- 1Fundamental Science on Nuclear Safety and Simulation Technology Laboratory, Harbin Engineering University, Harbin, China
- 2School of Mining and Civil Engineering, Liupanshui Normal University, Liupanshui, China
Pool scrubbing is a representative process for aerosol retention involved in severe LWR accidents and has proved to be a potential method for the removal of micron-sized aerosols. Bubble scrubbing, as the key component of pool scrubbing, has the advantages of a large gas-liquid contact area and long residence time. However, the filtration mechanism of submicron aerosol by bubble scrubbing is still not clear. Therefore, the goal of this research is to evaluate the filtration performance of submicron insoluble aerosol in a bubble column. Some preliminary experiments were carried out to investigate the influence of operational parameters on aerosol filtration efficiency, such as inlet aerosol concentration, gas flow rate and pool temperature. Mass method was used to analyze the aerosol filtration efficiency. The results show that the aerosol filtration efficiency is independent of inlet aerosol concentration when inlet aerosol concentration is in the range of 0.2–0.5 g/m3. Besides the aerosol filtration, efficiency is influenced by the superficial gas velocity. With the increase of superficial gas velocity, homogeneous bubbling, transition and a heterogeneous bubbling regime will appear in turn. The aerosol filtration efficiency decreases in the transition regime due to the appearance of large bubbles but increases in the heterogeneous bubbling regime account for intense gas-liquid interaction. Moreover, the aerosol filtration efficiency significantly decreases with pool temperature because the evaporation rate at the gas-liquid interface will significantly increase as the boiling point is approached, which gives retroaction to aerosol filtration efficiency.
Introduction
Since the Fukushima nuclear accident occurred, more attention has been paid to the issue of severe accidents. To prevent radioactive material from leaking into the environment, some dry or wet filtration methods are used. Pool scrubbing is a representative process for aerosol retention involved in severe LWR accidents. Examples include the core reflooding process in PWR, gas injection into suppression pool in BWR and also the wet scrubbing process in Filter Containment Venting Systems (FCVS) (Basu, 2014). The behavior of pool scrubbing can be divided into 4 parts, which include gas inject region, globule-breakup region, bubble swarm region and entrainment droplet region (Owczarski and Burk, 1991). The corresponding filtration mechanism of each region is different. The bubble scrubbing process is especially considered as the migration process of particles inside the bubble toward the liquid phase.
The deposition characteristics of aerosol in the rising bubble involve various mechanisms including gravitational settlement, Brownian diffusion, inertial impaction, and they also include thermophoresis and diffusiophoresis in the case of thermal non-equilibrium conditions. The calculation formula of deposition efficiency of particles in spherical bubbles was first proposed by Fuch (1964), which takes into account the gravitational settlement, Brownian diffusion, and inertial impaction. Additionally, the influence of each deposition mechanism is considered independent. The change of particle concentration in the bubble is equal to the algebraic superposition of concentration variation caused by each mechanism. From the model of Fuchs, the removal efficiency of particles due to Brownian diffusion decreases with the increase of particle diameter, while the removal efficiency due to gravitational sedimentation and inertial impaction increases with the increase of particle diameter. Because each removal mechanism has an effective size range, the particle size that has a minimum efficiency is in the nanometer size range, which is also called the most penetrating particle size (MPPS).
Experimental investigations on aerosol filtration performance by pool scrubbing have been conducted since the 1980s, and typical experiments include the Advanced Containment Experiments (ACE), the Battel Columbus Laboratories experiment (BCL), the Pool Atomic Energy Research Institute programme (JAERI), and the Pool Scrubbing Effect on Iodine Decontamination programme (POSEIDON), among others (Hakii et al., 1990; Kaneko et al., 1992; Escudero Berzal et al., 1995; Peyres and Herranz, 1996). Later, the POSEIDON-II experiment was carried out by Dehbi et al. (2001), which included 17 groups of different operating conditions with the aim of revealing the impact of various operation parameters on the decontamination process. The results showed that DF increased with the increase of submergence depth, steam fraction, and aerosol particle size. The author also studied the influence of several removal mechanisms by controlling the single change of entrance parameters, for example the effect of an inertial impaction mechanism can be verified by a single change of particle size. Kanai et al. (2016) studied the relationship between aerosol concentration and submerged depth by using an aerosol spectrometer, and a simple formula was proposed to calculate the decontamination factor (DF). Charvet et al. (2011) and Cadavid-Rodriguez et al. (2014) studied the influence of different operation parameters on nanoparticle removal efficiency, but the experimental gas flow rate was too small, which limits the generalization of experimental results. Some investigations indicate that the particle removal efficiency increases with the inlet particle concentration due to the coagulation of particles (Meikap and Biswas, 2004). However, Sun et al. (2018) experimentally investigated the relationship between DF and aerosol concentration with three measurement methods and drew the conclusion that DF increases with the decrease of aerosol concentration. The same experimental phenomenon also appeared in the study of Bandyopadhyay and Biswas (2006). Recently, Abe et al. (2018) visually studied the bubble dynamics with aerosol and flow structure during pool scrubbing and found that the interface oscillation of globules leads to the amount of aerosol collected by liquid phase.
Previous investigations confirmed pool scrubbing as a potential method to remove the micron-sized aerosol. Nonetheless, the influence of some experimental factors has not been clearly studied—for example, inlet aerosol concentration, gas flow rate, and subcooling degree. Besides, the aerosol removal mechanism is not clear and there is a lack of reliable experiment data to valid predictive models. Therefore, the goal of this paper is to study the effect of some operating parameters on the filtration efficiency of submicron aerosol by bubble scrubbing. Furthermore, this research is also expected to provide theoretical support for the design of pool scrubbing and source term analysis for severe nuclear accidents.
Experimental Facility
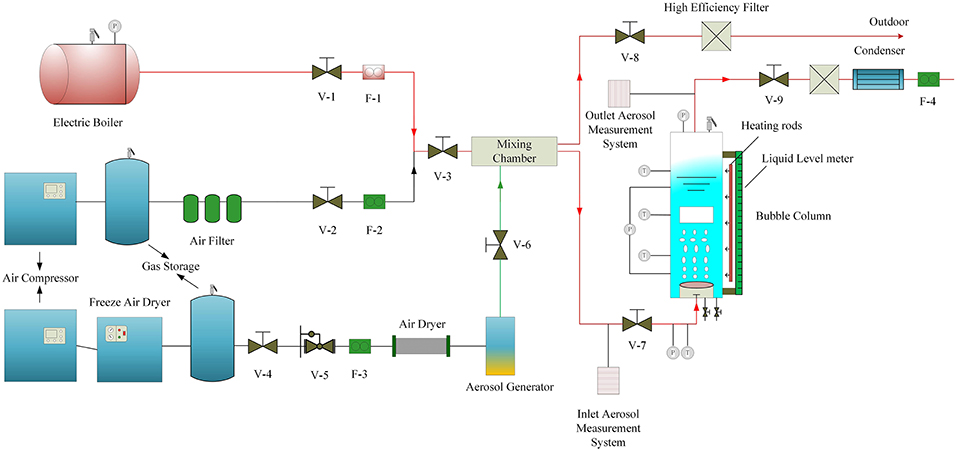
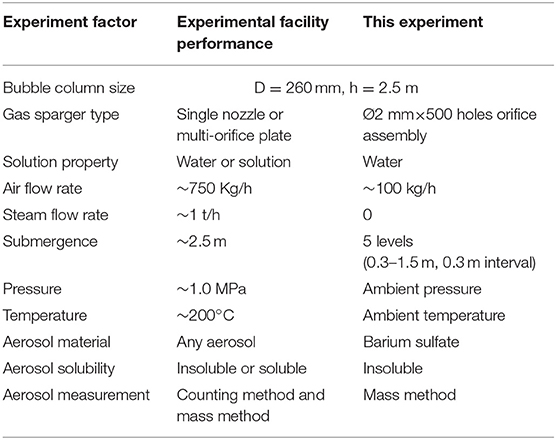
Figure 1 shows the schematic diagram of the aerosol experimental facility. The main test facility can be used for high-temperature and -pressure experiments, and the main test facility performance is listed in Table 1. The test section is made of stainless steel with an inner diameter of 260 mm. Additionally, the test vessel is 2.5 m in height. An air compressor and electric boiler provide air flow rate and steam flow rate, which can be adjusted to a desirable value. The air mass flowrate is measured by two mass flowmeters with operating ranges of 0–100 kg/h and a measurement accuracy of 0.2%. A uniform gas flow distribution is generated in the bubble column by a perforated stainless steel plate with 500 orifices, and the orifice diameter is 2 mm. Two heaters with different electric powers were used to heat up the water in the bubble column and maintain a constant pool temperature during the experiment process. Average gas hold-up was measured by a differential pressure transducer with an operating range of 0–20 KPa and a measurement accuracy of 0.055%. Pressure probes were installed at different positions away from the orifice plate and the pool surface to avoid fringe effect.
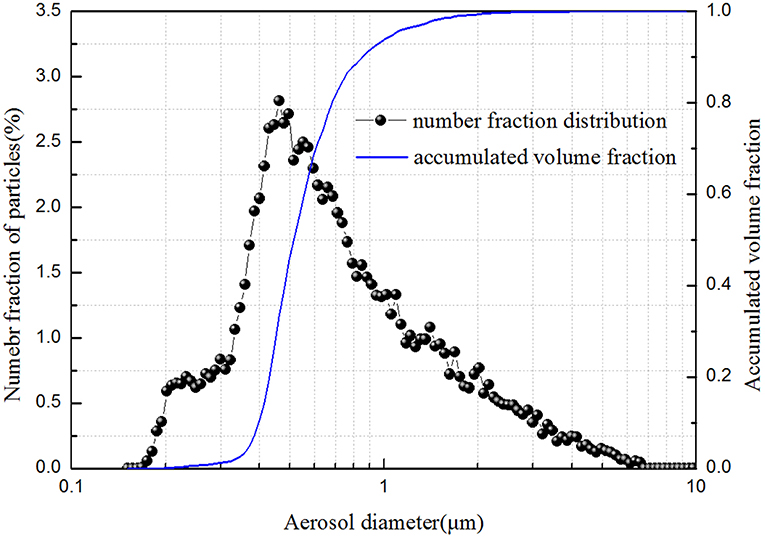
Insoluble barium sulfate (BaSO4) powder was used as an aerosol simulant material. Figure 2 gives the aerosol size distribution, which was measured by a welas 2000 laser particle size analyzer. The median diameter of the size distribution is 0.5 μm. Aerosol particles with a particle size <0.5 μm account for almost 45% of the total volume. The inlet aerosol concentration can be adjusted by switching the supplied gas flow rate through the aerosol generation. A mixed chamber was used to ensure the aerosol concentration was uniform and steady. Additionally, a bypass flow was set to guarantee the inlet aerosol concentration constant if the inlet laden gas flow rate was changed.
The mass method was used to analyze the removal efficiency of aerosol particles by passing sample gas through a glass fiber filter paper mounted on a filter hold. The mass of filter paper was measured by a high precision microbalance with an accuracy of 0.1 mg. No less than two measurements were conducted to reduce experimental error under each design condition. In order to prevent the measurement error of aerosol sampling caused by vapor condensation, the sampling pipeline needs to be heated and insulated, and isokinetic sampling should be guaranteed. The mass difference of the filter paper gave the total mass of aerosol particles collected, and the sample air volume was measured by two mass flowmeters, with operation ranges of 2–50 L/min and a relative deviation of 1%. Steam was removed in the snake condenser before entering the flowmeter when the sample gas contained water vapor, and the condensate water could be collected. Thus, the total aerosol mass removal efficiency was determined as follows:
Where is the mass of unremoved aerosol in outlet air retained on filter paper, g; is the mass of particles in inlet air retained on filter paper, g; is the outlet sample air volume, m3; and is the inlet sample air volume, m3.
Results and Discussion
Influence of Inlet Aerosol Concentration on Filtration Efficiency
The inlet aerosol concentration increases with the air flow rate supplied for aerosol generation, which is in the range of 0.006–0.5 g/m3. The test air flow rate is 10 kg/h and the submergence depth is 1.2 m. Figure 3A presents the aerosol filtration efficiency against inlet aerosol concentration. It can be seen that the aerosol filtration efficiency is almost equal to 100% when the inlet aerosol concentration is lower than 0.1 g/m3, but the aerosol filtration efficiency is independent of inlet aerosol concentration when inlet aerosol concentration is in the range of 0.2–0.5 g/m3. It can be explained that the inlet aerosol concentration is so low that the aerosol coagulation is limited. Because the outlet aerosol concentration is so low, there exists some measurement uncertainty by the mass method when inlet aerosol concentration is lower than 0.1 g/m3.
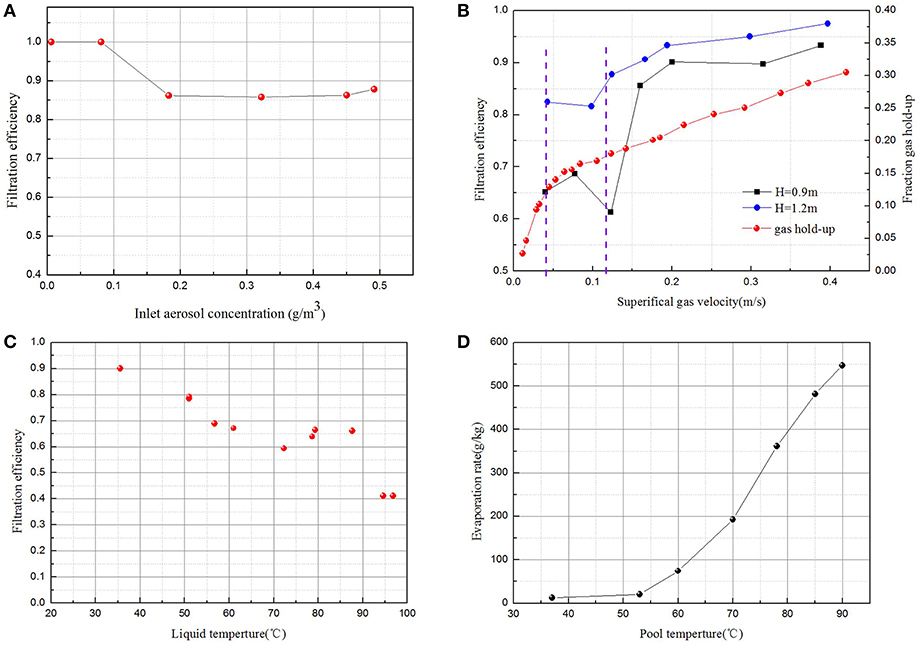
Figure 3. Influence of operation parameter on filtration efficiency (A) inlet aerosol concentration; (B) gas flow rate; (C) pool temperature; and (D) influence of pool temperature on evaporation rate.
Influence of Superficial Gas Velocity on Filtration Efficiency
In order to verify the effect of superficial gas velocity on aerosol filtration efficiency, control experiments were conducted at different superficial gas velocities (8–100 kg/h) and at constant submerged depths of 0.9 m and 1.2 m. Figure 3B shows the aerosol filtration efficiency achieved at different air flow rates. It can be seen that when superficial gas velocity is >0.12 m/s, the aerosol filtration efficiency increases with the increase of superficial gas velocity and tends to be stable when superficial gas velocity exceeds 0.2 m/s. Furthermore, the aerosol filtration efficiency with submerged depth of 1.2 m is higher than the aerosol filtration efficiency with submerged depth of 0.9 m at the same gas flow rate, which also indicates that the aerosol removal efficiency increases with the submerged depth.
Figure 3B also shows the relationship of fraction gas hold-up and superficial gas velocity. With the increase of gas flow rate, homogeneous bubbling, transition, and heterogeneous bubbling regimes will appear in turn. It shows that the regime is a homogeneous bubbling regime at superficial gas velocities lower than 0.04 m/s. There is a linear relationship between gas holdup and apparent gas velocity. The bubble size distribution is narrow and the gas hold-up is radially uniform. But the heterogeneous bubbling regime occurred when superficial gas velocity was higher than 0.12 m/s. The gas-liquid interaction is strong and the bubble size distribution is wide. These two flow patterns are separated by the transition regime, which is caused by local liquid circulation in the bubble column (Vial et al., 2000).
The two-phase flow pattern is a heterogeneous bubbling regime when the superficial gas velocity exceeds 0.12 m/s. When the superficial gas velocity increases, the fractional gas-holdup increases as well as the intensity of interface oscillation and bubble breakup and coalescence. The inertial impaction effect would become a dominant mechanism, which contributed positively to higher particle collection efficiency (Abe et al., 2018). However, when the superficial gas velocity is too large, the coalescence effect of the bubbles was enhanced, which would reduce the interfacial area between gas phase and liquid phase. Thus, the aerosol filtration efficiency tends to stabilize finally. When the superficial gas velocity is lower than 0.12 m/s, the two-phase flow pattern is the transition regime in this experimental study, and the aerosol filtration efficiency decreases with superficial gas velocity. The increase of superficial gas velocity causes increased fractioning of large bubbles, which further leads to the decrease of aerosol filtration efficiency. In this experiment, the two-phase flow pattern did not include a homogeneous bubbling regime, because an orifice plate with 2 mm orifice diameter was used.
Influence of Pool Temperature on Filtration Efficiency
In order to verify the effect of pool temperature on aerosol filtration efficiency, pure air experiments were conducted at different pool temperatures, a constant submerged depth of 0.7 m, and a gas flow rate of 10 kg/s. Figure 3C shows the aerosol filtration efficiency achieved at different pool temperatures. It can be seen that the aerosol filtration efficiency decreases with the increase of pool temperature. The pool temperature increases from 35 to 95°C, and the aerosol filtration efficiency significantly decreases from 90 to 40%. The aerosol filtration efficiency is somewhat anomalous between 75 and 90°C, which may require further repetitions of the experiment in order to clarify.
The increasing temperature leads to a decrease in the surface tension coefficient and kinematic viscosity. With the decrease of liquid surface tension with temperature, not only does the bubble detachment volume decrease (Bhavaraju et al., 1978), but the film drainage speed decreases (Lin et al., 2010) and thus leads to a decrease of bubble size. With the decrease of liquid viscosity, the bubble rise velocity increases, which results in a reduction of bubble residence time. However, the interface evaporation of gas-liquid phases leads to an increase of bubble size. Additionally, the gas density decreases with increasing temperature, which also increases the bubble size and decreases gas holdup. Thus, the total influence of pool temperature on bubble size and gas holdup depends on the competitive effect. As reported by Pohorecki et al. (2001) and Feng et al. (2019), the water temperature has a slight influence on bubble size, gas holdup, and interfacial area.
When the pool temperature increases, the subcooling degree decreases while the saturation vapor pressure increases, which influences the evaporation rate at the gas-liquid interface. Figure 3D gives the relationship between the evaporation rate and pool temperature. The evaporation rate is defined as the ratio of the mass of water vapor in the outlet gas to the mass of air. The mass of water vapor can be obtained by condensate water and the air mass can be achieved by the mass flowmeter installed at the sample line. It can be seen that the evaporation rate at the gas-liquid interface will significantly increase as the boiling point is approached, which gives retroaction to aerosol filtration efficiency. From the experimental results, it can be obtained that the interfacial evaporation of water vapor, which is called Stefan flow, plays a dominant role in collecting aerosol by bubbly scrubbing under low-subcooling conditions.
Conclusions
In this study, the filtration efficiency of insoluble aerosol in a bubble column was experimentally investigated under pure air condition, varying several operation conditions such as inlet aerosol concentration, gas flow rate, and pool temperature. The main conclusions are as follows:
(1) The aerosol filtration efficiency is independent of inlet aerosol concentration when inlet aerosol concentration is in the range of 0.2–0.5 g/m3.
(2) The aerosol filtration efficiency is influenced by the gas flow rate. With the increase of gas flow rate, homogeneous bubbling, transition, and heterogeneous bubbling regimes will appear in turn. The aerosol filtration efficiency decreases in the transition regime due to the occurrence of big bubbles but increases in the heterogeneous bubbling regime account for gas-liquid interaction.
(3) The aerosol filtration efficiency significantly decreases with pool temperature.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
YL and YZ carried out experiments and analyzed the experimental results as well as the writing of the paper. YL and KS assisted in experimental work and dealt with the experimental data. ZS and YZ set the guideline of this research and modified the language of the manuscript. HG and KS designed the experimental loop and guided the experimental work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors greatly appreciate support of the Natural Science Foundation of China (Grant No. 11675045). The author thanks the Key Supported Discipline of Guizhou Provence (Qian Xuewei He Zi ZDXK[2016]24), 2011 Collaborative Innovation Center of Guizhou Province (Qian Jiao he xietongchuangxin zi [2016]02). Additionally, the authors are thankful for the support of the Fundamental Science on Nuclear Safety and Simulation Technology Laboratory, Harbin Engineering University, China.
References
Abe, Y., Fujiwara, K., Saito, S., Yuasa, T., and Kaneko, A. (2018). Bubble dynamics with aerosol during pool scrubbing. Nucl. Eng. Des. 337, 96–107. doi: 10.1016/j.nucengdes.2018.06.017
Bandyopadhyay, A., and Biswas, M. N. (2006). Fly-ash scrubbing in a tapered bubble column scrubber. Process Safety Environ. Protect. 84, 54–62. doi: 10.1205/psep.05040
Bhavaraju, S. M., Russell, T. W. F., and Blanch, H. W. (1978). The design of gas sparged devices for viscous liquid systems. AIChE J. 24, 454–466.
Cadavid-Rodriguez, M. C., Charvet, A., Bemer, D., and Thomas, D. (2014). Optimization of bubble column performance for nanoparticle collection. J. Hazard. Mater. 271, 24–32. doi: 10.1016/j.jhazmat.2014.01.040
Charvet, A., Bardin-Monnier, N., and Thomas, D. (2011). Can bubble columns be an alternative to fibrous filters for nanoparticles collection? J. Hazard. Mater. 195, 432–439. doi: 10.1016/j.jhazmat.2011.08.064
Dehbi, A., Suckow, D., and Guentay, S. (2001). Aerosol retention in low-subcooling pools under realistic accident conditions. Nuclear Eng. Design 203, 229–241. doi: 10.1016/S0029-5493(00)00343-5
Escudero Berzal, M., Marcos Crespo, M. J., Swiderska-Kowalczyk, M., Martin Espigares, M., and Lopez Jimenez, J. (1995). State-of-the-Art Review on Fission Products Aerosol Pool Scrubbing Under Sever Accident Conditions (1018-5593). European Commission.
Feng, D., Ferrasse, J. –H., Soric, A., and Boutin, O. (2019). Bubble characterization and gas–liquid interfacial area in two phase gas–liquid system in bubble column at low Reynolds number and high temperature and pressure. Chem. Eng. Res. Design 144, 95–106. doi: 10.1016/j.cherd.2019.02.001
Hakii, J., Kaneko, I., Fukasawa, M., Yamashita, M., and Matsumoto, M. (1990). “Experimental study on aerosol removal efficiency for pool scrubbing under high temperature steam atmosphere,” in Proceedings of 21st DOE/NRC Nuclear Air Cleaning Conference (San Diego, CA).
Kanai, T., Furuya, M., Arai, T., and Nishi, Y. (2016). Development of an aerosol decontamination factor evaluation method using an aerosol spectrometer. Nuclear Eng. Des. 303, 58–67. doi: 10.1016/j.nucengdes.2016.04.011
Kaneko, I., Fukasawa, M., Naito, M., Miyata, K., and Matsumoto, M. (1992). “Experimental study on aerosol removal effect by pool scrubbing,” in 22nd DOE/NRC Nuclear Air Cleaning and Treatment Conference (Denver, CO).
Lin, T. J., Tsuchiya, K., and Fan, L. S. (2010). Bubble flow characteristics in bubble columns at elevated pressure and temperature. AIChE J. 44, 545–560. doi: 10.1002/aic.690440306
Meikap, B. C., and Biswas, M. N. (2004). Fly-ash removal efficiency in a modified multi-stage bubble column scrubber. Sep. Purif. Technol. 36, 177–190. doi: 10.1016/S1383-5866(03)00213-2
Owczarski, P. C., and Burk, K. W. (1991). SPARC-90: A Code for Calculating Fission Product Capture in Suppression Pools. U.S. Nuclear Regulatory Commission, Washington, DC.
Peyres, V., and Herranz, L. E. (1996). Analysis of dominant phenomena during aerosol scrubbing in saturated pools. J. Aerosol Sci. 27(Suppl. 1), 471–472.
Pohorecki, R., Zdrojkowski, A., Bielski, P., and Moniuk, W. (2001). Hydrodynamics of a pilot plant bubble column under elevated temperature and pressure. Chem. Eng. Sci. 56, 1167–1174. doi: 10.1016/S0009-2509(00)00336-5
Sun, H., Machida, S., Sibamoto, Y., Okagaki, Y., and Yonomoto, T. (2018). “Experimental investigation on dependence of decontamination factor on aerosol number concentration in pool scrubbing under normal temperature and pressure,” in 26th International Conference on Nuclear Engineering (London).
Keywords: insoluble aerosol, operation parameter, filtration efficiency, bubble column, pool scrubbing
Citation: Li Y, Shi K, Sun Z, Gu H and Zhou Y (2019) Preliminary Experimental Investigation on the Filtration Performance of Submicron Insoluble Aerosol in a Bubble Column. Front. Energy Res. 7:96. doi: 10.3389/fenrg.2019.00096
Received: 03 July 2019; Accepted: 26 August 2019;
Published: 11 September 2019.
Edited by:
Wenxi Tian, Xi'an Jiaotong University, ChinaReviewed by:
Jiejin Cai, South China University of Technology, ChinaShimpei Saito, Asahi Glass, Japan
Copyright © 2019 Li, Shi, Sun, Gu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingzhi Li, lyz1993@hrbeu.edu.cn; Yanmin Zhou, zhouyanmin@hrbeu.edu.cn
 Yingzhi Li
Yingzhi Li Kaiyi Shi
Kaiyi Shi Zhongning Sun1
Zhongning Sun1