- Centre for Ecological Sciences, Indian Institute of Science, Bangalore, India
Why animals commonly use multiple conspicuous and presumably costly signals is poorly understood. Tests of evolutionary hypotheses comprehensively covering the signaling repertoire in wild populations are crucial to establish biological relevance, yet are relatively rare. We tested a key hypothesis for the maintenance of multiple signals in a wild population of the lizard, Psammophilus dorsalis, specifically whether multiple signals are maintained as multiple messages directed at different receivers. In addition, we also examined patterns in covariation of signals as an initial test of an alternative hypothesis, that multiple signals may be maintained as redundant signals; such traits are proposed to convey and reinforce the same component of information and are expected to be strongly correlated. Breeding male P. dorsalis display from prominent rock perches within their territories, which overlap multiple female home ranges in rocky open habitats. We repeatedly measured the display behavior, covering the entire signaling repertoire, of individually-tagged wild males on their territories over their lifespans. We quantified patterns of covariation in multiple traits and their relationship with multiple receiver contexts, specifically competitors, mates and predators. We also examined the association between male signaling and indices of lifetime fitness. Males commonly used multiple signals, including behavioral signals and a rare dynamic color signal. These traits were strongly correlated and seemed largely directed toward females, suggesting that they were primarily maintained as redundant signals through female choice. However, other selection pressures also appeared to be important. One color trait seemed to be directed at competitors, providing limited support to the multiple receiver hypothesis. Several traits were reduced in the presence of predators, suggesting that they carry the cost of increased predation risk. Thus, multiple selection pressures, primarily female choice and predation risk, appear to affect male signaling. Finally, signaling traits appeared to influence a measure of lifetime reproductive success, providing rare evidence for the biological relevance of signaling traits under natural contexts.
Introduction
Animals often employ a diverse range of conspicuous traits to signal to conspecifics and occasionally, to heterospecifics (Brodie, 1977; Bradbury and Vehrencamp, 1998; Rek and Magrath, 2016). Given the large costs of signaling (Halfwerk et al., 2014), why do animals use multiple signals rather than a single signal to advertise their quality (Johnstone, 1996)? A key set of hypotheses explaining the evolution and maintenance of multiple signals within a population proposes that multiple signals represent uncorrelated independent pieces of information (multiple message and multiple receiver hypotheses; Moller and Pomiankowski, 1993; Johnstone, 1996). According to the “multiple message hypothesis,” multiple signals can evolve in a population if each signal conveys a different component of information about the overall quality of the signaler (Bókony et al., 2006; Bro-Jørgensen and Dabelsteen, 2008; Martín and López, 2009; Plasman et al., 2015). For example, in the Dickerson's collared lizard (Crotaphytus dickersonae), blue color of the skin appears to convey resource-holding potential while the blackness of the collar indicates immune condition (Plasman et al., 2015). In addition, multiple traits could be maintained if they are used in different contexts, or directed toward different receivers (Endler, 1992; Marchetti, 1998; Andersson et al., 2002; Loyau et al., 2005). In the wild, two common contexts in which individuals communicate are predation and mate-acquisition. Furthermore, within the mating context, individuals may use certain traits to signal to potential mates and others to signal to competitors. For example, a red carotenoid collar is reported to be involved in contest competition and an elongated tail in mate choice in the red-collared widowbird (Euplectes ardens) (Andersson et al., 2002). Such use of different traits might evolve either to avoid confusion regarding the intended receiver, and/or because different information may be communicated toward the different receivers. For example, individuals may convey information on their genetic quality to potential mates, their motivation to defend a territory/mate to potential competitors, and their ability to escape an attack to predators. Predation pressure can influence signal evolution, by favoring conspicuous displays directed specifically at the predator (Brodie, 1977; Caro, 1986) or by modifying the payoffs of signals functioning in other contexts, such as mate attraction (e.g., paler coloration in guppies from high-predation populations compared to those in low-predation populations; Endler, 1992).
While empirical support is arguably the greatest for the multiple-message hypothesis (Martín and López, 2009; Bro-Jørgensen, 2010; Plasman et al., 2015), alternative hypotheses have also been proposed for the maintenance of multiple signals. Several of these propose that multiple signals represent redundant pieces of information and are correlated (Moller and Pomiankowski, 1993; Candolin, 2003; Hebets and Papaj, 2005; Bro-Jørgensen, 2010). According to the “redundant signal” or “back-up signal” hypothesis, multiple signals convey, and reinforce the same component of information about the signaler's quality (Moller and Pomiankowski, 1993; Johnstone, 1996). For example, in the blue tit (Cyanistes caeruleus), two visual signals and an acoustic signal all appear to indicate the level of genetic diversity in a male (Ferrer et al., 2015). The probability of making a wrong decision and time taken to make a decision are lower if multiple traits, rather than a single trait, are evaluated (Smith and Evans, 2008). Red junglefowl (Gallus gallus) hens react faster to a rooster's food-alerting signal, if the hens are simultaneously exposed to rhythmic head-movements as well as vocalizations of the rooster (Smith and Evans, 2008). Alternatively, redundant signals may consist of an informative high-cost signal accompanied by less informative low-cost signals that improve the detectability and/or discriminability of the high-cost signal (Rowe, 1999). It is also possible that multiple mechanisms (e.g., both multiple message and redundancy in information) are simultaneously involved in the maintenance of multiple signals (Bro-Jørgensen and Dabelsteen, 2008).
An important aspect of examining these hypotheses for the maintenance of multiple traits is to examine the biological relevance of these traits, i.e., their relative contributions to fitness. Relationships of individual or a few traits with measures of fitness have been reported across a wide array of taxa [e.g., frillneck lizards (Hamilton et al., 2013), wolf spiders (Rundus et al., 2011), collared flycatcher (Qvarnstrom, 1997)]. However, where multiple signaling traits occur, the relationship between individual traits and the signaler's fitness may be complex (Candolin, 2003; Roberts et al., 2006). For example, the mate-attraction success of male ornate tree lizards (Urosaurus ornatus) could be explained only when male display-traits were considered in a multivariate rather than an individual trait analysis (Hamilton and Sullivan, 2005). Therefore, it is important to measure the entire signaling repertoire (Rek and Magrath, 2016), decipher the relationships among individual traits, and quantify their relative contributions to fitness. Furthermore, since behavioral signals are inherently variable, multiple measurements of signaling behavior, preferably distributed over an individual's lifetime, are needed to characterize well the level of signaling that the individual engages in.
In addition, much of our understanding of the ecology and evolution of signaling traits is based on work carried out in captive or semi-captive conditions (but see Baird, 2013). However, unlike in these controlled conditions, where individuals are typically exposed to a limited selection regime, individuals in wild populations experience diverse selection pressures. While there is considerable understanding of how traits evolve under a given selection pressure (such as sexual selection, predation), and under specific contexts (Zuk et al., 1992; Hamilton et al., 2013), information on how multiple selection pressures act simultaneously on signaling traits is scarce.
We studied the maintenance of multiple signaling traits in a wild population of Psammophilus dorsalis by investigating the relative importance of different selection pressures on these traits under natural ecological and social contexts, and the relationship of these traits with measures of lifetime fitness. P. dorsalis males are known to use visual signals—complex body postures and movements—for intraspecific communication (Radder et al., 2006). There is no evidence for olfactory or acoustic communication in this species, allowing us to study the entire signaling repertoire in this species. We investigated visual signaling in males in relation to (a) female mate choice, (b) male-male competition, and (c) predation risk. To understand the functions of these signals, we examined their associations with different contexts (mates, competitors, predators). These relationships allowed us to assess whether multiple signals may be maintained as multiple messages directed at different receivers. A stronger relationship of some signals with mates and others with competitors and/or predators would provide support for the multiple receiver hypothesis. As an initial evaluation of redundant signal hypotheses, we also examined the correlations amongst the multiple signals. A strongly correlated set of signals associated with a single context would indicate that multiple male signals are redundant. Such covariation in signals is not expected under the multiple receiver hypothesis (Candolin, 2003; Hebets and Papaj, 2005) since the presence of different receivers in the vicinity of the signaler is unlikely to be correlated. Finally, to evaluate the biological relevance of multiple signaling traits, we examined their relationship with measures of male lifetime fitness.
Methods
Study System
Psammophilus dorsalis is a diurnal, rock-dwelling, sexually dimorphic agamid lizard. Males are larger than females and display bright coloration during the breeding season (Deodhar and Isvaran, 2017), from May to September. Found exclusively on large flat rocks (henceforth sheet rocks), they perch on rocks and signal to conspecifics using body postures, movements and colors, and also reportedly react to heterospecifics (Radder et al., 2006). These lizards breed predominantly only during one breeding season (Deodhar and Isvaran, 2017). We performed this study in Rishi Valley, Andhra Pradesh, India (13° 32′N, 78° 28′E), from May 2011 to September 2013. The area experiences stark seasonality in temperature and precipitation (Deodhar and Isvaran, 2017) and primarily consists of thorny scrub vegetation and hilly terrain. At our study site, several predators such as common Indian monitor lizard (Varanus sp.), Indian fox (Vulpes bengalensis) and various species of snakes and birds of prey, have been observed to prey upon and interact with P. dorsalis (SD, personal observations).
Individual Identification
Adult males were tagged before the onset of the breeding season. Subsequently arriving adults and recruits were tagged as soon as possible. Lizards were captured by noosing and uniquely tagged using color-coded combinations of 4 ceramic beads. Beads were attached on the dorsal surface at the base of the lizard's tail using a procedure specifically developed for tagging lizards (Fisher and Muth, 1989). Body size (snout vent length) was measured using Vernier calipers (Mitutoyo) to the nearest millimeter. Handling time lasted a maximum of 15 min per individual. Lizards were released back at their capture-location. All animal handling and behavior sampling methods complied with the guidelines of the Institutional Animal Ethics Committee (Indian Institute of Science).
Behavior Sampling
The behavior of tagged animals was recorded using focal animal sampling in a repeated-measures design over their breeding lifespan. Using binoculars and a voice-recorder, during each sampling session, the switch from one behavioral state to another and every occurrence of selected behavioral events were continuously recorded. We recorded all the main display behavioral traits (e.g., headbob, pushup, gape, gular extension etc.), initially identified through previous work on P. dorsalis (Radder et al., 2005, 2006) and through preliminary observations at the study site (SD, unpublished data). We also recorded several behaviors which do not seem to be directly related to interacting with mates or competitors but likely related to maintaining body condition. These include foraging, moving (can be used to move toward resources, for thermoregulation, or to move away from predators) and alert behaviors (can be used for predator-detection); for a list of behaviors and their definitions, see Supplementary Table A. Male color was visually evaluated, classified as one of 8 mutually exclusive categories (states), and continuously monitored (Figure 1). Conspecifics within a 10 m radius were counted (once at the beginning, and subsequently, every 3–4 min during the session) and used to quantify two social contexts, namely the number of potential mates (females) and conspecific competitors (males) in the vicinity. Two ecological conditions, the presence/absence of predators and month (time during the breeding season), were recorded. The focal individual was followed for a minimum of 10 min and up to 30 min or till the individual disappeared from sight. Each focal session recording was later transcribed. For obtaining measures that are representative of the signaling behavior of an individual over the long-term, an individual was sampled regularly over its breeding lifespan. One to three focal sessions were conducted every month (not more than 1 session/day), over its breeding lifespan, until the animal was no longer seen at the study-site.

Figure 1. Photos of colors exhibited by males. Clockwise from top-left (A) pale (B) pale yellow (C) yellow (D) yellow ochre (E) orange (F) bright orange (G) crimson, and (H) fighting. To reduce the number of predictors, these 8 mutually exclusive states (see Supplementary Material Table A) of time spent in a given color were collapsed into 4 biologically meaningful levels, namely L1 (A+B), L2 (C+D), L3 (E+F+G), and “Fighting” colors.
Quantifying Male Fitness
Since male fitness could not be directly quantified with parentage assignment using genetic analyses, we used two proxies of male fitness: (a) “females per day” and (b) “breeding tenure” (see below). Similar measures have been used as proxies of male reproductive success in reptilian studies (Ruby, 1984; Lappin and Husak, 2005). To estimate proxies of male fitness, tagged individuals were regularly monitored till they disappeared (presumed dead, Deodhar and Isvaran, 2017). Sheet rocks and frequently used perches were mapped using a GPS (Garmin eTrexH). Locations of all lizards, tagged and untagged, were regularly recorded every time a sheet rock was visited for behavioral observations, and during censuses (at least fortnightly during the breeding season, and monthly during the non-breeding season) carried out as part of a long-term monitoring study. These data provided information on (a) the duration (in days) for which a male was resident on the sheet rock (henceforth, tenure) and (b) monthly home ranges of known individuals, which were calculated by drawing 95% minimum convex polygons. Based on long-term observations, which show that adult male movement between sheet rocks is rare (among the 208 lizards tagged between 2010 and 2013, only 6 instances of movement between sheet rocks were observed), adult males disappearing from a sheet rock were considered dead (Deodhar and Isvaran, 2017). Therefore, the measure of a male's tenure is likely to reflect his total adult lifespan. These data were used to calculate:
Females Per Day
This is an index of the number of mates that a male potentially had access to. Specifically, this proxy was calculated as the number of unique females present per day in a male's monthly home range, averaged over the months that a male was resident on the sheet rock. Using location data, we drew monthly 95% minimum convex polygons for each male, summed the number of unique females recorded in his monthly polygon during every observation session (behavioral session or census) in that month, and divided by the number of sessions/censuses during which that male's territory was surveyed. The estimates for the different months that a male was resident on the sheet rock were averaged to provide a lifetime “females per day” value for each male.
Breeding Tenure
The time for which a male was resident during the breeding season (May–Sep) alone was defined as the “breeding tenure” of a male. We assumed that the longer the breeding tenure the greater the access to potential mates. Since all males were followed till they disappeared from the study site (presumed dead) and since males typically experience only one breeding season, we obtained lifetime measures of breeding tenure.
Statistical Analyses
All analyses were carried out using R (Version 3.3.0) (R Core Team, 2016). For each focal sampling session, rates (counts per hour) of various behavioral events and proportions of time spent in various behavioral states were calculated (Supplementary Material Data sheet 4). To reduce the number of variables, the 8 mutually exclusive states of time spent in a given color were collapsed into 4 biologically meaningful levels (Pale:L1, Yellow:L2, Orange:L3 and “Fighting”; Figure 1, Supplementary Table A). To check for covariation in behaviors, we carried out a Principal Component Analysis (PCA) on selected, scaled behaviors (Supplementary Material Data Sheet 1). Prior to the PCA, we performed the Bartlett sphericity test and estimated the Keyser-Meyer-Olkin (KMO) measure to assess sampling adequacy (Budaev, 2010). The correlation matrix of behaviors is provided as Supplementary Material (Data sheets 5, 6) as recommended by Budaev (2010). We omitted rare behaviors (those seen in <20% of all focal sessions). To test for relationships of male behavior with social (number of mates and competitors in the vicinity) and ecological variables (season, predator-presence), we fitted linear mixed effects models, with composite behavioral variables (PC1a and PC2a, the first two principal components from the above PCA) as response variables (Supplementary Material Data Sheet 3). Since male behaviors loaded negatively on PC1a, we used (-PC1a) as the response variable for ease of interpretation (so that a large value of the response variable represented a higher rate/proportion of time spent in a state). The number of females (continuous) in the vicinity, number of males in the vicinity (continuous), season (factor with 6 levels, May–Oct) and predator presence (factor with two levels, present/absent) were included as fixed effects, and individual focal male ID as a random effect. Likelihood ratio tests were used to test the statistical significance of fixed effects. Following Nakagawa and Schielzeth (2013), we report both marginal and conditional , measures of the variance explained by the fixed effects and by the whole model (fixed and random effects), respectively. In order to compare the relative effects of each fixed effect, we report the change in the when each fixed effect is removed from the global model while retaining all remaining terms.
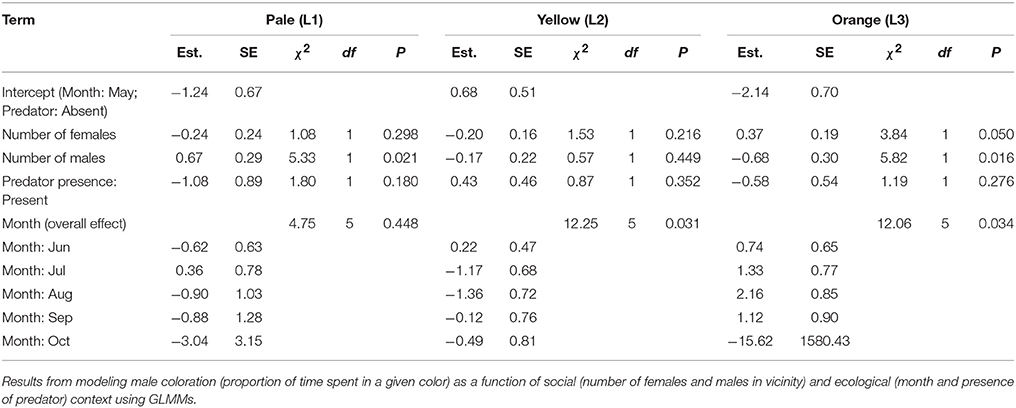
These mixed effects models represent a conservative test of the relationship between male behavior and predictors since we fitted only two models for the two composite behavioral variables. To supplement these analyses, we also fitted separate generalized linear mixed effects models (GLMMs) to examine the relationship of individual behaviors with social and ecological predictors. Depending on the nature of the individual response variables, suitable error structures were chosen. Firstly, for those behavioral events measured as rates and which were relatively rare (more than 40% of sampling sessions consisting of zeros) (viz. chase, crouch-shudder, change perch, forage: see Supplementary Table B), we used the occurrence of that event (present/absent) during the focal session as a binary response variable and used binomial error structure. Furthermore, for these rare behaviors, because of the large number of zeros, the effective degrees of freedom were relatively low. Therefore, we needed to reduce the number of parameters to be estimated in the statistical model, which was achieved by collapsing the levels for two of the predictor variables, viz. season and number of males in vicinity (this variable was chosen for collapsing over the number of females since the range in values was lower for the former rather than the latter). Thus, we included males in the vicinity as a categorical variable (present/absent), and season with a reduced number of levels (3 levels: May–Jun, Jul–Aug, Sep–Oct). Predator presence (present/absent) and number of females (continuous) were the other fixed effects in these models. Secondly, for behavioral events, measured as rates, which were common (<40% of sampling sessions consisting of zeros) (viz. headbob, move, pushup, reorient), we modeled the behavior as frequencies (number of counts per sampling session) and used negative binomial errors, and included the duration of the session (in seconds) as an offset to account for variation in sampling effort. For these behaviors, numbers of males and females in vicinity were included as continuous fixed effects and predator presence (present/absent) and season (6 levels, May:Oct) as categorical fixed effects. Thirdly, for modeling behaviors measured as proportion time spent in a state (viz. L1, L3), we used a quasibinomial error structure to account for overdispersion with the same fixed effects as those used for the common behavioral events. Individual ID was included as a random effect in all GLMMs.
Finally, we tested for the relationships of male traits with proxies of fitness. Since we were interested in individual-specific behavior unaffected by the immediate conditions experienced by an individual, we wished to obtain estimates of behavior after controlling for immediate social and ecological contexts. For this, we obtained adjusted behavioral rates and proportions of time spent in behavioral states by extracting random effects from the above GLMMs of individual behaviors. We first performed a PCA on adjusted behaviors to test for covariation. Since they showed strong covariation, we extracted the first two principal components (PC1b, PC2b). Next, we fitted linear models for each of the two proxies of male fitness, with composite behavioral variables (PC1b, PC2b) and additionally the log-transformed body size (measured at start of tenure) as predictors (Supplementary Material Data Sheet 2). We included male body size at the start of his tenure, since body size is known to affect male fitness in reptiles (Kingsolver and Raymond, 2008).
Results
We tagged 138 males and obtained 101 focal observation sessions on 41 males (mean = 2.5 sessions/male, SD = 1.4, range = 1:6) over their breeding life span. All traits varied widely (Supplementary Table B). Within a focal session, males changed colors frequently, with much variation among males in the time spent in the different color states. Such a behavior of dynamic color change is rare among lizards.
Relationship of Male Behavior With Social and Ecological Context
In the PCA of behavioral traits, most of the display-related behaviors (headbob, pushup, crouch-shudder, proportion of time spent in orange color) co-varied closely and loaded strongly on the first PC axis (PC1a). That is, lizards with higher rates of headbob also performed crouch-shudder display and pushup more frequently and spent more time in orange color. Since this axis largely represents correlated display traits (factor loadings displayed in Table 1), we henceforth call it “composite display-index.” Behaviors related to maintaining body condition (mainly foraging) strongly loaded on the second PC axis (PC2a; henceforth called “body-maintenance”) (Figure 2, Table 1). These two components cumulatively explained 50% of the variation in the data. Bartlett sphericity test [ = 350.68, p < 0.001] and KMO measure (KMO = 0.60) confirmed that sampling was adequate. Modeling each of these two axes as a function of social and ecological contexts revealed that variation among focal sessions in composite display-index was most strongly related to the number of females in the vicinity during a session, and to a lesser extent, to predator presence (Table 2, refer to Δ column). The composite display-index appeared to increase with the number of females in the vicinity (Figure 3A), indicating greater rates of displays and more time spent in orange color with more females in the vicinity. The display index appeared to decrease with predator presence (Figure 3B), suggesting overall reduced signaling in the presence of predators. The composite display-index was not consistently related to the number of males in the vicinity and varied marginally across months. Body-maintenance related behaviors appeared to decrease with predator presence (Figure 3C), varied marginally across months, but did not show clear relationships with either the number of females or males in the vicinity (Table 2).
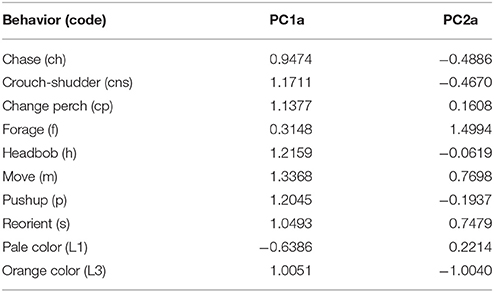
Table 1. Factor loadings of common behaviors in a Principal Component Analysis of common behaviors displayed across focal animal sampling sessions (n = 101) showing strong covariation in behaviors during a given session.
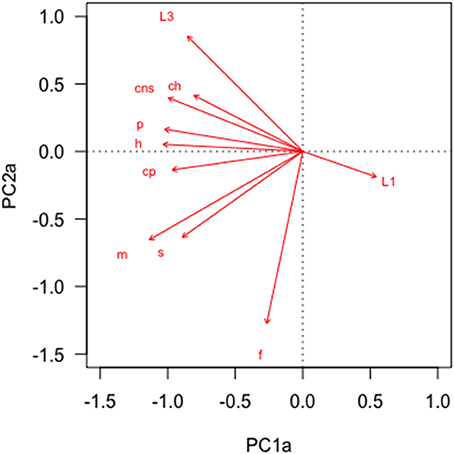
Figure 2. Principal Component Analysis of common behaviors across focal animal sampling sessions (n = 101) revealed strong covariation. Text-labels at the tip of each vector indicate behaviors (ch:Chase, cns:Crouch-shudder, cp:Change perch, f:Forage, h:Headbob, m:Move, p:Pushup, s:Reorient, L1:Pale color, L3:Orange color). See Table 1 for factor loadings.
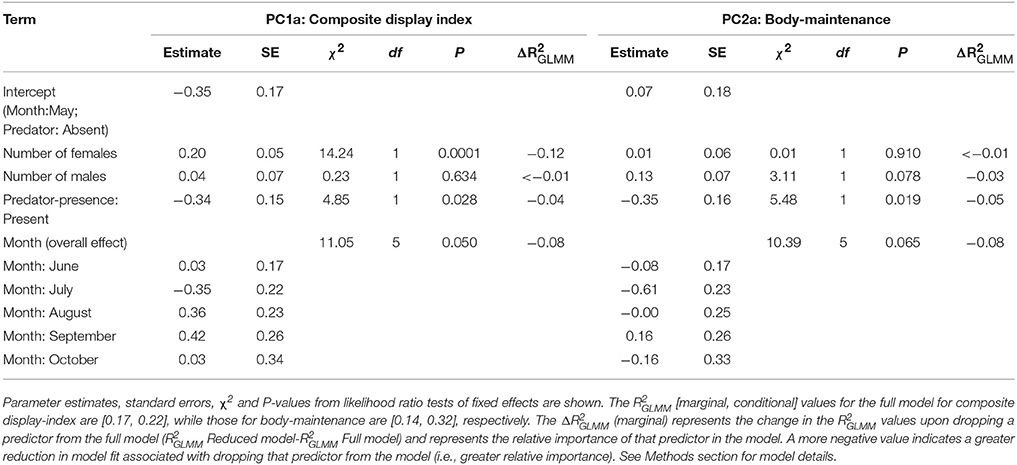
Table 2. Results from modeling composite behavioral variables, composite display-index, and body-maintenance, as a function of social (number of females and males in vicinity) and ecological (month and presence of predator) contexts using LMMs (n = 101 focal sessions).
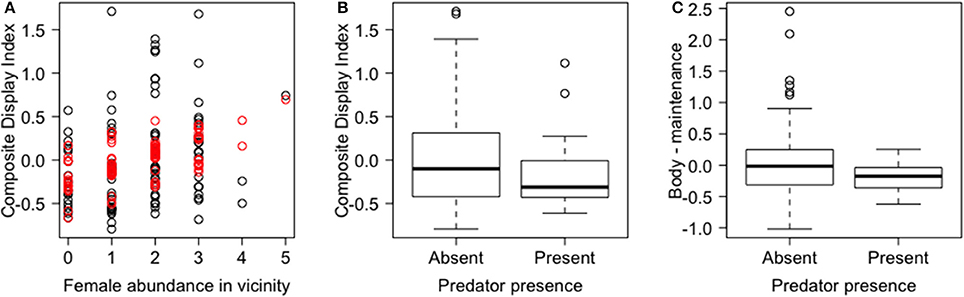
Figure 3. Relationships of composite behavioral variables (Principal Component axes) with social and ecological contexts. The main relationships detected in the linear mixed effects models of the two composite behavioral variables are shown. (A) Composite display-index, i.e., PC1a, (observed—black and predicted-red) is positively related to female abundance in the vicinity; (B) composite display-index is negatively related to predator presence; and (C) body-maintenance, i.e., PC2a, is negatively related to predator presence. Box plots show median and inter-quartile range.
Results of the Supplementary Models for individual behaviors closely supported those from analyses of composite behavioral variables (Tables 3, 4). Most display traits (e.g., headbob, crouch-shudder, proportion of time spent in orange color) were positively related to the number of females in the focal male's vicinity and negatively to predator presence. Only two traits—L1 color (positively) and L3 color (negatively)—were related to the number of males in the vicinity.
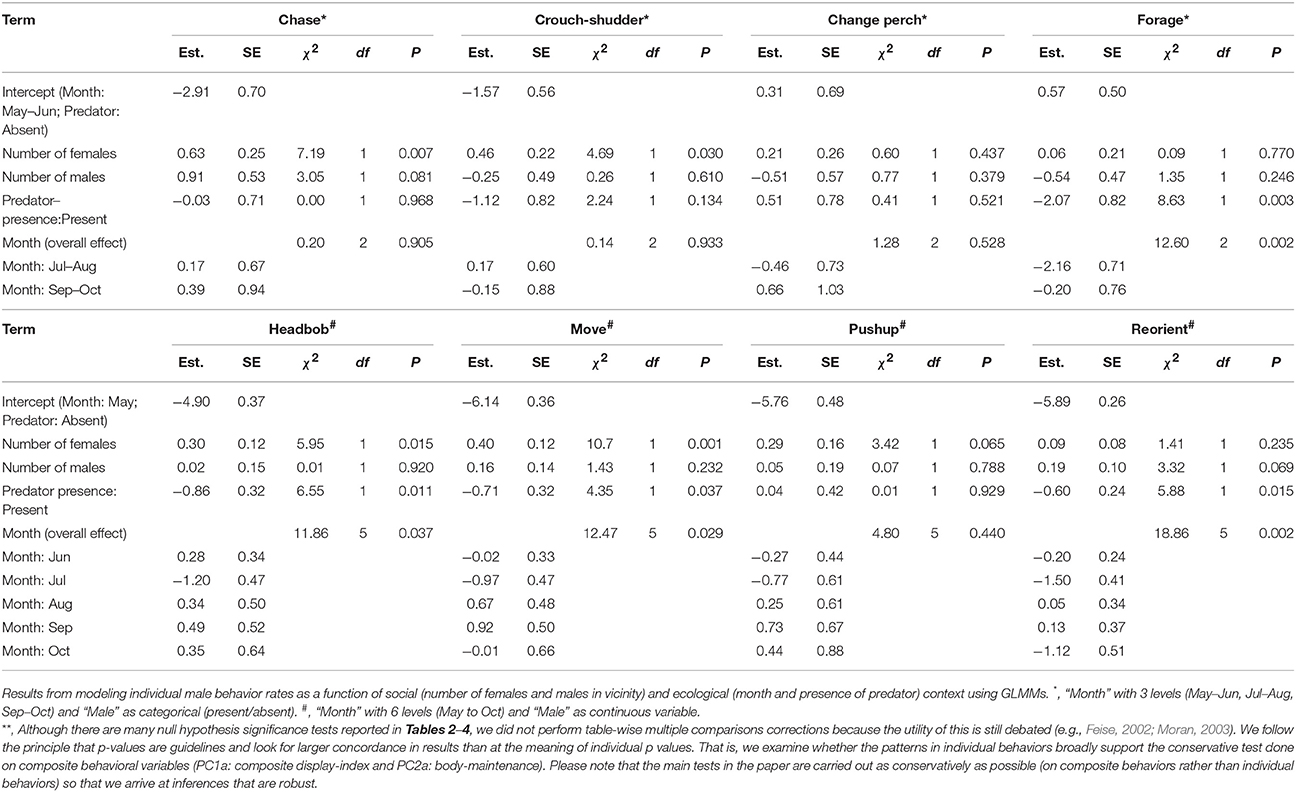
Table 3. Relationship of individual male behaviors (rates) with immediate social and ecological context **.
Relationship of Basal Behavior With Male Fitness Proxies
PCA revealed that behaviors adjusted for immediate social and ecological contexts still co-varied (Table 5, Supplementary Figure A). Bartlett sphericity test [ = 429.32, p < 0.001) and KMO measure (KMO = 0.69) confirmed that sampling was adequate. Display-related and body-maintenance behaviors loaded strongly on the first and second PC axes (PC1b, PC2b), respectively, which together explained 47% of the variation in the data. Lifetime breeding tenure was substantially related to composite display-index (adjusted) (R2 = 0.26; β[95% CI] = 38.05[13.69–62.42], F(1, 34) = 10.07, P = 0.003), but not detectably to body-maintenance (β[95% CI] = −16.69[−41.09 to 7.72], F(1, 34) = 1.93, P = 0.173) or size at start of tenure (β[95% CI] = −11.85[−160.33 to 136.64], F(1, 34) = 0.02, P = 0.872). Males that displayed more (i.e., higher values of composite display-index) had longer breeding tenures (Figure 4A). Females per day was consistently related to size at start of tenure (R2 = 0.34; β[95% CI] = −4.26[−7.52 to 0.99], F(1, 16) = 7.64, P = 0.014), but not detectably to composite display-index (β[95% CI] = 0.19[−0.36 to 0.73], F(1, 16) = 0.54, P = 0.474) or body-maintenance (β[95% CI] = 0.06[−0.43 to 0.56], F(1, 16) = 0.08, P = 0.786). Males that began their tenure at a smaller size appeared to have home ranges in areas with higher female densities (Figure 4B).
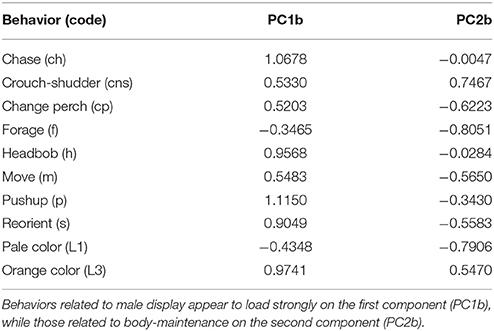
Table 5. Factor loadings from the Principal Component Analysis checking for covariation in adjusted behavior across males.
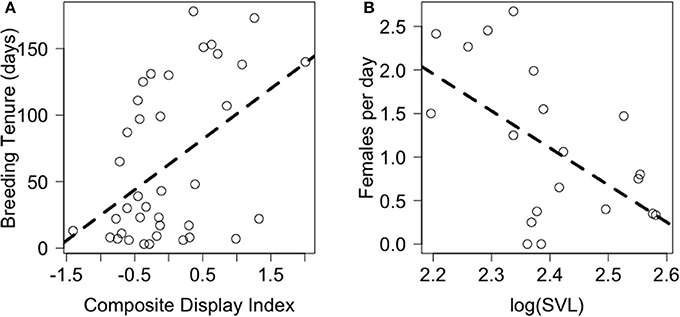
Figure 4. Relationship of proxies of male fitness, namely (A) breeding tenure and (B) females per day with (A) composite display-index and (B) body size at start of tenure, respectively. Dashed lines represent model predictions.
Discussion
We found that males regularly signal to conspecifics using multiple closely correlated traits, under natural ecological and social contexts. These traits appear to be primarily directed toward females, and to contribute toward male fitness. Our findings from a wild population provide rare evidence that these signaling traits are biologically relevant and appear to be influenced by multiple selection pressures.
Variation in Male Traits
Males typically used multiple behavioral traits while signaling, of which some (e.g., headbobs, pushups) were more common than others (e.g., gular extension, mounting). These stereotypical behaviors have been documented in other lizard species (Cowles, 1956; LeBas and Marshall, 2000; Radder et al., 2006). Apart from body postures and movements, P. dorsalis males were also observed dynamically changing their coloration from striking and conspicuous color patterns to paler and duller ones. Individual males modulated the color of their dorsal strip within a few seconds, with the lateral and ventral sides maintaining a dark color (see Figure 1). Additionally, during certain close-range male-male interactions, males showed a radically different color pattern (“Fighting” coloration), with red lateral and ventral sides and a yellow dorsal strip (Figure 1). Although such “dynamic color change” behavior has previously been reported in a few other species (Stuart-Fox and Moussalli, 2008; Kindermann et al., 2013; Teyssier et al., 2015) and recently in P. dorsalis (Batabyal and Thaker, 2017) there is relatively little information on this behavior from wild populations. We found that, like with other signals (e.g., headbob, pushup), males varied widely in the time they spent displaying in different coloration patterns.
What Maintains These Signaling Traits?
Male signaling behavior was dynamic and apparently sensitive to the costs and benefits associated with immediate social and ecological conditions. Our results suggest that the main benefits from signaling are related to attracting mates rather than to modulating male-male competition, and that predation risk is an important cost.
The frequency of most signaling traits increased with an increasing number of females in the vicinity, suggesting that they function in mate-choice. Similar patterns of males directing signals toward females (broadcasting and courtship), rather than toward males, have been reported from a few other lizards [collared lizard Crotyphytus collaris (Baird, 2013), brown anole Anolis sagrei (Driessens et al., 2014)]. Most signaling behaviors were not consistently associated with male abundance in the vicinity suggesting that their primary role may not lie in intrasexual competition. However, there was a weak relationship between the time spent in pale (+ve correlation) and orange (−ve correlation) colors and number of males, suggesting that the pale color pattern could be involved in male-male competition. Given that males can display only one color pattern at a time, if pale coloration is indeed directed toward males, and orange coloration toward females, this would indicate an interesting trade-off between intrasexual and intersexual signaling that could be pursued in future studies. Future work that experimentally simulates close encounters with competitors on male territories would help clarify the role of signaling traits in male-male competition.
Why Multiple Traits?
A striking result of this study is that males typically displayed using multiple signals, these multiple male signals covaried strongly, and increased simultaneously with increasing female abundance. These findings have implications for hypotheses of the maintenance of multiple signals. The clear covariation in signals in our study has rarely been reported (Candolin, 2003; Chaine and Lyon, 2015). The few previous studies that have explicitly examined for correlations in traits (Bro-Jørgensen and Dabelsteen, 2008; Chaine and Lyon, 2015; Ferrer et al., 2015) have mostly found weak or limited correlation in traits (but see Girard et al., 2015; Hegyi et al., 2015). The covariation of the main signaling traits in P. dorsalis and their relationship with female abundance suggests that the signals are redundant, and are perhaps maintained in the population because multiple redundant signals facilitate quicker and more accurate assessment of male phenotypic quality by females. Further work on the costs of these signals, the information they convey, and the response of receivers is needed to resolve how many of these signals are maintained because they are costly honest indicators (Moller and Pomiankowski, 1993; Johnstone, 1996) vs. non-informative low-cost signals that improve detectability and/or discriminability (Rowe, 1999).
Alternatively, each of these signaling traits could represent a different component of the male's quality and therefore be maintained as “multiple messages.” However, the strong correlations in these traits suggest that the different male-quality components are then strongly correlated. If this is the case, selection should result in the reduction of multiple signals to a single or a few traits that represent the correlated quality traits. Of the range of traits measured, a color trait (viz. time spent in pale color) showed a weak relationship with number of conspecific males in vicinity, suggesting that some of the traits could be maintained as multiple messages directed to multiple receivers. A study of Taurotragus oryx similarly suggested that both multiple message and redundant signal mechanisms might be involved in the maintenance of multiple signals (Bro-Jørgensen and Dabelsteen, 2008).
To summarize, the strong correlation between most of the traits measured suggests that these traits are maintained because of the greater effectiveness of multiple redundant traits in communicating content compared with a single trait. Further work on the information conveyed by these signals and the response of receivers to these signals is needed to confirm that these signals are redundant and to obtain a detailed understanding of the mechanisms maintaining these signals. Regardless of whether they are redundant signals, or whether they might represent multiple, correlated aspects of male quality, we find that these multiple traits appear to be primarily maintained in this population through sexual selection via female choice. Our findings highlight the insights that can be gained from comprehensively measuring the communication repertoire under multiple contexts.
Apart from social factors, we found that predation likely contributes toward maintaining variation in signals. None of the displays increased in the presence of predators suggesting that in P. dorsalis, these traits are not directed toward predators as a means to deter predation attempts. Rather, several displays appeared to reduce in the presence of predators, which suggests that these signals carry the cost of increased predation risk. Examining the evidence from other lizards, studies of Anolis lizards report contrasting results for the role of predation on male signals (Driessens et al., 2014). More generally, there is evidence from a wide range of taxa that predation risk is a common cost of conspicuous sexually-selected display traits (Tuttle and Ryan, 1981; Endler, 1992; Mougeot and Bretagnolle, 2000; Jones et al., 2002; Godin and McDonough, 2003; Stuart-Fox et al., 2003; Husak et al., 2006; Halfwerk et al., 2014).
Effect of Male Traits on Fitness
Our findings suggest that signaling can have important fitness consequences. Males that signaled more had longer breeding tenures. We assume that longer tenures are associated with increased mating opportunities. Previous studies, mostly covering a part of the lifespan, have also found that signaling has fitness consequences and is likely to experience strong selection (Bradbury and Vehrencamp, 1998; Girard et al., 2015); however, information on the relationship between signaling and lifetime measures of reproductive success is scarce.
Further, we found that males that were smaller in size at the start of their tenure were able to establish a territory in areas with higher female densities. This was unexpected, since we predicted that larger males would have their territories in female-dense areas. Since body size is correlated with age in many reptiles, one possible explanation is that individuals who are able to begin defending territories when younger are of higher quality, and correspondingly able to defend territories in female-dense areas, compared with males who establish territories when older (larger).
Conclusions
By tracking known individuals over their breeding lifespans in a wild population and comprehensively studying the signaling repertoire of breeding males, we found that multiple selection pressures (namely, intersexual selection and predation risk) appeared to affect male signaling traits. Most signaling traits appeared to be strongly correlated and directed toward females, providing support for the redundant signal hypothesis. A few traits seemed to be directed at conspecific males, providing limited support for the multiple message hypothesis. Finally, we found that the strongly correlated set of male behavioral signals, together with a morphological trait, may influence lifetime reproductive success, highlighting the biological relevance of these signaling traits.
Data Availability
Data and codes of statistical analyses are provided in the Supplementary Material. All descriptions of data sheets are included in the code.
Author Contributions
SD and KI were equally involved in designing the study, performing statistical analyses, and writing the manuscript. SD performed all fieldwork.
Funding
We thank funding agencies [Indian Institute of Science (IISc), Department of Biotechnology-IISc Partnership Grant, Department of Science and Technology FIST Grant]. Ministry of Human Resource Development for SD's fellowship.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to P. Somnath for providing support in fieldwork and two reviewers for constructive comments on the manuscript. We thank National Centre for Biological Sciences, Nature Conservation Foundation, and Rishi Valley for logistic support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00075/full#supplementary-material
References
Andersson, S., Pryke, S. R., Ornborg, J., Lawes, M. J., and Andersson, M. (2002). Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am. Nat. 160, 683–691. doi: 10.1086/342817
Baird, T. A. (2013). Male collared lizards, Crotaphytus collaris (Sauria: Crotaphytidae), signal females by broadcasting visual displays. Biol. J. Linn. Soc. 108, 636–646. doi: 10.1111/bij.12003
Batabyal, A., and Thaker, M. (2017). Signalling with physiological colours: high contrast for courtship but speed for competition. Anim. Behav. 129, 229–236. doi: 10.1016/j.anbehav.2017.05.018
Bókony, V., Lendvai, Á. Z., and Liker, A. (2006). Multiple cues in status signalling: the role of wingbars in aggressive interactions of male house sparrows. Ethology 112, 947–954. doi: 10.1111/j.1439-0310.2006.01246.x
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles of Animal Communication. Sunderland, MA: Sinauer Associates Inc.
Bro-Jørgensen, J. (2010). Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol. Evol. 25, 292–300. doi: 10.1016/j.tree.2009.11.003
Bro-Jørgensen, J., and Dabelsteen, T. (2008). Knee-clicks and visual traits indicate fighting ability in eland antelopes: multiple messages and back-up signals. BMC Biol. 6:47. doi: 10.1186/1741-7007-6-47
Budaev, S. V. (2010). Using principal components and factor analysis in animal behaviour research: caveats and guidelines. Ethology 116, 472–480. doi: 10.1111/j.1439-0310.2010.01758.x
Candolin, U. (2003). The use of multiple cues in mate choice. Biol. Rev. 78, 575–595. doi: 10.1017/S1464793103006158
Caro, T. M. (1986). The functions of stotting in Thomson's gazelles: some tests of the predictions. Anim. Behav. 34, 663–684.
Chaine, A. S., and Lyon, B. E. (2015). Signal architecture: temporal variability and individual consistency of multiple sexually selected signals (T Williams, Ed). Funct. Ecol. 29, 1178–1188. doi: 10.1111/1365-2435.12410
Cowles, R. B. (1956). Notes on natural history of a South African agamid lizard. Herpetologica 12, 297–302.
Deodhar, S., and Isvaran, K. (2017). Breeding phenology of Psammophilus dorsalis: patterns in time, space and morphology. Curr. Sci. 113:2120. doi: 10.18520/cs/v113/i11/2120-2126
Driessens, T., Vanhooydonck, B., and Van Damme, R. (2014). Deterring predators, daunting opponents or drawing partners? Signaling rates across diverse contexts in the lizard Anolis sagrei. Behav. Ecol. Sociobiol. 68, 173–184. doi: 10.1007/s00265-013-1669-4
Endler, J. A. (1992). Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. doi: 10.1086/285308
Feise, R. J. (2002). Do multiple outcome measures require p-value adjustment? BMC Med. Res. Methodol. 2:8. doi: 10.1186/1471-2288-2-8
Ferrer, E. S., García-Navas, V., Bueno-Enciso, J., José Sanz, J., and Ortego, J. (2015). Multiple sexual ornaments signal heterozygosity in male blue tits. Biol. J. Linn. Soc. 115, 362–375. doi: 10.1111/bij.12513
Fisher, M., and Muth, A. (1989). A technique for permanently marking lizards. Herpetol. Rev. 20, 45–46.
Girard, M. B., Elias, D. O., and Kasumovic, M. M. (2015). Female preference for multi-modal courtship: multiple signals are important for male mating success in peacock spiders. Proc. R. Soc. B Biol. Sci. 282:20152222. doi: 10.1098/rspb.2015.2222
Godin, J.-G. J., and McDonough, H. E. (2003). Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behav. Ecol. 14, 194–200. doi: 10.1093/beheco/14.2.194
Halfwerk, W., Dixon, M. M., Ottens, K. J., Kristina, J., Taylor, R. C., Ryan, M. J., et al. (2014). Risks of multimodal signaling: bat predators attend to dynamic motion in frog sexual displays. J. Exp. Biol. 217, 3038–3044. doi: 10.1242/jeb.107482
Hamilton, D. G., Whiting, M. J., and Pryke, S. R. (2013). Fiery frills: carotenoid-based coloration predicts contest success in frillneck lizards. Behav. Ecol. 24, 1138–1149. doi: 10.1093/beheco/art041
Hamilton, P. S., and Sullivan, B. K. (2005). Female mate attraction in ornate tree lizards, Urosaurus ornatus: a multivariate analysis. Anim. Behav. 69, 219–224. doi: 10.1016/j.anbehav.2004.03.011
Hebets, E. A., and Papaj, D. R. (2005). Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. doi: 10.1007/s00265-004-0865-7
Hegyi, G., Laczi, M., Nagy, G., Szász, E., Kötél, D., Török, J., et al. (2015). Stable correlation structure among multiple plumage colour traits: can they work as a single signal? Biol. J. Linn. Soc. 114, 92–108. doi: 10.1111/bij.12412
Husak, J. F., Macedonia, J. M., Fox, S. F., and Sauceda, R. C. (2006). Predation cost of conspicuous male coloration in collared Lizards (Crotaphytus collaris): an experimental test using clay-covered model lizards. Ethology 112, 572–580. doi: 10.1111/j.1439-0310.2005.01189.x
Johnstone, R. A. (1996). Multiple displays in animal communication: “backup signals” and “multiple messages.” Philos. Trans. R. Soc. B Biol. Sci. 351, 329–338.
Jones, G., Barabas, A., Elliot, W., and Stuart, P. (2002). Female greater wax moths reduce sexual display behavior in relation to the potential risk of predation by echolocating bats. Behav. Ecol. 13, 375–380. doi: 10.1093/beheco/13.3.375
Kindermann, C., Narayan, E. J., Wild, F., Wild, C. H., and Hero, J. M. (2013). The effect of stress and stress hormones on dynamic colour-change in a sexually dichromatic Australian frog. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 165, 223–227. doi: 10.1016/j.cbpa.2013.03.011
Kingsolver, J. G., and Raymond, H. M. (2008). Size, temperature, and fitness: three rules. Evol. Ecol. Res. 10, 251–268. Available online at: http://www.evolutionary-ecology.com/abstracts/v10/2242.html
Lappin, A. K., and Husak, J. F. (2005). Weapon performance, not size, determines mating success and potential reproductive output in the collared lizard (Crotaphytus collaris). Am. Nat. 166, 426–436. doi: 10.1086/432564
LeBas, N. R., and Marshall, N. J. (2000). The role of colour in signalling and male choice in the agamid lizard Ctenophorus ornatus. Proc. R. Soc. B Biol. Sci. 267, 445–452. doi: 10.1098/rspb.2000.1020
Loyau, A., Jalme, M. S., and Sorci, G. (2005). Intra- and Intersexual Selection for Multiple Traits in the Peacock (Pavo cristatus). Ethology 111, 810–820. doi: 10.1111/j.1439-0310.2005.01091.x
Marchetti, K. (1998). The evolution of multiple male traits in the yellow-browed leaf warbler. Anim. Behav. 55, 361–376. doi: 10.1006/anbe.1997.0586
Martín, J., and López, P. (2009). Multiple color signals may reveal multiple messages in male Schreiber's green lizards, Lacerta schreiberi. Behav. Ecol. Sociobiol. 63, 1743–1755. doi: 10.1007/s00265-009-0794-6
Moller, A. P., and Pomiankowski, A. (1993). Why have birds got multiple sexual ornaments? Behav. Ecol. Sociobiol. 32, 167–176. doi: 10.1007/BF00173774
Moran, M. D. (2003). Arguments for rejecting the sequential bonferroni in ecological studies. Oikos 100, 403–405. doi: 10.1034/j.1600-0706.2003.12010.x
Mougeot, F., and Bretagnolle, V. (2000). Predation risk and moonlight avoidance in nocturnal seabirds. J. Avian Biol. 31, 376–386. doi: 10.1034/j.1600-048X.2000.310314.x
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R-squared values from generalized linear mixed-effects models (RB O'Hara, Ed). Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Plasman, M., Reynoso, V. H., Nicolás, L., and Torres, R. (2015). Multiple colour traits signal performance and immune response in the Dickerson's collared lizard Crotaphytus dickersonae. Behav. Ecol. Sociobiol. 69, 765–775. doi: 10.1007/s00265-015-1892-2
Qvarnstrom, A. (1997). Experimentally increased badge size increases male competition and reduces male parental care in the collared flycatcher. Proc. R. Soc. B Biol. Sci. 264, 1225–1231. doi: 10.1098/rspb.1997.0169
Radder, R. S., Saidapur, S. K., and Shanbhag, B. A. (2005). Population density, microhabitat use and activity pattern of the Indian rock lizard, Psammophilus dorsalis (Agamidae). Curr. Sci. 89, 560–566. Available online at: http://www.currentscience.ac.in/Downloads/article_id_089_03_0560_0566_0.pdf
Radder, R. S., Saidapur, S. K., Shine, R., and Shanbhag, B. A. (2006). The language of lizards: interpreting the function of visual displays of the Indian rock lizard, Psammophilus dorsalis (Agamidae). J. Ethol. 24, 275–283. doi: 10.1007/s10164-006-0192-8
R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rek, P., and Magrath, R. D. (2016). Multimodal duetting in magpie-larks: how do vocal and visual components contribute to a cooperative signal's function? Anim. Behav. 117, 35–42. doi: 10.1016/j.anbehav.2016.04.024
Roberts, J. A., Taylor, P. W., and Uetz, G. W. (2006). Consequences of complex signaling: predator detection of multimodal cues. Behav. Ecol. 18, 236–240. doi: 10.1093/beheco/arl079
Rowe, C. (1999). Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921–931. doi: 10.1006/anbe.1999.1242
Ruby, D. E. (1984). Male breeding success and differential access to females in Anolis carolinensis. Herpetologica 40, 272–280.
Rundus, A. S., Sullivan-Beckers, L., Wilgers, D. J., and Hebets, E. A. (2011). Females are choosier in the dark: environment-dependent reliance on courtship components and its impact on fitness. Evolution 65, 268–282. doi: 10.1111/j.1558-5646.2010.01125.x
Smith, C. L., and Evans, C. S. (2008). Multimodal signaling in fowl, Gallus gallus. J. Exp. Biol. 211, 2052–2057. doi: 10.1242/jeb.017194
Stuart-Fox, D., and Moussalli, A. (2008). Selection for social signalling drives the evolution of chameleon colour change (FBM de Waal, Ed). PLoS Biol. 6:e25. doi: 10.1371/journal.pbio.0060025
Stuart-Fox, D., Moussalli, A., Marshall, N. J., and Owens, I. P. F. (2003). Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 66, 541–550. doi: 10.1006/anbe.2003.2235
Teyssier, J., Saenko, S. V., van der Marel, D., and Milinkovitch, M. C. (2015). Photonic crystals cause active colour change in chameleons. Nat. Commun. 6:6368. doi: 10.1038/ncomms7368
Tuttle, M. D., and Ryan, M. J. (1981). Bat predation and the evolution of frog vocalizations in the neotropics. Science 214, 677–678. doi: 10.1126/science.214.4521.677
Keywords: communication, multiple signals, redundant signal, multiple message hypothesis, sexual selection, reptiles
Citation: Deodhar S and Isvaran K (2018) Why Do Males Use Multiple Signals? Insights From Measuring Wild Male Behavior Over Lifespans. Front. Ecol. Evol. 6:75. doi: 10.3389/fevo.2018.00075
Received: 11 March 2018; Accepted: 15 May 2018;
Published: 06 June 2018.
Edited by:
Varvara Yu. Vedenina, Institute for Information Transmission Problems (RAS), RussiaReviewed by:
Eduardo S. A. Santos, Universidade de São Paulo, BrazilKeith Tarvin, Oberlin College, United States
Copyright © 2018 Deodhar and Isvaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shreekant Deodhar, c2hyZWVrYW50LmRlb2RoYXJAZ21haWwuY29t
 Shreekant Deodhar
Shreekant Deodhar Kavita Isvaran
Kavita Isvaran