas (Manriquez et al., 2014) and cuttlefish hatchlings Sepia officinalis (Maneja et al., 2011) and even undergoes a change in composition in the squid larvae Loligo vulgaris and Doryteuthis opalescens (Lacoue-Labarthe et al., 2011; Navarro et al., 2014). At present, there is no information about the effects of statolith size or composition on the acoustic behavior of invertebrates.
In general, the effects of CO2 on the auditory abilities of marine organisms remain poorly understood, with only a few studies performed in bony fishes (Ashur et al., 2017). There is even less known about the impacts of acidification on mechanotransduction mechanisms in invertebrates and cartilaginous fishes (sharks, rays, skates), reptiles and marine mammals. Only one study has examined the hearing system (otolith size) in freshwater organisms, where Chinook salmon, O. tshawytscha, larvae showed incremental decreases in otolith width at reduced water pH (Geen et al., 1985). Although the same physiological impacts on the auditory system that are found in marine environments might be expected to apply to freshwater animals (Ishimatsu et al., 2008), extrapolations must be made with caution, especially as the effects may be species-specific rather than environment-dependent.
Noise Pollution and Hearing Impairment
Human-generated sound, or anthropogenic noise, is a relatively recent addition to the aquatic soundscape, driven by a range of sources, such as shipping, pile driving, seismic surveys, explosions, sonar, deep-sea mining activities, dredging and motor-powered recreational and commercial craft (Hildebrand, 2009). The sounds produced by these activities have been found to elicit behavioral reactions and shifts in many aquatic species, changes in whole populations of organisms and even physical injury (Kunc et al., 2016). The level of noise in the sea has been linked to the global economy (Frisk, 2012), whereby shipping constitutes 90% of the method of trade between different countries, and it is certain to continue to increase as the ocean becomes more industrialized. Many excellent reviews exist on the effects of aquatic noise on marine mammals, bony fishes and invertebrates (Nowacek et al., 2007; Weilgart, 2007; Popper and Hastings, 2009; Slabbekoorn et al., 2010; Hawkins and Popper, 2014; Hawkins et al., 2014a,b; Radford et al., 2014; Whitfield and Becker, 2014; Braun, 2015; Peng et al., 2015; Williams et al., 2015; Zakon, 2015; de Soto, 2016; de Soto and Kight, 2016; Gomez et al., 2016; Kunc et al., 2016; Juanes et al., 2017), so the following information represents only a short synopsis.
The hearing abilities of aquatic organisms vary in their absolute sensitivity and frequency range. Most fishes are sensitive to low frequencies, from 50 Hz to about 5 kHz (Ladich and Fay, 2013), while marine mammals exhibit much larger hearing ranges, between 1 and 150 kHz (Dehnhardt, 2002; Weilgart, 2007). Almost nothing is known about hearing in aquatic invertebrates, although they most likely can only detect very low frequency sounds (Hawkins and Popper, 2014; Hawkins et al., 2014a). Similarly, there is little information on marine reptiles, although sea turtles have a peak sensitivity at about 500 Hz (Willis, 2016). Different sources of anthropogenic noise will affect each taxon differently, depending on the temporal, spatial, and frequency signature of the sound sources. For example, noise from shipping contains mainly low frequency components (<1,000 Hz) (Peng et al., 2015) and may, therefore, be more detrimental to organisms with a peak sensitivity that lies within this range, like bony fishes, and invertebrates.
Anthropogenic noise can have physiological, developmental and behavioral consequences on the hearing systems and acoustic behavior of an individual (Kunc et al., 2016). Several studies have reported damage of sensory hair cells, barotrauma and hearing loss in both freshwater and marine bony fishes (McCauley et al., 2003; Popper et al., 2005; Halvorsen et al., 2012a,b,c; Casper et al., 2013; Smith and Monroe, 2016), damage to the statocysts of several squid species (Guerra et al., 2004; André et al., 2011), as well as hearing loss and inner ear injuries in marine mammals (Ketten et al., 1993; Ketten, 1995; Southall et al., 2007; Weilgart, 2007). Seismic surveys, which blast compressed air to produce pulses of sound that can probe the sea floor for natural resources, have also caused extensive damage to the inner ears of pink snapper (Pagrus auratus) (McCauley et al., 2003). The operation of airguns has also been found to elicit behavioral changes in marine fishes, including movement to the bottom of the water column and fast swimming (Fewtrell and McCauley, 2012). Similarly, squid (Sepiotheuthis australias) responded to the sounds of airguns with alarm responses, changes in swimming patterns and vertical migration (Fewtrell and McCauley, 2012). McCauley et al. (2017) recently showed that seismic surveys killed zooplankton up to 1.2 km away from the airgun source.
Noise pollution can also cause changes in acoustic behavior when it masks essential information, distracts individuals or alters intraspecific communication (Kunc et al., 2016). Sound plays an important role in communication for aquatic organisms given the sound transmission properties of water and the inevitable constraints of light attenuation, which inhibit visual communication in some environments. The underwater soundscape contains essential information for survival of all taxa including cues to aid in migration, orientation, settlement, predator-prey interactions, and locating reproductive partners. These acoustic cues have the potential to be masked by anthropogenic noise, causing disruption of normal acoustic behaviors (see reviews by Radford et al., 2014; Erbe et al., 2016). For example, the amplitude of acoustic communication signals emitted by killer (Orcinus orca) and beluga (Delphinapterus leucas) whales has been shown to increase in the presence of ship noise (Scheifele et al., 2005; Holt et al., 2011). The tendency for signal producers to enhance the amplitude of communication signals under noisy conditions has been shown in marine mammals and fishes (Radford et al., 2014), but it is not clear whether sensory systems can be altered (e.g., increased sensitivity) to enhance signal reception. It has been suggested that marine mammals, such as pinnipeds, have evolved low signal-to-noise ratios as an adaptation for sound detection in noisy marine environments (Southall et al., 2000), but it is unclear whether this is the case in fishes. The response of fish larvae to natural reef sounds is also disrupted by boating noise, with implications for settlement and populations in coral reef habitats (Holles et al., 2013). Playback recordings of ship noise even increased the risks of starvation and predation for shore crabs (Carcinus maenus) in captivity (Wale et al., 2013), and damselfish (P. amboinensis) were more readily captured by their natural predators during exposure to motorboat noise (Simpson et al., 2016). Moreover, anthropogenic noise can also distract individuals: i.e., hermit crabs (Coenobia clypeatus) making them more vulnerable to predation (Chan et al., 2010a,b). Interestingly, noise can affect behavior across sensory modalities i.e., cuttlefish (S. officinalis), although not reliant on acoustic communication, changed their visual signals (body coloration) when exposed to anthropogenic noise (Kunc et al., 2014). Considering multiple sensory channels may be important to understand the broad implications of anthropogenic sound on aquatic organisms.
Degraded Optical Habitats and Behavior
Human activities such as agriculture, forestry, urbanization, and resource extraction often cause a change in water quality, such as increased turbidity, that can have a major effect on behavioral traits and individual fitness. Many aquatic vertebrates rely on vision for basic behavioral tasks such as finding food, selecting mates and avoiding predators (Guthrie, 1986). However, shifts in the optical quality of water can have a critical effect on population survival, species composition and ecosystem biodiversity. Light attenuates with depth depending on the particular absorbance properties of the water and the presence of dissolved organic matter (e.g., “humic substances,” such as tannins), phytoplankton, and particulate matter (Kirk, 1994). The nature of the habitat disruption will therefore determine how light is scattered and absorbed. For example, the introduction of suspended particles due to soil erosion increases the scattering of light, while eutrophication increases the algal load of the water and promotes the absorption of light with depth (Kirk, 1994).
Changes in the specific optical properties of the water, can lead to shifts in a particular behavior and this is particularly well known in freshwater ecosystems, where light environments tend to be more dynamic than in oceanic or coastal ecosystems. For example, increased turbidity can decrease the reaction distance of pike (Esox Lucius) predators, but increase the escape distance of roach (Rutilus rutilus) prey, suggesting that changes in the visual range alter the outcome of predator-prey interactions (Ranaker et al., 2012). Shoaling brings important anti-predator benefits that may be diminished under turbid conditions; shoals are less cohesive and individuals behave more like lone fish when visual contact among shoal members is reduced (Kimbell and Morrell, 2015). Shoaling behavior is also affected by changes in the spectral composition of water. For example, in western rainbowfish (Melanotaenia australis), individuals in environments rich in organic matter (which selectively absorbs short wavelength light) shoal further apart than those in water with full spectrum lighting (Kelley et al., 2012). In this study, rainbowfish increased the area and brightness of their coloration, allowing them to compensate for a change in signaling conditions and maintain visual communication among conspecifics (Kelley et al., 2012).
Several studies have revealed how turbidity can affect mate choice, and the signals used to convey male quality. In three-spine sticklebacks (G. aculeatus), poor quality males are less likely to curtail their courtship display in the presence of a competitor in turbid water than when in clear water, suggesting that courtship effort is a less reliable indicator of male quality under compromised visual conditions. Female behavior is also affected by turbidity in this species, where females are less attracted to males in turbid water than in clear water, and males compensate for this by heightening their courtship activity in turbid water (Engström-Öst and Candolin, 2006). Although the transmission of visual signals is clearly compromised in these environments, it is not clear whether turbidity causes short term changes in visual sensitivity (but see Plasticity in sensory systems, below) However, the reduced availability of visual cues does not necessarily mean that individuals will become increasingly reliant on other modalities as a compensation mechanism. For example, in broad-nosed pipefish (Syngnathus typhle), males spent longer assessing females in clear water than in turbid water, and the presence of olfactory cues did not compensate for the reduced availability of visual cues (Sundin et al., 2010). In addition, the optical properties of the water, such as the presence of humic substances, can also alter the water's chemical attributes. This can significantly disrupt chemical communication and facilitate hybridization between fish species, as has been described in swordtails, Xiphophorus spp. (Fisher et al., 2006). Similarly, if turbidity causes a breakdown in the cues used for mate choice, as in the case of African cichlids, sexual selection is relaxed and there is a loss of species diversity (Seehausen et al., 1997). Collectively, these studies illustrate the complex nature of the relationship between the optical and chemical properties of the water and the role of the light environment in mediating behavior.
Adaptive Evolution of Sensory Systems
Variation in the environmental conditions (i.e., predation risk, habitat structure) can result in strong selection on animal sensory systems (Endler, 1991). Threespine sticklebacks (G. aculeatus) that inhabit a diversity of aquatic habitats provide an excellent illustration of evolutionary divergence among populations of a single species. For example, differences in the mechanosensory lateral line system of lake-inhabiting sticklebacks varies depending on whether populations are benthic or limnetic (Wark and Peichel, 2010) and is largely based on heritable (genetic) variation (Wark et al., 2012). Although sticklebacks occupy lake habitats with a variety of photic conditions, there is limited variation in their visual pigment protein (opsin) sequences, with no amino acid differences occurring at functionally relevant tuning sites (Flamarique et al., 2013). However, adaptive divergence of the visual system has occurred between marine and freshwater populations via shifts in the levels of opsin gene expression, which have been linked with changes in photic conditions associated with marine sticklebacks' colonization of freshwater habitats (Rennison et al., 2016). Classic work with Lake Victoria cichlids has revealed how human impacts, such as eutrophication, can alter sensory-mediated processes of selection; turbidity relaxes color-mediated mate choice causing a breakdown in assortative mating and a loss of species diversity (Seehausen et al., 1997). While the genetic basis of senses other than vision is yet to be resolved, it is likely that other sensory systems will exhibit rapid evolutionary responses to human disturbances. Indeed, it is becoming apparent that sensory pollution, such as light and sound, can cause rapid evolutionary change in a variety of physiological and behavioral traits that are ultimately underpinned by sensory system responses (Swaddle et al., 2015).
Plasticity in Sensory Systems
It is becoming increasingly apparent that plasticity in animal sensory systems can determine fitness and have important implications for selection (Ronald et al., 2012). The CNS of fishes exhibits indeterminate growth, potentially allowing these taxa a critical advantage when dealing with environmental challenges. For example, fishes often exhibit changes in their retinal morphology across different life history stages (Shand et al., 1999, 2008) and show seasonal shifts in peripheral auditory frequency sensitivity associated with reproductive activities (Sisneros and Bass, 2003). Gymnotiform fishes, such as Brachyhypopomus gauderio, exhibit variation in electrocommunication signals that is dependent on sex, body condition and social experience (Salazar and Stoddard, 2008, 2009). While freshwater threespine sticklebacks (G. aculeatus) exhibit limited plasticity in the expression of their opsin genes (Flamarique et al., 2013; Rennison et al., 2016), studies with the bluefin killifish, Lucania goodie, have revealed rapid (after 1–3 days) switches in opsin expression corresponding with changes in lighting conditions (clear water vs. tea-stained treatments) (Fuller and Claricoates, 2011). Indeed, shifts in opsin expression, along with changes in chromatophore use (vitamin A1:A2 ratios), provide a mechanism for fishes to adapt to developmental stages and life history patterns (Temple et al., 2006; Shand et al., 2008).
There is also some evidence for plasticity in the development of the lateral line system in response to environmental variation. For example, exposure to altered water flows can cause a shift in the abundance of neuromasts (specialized cells for detecting water motion) over particular regions of the body of rainbowfishes (M. australis) (Kelley et al., 2017). Understanding the plasticity of sensory systems such as the mechanosensory lateral line is crucial for understanding and managing how fishes respond to physical hydrodynamic changes in the environment, i.e., altered patterns of migration due to dam construction (Goodwin et al., 2014). Given the numerous studies showing disruption of sensory systems and associated behaviors in aquatic systems, we understand surprisingly little of the role of rapid evolutionary change and phenotypic plasticity in facilitating species' responses to human impacts in these environments.
Sensory Switching Under Environmental Change
Sensory switching, or compensation, occurs when animals rely on alternative sensory modes (compensatory plasticity hypothesis), due to information limitation or sensory masking (e.g., turbidity and background noise), or because the sense organs become damaged. Sensory compensation is thus a form of plasticity that allows individuals to switch between modalities and gain the most information in a given sensory environment. In threespine sticklebacks (G. aculeatus), females rely on visual cues to select males in clear water, but switch to olfactory cues under turbid conditions (Heuschele et al., 2009). Furthermore, female preferences for male size are dependent on which sensory cues are available (Heuschele et al., 2009), suggesting that processes such as eutrophication can alter selection on courtship displays (Engström-Öst and Candolin, 2006; Wong et al., 2007). Some fishes migrate between water bodies of varying salinity, providing an opportunity to examine whether individuals rely on different sensory modalities to maintain important behaviors such as shoal cohesion. The Pacific blue-eye (Pseudomugil signifier), which occupies habitats ranging in salinity, responds to chemical cues from conspecifics, and forms tighter shoals in freshwater than in saltwater (Herbert-Read et al., 2010).
Summary and Conclusions
In this review, we find evidence that different forms of habitat disturbance can disrupt the sensory systems of aquatic vertebrates and invertebrates, with specific types of disruptor affecting species-specific behaviors. This makes it challenging to predict and manage these impacts at the community level, because species may exhibit different or opposing responses (or show no overt change in behavior), leading to altered ecological interactions, such as predator-prey relationships. Nonetheless, we suggest that significant insights will be gained when the sensory cues underlying a specific fitness-related behavior are known, although this is rarely likely to be the case. In some instances, the effects of human-induced environmental change on sensory systems are relatively well studied, such as the effect of ocean acidification on olfactory discrimination and predator recognition. Importantly, progress in this area has revealed that altered function of the GABAA receptors in the brain explains at least some of these behavioral impairments. Nonetheless, this research is in its infancy, and it is also possible that other neurotransmitters or neurological pathways are affected but are yet to be discovered (Tresguerres and Hamilton, 2017).
We have shown that many studies on species' responses to human-induced environmental change are focused on behavior. This is because a change in behavior is typically the first observed response to an environmental disruption (Tuomainen and Candolin, 2011), and is probably also the easiest to measure in situ. However, neurophysiological studies and knowledge of a species' sensory biology can provide essential information on functional and mechanistic responses at all scales, which can ultimately inform conservation strategies (Cooke et al., 2013). With the development of biosensors to directly monitor animal physiology, it is now easier to assess variation in function and tolerance among individuals, populations and species and more studies of this nature are required to understand the effect of environmental change on sensory physiology. In particular, most of the work has focussed on aquatic vertebrates and there is a clear lack of information concerning the response of invertebrates to human induced environmental change.
Our review of the literature has revealed many areas where knowledge of a species' sensory biology and basic biology are lacking. For example, light is not only essential for visual processes, but also guides behaviors that do not rely on directional monitoring of light, such as photokinesis (locomotory responses to light) and the entrainment of circadian rhythms (Land and Nilsson, 2012). A reduction in water quality that results in reduced light intensity, or a change in spectral composition, could disrupt fundamental biological processes such as sleep, activity patterns, and body colouration (Collin and Hart, 2015). This might be more likely in environments with reduced availability of short wavelength light (e.g., water rich in organic matter), because of the role of non-visual pigments such as melanopsin (with a sensitivity of ~475–500 mn), but studies are yet to be conducted (Collin and Hart, 2015). Similarly, light pollution has the potential to disrupt basic biological functioning in many organisms, but research tends to focus on particular taxa, such the impacts of artificial light on the movement trajectories of green turtle (Chelonia mydas) hatchlings (Thums et al., 2016).
There is a particular need to understand the impacts of human activities on species with sensory modalities that are less well understood. For example, we know little of the potential effects of electromagnetic fields (such as those produced by underwater cables) on the behavior of electroreceptive fishes and sharks (Orr, 2016), although there is evidence that diadromous eels, Anguilla spp., that use magnetic fields for migration, display altered swimming patterns when passing over subsea cables (Gill et al., 2012). More research is required on the effects of aquatic pollutants on fish taste buds and chemoreceptive systems (in marine and freshwater systems), particularly in species where olfactory cues play an important part in behaviors such as larval settlement and habitat choice. While ocean noise, such as seismic surveys, is known to affect acoustic communication in cetaceans (e.g., Di Iorio and Clark, 2010), less is known about these effects on other animals, such as teleost fishes. Many sensory systems operate under specific physiochemical thresholds, yet it is unclear how the increased warming of the oceans, for example, will affect these sensory thresholds.
An understanding of animal sensory biology is not only required to understand how species respond to human-induced environmental change, but also has the potential to be an important tool for conservation management. Such knowledge may partly explain the invasion success of exotic species, and hence be used to facilitate management strategies. For example, invasive Western mosquitofish (Gambusia affinis) are able to maintain their foraging efficiency across a range of optical water conditions, while native species, such as the New Zealand Inanga (Galaxias maculatus), display highly impaired foraging efficiency under turbid conditions (Abrahams et al., 2017). Thus, an understanding of sensory biology, combined with knowledge of the role of the senses in fitness-related behaviors (and plasticity in these traits), can be used to manage human impacts on marine and freshwater organisms.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge logistical support from The University of Western Australia (JK). The work was financially supported by Australian Research Council grants to WD (FT110100176 and DP140102117) and SC (LP120200002).
References
Abrahams, M. V., Bassett, D. K., and Montgomery, J. C. (2017). Sensory biology as a risk factor for invasion success and native fish decline. Trans. Am. Fish. Soc. 146, 1238–1244. doi: 10.1080/00028487.2017.1353545
André, M., Solé, M., Lenoir, M., Durfort, M., Quero, C., Mas, A., et al. (2011). Low-frequency sounds induce acoustic trauma in cephalopods. Front. Ecol. Environ. 9, 489–493. doi: 10.1890/100124
Ashur, M. M., Johnston, N. K., and Dixson, D. L. (2017). Impacts of ocean acidification on sensory function in marine organisms. Integr. Comp. Biol. 57, 63–63. doi: 10.1093/icb/icx010
Atchison, G. J., Henry, M. G., and Sandheinrich, M. B. (1987). Effects of metals on fish behavior: a review. Environ. Biol. Fishes 18, 11–25. doi: 10.1007/BF00002324
Bardach, J. E., Fujiya, M., and Holl, A. (1965). Detergents: effects on the chemical senses of the fish Ictalurus natalis (le Sueur). Science 148, 1605–1607. doi: 10.1126/science.148.3677.1605
Bignami, S., Enochs, I. C., Manzello, D. P., Sponaugle, S., and Cowen, R. K. (2013a). Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc. Natl. Acad. Sci. U.S.A. 110, 7366–7366. doi: 10.1073/pnas.1301365110
Bignami, S., Sponaugle, S., and Cowen, R. K. (2013b). Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Glob. Chang. Biol. 19, 996–996. doi: 10.1111/gcb.12133
Bignami, S., Sponaugle, S., and Cowen, R. K. (2014). Effects of ocean acidification on the larvae of a high-value pelagic fisheries species, mahi-mahi Coryphaena hippurus. Aquat. Biol. 21, 249–249. doi: 10.3354/ab00598
Braun, C. B. (2015). Signals and noise in the octavolateralis systems: what is the impact of human activities on fish sensory function? Integr. Zool. 10, 4–14. doi: 10.1111/1749-4877.12092
Brodin, T., Piovano, S., Fick, J., Klaminder, J., Heynen, M., and Jonsson, M. (2014). Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 369:20130580. doi: 10.1098/rstb.2013.0580
Brown, G. E. (2003). Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 4, 227–234. doi: 10.1046/j.1467-2979.2003.00132.x
Brown, G. E., Adrian, J. C., Smyth, E., Leet, H., and Brennan, S. (2000). Ostariophysan alarm pheromones: laboratory and field tests of the functional significance of nitrogen oxides. J. Chem. Ecol. 26, 139–154. doi: 10.1023/A:1005445629144
Brown, G. E., Adrian, J., James, C., Lewis, M. G., and Tower, J. M. (2002). The effects of reduced pH on chemical alarm signalling in ostariophysan fishes. Canad. J. Fish. Aquat. Sci. 59, 1331–1338. doi: 10.1139/f02-104
Brown, G. E., and Smith, R. J. (1994). Fathead minnows use chemical cues to discriminate natural shoalmates from unfamiliar conspecifics. J. Chem. Ecol. 20, 3051–3061. doi: 10.1007/BF02033710
Budelmann, B. U., and Williamson, R. (1994). Directional sensitivity of hair cell afferents in the Octopus statocyst. J. Exp. Biol. 187, 245–259.
Casper, B. M., Smith, M. E., Halvorsen, M. B., Sun, H., Carlson, T. J., and Popper, A. N. (2013). Effects of exposure to pile driving sounds on fish inner ear tissues. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 166, 352–360. doi: 10.1016/j.cbpa.2013.07.008
Chan, A. A. -H., David Stahlman, W., Garlick, D., Fast, C. D., Blumstein, D. T., and Blaisdell, A. P. (2010a). Increased amplitude and duration of acoustic stimuli enhance distraction. Anim. Behav. 80, 1075–1079. doi: 10.1016/j.anbehav.2010.09.025
Chan, A. A. Y, -H., Giraldo-Perez, P., Smith, S., and Blumstein, D. T. (2010b). Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461. doi: 10.1098/rsbl.2009.1081
Charmantier, A., Mccleery, R. H., Cole, L. R., Perrins, C., Kruuk, L. E., and Sheldon, B. C. (2008). Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. doi: 10.1126/science.1157174
Checkley, D. M., Dickson, A. G., Takahashi, M., Radich, J. A., Eisenkolb, N., and Asch, R. (2009). Elevated CO2 enhances otolith growth in young fish. Science 324, 1683–1683. doi: 10.1126/science.1169806
Chung, W.-S., Marshall, N. J., Watson, S.-A., Munday, P. L., and Nilsson, G. E. (2014). Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. J. Exp. Biol. 217, 323–326. doi: 10.1242/jeb.092478
Collin, S. P., and Hart, N. S. (2015). Vision and photoentrainment in fishes: the effects of natural and anthropogenic perturbation. Integr. Zool. 10, 15–28. doi: 10.1111/1749-4877.12093
Collin, S. P., and Marshall, N. J. (2003). Sensory Processing in Aquatic Environments. New York, NY: Springer-Verlag.
Cooke, S. J., Sack, L., Franklin, C. E., Farrell, A. P., Beardall, J., Wikelski, M., et al. (2013). What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv. Physiol. 1:cot001. doi: 10.1093/conphys/cot001
de Soto, N. A. (2016). “Peer-reviewed studies on the effects of anthropogenic noise on marine invertebrates: from scallop larvae to giant squid,” in The Effects of Noise on Aquatic Life II, eds A. N. Popper and A. Hawkins (New York, NY: Springer), 17–26.
de Soto, N. A., and Kight, C. (2016). “Physiological effects of noise on aquatic animals,” in Stressors in the Marine Environment, eds M. Solan and N. M. Whiteley (New York, NY: Oxford Univ Press), 135–158. doi: 10.1093/acprof:oso/9780198718826.003.0008
Dehnhardt, G. (2002). “Sensory systems,” in Marine Mammal Biology, ed A. R. Hoelzel (Oxford: Blackwell Science Ltd.), 116–141.
Di Iorio, L., and Clark, C. W. (2010). Exposure to seismic survey alters blue whale acoustic communication. Biol. Lett. 6, 51–54. doi: 10.1098/rsbl.2009.0651
Dixson, D. L., Munday, P. L., and Jones, G. P. (2010). Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75. doi: 10.1111/j.1461-0248.2009.01400.x
Endler, J. A. (1991). Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vision Res. 31, 587–608. doi: 10.1016/0042-6989(91)90109-I
Engström-Öst, J., and Candolin, U. (2006). Human-induced water turbidity alters selection on sexual displays in sticklebacks. Behav. Ecol. 18, 393–398. doi: 10.1093/beheco/arl097
Erbe, C., Reichmuth, C., Cunningham, K., Lucke, K., and Dooling, R. (2016). Communication masking in marine mammals: a review and research strategy. Mar. Pollut. Bull. 103, 15–38. doi: 10.1016/j.marpolbul.2015.12.007
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432. doi: 10.1093/icesjms/fsn048
Ferrari, M. C. O., Mccormick, M. I., Munday, P. L., Meekan, M. G., Dixson, D. L., Lönnstedt, O., et al. (2012b). Effects of ocean acidification on visual risk assessment in coral reef fishes. Funct. Ecol. 26, 553–558. doi: 10.1111/j.1365-2435.2011.01951.x
Ferrari, M. C., Manassa, R. P., Dixson, D. L., Munday, P. L., Mccormick, M. I., Meekan, M. G., et al. (2012a). Effects of ocean acidification on learning in coral reef fishes. PLoS ONE 7:e31478. doi: 10.1371/journal.pone.0031478
Fewtrell, J. L., and McCauley, R. D. (2012) Impact of air gun noise on the behaviour of marine fish squid. Mar. Pollut. Bull. 64, 984–993. doi: 10.1016/j.marpolbul.2012.02.009
Fisher, H. S., and Rosenthal, G. G. (2006). Female swordtail fish use chemical cues to select well-fed mates. Anim. Behav. 72, 721–725. doi: 10.1016/j.anbehav.2006.02.009
Fisher, H. S., Wong, B. B., and Rosenthal, G. G. (2006). Alteration of the chemical environment disrupts communication in a freshwater fish. Proc. R. Soc. B Biol. Sci. 273, 1187–1193. doi: 10.1098/rspb.2005.3406
Flamarique, I. N., Cheng, C. L., Bergstrom, C., and Reimchen, T. E. (2013). Pronounced heritable variation and limited phenotypic plasticity in visual pigments and opsin expression of threespine stickleback photoreceptors. J. Exp. Biol. 216, 656–667. doi: 10.1242/jeb.078840
Franke, A., and Clemmesen, C. (2011). Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.). Biogeosci. Discuss. 8, 7097–7097. doi: 10.5194/bgd-8-7097-2011
Frisk, G. V. (2012). Noiseonomics: the relationship between ambient noise levels in the sea and global economic trends. Sci. Rep. 2, 437–437. doi: 10.1038/srep00437
Frommel, A. Y., Schubert, A., Piatkowski, U., and Clemmesen, C. (2012). Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar. Biol. 160, 1825–1825. doi: 10.1007/s00227-011-1876-3
Fuller, R. C., and Claricoates, K. M. (2011). Rapid light-induced shifts in opsin expression: finding new opsins, discerning mechanisms of change, and implications for visual sensitivity. Mol. Ecol. 20, 3321–3335. doi: 10.1111/j.1365-294X.2011.05180.x
Gagliano, M., Depczynski, M., Simpson, S. D., and Moore, J. A. (2008). Dispersal without errors: symmetrical ears tune into the right frequency for survival. Proc. R. Soc. B Biol. Sci. 275, 527–527. doi: 10.1098/rspb.2007.1388
Geen, G. H., Neilson, J. D., and Bradford, M. (1985). Effects of pH on the early development and growth and otolith microstructure of chinook salmon, Oncorhynchus tshawytscha. Can. J. Zool. 63, 22–22. doi: 10.1139/z85-005
Gienapp, P., Teplitsky, C., Alho, J. S., Mills, J. A., and Merila, J. (2008). Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. doi: 10.1111/j.1365-294X.2007.03413.x
Gill, A. B., Bartlett, M., and Thomsen, F. (2012). Potential interactions between diadromous fishes of U.K. conservation importance and the electromagnetic fields and subsea noise from marine renewable energy developments. J. Fish Biol. 81, 664–695. doi: 10.1111/j.1095-8649.2012.03374.x
Gomez, C., Lawson, J. W., Wright, A. J., Buren, A. D., Tollit, D., and Lesage, V. (2016). A systematic review on the behavioural responses of wild marine mammals to noise: the disparity between science and policy. Can. J. Zool. 94, 801–819. doi: 10.1139/cjz-2016-0098
Goodwin, R. A., Politano, M., Garvin, J. W., Nestler, J. M., Hay, D., Anderson, J. J., et al. (2014). Fish navigation of large dams emerges from their modulation of flow field experience. Proc. Natl. Acad. Sci. U.S.A. 111, 5277–5282. doi: 10.1073/pnas.1311874111
Guerra, A., Gonzales, A. F., and Rocha, F. (2004). “A review of records of giant squid in the Northeastern Atlantic and severe injuries in Architeuthis dux stranded after acoustic exploration,” in ICES Annual Science Conference (Vigo), 1–17.
Guthrie, D. M. (1986). “Role of vision in fish behaviour,” in The Behaviour of Teleost Fishes, ed T. J. Pitcher (Beckenham: Crrom Helm Ltd.), 553.
Halvorsen, M. B., Casper, B. M., Matthews, F., Carlson, T. J., and Popper, A. N. (2012a). Effects of exposure to pile-driving sounds on the lake sturgeon, Nile tilapia and hogchoker. Proc. R. Soc. B Biol. Sci. 279, 4705–4714. doi: 10.1098/rspb.2012.1544
Halvorsen, M. B., Casper, B. M., Woodley, C. M., Carlson, T. J., and Popper, A. N. (2012b). Threshold for onset of injury in chinook salmon from exposure to impulsive pile driving sounds. PLoS ONE 7:e38968. doi: 10.1371/journal.pone.0038968
Halvorsen, M. B., Zeddies, D. G., Ellison, W. T., Chicoine, D. R., and Popper, A. N. (2012c). Effects of mid-frequency active sonar on hearing in fish. J. Acoust. Soc. Am. 131, 599–607. doi: 10.1121/1.3664082
Hara, T. J., and Thompson, B. E. (1978). Reaction of whitefish, coregonus-clupeaformis, to anionic detergent sodium lauryl sulfate and its effects on their olfactory responses. Water Res. 12, 893–897. doi: 10.1016/0043-1354(78)90042-8
Hawkins, A. D., and Popper, A. N. (2014). Assessing the impacts of underwater sounds on fishes and other forms of marine life. Acoust. Today 10, 30–41.
Hawkins, A. D., Pembroke, A. E., and Popper, A. N. (2014a). Information gaps in understanding the effects of noise on fishes and invertebrates. Rev. Fish Biol. Fish. 25, 39–64. doi: 10.1007/s11160-014-9369-3
Hawkins, A. D., Roberts, L., and Cheesman, S. (2014b). Responses of free-living coastal pelagic fish to impulsive sounds. J. Acoust. Soc. Am. 135, 3101–3116. doi: 10.1121/1.4870697
Hellström, G., Klaminder, J., Finn, F., Persson, L., Alanärä, A., Jonsson, M., et al. (2016). GABAergic anxiolytic drug in water increases migration behaviour in salmon. Nat. Commun. 7:13460. doi: 10.1038/ncomms13460
Herbert-Read, J. E., Logendran, D., and Ward, A. J. W. (2010). Sensory ecology in a changing world: salinity alters conspecific recognition in an amphidromous fish, Pseudomugil signifer. Behav. Ecol. Sociobiol. 64, 1107–1115. doi: 10.1007/s00265-010-0925-0
Heuer, R. M., and Grosell, M. (2014). Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1061–R1084. doi: 10.1152/ajpregu.00064.2014
Heuer, R. M., Welch, M. J., Rummer, J. L., Munday, P. L., and Grosell, M. (2016). Altered brain ion gradients following compensation for elevated CO(2) are linked to behavioural alterations in a coral reef fish. Sci. Rep. 6:33216. doi: 10.1038/srep33216
Heuschele, J., Mannerla, M., Gienapp, P., and Candolin, U. (2009). Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 20, 1223–1227. doi: 10.1093/beheco/arp123
Hildebrand, J. A. (2009). Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 395, 5–20. doi: 10.3354/meps08353
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Hoffmann, A. A., and Sgrò, C. M. (2011). Climate change and evolutionary adaptation. Nature 470, 479–485. doi: 10.1038/nature09670
Holles, S. H., Simpson, S. D., Radford, A. N., Berten, L., and Lecchini, D. (2013). Boat noise disrupts orientation behaviour in a coral reef fish. Mar. Ecol. Prog. Ser. 485, 295–300. doi: 10.3354/meps10346
Holt, M. M., Noren, D. P., and Emmons, C. K. (2011). Effects of noise levels and call types on the source levels of killer whale calls. J. Acoust. Soc. Am. 130, 3100–3106. doi: 10.1121/1.3641446
Hurst, T. P., Fernandez, E. R., Mathis, J. T., Miller, J. A., Stinson, C. M., and Ahgeak, E. F. (2012). Resiliency of juvenile walleye pollock to projected levels of ocean acidification. Aquat. Biol. 17, 247–259. doi: 10.3354/ab00483
Irie, K., Kawaguchi, M., Mizuno, K., Song, J. Y., Nakayama, K., Kitamura, S., et al. (2011). Effect of heavy oil on the development of the nervous system of floating and sinking teleost eggs. Mar. Pollut. Bull. 63, 297–302. doi: 10.1016/j.marpolbul.2011.04.018
Ishimatsu, A., Hayashi, M., and Kikkawa, T. (2008). Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–295. doi: 10.3354/meps07823
Johansen, J. L., Allan, B. J. M., Rummer, J. L., and Esbaugh, A. J. (2017). Oil exposure disrupts early life-history stages of coral reef fishes via behavioural impairments. Nat. Ecol. Evol. 1, 1146–1152. doi: 10.1038/s41559-017-0232-5
Juanes, F., Cox, K., Brennan, L., and Dudas, S. (2017). The effect of anthropogenic and biological noise on fish behavior and physiology: a meta-analysis. J. Acoust. Soc. Am. 141, 3862–3862. doi: 10.1121/1.4988626
Kaplan, M. B., Mooney, T. A., Mccorkle, D. C., and Cohen, A. L. (2013). Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PLoS ONE 8:e63714. doi: 10.1371/journal.pone.0063714
Kawashima, H., and Kumashiro, I. (1969). Studies of Purine N-Oxides. III. The synthesis of Purine 3-N-Oxides. Bull. Chem. Soc. Jpn. 42, 750–755.
Kelley, J. L., and Magurran, A. E. (2003). Learned predator recognition and antipredator responses in fishes. Fish Fish. 4, 216–226. doi: 10.1046/j.1467-2979.2003.00126.x
Kelley, J. L., Grierson, P. F., Collin, S. P., and Davies, P. M. (2018). Habitat disruption and the identification and management of functional trait changes. Fish Fish. 19, 716–728. doi: 10.1111/faf.12284
Kelley, J. L., Grierson, P. F., Davies, P. M., and Collin, S. P. (2017). Water flows shape lateral line morphology in an arid zone freshwater fish. Evolu. Ecol. Res. 18, 411–428.
Kelley, J. L., Phillips, B., Cummins, G. H., and Shand, J. (2012). Changes in the visual environment affect colour signal brightness and shoaling behaviour in a freshwater fish. Anim. Behav. 83, 783–791. doi: 10.1016/j.anbehav.2011.12.028
Ketten, D. R. (1995). “Estimates of blast injury and acoustic trauma zones for marine mammals from underwater explosions,” in Sensory Systems of Aquatic Mammals, eds R. A. Kastelein, J. A. Thomas, and P. E. Nachtigall (Woerden: De Spil Publishers), 391–408.
Ketten, D. R., Lien, J., and Todd, S. (1993). Blast injury in humpback whale ears: evidence and implications. J. Acoust. Soc. Am. 94, 1849–1850. doi: 10.1121/1.407688
Kimbell, H. S., and Morrell, L. J. (2015). Turbidity influences individual and group level responses to predation in guppies, Poecilia reticulata. Anim. Behav. 103, 179–185. doi: 10.1016/j.anbehav.2015.02.027
Kunc, H. P., Lyons, G. N., Sigwart, J. D., Mclaughlin, K. E., and Houghton, J. D. (2014). Anthropogenic noise affects behavior across sensory modalities. Am. Nat. 184, E93–E100. doi: 10.1086/677545
Kunc, H. P., Mclaughlin, K. E., and Schmidt, R. (2016). Aquatic noise pollution: implications for individuals, populations, and ecosystems. Proc. R. Soc. B 283:20160839. doi: 10.1098/rspb.2016.0839
Lacoue-Labarthe, T., Reveillac, E., Oberhansli, F., Teyssie, J. L., Jeffree, R., and Gattuso, J. P. (2011). Effects of ocean acidification on trace element accumulation in the early-life stages of squid Loligo vulgaris. Aquat. Toxicol. 105, 166–176. doi: 10.1016/j.aquatox.2011.05.021
Ladich, F., and Fay, R. R. (2013). Auditory evoked potential audiometry in fish. Rev. Fish Biol. Fish 23, 317–364. doi: 10.1007/s11160-012-9297-z
Leduc, A. O. H. C., Ferrari, M. C. O., Kelly, J. M., and Brown, G. E. (2004b). Learning to recognize novel predators under weakly acidic conditions: the effects of reduced pH on acquired predator recognition by juvenile rainbow trout. Chemoecology 14, 107–112. doi: 10.1007/s00049-003-0268-7
Leduc, A. O. H. C., Roh, E., and Brown, G. E. (2009). Effects of acid rainfall on juvenile Atlantic salmon (Salmo salar) antipredator behaviour: loss of chemical alarm function and potential survival consequences during predation. Mar. Freshw. Res. 60, 1223–1230. doi: 10.1071/MF08323
Leduc, A. O. H. C., Roh, E., Macnaughton, C. J., Benz, F., Rosenfeld, J., and Brown, G. E. (2010). Ambient pH and the Response to chemical alarm cues in juvenile Atlantic Salmon: mechanisms of reduced behavioral responses. Trans. Am. Fish. Soc. 139, 117–128. doi: 10.1577/T09-024.1
Leduc, A. O., Kelly, J. M., and Brown, G. E. (2004a). Detection of conspecific alarm cues by juvenile salmonids under neutral and weakly acidic conditions: laboratory and field tests. Oecologia 139, 318–324. doi: 10.1007/s00442-004-1492-8
Leduc, A. O., Munday, P. L., Brown, G. E., and Ferrari, M. C. (2013). Effects of acidification on olfactory-mediated behaviour in freshwater and marine ecosystems: a synthesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 368:20120447. doi: 10.1098/rstb.2012.0447
Leduc, A., Noseworthy, M., Adrian, J., and Brown, G. (2003). Detection of conspecific and heterospecific alarm signals by juvenile pumpkinseed under weak acidic conditions. J. Fish Biol. 63, 1331–1336. doi: 10.1046/j.1095-8649.2003.00230.x
Leis, J. M., Siebeck, U., and Dixson, D. L. (2011). How Nemo finds home: the neuroecology of dispersal and population connectivity in larvae of marine fishes. Integr. Comp. Biol. 51, 826–843. doi: 10.1093/icb/icr004
Liley, N. R. (1982). Chemical communication in fish. Can. J. Fish. Aquat. Sci. 39, 22–35. doi: 10.1139/f82-005
Linbo, T. L., Stehr, C. M., Incardona, J. P., and Scholz, N. L. (2006). Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ. Toxicol. Chem. 25, 597–603. doi: 10.1897/05-241R.1
Maneja, R. H., Frommel, A. Y., Geffen, A. J., Folkvord, A., Piatkowski, U., Chang, M. Y., et al. (2013). Effects of ocean acidification on the calcification of otoliths of larval Atlantic cod Gadus morhua. Mar. Ecol. Prog. Ser. 477, 251–258. doi: 10.3354/meps10146
Maneja, R. H., Piatkowski, U., and Melzner, F. (2011). Effects of ocean acidification on statolith calcification and prey capture in early life cuttlefish, Sepia officinalis. J. Shellfish Res. 30:1011.
Manriquez, P. H., Jara, M. E., Mardones, M. L., Torres, R., Lagos, N. A., Lardies, M. A., et al. (2014). Effects of ocean acidification on larval development and early post-hatching traits in Concholepas concholepas (loco). Mar. Ecol. Prog. Ser. 514, 87–87. doi: 10.3354/meps10951
Maturana, H. R., and Sperling, S. (1963). Unidirectional response to angular acceleration recorded from the middle cristal nerve in the Statocyst of Octopus vulgaris. Nature 197, 815–816. doi: 10.1038/197815b0
McCauley, R. D., Day, R. D., Swadling, K. M., Fitzgibbon, Q. P., Watson, R. A., and Semmens, J. M. (2017). Widely used marine seismic survey air gun operations negatively impact zooplankton. Nat. Ecol. Evol. 1:0195. doi: 10.1038/s41559-017-0195
McCauley, R. D., Fewtrell, J. L., and Popper, A. N. (2003). High intensity anthropogenic sound damages fish ears. J. Acoust. Soc. Am. 113, 638–642. doi: 10.1121/1.1527962
McNeil, P. L., Boyle, D., Henry, T. B., Handy, R. D., and Sloman, K. A. (2014). Effects of metal nanoparticles on the lateral line system and behaviour in early life stages of zebrafish (Danio rerio). Aquat. Toxicol. 152, 318–323. doi: 10.1016/j.aquatox.2014.04.022
Merilä, J., and Hendry, A. P. (2014). Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. doi: 10.1111/eva.12137
Montgomery, J. C., Bleckmann, H., and Coombs, S. (2014). “Sensory ecology and neuroethology of the lateral line,” in The Lateral Line System, eds S. Coombs, H. Bleckmann, R. R. Fay, and A. N. Popper (New York, NY: Springer), 121–150.
Montgomery, J. C., Tolimieri, N., and Haine, O. S. (2001). Active habitat selection by pre-settlement reef fishes. Fish Fish. 2, 261–277. doi: 10.1046/j.1467-2960.2001.00053.x
Moore, A. (1994). An electrophysiological study on the effects of pH on olfaction in mature male Atlantic salmon (Salmo salar) parr. J. Fish Biol. 45, 493–502. doi: 10.1111/j.1095-8649.1994.tb01331.x
Moritz, C., Patton, J. L., Conroy, C. J., Parra, J. L., White, G. C., and Beissinger, S. R. (2008). Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264. doi: 10.1126/science.1163428
Mu, J., Jin, F., Wang, J., Zheng, N., and Cong, Y. (2015). Effects of CO2-driven ocean acidification on early life stages of marine medaka Oryzias melastigma. Biogeosciences 12, 3861–3861. doi: 10.5194/bg-12-3861-2015
Munday, P. L., Dixson, D. L., Donelson, J. M., Jones, G. P., Pratchett, M. S., Devitsina, G. V., et al. (2009). Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. U.S.A. 106, 1848–1852. doi: 10.1073/pnas.0809996106
Munday, P. L., Dixson, D. L., Mccormick, M. I., Meekan, M., Ferrari, M. C., and Chivers, D. P. (2010). Replenishment of fish populations is threatened by ocean acidification. Proc. Natl. Acad. Sci. U.S.A. 107, 12930–12934. doi: 10.1073/pnas.1004519107
Munday, P. L., Gagliano, M., Donelson, J. M., Dixson, D. L., and Thorrold, S. R. (2011a). Ocean acidification does not affect the early life history development of a tropical marine fish. Mar. Ecol. Prog. Ser. 423, 211–211. doi: 10.3354/meps08990
Munday, P. L., Hernaman, V., and Dixson, D. L. (2011b). Effect of ocean acidification on otolith development in larvae of a tropical marine fish. Biogeosciences 8, 1631–1641. doi: 10.5194/bg-8-1631-2011
Navarro, M. O., Kwan, G. T., Batalov, O., Choi, C. Y., Pierce, N. T., and Levin, L. A. (2016). Development of embryonic market squid, Doryteuthis opalescens, under chronic exposure to low environmental pH and [O2]. PLoS ONE 11:e0167461. doi: 10.1371/journal.pone.0167461
Navarro, M., Bockmon, E., Frieder, C., Gonzalez, J., and Levin, L. (2014). Environmental pH, O2 and capsular effects on the geochemical composition of statoliths of embryonic squid Doryteuthis opalescens. Water 6, 2233–2233. doi: 10.3390/w6082233
Nilsson, G. E., Dixson, D. L., Domenici, P., Mccormick, M. I., Sorensen, C., Watson, S. A., et al. (2012). Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2, 201–204. doi: 10.1038/nclimate1352
Nowacek, D. P., Thorne, L. H., Johnston, D. W., and Tyack, P. L. (2007). Responses of cetaceans to anthropogenic noise. Mamm. Rev. 37, 81–115. doi: 10.1111/j.1365-2907.2007.00104.x
Olsen, K. H., and Hoglund, L. B. (1985). Reduction by a surfactant of olfactory mediated attraction between juveniles of arctic charr, Salvelinus-alpinus (L). Aquat. Toxicol. 6, 57–69. doi: 10.1016/0166-445X(85)90020-7
Orr, M. (2016). The Potential Impacts of Submarine Cables on Benthic Elasmobranchs. Ph.D. thesis, University of Auckland.
Ou, M., Hamilton, T. J., Eom, J., Lyall, E. M., Gallup, J., Jiang, A., et al. (2015). Responses of pink salmon to CO2-induced aquatic acidification. Nat. Clim. Change 5, 950–955. doi: 10.1038/nclimate2694
Partan, S. R. (2017). Multimodal shifts in noise: switching channels to communicate through rapid environmental change. Anim. Behav. 124, 325–337. doi: 10.1016/j.anbehav.2016.08.003
Peng, C., Zhao, X., and Liu, G. (2015). Noise in the sea and its impacts on marine organisms. Int. J. Environ. Res. Public Health 12, 12304–12323. doi: 10.3390/ijerph121012304
Perry, D. M., Redman, D. H., Widman, J. C., Meseck, S., King, A., and Pereira, J. J. (2015). Effect of ocean acidification on growth and otolith condition of juvenile scup, Stenotomus chrysops. Ecol. Evol. 5, 4187–4187. doi: 10.1002/ece3.1678
Popper, A. N., and Hastings, M. C. (2009). The effects of anthropogenic sources of sound on fishes. J. Fish Biol. 75, 455–489. doi: 10.1111/j.1095-8649.2009.02319.x
Popper, A. N., and Lu, Z. M. (2000). Structure-function relationships in fish otolith organs. Fish. Res. 46, 15–25. doi: 10.1016/S0165-7836(00)00129-6
Popper, A. N., Smith, M. E., Cott, P. A., Hanna, B. W., Macgillivray, A. O., Austin, M. E., et al. (2005). Effects of exposure to seismic airgun use on hearing of three fish species. J. Acoust. Soc. Am. 117, 3958–3971. doi: 10.1121/1.1904386
Radford, A. N., Kerridge, E., and Simpson, S. D. (2014). Acoustic communication in a noisy world: can fish compete with anthropogenic noise? Behav. Ecol. 25, 1022–1030. doi: 10.1093/beheco/aru029
Ranaker, L., Jonsson, M., Nilsson, P. A., and Bronmark, C. (2012). Effects of brown and turbid water on piscivore-prey fish interactions along a visibility gradient. Freshw. Biol. 57, 1761–1768. doi: 10.1111/j.1365-2427.2012.02836.x
Rennison, D. J., Owens, G. L., Heckman, N., Schluter, D., and Veen, T. (2016). Rapid adaptive evolution of colour vision in the threespine stickleback radiation. Proc. R. Soc. B Biol. Sci. 283:20160242. doi: 10.1098/rspb.2016.0242
Reusch, T. B. H. (2014). Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evol. Appl. 7, 104–122. doi: 10.1111/eva.12109
Réveillac, E., Lacoue-Labarthe, T., Oberhänsli, F., Teyssié, J.-L., Jeffree, R., Gattuso, J.-P., et al. (2015). Ocean acidification reshapes the otolith-body allometry of growth in juvenile sea bream. J. Exp. Mar. Biol. Ecol. 463, 87–87. doi: 10.1016/j.jembe.2014.11.007
Ronald, K. L., Fernández-Juricic, E., and Lucas, J. R. (2012). Taking the sensory approach: how individual differences in sensory perception can influence mate choice. Anim. Behav. 84, 1283–1294. doi: 10.1016/j.anbehav.2012.09.015
Rossi, T., Connell, S. D., and Nagelkerken, I. (2016a). Silent oceans: ocean acidification impoverishes natural soundscapes by altering sound production of the world's noisiest marine invertebrate. Proc. R. Soc. B Biol. Sci. 283:20153046. doi: 10.1098/rspb.2015.3046
Rossi, T., Nagelkerken, I., Pistevos, J. C., and Connell, S. D. (2016b). Lost at sea: ocean acidification undermines larval fish orientation via altered hearing and marine soundscape modification. Biol. Lett. 12:20150937. doi: 10.1098/rsbl.2015.0937
Rossi, T., Nagelkerken, I., Simpson, S. D., Pistevos, J. C., Watson, S.-A., Merillet, L., et al. (2015). Ocean acidification boosts larval fish development but reduces the window of opportunity for successful settlement. Proc. R. Soc. B Biol. Sci. 282:20151954. doi: 10.1098/rspb.2015.1954
Salazar, V. L., and Stoddard, P. K. (2008). Sex differences in energetic costs explain sexual dimorphism in the circadian rhythm modulation of the electrocommunication signal of the gymnotiform fish Brachyhypopomus pinnicaudatus. J. Exp. Biol. 211, 1012–1020. doi: 10.1242/jeb.014795
Salazar, V. L., and Stoddard, P. K. (2009). Social competition affects electric signal plasticity and steroid levels in the gymnotiform fish Brachyhypopomus gauderio. Horm. Behav. 56, 399–409. doi: 10.1016/j.yhbeh.2009.07.009
Schade, F. M., Clemmesen, C., and Wegner, K. M. (2014). Within- and transgenerational effects of ocean acidification on life history of marine three-spined stickleback (Gasterosteus aculeatus). Mar. Biol. 161, 1667–1667. doi: 10.1007/s00227-014-2450-6
Scheifele, P. M., Andrew, S., Cooper, R. A., Darre, M., Musiek, F. E., and Max, L. (2005). Indication of a Lombard vocal response in the St. Lawrence river beluga. J. Acoust. Soc. Am. 117, 1486–1492. doi: 10.1121/1.1835508
Scott, G. R., and Sloman, K. A. (2004). The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 68, 369–392. doi: 10.1016/j.aquatox.2004.03.016
Seehausen, O., Vanalphen, J. J. M., and Witte, F. (1997). Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808–1811. doi: 10.1126/science.277.5333.1808
Shand, J., Archer, M. A., and Collin, S. P. (1999). Ontogenetic changes in the retinal photoreceptor mosaic in a fish, the black bream, Acanthopagrus bucheri. J. Comp. Neurol. 412, 203–217.
Shand, J., Davies, W. L., Thomas, N., Balmer, L., Cowing, J. A., Pointer, M., et al. (2008). The influence of ontogeny and light environment on the expression of visual pigment opsins in the retina of the black bream, Acanthopagrus butcheri. J. Exp. Biol. 211, 1495–1503. doi: 10.1242/jeb.012047
Simpson, S. D., Munday, P. L., Wittenrich, M. L., Manassa, R., Dixson, D. L., Gagliano, M., et al. (2011). Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–917. doi: 10.1098/rsbl.2011.0293
Simpson, S. D., Radford, A. N., Nedelec, S. L., Ferrari, M. C., Chivers, D. P., Mccormick, M. I., et al. (2016). Anthropogenic noise increases fish mortality by predation. Nat. Commun. 7:10544. doi: 10.1038/ncomms10544
Sisneros, J. A., and Bass, A. H. (2003). Seasonal plasticity of peripheral auditory frequency sensitivity. J. Neurosci. 23, 1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003
Slabbekoorn, H., Bouton, N., Van Opzeeland, I., Coers, A., Ten Cate, C., and Popper, A. N. (2010). A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427. doi: 10.1016/j.tree.2010.04.005
Smale, D. A., and Wernberg, T. (2013). Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B 280:20122829. doi: 10.1098/rspb.2012.2829
Smith, J. J., Leduc, A. O. H. C., and Brown, G. E. (2008). Chemically mediated learning in juvenile rainbow trout. Does predator odour pH influence intensity and retention of acquired predator recognition? J. Fish Biol. 72, 1750–1760. doi: 10.1111/j.1095-8649.2008.01849.x
Smith, M. E., and Monroe, J. D. (2016). Causes and consequences of sensory hair cell damage and recovery in fishes. Adv. Exp. Med. Biol. 877, 393–417. doi: 10.1007/978-3-319-21059-9_17
Southall, B. L., Bowles, A. E., Ellison, W. T., Finneran, J. J., Gentry, R. L., et al. (2007). Marine mammal noise exposure criteria: initial scientific recommendations. Aquat. Mam. 33, 1–121.
Southall, B. L., Schusterman, R. J., and Kastak, D. (2000). Masking in three pinnipeds: underwater, low-frequency critical ratios. J. Acoust. Soc. Am. 108, 1322–1326. doi: 10.1121/1.1288409
Sundin, J., Berglund, A., and Rosenqvist, G. (2010). Turbidity hampers mate choice in a pipefish. Ethology 116, 713–721. doi: 10.1111/j.1439-0310.2010.01787.x
Sutherland, W. J., Freckleton, R. P., Godfray, H. C. J., Beissinger, S. R., Benton, T., Cameron, D. D., et al. (2013). Identification of 100 fundamental ecological questions. J. Ecol. 101, 58–67. doi: 10.1111/1365-2745.12025
Swaddle, J. P., Francis, C. D., Barber, J. R., Cooper, C. B., Kyba, C. C., Dominoni, D. M., et al. (2015). A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550–560. doi: 10.1016/j.tree.2015.06.009
Temple, S. E., Plate, E. M., Ramsden, S., Haimberger, T. J., Roth, W. M., and Hawryshyn, C. W. (2006). Seasonal cycle in vitamin A1/A2-based visual pigment composition during the life history of coho salmon (Oncorhynchus kisutch). J. Comp. Physiol. A 192, 301–313. doi: 10.1007/s00359-005-0068-3
Thums, M., Whiting, S. D., Reisser, J., Pendoley, K. L., Pattiaratchi, C. B., Proietti, M., et al. (2016). Artificial light on water attracts turtle hatchlings during their near shore transit. R. Soc. Open Sci. 3:160142. doi: 10.1098/rsos.160142
Tierney, K. B., Baldwin, D. H., Hara, T. J., Ross, P. S., Scholz, N. L., and Kennedy, C. J. (2010). Olfactory toxicity in fishes. Aquat. Toxicol. 96, 2–26. doi: 10.1016/j.aquatox.2009.09.019
Tohse, H., and Mugiya, Y. (2001). Effects of enzyme and anion transport inhibitors on in vitro incorporation of inorganic carbon and calcium into endolymph and otoliths in salmon Oncorhynchus masou. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 128, 177–184. doi: 10.1016/S1095-6433(00)00287-7
Tohse, H., Ando, H., and Mugiya, Y. (2004). Biochemical properties and immunohistochemical localization of carbonic anhydrase in the sacculus of the inner ear in the salmon Oncorhynchus masou. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 137, 87–94. doi: 10.1016/S1095-6433(03)00272-1
Tresguerres, M., and Hamilton, T. J. (2017). Acid base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 220, 2136–2148. doi: 10.1242/jeb.144113
Tuomainen, U., and Candolin, U. (2011). Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. doi: 10.1111/j.1469-185X.2010.00164.x
Wale, M. A., Simpson, S. D., and Radford, A. N. (2013). Noise negatively affects foraging and antipredator behaviour in shore crabs. Anim. Behav. 86, 111–118. doi: 10.1016/j.anbehav.2013.05.001
Ward, A. J., Duff, A. J., Horsfall, J. S., and Currie, S. (2008). Scents and scents-ability: pollution disrupts chemical social recognition and shoaling in fish. Proc. R. Soc. B Biol. Sci. 275, 101–105. doi: 10.1098/rspb.2007.1283
Wark, A. R., and Peichel, C. L. (2010). Lateral line diversity among ecologically divergent threespine stickleback populations. J. Exp. Biol. 213, 108–117. doi: 10.1242/jeb.031625
Wark, A. R., Mills, M. G., Dang, L. H., Chan, Y. F., Jones, F. C., Brady, S. D., et al. (2012). Genetic architecture of variation in the lateral line sensory system of threespine sticklebacks. G3 (Bethesda). 2, 1047–1056. doi: 10.1534/g3.112.003079
Weilgart, L. S. (2007). The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 85, 1091–1116. doi: 10.1139/Z07-101
Whitfield, A. K., and Becker, A. (2014). Impacts of recreational motorboats on fishes: a review. Mar. Pollut. Bull. 83, 24–24. doi: 10.1016/j.marpolbul.2014.03.055
Williams, R., Wright, A. J., Ashe, E., Blight, L. K., Bruintjes, R., Canessa, R., et al. (2015). Impacts of anthropogenic noise on marine life: publication patterns, new discoveries, and future directions in research and management. Ocean Coastal Manage. 115, 17–24. doi: 10.1016/j.ocecoaman.2015.05.021
Willis, K. L. (2016). Underwater hearing in turtles. Adv. Exp. Med. Biol. 875, 1229–1235. doi: 10.1007/978-1-4939-2981-8_154
Wisenden, B. D. (2003). “Chemically mediated strategies to counter predation,” in Sensory Processing in Aquatic Environments, eds S. P. Collin and N. J. Marshall (New York, NY: Springer-Verlag), 236–251.
Wong, B. B., Candolin, U., and Lindstrom, K. (2007). Environmental deterioration compromises socially enforced signals of male quality in three-spined sticklebacks. Am. Natur. 170, 184–189. doi: 10.1086/519398
Wong, B. B., Fisher, H. S., and Rosenthal, G. G. (2005). Species recognition by male swordtails via chemical cues. Behav. Ecol. 16, 818–822. doi: 10.1093/beheco/ari058
Young, A., Kochenkov, V., Mcintyre, J. K., Stark, J. D., and Coffin, A. B. (2018). Urban stormwater runoff negatively impacts lateral line development in larval zebrafish and salmon embryos. Sci. Rep. 8:2830. doi: 10.1038/s41598-018-21209-z
Keywords: climate change, sensory ecology, ocean acidification, sensory plasticity, contemporary evolution
Citation: Kelley JL, Chapuis L, Davies WIL and Collin SP (2018) Sensory System Responses to Human-Induced Environmental Change. Front. Ecol. Evol. 6:95. doi: 10.3389/fevo.2018.00095
Received: 08 March 2018; Accepted: 19 June 2018;
Published: 09 July 2018.
Edited by:
Martin Stevens, University of Exeter, United KingdomReviewed by:
Thomas Cronin, University of Maryland, Baltimore County, United StatesAdelino V. M. Canario, University of Algarve, Portugal
Copyright © 2018 Kelley, Chapuis, Davies and Collin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer L. Kelley, amVubmlmZXIua2VsbGV5QHV3YS5lZHUuYXU=
†Joint last (senior) authors.
 Jennifer L. Kelley
Jennifer L. Kelley Lucille Chapuis
Lucille Chapuis Wayne I. L. Davies
Wayne I. L. Davies Shaun P. Collin
Shaun P. Collin