
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Energy Res. , 27 May 2020
Sec. Bioenergy and Biofuels
Volume 8 - 2020 | https://doi.org/10.3389/fenrg.2020.00090
This article is part of the Research Topic Energy and Resource Valorisation of Biomass and Waste towards Sustainable Environment via Thermochemical and Biological Application View all 11 articles
Cinnamomum camphora is an evergreen tree native to China and distributed to some Asian countries. Its leaves are rich in bioactive compounds and their pharmacological effects are well-known. A potent method to extract and preserve the active biological substances is crucial for latter application in bioenergy and as chemical raw material. In this study, the metabolite profiles of C. camphora were comprehensively analyzed by using FT-IR and GC-MS after extracted by three type of solvent: ethanol, benzene, and acetone. The identified chemical compounds were then classified into different functional groups based on previous study. Our result proposes the potential application of C. camphora into biomedicine, bioenergy, and chemical raw materials industry.
Increment of human population has huge impact to energy consumption that mainly depends on non-renewable energy such as fossil fuel, oil, coal, and natural gas to sustain the human need. To date, only 11% of energy is generated from renewable energy (https://www.iea.org/weo2018/) due to several shortages such as efficiency and resources. Therefore, researcher started to explore resource like Cinnamomum camphora as alternative to fossil fuel.
Cinnamomum camphora (Lauraceae) is commonly known as camphor tree, are widely distributed in subtropical zones, including southeastern China and northeastern Australia (Yakefu et al., 2018). C. camphora is famous for its ornamental, economic, and medicinal value (Chen and Dai, 2015). In particular, C. camphora contain oil gland cells that can be used to extract camphor oil (Afrin et al., 2019). The essential oil has antifungal activity (Sattar et al., 1991), insecticidal and insect repellent properties (Liu L. et al., 2018), antioxidant (Fu et al., 2016), anti-aging, anti-bacterial, and anti-inflammatory effects (Chen C. et al., 2018; He et al., 2018; Chen et al., 2019). The bark is used in treating limb ulcer and the fruit of C. camphora has properties to relieve fever, treat common flu, and dysentery (Satyal et al., 2013). The root of C. camphora promote blood circulation, improving rheumatism, treating stomach diseases, and bruises (Jiang et al., 2016; Chen S. et al., 2018; Liu X. et al., 2019).
The application of camphor oil is not limited to medical application but also as flavors, soap, painting materials, mineral processing, plastics polymer, explosives, and as preservative (Guo et al., 2016). The leaf powder of C. camphora was reported to absorb and remove dye (Wang et al., 2014), copper (II) ions (Chen et al., 2010a), and Pb (II) (Chen et al., 2010b) from aqueous solution. The broth of C. camphora represents new green material in palladium nanoparticles formation to replace synthetic nanoparticles (Yang et al., 2010).
To tackle the problem of resources, supply in application as renewable energy, the large-scale production of C. camphora has been established via in vitro micropropagation technique (Shi et al., 2016). To fully utilize the resources of C. camphora, the biosynthesis pathway of secondary metabolomics production is also well explored (Chen et al., 2017). Volatile constituents from C. camphora have been detected and identified such as camphor, eucalyptol, linalool, nerolidol, limonene, β-pinene, -terpineol, and other volatile substances (Yang et al., 2016; Xu Y. et al., 2018). In particular, the biosynthesis pathway of secondary metabolomics production is also well explored (Chen et al., 2017). The constituent of oil composition was also reported (Jiang et al., 2016; Liu Z. et al., 2018). However, there is no complete list of metabolite profile available for different solvent types and hence it is difficult to suggest the optimal extraction method for C. camphora. As we know different solvent type has affinity to different type of metabolites and results to the extraction of different functional groups, we tested three types of solvents and listed the metabolite profile from the three solvents.
An amount of 500 g of C. camphora leaves were cleaned, dried, and crushed into fine powder. The powder is subsequently sieved through a 200 mesh, the sample was divided into three parts and soaked with three types of solvents: ethanol, acetone, and benzene with ratio of material to liquid 1:15 (Xu K. et al., 2018; Figure 1). The mixture was boiled in water baths for 4 h and the temperature were set to the boiling point of each solvent. After removal from water bath, the solution were filtered by pump filter and then dried up using rotary evaporator. The solvent was collected once it reach approximately amount of 20 mL (Kumar and Kumari, 2019).
After 200 mesh screening, the solid powder was taken as the sample. 0.5–2 mg of sieved powder was mixed with an appropriate amount of potassium bromide. It was then ground into fine and uniform particles in a mortar. A sample of this powder was taken and pressed to flatten the sample into an evenly transparent sheet. This sheet was then dried in an oven.
Solvents of ethanol, acetone, and benzene were pipetted out by using manual pipette and dropped evenly in the center of pure potassium bromide thin films. The film was then oven dried before it was measured at 4,000–400 cm−1 by Fourier transform infrared spectroscopy (Shimadzu, IR affinity-1) (Wu et al., 2019).
The program was set as initial temperature at 5°C without retention, increased and remained from 13 to 18°C, and then rose to 30°C for 5 min. The inlet temperature was set to 28°C with column flow of 1.0 mL/min, and split ratio was 20:1. A fused silica HP-5ms capillary GC column (30 m × 0.25 mm × 0.25 μm) was used. Mass spectrometry proceeded under the following conditions: ionization mode was EI, electron energy of 70 eV, ion source temperature of 23°C, scanning starting point of 30–60°C (Liu Z. et al., 2019; Ma et al., 2019).
The raw MS spectra data were first normalized by peak area and based line corrected. The chemicals composition detected were then identified by in house databased program and were grouped into similar functional groups based on previous study.
The FTIR result of the fine powder sample show peaks and variation observed in the regions of 3,730–3,050 cm−1, 3,050–2,500 cm−1, 2,000–1,500 cm−1, and 1,500–500 cm−1 (Figure 2). According to the infrared functional group comparison table, there is a strong absorption peak in the region of 1,500–500 cm−1 represents to ether bond, which can be identified as aliphatic ether and aromatic ether. The region observed at 3,050–2,500 cm−1 represents mainly olefins, alkynes, and aromatic compounds, resulted by the stretching vibration absorption of unsaturated carbon C-H.
Benzene solvent causing a sharp peak observed at 650 cm−1. The presence of organic halides could be determined due to the expansion and contraction of aliphatic C-Br. In the 2,000–1,500 cm−1 region, there are olefins due to the vibrations of the bonds of unsaturated carbon, which are consistent with the compounds predicted in the same interval of raw powder sample. In the acetone solvent, the region around 1,500–500 cm−1 may represent alcohol, phenol, aromatic ether, and ester. Finally, the solvents of ethanol may contain functional group of olefins and amines as shown in the region 1,500–500 cm−1 and aromatic ethers as observed at region 1,050 cm−1.
These results show that the infrared spectra of the ethanol as solvent are similar to those of raw powder, indicating that ethanol is a better solvent for extracting bioactive components from C. camphora leaves than benzene and acetone.
A total of 120 compounds were detected from the acetone solvent by GC-MS (Appendix Figure 1, Appendix Table 1). Amongst them, 94 were identified and group into functional groups of amines (13.18%), ketone (10.77%), alkenes (6.61%), alcohol (5.53%), and esters (4.14%) (Figure 3). There are 58 of chemical compound were detected in ethanol solvent, in which 48 are organic compounds (Appendix Figure 2, Appendix Table 2). These compounds were classified into amines group (27.70%), Tetrazol-5-amine and N-(3, 4-dimethoxybenzyl) (25.44%), alcohols (24.05%), alkenes (19.80%), ketones (16.33%), and esters (6.99%). In benzene solvent, only 12 chemical compounds were detected and 4 substances were identified and classified into esters (52.95%), amines (41.99%), and alcohols (5.06%) (Appendix Figure 3, Appendix Table 3).
The results showed that the three solvents for C. camphora leaves have outstanding functional properties that serve as chemical raw materials for different green application including cosmetics, spices, food additives, chemicals raw materials, and biomedicine. The percentage of the bioactive components detected are highest in benzene solvent (41.99%), followed by ethanol solvent (33.53%), and acetone solvent (28.23%). For cosmetics and food additives application, the best extraction method is ethanol as solvent which enables recovery of 2 and 10.44% of active compounds, respectively. The functional content of spices was highest when acetone as solvent (15.51%) (Figure 4).
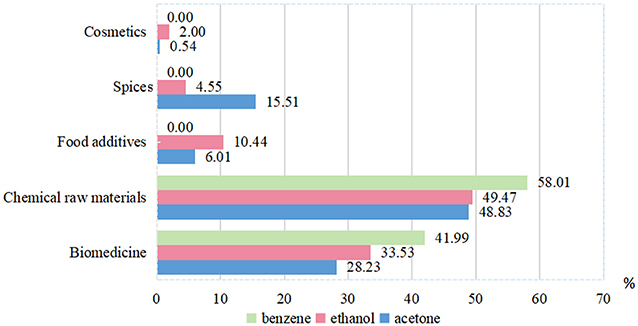
Figure 4. Potential application based on thefunctional groups constitute of three solvents from C. camphora leaves.
In order to enhance the application value of C. camphora leaves, the effects of different extraction methods on the bioactive substances in C. camphora leaves were investigated. Three common solutions of ethanol, acetone, and benzene were used to extract the substances in C. camphora leaves. There were significant differences in the types and proportions of bioactive compounds obtained by the three extraction methods. The FT- IR results showed that ethanol can effectively extract the bioactive compounds in C. camphora leaves. The GC-MS results indicated that more amines and alcohols were obtained from C. camphora using ethanol as solvent. The amines can be used in cosmetics and biomedicine, and the alcohols can be widely used in industrial fields. Aldehyde, ketones, alkene, and esters in ethanol solvent can also be applied in the fields of chemical raw materials, biomedicine, bioenergy, and spices.
All datasets generated for this study are included in the article/Supplementary Material.
DZ contributed to the integrity of the entire research, research concepts, and research design. YC contributed to the literature research, manuscript editing, and critically revision. ZZ and XW did the experimental studies, data acquisition, data analysis, and manuscript preparation. YL and XL gave guidance and participated in the experiments.
This project was supported by the talent project (DZ) of Henan Agricultural University, China, and the Project of Henan Provincial Science Research, China (192102110174).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2020.00090/full#supplementary-material
Afrin, F., Chouhan, G., Islamuddin, M., Want, M. Y., Ozbak, H. A., and Hemeg, H. A. (2019). Cinnamomum cassia exhibits antileishmanial activity against Leishmania donovani infection in vitro and in vivo. PLoS Negl. Trop. Dis. 13:e0007227. doi: 10.1371/journal.pntd.0007227
Chen, C., Zheng, Y., Liu, S., Zhong, Y., Wu, Y., Li, J., et al. (2017). The complete chloroplast genome of Cinnamomum camphora and its comparison with related Lauraceae species. Peer J. 5:e3820. doi: 10.7717/peerj.3820
Chen, C., Zheng, Y., Zhong, Y., Wu, Y., Li, Z., Xu, L. A., et al. (2018). Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genomics. 19:550. doi: 10.1186/s12864-018-4941-1
Chen, H., Dai, G., Zhao, J., Zhong, A., Wu, J., and Yan, H. (2010a). Removal of copper (II) ions by a biosorbent—Cinnamomum camphora leaves powder. J. Hazardous Mater. 177, 228–236. doi: 10.1016/j.jhazmat.2009.12.022
Chen, H., Zhao, J., Dai, G., Wu, J., and Yan, H. (2010b). Adsorption characteristics of Pb (II) from aqueous solution onto a natural biosorbent, fallen Cinnamomum camphora leaves. Desalination 262, 174–182. doi: 10.1016/j.desal.2010.06.006
Chen, S., Zheng, T., Ye, C., Huannixi, W., Yakefu, Z., Meng, Y., et al. (2018). Algicidal properties of extracts from Cinnamomum camphora fresh leaves and their main compounds. Ecotoxicol. Environ. Saf. 163, 594–603. doi: 10.1016/j.ecoenv.2018.07.115
Chen, Y., and Dai, G. (2015). Acaricidal activity of compounds from Cinnamomum camphora (L.) Presl against the carmine spider mite, Tetranychus cinnabarinus. Pest Manage. Sci. 71, 1561–1571. doi: 10.1002/ps.3961
Chen, Y., Weng, Y., Zhou, M., Meng, Y., Liu, J., Yang, L., et al. (2019). Linalool-and α-terpineol-induced programmed cell death in Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 167, 435–440. doi: 10.1016/j.ecoenv.2018.10.062
Fu, J., Zeng, C., Zeng, Z., Wang, B., and Gong, D. (2016). Cinnamomum camphora seed kernel oil ameliorates oxidative stress and inflammation in diet-induced obese rats. J. Food Sci. 81, H1295–H1300. doi: 10.1111/1750-3841.13271
Guo, S., Geng, Z., Zhang, W., Liang, J., Wang, C., Deng, Z., et al. (2016). The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 17:1836. doi: 10.3390/ijms17111836
He, H., Qin, J., Cheng, X., Xu, K., Teng, L., and Zhang, D. (2018). Effects of exogenous 6-BA and NAA on growth and contents of medicinal ingredient of Phellodendron chinense seedlings. Saudi J. Biol. Sci. 25, 1189–1195. doi: 10.1016/j.sjbs.2017.11.037
Jiang, H., Wang, J., Song, L., Cao, X., Yao, X., Tang, F., et al. (2016). GC × GC-TOFMS analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora L. Presl and their insecticidal and repellent activities. Molecules 21:423. doi: 10.3390/molecules21040423
Kumar, S., and Kumari, R. (2019). Cinnamomum: review article of essential oil compounds, ethnobotany, antifungal and antibacterial effects. Open Access J. Sci. 3, 13–16. doi: 10.15406/oajs.2019.03.00121
Liu, L., Cheng, X., Zhao, W. W., Wang, Y. H., Dong, X., Chen, L., et al. (2018). Systematic characterization of volatile organic components and pyrolyzates from Camellia oleifera seed cake for developing high value-added products. Arab. J. Chem. 6, 802–814. doi: 10.1016/j.arabjc.2017.12.031
Liu, X., Meng, Y., Zhang, Z., Wang, Y., Geng, X., Li, M., et al. (2019). Functional nano-catalyzed pyrolyzates from branch of Cinnamomum camphora. Saudi J. Biol. Sci. 26, 1227–1246. doi: 10.1016/j.sjbs.2019.06.003
Liu, Z., Deng, B., Li, S., and Zou, Z. (2018). Optimization of solvent-free microwave assisted extraction of essential oil from Cinnamomum camphora leaves. Industr. Crops Products 124, 353–362.
Liu, Z., Kong, L., Lu, S., and Zou, Z. (2019). Application of a combined homogenate and ultrasonic cavitation system for the efficient extraction of flavonoids from Cinnamomum camphora leaves and evaluation of their antioxidant activity in vitro. J. Analyt. Methods Chem. 2019:4892635. doi: 10.1155/2019/4892635
Ma, X., Hu, Z., Mao, J., Xu, Y., Zhu, X., and Xiong, H. (2019). Synthesis of cocoa butter substitutes from Cinnamomum camphora seed oil and fully hydrogenated palm oil by enzymatic interesterification. J. Food Sci. Technol. 56, 835–845. doi: 10.1007/s13197-018-3543-x
Sattar, A., Gilani, A. M., and Saeed, M. A. (1991). Gas chromatographic examination of the essential oil of cinnamomum camphora. Pak. J. Sci. Ind. Res. 34, 135–136.
Satyal, P., Paudel, P., Poudel, A., Dosoky, N. S., Pokharel, K. K., and Setzer, W. N. (2013). Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Natural Product Commun. 8, 1777–1784. doi: 10.1177/1934578x1300801232
Shi, X., Zhang, C., Liu, Q., Zhang, Z., Zheng, B., and Bao, M. (2016). De novo comparative transcriptome analysis provides new insights into sucrose induced somatic embryogenesis in camphor tree (Cinnamomum camphora L.). BMC Genomics. 17:26. doi: 10.1186/s12864-015-2357-8
Wang, H., Yuan, X., Wu, Z., Wang, L., Peng, X., Leng, L., et al. (2014). Removal of basic dye from aqueous solution using Cinnamomum camphora sawdust: kinetics, isotherms, thermodynamics, and mass-transfer processes. Separ. Sci. Technol. 49, 2689–2699. doi: 10.1080/01496395.2014.940590
Wu, L., Xiong, W., Hu, J. W., Wu, J., Li, Z. J., Gao, Y., et al. (2019). Secondary metabolites from the twigs of Cinnamomum camphora. Chem. Natural Comp. 55, 345–347. doi: 10.1007/s10600-019-02686-8
Xu, K., He, G., Qin, J., Cheng, X., He, H., Zhang, D., et al. (2018). High-efficient extraction of principal medicinal components from fresh Phellodendron bark (Cortex phellodendri). Saudi J. Biol. Sci. 25, 811–815. doi: 10.1016/j.sjbs.2017.10.008
Xu, Y., Xiao, H., Guan, H., and Long, C. (2018). Monitoring atmospheric nitrogen pollution in Guiyang (SW China) by contrasting use of Cinnamomum Camphora leaves, branch bark and bark as biomonitors. Environ. Pollut. 233, 1037–1048. doi: 10.1016/j.envpol.2017.10.005
Yakefu, Z., Huannixi, W., Ye, C., Zheng, T., Chen, S., Peng, X., et al. (2018). Inhibitory effects of extracts from Cinnamomum camphora fallen leaves on algae. Water Sci. Technol. 77, 2545–2554. doi: 10.2166/wst.2018.199
Yang, D., Zhang, H., Peng, K., Chen, L., He, H., Huang, X., et al. (2016). Differential gene regulation of lipid synthesis in the developing seeds of two biodiesel tree species, Jatropha and Vernicia. Int. J. Agric. Biol. 18, 1143–1152. doi: 10.17957/IJAB/15.0218
Keywords: renewable biomass, Cinnamomum camphora leaves, extract, bioactive components, bioenergy
Citation: Zhang Z, Wu X, Lai Y, Li X, Zhang D and Chen Y (2020) Efficient Extraction of Bioenergy From Cinnamomum camphora Leaves. Front. Energy Res. 8:90. doi: 10.3389/fenrg.2020.00090
Received: 10 November 2019; Accepted: 28 April 2020;
Published: 27 May 2020.
Edited by:
Su Shiung Lam, University of Malaysia Terengganu, MalaysiaReviewed by:
Peter Nai Yuh Yek, University College of Technology Sarawak, MalaysiaCopyright © 2020 Zhang, Wu, Lai, Li, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dangquan Zhang, emhhbmdkYW5ncXVhbkAxNjMuY29t; Yuanyuan Chen, Y3l1YW4wOTFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.