- 1Ministry of Education-Shanghai Key Laboratory of Children's Environmental Health, and Department of Pediatrics, Xinhua Hospital, Shanghai Jiao-Tong University School of Medicine, Shanghai, China
- 2Department of Obstetrics and Gynecology, Faculty of Medicine, Dalla Lana School of Public Health, Lunenfeld-Tanenbaum Research Institute, Prosserman Center for Population Health Research, Mount Sinai Hospital, and Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada
- 3Department of Clinical Assay Laboratory, Xinhua Hospital, Shanghai Jiao-Tong University School of Medicine, Shanghai, China
- 4Department of Obstetrics and Gynecology, Xinhua Hospital, Shanghai Jiao-Tong University School of Medicine, Shanghai, China
- 5Department of Pediatric, International Peace Maternity and Child Health Hospital, Shanghai Jiao-Tong University School of Medicine, Shanghai, China
Fetuin-A is a multifunctional glycoprotein that has been implicated in insulin resistance and bone metabolism. We assessed whether fetuin-A is associated with poor or excessive fetal growth. In the Shanghai Birth Cohort, we conducted a nested case-control study of 60 trios of small-for-gestational-age (SGA, birth weight <10th percentile), optimal-for-gestational-age (OGA, 25–75th, the reference) and large-for-gestational-age (LGA, >90th percentile) infants matched by sex and gestational age. Cord plasma concentrations of fetuin-A and fetal growth factors [insulin, proinsulin, insulin-like growth factor (IGF)-I and IGF-II] were measured. Cord plasma fetuin-A concentrations were higher in SGA (809.4 ± 306.9 μg/ml, P = 0.026) and LGA (924.2 ± 375.9 μg/ml, P < 0.001) relative to OGA (680.7 ± 262.1 μg/ml) newborns, and were not correlated to insulin, proinsulin, IGF-I and IGF-II (all P > 0.2). Higher fetuin-A concentrations were associated with increased risks of SGA [OR = 1.67 (1.08–2.58) per SD increment, P = 0.024] and LGA [OR = 2.36 (1.53–3.66), P < 0.001]. Adjusting for maternal and neonatal characteristics and fetal growth factors, the elevated risk changed little for LGA [adjusted OR = 2.28 (1.29–4.01), P = 0.005], but became non-significant for SGA (P = 0.202). Our study is the first to demonstrate that fetuin-A may be involved in excessive fetal growth. This association is independent of fetal growth factors.
Introduction
Fetuin-A or α2-HS-glycoprotein (AHSG) is a liver-derived glycoprotein, and has been implicated in insulin resistance and metabolic syndrome related disorders (1–4). Fetuin-A inhibits insulin receptor signaling by binding to insulin receptor tyrosine kinase (5, 6). AHSG knockout mice manifest improved insulin sensitivity (3). Human studies have associated elevated circulating fetuin-A concentrations with diabetes, obesity and non-alcoholic fatty liver disease in adults (2, 7).
Abnormal (poor or excessive) fetal growth is associated with elevated risks of metabolic syndrome related disorders in adulthood (8, 9). A variety of maternal and fetal elements may contribute to abnormal fetal growth (10, 11). Fetuin-A has been implicated in the regulation of bone growth (12). Animal studies showed that AHSG knockout mice had stunted femur growth (13). However, growth restricted pigs had higher plasma fetuin-A concentrations compared with normal sized littermate at birth (14), suggesting fetuin-A may have different implications for fetal growth across species.
Both insulin resistance and bone growth are relevant for fetal growth (15, 16), suggesting that fetuin-A may be implicated in abnormal fetal growth. A negative association has been observed between maternal circulating fetuin-A level and fetal growth (17, 18). Data are scarce concerning whether cord blood fetuin-A is associated with fetal growth. A small study reported no significant differences in cord serum fetuin-A concentrations between newborns with fetal growth restriction (n = 20) vs. normal birth weight (19), while another small study reported marked defects in the glycosylation of fetuin-A in small-for-gestation-age (n = 10) newborns (20). We are unaware of any study on cord blood fetuin-A concentration in excessive fetal growth. The aim of the present study was to evaluate whether cord blood fetuin-A concentration is associated with poor or excessive fetal growth.
Materials and Methods
Study Design, Population and Specimens
We performed a nested matched case-control study based on the recently described Shanghai Birth Cohort (SBC) (21). Briefly, the SBC is a prospective birth cohort study including 4,127 pregnant women in Shanghai, 2013–2016. Data on maternal, pregnancy and delivery characteristics were collected. Umbilical cord blood samples [in multiple tubes for serum (non-coagulant) and plasma (EDTA)] were collected in a standardized protocol by trained research staff immediately after delivery. Serum and plasma samples were obtained by centrifugation (centrifuge: BECKMAN COULTER Allegra X-15R, USA) at 4°C, 4,000 rpm for 10 min, and were stored in multiple aliquots at −80°C until assays. The study was approved by the research ethics committees of Xinhua Hospital (ref no. M2013-010) and all participating hospitals, Shanghai, China. Written informed consent was obtained from all study participants. The study adhered to the guidelines of the Declaration of Helsinki.
There were a total of 3,692 singleton live births with data available on birth weight and gestational age. Birth weight was measured by an electronic weighing device to the nearest gram. Gestational age (weeks) was calculated based on the date of last menstruation period (LMP) and confirmed by first trimester ultrasound dating. If the ultrasound dating was more than 2 weeks from the LMP-based estimate, the ultrasound dating-based gestational age was used. Infants were classified as small-for-gestational-age (SGA, <10th percentile), appropriate-for-gestational-age (AGA, between 10 and 90th percentile) and large-for-gestational-age (LGA, >90th percentile) according to the 2015 Chinese sex- and gestational age-specific birth weight standards (22). Among the AGA infants, we defined optimal-for-gestational-age (OGA) as birth weight between 25 and 75th percentiles, for the purpose of maximizing the contrasts to infants with poor (SGA) or excessive (LGA) fetal growth.
The present study is a random sample of 60 trios of SGA, OGA and LGA infants matched by sex (the same) and gestational age at birth (within 1 week). Eligible study subjects must meet all the following criteria: (1) Han ethnicity (the majority ethnic group, >98%); (2) maternal age 20–45 years; (3) natural conception; (4) singleton pregnancy; (5) free of maternal severe chronic disease or severe pregnancy complications (e.g., diabetes, heart disease, preeclampsia/eclampsia); (6) no birth defects; (7) 5-min Apgar score > = 7; (8) cord blood specimens available for biomarker assays. Therefore, the study sample included 180 singleton newborns (60 × 3). Their mothers were all non-smokers. Figure 1 presents the flowchart in the selection of study subjects. The study had a power of 85% to detect a 0.6 SD or greater difference in cord plasma fetuin-A concentration between SGA vs. OGA, or LGA vs. OGA infants, accounting for multiple tests.
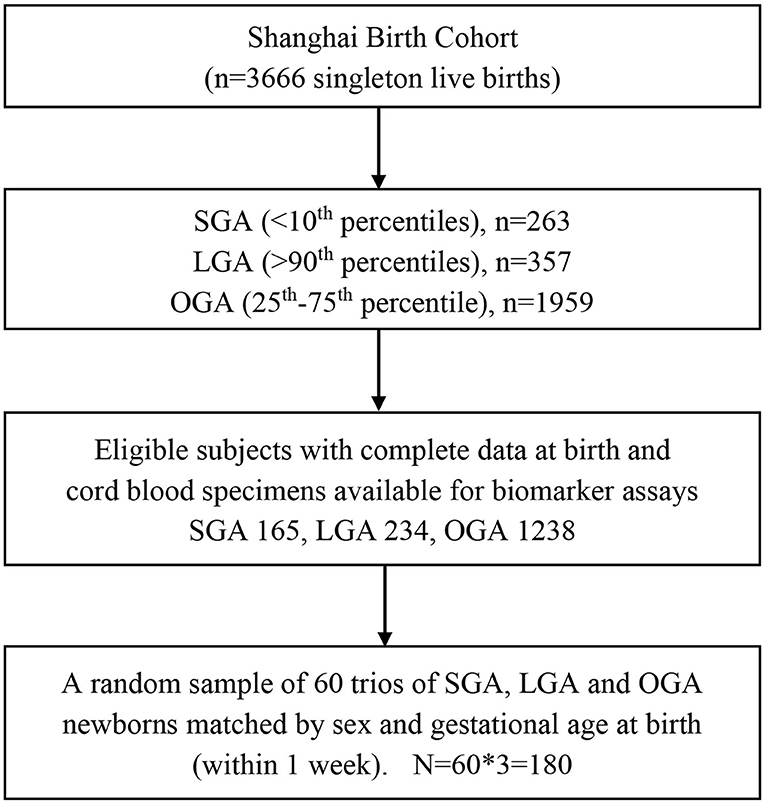
Figure 1. Flowchart in the selection of study subjects in a nested matched case control study of SGA, LGA, and OGA newborns in the Shanghai Birth Cohort. SGA, small-for-gestational-age (birth weight <10th percentile); LGA, large-for-gestational-age (>90th percentile); OGA, optimal-for-gestational-age (25–75th percentiles).
Biochemical Assays
Plasma fetuin-A (R&D system, Minnesota, USA), proinsulin (Mercodia, Uppsala, Sweden), and IGF-II (R&D system, MI, USA) were measured by enzyme-linked immunosorbent assay (ELISA) kits, and the absorbance was determined using a microplate spectrophotometer (Beckman CX7, USA). Serum insulin and insulin-like growth factor 1 (IGF-I) concentrations were detected by automated chemiluminescent assays (ADVIA Centaur and Immulite 2000, SIEMENS, Germany). The minimal detectable concentrations were 0.62 ng/ml for fetuin-A, 3.5 pmol/L for insulin, 1.7 pmol/L for proinsulin, 25 ng/ml for IGF-I and 1.88 pg/ml for IGF-II, respectively. The intra-assay and inter-assay coefficients of variation were in the ranges of 0.5–8.3% for fetuin-A, 2.0–6.5% for insulin and IGF-I, 0.34–5.0% for proinsulin, and 2.4–9.3% for IGF-II, respectively. We measured not only insulin but also proinsulin because both are positively associated with birth weight (23).
Statistical Analysis
Continuous variable data were presented as Mean ± SD. Categorical variable data were presented as n (%). Biomarker data were log-transformed for I-tests and correlation analyses. Paired Student's t-test and Chi-squared test were used to evaluate the differences between two groups (SGA vs. OGA; LGA vs. OGA). Pearson partial correlation coefficients were calculated to evaluate the associations of cord blood fetuin-A with fetal growth factors adjusted for gestational age at delivery. Multinomial logistic regression models were fitted to assess the associations of cord blood fetuin-A with abnormal fetal growth (SGA, LGA) adjusted for maternal and neonatal characteristics. Z (standard deviation) scores of biomarker data were used in multinomial logistic regression models to facilitate the comparisons of effect sizes. There were no significant interactions between predictor variables affecting the primary effect estimates of interest. P < 0.025 was considered statistically significant in testing the primary hypothesis on the differences in fetuin-A concentrations between SGA vs. OGA, or LGA vs. OGA infants (Bonferroni correction for 2 tests).
Data analyses were performed using R, Version 3.5.1. Packages PPCOR and NNET were used in partial correlation and multinomial logistic regression analyses, respectively. The frequencies of missing values in co-variables were low (<7%). In the multinomial logistic regression analyses, multiple imputations were conducted using the MICE package in R. We created 25 datasets with imputations on missing data, and presented the results on the pooled regression coefficient statistics. We conducted sensitivity analysis to examine the multinomial logistic regression results without data imputations on missing values.
Results
Maternal and neonatal characteristics are presented in Table 1. LGA newborns had higher maternal pre-pregnancy BMI, and were more frequently delivered by cesarean section than OGA newborns. As expected, mothers of LGA infants had higher blood glucose concentrations in the 75 g 2-h oral glucose tolerance tests (fasting, 1-h and 2-h) at 24–28 weeks of gestation. SGA infants had lower maternal pre-pregnancy BMI, but did not differ significantly from OGA infants in other characteristics.
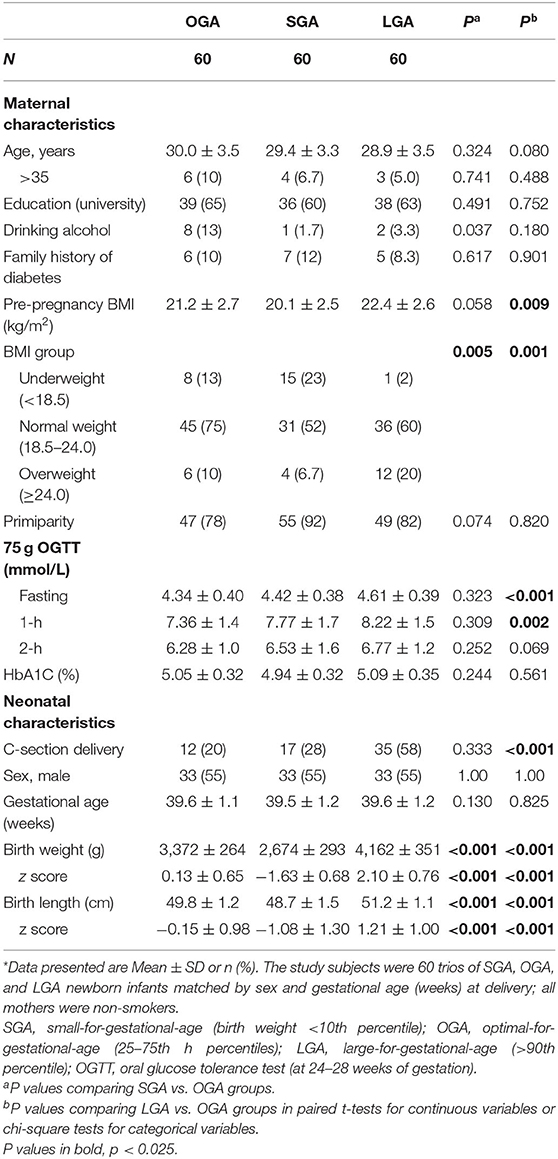
Table 1. Maternal and neonatal characteristics in a matched study of SGA, OGA, and LGA singleton newborns in Shanghai Birth Cohort*.
Compared to OGA infants, cord plasma fetuin-A concentrations were significantly higher in both SGA (P = 0.024) and LGA (P < 0.001) infants (Table 2 and Figure 2). For all fetal growth factors (insulin, IGF-I, and IGF-II), cord blood concentrations were the lowest in SGA infants, and the highest in LGA infants. IGF-I and proinsulin concentrations were significantly lower in SGA infants, and higher in LGA infants than in OGA infants. Insulin concentrations were significantly lower in SGA infants.
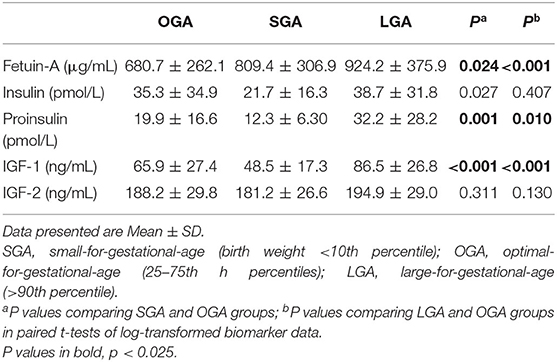
Table 2. Cord blood concentrations of fetuin-A and fetal growth factors in SGA, OGA, and LGA infants.
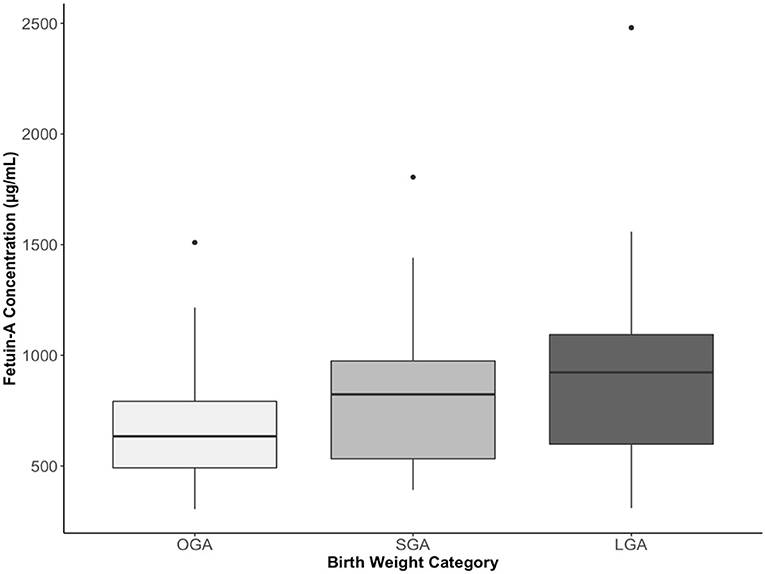
Figure 2. Cord plasma fetuin-A concentrations in small-, optimal-, or large-for-gestational-age (SGA, OGA, LGA) infants. The boxes represent the medians and interquartile ranges, the dots represent the maximal values. P = 0.024 comparing SGA vs. OGA, and P < 0.001 comparing LGA vs. OGA.
Adjusting for gestational age at delivery/specimen sampling, cord blood Fetuin-A was not correlated with insulin, proinsulin, IGF-I and IGF-II or maternal fasting blood glucose (all P > 0.2, Table 3).
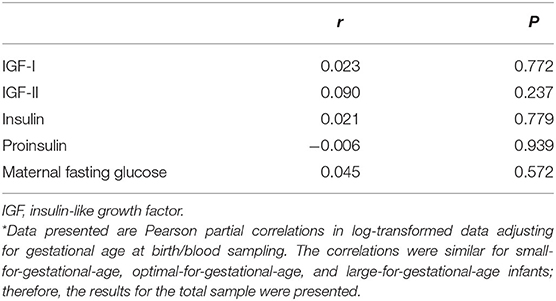
Table 3. Pearson partial correlation coefficients * of cord blood fetuin-A with fetal growth factors and maternal fasting glucose (24–28 weeks of gestation).
Without any adjustment, higher cord plasma fetuin-A concentrations were associated with increased odds of both LGA and SGA (Table 4). Adjusted for maternal and neonatal characteristics, the OR for SGA became no longer statistically significant, while the OR remained highly significant for LGA (OR = 2.42, 95% CI 1.47–3.97). Among the fetal growth factors, higher cord blood IGF-I and proinsulin concentrations were strongly associated with a lower odds of SGA and a higher odds of LGA. Higher cord blood insulin concentrations were associated with a lower odds of SGA (OR = 0.45, 95% CI 0.25–0.81). Cord plasma IGF-II was not associated with SGA or LGA.
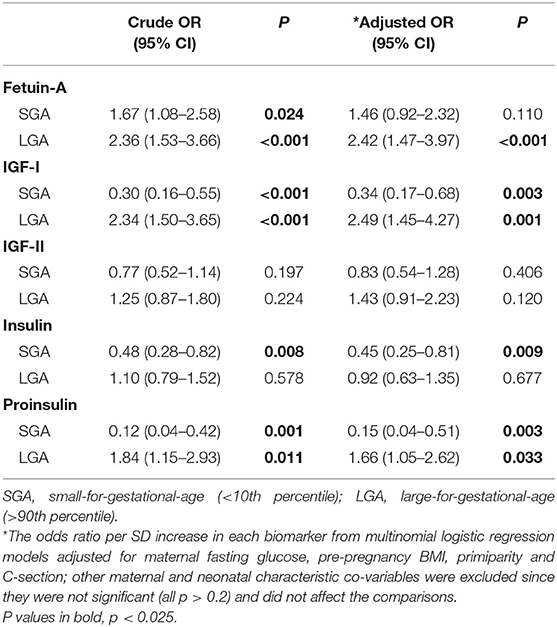
Table 4. Associations of cord blood fetuin-A and fetal growth factors (insulin, proinsulin, IGF-1, IGF-2) with the risks of SGA and LGA.
In the fully adjusted model including maternal, neonatal characteristics and fetal growth factors (Table 5), the OR for fetuin-A in association with LGA or SGA changed little compared to the OR adjusted for maternal and neonatal characteristics only (Table 4). The elevated risk after the adjustment remained significant for LGA (OR = 2.28, P = 0.005), and became not statistically significant for SGA (OR = 1.41, P = 0.202). Similar findings were observed in the multinomial logistic regression analyses without imputations for missing data (results not shown).
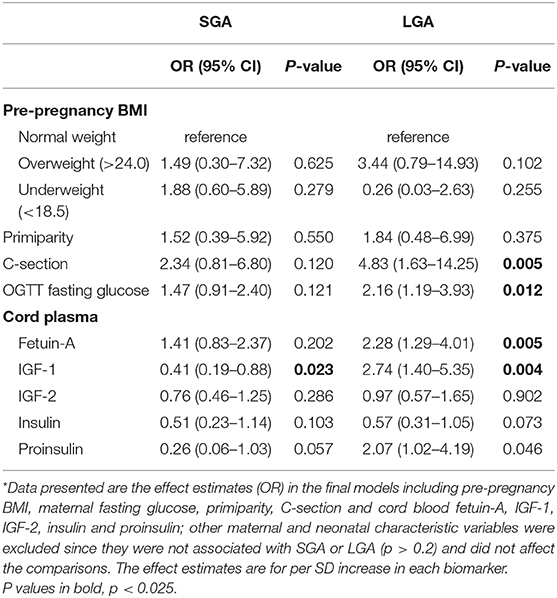
Table 5. Associations of maternal characteristics, cord plasma fetuin-A and fetal growth factors with the risks of SGA and LGA in the fully adjusted models*.
Discussion
We observed elevated cord blood fetuin-A concentrations in infants with poor (SGA) or excessive (LGA) fetal growth relative to infants with optimal fetal growth (OGA). Higher cord blood fetuin-A concentrations were associated with an increased risk of LGA independent of fetal growth factors.
Our study is the first to report a strong positive association between cord blood fetuin-A and excessive fetal growth. The association is independent of IGF-I, IGF-II, proinsulin and insulin, suggesting it may be mediated by pathways other than fetal growth factors. Our data confirmed that cord blood IGF-I and proinsulin concentrations are strongly associated with fetal growth (23, 24).
We observed higher cord blood fetuin-A concentrations in SGA vs. OGA infants, but the adjusted OR became not statistically significant, suggesting that the association may be an artifact of confounding factors. Similarly, a small study reported that fetuin-A concentrations were not different in infants with fetal growth restriction (n = 20) compared to normal birth weight infants (19). It should be cautioned that both our and previous studies were not powered to detect relatively small differences. It is unclear whether the higher cord blood fetuin-A concentrations in SGA infants are related to their increased insulin resistance in childhood (25). Long-term follow-up studies are required to address this question.
We are unaware of any research data on cord blood fetuin-A concentration in excessive fetal growth. We observed that LGA - an indicator of excessive fetal growth, was associated with substantially elevated cord blood fetuin-A concentrations. Interestingly, a study in prepubertal children observed higher fetuin-A concentrations in overweight/obese relative to underweight/normal weight children (4), consistent with our finding to some extent but at a different life stage. Moreover, studies in children and adults have associated elevated circulating fetuin-A concentrations with obesity and other metabolic disorders (26, 27). We might speculate that elevated fetuin-A concentrations in LGA infants may play a role in early life development of the vulnerability to metabolic syndrome related disorders.
We could not determine whether the elevated fetuin-A concentration in LGA infants is a cause or consequence of excessive fetal growth. A previous study using bidirectional Mendelian randomization analysis suggests that AHSG (the gene coding fetuin-A) is casually related to BMI (28), lending some support in fetuin-A's role in the development of fetal overgrowth. The pathways relating fetuin-A to fetal growth are unknown. Studies have linked fetuin-A with insulin resistance (4). The production of pro-inflammatory cytokines as well as the mobilization of free fatty acids via toll-like-receptor-4 (1, 29). Some nutrients such as curcumin and niacin have been associated with decreased serum fetuin-A concentrations (30, 31). It remains to be explored whether these nutrients may be related to the association between fetuin-A and LGA.
The matched study is a powerful design to detect differences in cord blood biomarkers between groups. All study subjects are Chinese Han ethnicity, eliminating the potential confounding effect of ethnic difference in fetal growth. We used OGA (25–75th percentiles) rather than AGA (10–90th percentiles) infants as the comparison (control) group. This study approach may be more powerful in identifying the differences between infants with extreme (poor or excessive) vs. normal fetal growth due to stronger contrasts than a study using AGA as the comparison group (24). A main limitation is the lack of data on fetuin-A isoforms. We did not have the data on glycosylation and/or phosphorylation isoforms of fetuin-A. Future studies may further explore whether there are elevations in specific isoforms of cord blood fetuin-A in LGA infants. Our study was powered to detect moderate to large differences (>0.6 SD), but not powered to detect small differences. This is an observational study, and causality could not be affirmed. Reverse causality could not be ruled out. The study sample was restricted to Chinese infants. More studies in other ethnic groups/populations are required to confirm the generalizability of the study findings.
In conclusion, cord blood fetuin-A concentrations were elevated in SGA and LGA infants. Fetuin-A may be involved in excessive fetal growth independent of fetal growth factors (insulin, IGF-I and IGF-II).
Data Availability Statement
The datasets presented in this article are not readily available because Access to the de-identified participant research data must be approved by the research ethics board on a case-by-case basis, please contact the corresponding author for assistance in data access request. Requests to access the datasets should be directed to Zhong-Cheng Luo, emNsdW8mI3gwMDA0MDtsdW5lbmZlbGQuY2E=; Fengxiu Ouyang, b3V5YW5nZmVuZ3hpdSYjeDAwMDQwO3hpbmh1YW1lZC5jb20uY24=.
Ethics Statement
The Institutional Review Board of Xinhua Hospital, Shanghai Jiao-Tong University School of Medicine approved this study (ref no. M2013-010, date of approval: August 23, 2013). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Z-CL, JZ, and FO conceived the study with inputs from all co-authors. W-JW, M-NY, YD, HH, FF, RH, X-GY, G-HZ, XZ, TZ, X-YH, JZ, FO, and Z-CL contributed to the acquisition of research data. W-JW and SW conducted the literature review, data analysis, and drafted the manuscript. Z-CL is the guarantor of this work, has full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed in revising the article critically for important intellectual content and approved the final version for publication.
Funding
This work was supported by research grants from the Ministry of Science and Technology of China (2019YFA0802501 and 2017YFE0124700), the National Natural Science Foundation of China (81673178 and 81961128023), the Canadian Institutes of Health Research (158616), the Shanghai Municipal Health and Family Planning Commission (2020CXJQ01), and the National Human Genetic Resources Sharing Service Platform (2005DKA21300). The funders have no role in all aspects of the study, including study design, data collection and analysis, the preparation of the manuscript, and the decision for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledged all research staff who had contributed to patient recruitment and data collection in the Shanghai Birth Cohort.
Abbreviations
LGA, large-for-gestational-age; OGA, optimal-for-gestational-age; SGA, small-for-gestational-age; IGF, insulin-like growth factor; OR, odds ratio.
References
1. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. (2012) 18:1279–85. doi: 10.1038/nm.2851
2. Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. (2006) 29:853–7. doi: 10.2337/diacare.29.04.06.dc05-1938
3. Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. (2002) 51:2450–8. doi: 10.2337/diabetes.51.8.2450
4. Shim YS Kang MJ Oh YJ Baek JW Yang S Hwang IT. Fetuin-A as an alternative marker for insulin resistance and cardiovascular risk in prepubertal children. J Atheroscler Thromb. (2017) 24:1031–8. doi: 10.5551/jat.38323
5. Mathews ST, Chellam N, Srinivas PR, Cintron VJ, Leon MA, Goustin AS, et al. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol Cell Endocrinol. (2000) 164:87–98. doi: 10.1016/S0303-7207(00)00237-9
6. Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. (1989) 58:631–40. doi: 10.1016/0092-8674(89)90098-6
7. Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. (2017) 13:509–20. doi: 10.1038/nrendo.2017.56
8. Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. (2014) 348:g14. doi: 10.1136/bmj.g14
9. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. (2005) 115:e290–6. doi: 10.1542/peds.2004-1808
10. McGrath RT, Glastras SJ, Hocking SL, Fulcher GR. Large-for-gestational-age neonates in type 1 diabetes and pregnancy: contribution of factors beyond hyperglycemia. Diabetes Care. (2018) 41:1821–8. doi: 10.2337/dc18-0551
11. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. (2017) 317:2207–25. doi: 10.1001/jama.2017.3635
12. Brylka L, Jahnen-Dechent W. The role of fetuin-A in physiological and pathological mineralization. Calcif Tissue Int. (2013) 93:355–64. doi: 10.1007/s00223-012-9690-6
13. Seto J, Busse B, Gupta HS, Schafer C, Krauss S, Dunlop JW, et al. Accelerated growth plate mineralization and foreshortened proximal limb bones in fetuin-A knockout mice. PLoS ONE. (2012) 7:e47338. doi: 10.1371/journal.pone.0047338
14. Ramsay TG, Stoll MJ, Blomberg LA, Caperna TJ. Regulation of fetuin A gene expression in the neonatal pig liver. Animal. (2018) 12:288–94. doi: 10.1017/S1751731117001410
15. Pfab T, Slowinski T, Godes M, Halle H, Priem F, Hocher B. Low birth weight, a risk factor for cardiovascular diseases in later life, is already associated with elevated fetal glycosylated hemoglobin at birth. Circulation. (2006) 114:1687–92. doi: 10.1161/CIRCULATIONAHA.106.625848
16. Schweizer R, Martin DD, Haase M, Roth J, Trebar B, Binder G, et al. Similar effects of long-term exogenous growth hormone (GH) on bone and muscle parameters: a pQCT study of GH-deficient and small-for-gestational-age (SGA) children. Bone. (2007) 41:875–81. doi: 10.1016/j.bone.2007.06.028
17. Zbucka-Kretowska M, Kuzmicki M, Telejko B, Goscik J, Ciborowski M, Lipinska D, et al. First-trimester irisin and fetuin-a concentration in predicting macrosomia. J Matern Fetal Neonatal Med. (2019) 32:2868–73. doi: 10.1080/14767058.2018.1450859
18. Kalabay L, Cseh K, Pajor A, Baranyi E, Csakany GM, Melczer Z, et al. Correlation of maternal serum fetuin/alpha2-HS-glycoprotein concentration with maternal insulin resistance and anthropometric parameters of neonates in normal pregnancy and gestational diabetes. Eur J Endocrinol. (2002) 147:243–8. doi: 10.1530/eje.0.1470243
19. Briana DD, Boutsikou M, Gourgiotis D, Boutsikou T, Baka S, Marmarinos A, et al. Serum fetuin-A/alpha2-HS-glycoprotein in human pregnancies with normal and restricted fetal growth. J Matern Fetal Neonatal Med. (2008) 21:826–30. doi: 10.1080/14767050802326255
20. Karamessinis PM, Malamitsi-Puchner A, Boutsikou T, Makridakis M, Vougas K, Fountoulakis M, et al. Marked defects in the expression and glycosylation of alpha2-HS glycoprotein/fetuin-A in plasma from neonates with intrauterine growth restriction: proteomics screening and potential clinical implications. Mol Cell Proteomics. (2008) 7:591–9. doi: 10.1074/mcp.M700422-MCP200
21. Zhang J, Tian Y, Wang W, Ouyang F, Xu J, Yu X, et al. Cohort profile: the shanghai birth Cohort. Int J Epidemiol. (2019) 48:21. doi: 10.1093/ije/dyy277
22. Zhu L, Zhang R, Zhang S, Shi W, Yan W, Wang X, et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua Er Ke Za Zhi. (2015) 53:97–103. doi: 10.1353/bkb.2015.0019
23. Luo ZC, Nuyt AM, Delvin E, Audibert F, Girard I, Shatenstein B, et al. Maternal and fetal IGF-I and IGF-II levels, fetal growth, and gestational diabetes. J Clin Endocrinol Metab. (2012) 97:1720–8. doi: 10.1210/jc.2011-3296
24. Yang MN, Zhang GH, Du K, Wang WJ, Dong Y, He H, et al. Retinol-binding protein 4, fetal overgrowth and fetal growth factors. Pediatr Res. (2020) 87:946–51. doi: 10.1038/s41390-019-0685-0
25. Chiavaroli V, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, Mohn A. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics. (2009) 124:695–702. doi: 10.1542/peds.2008-3056
26. Chung HS, Lee HJ, Hwang SY, Choi JH, Yoo HJ, Seo JA, et al. Relationship of circulating fetuin-a levels with body size and metabolic phenotypes. Int J Endocrinol. (2018) 2018:7918714. doi: 10.1155/2018/7918714
27. Xu Y, Xu M, Bi Y, Song A, Huang Y, Liu Y, et al. Serum fetuin-A is correlated with metabolic syndrome in middle-aged and elderly Chinese. Atherosclerosis. (2011) 216:180–6. doi: 10.1016/j.atherosclerosis.2011.01.020
28. Thakkinstian A, Chailurkit L, Warodomwichit D, Ratanachaiwong W, Yamwong S, Chanprasertyothin S, et al. Causal relationship between body mass index and fetuin-A level in the Asian population: a bidirectional mendelian randomization study. Clin Endocrinol. (2014) 81:197–203. doi: 10.1111/cen.12303
29. Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS ONE. (2008) 3:e1765. doi: 10.1371/journal.pone.0001765
30. Oner-Iyidogan Y, Kocak H, Seyidhanoglu M, Gurdol F, Gulcubuk A, Yildirim F, et al. Curcumin prevents liver fat accumulation and serum fetuin-A increase in rats fed a high-fat diet. J Physiol Biochem. (2013) 69:677–86. doi: 10.1007/s13105-013-0244-9
Keywords: insulin-like growth factor, large-for-gestational-age, small-for-gestational-age, insulin, fetuin A
Citation: Wang W-J, Wang S, Yang M-N, Dong Y, He H, Fang F, Huang R, Yu X-G, Zhang G-H, Zhao X, Zheng T, Huang X-Y, Zhang J, Ouyang F and Luo Z-C (2020) Fetuin-A in Infants Born Small- or Large-for-Gestational-Age. Front. Endocrinol. 11:567955. doi: 10.3389/fendo.2020.567955
Received: 31 May 2020; Accepted: 02 September 2020;
Published: 30 September 2020.
Edited by:
Eli Hershkovitz, Soroka Medical Center, IsraelReviewed by:
George Paltoglou, National and Kapodistrian University of Athens, GreeceJarmo Jääskeläinen, Kuopio University Hospital, Finland
Copyright © 2020 Wang, Wang, Yang, Dong, He, Fang, Huang, Yu, Zhang, Zhao, Zheng, Huang, Zhang, Ouyang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong-Cheng Luo, emNsdW8mI3gwMDA0MDtsdW5lbmZlbGQuY2E=; Fengxiu Ouyang, b3V5YW5nZmVuZ3hpdSYjeDAwMDQwO3hpbmh1YW1lZC5jb20uY24=
†These authors have contributed equally to this work
 Wen-Juan Wang1,2†
Wen-Juan Wang1,2† Xiao-Yi Huang
Xiao-Yi Huang Fengxiu Ouyang
Fengxiu Ouyang Zhong-Cheng Luo
Zhong-Cheng Luo