
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 15 September 2020
Sec. Pediatric Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00609
This article is part of the Research Topic Modern Management and Monitoring of Childhood Diabetes View all 9 articles
Severe hypoglycemia is defined as a condition with serious cognitive dysfunction, such as a convulsion and coma, requiring external help from other persons. This condition is still lethal and is reported to be the cause of death in 4–10% in children and adolescents with type 1 diabetes. The incidence of severe hypoglycemia in the pediatric population was previously reported as high as more than 50–100 patient-years; however, there was a decline in the frequency of severe hypoglycemia during the past decades, and relationship with glycemic control became weaker than previously reported. A lot of studies have shown the neurological sequelae with severe hypoglycemia as cognitive dysfunction and abnormalities in brain structure. This serious condition also provides negative psychosocial outcomes and undesirable compensatory behaviors. Various possible factors, such as younger age, recurrent hypoglycemia, nocturnal hypoglycemia, and impaired awareness of hypoglycemia, are possible risk factors for developing severe hypoglycemia. A low HbA1c level is not a predictable value for severe hypoglycemia. Prevention of severe hypoglycemia remains one of the most critical issues in the management of pediatric patients with type 1 diabetes. Advanced technologies, such as continuous glucose monitoring (CGM), intermittently scanned CGM, and sensor-augmented pump therapy with low-glucose suspend system, potentially minimize the occurrence of severe hypoglycemia without worsening overall glycemic control. Hybrid closed-loop system must be the most promising tool for achieving optimal glycemic control with preventing the occurrence of severe hypoglycemia in pediatric patients with type 1 diabetes.
Hypoglycemia is a commonly observed acute complication in the management of type 1 diabetes. It is a major barrier to achieve optimal glycemic control (1) and may affect quality of life in the patients (2). Minimizing hypoglycemia is an important objective in the management of type 1 diabetes, and this can be attained by evaluating the risk factors and preventing them, although intensive glycemic management (3).
Severe hypoglycemia is defined as a condition with serious cognitive dysfunction, such as a convulsion and coma, requiring external help from other persons to provide glucose and glucagon or take other correction assistance. Severe hypoglycemic coma is defined as the subgroup of severe hypoglycemia related to a convulsion or unconsciousness (4). Severe hypoglycemia is still lethal and is reported to be the cause of death in 4% to 10% (5–7). It may be associated with permanent brain damage and is related to cognitive dysfunction and abnormalities in brain structure particularly in young children with type 1 diabetes (8–15). The International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines 2018 has recommended that a target of HbA1c should be <7.0% (53 mmol/mol) in patients who can access contemporary technologies of insulin treatment and the potency to regular self-checking blood glucose and/or the use of continuous glucose monitoring (CGM) (16). Whereas, careful attention must be poured to avoid severe hypoglycemia, glucose targets must be increased in patients with the risk factors for severe hypoglycemia (4, 17).
The main purpose of this review is to evaluate possible risk factors and the neurological sequelae in severe hypoglycemia and to introduce the advanced technologies to minimize the occurrence of severe hypoglycemia in children and adolescents with type 1 diabetes.
Briefly, we performed the literature research by MEDLINE and EMBASE covering the period between 1980 and 2019. The search terms were “type 1 diabetes,” “children,” “adolescents,” and “hypoglycemia” or “severe hypoglycemia.” Language restriction was applied in English.
High incidence of severe hypoglycemia was shown by the Diabetes Control and Complications Trial (DCCT) in 1997 (18); i.e., the incidence of hypoglycemia that needed treatment assistance was 61.2 per 100 patient-years in patients receiving intensive treatment and 18.7 per 100 patient-years in those receiving conventional treatment, respectively, with a relative risk of 3.28. The relative risk for coma and/or seizure was 3.02 for intensive treatment. High incidence was also demonstrated in large pediatric cohorts in Australia (19) and Colorado (20) in the early 2000s. However, the incidence has been decreasing over time. A population-based cohort of Western Australia demonstrated that the incidence of severe hypoglycemia was 17.3 per 100 patient-years in 2001 and 5.8 per 100 patient-years in 2006, and a 12% annual rate of decrease was observed during the study period (21). A similar decreased trend was also observed in children and adolescents in Germany and Australia (22) and in Japan (23). A recent Italian study conducted in 29 diabetes centers during 2011–2012 reported less incidence of 7.7 per 100 patient-years (24), whereas another Italian-center study showed higher incidence of 12.6 per 100 patient-years in 1990 and 16.5 per 100 patient-years in 2010, respectively (Table 1). Development of treatment regimens might contribute to decrease in the incidence of severe hypoglycemia; however, despite the advent of new insulin regimens, severe hypoglycemia still remained a relevant risk and a current threat for patients with type 1 diabetes and their family members (25).
On the other hand, previous studies showed that high incidence of severe hypoglycemia was related to a lower HbA1c level (18, 26); however, this association has recently weakened as reported in large longitudinal cohorts (21, 27, 28). A cross-sectional analysis of 3 contemporary pediatric diabetes registry databases showed no inverse correlation between a mean HbA1c level and risk factors for severe hypoglycemia in children and adolescents with type 1 diabetes (29). It is possible that the advanced technologies over the past decades could be enabling better glycemic control without increase in the risk of severe hypoglycemia. Such advances could include the introduction and increased use of insulin analogs, insulin pump therapy, increased frequency of self-monitoring of blood glucose, and use of CGM.
Resent meta-analyses of the literature indicated that young patients with type 1 diabetes tended to show mildly lower overall intellectual function than healthy controls and that the domains of executive functions, learning, memory, and processing speed were also impaired (30, 31). On the other hand, larger difference in cognitive function was found in the subset of young patients with certain risk factors, including younger onset age and greater exposures to both severe hypoglycemia and hyperglycemia (32).
Several studies in pediatric patients with type 1 diabetes demonstrated frequent episodes of severe hypoglycemia were related to worse performance than healthy controls on certain attention tasks, such as overall cognitive function, and verbal and visual memory. Particularly, children with certain risk factors, including younger onset age and frequent episodes of severe hypoglycemia, tended to develop cognitive dysfunction. Lin et al. (10) found that severe hypoglycemia with early onset of type 1 diabetes below 6 years of age adversely affected verbal abilities, working memory, and processing speed later in life than healthy controls. Another study also showed that younger onset age (<5 years) was related to deficit of cognitive function (8). On the other hand, other studies demonstrated that frequent episodes of severe hypoglycemia particularly affected distinct memory function, when these episodes appeared before 5 years of age, and were related to full-scale IQ scores, processing speed, working memory, and perceptual reasoning (11, 12). Furthermore, severe hypoglycemia with a convulsion was associated with greater performance deficits, including attention tasks, overall cognitive function, and verbal and visual memory (33). Blasetti et al. (34) indicated that prior episodes of severe and frequent hypoglycemia were mostly related to decreased learning and memory in young patients with type 1 diabetes using a meta-analysis.
On the other hand, some studies have demonstrated that severe hypoglycemia is unlikely to affect cognitive function. The Epidemiology of Diabetes Interventions and Complications follow-up study, conducted 18 years after the DCCT, reported that cognitive function did not decrease over the extended period in the youngest patients, although relatively high frequencies of severe hypoglycemia (35). A cross-sectional (36) and longitudinal follow-up research in the same population-based cohort (37) also did not elucidate a decrease in full-scale IQ scores, although executive function and fluid intelligence may be insufficient. On the other hand, other studies demonstrated that cognitive dysfunction also occurred with hyperglycemia other than hypoglycemia (9, 38–40). A large study of younger children (4–10 years of age) with a short period of type 1 diabetes (mean 2.5 years) reported cognitive differences than age-matched healthy children (40). There were differences in full-scale IQ scores and executive functioning even after adjustment of parent IQ scores and internalizing mood symptom levels. The degree of exposure to hyperglycemia was relatively related to performance in these domains. The long-duration impact of hyperglycemia may play an additional role for cognitive outcomes in pediatric patients with type 1 diabetes.
The association of structural abnormalities of brain accompanied by severe hypoglycemia has been shown, although there is increasing evidence that the brain changes were observed even without significant episodes of hypoglycemia among young patients with type 1 diabetes. Pell et al. (41) reported an interaction between age and brain volume with youth with type 1 diabetes but with the occurrence of dysglycemia. Greater hippocampal volumes (14) and decreased gray and white matter volumes were observed in children experienced hypoglycemic seizures (12). However, another study showed that brain changes were observed both with hypoglycemia and with hyperglycemia. Episodes of severe hypoglycemia were related to decreased gray matter volume in the left superior temporal region, whereas frequent episodes of hyperglycemia were related to decreased gray matter volume in the right cuneus and precuneus, decreased white matter volume in the right posterior parietal region, and increased gray matter volume in the right prefrontal region (15) (Figure 1). These findings suggest that regional differences of brain volume might be related to both hypoglycemia and hyperglycemia.
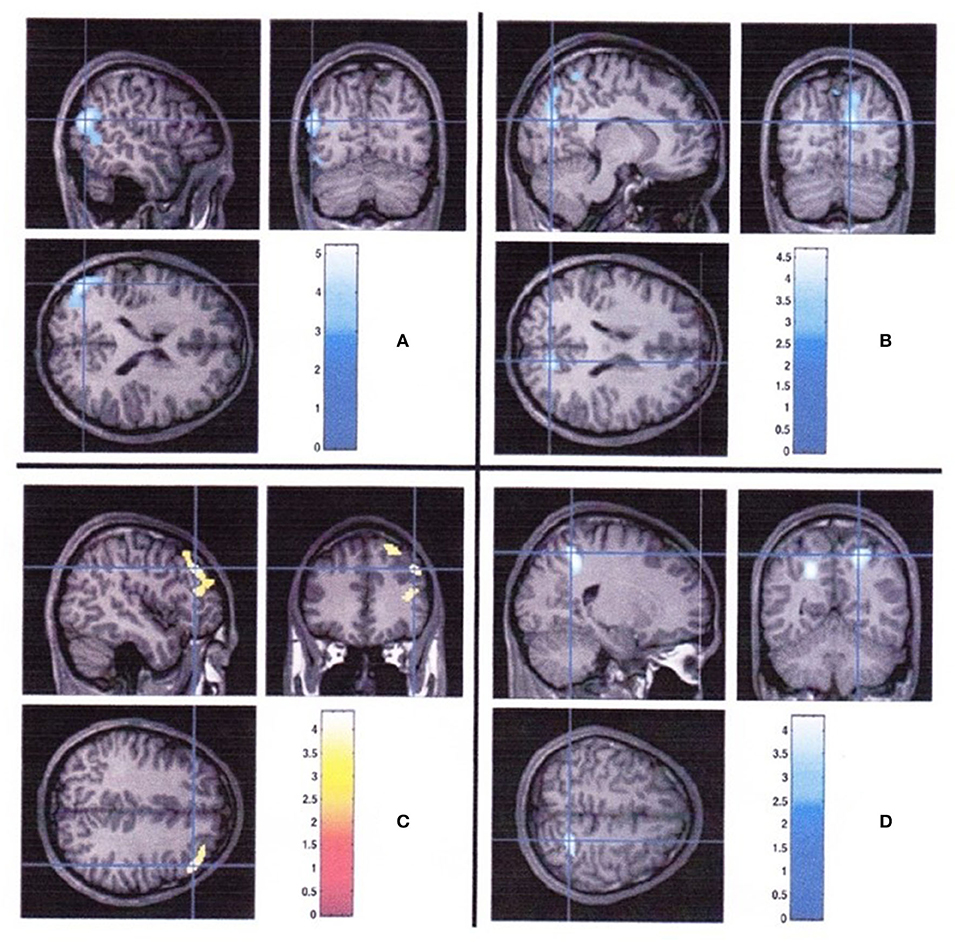
Figure 1. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes (15). (A) Regions with smaller gray matter volume in diabetic youth with severe hypoglycemia compared with those in diabetic youth without severe hypoglycemia. (B) Regions of less gray matter, (C) more gray matter, and (D) less white matter associated with greater hyperglycemia exposure.
Recent reports also indicated the relation between frequent episodes of severe hypoglycemia and the increased risk of later-onset epilepsy (42, 43), and although the causative mechanisms were not elucidated, metabolic brain adaptations to frequent severe hypoglycemia might be the cause of later-onset epilepsy (44).
Severe hypoglycemia is likely to provide negative psychosocial outcomes and undesirable compensatory behaviors (2, 45). The fear toward severe hypoglycemia may induce anxiety, and in many children and family members, significant degrees of anxiety possibly lead to confusion in daily living activities and inadequate management of diabetes (46). Children with type 1 diabetes and family members have risks of increased anxiety, insufficient sleep, and impaired quality of life (2, 47, 48). Fear of severe hypoglycemia, particularly during night, must be the most serious problem in family members of younger children with type 1 diabetes (49). This fear may lead them to accept high blood glucose levels, and suboptimal glycemic control with behaviors avoiding hypoglycemia resulted in inadequate glycemic control (2, 50, 51). The use of CGM and sensor-augmented pump can decrease the fear of hypoglycemia, although the studies conducted in children are limited (46, 52).
Various factors can affect severe hypoglycemia. Possible risk factors for developing severe hypoglycemia are shown in Table 1.
Various studies have indicated that younger children tend to have more frequent and/or more serious episodes of hypoglycemia than later-onset patients with type 1 diabetes (53–57). Younger children are likely to have more physical activities and less consumption of food, showing fluctuating blood glucose profiles, which increase risks for developing hypoglycemia (4). They lack counterregulatory hormone responses to subsequent hypoglycemia via autonomic function (56). Deficits of counterregulatory hormone responses also cause autonomic failure. Consequently, younger children are at risk for frequent and severe hypoglycemia. Neurological damages accompanied by severe hypoglycemia are more common and more severe in younger children with type 1 diabetes (13, 14). The onset age of diabetes might play a role for early exposure to severe hypoglycemia, as earlier exposure can occur in those with younger onset of type 1 diabetes (31).
The counterregulatory hormone responses to hypoglycemia attenuate during sleep (56, 58), and patients with type 1 diabetes tend to be less awakened by hypoglycemia compared with healthy subjects (56). The fear of nocturnal hypoglycemia often provides anxiety and emotional stress, interfering sleep and lowering quality of life in family members, which is the most common cause of distress in family members (59).
Earlier reports showed a high frequency of nocturnal hypoglycemia up to 40% during any nights in pediatric patients with type 1 diabetes (60–63), whereas recent studies have demonstrated lowering frequencies of 15–25% during any nights (63, 64). Half of these hypoglycemic events were undetected by patients themselves, families, and caregivers (60, 65). The Juvenile Diabetes Research Foundation (JDRF) found recurrent and prolonged nocturnal hypoglycemia during 8.5% of nights in both children and adults, but more extended in children (66). The use of insulin pump can decrease nocturnal hypoglycemia (67), and this is further decreased using sensor-augmented pump with the control algorithms, which can suspend basal insulin delivery with sensor-detected (68) or sensor-predicted hypoglycemia (69).
Impaired awareness of hypoglycemia is an acquired complication of insulin treatment, whereby the potency to detect the onset of hypoglycemia is decreased or absent (70). Deficit of the counterregulatory hormone responses to hypoglycemia frequently coexist. Development of impaired awareness of hypoglycemia increases the risk for severe hypoglycemia more. This condition was reported to exist in approximately a quarter of adults with type 1 diabetes. Children and adolescents had similar prevalence of 19–37% (2, 71, 72). However, a recent study has shown reduction in the prevalence over time, i.e., 33% in 2002 vs. 21% in 2015 in the same population-based cohort (73). Although the prevalence of impaired awareness of hypoglycemia has decreased, it is still a major risk factor for developing severe hypoglycemia. Patients with impaired awareness of hypoglycemia have a 6-fold increase in the prevalence of severe hypoglycemia (74).
It has been known that impaired awareness of hypoglycemia is related to decrease in glycemic thresholds for the release of counterregulatory hormones and induction of adrenergic warning signs. Korytkowski et al. (75) reported that a 2- to 3-fold decrease in the epinephrine responses was related to the loss of adrenergic warning symptoms against hypoglycemia. On the other hand, loss of autonomic symptoms precedes the neuroglycopenic symptoms, and patients are less likely to recognize hypoglycemia. Hypoglycemia tends to be prolonged when the awareness of low blood glucose is impaired. Patients can develop hypoglycemic seizures if unrecognized and prolonged conditions continue for more than 2–4 h (76).
Most events of severe hypoglycemia occur during nighttime, because sleep more strongly impairs the counterregulatory hormone responses to hypoglycemia in patients with type 1 diabetes as well as normal subjects (58). On the other hand, the glycemic threshold for neuroglycopenia does not change as much with the intensity of treatment, glycemic control, or with prior hypoglycemia (77–79).
Avoidance of severe hypoglycemia for 2–3 weeks can reverse impaired awareness of hypoglycemia (17), which is difficult to achieve in practice with current intensive insulin treatment in children with type 1 diabetes. Advanced technologies, such as the use of CGM (80) or sensor-augmented pump with control algorithms including suspend functions (68, 81), could reduce the rate of severe hypoglycemia in patients showing impaired awareness of hypoglycemia.
Most children with type 1 diabetes have isolated episodes of severe hypoglycemia; however, a few experience recurrent episodes of severe hypoglycemia. Frequent episodes of hypoglycemia are related to defective counterregulatory hormone responses to subsequent decrease in blood glucose concentrations. Therefore, prior episodes of frequent hypoglycemia are considered as an important risk factor for subsequent severe hypoglycemia (4). Both defective counterregulatory hormone responses and impaired awareness of hypoglycemia cause hypoglycemia-associated autonomic failure related to recurrent hypoglycemia, resulting in subsequent severe hypoglycemia (82–84). In DCCT, analysis of 424 intensively treated patients found that longer duration of diabetes, glycemic control, and prior severe hypoglycemia were related to the occurrence of severe hypoglycemia (85). JDRF reported that the higher rate of severe hypoglycemia was related to severe hypoglycemia that occurred in the last 6 months (86). Therefore, prior episodes of recurrent hypoglycemia can be one of the important predictors of subsequent severe hypoglycemia.
In the 1990s, strict glycemic control was evaluated to affect the frequency of severe hypoglycemia (18), particularly in younger children (19, 24). However, data from 2000 to 2009 in the Western Australian Children's Diabetes Database were analyzed, and there was a decline in the frequency of severe hypoglycemia, and relationship with glycemic control became weaker than previously reported (19, 21, 24). The reduction in the severe hypoglycemia may have resulted from improvement in management of diabetes during the past decades. The correlation between glycemic control and the risk of severe hypoglycemia seems to be weaker, without increased risk of severe hypoglycemia associated with improvement of glycemic control (21, 26–28). The association of a low HbA1c level is not a predictable value for severe hypoglycemia in pediatric patients with type 1 diabetes (27). Therefore, adequate glycemic control can be attained without increasing episodes of severe hypoglycemia.
Coexisting morbidities, including hypothyroidism (87), celiac disease (88), and Addison disease (89, 90), have been reported to be possible risk factors for severe hypoglycemia. The use of a gluten-free diet and adequate treatment of Addison disease and hypothyroidism can decrease the rate of severe hypoglycemia. Rarely, intentional self-administration of insulin to cause hypoglycemia, i.e., factitious hypoglycemia, can introduce recurrent and serious hypoglycemia and should be diagnosed as having psychological problems including eating disorders or psychiatric disease (91).
Urgent treatment is required when severe hypoglycemia occurs and can be effectively reversed by injection of glucagon, which can be administered intravenously, intramuscularly, or subcutaneously (92, 93). Family members and caregivers have difficulties in preparation and administration of glucagon, because glucagon reconstitution with sterile water is required in the current preparations. To resolve these problems, an intranasal glucagon preparation has been tried in children (94) and adults (95) with type 1 diabetes and was revealed to be a promising alternative to intramuscular glucagon. Glucagon cannot be available in areas with limited resources, and in the areas where glucagon may not be available, glucose gel or in powder form is used.
On the other hand, glucose must be administered intravenously more than a few minutes to reverse hypoglycemia. Rapid infusion or excessive concentration (i.e., 50%) can cause an excessive osmotic alteration, leading to hyperosmolar injury of brain (96).
Prevention of severe hypoglycemia remains one of the most critical issues in the management of pediatric patients with type 1 diabetes. Closed-loop system is probably the best technology for prevention of hypoglycemia; however, in the initial step toward closed-loop system, CGM or integrated CGM and insulin pump have enabled patients with type 1 diabetes to further decrease hypoglycemia (4). Possible technologies to prevent the occurrence of severe hypoglycemia and to decrease in the risk factors for developing severe hypoglycemia are shown in Table 1.
Several studies have reported that CGM can reduce hypoglycemic events with a concomitant improvement in HbA1c in patients with type 1 diabetes, regardless of age (97–99). A randomized controlled multicenter study demonstrated reduction in time spent in hypoglycemia concomitant with a decrease in HbA1c in both children and adults with type 1 diabetes (98). A multicenter analysis of 3,553 subjects from the German-Austrian-Swiss-Luxembourgian Diabetes Prospective Follow-up registry demonstrated that initiation and regular use of CGM in children and adolescents with type 1 diabetes were associated with reduction in both diabetic ketoacidosis and severe hypoglycemia with modest improvement in glycemic control (100). Although the use of CGM can decrease the episodes of severe hypoglycemia in adult patients (80, 101), this effect is not elucidated in pediatric patients (102). Moreover, JDRF (66) reported frequent and often prolonged hypoglycemia, particularly during nighttime, in pediatric patients with type 1 diabetes, although using CGM; i.e., hypoglycemic events occurred in 8.5% during nights, and the duration of hypoglycemia over 2 h was 23% of the nights. Adolescents have a high acoustic arousal threshold from sleep (103) and therefore could have severe hypoglycemic events during nighttime (76). Buckingham et al. (104) reported that 71% of youth wearing CGM did not respond to nighttime alarms. On the other hand, Ly et al. (105) reported that CGM with preset alarms improved epinephrine response in adolescents with type 1 diabetes, who had impaired awareness of hypoglycemia and a risk for nocturnal hypoglycemia. This study suggests that CGM might be a useful tool to relieve impaired awareness of hypoglycemia and potently avoid severe hypoglycemia in adolescents with type 1 diabetes.
Intermittently scanned CGM (isCGM; FreeStyle Libre; Abbott Diabetes Care, Alameda, CA, USA) has similar methodology to show continuous glucose measurements as ambulatory glucose profiles retrospectively at the time of checking. Glucose trend can be observed after intermittently scanning the sensor. IsCGM is approved in a number of countries for use, but there were a few clinical studies showing the effect on glycemic control in pediatric patients (106–109). These studies on isCGM have demonstrated a similar effect on maintaining adequate glycemic control as when using CGM, but decrease in the time spent in hypoglycemia seems difficult on multiple daily injections of insulin without using the advanced technologies, such as a sensor-augmented pump with low-glucose suspension or a hybrid closed-loop system (109).
Sensor-augmented pump with low-glucose suspension further decreases the time spent in hypoglycemia and the occurrence of severe hypoglycemia. If the users ignore the alarm sounds, a low-glucose suspend system automatically suspends basal insulin delivery for up to 2 h in response to sensor-detected hypoglycemic events, after which basal insulin delivery is resumed at the programmed rate. A low-glucose suspend system can reduce the time of hypoglycemia, particularly during nighttime (81, 110, 111). This function also decreases moderate to severe hypoglycemic events, particularly in patients with impaired awareness of hypoglycemia (81). Furthermore, hyperglycemia, deterioration in overall glycemic control, and development to ketoacidosis are low frequencies (97, 111).
Predictive low-glucose management system, the MiniMed 640G System (Medtronic, Northridge, CA, USA), suspends basal insulin delivery with the hypoglycemia prediction algorithm. Basal insulin delivery is usually suspended when sensor glucose level is within 3.9 mmol/L (70 mg/dL) above the patient-set low limit and is predicted to be 1.1 mmol/L (20 mg/dL) above this low limit for 30 min. When patients do not interfere, accompanied by the pump suspension, the insulin delivery resumes after the suspend period of 2 h or less at the programmed rate (Figure 2). The use of predictive low-glucose suspension more effectively decreases the rate of hypoglycemia and the risk of severe hypoglycemia in patients with type 1 diabetes (68, 68, 112–117). Buckingham et al. (117) reported that predictive low-glucose suspension prevented hypoglycemia on 75% of nights and in 84% of predicted events in adolescents and young adults with type 1 diabetes. In the study, there was mild ketosis in a few cases when the insulin pump was suspended for 1.5–2 h, and serum ketone bodies returned to normal range with resumption of basal insulin delivery. On the other hand, Abraham et al. (68) demonstrated that predictive low-glucose suspension was related to reduction in hypoglycemia than single use of sensor-augmented pump in a 6-month, multicenter, randomized controlled trial for pediatric patients with type 1 diabetes. This decline was observed both during daytime and nighttime. Episodes of hypoglycemia with a sensor-glucose value <3.5 mmol/L (63 mg/dL) for over 20 min also reduced with predictive low-glucose suspension than single use of sensor-augmented pump. Deterioration of glycemic control was not found in the use of the predictive low-glucose suspension. These findings suggest that a sensor-augmented pump therapy with low-glucose management, especially with predictive low-glucose suspension, is an important promising tool to decrease the frequency in the occurrence of hypoglycemia and the risks for severe hypoglycemia without worsening overall glycemic control in pediatric patients with type 1 diabetes.
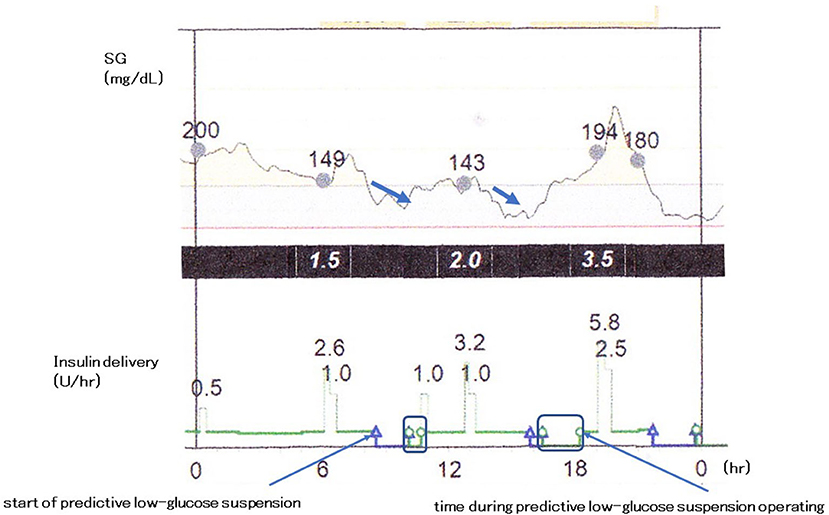
Figure 2. Predictive low-glucose management system. The MiniMed 640G System (Medtronic, Northridge, CA, USA).
In 2016, the Food and Drug Administration approved the first closed-loop system, the MiniMed 670G System (Medtronic), for patients 14 years or older in the United States. Hybrid closed-loop system, commonly referred to as an artificial pancreas, is an automated insulin delivery management, combined with CGM and insulin delivery without patient intervention. The system uses a proprietary proportional-integral-derivative controller with insulin feedback to calculate insulin dosages continually according to CGM levels (118–120). Several studies have been handled on the closed-loop systems and demonstrate improvement of glycemic control with decrease in the rate of hypoglycemia and the occurrence of severe hypoglycemia in both adults and children, especially at nighttime (121–125). In an open-label, randomized, crossover study design, the use of a closed-loop system significantly increased the time spent in the target range [3.9–10 mmol/L (70–180 mg/dL)], whereas the time spent in hypoglycemic range significantly decreased [<3.9 mmol/L (<70 mg/dL)] during both daytime and nighttime in adolescents with type 1 diabetes (Figure 3) (125). The use of hybrid closed-loop system is in general effective and safe particularly at nighttime, and allows enough sleep and reduces the burden of diabetes management during overnight. The closed-loop system must be one of the most promising technologies to attain optimal glycemic control with minimizing the episodes of hypoglycemia, as well as occurrence of severe hypoglycemia. Although the majority of the systems include single-hormone insulin, dual-hormone systems, which infuse both insulin and glucagon, have also been in the research phase (126, 127).
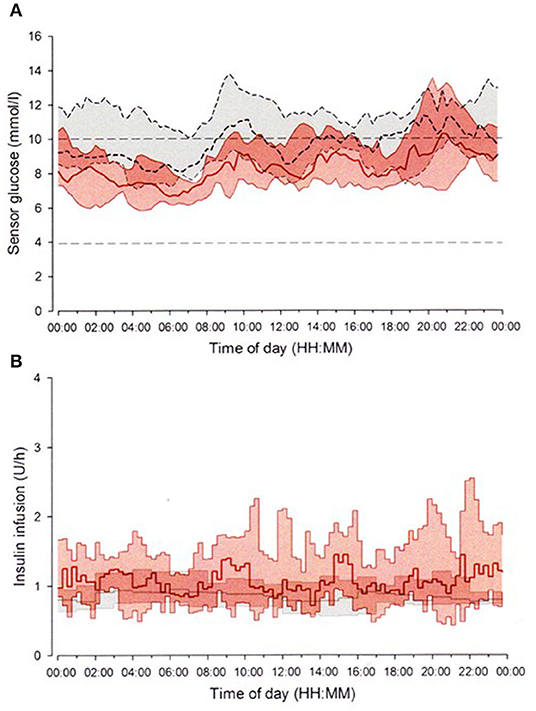
Figure 3. Closed-loop system. The FlorenceD2A closed-loop system (University of Cambridge, Cambridge, UK) (125). Median sensor glucose (A) and insulin delivery (B) during closed-loop insulin delivery period (solid red line and red-shaped area) and sensor-augmented pump period (dashed black line and gray-shaped area) from midnight to midnight. The glucose range 3.9–10.0 mmol/L (70–180 mg/dL) is denoted by horizontal dashed lines (A).
In summary, the incidence of severe hypoglycemia has been markedly declined in recent years, but still a lethal condition. Minimizing the risk factors for development of severe hypoglycemia is an important objective to prevent the occurrence of severe hypoglycemia in pediatric patients with type 1 diabetes. The new concept of time spent within target glucose range (time in range) will be in general used to evaluate the glucose trend and the quality of metabolic control (128, 129). Achieving the target range [3.9–10 mmol/L (70–180 mg/dL)] more than 70% with minimizing severe hypoglycemia <1% is crucial in the management of not only adults but also children and adolescents with type 1 diabetes (128). This can be achieved through advanced diabetes technologies even in pediatric patients.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that this study received funding from Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., Terumo Corp., and JCR Pharmaceuticals Co., Ltd. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endoc Pract. (2008) 14:750–6. doi: 10.4158/EP.14.6.750
2. Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycemia, fear of hypoglycemia and quality of life in children with type 1 diabetes and their parents. Diabet Med. (2013) 30:1126–31. doi: 10.1111/dme.12247
3. American Diabetes Association. Minimizing hypoglycemia in diabetes. Diabetes Care. (2015) 38:1583–91. doi: 10.2337/dc15-0279
4. Abraham MB, Jones TW, Naranjo D, Karges B, Oduwole A, Tauschmann M. ISPAD Clinical Practice Consensus Guidelines 2018: Assessment and management of hypoglycemia in children and adolescents with Diabetes. Pediatr Diabetes. (2018) 19 (Suppl. 27):178–92. doi: 10.1111/pedi.12698
5. Patterson CC, Dahlquist G, Harjutsalo V, Joner G, Feltbower RG, Svensson G, et al. Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia. (2007) 50:2439–42. doi: 10.1007/s00125-007-0824-8
6. Feltbower RG, Bodansky HJ, Patterson CC, Parslow RC, Stephenson CR, Reynolds C, et al. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire register of diabetes in children and young adults. Diabetes Care. (2008) 31:922–6. doi: 10.2337/dc07-2029
7. Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. (2006) 49:298–305. doi: 10.1007/s00125-005-0082-6
8. Ryan CM. Why is cognitive dysfunction associated with the development of diabetes early in life? The diathesis hypothesis. Pediatr Diabetes. (2006) 7:289–97. doi: 10.1111/j.1399-5448.2006.00206.x
9. Gonder-Frederick LA, Zrebiec JF, Bauchowitz AU, Ritterband LM, Magee JC, Cox DJ, et al. Cognitive function is disrupted by both hypo- and hyperglycemia in school-aged children with type 1 diabetes: a field study. Diabetes Care. (2009) 32:1001–6. doi: 10.2337/dc08-1722
10. Lin A, Northam EA, Rankins D, Werther GA, Cameron FJ. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatr Diabetes. (2010) 11:235–43. doi: 10.1111/j.1399-5448.2009.00588.x
11. Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. (2005) 28:2372–7. doi: 10.2337/diacare.28.10.2372
12. Aye T, Reiss AL, Kesler S, Hoang S, Drobny J, Park Y, et al. The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes Care. (2011) 34:1458–62. doi: 10.2337/dc10-2164
13. Ho MS, Weller NJ, Ives FJ, Carne CL, Murray K, vanden Driesen RI, et al. Prevalence of structural central nervous system abnormalities in early-onset type 1 diabetes mellitus. J Pediatr. (2008) 153:385–90. doi: 10.1016/j.jpeds.2008.03.005
14. Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes. (2010) 59:236–41. doi: 10.2337/db09-1117
15. Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, et al. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. (2007) 30:2331–7. doi: 10.2337/dc07-0351
16. DiMeglio LA, Acerini C, Codner E, Craig EM, Hofer SE, Pillay K, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediat Diabetes. (2018) 19 (Suppl. 27):105–14. doi: 10.1111/pedi.12737
17. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin dependent diabetes. Lancet. (1994) 344:283–7. doi: 10.1016/S0140-6736(94)91336-6
18. The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. (1997) 46:271–86. doi: 10.2337/diab.46.2.271
19. Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. (2004) 27:2293–8. doi: 10.2337/diacare.27.10.2293
20. Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. (2002) 287:2511–8. doi: 10.1001/jama.287.19.2511
21. O'Connell SM, Cooper MN, Bulsara MK, Davis EA, Jones TW. Reducing rates of severe hypoglycemia in a population-based cohort of children and adolescents with type 1 diabetes over the decade 2000-2009. Diabetes Care. (2011) 34:2379–80. doi: 10.2337/dc11-0748
22. Karges B, Rosenbauer J, Kapellen T, Wagner VM, Schober E, Karges W, et al. Hemoglobin A1c levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med. (2014) 11:e1001742. doi: 10.1371/journal.pmed.1001742
23. Urakami T, Habu M, Suzuki J. DKA and severe hypoglycemia in management of type 1 diabetes during 2003-2013. Pediatr Int. (2014) 56:940. doi: 10.1111/ped.12521
24. Cherubini V, Pintaudi B, Rossi MC, Lucisano G, Pellegrini F, Chiumello G, et al. Severe hypoglycemia and ketoacidosis over one year in Italian pediatric population with type 1 diabetes mellitus: a multicenter retrospective observational study. Nutr Metab Cardiovasc Dis. (2014) 24:538–46. doi: 10.2337/diacare.20.1.22
25. Maltoni G, Zucchini S, Scipione M, Rollo A, Balsamo C, Bertolini C, et al. Severe hypoglycemic episodes: a persistent threat for children with type 1 diabetes mellitus and their families. J Endocrinol Invest. (2013) 36:617–21. doi: 10.3275/8896
26. Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore Study Group on childhood diabetes. Diabetes Care. (1997) 20:714–20. doi: 10.2337/diacare.20.5.714
27. Fredheim S, Johansen A, Thorsen SU, Kremke B, Nielsen LB, Olsen BS, et al. Nationwide reduction in the frequency of severe hypoglycemia by half. Acta Diabetol. (2015) 52:591–9. doi: 10.1007/s00592-014-0697-5
28. Karges B, Kapellen T, Wagner VM, Steigleder-Schweiger C, Karges W, Holl RW, et al. Glycated hemoglobin A1c as a risk factor for severe hypoglycemia in pediatric type 1 diabetes. Pediatr Diabetes. (2017) 18:51–8. doi: 10.1111/pedi.12348
29. Haynes A, Hermann JM, Miller KM, Hofer SE, Jones TW, Beck RW, et al. Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes. (2017) 18:643–50. doi: 10.1111/pedi.12477
30. Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care. (2008) 31:1892–7. doi: 10.2337/dc07-2132
31. Neguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes: a meta-analysis. J Pediatr Psychol. (2009) 34:271–82. doi: 10.1093/jpepsy/jsn074
32. Cato A, Hershey. Cognition and type 1 diabetes in children and adolescents. Diabetes Spectr. (2016) 29:197–202. doi: 10.2337/ds16-0036
33. Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study. J Pediatr. (1999) 134:503–6. doi: 10.1016/S0022-3476(99)70211-8
34. Blasetti A, Chiuri RM, Tocco AM, Di Giulio C, Mattei PA, Ballone E, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol. (2011) 26:1383–91. doi: 10.1177/0883073811406730
35. Musen G, Jacobson AM, Ryan CM, Cleary PA, Wabersky BH, Weinger K, et al. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the diabetes control and complications trial. Diabetes Care. (2008) 31:1933–8. doi: 10.2337/dc08-0607
36. Strudwick SK, Carne C, Gardiner J, Foster JK, Davis EA, Jones TW. Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr. (2005) 147:680–5. doi: 10.1016/j.jpeds.2005.06.010
37. Ly TT, Anderson M, McNamara KA, Davis EA, Jones TW. Neurocognitive outcomes in young adults with early-onset type 1 diabetes: a prospective follow-up study. Diabetes Care. (2011) 34:2192–7. doi: 10.2337/dc11-0697
38. Schoenle EJ, Schoenle D, Molinari L, Largo RH. Impaired intellectual development in children with type 1 diabetes: association with HbA(1c), age at diagnosis and sex. Diabetologia. (2002) 45:108–14. doi: 10.1007/s125-002-8250-6
39. Semenkovich K, Patel PP, Pollock AB, Beach KA, Neison S, Masterson JJ, et al. Academic abilities and glycaemic control in children and young people with type 1 diabetes mellitus. Diabet Med. (2016) 33:668–73. doi: 10.1111/dme.12854
40. Cato MA, Mauras N, Ambrosino J, Bondurant A, Conrad AI, Kollman C, et al. Diabetes Research in Children Network. Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Soe. (2014) 20:238–47. doi: 10.1017/S1355617713001434
41. Pell GS, Lin A, Wellard RM, Werther GA, Cameron FJ, Finch SJ, et al. Age-related loss of brain volume and T2 relaxation time in youth with type 1 diabetes. Diabetes Care. (2012) 35:513–9. doi: 10.2337/dc11-1290
42. Dafoulas GE, Toulis KA, McCorry D, Kumarendran B, Thomas GN, Willis BH, et al. Type 1 diabetes mellitus and risk of incident epilepsy: a population-based, open-cohort study. Diabetologia. (2017) 60:258–61. doi: 10.1007/s00125-016-4142-x
43. Chou IC, Wang CH, Lin WD, Tsai FJ, Lin CC, Kao CH. Risk of epilepsy in type 1 diabetes mellitus: a population-based cohort study. Diabetologia. (2016) 59:1196–203. doi: 10.1007/s00125-016-3929-0
44. Trico D, Herzog RI. Metabolic brain adaptations to recurrent hypoglycaemia may explain the link between type 1 diabetes mellitus and epilepsy and point towards future study and treatment options. Diabetologia. (2017) 60:938–9. doi: 10.1007/s00125-017-4231-5
45. Harris SB, Khunti K, Landin-Olsson M, Galbo-Jørgensen CB, Bøgelund M, Chubb B, et al. Descriptions of health states associated with increasing severity and frequency of hypoglycemia: a patient-level perspective. Patient Prefer Adherence. (2013) 7:925–36. doi: 10.2147/PPA.S46805
46. Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep. (2016) 16:77. doi: 10.1007/s11892-016-0762-2
47. Barnard KD, Wysocki T, Allen JM, Elleri D, Thabit H, Leelarathna L, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care. (2014) 2:e000025. doi: 10.1136/bmjdrc-2014-000025
48. Martyn-Nemeth P, Schwarz Farabi S, Mihailescu D, Nemeth J, Quinn L. Fear of hypoglycemia in adults with type 1 diabetes: impact of therapeutic advances and strategies for prevention – a review. J Diabetes Complicat. (2016) 30:167–77. doi: 10.1016/j.jdiacomp.2015.09.003
49. Van Name MA, Hilliard ME, Boyle CT, Miller KM, DeSalvo DJ, Anderson BJ, et al. Nighttime is the worst time: parental fear of hypoglycemia in young children with type 1 diabetes. Pediatr Diabetes. (2018) 19:114–20. doi: 10.1111/pedi.12525
50. Haugstvedt A, Wentzel-Larsen T, Graue M, Sovik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med. (2010) 27:72–8. doi: 10.1111/j.1464-5491.2009.02867.x
51. Hawkes CP, McDarby V, Cody D. Fear of hypoglycemia in parents of children with type 1 diabetes. J Paediatr Child Health. (2014) 50:639–42. doi: 10.1111/jpc.12621
52. Abraham MB, Heels K, Nicholas JA, Cole C, Gebert R, Klimek G, et al. Unexpected management behaviors in adolescents with type 1 diabetes using sensor-augmented pump therapy. J Diabetes Sci Technol. (2018) 12:592–8. doi: 10.1177/1932296817752188
53. Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics. (1985) 75:921–7.
54. Hagen JW, Barklay CR, Anderson BJ, Feeman DJ, Segal SS, Bacon G, et al. Intellective functioning and strategy use in children with insulin-dependent diabetes mellitus. Child Dev. (1990) 61:1713–27. doi: 10.2307/1130833
55. Holmes CS, Richman LC. Cognitive profiles of children with insulin-dependent diabetes. J Dev Behav Pediatr. (1985) 6:323–6. doi: 10.1097/00004703-198512000-00001
56. Matyka KA, Crowne EC, Havel PJ, Macdonald IA, Matthews D, Dunger DB. Counterregulation during spontaneous nocturnal hypoglycemia in prepubertal children with type 1 diabetes. Diabetes Care. (1999) 22:1144–50. doi: 10.2337/diacare.22.7.1144
57. Ly TT, Jones TW. Managing hypoglycemia in children: what the clinician needs to know before advising parents. Diabetes Manage. (2012) 2:503–12. doi: 10.2217/dmt.12.73
58. Jones TW, Porter P, Sherwin RS, Davis EA, O'Leary P, Frazer F, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. (1998) 338:1657–62. doi: 10.1056/NEJM199806043382303
59. Monaghan MC, Hilliard ME, Cogen FR, Streisand R. Nighttime caregiving behaviors among parents of young children with type 1 diabetes: associations with illness characteristics and parent functioning. Fam Syst Health. (2009) 27:28–38. doi: 10.1037/a0014770
60. Beregszaszi M, Tubiana-Rufi N, Benali K, Noel M, Bloch J, Czernichow P. Nocturnal hypoglycemia in children and adolescents with insulin-dependent diabetes mellitus: prevalence and risk factors. J Pediatr. (1997) 131:27–33. doi: 10.1016/S0022-3476(97)70121-5
61. Matyka KA, Wigg L, Pramming S, Stores G, Dunger DB. Cognitive function and mood after profound nocturnal hypoglycaemia in prepubertal children with conventional insulin treatment for diabetes. Arch Dis Child. (1999) 81:138–42. doi: 10.1136/adc.81.2.138
62. Kaufman FR, Austin J, Neinstein A, Jeng L, Halvorson M, Devoe DJ, et al. Nocturnal hypoglycemia detected with the continuous glucose monitoring system in pediatric patients with type 1 diabetes. J Pediatr. (2002) 141:625–30. doi: 10.1067/mpd.2002.129175
63. Wilson DM, Calhoun PM, Maahs DM, Chase HP, Masser L, Buckingham BA, et al. Factors associated with nocturnal hypoglycemia in at-risk adolescents and young adults with type 1 diabetes. Diabetes Technol Ther. (2015) 17:385–91. doi: 10.1089/dia.2014.0342
64. Buckingham BA, Cameron F, Calhoun P, Maahs DM, Wilson DM, Chase HP, et al. Outpatient safety assessment of an in-home predictive low-glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther. (2013) 15:622–7. doi: 10.1089/dia.2013.0040
65. Porter PA, Keating B, Byrne G, Jones TW. Incidence and predictive criteria of nocturnal hypoglycemia in young children with insulin-dependent diabetes mellitus. J Pediatr. (1997) 130:366–72. doi: 10.1016/S0022-3476(97)70197-5
66. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care. (2010) 33:1004–8. doi: 10.2337/dc09-2081
67. Willi SM, Planton J, Egede L, Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr. (2003) 143:796–801. doi: 10.1067/S0022-3476(03)00579-1
68. Abraham MB, Nicholas JA, Smith GJ, Fairchild JM, King BR, Ambler GR, et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care. (2018) 41:303–10. doi: 10.2337/dc17-1604
69. Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. (2013) 369:224–32. doi: 10.1056/NEJMoa1303576
70. Graveling AJ, Frier BM. Impaired awareness of hypoglycaemia: a review. Diabetes Metab. (2010) 36 (Suppl. 3):S64–74. doi: 10.1016/S1262-3636(10)70470-5
71. Barkai L, Vamosi I, Lukacs K. Prospective assessment of severe hypoglycaemia in diabetic children and adolescents with impaired and normal awareness of hypoglycaemia. Diabetologia. (1998) 41:898–903. doi: 10.1007/s001250051005
72. Ly TT, Gallego PH, Davis EA, Jones TW. Impaired awareness of hypoglycemia in a population-based sample of children and adolescents with type 1 diabetes. Diabetes Care. (2009) 32:1802–6. doi: 10.2337/dc09-0541
73. Abraham MB, Gallego PH, Brownlee WM, Smith GJ, Davis EA, Jones TW. Reduced prevalence of impaired awareness of hypoglycemia in a population-based clinic sample of youth with type 1 diabetes. Pediatr Diabetes. (2017) 18:729–33. doi: 10.1111/pedi.12460
74. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. (1994) 17:697–703. doi: 10.2337/diacare.17.7.697
75. Korytkowski MT, Mokan M, Veneman TF, Mitrakou A, Cryer PE, Gerich JE. Reduced beta-adrenergic sensitivity in patients with type 1 diabetes and hypoglycemia unawareness. Diabetes Care. (1998) 21:1939–43. doi: 10.2337/diacare.21.11.1939
76. Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of nocturnal hypoglycemia before seizures. Diabetes Care. (2008) 31:2110–2. doi: 10.2337/dc08-0863
77. Jones TW, Boulware SD, Kraemer DT, Caprio S, Sherwin RS, Tamborlane WV. Independent effects of youth and poor diabetes control on responses to hypoglycemia in children. Diabetes. (1991) 40:358–63. doi: 10.2337/diab.40.3.358
78. Amiel SA, Pottinger RC, Archibald HR, Chusney G, Cunnah DT, Prior PF, et al. Effect of antecedent glucose control on cerebral function during hypoglycemia. Diabetes Care. (1991) 14:109–18. doi: 10.2337/diacare.14.2.109
79. Amiel SA, Gale E. Physiological responses to hypoglycemia. Counterregulation and cognitive function. Diabetes Care. (1993) 16 (Suppl. 3):48–55. doi: 10.2337/diacare.16.3.48
80. van Beers CA, DeVries JH, Kleijer SJ, Smits MM, Geelhoed-Duijvestijn PH, Kramer MH, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. (2016) 4:893–902. doi: 10.1016/S2213-8587(16)30193-0
81. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. (2013) 310:1240–7. doi: 10.1001/jama.2013.277818
82. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. (2005) 54:3592–601. doi: 10.2337/diabetes.54.12.3592
83. Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic human. Diabetes. (1991) 40:223–6. doi: 10.2337/diab.40.2.223
84. Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recurrent antecedent hypoglycemia reduces autonomic response to symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. (1993) 91:819–28. doi: 10.1172/JCI116302
85. The DCCT Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med. (1991) 90:450–9.
86. Juvenile Diabetes Research Foundations Continuous Glucose Monitoring Study Group. Factors predictive of severe hypoglycemia in type 1 diabetes. Diabetes Care. (2011) 34:586–90. doi: 10.2337/dc10-1111/-/DCI
87. Mohn A, Di Michele S, Di Luzio R, Tumini S, Chiarelli F. The effect of subclinical hypothyroidism on metabolic control in children and adolescents with type 1 diabetes mellitus. Diabet Med. (2002) 19:70–3. doi: 10.1046/j.1464-5491.2002.00635.x
88. Mohn A, Cerruto M, Iafusco D, Prisco F, Tumini S, Stoppoloni O, et al. Celiac disease in children and adolescents with type I diabetes: importance of hypoglycemia. J Pediatr Gastroenterol Nutr. (2001) 32:37–40. doi: 10.1097/00005176-200101000-00012
89. McAulay V, Frier BM. Addison's disease in type 1 diabetes presenting with recurrent hypoglycaemia. Postgrad Med J. (2000) 76:230–2. doi: 10.1136/pmj.76.894.230
90. Likhar T, Maqzoub S, Griffiths MJ, Buch HN. Screening for Addison's disease in patients with type 1 diabetes mellitus and recurrent hypoglycaemia. Postgrad Med J. (2007) 83:420–1. doi: 10.1136/pgmj.2007.058321
91. Boileau P, Aboumrad B, Bougneres P. Recurrent comas due to secret self-administration of insulin in adolescents with type 1 diabetes. Diabetes Care. (2006) 29:430–1. doi: 10.2337/diacare.29.02.06.dc05-1845
92. Chung ST, Haymond MW. Minimizing morbidity of hypoglycemia in diabetes: a review of mini-dose glucagon. J Diabetes Sci Technol. (2015) 9:44–51. doi: 10.1177/1932296814547518
93. Pearson T. Glucagon as a treatment of severe hypoglycemia: safe and efficacious but underutilized. Diabetes Educ. (2008) 34:128–34. doi: 10.1177/0145721707312400
94. Sherr JL, Ruedy KJ, Foster NC, Piché CA, Dulude H, Rickels MR, et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. (2016) 39:555–62. doi: 10.2337/dc15-1606
95. Rickels MR, Ruedy KJ, Foster NC, Piché CA, Dulude H, Sherr JL, et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. (2016) 39:264–70. doi: 10.2337/dci16-0025
96. Wood SP. Is D50 too much of a good thing? A reappraisal of the safety of 50% dextrose administration in patients with hypoglycemia. JEMS. (2007) 32:103–6. doi: 10.1016/S0197-2510(07)70090-6
97. Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Eng J Med. (2010) 363:311–20. doi: 10.1056/NEJMoa1002853
98. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. (2011) 34:795–800. doi: 10.2337/dc10-1989
99. Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. (2012) 55:3155–62. doi: 10.1007/s00125-012-2708-9
100. Tauschmann M, Hermann JM, Freiberg C, Papsch M, Thon A, Heidtmann B, et al. Reduction in diabetic ketoacidosis and severe hypoglycemia in pediatric type 1 diabetes during the first year of continuous glucose monitoring: A multicenter analysis of 3,553 subjects from the DPV registry. Diabetes Care. (2020) 43:e40–2. doi: 10.2337/dc19-1358
101. Choudhary P, Ramasamy S, Green L, Gallen G, Pender S, Brackenridge A, et al. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care. (2013) 36:4160–2. doi: 10.2337/dc13-0939
102. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. (2008) 359:1464–76. doi: 10.1056/NEJMoa0805017
103. Ly TT, Jones TW, Griffiths A, Dart J, Davis EA, Stick S, et al. Hypoglycemia does not change the threshold for arousal from sleep in adolescents with type 1 diabetes. Diabetes Technol Ther. (2012) 14:101–4. doi: 10.1089/dia.2011.0144
104. Buckingham B, Block J, Burdick J, Kalajian A, Kollman C, Choy M, et al. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. (2005) 7:440–7. doi: 10.1089/dia.2005.7.440
105. Ly TT, Hewitt J, Davev RJ, Lim EM, Davis EA, Jones TW. Improving epinephrine responses in hypoglycemia unawareness with real-time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care. (2011) 34:50–2. doi: 10.2337/dc10-1042
106. Campbell F, Kordonouri O, Murphy N, Stewart C. FreeStyle libre use for self-management of diabetes in children and adolescents. In: Program of 77th American Diabetes Association Scientific Sessions. 110-LB. San Diego, CA (2017).
107. Dunn TC, Xu Y, Hayter G, Ajjan R. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. (2018) 37:37–45. doi: 10.1016/j.diabres.2017.12.015
108. Edge J, Acerini C, Campbell F, Hamilton-Shield J, Moudiotis C, Rahman S, et al. An alternative sensor-based method for glucose monitoring in children and young people with diabetes. Arch Dis Child. (2017) 102:543–9. doi: 10.1136/archdischild-2016-311530
109. Urakami T, Yoshida K, Kuwabara R, Mine Y, Aoki M, Suzukli J, et al. Individualization of recommendations from the international consensus on continuous glucose monitoring-derived metrics in Japanese children and adolescents with type 1 diabetes. Endocr J. (2020). doi: 10.1507/endocrj.EJ20-0193
110. Choudhary P, Shin J, Wang Y, Evans MN, Hammond PJ, Kerr D, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. (2011) 34:2023–5. doi: 10.2337/dc10-2411
111. Ly TT, Nicholas JA, Retterath A, Davis EA, Jones TW. Analysis of glucose responses to automated insulin suspension with sensor-augmented pump therapy. Diabetes Care. (2012) 35:1462–5. doi: 10.2337/dc12-0052
112. Abraham MB, Davey R, O'Grady MJ, Ly TT, Paramalingam N, Fournier PA, et al. Effectiveness of a predictive algorithm in the prevention of exercise-induced hypoglycemia in type 1 diabetes. Diabetes Technol Ther. (2016) 18:543–50. doi: 10.1089/dia.2016.0141
113. Abraham MB, de Bock M, Paramalingam N, O'Grady MJ, Ly TT, George C, et al. Prevention of insulin-induced hypoglycemia in type 1 diabetes with predictive low glucose management system. Diabetes Technol Ther. (2016) 18:436–43. doi: 10.1089/dia.2015.0364
114. Danne T, Tsioli C, Kordonouri O, Blaesig S, Remus K, Roy A, et al. The PILGRIM study: in silico modeling of a predictive low glucose management system and feasibility in youth with type 1 diabetes during exercise. Diabetes Technol Ther. (2014) 16:338–47. doi: 10.1089/dia.2013.0327
115. Biester T, Kordonouri O, Holder M, Remus K, Kieninger-Baum D, Wadien T, et al. “Let the algorithm do the work”: reduction of hypoglycemia using sensor-augmented pump therapy with predictive insulin suspension (SmartGuard) in pediatric type 1 diabetes patients. Diabetes Technol Ther. (2017) 19:173–82. doi: 10.1089/dia.2016.0349
116. Choudhary P, Olsen BS, Conget I, Welsh JB, Vorrink L, Shin JJ. Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther. (2016) 18:288–91. doi: 10.1089/dia.2015.0324
117. Buckingham B, Chase H, Dassau E, Cobry E, Clinton P, Gage V, et al. Prevention of nocturnal hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Care. (2010) 33:1013–7. doi: 10.2337/dc09-2303
118. Shah VN, Shoskes A, Tawfik B, Garg SK. Closed-loop system in the management of diabetes: past, present, and future. Diabetes Technol Ther. (2014) 16:477–90. doi: 10.1089/dia.2014.0193
119. Kropff J, DeVries JH. Continuous glucose monitoring, future products, and update on worldwide artificial pancreas projects. Diabetes Technol Ther. (2016) 18 (Suppl. 2):S253–63. doi: 10.1089/dia.2015.0345
120. Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. (2016) 59:1795–805. doi: 10.1007/s00125-016-4022-4
121. Ruiz JL, Sherr JL, Cengiz E, Carria L, Roy A, Voskanyan G, et al. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol. (2012) 6:1123–30. doi: 10.1177/193229681200600517
122. Steil GM. Algorithms for a closed-loop artificial pancreas: the case for proportional-integral-derivative control. J Diabetes Sci Technol. (2013) 7:1621–31. doi: 10.1177/193229681300700623
123. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. (2008) 31:934–9. doi: 10.2337/dc07-1967
124. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Kovatchev BA, Kudva Y, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. (2019) 381:1707–17. doi: 10.1056/NEJMoa1907863
125. Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. (2016) 39:1168–74. doi: 10.2337/dc15-2078
126. Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. (2014) 371:313–25. doi: 10.1056/NEJMoa1314474
127. Russell SJ, Hillard MA, Balliro C, Magyar KL, Selagamsetty R, Sinha M, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. (2016) 4:233–43. doi: 10.1016/S2213-8587(15)00489-1
128. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. (2019) 42:1593–603. doi: 10.2337/dci19-0028
Keywords: severe hypoglycemia, children and adolescents, type 1 diabetes, risk factor, advanced technology
Citation: Urakami T (2020) Severe Hypoglycemia: Is It Still a Threat for Children and Adolescents With Type 1 Diabetes? Front. Endocrinol. 11:609. doi: 10.3389/fendo.2020.00609
Received: 31 January 2020; Accepted: 27 July 2020;
Published: 15 September 2020.
Edited by:
Valentino Cherubini, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyReviewed by:
Stefano Zucchini, Sant'Orsola-Malpighi Polyclinic, ItalyCopyright © 2020 Urakami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tatsuhiko Urakami, dXJha2FtaS50YXRzdWhpa29Abmlob24tdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.