- 1Key Laboratory of Low-grade Energy Utilization Technologies and Systems, Chongqing University, Ministry of Education, Chongqing, China
- 2Institute of Engineering Thermophysics, Chongqing University, Chongqing, China
White-rot fungi is capable of producing extracellular enzymes that degrade lignin structure and facilitate biofuel production from lignocellulosic biomass wastes. However, fungal monocultures are constrained by low activities of the lignin-degrading enzyme system, leading to poor treatment efficiency and a long duration, which are not advantageous for large-scale applications. To improve enzyme production and enhance lignin degradation, a novel coculture system was proposed using the white-rot fungi Phanerochaete chrysosporium and Irpex lacteus CD2. The degradation efficiency of the alkali lignin by the fungal coculture was 26.4%, which was higher than that of the fungal monocultures. It was due to the production of lignin degrading enzymes was promoted in the liquid medium. Scanning electron microscopy, Fourier transform infrared, thermogravimetric and mercury porosimeter analyses results revealed that the alkali lignin treated with the fungal coculture had the largest porosity, and the degree of destruction of the alkali lignin structure by the fungal coculture was higher than that of the fungal monocultures. Meanwhile, the nonproductive adsorption of enzymes on alkali lignin was significantly reduced by 61.0% when the biomass was treated with the fungal coculture. As a result, the nonproductive adsorption was remarkably reduced, while it significantly improved the cellulase catalysis efficiency. These results demonstrated the synergistic effects of the fungal coculture for biomass treatment and provided a new approach for increasing lignin degradation while improving enzymatic catalysis and biofuel production through fungal coculture.
Introduction
Lignocellulosic biomass from agricultural residues is an abundant resource in China, with an annual productivity of 980 million tons, which is equal to approximately 490 million tons of coal (Isroi et al., 2011). However, a mass of lignocellulosic biomass is incinerated in the field, it not only pollutes the environment but also wastes many resources. Converting lignocellulosic biomass to biofuels, such as bioethanol, biohydrogen and biomethane, has significant energy savings and emission reduction benefits (Xia et al., 2016).
Lignocellulose is mainly composed of three components: lignin, cellulose and hemicellulose. Cellulose and hemicellulose are polysaccharide structures that can be directly hydrolyzed by enzymes into available reducing sugars. However, lignin is a complex cross-linked phenolic polymer, which is composed of guaiacyl propane units (G), syringyl propane units (S) and p-hydroxyphenyl propane units (H) at different ratios (Christopher et al., 2014). The presence of lignin makes it difficult to directly utilize the polysaccharides in lignocellulosic biomass. The removal of lignin improves the accessibility of the polysaccharides, resulting in high biomass conversion efficiency and an economically feasible production process. Without an appropriate pretreatment method, only 20% of the theoretical maximum yield can be obtained by enzymatic hydrolysis (Suksong et al., 2020).
Biological pretreatment methods have attracted interest because of their potential advantages over other pretreatment methods, such as lower energy consumption, no chemical reagents and greater substrate and reaction specificity (Sindhu et al., 2016). The most promising microorganisms are white-rot fungi (WRF), which is the only group known to be able to completely degrade lignin into CO2 and H2O at a normal temperature and pressure (Zhang et al., 2019). Several WRF, such as Phanerochaete chrysosporium, Pleurotus ostreatus, and Irpex lacteus, have been identified for their efficient degradation of lignin in a variety of lignocellulosic biomass materials (Isroi et al., 2011). The high lignin degradation ability of WRF is due to their unique extracellular lignin degrading enzymes (LDEs) system, such as lignin peroxidases (LiP), laccase (Lac), and manganese peroxidases (MnP) (Sharma et al., 2019). When reactive oxygen is used as an intermediary condition for lignin degradation, LDEs degrade lignin into a pool of heterogeneous aromatics, which are ultimately metabolized by the secreting organism or other microbes (Ren et al., 2016).
At present, most studies have improved the selective degradation efficiency of lignin by optimizing monoculture conditions or by screening natural strains. For example, Dong et al. (2013) introduced the characteristics and process of using three WRF to degrade sugarcane bagasse, and found that P. chrysosporium was the most effective lignin-degrading strain. Salvachúa et al. (2013) proved that I. lacteus has the ability to selectively and efficiently degrade lignin in wheat straw. By comparing the extracellular secretases of I. lacteus and P. chrysosporium, it was found while both have the ability to efficiently degrade lignin, the LDEs secreted by the two WRF are different. Zeng et al. (2013) detected the activity of Lac and MnP in stationary cultures of I. lacteus. In contrast, stationary cultures of a highly degradative strain of P. chrysosporium only exhibited activities of LiP and MnP. However, the fungal monocultures produce few types of LDEs, resulting in poor effects and low efficiency in the lignin treatment process. As a result, the duration of the biological pretreatment is too long to be applied at a large scale.
In wood and many other microenvironments, fungi typically live and grow in close proximity to each other, and these fungi may display synergistic interactions (Yadav and Chandra, 2015). The fungal coculture could lead to higher enzyme production through synergistic interactions, and this result depends on the particular species in combination or the mode of interaction between species (Chen et al., 2019). In a coculture system, the evaluation of antagonistic effects of the selected fungal cocultures is important for producing LDEs (Tesfaw and Assefa, 2014). Experimental evidence have suggested that the competition for space and nutrients may result in the enhanced degradation of lignin and in elevated production of LDEs such as MnP and Lac (Dong et al., 2014; Wang et al., 2014; Li et al., 2019). Additionally, in the different WRF coculture systems, the LDEs that play a major role in the lignin degradation process are also different. Lac might play the central role in the lignin degradation process in one coculture system, while in others, MnP or LiP or even Lac-MnP or Lac-LiP-MnP might be responsible for such a process (Sun et al., 2011). In recent years, some researchers have obtained fungal cocultures that efficiently degrade lignin by screening WRF in natural environments (Mishra et al., 2017; Chen et al., 2019). P. chrysosporium and I. lacteus are two types of WRF with strong abilities to degrade lignin in nature. However, under standard culture conditions, the LDEs system secreted by P. chrysosporium and I. lacteus are different. The complementarity nature of the LDEs produced by different WRF and their impacts on the subsequent enzymatic hydrolysis process during fungal coculture have not yet been reported in the literatures.
The innovation of this study is to propose a new coculture system of P. chrysosporium and I. lacteus CD2, based on their complementarity LDEs, to treat alkali lignin as a model compound derived from lignocellulose biomass. The objectives of this study are to study the degradation efficiency of fungal coculture on alkali lignin, and to assess the effects of alkali lignin modification from fungi treatment on enzymatic hydrolysis and adsorption.
Materials and Methods
Strains, Enzymes, and Chemicals
The fungus P. chrysosporium was obtained from the Guangdong Culture Collection Center (Guangdong, China). The fungus I. lacteus CD2 was obtained from the China General Microbiological Culture Collection Center (Beijing, China). The strains stored at 4°C were inoculated in fresh Potato dextrose agar (PDA) slant culture medium. The PDA slant culture medium was prepared as follows: peeled potato blocks were boiled for 20 min and filtered, then 2% (w/v) of glucose and 2% (w/v) of agar was added to the filtrate and its pH was regulated to 7.6. The culture medium was then steam-sterilized at 121°C for 20 min using a high-pressure steam sterilizer. The inoculations were cultured for 7 days at 28°C. The spores of the two fungi were removed from the PDA slant culture medium and stored at 4°C until use.
The alkali lignin and all of the chemicals used in this work were purchased from Sigma-Aldrich and were of analytical reagent grade. The enzyme used in the experiment was a commercial complex enzyme called Cellic CTec2 (Novozymes, China) with an activity of 70 filter paper unit (FPU)/ml. The FPU was measured by following the National Renewable Energy Laboratory standard biomass analytical procedures (Lindedam et al., 2014).
Antagonistic Experiment
The inoculation ring was dipped into the spores, and the two fungi were inoculated on the PDA solid plate medium. The inverted plates were cultured at 28°C in a constant temperature incubator, and the growth of fungi was observed every day (Schoneberg et al., 2015).
Degradation of Alkali Lignin by Fungal Monoculture and Coculture
In Erlenmeyer flasks, 0.2 g of alkali lignin and 40 ml of the liquid medium were steam-sterilized at 121°C for 20 min. The substrates were inoculated with spores to achieve an initial content of 106 spores/ml. The treatment experiment was conducted at 180 rpm and 28°C for 21 days in a shaker bath. Samples were taken by different time intervals (samples were taken every day in the first week, and every other day from the second week to the third week) and were diluted five times, centrifuged at 8,000 rpm for 10 min, and filtered, then the absorbance of the alkali lignin was measured at optical density 280 nm (Hao et al., 2015). The degradation rate was calculated by weight. All of the experiments and analyses were conducted in triplicate.
The fungi degradation medium components were glucose (2 g/L), MgSO4·7H2O (0.1 g/L), KH2PO4 (1 g/L), ammonium tartrate (0.4 g/L), Tween 80 (0.5 ml/L), CaCl2 (0.1 g/L), veratryl alcohol (0.57 ml/L), and 1 ml of trace element solution per liter of medium solution. The trace element solution contained MnSO4 (0.5 g/L), Al(K(SO4)2)·12H2O (0.02 g/L), FeSO4·7H2O (0.1 g/L), H3BO3 (0.01 g/L), CoCl2·6H2O (0.1 g/L), ZnSO4·7H2O (0.1 g/L), and CuSO4·5H2O (0.01 g/L) (Asina et al., 2016).
Lignin Degrading Enzymes Activity Assays
Lignin-degrading enzyme activities were assayed from the liquid cultures. The enzyme assays were conducted in a 3 ml reaction mixture at 25°C by using the extracellular medium of the fungal culture. The Lac activity was measured spectrophotometrically by the oxidation of ABTS [2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate)] (Sigma Aldrich) (More et al., 2011). The reaction mixture contained 0.5 mM ABTS, 2.8 ml of 0.1 M sodium acetate (pH = 4.5), and 0.1 ml of the culture supernatant and was incubated for 5 min. The absorbance was read at 420 nm in a spectrophotometer with deionized water as the blank. One unit was defined as the amount of Lac that oxidized 1 μmol of ABTS substrate per min. The MnP activity was determined by the method of Wiberth (Wiberth et al., 2018). The 3 ml reaction mixture contained 50 mM sodium malonate (pH = 4.5), 2 mM MnSO4, 0.1 mM H2O2, and 0.5 ml of the enzyme solution. The reaction was initiated at 37°C, and the initial rate of Mn(III) malonate formation was determined using a spectrophotometer by following the initial increase in absorbance at 270 nm (ε = 11,590 M/cm). One unit of MnP activity was defined as the amount of MnP that catalyzed the formation of 1 μmol of Mn(III) per min. The activity of LiP was measured at 310 nm using veratryl alcohol as the substrate (Yang et al., 2013). The standard reaction mixture (3 ml) contained 0.2 mM veratryl alcohol, 0.15 mM H2O2 and the enzyme solution in 100 mM sodium tartrate buffer (pH = 3.0). One unit of LiP was defined as the amount of enzyme necessary to oxidize 1.0 mmol veratryl alcohol per min. One-way ANOVA analysis was performed to assess significant differences among groups.
Chemical Structure Analysis of Alkali Lignin
The Fourier transform infrared (FTIR) spectra was used to demonstrated the chemical group changes before and after fungi treatment. FTIR scans were conducted at 400–4,000 cm−1. Background scanning was performed for correction before data collection. Scanning electron microscope (SEM) was used to evaluate the microstructural changes in the alkali lignin before and after treatment. The porosity test was carried out by the mercury intrusion porosimetry method, and. The tests were performed at a pressure of up to 33,000 psia. The thermogravimetric (TG) properties of all the samples were obtained to thermal stability changes before and after treatment, and the element analysis was used to determine the content of C, H, N, O, and S in the samples. The alkali lignin samples were placed in the oven to dry at 105°C for 2–3 h to remove moisture from the samples before the TG analysis.
Enzymes Hydrolysis and Adsorption
To evaluate the effect of the alkali lignin treated by fungi on cellulose hydrolysis, the enzymatic hydrolysis of Avicel was conducted in a flask containing sodium citrate buffer (0.1 M, pH = 4.8) for 72 h at 50°C. Specifically, the solid substrate loading rate was 1% (w/w), and the mixture was stirred in a shaker bath at 160 rpm. The dose of the hydrolysis enzyme (SAE0020) was 50 FPU/g substrate. The hydrolysis efficiency of Avicel was evaluated by the yield of reducing sugar. The yield of reducing sugar was determined by the 3,5-dinitrosalicylic acid method (Lee et al., 2020).
Studying the isothermal adsorption line of enzymes on alkali lignin before and after fungi treatment, the concentration of the different enzyme proteins was 0.1–2.0 mg/ml. Alkali lignin and the enzyme were incubated in a flask that contained 0.1 M sodium citrate buffer (pH = 4.8) at 50°C with shaking (160 rpm) for 3 h to reach equilibrium (Deng et al., 2019). The amount of protein adsorbed on the alkali lignin was calculated by weight. The free protein concentration was determined using the Bio-Rad protein assay, which is a Bradford-based colorimetric method, and bovine serum albumin (Sigma-Aldrich) was used as a standard (Ku et al., 2013).
According to published literature (Yang and Fang, 2015), the adsorption of enzymes on the alkali lignin can be described by the following Langmuir equation (Eq. 1):
where Eads is the amount of enzyme adsorbed on the lignin (mg/g lignin), Efree is the free enzyme concentration (mg/ml) in the suspension, Emax is the maximum enzyme adsorption capacity of lignin (mg/g lignin), and K is the Langmuir adsorption constant (ml/mg enzyme).
Machine and Equipment
The culture medium was sterilized by using a high-pressure steam sterilizer (YXQ-LS, BOXUN, China). All the treatments were conducted in shaker bath (ZWYR-2102, ZHICHENG, China). The FTIR spectra were obtained using an FTIR Microscope (Nicolet iN10, Thermo Scientific, United States) equipped with a deuterated triglycine sulfate detector. A SEM (VEGA 3 LMH, TESCAN, Czech Republic) was used to obtain the surface morphology of the alkali lignin at a voltage of 10 kV. The porosity was performed using the Micromertics Instruments Corporation AutoPore IV 9500. The thermal stability properties were analyzed via a TG analyzer (STA409PC, NETZSCH, Germany) at a constant heating rate of 10°C/min. The atmosphere was N2, and the flow rate was 100 ml/min. The contents of C, H, N, O and S were determined by an element analyzer (FLASH 2000, Thermo Scientific, America).
Results and Discussion
Antagonistic Experiment
To analyze whether P. chrysosporium and I. lacteus are suited to simultaneous culture, the antagonistic characteristics between the fungi was tested. Supplementary Figure 1 displays the coculture of the two fungi in a PDA solid plate medium on the seventh day. P. chrysosporium were the white spherical particles on the PDA solid plate medium, and I. lacteus were the white hyphae. The two types of WRF grew evenly on the surface of the PDA solid plate medium, and there was no blank in the middle of the plate (Yao et al., 2016). The results implied the two fungi are suited to synchronous coculture, and it is predicted that this coculture of fungi could synergistically degrade alkali lignin.
Fungal Degradation Characteristics of Alkali Lignin
Figure 1 shows the degradation of alkali lignin by fungi cultivated under different methods. The degradation effects of different fungi on lignin showed significant differences compared with control (p < 0.01). During the first 3 days, the fungi initially used the glucose in the medium as the main source of carbon for growth, so the concentration of alkali lignin changed only slightly. After that, the fungal growth reached the logarithmic phase, and the fungi began to use the alkali lignin in the medium as the main carbon source, resulting in a gradual decrease in the concentration of alkali lignin.
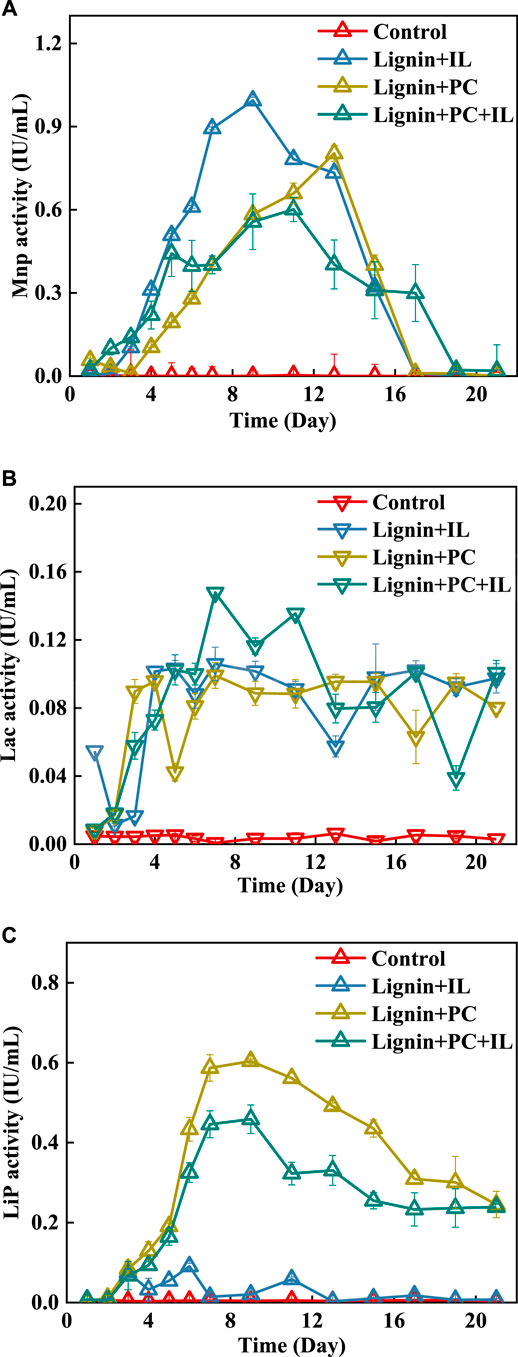
The extracellular lignolytic enzymes from the fungi synergistically cleave the Cα–Cβ bonds and oxidize Cα, and the major route of lignin degradation is the cleavage of the Cα–Cβ bonds by LiP, MnP, and Lac (Harmanpreet et al., 2017). Figure 2 shows the LDEs activity during the fungal degradation of alkali lignin. The activity of Lac and Lip showed significant differences compared with control (p < 0.05). Because P. chrysosporium and I. lacteus could not fully secrete these three lignin-degrading enzymes (Arora and Sharma, 2010), the Lac activity during alkali lignin degradation was lower than the LiP and MnP activities. In the initial stage of alkali lignin degradation, the MnP activity produced by the monoculture of I. lacteus was much higher than that produced by the other two fungal culture methods, but I. lacteus produced almost no LiP enzyme. It was speculated that the degradation of alkali lignin was dominated by MnP. MnP was considered to be the main enzyme involved in the partial mineralization of a broad spectrum of aromatic substances (Parikh et al., 2014).
The MnP production over time for the fungal coculture and monocultures is summarized in Figure 2A. Because the control group did not have LDEs, the degradation efficiency of the alkali lignin was unchanged. For the monoculture of I. lacteus, the time for the biomass to reach the logarithmic phase was shorter than that of the fungal coculture. The rapid increase in the demand for a carbon source for I. lacteus has resulted in the production of more LDEs. The MnP activity produced by I. lacteus was the highest and showed a stable increased in expression during the first week. The MnP activity for I. lacteus reached 0.99 IU/ml on the seventh day, which was 41.3 and 44% higher than that of P. chrysosporium and the fungal coculture, respectively. Therefore, the degradation efficiency of alkali lignin treated by I. lacteus was the highest, reaching 16.6% in the first week, compared with P. chrysosporium (6.7%) and the fungal coculture (15.6%). P. chrysosporium produced MnP and very little Lac during the treatment, and the MnP activity was lower than that of I. lacteus and the fungal coculture, so P. chrysosporium had the lowest alkali lignin degradation efficiency.
Limited by fungal biomass, the highest activity of MnP produced in the fungal coculture occurred later than that of I. lacteus. After the first week, the MnP activity produced by I. lacteus and P. chrysosporium decreased significantly, but that produced by the fungal coculture still increased. On the 17th day, the activity of MnP was almost absent in the fungal monocultures, whereas in the fungal coculture, the activity of MnP was still 0.3 IU/ml. In addition, the enzymes produced by the fungal coculture included not only MnP and Lac but also LiP produced by P. chrysosporium. Thus, a high MnP activity and the synergistic effects of three major LDEs resulted a higher degradation rate for the fungal coculture compared to the fungal monocultures during the last week. At the end of the experiment, the degradation efficiency of alkali lignin by the fungal coculture was the highest at 29.8%, compared with P. chrysosporium (21.9%) and I. lacteus (23.0%). As evaluated above, the coculture of P. chrysosporium and I. lacteus could effectively accelerate the degradation of alkali lignin and promoted the production of LDEs.
Chemical Structure of Alkali Lignin in Fungi Treatment
Fourier Transform Infrared Analysis
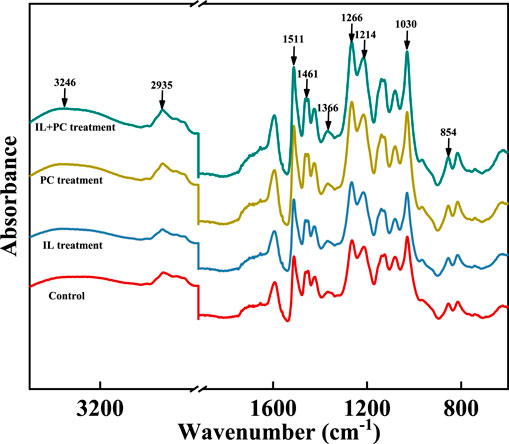
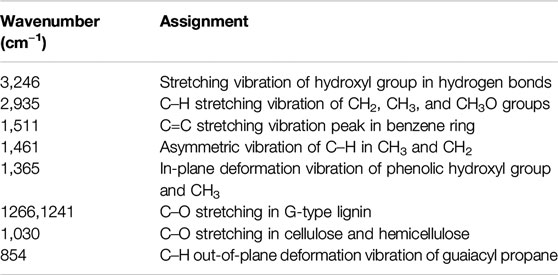
The FTIR spectra of the alkali lignin before and after the fungi treatments are shown in Figure 3, and the major bands are assigned in Table 1 (Kobayashi et al., 2009). The alkali lignin treated by fungi has changed significantly in the fingerprint region (1,300–600 cm−1), corresponding to the stretching vibrations of different groups in the lignin (Ying et al., 2018). This suggested that the overall structure of the alkali lignin had been destroyed after fungi treatment.
In the range of 3,200–3,400 cm−1, the peaks of the autoclave-sterilized lignin appeared at 3,246 cm−1, and the peaks of lignin after fungi treatment appeared at 3,362 cm−1 (P. chrysosporium), 3,370 cm−1 (I. lacteus), and 3,354 cm−1 (fungal coculture). This indicated that the degree of O–H association between the alkali lignin molecules was reduced and the molecular structure of the alkali lignin was ruptured during the treatment (Sun et al., 2016). The signal intensity of the alkali lignin at 2,935 cm−1 decreased, indicating that some of the guaiacyl and syringyl methoxy groups in the alkali lignin were broken during the fungi treatment. After fungi treatment, there was a decrease in the intensity of the stretching vibration peak (1,461 cm−1) of the alkali lignin, which was derived from methylene, indicating that some macromolecular lignin was degraded (Sun et al., 2018). After fungi treatment, the peaks at 1,600 and 1,511 cm−1 increased, which represent the vibration of C=C on the benzene ring, indicating the depolymerization of macromolecular lignin (Liu et al., 2014). The fingerprint characteristic absorption peak of syringyl increased after fungi treatment, suggesting that syringyl lignin was further degraded and more syringyl units were released (Singh et al., 2015).
Scanning Electron Microscope and Porosity Analysis
Figure 4 shows the microstructure of the alkali lignin treated by the fungal monocultures and coculture. It can be observed that the shape of the autoclaved alkali lignin (Figure 5A) was irregular polygons and was composed of many irregular spherical particles. After fungi treatment, the surface gradually became rough and many pores were formed due to fungi degradation (Figure 4B,C). In addition, for the alkali lignin treated by the fungal coculture (Figure 4D), many micropores adhering to the surfaces of large pores could be found compared to alkali lignin treated by the fungal monocultures. The destruction of alkali lignin treated by the fungal coculture was greater than that by the fungal monocultures.
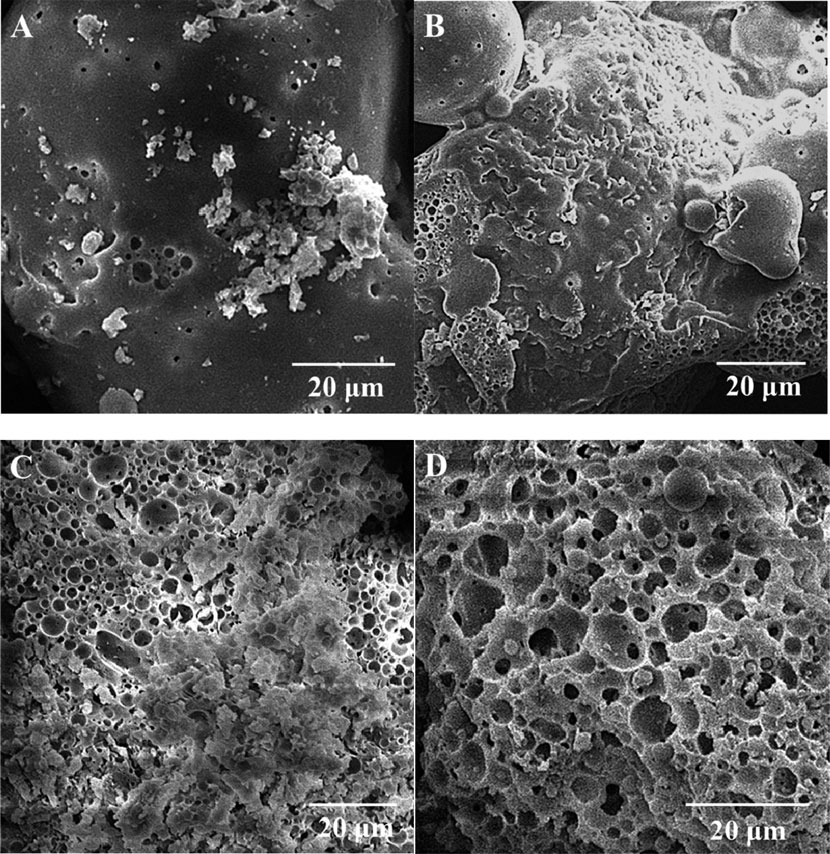
FIGURE 4. Scanning electron microscopy images of (A) autoclaved alkali lignin, (B) I. lacteus treatment, (C) P. chrysosporium treatment, and (D) fungal coculture treatment.
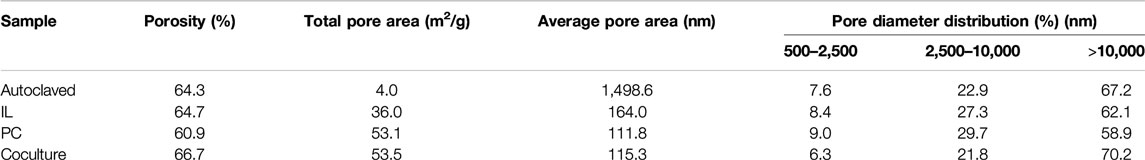
The porosity and pore diameter distribution of the alkali lignin are shown in Table 2. Megapores (>10,000 nm) increased 3.0% after the alkali lignin treated by the fungal coculture, but the macropores (2,500–10,000 nm) of the alkali lignin treated by the fungal monocultures increased 4.36 (I. lacteus) and 6.72% (P. chrysosporium). These results indicated that the damage to the alkali lignin by the fungal coculture was greater than that by the monocultures, which was confirmed by the SEM results. The porosity of the alkali lignin increased after being treated with I. lacteus and the fungal coculture. The total pore area was greatly increased after the fungi treatment, which proved that the alkali lignin formed more porous structures after fungi treatment and that the macromolecular lignin was depolymerized.
Thermogravimetric/Differential Thermogravimetric Analysis
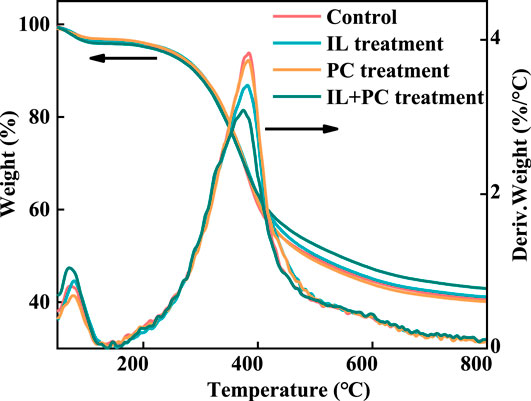
Figure 5 shows the TG and differential TG curves of the autoclaved and fungi-treated alkali lignin samples. The pyrolysis process of the alkali lignin samples could be divided into dehydration and lignin degradation stages. According to the TG curves, the residue char contents of the alkali lignin were 39.9% (Control), 40.5% (I. lacteus), 39.6% (P. chrysosporium), and 42.1% (fungal coculture) (Park et al., 2017). The residual char content is related to the ratio of G and S in alkali lignin (Yang et al., 2010). G has better thermal stability than the other two lignin monomers; therefore, the larger the proportion of G, the more char that remains (Shen et al., 2011). It could be speculated that some of the G-lignin was consumed during the fungal coculture treatment, which was confirmed by previous FTIR results. From the differential TG curve, the maximum decomposition rate of the autoclave-sterilized alkali lignin appeared at 385°C, but the temperature peak of the alkali lignin after fungi treatment was shifted left. This suggested that the alkali lignin was converted to small molecule aromatic compounds with poor thermal stability during the fungi treatment process (Cao and Aita, 2013). It is worth mentioning that the temperature peak of the alkali lignin treated with the fungal coculture was advanced by 11°C (374°C), indicating that the alkali lignin had the poorest thermal stability after the fungal coculture treatment.
Element Analysis
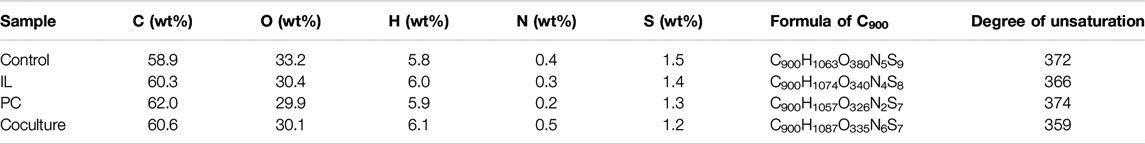
The element analysis and degree of unsaturation of the autoclaved and fungi-treated alkali lignins are shown in Table 3. The degree of unsaturation of the alkali lignin was further reduced by fungi treatment. This indicated that the dense structure of the alkali lignin was depolymerized during fungi treatment, and some of the macromolecular lignin was degraded. The degree of unsaturation of alkali lignin decreased 3.5% after the fungal coculture treatment compared to the autoclaved alkali lignin. After fungi treatment, the mass percentage of elemental sulfur decreased in alkali lignin, suggesting that the elemental sulfur in alkali lignin was dissolved during the treatment (Xu et al., 2013).
Effects of Alkali Lignin Modification on Enzymatic Hydrolysis and Adsorption
Nonproductive Adsorption of Enzymes
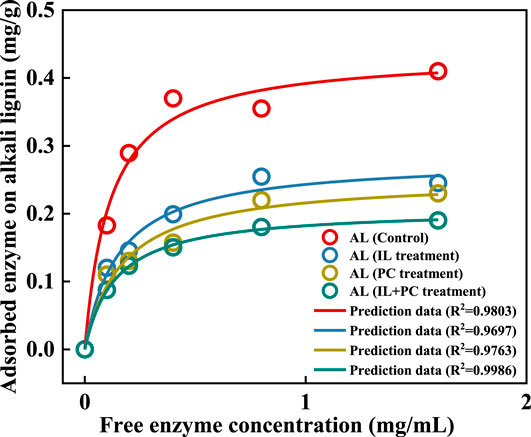
Figure 6 shows the isothermal adsorption of cellulase by alkali lignin at different free enzyme concentrations. The Langmuir adsorption isotherm equation could accurately describe the adsorption of cellulase on alkali lignin. It could be seen that as the free enzyme concentration increased, the adsorption capacity becomes saturated, and the autoclaved alkali lignin had the largest adsorption capacity. After the fungi treatment of alkali lignin, the adsorption capacity decreased by 36.4% (I. lacteus treatment), 43.2% (P. chrysosporium treatment), and 52.3% (fungal coculture treatment) compared with the autoclaved alkali lignin.
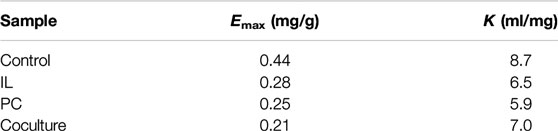
Table 4 shows the adsorption parameters, which were estimated by fitting the enzymatic adsorption data to the Langmuir model. The maximum adsorption capacities (Emax) of lignin were 0.44 (Control), 0.28 (I. lacteus treatment), 0.25 (P. chrysosporium treatment), and 0.21 (fungal coculture treatment) mg/g lignin. The autoclaved alkali lignin had the strongest adsorption capacity, and could adsorb approximately 40–50% more protein than the alkali lignin treated with the fungal coculture. The autoclaved alkali lignin had the highest affinity (K) for the enzyme (8.7 ml/mg protein), while the affinities for the enzyme were 25.3, 32.2, and 20.0% lower than that of the autoclaved alkali lignin for the alkali lignin treated with I. lacteus, P. chrysosporium, and the fungal coculture, respectively. The strength of the absorption between the alkali lignin and the enzyme was characterized by the coefficient K, which was calculated using the maximum adsorption capacity multiplied by the Langmuir adsorption constant (Deng et al., 2019). This is consistent with the results of previous hydrolysis experiments. It was speculated that the fungi treatment of alkali lignin degraded the macromolecular alkali lignin, which generated more exposed carboxylic groups. This could make the lignin more hydrophilic, which would decrease the hydrophobic interaction between the alkali lignin and enzymes and reduce the nonproductive adsorption of cellulase (Yuan et al., 2018).
Enzymatic Hydrolysis of Avicel
The decreased nonproductive adsorption of cellulase on alkali lignin would lead to an increase in the yield of reducing sugar during the hydrolysis of Avicel. Figure 7 presents the enzymatic hydrolysis of Avicel with or without the addition of alkali lignin. All alkali lignins had an inhibitory effect on the reducing sugar yield. The 72 h glucan conversion efficiencies with the addition of differently treated alkali lignin were 50.6% (I. lacteus treatment), 50.3% (P. chrysosporium treatment), and 54.1% (fungal coculture treatment). The Avicel without the addition of alkali lignin had the maximum reducing sugar yield at 72 h of 56.5%.
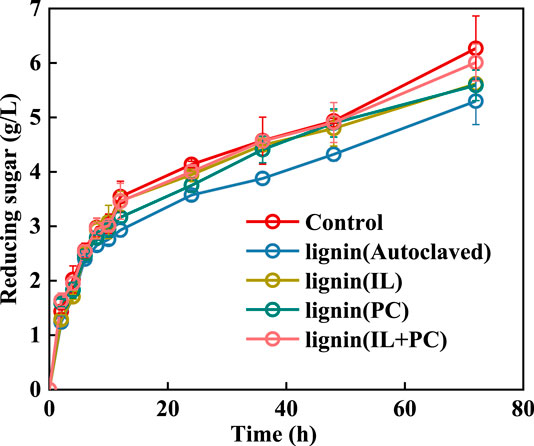
FIGURE 7. Effects of fungi-treated lignin on reducing sugar yield in the enzymatic saccharification of Avicel.
Although the addition of alkali lignin had an inhibitory effect on the hydrolysis saccharification process of Avicel, the fungi-treated lignin had less of a negative effect than the autoclaved alkali lignin. After adding alkali lignin that was treated with the fungal coculture, the reducing sugar yield could reach 95.8% of that without alkali lignin, which was the highest yield among all of the fungi treatments. This result was due to the decrease in absorption capacity of the alkali lignin for the hydrolysis enzyme after fungi treatment.
Practical Implications
The results obtained in this study may provide an efficient approach for the biological pretreatment of lignocellulosic biomass for biofuel production. To further improve the feasibility of fungal coculture system for a large-scale application in the future, more research should be devoted to revealing the complex interaction between mixed enzymes generated by fungal coculture system during the biodegradation of lignocellulosic biomass, and developing advanced bioreactors for the efficient fungal treatment process for biomass conversion. Also, technical-economic assessment and life cycle assessment should be carried out to demonstrate the feasibility of such a pretreatment system based on the long-term operation performance.
Conclusion
The limitations of fossil fuels have led to an increase production of biofuels from renewable lignocellulosic biomass sources. Therefore, improving the efficiency of biological pretreatment of lignocellulosic biomass has become the most important point for the development of biofuels. This study opens a new opportunity to improve the efficiency of biological pretreatment and biomass conversion. The degradation efficiency of alkali lignin treated by the fungal coculture reached 26.4%, which is 3.4 and 5.2% higher than the monocultures of I. lacteus and P. chrysosporium, respectively. Physicochemical analyses showed that the chemical groups of the alkali lignin were changed significantly after fungal treatment. The alkali lignin generated more carboxylic groups, which increased its hydrophilicity and reduced the absorption of alkali lignin to cellulase. The nonproductive adsorption of cellulase on alkali lignin decreased by 40–50% compared with the control group.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
RL: Methodology, investigation, writing—original draft preparation. QL and AX: Conceptualization, supervision. YH, ZD, and XiZ: Writing—review and editing. XuZ: Project administration.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 51836001 and 51876016), the Venture and Innovation Support Program for Chongqing Overseas Returnees (No. cx2019040), the Fundamental Research Funds for the Central Universities (Nos. 2020CDJQY-A054 and 2018CDPTCG0001/18) and the Young Elite Scientists Sponsorship Program by CAST (2018QNRC001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2020.575371/full#supplementary-material
References
Arora, D. S., and Sharma, R. K. (2010). Ligninolytic fungal laccases and their biotechnological applications. Biotechnol. Appl. Biochem. 160 (6), 1760–1788. doi:10.1007/s12010-009-8676-y
Asina, F., Brzonova, I., Voeller, K., Kozliak, E., Kubátová, A., Yao, B., et al. (2016). Biodegradation of lignin by fungi, bacteria and laccases. Bioresour. Technol. 220, 414–424. doi:10.1016/j.biortech.2016.08.016
Cao, S., and Aita, G. M. (2013). Enzymatic hydrolysis and ethanol yields of combined surfactant and dilute ammonia treated sugarcane bagasse. Bioresour. Technol. 131, 357–364. doi:10.1016/j.biortech.2012.12.170
Chen, K.-J., Tang, J.-C., Xu, B.-H., Lan, S.-L., and Cao, Y. (2019). Degradation enhancement of rice straw by co-culture of Phanerochaete chrysosporium and Trichoderma viride. Sci. Rep. 9 (1), 1–7. doi:10.1038/s41598-019-56123-5
Christopher, L. P., Yao, B., and Ji, Y. (2014). Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2, 12. doi:10.3389/fenrg.2014.00012
Deng, Z., Xia, A., Liao, Q., Zhu, X., Huang, Y., and Fu, Q. (2019). Laccase pretreatment of wheat straw: effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol. Biofuels 12 (1), 159. doi:10.1186/s13068-019-1499-3
Dong, X. Q., Yang, J. S., Zhu, N., Wang, E. T., and Yuan, H. L. (2013). Sugarcane bagasse degradation and characterization of three white-rot fungi. Bioresour. Technol. 131, 443–451. doi:10.1016/j.biortech.2012.12.182
Dong, Y.-C., Dai, Y.-N., Xu, T.-Y., Cai, J., and Chen, Q.-H. (2014). Biodegradation of chestnut shell and lignin-modifying enzymes production by the white-rot fungi Dichomitus squalens, Phlebia radiata. Bioproc. Biosyst. Eng. 37 (5), 755–764. doi:10.1007/s00449-013-1045-9
Hao, X., Quansheng, Y., Dan, S., Honghui, Y., Jidong, L., Jiangtao, F., et al. (2015). Fabrication and characterization of PbO2 electrode modified with [Fe(CN)6]3− and its application on electrochemical degradation of alkali lignin. J. Hazard Mater. 286, 509–516. doi:10.1016/j.jhazmat.2014.12.065
Isroi, I., Millati, R., Niklasson, C., Taherzadeh, M. J., and Lundquist, K. (2011). Biological treatment of Lignocelluloses with white-rot funghi and its applications: review. BioResources 6 (4), 5224–5259. doi:10.2172/1830
Kobayashi, N., Okada, N., Hirakawa, A., Sato, T., Kobayashi, J., Hatano, S., et al. (2009). Characteristics of solid residues obtained from hot-compressed-water treatment of woody biomass. J. Ind. Eng. Chem. Res. 48 (1), 373–379. doi:10.1021/ie800870k
Ku, H.-K., Lim, H.-M., Oh, K.-H., Yang, H.-J., Jeong, J.-S., and Kim, S.-K. (2013). Interpretation of protein quantitation using the Bradford assay: comparison with two calculation models. Anal. Biochem. 434 (1), 178–180. doi:10.1016/j.ab.2012.10.045
Lee, D.-S., Lee, Y.-G., Song, Y., Cho, E.-J., and Bae, H.-J. (2020). Hydrolysis Patterns of xylem tissues of hardwood pretreated with acetic acid and hydrogen peroxide. Front. Energy Res. 8, 34. doi:10.3389/fenrg.2020.00034
Li, X., He, Y., Zhang, L., Xu, Z., Ben, H., Gaffrey, M. J., et al. (2019). Discovery of potential pathways for biological conversion of poplar wood into lipids by co-fermentation of Rhodococci strains. Biotechnol. Biofuels 12 (1), 60. doi:10.1186/s13068-019-1395-x
Lindedam, J., Bruun, S., Jørgensen, H., Decker, S. R., Turner, G. B., DeMartini, J. D., et al. (2014). Evaluation of high throughput screening methods in picking up differences between cultivars of lignocellulosic biomass for ethanol production. Biomass Bioenergy 66, 261–267. doi:10.1016/j.biombioe.2014.03.006
Liu, Y., Hu, T., Wu, Z., Zeng, G., Huang, D., Shen, Y., et al. (2014). Study on biodegradation process of lignin by FTIR and DSC. Environ. Sci. Pollut. Res. 21 (24), 14004–14013. doi:10.1007/s11356-014-3342-5
Meehnian, H., Jana, A. K., and Jana, M. M. (2017). Pretreatment of cotton stalks by synergistic interaction of Daedalea flavida and Phlebia radiata in co-culture for improvement in delignification and saccharification. Int. Biodeterior. Biodegrad. 117, 68–77. doi:10.1016/j.ibiod.2016.11.022
Mishra, V., Jana, A. K., Jana, M. M., and Gupta, A. (2017). Enhancement in multiple lignolytic enzymes production for optimized lignin degradation and selectivity in fungal pretreatment of sweet sorghum bagasse. Bioresour. Technol. 236, 49–59. doi:10.1016/j.biortech.2017.03.148
More, S. S., Renuka, P. S., Pruthvi, K., Swetha, M., Malini, S., and Veena, S. M. (2011). Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzym. Res. 2011, 248735. doi:10.4061/2011/248735
Parikh, J., Nadagouda, V., and Shukla, M. D. (2014). Production of lignin degrading enzymes namely Laccase, Manganese Peroxidase (MnP) and Lignin Peroxidase (LiP) of selected white-rot fungus in solid state fermentation (solid media). Res. J. Biotechnol. 9 (10), 80–82. doi:10.5897/ajb2014.14331
Park, S. Y., Kim, J.-Y., Youn, H. J., and Choi, J. W. (2018). Fractionation of lignin macromolecules by sequential organic solvents systems and their characterization for further valuable applications. Int. J. Biol. Macromol. 106, 793–802. doi:10.1016/j.ijbiomac.2017.08.069
Ren, N.-Q., Zhao, L., Chen, C., Guo, W.-Q., and Cao, G.-L. (2016). A review on bioconversion of lignocellulosic biomass to H2: key challenges and new insights. Bioresour. Technol. 215, 92–99. doi:10.1016/j.biortech.2016.03.124
Salvachúa, D., Martínez, A. T., Tien, M., López-Lucendo, M. F., García, F., de los Ríos, V., et al. (2013). Differential proteomic analysis of the secretome of Irpex lacteus and other white-rot fungi during wheat straw pretreatment. Biotechnol. Biofuels 6 (1), 115. doi:10.1186/1754-6834-6-115
Schöneberg, A., Musa, T., Voegele, R. T., and Vogelgsang, S. (2015). The potential of antagonistic fungi for control of fusarium graminearumandFusarium crookwellensevaries depending on the experimental approach. J. Appl. Microbiol. 118 (5), 1165–1179. doi:10.1111/jam.12775
Sharma, H. K., Xu, C., and Qin, W. (2019). Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valoriz. 10 (2), 235–251. doi:10.1007/s12649-017-0059-y
Shen, Q., Zhang, T., Zhang, W.-X., Chen, S., and Mezgebe, M. (2011). Lignin-based activated carbon fibers and controllable pore size and properties. J. Appl. Polym. Sci. 121 (2), 989–994. doi:10.1002/app.33701
Sindhu, R., Binod, P., and Pandey, A. (2016). Biological pretreatment of lignocellulosic biomass – an overview. Bioresour. Technol. 199, 76–82. doi:10.1016/j.biortech.2015.08.030
Singh, S., Cheng, G., Sathitsuksanoh, N., Wu, D., Varanasi, P., George, A., et al. (2015). Comparison of different biomass pretreatment techniques and their impact on chemistry and structure. Front. Energy Res. 2, 62. doi:10.3389/fenrg.2014.00062
Suksong, W., Wongfaed, N., Sangsri, B., Kongjan, P., Prasertsan, P., Podmirseg, S. M., et al. (2020). Enhanced solid-state biomethanisation of oil palm empty fruit bunches following fungal pretreatment. Ind. Crop. Prod. 145, 112099. doi:10.1016/j.indcrop.2020.112099
Sun, C., Xia, A., Liao, Q., Fu, Q., Huang, Y., Zhu, X., et al. (2018). Improving production of volatile fatty acids and hydrogen from microalgae and rice residue: effects of physicochemical characteristics and mix ratios. Appl. Energy 230, 1082–1092. doi:10.1016/j.apenergy.2018.09.066
Sun, F.-h., Li, J., Yuan, Y.-x., Yan, Z.-y., and Liu, X.-f. (2011). Effect of biological pretreatment with Trametes hirsuta yj9 on enzymatic hydrolysis of corn stover. Int. Biodeterior. Biodegrad. 65 (7), 931–938. doi:10.1016/j.ibiod.2011.07.001
Sun, S., Huang, Y., Sun, R., and Tu, M. (2016). The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 18 (15), 4276–4286. doi:10.1039/c6gc00685j
Tesfaw, A., and Assefa, F. (2014). Co-culture: a great promising method in single cell protein production. Biotechnol. Mol. Biol. Rev. 9 (2), 12–20. doi:10.5897/bmbr2014.0223
Wang, W., Yuan, T., and Cui, B. (2014). Biological pretreatment with white rot fungi and their co-culture to overcome lignocellulosic recalcitrance for improved enzymatic digestion. Bioresources 9 (3), 3968–3976. doi:10.15376/biores.9.3.3968-3976
Wiberth, C.-C., Casandra, A.-Z. C., Zhiliang, F., and Gabriela, H. (2018). Oxidative enzymes activity and hydrogen peroxide production in white-rot fungi and soil-borne micromycetes co-cultures. Ann. Microbiol. 69 (2), 171–181. doi:10.1007/s13213-018-1413-4
Xia, A., Jacob, A., Tabassum, M. R., Herrmann, C., and Murphy, J. D. (2016). Production of hydrogen, ethanol and volatile fatty acids through co-fermentation of macro- and micro-algae. Bioresour. Technol. 205, 118–125. doi:10.1016/j.biortech.2016.01.025
Xu, G., Wang, L., Liu, J., and Wu, J. (2013). FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 280, 799–805. doi:10.1016/j.apsusc.2013.05.065
Yadav, S., and Chandra, R. (2015). Syntrophic co-culture of Bacillus subtilis and Klebsiella pneumonia for degradation of kraft lignin discharged from rayon grade pulp industry. J. Environ. Sci. 33, 229–238. doi:10.1016/j.jes.2015.01.018
Yang, C.-Y., and Fang, T. J. (2015). Kinetics of enzymatic hydrolysis of rice straw by the pretreatment with a bio-based basic ionic liquid under ultrasound. Process Biochem. 50 (4), 623–629. doi:10.1016/j.procbio.2015.01.013
Yang, Q., Zhang, H., Li, X., Wang, Z., Xu, Y., Ren, S., et al. (2013). Extracellular enzyme production and phylogenetic distribution of yeasts in wastewater treatment systems. Bioresour. Technol. 129, 264–273. doi:10.1016/j.biortech.2012.11.101
Yang, X., Ma, F., Zeng, Y., Yu, H., Xu, C., and Zhang, X. (2010). Structure alteration of lignin in corn stover degraded by white-rot fungus Irpex lacteus CD2. Int. Biodeterior. Biodegrad. 64 (2), 119–123. doi:10.1016/j.ibiod.2009.12.001
Yao, L., Zhu, L.-P., Xu, X.-Y., Tan, L.-L., Sadilek, M., Fan, H., et al. (2016). Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Sci. Rep. 6 (1), 33237. doi:10.1038/srep33237
Ying, W., Shi, Z., Yang, H., Xu, G., Zheng, Z., and Yang, J. (2018). Effect of alkaline lignin modification on cellulase-lignin interactions and enzymatic saccharification yield. Biotechnol. Biofuels 11 (1), 214. doi:10.1186/s13068-018-1217-6
Yuan, Y., Zhai, R., Li, Y., Chen, X., and Jin, M. (2018). Developing fast enzyme recycling strategy through elucidating enzyme adsorption kinetics on alkali and acid pretreated corn stover. Biotechnol. Biofuels 11 (1), 316. doi:10.1186/s13068-018-1315-5
Zeng, G.-M., Zhao, M.-H., Huang, D.-L., Lai, C., Huang, C., Wei, Z., et al. (2013). Purification and biochemical characterization of two extracellular peroxidases from Phanerochaete chrysosporium responsible for lignin biodegradation. Int. Biodeterior. Biodegrad. 85, 166–172. doi:10.1016/j.ibiod.2013.07.005
Keywords: biomass hydrolysis, white-rot fungi, coculture, lignin, enzyme absorption
Citation: Luo R, Liao Q, Xia A, Deng Z, Huang Y, Zhu X and Zhu X (2020) Synergistic Treatment of Alkali Lignin via Fungal Coculture for Biofuel Production: Comparison of Physicochemical Properties and Adsorption of Enzymes Used As Catalysts. Front. Energy Res. 8:575371. doi: 10.3389/fenrg.2020.575371
Received: 23 June 2020; Accepted: 18 August 2020;
Published: 08 September 2020.
Edited by:
Mohammad Rehan, King Abdulaziz University, Saudi ArabiaReviewed by:
Muhammad Amjad, University of Engineering and Technology, Lahore, PakistanMd Mofijur Rahman, University of Technology Sydney, Australia
Copyright © 2020 Luo, Liao, Xia, Deng, Huang, Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Liao, bHF6eEBjcXUuZWR1LmNu Ao Xia, YW94aWFAY3F1LmVkdS5jbg==
Abbreviations:
SEM, scanning electron microscope; FTIR, fourier transform infrared; TG/DTG, thermogravimetric/differential thermogravimetric; WRF, white-rot fungi; LDEs, lignin degrading enzymes system; Lac, laccase; MnP, manganese peroxidases; LiP, lignin peroxidases; PDA, potato dextrose agar. Ruhong Luo1,2
Ruhong Luo1,2