
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 19 August 2020
Sec. Toxicology, Pollution and the Environment
Volume 8 - 2020 | https://doi.org/10.3389/fenvs.2020.00138
This article is part of the Research TopicMicroplastics in the Marine Environment: Sources, Distribution, Biological Effects and Socio-Economic ImpactsView all 24 articles
For this study, the transfer of plastic additives to stomach oil of northern fulmars (Fulmarus glacialis) has been investigated. Procellariiform seabirds retain oily components of their prey in theirs stomach as a means to store energy. A marine litter-derived microplastic reference mixture and separately a marine litter-derived polystyrene sample were added to stomach oils in an experiment. A total of 15 additives, including plasticizers, antioxidants, UV stabilizers, flame retardants, and preservatives, were identified in the original plastic mixtures and monitored in the leachates. These substances include those known for endocrine disruptive, carcinogenic, and/or other negative effects on organisms. Stomach oil was exposed to these plastic materials and was sampled during a long-term experiment (0, 14, and 90 days’ exposure of plastic particles in stomach oil) and a subsequent short-term detailed study (8 h and 1, 2, 4, 8, and 21 days). Five of the monitored substances were shown to strongly leach from the microplastic reference mixture into the stomach oil during the experiment. Four substances were identified in a marine litter-derived polystyrene foam, of which two leached into stomach oil. Leaching of harmful plastic additives to the stomach oil of fulmars may be of concern, as fulmars regularly ingest plastics that are retained and gradually ground in the gizzard before passage to the intestines and excretion.
Plastic pollution in the marine environment is ubiquitous in all ecosystems (Galgani et al., 2015; van Sebille et al., 2015) and represents a size continuum of items from macroplastic (> 5 mm), through microplastic (5 mm to 1 μm) to nanoplastic (< 1 μm; Arthur et al., 2009; Gigault et al., 2018). In addition, plastic pollution also represents a diverse range of polymer types that can contain a broad range of additive chemicals that provide specific properties and functionalities to plastic products (Rochman, 2015; Rochman et al., 2019). Northern fulmars (Fulmarus glacialis) are seabirds of the order of tubenoses (Procellariiformes). They are non-specialized foragers, opportunistically hunting for fish, squid, crustaceans, and jellyfish at or close to the seawater surface (Camphuysen and Van Franeker, 1997; Ojowski et al., 2001; Byrkjedal and Langhelle, 2019), but they also scavenge on ship offal and carrion (Camphuysen and Garthe, 1997). Fulmars regularly ingest plastics in high quantities, with 95% of birds studied in the North Sea containing an average of 31 plastic particles at an average mass of 0.28 g (OSPAR, 2019). These plastic loads decrease with latitude and reflect local abundances of plastics in the environment (Mallory, 2008; Van Franeker et al., 2011; Kühn and Van Franeker, 2012; Trevail et al., 2015). In contrast to other seabirds, most Procellariiformes store energy reserves both in adipose fat and in oil accumulated in the proventriculus of the bird (Wang et al., 2007). This light yellow– to dark orange–colored oil is produced from dietary remains and is not a product of stomach excretions (Lewis, 1969; Clarke and Prince, 1976; Imber, 1976). The composition of stomach oils can vary greatly, depending on prey species and the most recent diet, but typically consists of different types of wax esters, diacyl glycerol ethers, and triglycerides (Lewis, 1969; Imber, 1976). Stomach oil is found in all life stages of Procellariiform seabirds and is an efficient way to store highly concentrated caloric food in low volumes (Place et al., 1989). According to Place et al. (1989), this is more efficient and corresponds more flexibly to the energy demands of the birds as metabolism of fatty acids to the adipose fat reserves is unnecessary. When threatened, fulmars also spit out stomach oil as an effective deterrent. Hydrophobic organic pollutants such as petroleum hydrocarbons (Clarke and Prince, 1976), polychlorinated biphenyls (PCBs), and pesticides (Foster et al., 2010) are lipophilic, meaning that they preferentially partition and dissolve in stomach oil. As hydrophobic organic pollutants can be obtained via contaminated natural food, stomach oil has been suggested as a suitable monitoring medium for marine pollution (Clarke and Prince, 1976).
Plastic debris can contain a broad range of additive and adsorbed chemicals with associated degradation products (Andrady and Neal, 2009). There are two main pathways in which chemicals are associated with plastic debris. First is the addition of chemicals during their production process to enhance specific characteristics of the plastics (e.g., flexibility, flame resistance, or color) and residual chemicals from this production process (Rani et al., 2015). The other pathway occurs when plastics are exposed to dissolved chemicals already present in the (marine) environment (Teuten et al., 2009). Substances can be adsorbed to the plastic surface, especially smaller plastic items, which exhibit a comparably larger surface-to-volume ratio (Barnes et al., 2009). Weathering of plastics in the marine environment may enhance this adsorption process (Jahnke et al., 2017). The bioavailability of these plastic-associated chemicals following ingestion of plastic debris by marine organisms remains unclear, but has been suggested to be a function of gut conditions, gut residence time, environmental concentrations, and previous exposure/existing chemical accumulation in individual organisms (Koelmans et al., 2016; Sørensen et al., 2020).
The combination of both chemical exposure pathways, together with the physical characteristics of plastic, may harm marine wildlife when plastic debris is ingested. The capacity for wildlife to take up a range of plastic associated chemicals has been demonstrated in laboratory experimental setups (e.g., Teuten et al., 2009; Browne et al., 2015; Hermabessiere et al., 2017; Tanaka et al., 2018; Roman et al., 2019a). However, unrealistic exposure scenarios are often applied during experiments with regard to the type and shape of plastic, degree of degradation, and associated toxic substances, as most studies use homogeneous-shaped, pristine plastics (Phuong et al., 2016; Sørensen et al., 2020). Furthermore, most studies lack environmental relevance because of artificially loading the plastic materials with high concentrations of chemicals prior to study, and very few studies consider factors such as background levels of chemicals or existing chemical levels in organisms (Heinrich et al., 2020).
In 2020, the ingestion of plastics by marine organisms had been reported in at least 701 species (Kühn and van Franeker, 2020). Procellariiform seabirds, in particular, were found to regularly ingest plastics, possibly confusing them with natural diet items (Kühn et al., 2015; Ryan, 2016). Of 144 Procellariiform seabird species, 63.2% have been recorded with ingested plastics (Kühn and van Franeker, 2020), sometimes in frequencies of occurrence higher than 90% (e.g., Van Franeker et al., 2011; Roman et al., 2016; Rapp et al., 2017; Rodríguez et al., 2018). Procellariiformes ingest a wide variety of plastics, including different shapes (Van Franeker et al., 2011) and colors (Kühn et al., 2015), with the size of the ingested plastics related to body size (Roman et al., 2019b).
The first prolonged contact of ingested plastic (and natural food) with Procellariiform seabirds occurs in the proventricular stomach where gastric juices are produced to initiate the digestion process. Once in the smaller and muscular gizzard, plastic items are gradually worn, and pieces small enough to pass to the intestines are excreted (Fisher, 1952; Warham, 1996). However, the intensity and pace of the wearing process are not fully understood. In northern fulmars, hard plastic particles have to be reduced to just a few millimeters in size before they can pass from the gizzard to the intestines (Bravo Rebolledo, 2011; Terepocki et al., 2017). Terepocki et al. (2017) indicated different sizes of plastics along the digestive system from the proventriculus, via the gizzard, to the gut. The average particle mass reduced from 66 to 25 mg and finally 7 mg, respectively. The retention time of plastic in seabirds is unknown and may vary between species and by type of plastic item (size, shape, and flexibility). For the northern fulmar and its close relatives, Van Franeker and Law (2015) have suggested 75% of ingested plastic disappears within a month. However, for other species, retention times of several months to even years have been suggested (Ryan, 2015).
The leaching of chemical components from particulates to the surrounding medium is known to increase as a function of decreasing particle size and the corresponding increase in surface area (Rochman, 2015). The grinding process in seabird digestive systems increases the surface area of ingested plastics in the stomach, which consequently increases the available surface area for additive chemicals to partition into the stomach oil. As the medium in this case is an oil, it is expected that hydrophobic chemicals in particular will preferentially partition from the plastic (Tanaka et al., 2015). This process has been described previously by Tanaka et al. (2015), where the uptake of polybrominated flame retardants to stomach oil of Procellariiformes has been recorded. Once plastic particles have been reduced to a size that they can be easily excreted, it is likely that any further partitioning of additive chemicals is limited owing the short residence times of particles < 1 mm.
There has been limited focus in previous studies on the partitioning of additive chemicals. The current study aims to document the potential uptake of harmful plastic additive chemicals into fulmar stomach oil using plastic debris sampled from the marine environment as test materials. The mechanism of uptake of substances from plastic into seabirds is a crucial step to understand potential harm of plastic on seabirds, both at the individual and population levels.
Plastic debris was collected and carefully characterized in terms of polymer type, shape, and size and milled into a microplastic mixture (Kühn et al., 2018). A comparable sample was prepared using beached polystyrene foam only. Stomach oil collected from fulmars was exposed to these plastics under realistic gut conditions. The additive chemical profiles were determined in the source materials and the stomach oil samples after exposure.
A marine litter-derived microplastic reference material (PTX001) was used. The collection and production of the material have been described in detail in Kühn et al. (2018). Briefly, 351 macroplastic litter items were collected from a Dutch beach (Texel, April to August 2016), equaling the mass composition of plastics during an earlier large beach clean-up. The sample comprised a mixture of rigid and flexible items (ca. 37 and 63%, respectively). The collected material was cryomilled (Retsch ZM 200, Carat GmbH) to a mix of variable sizes less than 3.0 mm in diameter. The size distribution of these particles was determined by sieving the mixture through a stacked sieve system. One gram of the PTX001 material contained around 400,000 plastic particles. The produced microplastics are irregular in shape and exhibit a broad size distribution, being more environmentally representative than the uniform spherules often used in exposure studies (Phuong et al., 2016). The polymer composition comprised mainly polyethylene (PE; 60.9%) and polypropylene (PP; 27.7%), but with many other polymers present in small amounts that provide a distribution similar to that of global polymer production (Geyer et al., 2017) and that found in seabirds (e.g., Tanaka et al., 2019). A detailed chemical analyses confirmed the presence of various heavy metals (Cd, Cr, Cu, Fe, Ga, In, Mn, Mo, Ni, Pb, Pd, Sn, Zn) and light metals (Al, Ba, Ca, K, Mg, Na, Sr, Ti), as well as additive chemicals and chemicals associated with plastic production processes (Kühn et al., 2018). A summary of the plasticizer, UV stabilizer, and flame retardant additives found in the mixture is listed in Supplementary Table 5. To determine the contribution of a different plastic type to the total process of additive leaching, three pieces of weathered expanded polystyrene foam (PS) were collected at the same time from the same beach as the macroplastic litter items used in production of the PTX001 mixture. The PS foam was cut manually to small particles of ∼0.5 mm in size, because cryomilling of the foamed material proved unsuccessful.
Stomach oil was collected from northern fulmars on the Faroe Islands in the north Atlantic Ocean, where fulmar fledglings are harvested for human consumption (Jensen, 2012). Fledglings are caught from the sea surface with a long-handled net (“fleygg”). Shortly after fledging, most of the young birds are too heavy to take off and are easily caught. Once caught, birds are immediately killed by breaking the neck. The fledglings not only have large fat deposits but often also contain considerable quantities of stomach oil (some tens to well over 100 mL). For this project, hunters prevented the loss of the oil by tying a small rope around the neck and provided us with the undamaged stomachs. The oil was drained directly from the stomach into glass bottles and frozen at −20°C. The stomach oil used in the current study was a homogenate combined from more than 50 different chicks collected between 2014 and 2016. As natural foods may contain contaminants, and as chicks already contain plastics transferred by their parents, a basic load of chemicals in the stomach oil was already expected at the start of the experiments.
Two exposure experiments were conducted: a long-term experiment (LTE) with three sampling points (0, 14, and 90 days) and a detailed short-term study with sampling at 8 h and 1, 2, 4, 8 and 21 days. The procedure and setting for both studies were the same, and the same batches of homogenized stomach oil and plastic test materials (PTX001 and PS) were used in both studies. Plastics were added to the stomach oil and stirred continuously at 120 revolutions/min in a shaking bath (Julabo SW23) to mimic stomach contractions and to keep the plastics in suspension. The oil was kept in amber glass vials at 40°C, the common body temperature in Procellariiform seabirds (Warham, 1996). The exposure design comprised two bottles of the PTX001 microplastic mixture, two bottles of PS, and two control bottles without added plastic, with each exposure and control sample containing 40 mL of stomach oil. The added quantity of plastics was 1.0 g for the PTX001 samples (25 g/L) and 0.3 g for the PS (7.5 g/L). A lower exposure concentration was used for the PS foam (7.5 g/L) because the particles had a very low density by high volume relative to the available volume of stomach oil. A 5 mL aliquot of oil was removed from each bottle at each sampling time point. Care was taken to ensure oil and plastics were removed in proportional quantities by using a large glass pipette where plastics were retained together with the oil. The oil was then vacuum-filtered through a glass microfiber filter (GFF, pore size 0.7 μm; GE Whatman) in a glass filtration system to remove the plastic particles (Figure 1). The filtered oil from each 5-mL sample was then divided between three glass vials of ~1 mL each and directly frozen at −20°C until further analysis. This corresponds to 3 × 1-mL test vials being retrieved at each time point from each duplicate bottle, resulting in a total of six subsamples per plastic type and time point. All laboratory materials and Teflon-capped sample containers were carefully rinsed with hexane prior to use to reduce contamination.

Figure 1. (A) Marine derived plastic litter (PTX001) in stomach oil before filtration. (B) PTX001 on a GFF filter (diameter 25 mm) after filtration. Note the different plastic shapes, sizes, and colors.
In total, three sets of chemical analyses were conducted:
1. The LTE: Oil samples collected and analyzed on day 0 (control only) and next after 14 and 90 days, with controls repeated at both dates. The sampling point of 90 days was chosen as the retention time of plastics in bird species has been reported to be between 2 months (Terepocki et al., 2017) and many months (Ryan and Jackson, 1987; Ryan, 2015).
2. The short-term detailed experiment (STD): Replicated the LTE setup, but over a shorter timescale. STD oil samples were taken on day 0 (control) and next after 8 h and 1, 2, 4, 8, and 21 days. Controls were measured in the first three and last samples.
3. The long-term replicate (LTR): These analyses represented a check on the replicability of the initial (LTE) methods. The 14-day, 90-day, and control samples from the LTE were reanalyzed.
Samples of the plastic materials (∼500 mg PTX001 mixture, ∼18–30 mg PS foam) were solvent extracted in triplicate with two different solvents; dichloromethane (DCM, Rathburn) and ethyl acetate (EtOAc, Fluka). In each case, 4 mL of solvent and an internal standard mixture (0.2508 μg naphthalene-d8, 0.0500 μg phenanthrene-d10, 0.0486 μg chrysene-d12) were added to each sample prior to bath sonication for 30 min (Bandelin Sonorex Super RK 510 H, 640 W, 35 kHz) at either room temperature (DCM) or 65°C (EtOAc). The solvent extract was then filtered through a pipette packed with Bilsom cotton to remove plastic particles and a small amount of anhydrous Na2SO4 to remove any moisture. The extracts were then concentrated by solvent evaporation (40°C under a gentle flow of N2) to ~500 μL, and a recovery internal standard (0.0984 μg fluorene-d10 and 0.1064 μg acenapthene-d10) was added prior to gas chromatography–mass spectrometry (GC-MS) analysis. Prior to clean-up by gel permeation chromatography (GPC), samples extracted by DCM were readjusted to 1 mL volume with additional DCM.
Samples of fulmar oil (50 mg in the LTE and 100 mg in the LTR and STD) were transferred from the 1-mL vials to a glass tube and dissolved in 1 mL DCM:n-hexane (1:1). An internal standard mixture (0.2508 μg naphthalene-d8, 0.0500 μg phenanthrene-d10, 0.0486 μg chrysene-d12) was added, and the sample vortexed (30 s). The sample volume was then adjusted to 1 mL by solvent evaporation (40°C under a gentle flow of N2).
Both fulmar oil extracts and DCM polymer extracts were subject to instrumental clean-up by GPC (Agilent). Samples (500 μL) were injected with DCM as the mobile phase (0.5 mL/min in the LTE, 5 mL/min in the LTR and STD), and components separated using either an Agilent PLGel column (7.5 × 300 mm, 5 μm; LTE) or a Waters Envirogel column (19 × 300 mm, 15 μm; LTR and STD). Chromatograms were monitored at 210, 254, and 280 nm UV. After initial optimization, analyte fractions were collected from 16 to 35 min (LTE) or 10.5 to 15 min (STD) with preadded n-hexane in the collection vials as a keeper. The sample volume was adjusted to 0.5 mL by solvent evaporation (40°C under a gentle flow of N2), and recovery internal standards (0.0984 μg fluorene-d10 and 0.1064 μg acenapthene-d10) were added prior to GC-MS analysis.
The GC-MS system comprised an Agilent 7890A GC equipped with an Agilent 5975C Mass Selective Detector. The inlet was set to 250°C, the transfer line to 300°C, the ion source to 230°C, and the quadrupole to 150°C. The carrier gas was helium at a constant flow of 1.1 mL/min. Samples of 1 μL were injected by pulsed splitless injection. The GC column was an Agilent DB5-MS ultra-inert column (30 m, 0.25-μm film thickness, 0.25-mm internal diameter). The GC oven was held at 40°C (2 min), ramped at 6°C/min to 320°C, and held at that temperature for 20 min. Mass spectra were recorded in full scan mode over the mass range 50 to 500 m/z, after a 12-min hold time.
Using compounds identified from the full-scan analysis of the PTX001 and PS material extracts, a selected ion monitoring (SIM) method was developed to enable a more detailed, targeted analysis of chemicals present in the stomach oil extracts. This approach increases the sensitivity of the analysis and helps to reduce background noise and interference from biogenic compounds derived from the stomach oil. The same GC-MS system and instrumental conditions as above were applied. Selected ions representative of the tentatively identified organic additive compounds were monitored according to Supplementary Table 1.
For non-target screening, chromatograms and mass spectra were recorded using Chemstation software, investigated in Masshunter Qualitative Navigator B.08.00, further processed using Masshunter Unknowns Analysis (“Unknowns”) followed by export to csv format using Python and data processed in R. After initial inspection of chromatograms, peaks were deconvoluted using Unknowns algorithms, and best hits from NIST 2017 library were extracted. Compounds were filtered based on their observed presence in at least three of six replicates for each polymer and a > 90% match to NIST 2017 library mass spectra. Biogenic compounds, or compounds of possible biogenic origin, were removed from the data set. All compounds found in the control samples were also removed from the data set, leaving only those that could be confidently attributed to coming from the PTX001 and PS materials.
For targeted analysis, the selected tentatively identified compounds were recorded by their retention time and major ions (Supplementary Table 1) in GC-MS SIM mode. Masshunter Quantitative Analysis was further used to integrate peak areas of the selected compounds and the added internal standards. The area of each tentatively identified compound was normalized by dividing by the area of one internal standard in each sample and the normalized relative intensities used to compare samples.
Given the exploratory and non-quantitative nature of the analysis, there was a lack of reference standard chemicals for the identified additives, and therefore, it was not possible to establish individual calibration curves for each chemical. Control-derived limits of detection (LOD) were established based on control measurements for each of the three treatments separately. The LOD was calculated as the average of the controls of each treatment plus three standard deviations. As expected, results showed that even the unexposed control stomach oil samples contained some level of additive chemicals already present when the oil was harvested.
In the current study, non-target screening with a 90% confidence match to library spectra (>90% match to NIST 2017 library) permitted identification of 15 different organic chemical additives in the solvent extracts produced from the two test materials, with 14 identified in PTX001 and 4 identified in the PS (Table 1). Three of the identified compounds were found in both materials (acetophenone, propanediylbisbenzene, and triphenylbenzene). Full names for each compound are given in Table 1. These substances include common additives such as plasticizers, antioxidants, UV stabilizers, flame retardants, and preservatives (Table 1). For some chemicals identified in the samples, however, the use or origin is unclear. Chemical properties and estimated biodegradability, bioaccumulation, and biotransformation rates of these compounds are given in Supplementary Table 6. Estimates have been calculated using the BIOWINTM and BCFBAFTM packages of EpiSuite (US EPA, 2012). For both the long-term experiments (LTE, LTR) and the short-term experiment (STD), the temporal concentration trends of each target compound in the stomach oil are shown in Supplementary Material (Chapters 3.1–3.15).
Stomach oil extract samples from long-term exposures to PTX001 and PS (0, 14, and 90 days) were analyzed twice (LTE and LTR) to evaluate analytical reproducibility, because the LTE and STD results were generated using two different GC-MS analysis methods. The results of the LTE and LTR analyses showed mostly consistent responses relative to the internal standard (phenanthrene-d10) and how it relates to the LOD. For five substances (acetophenone, propanediylbisbenzene, triphenylbenzene, DEHP, and di-(2-ethylhexyl) terephthalate), the response scale increased for the LTR. The relative LOD was comparable for all substances during the LTE and the LTR.
For most samples, the two bottles representing identical treatments (bottles A and B; indicated in Supplementary Material graphs by two samples at the same time point) show similar averaged results, indicating the replicability of the chosen approach. The highest variation between A and B samples was observed for the compound TCPP (3:1) for PTX001 and PS (but not for the controls) during the LTE and the LTR experiments (Supplementary Material Chapter 3.7, page 13). Within these bottles, the replicability was generally good (indicated in the graphs with error bars per sample). Different results between pairs of A and B sample bottles were mainly observed in the STD experiment. For PTX001, a high deviation was observed in TCEP and bumetrizole, whereas for PS, a high deviation was observed in 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione and DBP. Among control bottles, the difference between the A and B samples was pronounced for only one compound, di-(2-ethylhexyl) terephthalate.
Another indication of the reproducibility of the measurements is demonstrated by the control values, which remained relatively stable over time during all measurements with only a few exceptions. Variable control values over time were observed mainly during the first days of the STD experiment, with the strongest variation found in DEHP (Supplementary Material Chapter 3.13 page 19).
All experimental results are summarized in Table 2 and presented in detail in the graphs in Supplementary Material Chapters 3.1–3.15. A selection of results is shown and discussed here, subdivided into (i) additive compounds that exhibited clear signs of leaching to the stomach oil and (ii) compounds for which there was no evidence of leaching. Other substances showed weak or inconclusive results (Table 2). For improved visual interpretation, graphs for the LTE show connection lines from the 0-day measurement (control) to the 14- and 90-day measurements. STD graphs include linear trendlines and standard deviations of the duplicate measurements. In Supplementary Material, all graphs include linear trendlines and standard deviation.
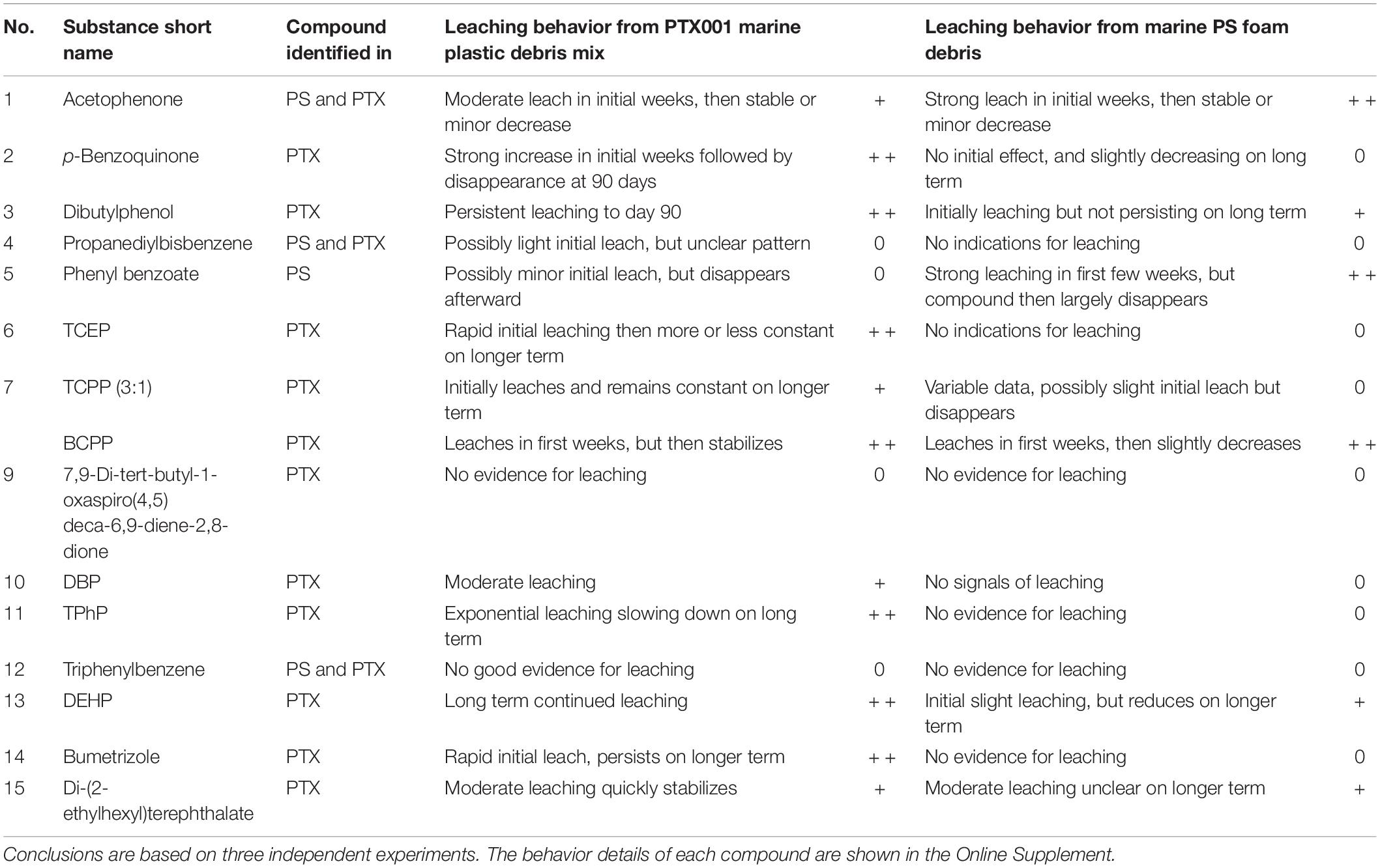
Table 2. Summary of the leaching results for different substances detected in marine litter-derived microplastic reference mixture (PTX001) and marine litter-derived polystyrene foam (PS) to stomach oil of northern fulmars (0 = no, + moderate, + + strong leaching).
Fast leaching of acetophenone (a precursor to resins and copolymers) from both PTX001 and PS foam to the stomach oil was evident during the first 2 weeks (Figure 2 and Supplementary Material Chapter 3.1 page 7), with PS foam especially exhibiting a high level of leaching. STD results show that acetophenone started leaching from PS almost immediately, followed by strong increase in relative concentration up to day 8 and reached the highest level by day 21. The LTE experiment supports that pattern, showing a high increase during the first 14 days followed by a stabilization until day 90. For PTX001 in the LTE experiment, the acetophenone level increased above the level in the control samples after 14 days and subsequently decreased slightly at day 90. During the STD experiment, acetophenone showed gradual leaching from PTX001 until day 21, however, leaching was less pronounced than in PS.
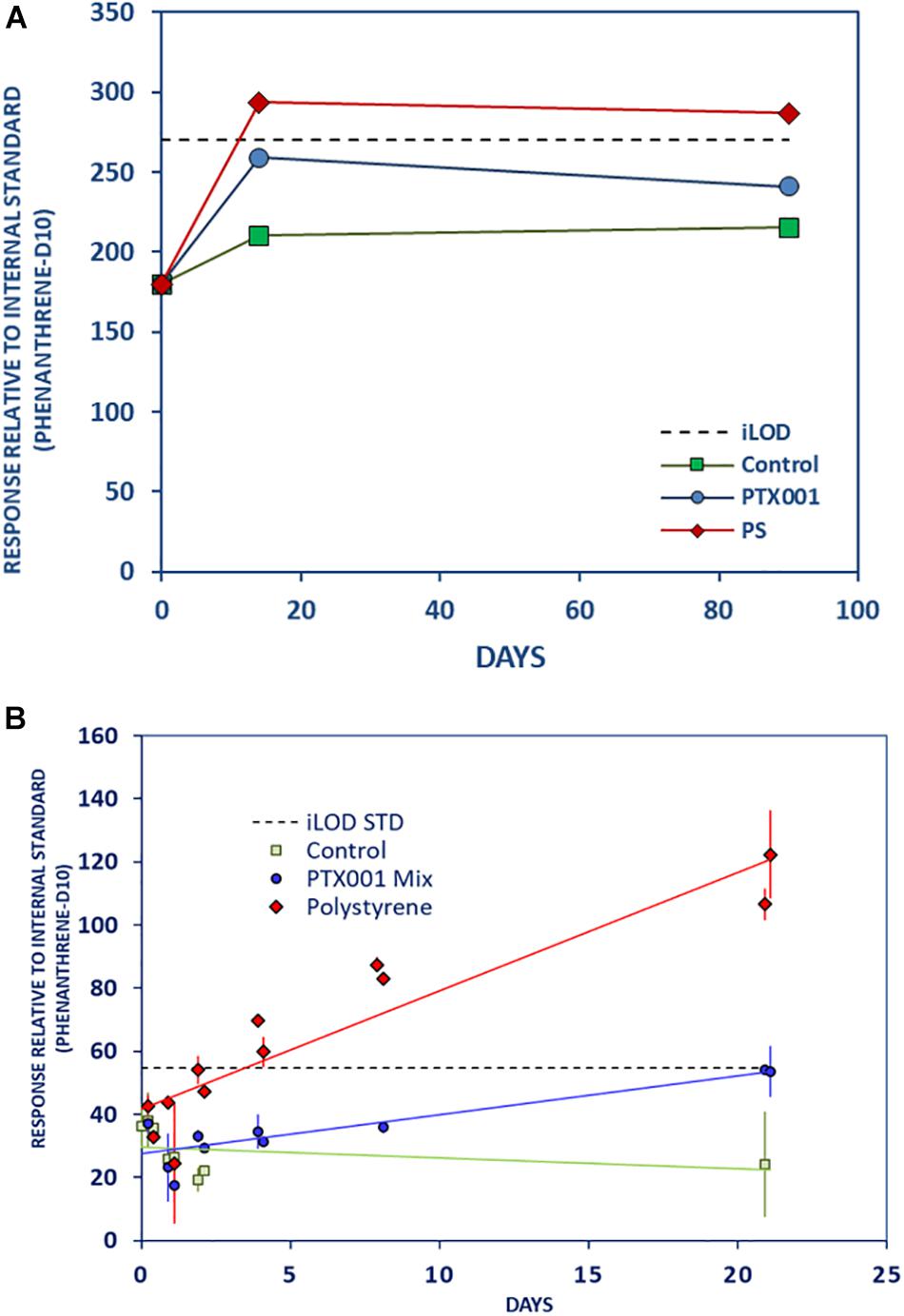
Figure 2. Strong leaching of acetophenone from PS and moderate leaching from PTX001 to stomach oil. (A) long-term experiment (LTE) with connecting lines and (B) short-term detail experiment (STD) with linear trendlines and standard deviations. Both lines are only used for visualization of data and do not imply that leaching patterns are linear.
Strong leaching of dibutylphenol, an antioxidant additive, was observed to occur from the PTX001 plastics (Figure 3). LTE and LTR results suggest gradual leaching for up to 3 months. Results from the STD experiment are rather variable but do support leaching also in the initial weeks. Some leaching of dibutylphenol may also occur from PS, but results are rather variable (Supplementary Material Chapter 3.3, page 9).
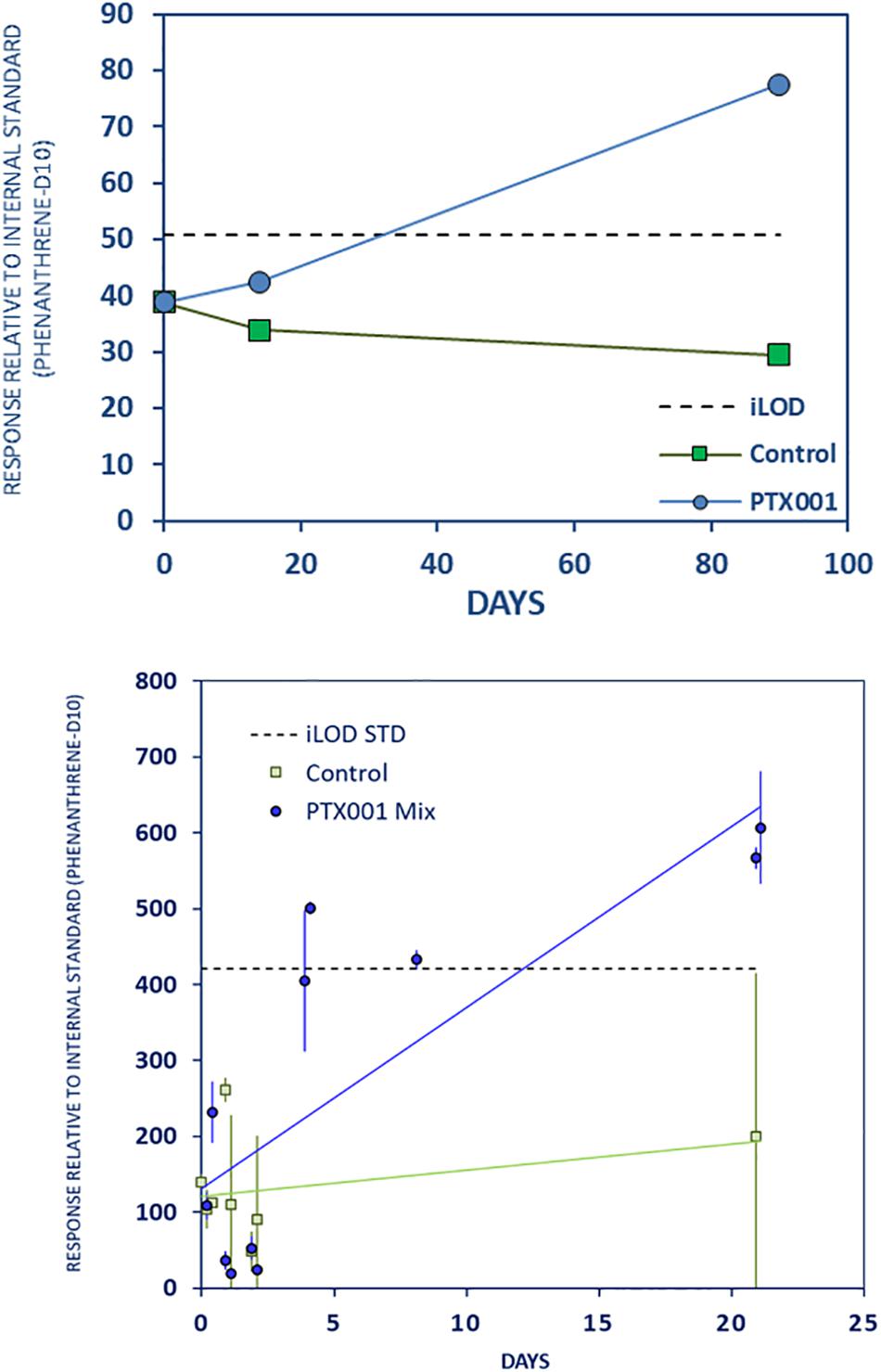
Figure 3. Strong gradual leaching of dibutylphenol from PTX001 to stomach oil. Further caption details as in Figure 2.
Phenyl benzoate, a preservative, showed an initial strong leaching from PS foam to the stomach oil in all three experiments (Figure 4). Results from the LTE and the LTR experiments indicated a decrease of phenyl benzoate leachate concentrations between 14 and 90 days, although the substance was still above the control levels at 90 days (Supplementary Material Chapter 3.5, page 11).
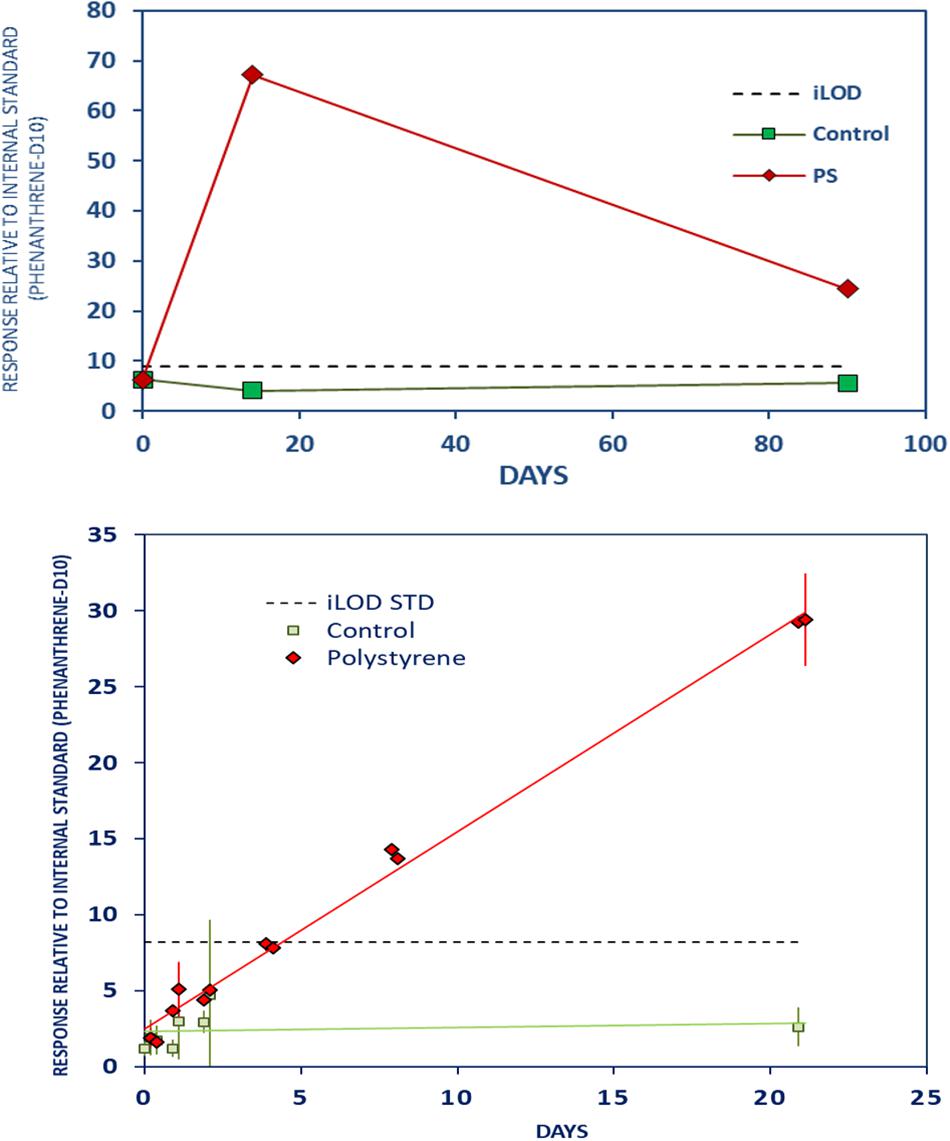
Figure 4. Strong initial leaching of phenyl benzoate from PS to stomach oil with decrease in leachate concentrations between 14 and 90 days. Further caption details as in Figure 2.
TCEP, a plasticizer, flame retardant, and viscosity regulator, showed an initial rapid leaching from PTX001 during the LTE and the LTR experiments, which decreased only slightly after 90 days (Figure 5 and Supplementary Material Chapter 3.6, page 12). During the STD experiment, high variation within both replicate samples (bottles A and B) was observed, and although all data points are above the control level, the results are probably less reliable.
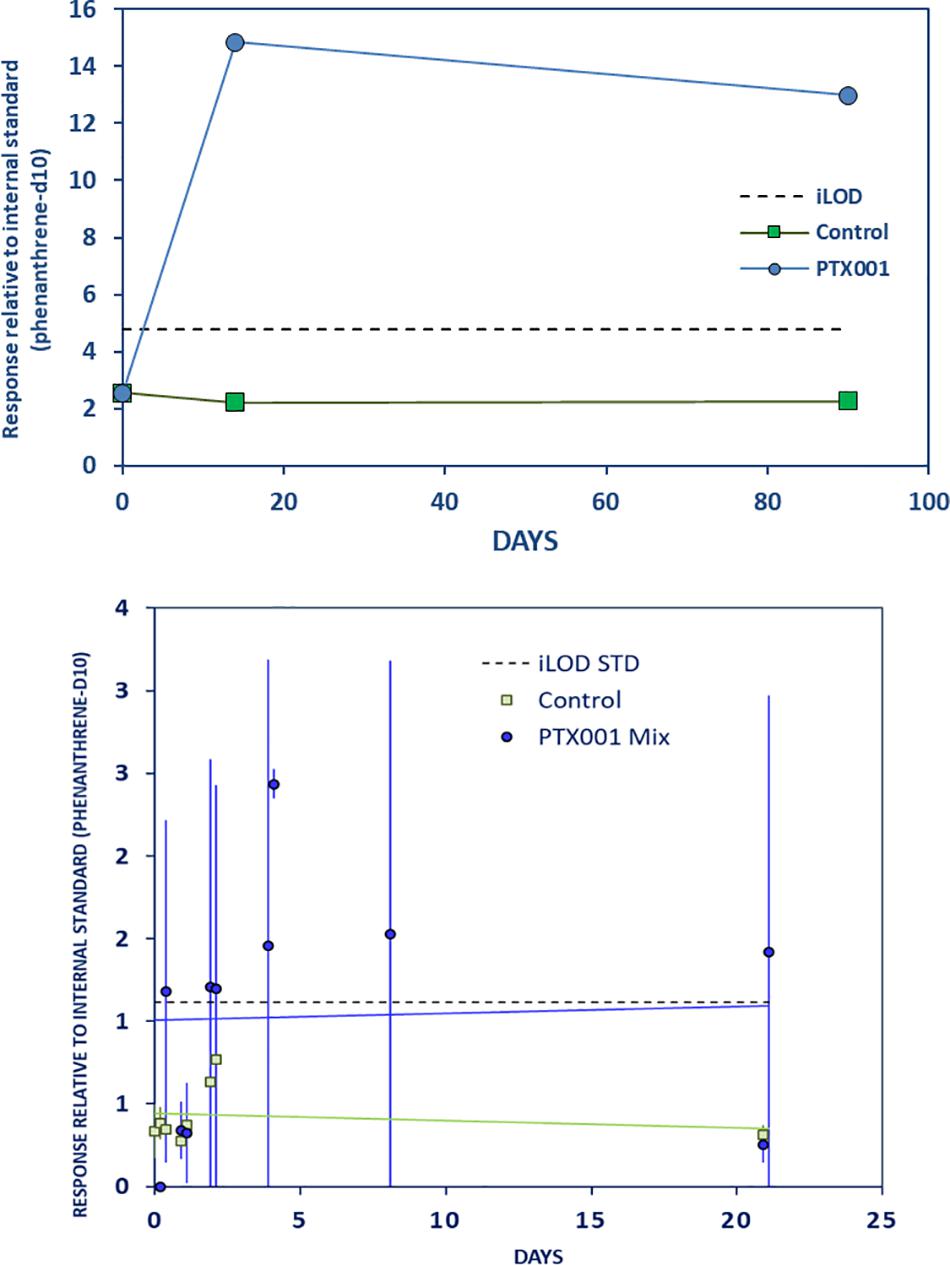
Figure 5. Strong initial leaching of TCEP from PTX001 to stomach oil. Left: long-term experiment. Further caption details as in Figure 2.
DEHP, a plasticizer (Figure 6 and Supplementary Material Chapter 3.13, page 19), showed strong leaching from PTX001 during both the LTE and LTR experiments, with concentrations continuing to increase strongly until day 90. These findings are not supported in the STD experiment, where the control samples showed a highly varied pattern over the initial days and thus indicating lower reliability of the results.
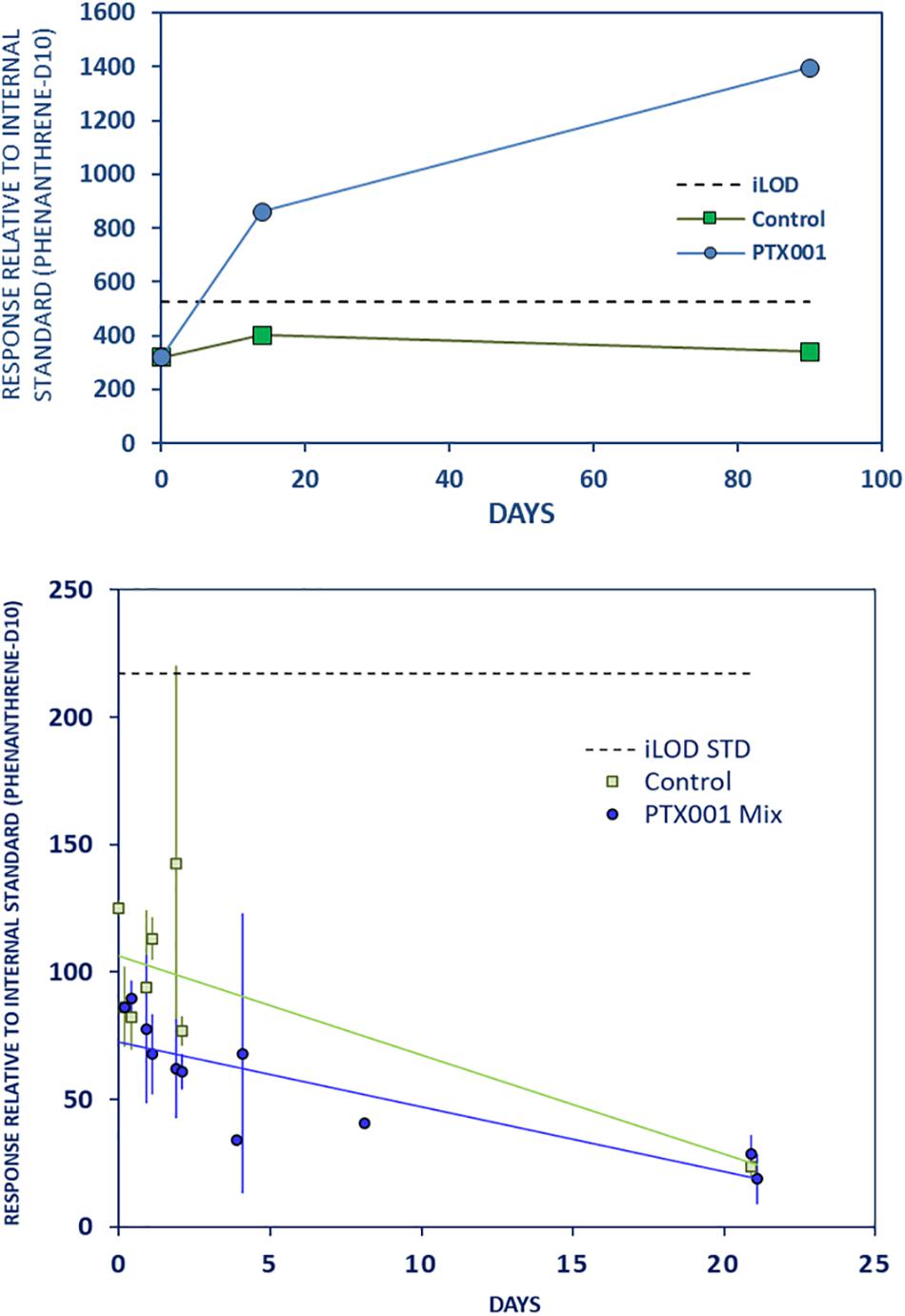
Figure 6. Strong long-term leaching of DEHP from PTX001 to stomach oil. Further caption details as in Figure 2.
Bumetrizole, a UV stabilizer also known as Tinuvin 326 (Figure 7 and Supplementary Material Chapter 3.14, page 20), rapidly leached from PTX001 to a great extent during the first days of exposure in the LTE and LTR experiments. The levels appear to stabilize after 14 days of exposure, demonstrated by results from all three experiments. Data from the STD experiment indicate that leaching to the maximum observed concentration occurs almost instantly after the plastic is exposed to the stomach oil.
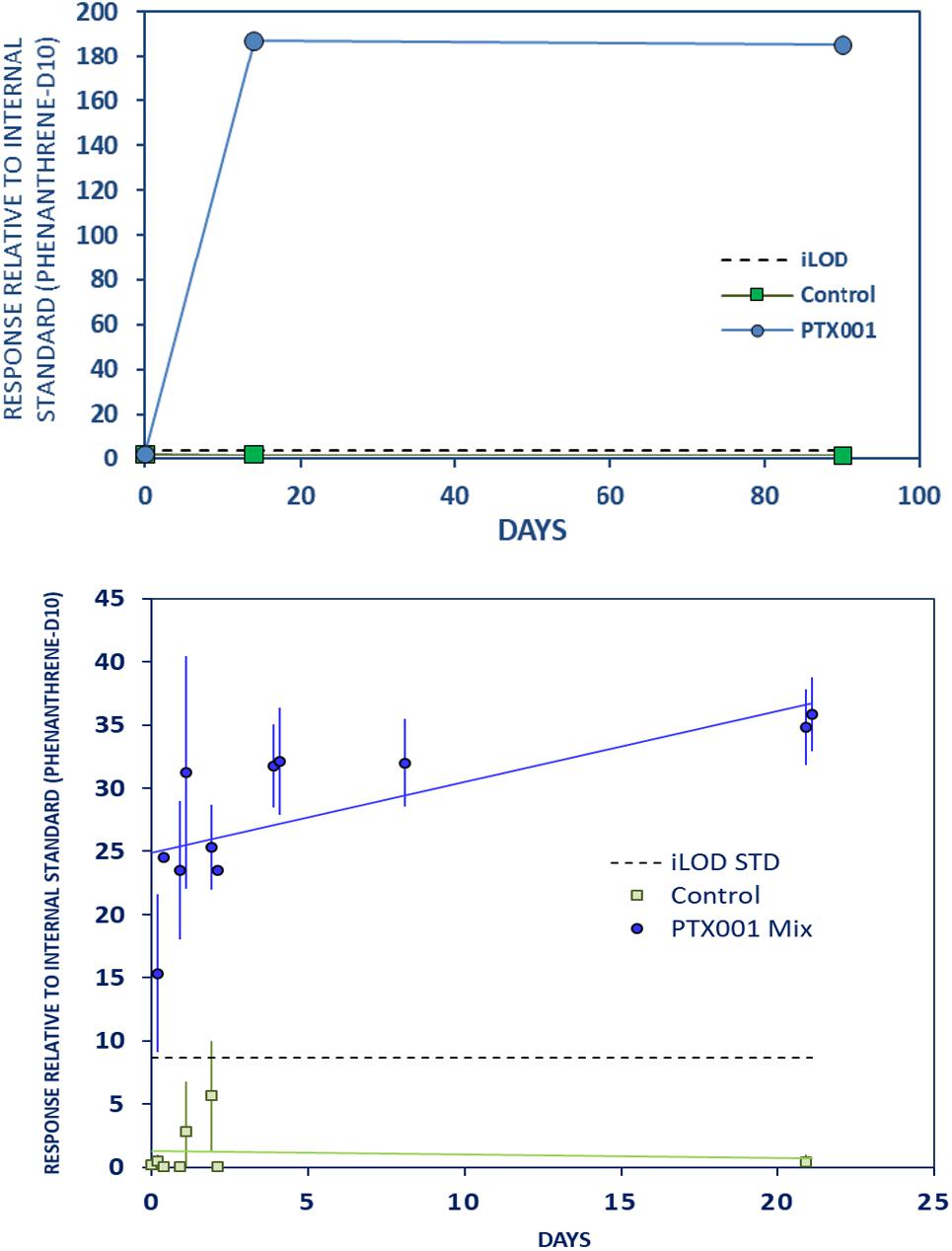
Figure 7. Strong initial leaching of bumetrizole from PTX001 to stomach oil. Further caption details as in Figure 2.
Although identified in the solvent extracts of the PTX001 and PS test materials, some of the tentatively identified additive chemicals were not found in the corresponding stomach oil leachates in any of the studies. In the case of PTX001, no detectable leaching was measured for phenyl benzoate (preservative; Supplementary Material Chapter 3.5, page 11), propanediylbisbenzene (unknown use; Supplementary Material Chapter 3.4, page 10), 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (antioxidant; Supplementary Material Chapter 3.9, page 15), DBP (plasticizer; Supplementary Material Chapter 3.10, page 15), and triphenylbenzene (packaging migration residue; Figure 8 and Supplementary Material Chapter 3.12, page 18). For the PS foam, propanediylbisbenzene and triphenylbenzene (Figure 8) were only found in the parent material and not in the corresponding leachates.
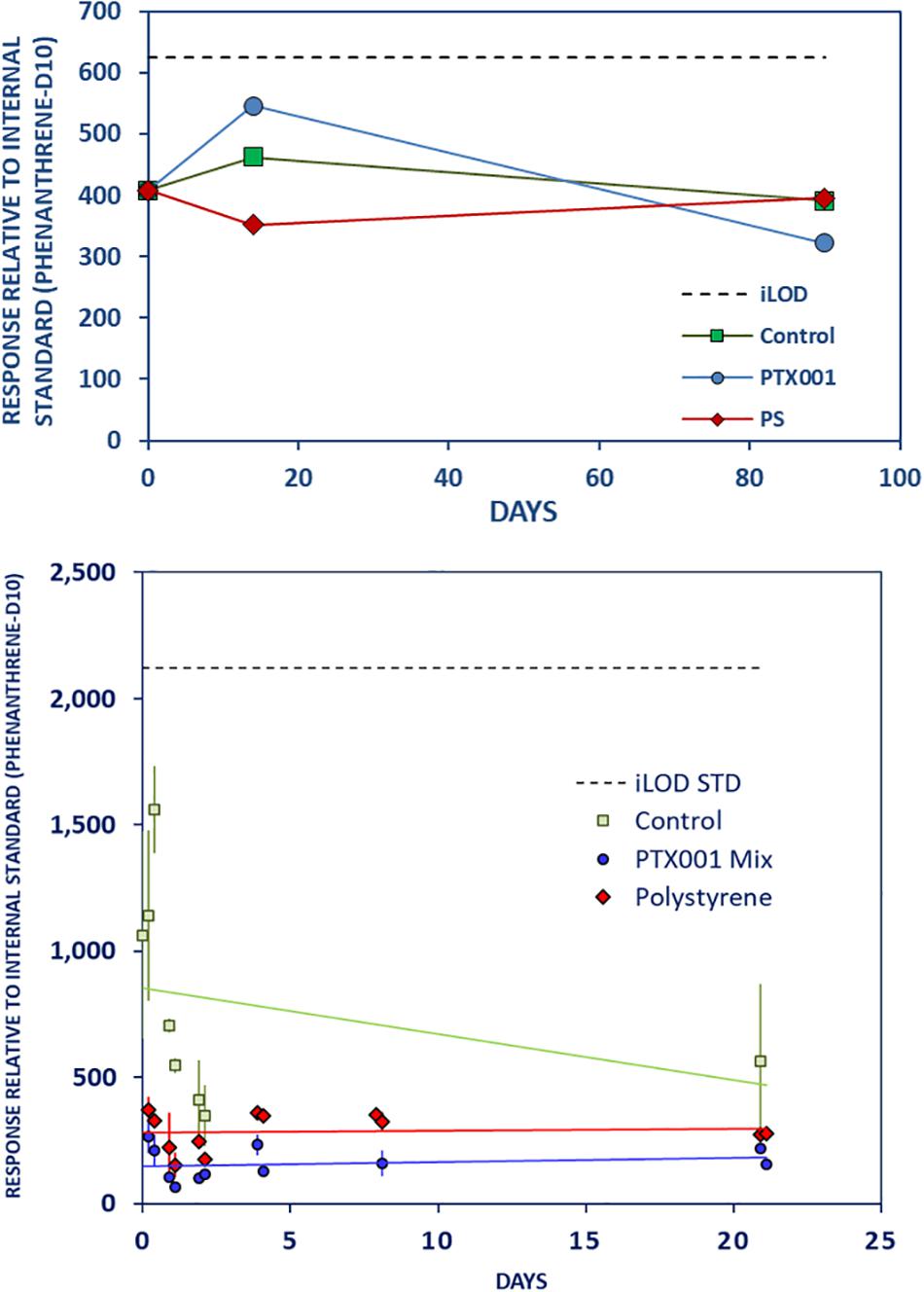
Figure 8. No leaching of triphenylbenzene from PTX001 or PS to stomach oil. Further caption details as in Figure 2.
Results from this study confirmed that different types of additives leached from the marine litter-derived microplastic test materials to the fulmar stomach oil. The relative amounts varied across the different chemicals and reflected their individual properties. As a result, the additive chemical profiles of the test materials and leachates exhibited some substantial differences to each other. For the microplastic mixture (PTX001), leached chemicals included precursors to resins and copolymers, antioxidants, plasticizers, flame retardants, and UV stabilizers. For PS, leaching of precursors to resins and copolymers, antioxidants, and preservatives was observed. According to Smedes et al. (2017), increasing lipophilicity (log KOW) of organic hydrophobic contaminants would lead to increased lipid–polymer partition coefficients. In the current study, we observe no clear pattern between log KOW and which compounds leach to a greater extent. The higher number of chemicals present in the PTX001 material reflects its composition of multiple plastic types, each with its own additive chemical profiles. Three of the chemicals were present in both materials, suggesting these may derive from the PS component of PTX001 or that different polymer types sometimes contain the same additives.
Many studies looking at the leaching of additives from plastic have utilized chemical analyses to investigate the concentrations of specific target chemicals and chemical groups. While this approach can provide useful information, it risks filtering out other compounds that may be present and contributing to any observed leachate toxicity. The current study used non-target screening as start point for identifying as many chemicals as possible in marine litter-derived microplastic test materials (PTX001 and PS foam). This enabled the development of analytical methods for quantifying these same chemicals in the corresponding leachates generated in a series of exposure studies (LTE and STD) conducted with northern fulmar stomach oil.
In the STD experiment, some of the substances showed high variation and unclear patterns of leaching during the first days (0–4 days) of exposure. For example, p-benzoquinone (Supplementary Material Chapter 3.2, page 8) and TCPP (3:1) (Supplementary Material Chapter 3.7, page 13) show varying concentration patterns for all measurements during 8 h and days 1 and 2 stomach oil leachate samples (high variation between bottles A and B and high variation within each bottle). This could be caused by compound instability in the sample matrix during processing, storage, or transportation. In addition, minor differences in sampling time and sample treatment may influence the data more significantly in the early stages of the exposure. Despite the sometimes erratic concentration patterns during the first days of the STD experiment, the longer-term results and trends overlap between the three experiments (LTE, LTR, and STD), providing the necessary confidence in the results presented.
Some of the additive chemicals (e.g., p-benzoquinone and triphenylbenzene for PTX001 and phenyl benzoate for PS) exhibited an initial increase in stomach oil followed by a subsequent decrease at 90 days. The data for such chemicals suggest that equilibrium has been reached prior to the sampling point at 90 days, but that a secondary process is occurring that results in the concentration decrease observed between 14 and 90 days. It is not possible to identify exactly what process or processes have contributed to this trend, but a number of viable mechanisms are possible given the long timescales used in the LTE experiment. Several of the identified compounds are readily biodegradable according to estimates made using BIOWINTM (Supplementary Table 6; US EPA, 2012). However, as some of the compounds that are readily biodegradable do not see a decrease in relative concentrations of the duration of the experiments, it is unlikely that biotic degradation has influenced the results of the current study. It is important to consider that the exposure systems were not sterile and that microbial biotransformation and biodegradation of additive chemicals might occur once they have partitioned to the stomach oil. These processes only require a small modification to the chemical structure (e.g., partial degradation) and the resulting biotransformation products would not be measured using the targeted analytical chemical methods developed for leachate characterization and quantification. It is possible that specific chemicals will be more or less susceptible to such different processes, but also that more than one of these mechanisms may act on an individual additive chemical at the same time.
In our attempts to document the leaching of different organic chemical additives from marine litter-derived microplastic test materials into the stomach oil of northern fulmars, we encountered issues with the comparability of data from the three experiments. The results in the LTE experiment and the STD experiment were sometimes inconsistent, and we are unable to propose a satisfactory explanation for such discrepancies. Long-term samples were analyzed twice, initially as part of the LTE experiment and then reanalyzed (LTR) together with the samples generated in the STD experiment. All GC-MS analyses were performed in randomized order, and blank samples were frequently analyzed to ensure there was no carryover of chemicals between samples and analyses. Subsamples of fulmar stomach oil, PTX001, and PS foam samples from the same batches (common source) were used in both the long- and short-term studies, and it is suggested that any inhomogeneity in oil or plastic materials is relatively small and not responsible for the observed differences in the leachates as the comparability of A and B samples demonstrates. The stomach oil was stored frozen prior to the LTE and quickly refrozen for storage between the LTE and STD exposure experiments. Furthermore, there were no changes in the sampling protocol between the two studies. Therefore, we have chosen to regard the LTE experiment and STD experiment as separate studies representing short- and long-term exposures. Despite the inconsistencies described, the strong similarities between many of the leaching profiles from the LTE experiment and the STD experiment samples, together with the stable levels of contamination (in most cases) observed in the control samples and good overlap of A and B samples, suggest sufficient reliability in the reported outcomes of this study.
The results in the current study clearly show that several additive substances can leach from plastics ingested by fulmars into stomach oil under environmentally relevant conditions and over timescales estimated to be within the gut residence time (Ryan and Jackson, 1987; Van Franeker and Law, 2015; Ryan, 2015; Terepocki et al., 2017). Once leached into the stomach oil, there are well-established mechanisms that can facilitate the uptake of some of these chemicals by the birds (Galloway, 2015; Garvey, 2019; Tanaka et al., 2020). However, whether an individual additive chemical is subsequently transferred into specific organs or tissues and whether it will accumulate are influenced by a range of factors. The properties of a specific chemical will determine the partitioning between uptake or excretion in the feces (Tourinho et al., 2019; Ribeiro et al., 2019). For chemicals that are absorbed, some may undergo metabolization prior to or during storage and accumulation in specific tissues or removal from internal organs via kidney function or to feathers (Letcher et al., 2010; Provencher et al., 2018). It must be emphasized that leaching of chemicals to stomach oil in the current study was observed to occur in stomach oil that was already contaminated with chemicals from food and plastics ingested by the fulmar chicks during the 7-week nestling period. The basic load of selected additives can be seen in the 0-measurements in this study, as these represent the oil before any treatment.
It is difficult to find relevant toxicity data for the specific 15 chemicals identified in the current study. However, several of the compounds that were observed to leach into the stomach oil belong to groups of chemicals that may have serious impacts on the health of the animals (Zimmermann et al., 2019). For example, certain UV stabilizers (e.g., UV320, UV326-328) have been shown to bioaccumulate, act as endocrine disruptors, and cause mutagenic toxicity responses (Rani et al., 2015). Phthalates, widely used as plasticizers, have been found to be endocrine disruptors, as well as affecting reproduction (Oehlmann et al., 2009; Geueke and Muncke, 2017). DEHP is very common in the environment (Hermabessiere et al., 2017), but our results indicate that it can also leak directly from marine plastic litter to the stomach oil of fulmars, offering an additional pathway of uptake. The uptake of DEHP from plastics is enhanced by the natural conditions in seabirds’ digestive systems, such as high temperatures and low pH values (Bakir et al., 2014). Negative effects of plastic-associated substances on other organisms have been observed in other experimental studies (e.g., Lithner et al., 2009; Capolupo et al., 2020). Coffin et al. (2019) provided strong experimental evidence of increase of the biological estrogenicity of cells from ingestion of some plastic items by both birds and fishes.
In our study, leaching of chemicals from the plastic test materials is presented relative to the initial occurrence. We cannot assess if the leached quantities of chemicals would lead to direct health impacts in fulmars, and this should be a focus of future studies. Importantly, any effects from plastic-associated chemicals on marine organisms are likely to be influenced by the complex interplay of multiple intrinsic and extrinsic factors, including environmental conditions, specific chemical profiles associated with individual plastic items, polymer type, amount of plastic ingested, form and origin of the plastic ingested, and preexisting contaminant levels in organisms and the surrounding environment. Although the sublethal effects of plastic-related compounds on the health of populations or species remain difficult to substantiate (Browne et al., 2015; Werner et al., 2016), our results give grounds for concern.
Tanaka et al. (2019) showed that plastics ingested by fulmars and albatrosses contain UV stabilizers, flame retardants, and styrene oligomers, similar to those found in the PTX001 and PS foam test materials. PE and PP were the most common plastic types encountered in the studies by Tanaka et al. (2019) and in the PTX001 samples (Kühn et al., 2018). Tanaka et al. (2013, 2015) documented that specific congeners of polybrominated flame retardants leached from ingested plastic to the stomach fluids and were subsequently transferred to tissues in short-tailed shearwaters (Ardenna tenuirostris). This organism-level detection of polybrominated flame retardants shows a pathway that may occur in fulmars and other seabirds, comparable to the stomach oil leaching mechanism in fulmars as described in our experiment. Recently, Tanaka et al. (2020) fed artificially spiked plastic pellets to streaked shearwater (Calonectris leucomelas) chicks and concluded that in seabird species that consume plastics as frequently as fulmars, leaching of additives represented a considerably more important pathway of specific pollutants to the bird tissues than through accumulation of pollutants in food. Importantly, in the experiment of Tanaka et al. (2020), additives were built in the polymer matrix, and not just added to the surface. Together with organism-level detection of such chemicals by Tanaka et al. (2020), our results provide the evidence that such leaching of additives or their degradation products from degrading plastic litter actually occurs in the marine environment, from plastics ingested by a range of marine wildlife.
The studies by Tanaka et al. (2013, 2019, 2018, 2020) and our work on embedded additive leaching cannot be compared to some published model approaches and seabird investigations focusing on plastic surface adsorption and desorption of persistent organic pollutants. Model approaches by, e.g., Gouin et al. (2011), Koelmans et al. (2014, 2016), and Bakir et al. (2016) have indicated that plastics ingested by seabirds are not acting as a relevant source of pollutants in comparison to food. It has even been implied that during gut passage plastics could act as passive samplers for pollutants already present in the organisms, thereby reducing contaminant concentrations in the body. Seabird studies by Herzke et al. (2016), Provencher et al. (2018), and Provencher et al. (2020) tend to be seen as support for such models because no correlation could be demonstrated of selected pollutants on plastics in the stomachs of individual birds and the concentration of such substances in their tissues. As such, the models and seabird studies represent a quite different process to that of the leaching of a wide range of plastic additives embedded in the polymer matrix, and results should not be compared.
In the current approach, we attempted to avoid inclusion of foodweb-related chemical pollutants, either additives or adsorbed, by excluding substances found in the untreated stomach oils. The relative importance of both pathways seems difficult to quantify. As additive chemicals are distributed throughout the entire polymer matrix and not just at the surface, grinding of plastics in seabird stomachs makes such substances increasingly available for leaching due to the increased surface area. Although modeling studies can provide a useful indication of the bioavailability of plastic additive and adsorbed chemicals to organisms following ingestion (Koelmans et al., 2014; Koelmans, 2015), they are not necessarily able to consider the ingestion of a broad spectrum of highly variable consumer debris items in combination with the unique gastric environment in Procellariiformes, with high temperatures, low pH values, the occurrence of stomach oil, and the grinding activity in the gizzard. Our results clearly add evidence to earlier studies by Tanaka et al. (2015, 2020). Results of both studies suggest major value in further work to evaluate impacts at the cellular, tissue, and organism levels. Plastic ingested by Procellariiformes can be a vector of several harmful additive chemical compounds (e.g., plasticizers, flame retardants, etc.) over environmentally relevant gut residence times.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
This animal study was reviewed and approved by the Environment Agency of the Faroe Islands (Reference number: 18/00440-1).
JF conceptualized the study and collected the fulmar stomach oil on the Faroe Islands. AO and colleagues at Carat GmbH developed the grinding technology and provided the microplastic mixtures used for the experiments. SK executed the exposure experiment and wrote the first draft of the manuscript. LS and AB analyzed the samples and wrote sections of the manuscript. All authors contributed to manuscript revisions and read and approved the submitted version. A previous version of this manuscript is included in the Ph.D. thesis of SK (https://doi.org/10.18174/509638).
SK was funded by the Dutch Science Foundation (project ALW-NWO 856.15.001) under EU Joint Program Initiative (JPI) “Oceans” within the PLASTOX project (Direct and Indirect Ecotoxicological Impacts of Microplastics on Marine Organisms).
AB and LS were employed by the research institute SINTEF Ocean. AO was employed at the company CARAT GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
André Meijboom (Wageningen Marine Research) helped with the execution of the experiments. The collection of stomach oil of hunted fledgling fulmars on the Faroe Islands was supported by many persons including Bergur Olsen, Høgni Arnbjarnarson, Bjarni Mikkelsen, Johannis Danielsen, Jens-Kjeld Jensen, Maria Dam, Atli Poulsen, and many of their local friends. Itsasne Beitia Aguirre, Marianne Aas, Marianne Molid, and Lisbet Støen (SINTEF) are acknowledged for assistance with sample preparation for chemical analysis. We want to thank the two reviewers who contributed to improving the original manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2020.00138/full#supplementary-material
Andrady, A. L., and Neal, M. A. (2009). Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 1977–1984.
Arthur, C., Baker, J., and Bamford, H. (2009). Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, September 9-11, 2008, eds C. Arthur, J. Baker, and H. Bamford (Tacoma, WA: University of Washington Tacoma), 49.
Bakir, A., O’Connor, I. A., Rowland, S. J., Hendriks, A. J., and Thompson, R. C. (2016). Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 219, 56–65. doi: 10.1016/j.envpol.2016.09.046
Bakir, A., Rowland, S. J., and Thompson, R. C. (2014). Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 185, 16–23. doi: 10.1016/j.envpol.2013.10.007
Barnes, D. K., Galgani, F., Thompson, R. C., and Barlaz, M. (2009). Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 1985–1998. doi: 10.1098/rstb.2008.0205
Bravo Rebolledo, E. L. (2011). Threshold Levels and Size Dependent Passage of Plastic Litter in Stomachs of Fulmars. MSc thesis. Wageningen: Aquatic Ecology and Water Quality Management group.
Browne, M. A., Underwood, A., Chapman, M., Williams, R., Thompson, R. C., and van Franeker, J. A. (2015). Linking effects of anthropogenic debris to ecological impacts. Proc. R. Soc. Lon. B. Biol. Sci. 282:20142929. doi: 10.1098/rspb.2014.2929
Byrkjedal, I., and Langhelle, G. (2019). Selective feeding on jellyfish organs by Northern Fulmars Fulmarus glacialis. Orn. Norveg. 42, 15–18. doi: 10.15845/on.v42i0.1493
Camphuysen, C. J., and Van Franeker, J. A. (1997). Notes on the diet of northern fulmars Fulmarus glacialis from Bjornoya (Bear Island). Sula 11, 1–10.
Camphuysen, K., and Garthe, S. (1997). An evaluation of the distribution and scavenging habits of northern fulmars (Fulmarus glacialis) in the North Sea. ICES J. Mar. Sci. 54, 654–683. doi: 10.1006/jmsc.1997.0247
Capolupo, M., Sørensen, L., Jayasena, K. D. R., Booth, A. M., and Fabbri, E. (2020). Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 169:115270. doi: 10.1016/j.watres.2019.115270
Clarke, A., and Prince, P. A. (1976). The origin of stomach oil in marine birds: Analyses of the stomach oil from six species of subantarctic procellariiform birds. J. Exp. Mar. Biol. Ecol. 23, 15–30. doi: 10.1016/0022-0981(76)90082-4
Coffin, S., Huang, G.-Y., Lee, I., and Schlenk, D. (2019). Fish and Seabird Gut Conditions Enhance Desorption of Estrogenic Chemicals from Commonly-Ingested Plastic Items. Environ. Sci. Technol. 53, 4588–4599. doi: 10.1021/acs.est.8b07140
Foster, K. L., Wang, S. W., Mackay, D., Mallory, M. L., and Blais, J. M. (2010). Preliminary assessment of avian stomach oils: a vector of contaminants to chicks and potential for diet analysis and biomonitoring. Environ. Sci. Technol. 44, 6869–6874. doi: 10.1021/es1009983
Galgani, F., Hanke, G., and Maes, T. (2015). “Global distribution, composition and abundance of marine litter,” in Marine Anthropogenic Litter, eds M. Bergmann, L. Gutow, and M. Klages (Springer), 29–56.
Galloway, T. S. (2015). “Micro- and Nano-plastics and Human Health,” in Marine Anthropogenic Litter, eds M. Bergmann, L. Gutow, and M. Klages (Cham: Springer International Publishing), 343–366.
Garvey, M. (2019). Food pollution: a comprehensive review of chemical and biological sources of food contamination and impact on human health. Nutrire 44:1. doi: 10.1186/s41110-019-0096-3
Geueke, B., and Muncke, J. (2017). Substances of Very High Concern in Food Contact Materials: Migration and Regulatory Background. Pack. Technol. Sci. 31, 757–769. doi: 10.1002/pts.2288
Geyer, R., Jambeck, J. R., and Law, K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv. 3:e1700782. doi: 10.1126/sciadv.1700782
Gigault, J., Halle, A. T., Baudrimont, M., Pascal, P.-Y., Gauffre, F., Phi, T.-L., et al. (2018). Current opinion: What is a nanoplastic? Environ. Pollut. 235, 1030–1034. doi: 10.1016/j.envpol.2018.01.024
Gouin, T., Roche, N., Lohmann, R., and Hodges, G. (2011). A Thermodynamic Approach for Assessing the Environmental Exposure of Chemicals Absorbed to Microplastic. Environ. Sci. Technol. 45, 1466–1472. doi: 10.1021/es1032025
Heinrich, P., Hanslik, L., Kämmer, N., and Braunbeck, T. (2020). The tox is in the detail: technical fundamentals for designing, performing, and interpreting experiments on toxicity of microplastics and associated substances. Environ. Sci. Pollut. Res. 27, 22292–22318. doi: 10.1007/s11356-020-08859-1
Hermabessiere, L., Dehaut, A., Paul-Pont, I., Lacroix, C., Jezequel, R., Soudant, P., et al. (2017). Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere 182, 781–793. doi: 10.1016/j.chemosphere.2017.05.096
Herzke, D., Anker-Nilssen, T., Nøst, T. H., Götsch, A., Christensen-Dalsgaard, S., Langset, M., et al. (2016). Negligible Impact of ingested microplastics on tissue concentrations of persistent organic pollutants in Northern Fulmars off coastal Norway. Environ. Sci. Technol. 50, 1924–1933. doi: 10.1021/acs.est.5b04663
Jahnke, A., Arp, H. P. H., Escher, B. I., Gewert, B., Gorokhova, E., Kühnel, D., et al. (2017). Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ. Sci. Technol. 4, 85–90. doi: 10.1021/acs.estlett.7b00008
Koelmans, A. A. (2015). “Modeling the role of microplastics in Bioaccumulation of organic chemicals to marine aquatic organisms. A Critical Review,” in Marine Anthropogenic Litter, eds M. Bergmann, L. Gutow, and M. Klages (Springer), 309–324.
Koelmans, A. A., Bakir, A., Burton, G. A., and Janssen, C. R. (2016). Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 50, 3315–3326. doi: 10.1021/acs.est.5b06069
Koelmans, A. A., Besseling, E., and Foekema, E. M. (2014). Leaching of plastic additives to marine organisms. Environ. Pollut. 187, 49–54. doi: 10.1016/j.envpol.2013.12.013
Kühn, S., Bravo Rebolledo, E. L., and van Franeker, J. A. (2015). “Deleterious effects of litter on marine life,” in Marine Anthropogenic Litter, eds M. Bergmann, L. Gutow, and M. Klages (Springer), 75–116.
Kühn, S., and Van Franeker, J. A. (2012). Plastic ingestion by the northern fulmar (Fulmarus glacialis) in Iceland. Mar. Pollut. Bull. 64, 1252–1254. doi: 10.1016/j.marpolbul.2012.02.027
Kühn, S., and van Franeker, J. A. (2020). Quantitative overview of marine debris ingested by marine megafauna. Mar. Pollut. Bull. 151:110858. doi: 10.1016/j.marpolbul.2019.110858
Kühn, S., van Oyen, A., Booth, A. M., Meijboom, A., and van Franeker, J. A. (2018). Marine microplastic: Preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere 213, 103–113. doi: 10.1016/j.chemosphere.2018.09.032
Letcher, R. J., Bustnes, J. O., Dietz, R., Jenssen, B. M., Jørgensen, E. H., Sonne, C., et al. (2010). Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci. Tot. Environ. 408, 2995–3043. doi: 10.1016/j.scitotenv.2009.10.038
Lewis, R. (1969). Studies on the stomach oils of marine animals—II. Oils of some procellariiform birds. Comparat. Biochem. Physiol. 31, 725–731.
Lithner, D., Damberg, J., Dave, G., and Larsson, K. (2009). Leachates from plastic consumer products-screening for toxicity with Daphnia magna. Chemosphere 74, 1195–1200. doi: 10.1016/j.chemosphere.2008.11.022
Mallory, M. L. (2008). Marine plastic debris in northern fulmars from the Canadian high Arctic. Mar. Pollut. Bull. 56, 1501–1504. doi: 10.1016/j.marpolbul.2008.04.017
Oehlmann, J., Schulte-Oehlmann, U., Kloas, W., Jagnytsch, O., Lutz, I., Kusk, K. O., et al. (2009). A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2047–2062. doi: 10.1098/rstb.2008.0242
Ojowski, U., Eidtmann, C., Furness, R., and Garthe, S. (2001). Diet and nest attendance of incubating and chick-rearing northern fulmars (Fulmarus glacialis) in Shetland. Mar. Biol. 139, 1193–1200. doi: 10.1007/s002270100655
OSPAR (2019). OSPAR Committee Assessment: Plastic particles in fulmar stomachs in the North Sea., OSPAR Assessment Portal (OAP) Online Document, https://oap.ospar.org/en/ospar-assessments/committee-assessments/eiha-thematic-assessments/marine-litter/plastic-particles-in-fulmar-stomachs-north-sea/ (accessed October 30, 2019).
Phuong, N. N., Zalouk-Vergnoux, A., Poirier, L., Kamari, A., Châtel, A., Mouneyrac, C., et al. (2016). Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 211, 111–123. doi: 10.1016/j.envpol.2015.12.035
Place, A. R., Stoyan, N. C., Ricklefs, R. E., and Butler, R. G. (1989). Physiological Basis of Stomach Oil Formation in Leach’s Storm-Petrel (Oceanodroma leucorhoa). Auk 106, 687–699. doi: 10.1093/auk/106.4.687
Provencher, J. F., Avery-Gomm, S., Braune, B. M., Letcher, R. J., Dey, C. J., and Mallory, M. L. (2020). Are phthalate ester contaminants in northern fulmar preen oil higher in birds that have ingested more plastic? Mar. Pollut. Bull. 150:110679. doi: 10.1016/j.marpolbul.2019.110679
Provencher, J. F., Avery-Gomm, S., Liboiron, M., Braune, B. M., Macaulay, J. B., Mallory, M. L., et al. (2018). Are ingested plastics a vector of PCB contamination in northern fulmars from coastal Newfoundland and Labrador? Environ. Res. 167, 184–190. doi: 10.1016/j.envres.2018.07.025
Rani, M., Shim, W. J., Han, G. M., Jang, M., Al-Odaini, N. A., Song, Y. K., et al. (2015). Qualitative analysis of additives in plastic marine debris and its new products. Arch. Environ. Contam. Toxicol. 69, 352–366. doi: 10.1007/s00244-015-0224-x
Rapp, D. C., Youngren, S. M., Hartzell, P., and Hyrenbach, K. D. (2017). Community-wide patterns of plastic ingestion in seabirds breeding at French Frigate Shoals, Northwestern Hawaiian Islands. Mar. Pollut. Bull. 123, 269–278. doi: 10.1016/j.marpolbul.2017.08.047
Ribeiro, F., O’Brien, J. W., Galloway, T., and Thomas, K. V. (2019). Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms. TrAC Trends Analytic. Chem. 111, 139–147. doi: 10.1016/j.trac.2018.12.010
Rochman, C., Brookson, C., Bikker, J., Djuric, N., Earn, A., Bucci, K., et al. (2019). Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 38, 703–711.
Rochman, C. M. (2015). “The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment,” in Marine Anthropogenic Litter, eds M. Bergmann, L. Gutow, and M. Klages (Springer), 117–140.
Rodríguez, A., Ramírez, F., Carrasco, M. N., and Chiaradia, A. (2018). Seabird plastic ingestion differs among collection methods: Examples from the short-tailed shearwater. Environ. Pollut. 243, 1750–1757. doi: 10.1016/j.envpol.2018.09.007
Roman, L., Lowenstine, L., Parsley, L. M., Wilcox, C., Hardesty, B. D., Gilardi, K., et al. (2019a). Is plastic ingestion in birds as toxic as we think? Insights from a plastic feeding experiment. Sci. Tot. Environ. 665, 660–667. doi: 10.1016/j.scitotenv.2019.02.184
Roman, L., Paterson, H., Townsend, K. A., Wilcox, C., Hardesty, B. D., and Hindell, M. A. (2019b). Size of marine debris items ingested and retained by petrels. Mar. Pollut. Bull. 142, 569–575. doi: 10.1016/j.marpolbul.2019.04.021
Roman, L., Schuyler, Q. A., Hardesty, B. D., and Townsend, K. A. (2016). Anthropogenic Debris Ingestion by Avifauna in Eastern Australia. PLoS One 11:e0158343. doi: 10.5061/dryad.p48f7
Ryan, P. G. (2015). How quickly do albatrosses and petrels digest plastic particles? Environ. Pollut. 207, 438–440. doi: 10.1016/j.envpol.2015.08.005
Ryan, P. G. (2016). “Ingestion of Plastics by Marine Organisms,” in Hazardous Chemicals Associated with Plastics in the Marine Environment, eds H. Takada and H. K. Karapanagioti (Cham: Springer International Publishing), 235–266.
Ryan, P. G., and Jackson, S. (1987). The lifespan of ingested plastic particles in seabirds and their effect on digestive efficiency. Mar. Pollut. Bull. 18, 217–219. doi: 10.1016/0025-326X(87)90461-9
Smedes, F., Rusina, T. P., Beeltje, H., and Mayer, P. (2017). Partitioning of hydrophobic organic contaminants between polymer and lipids for two silicones and low density polyethylene. Chemosphere 186, 948–957. doi: 10.1016/j.chemosphere.2017.08.044
Sørensen, L., Rogers, E., Altin, D., Salaberria, I., and Booth, A. M. (2020). Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ. Pollut. 258:113844. doi: 10.1016/j.envpol.2019.113844
Tanaka, K., Takada, H., Yamashita, R., Mizukawa, K., Fukuwaka, M.-A., and Watanuki, Y. (2013). Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 69, 219–222. doi: 10.1016/j.marpolbul.2012.12.010
Tanaka, K., Takada, H., Yamashita, R., Mizukawa, K., Fukuwaka, M.-A., and Watanuki, Y. (2015). Facilitated leaching of additive-derived PBDEs from plastic by seabirds’ stomach oil and accumulation in tissues. Environ. Sci. Technol. 49, 11799–11807. doi: 10.1021/acs.est.5b01376
Tanaka, K., van Franeker, J. A., Deguchi, T., and Takada, H. (2019). Piece-by-piece analysis of additives and manufacturing byproducts in plastics ingested by seabirds: Implication for risk of exposure to seabirds. Mar. Pollut. Bull. 145, 36–41. doi: 10.1016/j.marpolbul.2019.05.028
Tanaka, K., Yamashita, R., and Takada, H. (2018). Transfer of Hazardous Chemicals from Ingested Plastics to Higher-Trophic-Level Organisms. The Handbook of Environmental Chemistry. Berlin: Springer, doi: 10.1007/698_2018_255
Tanaka, K., Watanuki, Y., Takada, H., Ishizuka, M., Yamashita, R., Kazama, M., et al. (2020). In vivo Accumulation of Plastic-Derived Chemicals into Seabird Tissues. Curr. Biol. press 30, e723–728. doi: 10.1016/j.cub.2019.12.037
Terepocki, A. K., Brush, A. T., Kleine, L. U., Shugart, G. W., and Hodum, P. (2017). Size and dynamics of microplastic in gastrointestinal tracts of Northern Fulmars (Fulmarus glacialis) and Sooty Shearwaters (Ardenna grisea). Mar. Pollut. Bull. 116, 143–150. doi: 10.1016/j.marpolbul.2016.12.064
Teuten, E. L., Saquing, J. M., Knappe, D. R., Barlaz, M. A., Jonsson, S., Bjorn, A., et al. (2009). Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2027–2045. doi: 10.1098/rstb.2008.0284
Tourinho, P. S., Kočí, V., Loureiro, S., and van Gestel, C. A. M. (2019). Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 252, 1246–1256. doi: 10.1016/j.envpol.2019.06.030
Trevail, A. M., Gabrielsen, G. W., Kühn, S., and Van Franeker, J. A. (2015). Elevated levels of ingested plastic in a high Arctic seabird, the northern fulmar (Fulmarus glacialis). Polar. Biol. 38, 975–981. doi: 10.1007/s00300-015-1657-4
US EPA (2012). Estimation Programs Interface SuiteTM for Microsoft®. Windows, v 4.11. Washington, DC: United States Environmental Protection Agency.
Van Franeker, J. A., Blaize, C., Danielsen, J., Fairclough, K., Gollan, J., Guse, N., et al. (2011). Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Pollut. 159, 2609–2615. doi: 10.1016/j.envpol.2011.06.008
Van Franeker, J. A., and Law, K. L. (2015). Seabirds, gyres and global trends in plastic pollution. Environ. Pollut. 203, 89–96. doi: 10.1016/j.envpol.2015.02.034
van Sebille, E., Wilcox, C., Lebreton, L. C. M., Maximenko, N., Hardesty, B. D., van Franeker, J. A., et al. (2015). A global inventory of small floating plastic debris. Environ. Res. Lett. 10:124006. doi: 10.1088/1748-9326/10/12/124006
Wang, S. W., Iverson, S. J., Springer, A. M., and Hatch, S. A. (2007). Fatty acid signatures of stomach oil and adipose tissue of northern fulmars (Fulmarus glacialis) in Alaska: implications for diet analysis of Procellariiform birds. J. Comparat. Physiol. B. 177, 893–903. doi: 10.1007/s00360-007-0187-y
Warham, J. (1996). The behaviour, population biology and physiology of the petrels. Cambridge, MA: Academic Press.
Werner, S., Budziak, A., van Franeker, J. A., Galgani, F., Hanke, G., Maes, T., et al. (2016). Harm caused by Marine Litter. Brussels: European Union.
Keywords: plastic ingestion, marine litter, additive leaching, gastric fluid, Fulmarus glacialis
Citation: Kühn S, Booth AM, Sørensen L, van Oyen A and van Franeker JA (2020) Transfer of Additive Chemicals From Marine Plastic Debris to the Stomach Oil of Northern Fulmars. Front. Environ. Sci. 8:138. doi: 10.3389/fenvs.2020.00138
Received: 11 May 2020; Accepted: 23 July 2020;
Published: 19 August 2020.
Edited by:
Juliana Assunção Ivar Do Sul, Leibniz Institute for Baltic Sea Research (LG), GermanyReviewed by:
Lauren Roman, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaCopyright © 2020 Kühn, Booth, Sørensen, van Oyen and van Franeker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanne Kühn, c3VzYW5uZS5rdWVobkB3dXIubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.