- 1Department of Epidemiology, School of Public Health, Fudan University, Shanghai, China
- 2Key Laboratory of Public Health Safety of Ministry of Education, Shanghai, China
- 3Department of Chronic Disease Control and Prevention, Deqing County Center for Disease Control and Prevention, Huzhou, China
- 4Department of Chronic Disease Control and Prevention, Yuhuan City Center for Disease Control and Prevention, Taizhou, China
- 5Department of Chronic Disease Control and Prevention, Minhang District Center for Disease Control and Prevention, Shanghai, China
- 6Department of Chronic Disease Control and Prevention, Haimen City Center for Disease Control and Prevention, Nantong, China
- 7School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
Objectives: Although the association between thyroid stimulating hormone (TSH) and obesity in children has been investigated in several cross-sectional studies, no study evaluated this association among girls during puberty, which were in a key period closely related to the fluctuations of thyroid hormones and development of obesity. Therefore, we conducted a cohort study to investigate the association of general and abdominal obesity with TSH in girls during puberty.
Setting and participants: A cohort study of 481 school-aged girls during puberty was conducted in four regions in east China, with a baseline survey in 2017 and a follow-up survey in 2019.
Outcome measures: Anthropometric indexes including height, weight and waist circumference (WC) were measured, and body mass index (BMI) was then calculated. Blood samples were collected to determine TSH and free thyroxine (FT4).
Results: Of the 474 girls at baseline survey, the prevalences of BMI-based general obesity and WC-based abdominal obesity were 19.8% (94/474) and 21.7% (103/474), respectively. Compared with normal weight girls, the median serum TSH level was significantly higher in general obese girls (P = 0.037), but not in central obese girls (P = 0.173). Multiple logistic regression models indicated that those in the highest tertile of serum TSH level had a significantly higher risk of BMI-based overweight/obesity (OR = 1.83, 95% CI 1.01 to 3.32) compared with the lowest tertile. Analyses from 435 girls prospectively followed-up for 2 years revealed that those with general or central obesity also had higher follow-up TSH level (P = 0.004 and P = 0.008, respectively). The TSH level for girls with general obesity at baseline but normal weight at follow-up was 0.45 mU/L (95% CI 0.11 to 0.79) higher than those with normal weight at baseline and follow-up.
Conclusions: TSH was positively associated with both general and abdominal obesity among girls during puberty.
Introduction
Overweight and obesity are defined as abnormal and excess fat accumulation due to an imbalance between calorie intake and expenditure. The prevalence of overweight and obesity among children and adolescents aged 5–19 years has increased dramatically from 4% in 1975 to over 18% in 2016 globally (1). Childhood obesity has been linked to increased risks of asthma (2), metabolic syndrome (3), cardiovascular diseases (CVD) (4) and premature death in adulthood (5). Meanwhile, overweight compared with normal weight children are more likely to be obese in adulthood (6). Obesity is related to multiple endocrine alterations including various hormones (7, 8). Identifying hormonal targets may help obesity prevention and control.
Thyroid hormones are primarily involved in metabolism and energy expenditure of individuals (9). Evidence suggests that thyroid dysfunction predispose to obesity, or vice versa (10). Overweight and obese children tend to have higher TSH levels (11). The association between adiposity and thyroid dysfunction may be modified by sex. Females are more likely to have thyroid abnormalities as compared with males in adults, but not in children aged 8–10 years (12). The synthesis and secretion of thyroid hormones are controlled by hypothalamic-pituitary-thyroid axis, which is usually reactivated during puberty (13). The incidence of thyroid disorder in females increases upon the onset of puberty, and decreases after menopause (14). On the other hand, women with earlier menarche experience a higher risk of obesity (15). Girls during puberty therefore, provide a good opportunity to investigate the fluctuations of thyroid hormones in association with development of obesity, but this issue cannot be addressed in cross-sectional studies (16, 17). In this longitudinal study conducted in east China, we explored the association of TSH with overweight or obesity among girls during puberty, and assessed the potential effect modification of covariates.
Subjects and Methods
Study Population
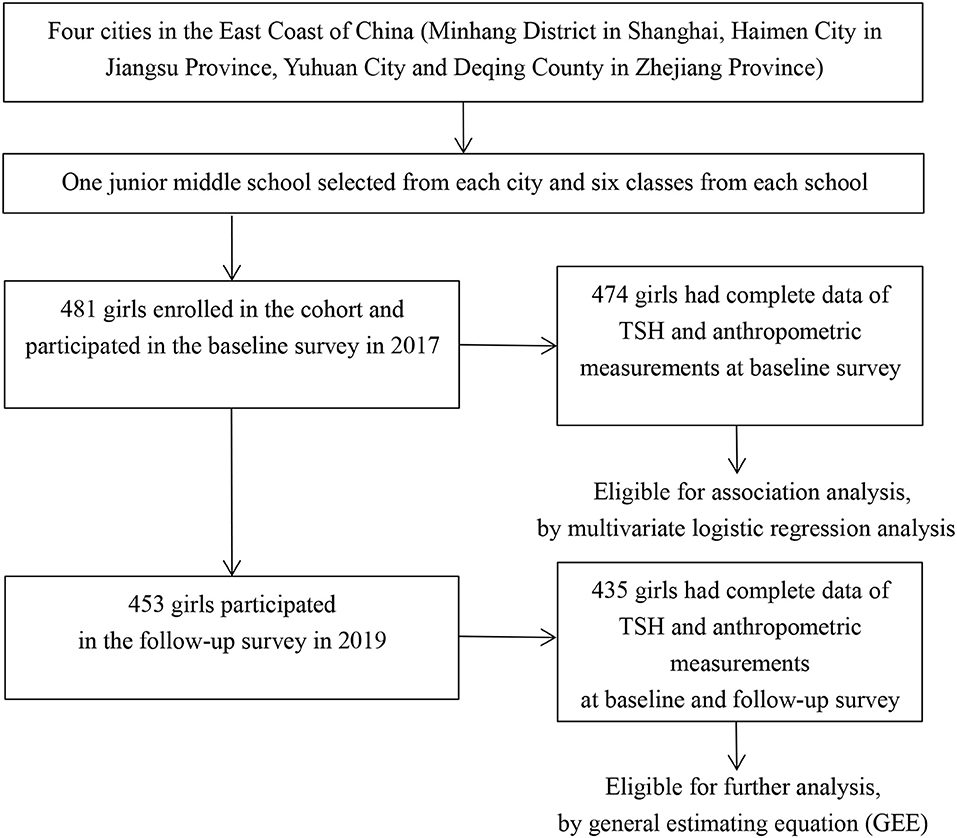
Four regions in East Coast of China (Minhang District in Shanghai, Haimen City in Jiangsu Province, Yuhuan City, and Deqing County in Zhejiang Province) were selected by purposive sampling. Previous studies have revealed an iodine-sufficient status along with distinguished iodized-salt consumption proportions among those sites (18–21). One junior middle school was randomly selected from each of the four regions and students were mainly local residents. A total of 481 girls from six classes of each school in grade 7 were enrolled into the cohort in October or November 2017 after excluding those with iodine supplement, thyroid disorder, pituitary abnormality or other conditions that affect thyroid hormone levels, and 474 of them completed both physical examinations and blood sample collections. Among 453 girls who participated in the follow-up investigation, 435 had complete data on thyroid function determinations and anthropometric measurements (Figure 1). Informed written consents were obtained from all the participants and their parents, and the study was approved by the ethical review board of the School of Public Health of Fudan University.
Patient and Public Involvement
No patient involved.
Information Collection and Variables Definition
Information on demographic and lifestyle factors were collected by a self-administrated questionnaire. Menarche (yes, no) and age at menarche were assessed. Important variables included monthly family income in Chinese Yuan (CNY) (≤3,000 CNY, >3,000 CNY), the highest education level for parents (junior high school or below, senior high school or above), daily bed time (≤8 h, >8 h) and daily activity time (≤1 h, >1 h).
Anthropometric Measurements
Anthropometric measurements, including standing height (cm), weight (kg), and waist circumferences (cm) were taken by local health professionals according to a standard protocol. Height and weight were respectively measured to the nearest 0.1 cm and 0.1 kg with the subjects standing without shoes and wearing light clothing only. Waist circumference (WC) was measured to the nearest 0.1 cm at the midpoint between the lower rib and the upper iliac crest. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2).
Thyroid Hormones Determination
Blood samples of ~5 mL were collected through antecubital vein puncture from each participant at both baseline and follow-up. Serum and blood clot were immediately centrifuged and kept at −80°C until analysis. Serum thyroid stimulating hormone (TSH) and free thyroid hormone (FT4) were determined by using an enzymatic cycling method.
Urine and Salt Samples Collection and Iodine Content Determination
In order to distinguish the influences of home diet and school canteen diet on urine iodine concentration (UIC), each participant provided morning urine samples on a Monday and a Thursday, and we used the method of inductively coupled plasma mass spectrometry (ICP-MS) to determine the UICs. Weighted daily urine iodine output was calculated as follows: (1) daily urine iodine output (μg) = MCr×1000×UIC÷UCr, (22) (MCr: daily urine creatinine output, mmol/day, MCr = EXP(0.0102×H-0.6854; H: body height, cm; UIC: urine iodine concentration, μg/L; UCr: urine creatinine content, μmol/L); and (2) weighted daily urine iodine output (μg) = 2/7× daily urine iodine output on Monday + 5/7 × daily urine iodine output on Thursday.
Participants were also asked to bring a salt sample of more than 20 g from home and salt iodine was then measured using a national standard method with a proper quality control (GB/T 13025.7-2012) (23). Iodized-salt consumption status was grouped into two categories: non-iodized salt (<5 mg/kg) and iodized salt (≥5 mg/kg) (24). In our study, the proportions of iodized-salt consumption was 88.98, 95.45, 85.96, and 94.50% in Minhang, Haimen, Yuhuan, and Deqing, respectively.
Statistical Analysis
Two patterns of obesity, general (peripheral) and central (abdominal) obesity, were evaluated by using body mass index (BMI) and waist circumference (WC), respectively (25). All participants were categorized into three groups of under/normal weight, overweight, and obesity according to the BMI growth reference values for Chinese children and adolescents aged 2–18 years suggested by Li et al. (26) and WC distribution of Chinese children and adolescents aged 7–18 years reported by Ji et al. (27). Participants were categorized into two groups (under/normal weight group, overweight/obesity) according to BMI due to small numbers for some subgroups.
Statistical description and cross-sectional analysis were performed based on data from 474 girls with complete information of serum TSH concentration and anthropometric measurements at baseline. Wilcoxon test and χ2 test were used to analyze continuous variables and categorical variables, respectively. Multiple logistic regression models were utilized to estimate the odds ratios (ORs) and 95% confidence intervals (95% CIs) for BMI- or WC-based overweight/obesity risks in relation to TSH levels (tertile 1, ≤1.53; mU/L, tertile 2, 1.54–2.37 mU/L; tertile 3, >2.37 mU/L). Major variables at baseline, including age, serum FT4 concentration, menarche or not, area with different proportions of iodized-salt consumption (≤90%, >90%) and weighted daily urine iodine output were taken into account. Stratified analysis was conducted according to age, menarche or not, area with different proportions of iodized-salt consumption, and their interactions with TSH levels were also assessed.
Girls who were lost to follow-up after 2 years or with missing data on TSH concentration or anthropometric measurements were excluded, leaving 435 girls with complete information for longitudinal data analysis. TSH levels at baseline or follow-up were compared between the under/normal weight and overweight/obesity groups. Four weight patterns were defined: (1) “BNFN”: under/normal weight at both baseline and follow-up; (2) “BNFO”: under/normal weight at baseline and overweight/obesity at follow-up; (3) “BOFN”: overweight/obesity at baseline and under/normal weight at follow-up; and (4) “BOFO”: overweight/obesity at both baseline and follow-up). General estimating equation (GEE) was used to evaluate the influences of the weight patterns, time and their interaction on serum TSH levels. The level of statistical significance was defined as α = 0.05 of two-side probability. All analyses were performed by using SPSS software for Windows (version 24.0, IBM Crop., Armonk, New York, USA). Figures were created by using GraphPad Prism software (version 7, California, USA).
Results
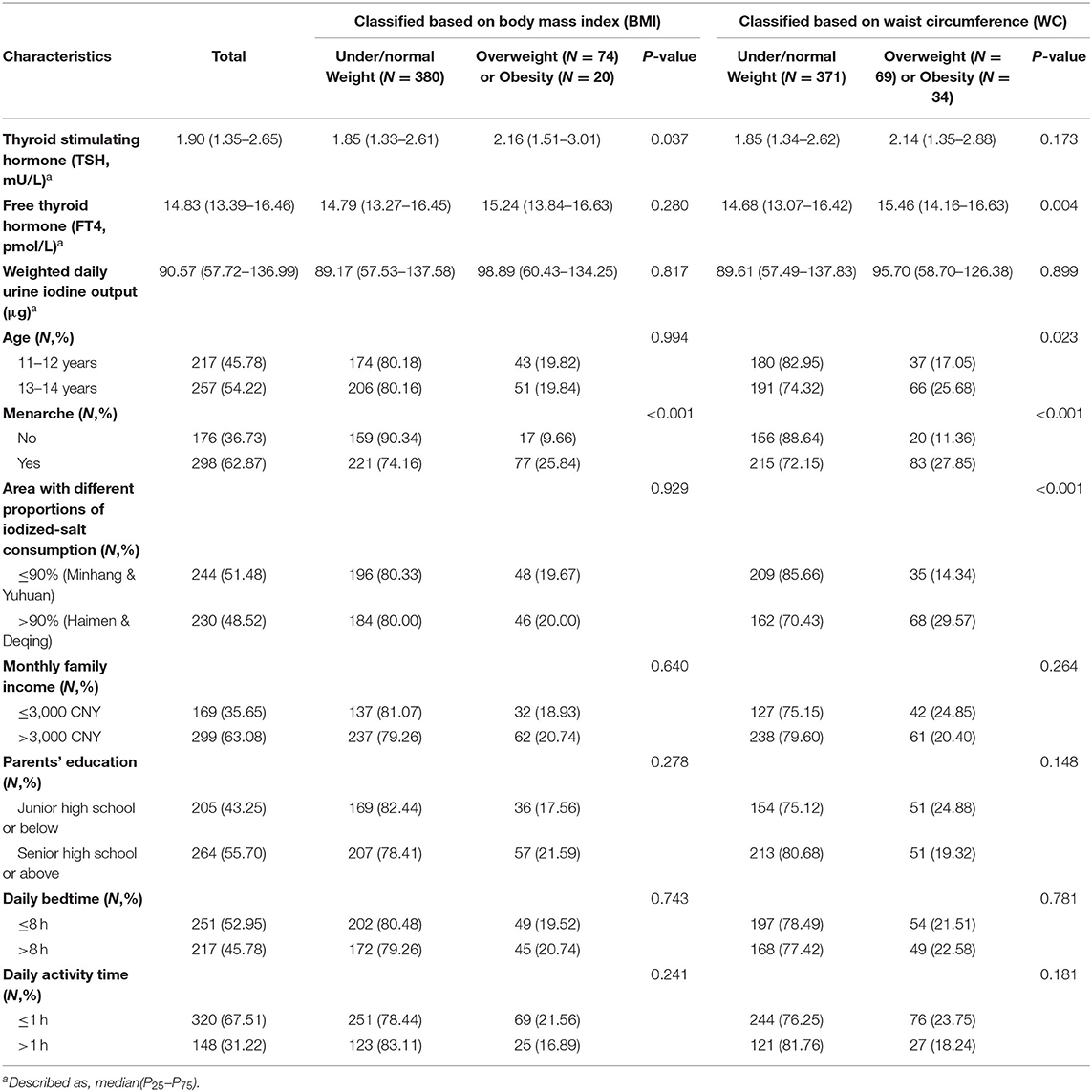
The mean age for the 474 girls was 12.48 (±0.69) years at baseline. Of the participants, the prevalence was 19.83% for general overweight/obesity based on BMI, and was 21.73% for central overweight/obesity based on WC (Table 1). The prevalence of central overweight/obesity increased with age (P = 0.023). Girls who had experienced menarche had a higher risk of overweight/obesity compared with those who did not (P < 0.001). Generally overweight/obese girls had a higher serum TSH concentration than under/normal weight girls (median: 2.16 vs. 1.85 mU/L, P = 0.037), but serum FT4 levels show no significant difference between the two groups (P = 0.280). Centrally overweight/obese girls showed higher serum FT4 (P = 0.004) but similar TSH (P = 0.173) levels as compared with under/normal weight girls.
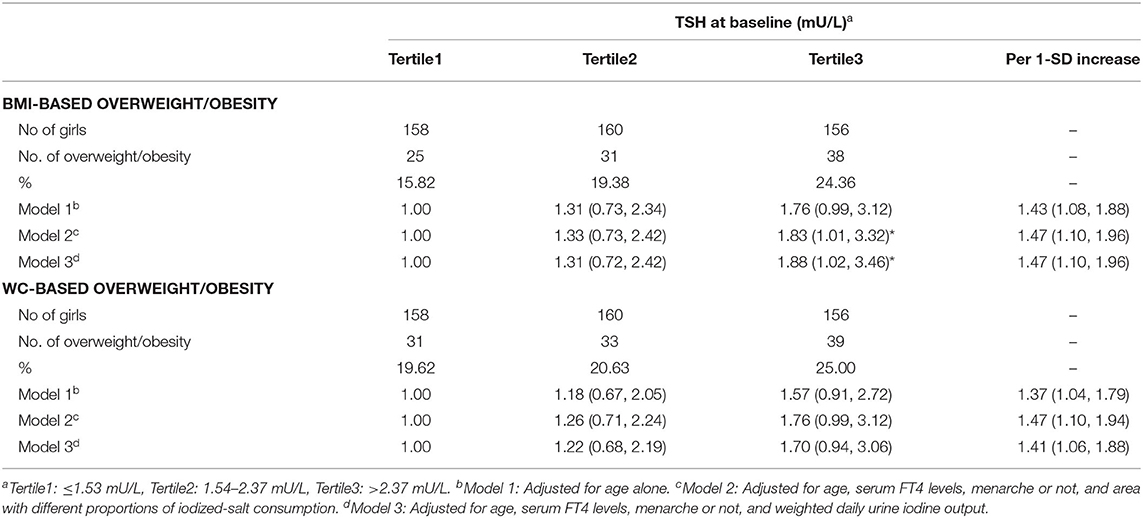
Multiple logistic regression analysis showed that girls in the highest tertile of serum TSH concentration had a significantly higher risk of BMI-based overweight/obesity compared with the lowest tertile after adjustment for potential confounders (OR = 1.83, 95% CI 1.01 to 3.32) (Table 2: Model 2), and that the adjusted OR 1.47 for both BMI-based and WC-based overweight/obesity risk in association with 1-SD increment in serum TSH (Table 2: Model 2).
In stratified analysis, serum TSH was positively related to the risks of BMI-based and WC-based overweight/obesity only among girls from area with the proportions of iodized-salt consumption more than 90% (BMI-based category: OR = 2.92, 95% CI 1.18 to 7.21; WC-based category: OR = 2.32, 95% CI 1.10 to 4.89) (Figures 2E,F). Among the older age group, the adjusted OR for BMI-based classification was 2.40 (95% CI 1.09 to 5.27) (Figure 2A).
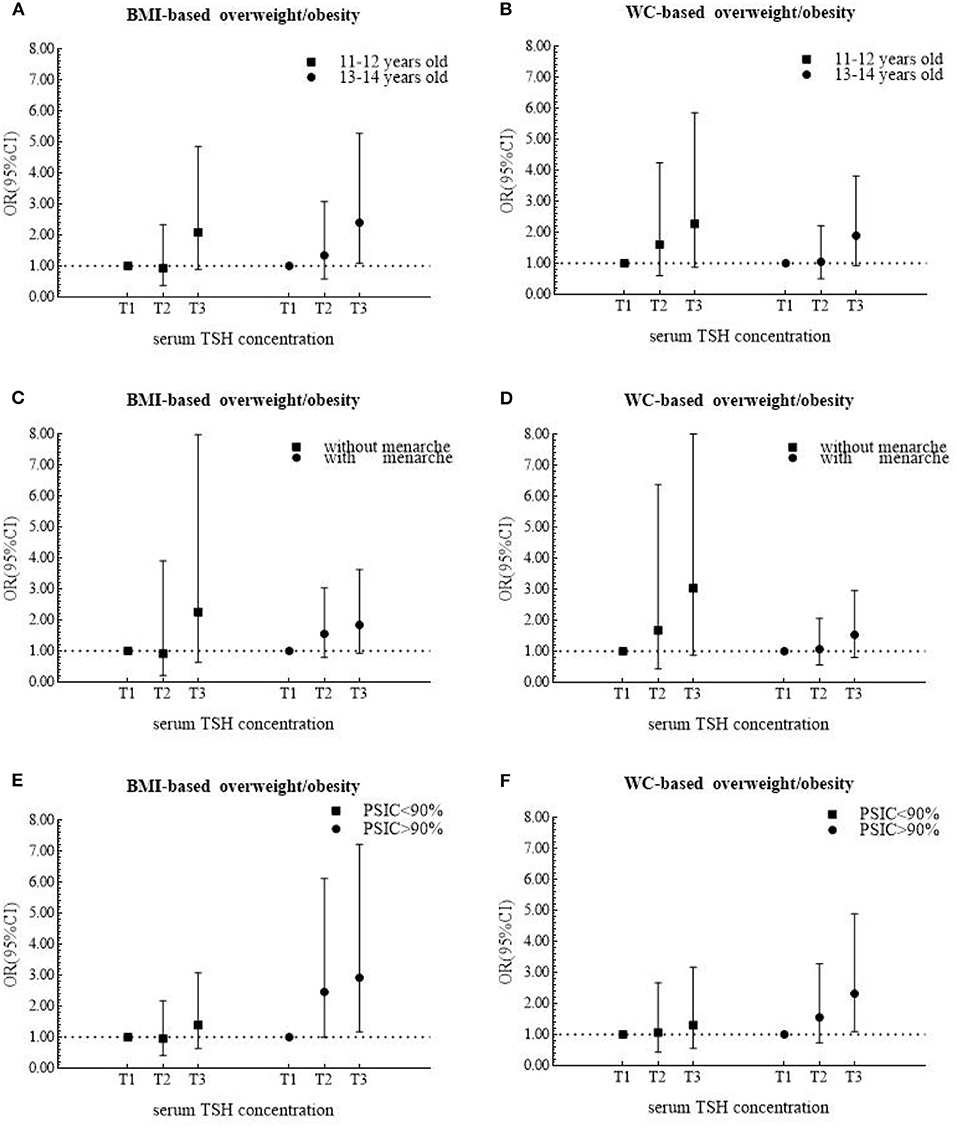
Figure 2. Odds ratios (ORs) and 95% confidence internals (95% CIs) for risk of overweight/obesity according to tertiles of serum TSH levels after being stratified by age, menarche and area in 474 girls.
A total of 435 girls were followed up for 2 years, and the serum TSH concentration significantly decreased (P < 0.001) (Median: 1.90 mU/L at baseline and 1.50 mU/L at follow-up). The BMI-based overweight/obesity group maintained a higher level of serum TSH than the under/normal weight group both at baseline (P = 0.018) and follow-up (P = 0.004) (Figure 3A), but the difference was only observed for WC-based measure at follow-up (Figure 3B).
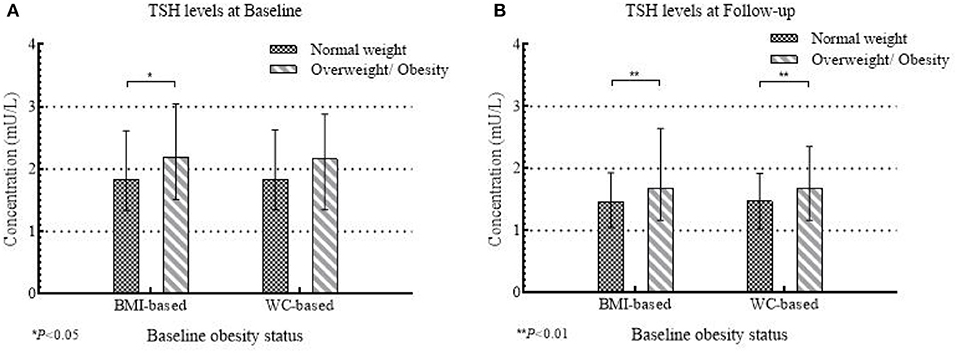
Figure 3. The concentrations of serum TSH at baseline and follow-up between normal weight and overweight/obese group according to baseline obesity status in 435 girls.
General estimating equation (GEE) indicated that there were main effects of BMI-based obesity development patterns (P = 0.016) and time (P < 0.001), but no interaction between them on the serum TSH levels (P > 0.05). Girls who experienced BMI-based overweight/obesity at baseline and then evolved to normal weight at follow-up (BOFN) had 0.42 mU/L (95% CI 0.07 to 0.77) higher serum TSH level than girls who kept normal weight (Table 3).
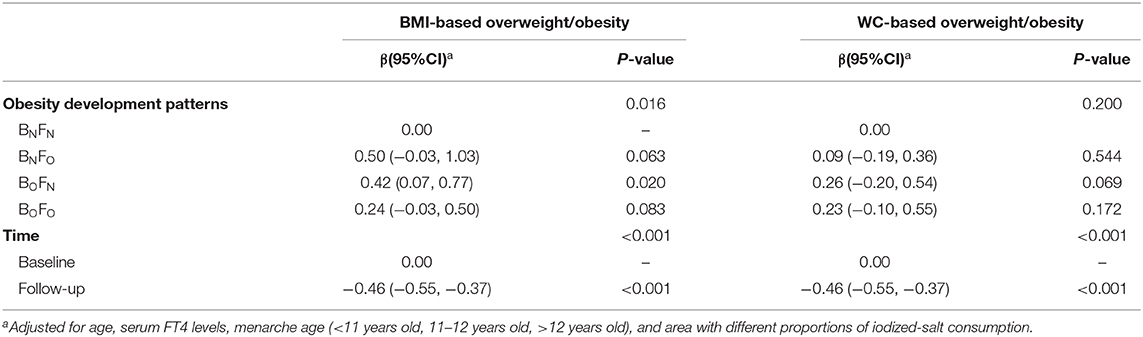
Table 3. Regression coefficients (βs) and 95% confidence internals (95% CIs) for serum TSH change over 2 years in 435 girls using general estimating equation (GEE).
Discussion
Our study indicated that in pubertal girls with a higher serum TSH level had a higher risk of overweight/obesity based on BMI or WC measures, and the association was more evident for the BMI measure. Previous studies demonstrated that TSH was positively related to BMI-based obesity in pediatric outpatients (11, 28), and WC-based obesity in adolescents (29, 30).
TSH, also known as thyrotropin, is produced by the anterior pituitary, regulated by the thyroid releasing hormone (TRH), and limited by the negative feedback from the thyroid hormones (THs) (31). THs primarily regulate basal metabolism, which constitutes approximately 66% of total daily energy expenditure (32). In addition, THs are involved in lipid and glucose metabolism with a mediator named sterol regulatory element-binding proteins (SREBP-2) (33, 34). Nader et al. found that increases in TSH levels within the reference range were associated with increases in insulin levels and homeostasis model assessment (HOMA) levels of insulin resistance(IR) (35), which may contribute to obesity in childhood (36). Insulin is a critical regulator of adipocyte biology, and promotes adipocyte triglyceride storage by some mechanisms, including transportation of glucose, differentiation of preadipocytes to adipocytes, and synthesis of triglyceride (lipogenesis) (37). Moreover, the positive relationship of TSH with triglyceride and non-HDL lipoproteins was indicated in a large population-based study among children and adolescents (38).
On the other hand, obesity may be a cause of elevated TSH levels (10). One plausible explanation is that adipose tissue secretes inflammatory cytokines into the general circulation, such as tumor necrosis factor (TNF-α) and interleukin (IL-1, IL-6) (39). These cytokines impede the expression of sodium iodine transporter mRNA and the activity of iodine uptake in human thyroid cells, which reduces the secretion of THs, and then leads a compensatory rise in TSH levels. Another hypothesis is that the production of leptin-mediated pro-thyrotropin-releasing hormone (pro-TRH) increases with weight gain (40). Leptin is predominantly released by adipocytes and stimulates TSH secretion by hypothalamic-pituitary axis (41). Non-synonymous mutations in thyroid stimulating hormone receptor (TSH-R) gene also plays a role in elevated TSH levels in relation to obesity (42).
In stratified analysis, the positive association between TSH and obesity was only observed in girls aged 13 to 14 years and from area with the proportions of iodized-salt consumption more than 90% (Haimen and Deqing), indicating that there may exist modification effect from age and iodine.
The strengths of our study include a cohort study enrolling school-aged girls, repeated measures of thyroid hormones and objectively anthropometric measurements following a standardized protocol collected at both baseline and follow-up. To our knowledge, rare studies have paid attention to girls around puberty for the relationship between thyroid function and adiposity based on two different evaluation criterion. However, there were several limitations. First, there is a lack of information on TPO-Ab (thyroid peroxidase antibody) that may be a better indicator of thyroid function. Moreover, our subjects were enrolled from sufficient-iodine and higher-economic areas in east China with a relatively small simple size, caution should be taken when extrapolating the results.
Conclusion
In this prospective cohort study of girls during puberty, we found that TSH was positively associated with the risk of general or abdominal adiposity. Our findings emphasize the importance of preventing excess adiposity in girls with thyroid dysfunction.
Strengths and Limitations of this Study
1. Main strengths include prospective cohort study design, targeting girls during puberty, repeated thyroid hormone measures and objectively anthropometric measurements following a standardized protocol collected at both baseline and follow-up.
2. Both central and abdominal adiposity were found to be association with increased levels of serum TSH.
3. Lack of information on TPO-Ab (thyroid peroxidase antibody), a better measure of thyroid function.
Ethics Statement
The study was approved by the ethical review board of the School of Public Health of Fudan University (#2012-03-0350S). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
XD, MS, DX, JQ, NW, and QJ contributed to the study design. YW, CF, FJ, RL, and NW contributed to data acquisition and collection. YW, NW, and YC contributed to data analysis and interpretation, and drafted the manuscript. All authors contributed to the preparation of the final document, read, and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81602806) and Shanghai Leading Academic Discipline Project of Public Health (Grant No. 15GWZK0801). Minhang District Natural Science Research Project (Grant No. 2018MHZ010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge all the staff who participated in this project, including health workers in Deqing County Center for Disease Control and Prevention, Yuhuan City Center for Disease Control and Prevention, Minhang District Center for Disease Control and Prevention, and Haimen City Center for Disease Control and Prevention, and those from local community health centers.
Abbreviations
TSH, thyroid stimulating hormone; TRH, thyroid releasing hormone; THs, thyroid hormones; FT4, free thyroxine; BMI, body mass index; WC, waist circumference; UIC, urine iodine concentration; ICP-MS, inductively coupled plasma mass spectrometry; GEE, general estimating equation.
References
1. World Health Organization. Obesity and Overweight 2017. Available online at: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed October 15, 2018).
2. Lang JE. Obesity and childhood asthma. Curr Opin Pulm Med. (2019) 25:34–43. doi: 10.1097/MCP.0000000000000537
3. Nehus E, Mitsnefes M. Childhood obesity and the metabolic syndrome. Pediatr Clin North Am. (2019) 66:31–43. doi: 10.1016/j.pcl.2018.08.004
4. Weihrauch-Blueher S, Schwarz P, Klusmann J-H. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metab Clin Exp. (2019) 92:147–52. doi: 10.1016/j.metabol.2018.12.001
5. Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev. (2016) 17:56–67. doi: 10.1111/obr.12316
6. Angel Rivera J, Gonzalez de Cossio T, Susana Pedraza L, Cony Aburto T, Georgina Sanchez T, Martorell R. Childhood and adolescent overweight and obesity in Latin America: a systematic review. Lancet Diabetes Endocrinol. (2014) 2:321–32. doi: 10.1016/S2213-8587(13)70173-6
7. Scacchi M, Pincelli AL, Cavagnini F. Growth hormone in obesity. Int J Obesity. (1999) 23:260–71. doi: 10.1038/sj.ijo.0800807
8. Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. (2004) 5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x
9. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. (2014) 94:355–82. doi: 10.1152/physrev.00030.2013
10. Garcia-Solis P, Garcia OP, Hernandez-Puga G, Sanchez-Tusie AA, Saenz-Luna CE, Hernandez-Montiel HL, et al. Thyroid hormones and obesity: a known but poorly understood relationship. Endokrynol Pol. (2018) 69:292–303. doi: 10.5603/EP.2018.0032
11. Ekinci F, Uzuner A, Demet MC, Turgut B, Tosun N. Thyroid hormones and BMI in obese children: one year follow up results. Marmara Med J. (2015) 28:129–34.
12. Wang N, Fang H, Fu CW, Huang PX, Su MF, Jiang F, et al. Associations of adiposity measurements with thyroid nodules in Chinese children living in iodine-sufficient areas: an observational study. BMJ Open. (2017) 7:e016706. doi: 10.1136/bmjopen-2017-016706
13. Peper JS, Brouwer RM, van Leeuwen M, Schnack HG, Boomsma ID, Kahn SR, et al. HPG-axis hormones during puberty: a study on the association with hypothalamic and pituitary volumes. Psychoneuroendocrinology. (2010) 35:133–40. doi: 10.1016/j.psyneuen.2009.05.025
14. Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer. (2014) 21:T273–83. doi: 10.1530/ERC-14-0053
15. Ong KK, Northstone K, Wells JCK. Earlier mother's age at menarche predicts rapid infancy growth and childhood obesity. PLoS Med. (2007) 4:e132. doi: 10.1371/journal.pmed.0040132
16. Ozer S, Butun I, Sonmezgoz E, Yilmaz R, Demir O. Relationships among thyroid hormones and obesity severity, metabolic syndrome and its components in Turkish children with obesity. Nutr Hosp. (2015) 32:645–51. doi: 10.3305/nh.2015.32.2.9212
17. Witkowska-Sedek E, Kucharska A, Ruminska M, Pyrzak B. Thyroid dysfunction in obese and overweight children. Endokrynol Pol. (2017) 68:54–60. doi: 10.5603/EP.2017.0007
18. Zhang YM, Wang YY, Wang XC, Dong XL, Jiang F, Fu CW, et al. Iodine nutrition and thyroid abnormality in adolescent girls in Deqing County. Chin J Sch Health. (2018) 39:1723–5. doi: 10.16835/j.cnki.1000-9817.2018.11.037
19. Su M, Wang CY, Li ST, Ying XH, Zhao Q, Fu CW, et al. Repeated cross-sectional studies on urinary iodine and iodine content of salt among school-aged children from 2012 to 2014 in Yuhuan County, Zhejiang Province. J Hyg Res. (2016) 45:14–8.
20. Liu P, Wang N, Fang H, Wang HX, Yan YJ, Fu CW, et al. Alteration on household salt consumption status and urinary iodine concentration of a primary school children in Shanghai, 2012-2014. Chin J Prev Med. (2016) 50:282–4. doi: 10.3760/cma.j.issn.0253-9624.2016.03.020
21. Huang PX, Jiang F, Feng X, Liu JX, Gui MF, Zhao Q, et al. A cross-sectional study of urinary iodine and salt iodine content among schoolchildren and their families in Haimen City, Jiangsu Province. Chin J Endemiol. (2014) 33:654–6. doi: 10.3760/cma.j.issn.2095-4255.2014.06.016
22. Wang HX, Tang CX, Yang JQ, Wang N, Jiang F, Xia QH, et al. Predictors of urinary antibiotics in children of Shanghai and health risk assessment. Environ Int. (2018) 121:507–14. doi: 10.1016/j.envint.2018.09.032
23. Zhang YP, Huang YH. Discussion of “GB/T 13025.7-2012 general test method for the determination of iodine in salt industry”. Chin J Endemiol. (2014) 33:346–8. doi: 10.3760/cma.j.issn.2095-4255.2014.03.028
24. Ministry of health of the People's Republic of China. Food Safety National Standard Edible Salt Iodine Content GB 26878-2011 [S]. Vol 1. Beijing: China Standard Publishing House (2011).
25. Wang YY, Jiang YG, Wang N, Zhu MY, Liu X, Wang RP, et al. Central but not general obesity is positively associated with the risk of hyperhomocysteinemia in middle-aged women. Nutrients. (2019) 11:1614. doi: 10.3390/nu11071614
26. Li H, Zhong XN, Ji CY, Mi J. Body mass index cut-offs for overweight and obesity in Chinese children and adolescents aged 2-18 years. Chin J Endemiol. (2010) 31:616–20. doi: 10.3760/cma.j.issn.0254-6450.2010.06.004
27. Ji CY, Sung RYT, Ma GS, Ma J, He ZH, Chen TJ. Waist circumference distribution of chinese school-age children and adolescents. Biomed Environ Sci. (2010) 23:12–20. doi: 10.1016/S0895-3988(10)60026-8
28. Radhakishun NNE, van Vliet M, von Rosenstiel IA, Weijer O, Beijnen JH, Brandjes DPM, et al. Increasing thyroid-stimulating hormone is associated with impaired glucose metabolism in euthyroid obese children and adolescents. J Pediatr Endocrinol Metab. (2013) 26:531–7. doi: 10.1515/jpem-2012-0302
29. Zhang JF, Jiang RH, Li L, Li P, Li X, Wang ZN, et al. Serum thyrotropin is positively correlated with the metabolic syndrome components of obesity and dyslipidemia in Chinese adolescents. Int J Endocrinol. (2014) 2014:289503. doi: 10.1155/2014/289503
30. Cerbone M, Capalbo D, Wasniewska M, Raso GM, Alfano S, Meli R, et al. Cardiovascular risk factors in children with long-standing untreated idiopathic subclinical hypothyroidism. J Clin Endocrinol Metab. (2014) 99:2697–703. doi: 10.1210/jc.2014-1761
31. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. (2003) 14:11–23. doi: 10.1016/s1042-3680(02)00017-7
32. Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. (2008) 18:141–4. doi: 10.1089/thy.2007.0266
33. Crunkhorn S, Patti ME. Links between thyroid hormone action, oxidative metabolism, and diabetes risk? Thyroid. (2008) 18:227–37. doi: 10.1089/thy.2007.0249
34. Shin DJ, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2). J Biol Chem. (2003) 278:34114–8. doi: 10.1074/jbc.M305417200
35. Nader NS, Bahn RS, Johnson MD, Weaver AL, Singh R, Kumar S. Relationships between thyroid function and lipid status or insulin resistance in a pediatric population. Thyroid. (2010) 20:1333–9. doi: 10.1089/thy.2010.0180
36. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol. (2008) 159(Suppl. 1):S67–74. doi: 10.1530/EJE-1508-0245
37. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. New Engl J Med. (2004) 350:2362–74. doi: 10.1056/NEJMoa031049
38. Witte T, Ittermann T, Thamm M, Riblet NBV, Voelzke H. Association between serum thyroid-stimulating hormone levels and serum lipids in children and adolescents: a population-based study of German Youth. J Clin Endocrinol Metab. (2015) 100:2090–7. doi: 10.1210/jc.2014-4466
39. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. J Endocrinol. (2011) 11:85–97. doi: 10.1038/nri2921
40. Mantzoros CS, Ozata M, Negrao AB, Suchard MA, Ziotopoulou M, Caglayan S, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. (2001) 86:3284–91. doi: 10.1210/jcem.86.7.7644
41. Betry C, Challan-Belval MA, Bernard A, Charrie A, Drai J, Laville M, et al. Increased TSH in obesity: evidence for a BMI-independent association with leptin. Diabetes Metab. (2015) 41:248–51. doi: 10.1016/j.diabet.2014.11.009
Keywords: thyroid stimulating hormone, general obesity, central obesity, school-aged girls, puberty, cohort study
Citation: Wang Y, Dong X, Fu C, Su M, Jiang F, Xu D, Li R, Qian J, Wang N, Chen Y and Jiang Q (2020) Thyroid Stimulating Hormone (TSH) Is Associated With General and Abdominal Obesity: A Cohort Study in School-Aged Girls During Puberty in East China. Front. Endocrinol. 11:620. doi: 10.3389/fendo.2020.00620
Received: 17 April 2020; Accepted: 29 July 2020;
Published: 30 September 2020.
Edited by:
Christoph Reiners, University Hospital Würzburg, GermanyReviewed by:
Takao Ando, Nagasaki University Hospital, JapanGiorgio Napolitano, University of Studies G. d'Annunzio Chieti and Pescara, Italy
Copyright © 2020 Wang, Dong, Fu, Su, Jiang, Xu, Li, Qian, Wang, Chen and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Wang, bmEud2FuZ0BmdWRhbi5lZHUuY24=; Yue Chen, WXVlLkNoZW5AdW90dGF3YS5jYQ==
 Yingying Wang
Yingying Wang Xiaolian Dong3
Xiaolian Dong3 Dongli Xu
Dongli Xu