
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 08 September 2020
Sec. Thyroid Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00511
This article is part of the Research Topic Risk-benefit Considerations and Staging of Differentiated Thyroid Cancer View all 16 articles
 Shuai Xue1†
Shuai Xue1† Li Zhang2†
Li Zhang2† Renzhu Pang1
Renzhu Pang1 Peisong Wang1
Peisong Wang1 Meishan Jin3
Meishan Jin3 Liang Guo3
Liang Guo3 Yuhua Zhou3
Yuhua Zhou3 Bingfei Dong1*
Bingfei Dong1* Guang Chen1*
Guang Chen1*Background: Papillary thyroid carcinoma (PTC) patients with anterior extrathyroidal extension (ETE) involving the strap muscle have a relatively better prognosis than those with posterior gross ETE involving the recurrent laryngeal nerve. Whether prophylactic central-compartment lymph node dissection (CLND) should be performed in PTCs with only strap muscle invasion (SMI) is still unclear.
Methods: A retrospective cohort study was conducted in clinical N0 (cN0) PTC patients with SMI who underwent thyroid surgery from 2009 to 2017. A total of 152 patients were included, and predictive factors of central-compartment lymph node metastasis (CLNM) were determined.
Results: Among the 281 PTCs patients with SMI, 152 (51.1%) did not clinically present with lymph node metastasis. Microscopic CLNM was identified in 77 (50.7%) cN0 PTC patients with SMI. According to the univariate and multivariate analyses, male patients and those aged <40 years were more likely to be diagnosed with CLNM than female patients and those aged >40 years (odds ratio [OR] = 6.22 [95% confidence interval (CI), 1.43–27.10], p = 0.02 vs. OR = 9.94 [95% CI, 2.79–35.44], p = 0.00). The CLNM positive rate of male patients aged <40 years was 87.5%, while that for female patients aged ≥55 years was 23.8%. However, risk factors associated with large-volume CLNM were not identified because of the small number of patients.
Conclusions: Taken together, nearly half of PTC patients with SMI did not clinically present with lymph node metastasis. Male sex and patients aged <40 years were identified as the predictive factors of CLNM in cN0 PTCs with SMI. Hence, the results of this single-center study raise the possibility that prophylactic CLND may be more often considered for younger male PTC patients with SMI.
The incidence of papillary thyroid carcinoma (PTC) has significantly increased worldwide during the past decades (1, 2). Central-compartment lymph node metastasis (CLNM), considered a poor clinical feature, is associated with the prognosis of PTC (3, 4). Patients with >5 lymph node metastasis (LNM), are associated with structural recurrence, distant metastasis, and mortality (4, 5), even despite being micrometastases. Therefore, prophylactic central-compartment lymph node dissection (CLND) was recommended for T3 or T4 primary tumors, or if the information of LNM would be used to plan further treatment strategies, like completion thyroidectomy or radioiodine ablation (5). However, the sensitivity and specificity of ultrasonography (US) for diagnosing CLNM is poor (6, 7). Therefore, several preoperative clinical factors of CLNM for PTC have been identified by several studies, suggesting that high-risk PTCs require aggressive treatment (8, 9). However, the conflicting results from these studies contribute to different therapeutic strategies for PTC (10).
Extrathyroidal extension (ETE) is defined as a tumor spread outside of the thyroid and into the surrounding tissues (11). The degree of extrathyroidal extension (ETE) in PTC plays a significant role in recurrence and mortality (11). The American Thyroid Association guidelines recommend CLND in PTCs with gross ETE (5). Along with the degree of gross ETE, the site of the tumor gross invasion is also associated with disease outcome. However, only strap muscle invasion (SMI) has an effect on the prognosis of PTC, which has already been confirmed by a substantial amount of evidence (12–15). It was reported that DTC patients with only gross SMI had the same recurrent rate as those with microscopic ETE (12–14). Patients with anterior SMI have a relatively better prognosis compared with patients with posterior gross ETE involving the recurrent laryngeal nerve or esophagus because SMI can be easily resected with negative margins (16, 17). Hence, whether prophylactic CLND should only be performed in PTCs with only SMI is still unclear.
This study aimed to evaluate CLNM in clinical N0 (cN0) PTC with only SMI by performing a retrospective analysis of our clinical PTC cohort and identifying the clinicopathological features to predict CLNM, which may guide physicians in planning further treatment strategies.
This retrospective study was approved by the Institutional Review Board of the First Hospital of Jilin University, and the need for informed consent was waived. A total of 9,866 consecutive PTC patients from January 2009 to July 2017 who underwent surgery at our department were analyzed retrospectively. The inclusion criteria for patient selection were as follows: (1) patient information found in a hospital database; (2) postoperative pathological diagnosis of conventional PTC with SMI (without other gross ETEs); and (3) absence of suspicious cervical lymph nodes observed during US, computed tomography (CT), and/or fine-needle aspiration (FNA) preoperatively (cN0). The exclusion criteria were as follows: (1): age <18 years, (2) history of neck radiotherapy, and (3) history of previous thyroid surgery. Finally, 152 PTC patients with SMI were enrolled in our study. The flowchart of patient selection is shown in Figure 1.
The majority of PTCs were identified by US examination, which was performed to evaluate thyroid tumor and neck lymph nodes by a trained radiologist (Y Yin) and surgeons preoperatively (SX, RP, and J Liu). FNA was recommended in patients with suspicious thyroid nodules ≥5 mm. For suspicious thyroid nodules <5 mm, after providing a full explanation of the potential risks and benefits of surgery, patients' decision on whether to undergo surgery was considered. Suspicious SMI was previously defined by US according to the following criteria: (1) a tumor was located in the anterior portion of the thyroid and (2) the thyroid capsule was disrupted by the growing tumor, or >25% of the tumor perimeter was abutting the thyroid capsule. All patients with suspicious SMI underwent neck-enhanced CT to further evaluate the SMI and cervical lymph nodes. FNA for suspicious lymph nodes was recommended when the largest diameter of the cervical lymph node was >0.8 cm and when patients presented with ≥1 malignant US/CT features (microcalcifications, cystic aspect, peripheral vascularity, hyperechogenicity, and rounded shape). The total thyroid and invaded strap muscle were dissected. Bilateral prophylactic CLND was performed (18). Radioactive iodine and thyroid-stimulating hormone-suppressive hormonal therapy were recommended to postoperative patients according to the established guidelines (5).
Histological specimens were examined and reviewed (19). Histopathological characteristics, including the largest tumor diameter (LTD), location of the tumor, ETE, presence of Hashimoto thyroiditis (HT), and LNM (the number and diameter), were recorded. The concordance rate between the two pathologists for the 152 enrolled patients in this study was 100%.
Nominal variables were described as a frequency with a percentage and a mean with a standard deviation for continuous variables. To identify the differences between groups for specific variables, the Statistical Package for the Social Sciences (SPSS) version 22 software (SPSS Inc., Chicago, IL) was used for statistical analysis. Nominal variables and continuous variables were assessed by performing Pearson's chi-squared test and Mann-Whitney U-test. A logistic regression model was used to evaluate the risk factors for CLNM and high-volume CLNM (≥5-mm metastatic central lymph nodes). A p < 0.05 was considered statistically significant (two-sided).
Among the 281 PTC patients with SMI, 152 (51.1%) did not clinically present with lymph node metastasis. The baseline clinicopathological and genetic characteristics of the 152 PTC patients with SMI are summarized in Table 1. A total of 136 (89.5%) patients were female, and the average age of all patients was 46.9 years. Ninety-five (62.5%) patients presented with a solitary tumor, while a total of 57 (37.5%) patients presented with multifocal tumors. Microscopic CLNM was identified in 77 (50.7%) cN0 PTC patients with SMI.
To identify the risk factors for CLNM in cN0 PTCs with SMI, univariate analysis for variables associated with CLNM was performed, including sex, age, bilaterality, multifocality, location of the tumor, LTD, and HT. According to the univariate and multivariate analyses, males and patients aged <40 years were more likely to be diagnosed with CLNM (odds ratio [OR] = 6.22 [95% confidence interval (CI), 1.43–27.10], p = 0.02; OR = 9.94 [95% CI, 2.79–35.44], p = 0.00) than females and patients aged >40 years, as shown in Table 2. The CLNM positive rate of male patients aged <40 years was 87.5%, while it was only 23.8% in female patients aged ≥55 years, as indicated in Figure 2.
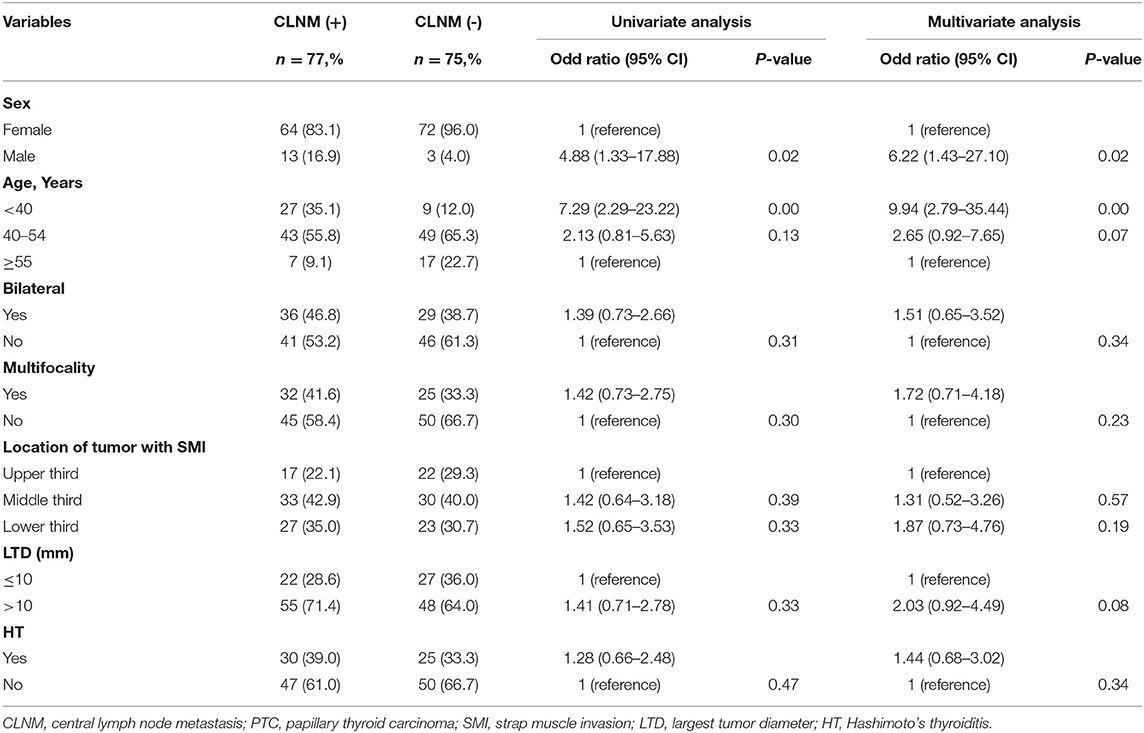
Table 2. Univariate and multivariate analysis of clinicopathological characteristics for CLNM in cN0 PTCs with SMI.
To identify the risk factors for large-volume CLNM in cN0 PTCs with SMI, univariate analysis for variables associated with large-volume CLNM was performed, including sex, age, bilaterality, multifocality, location of the tumor, LTD, and HT. According to the univariate and multivariate analyses, risk factors associated with large-volume CLNM were not identified, as shown in Table 3.
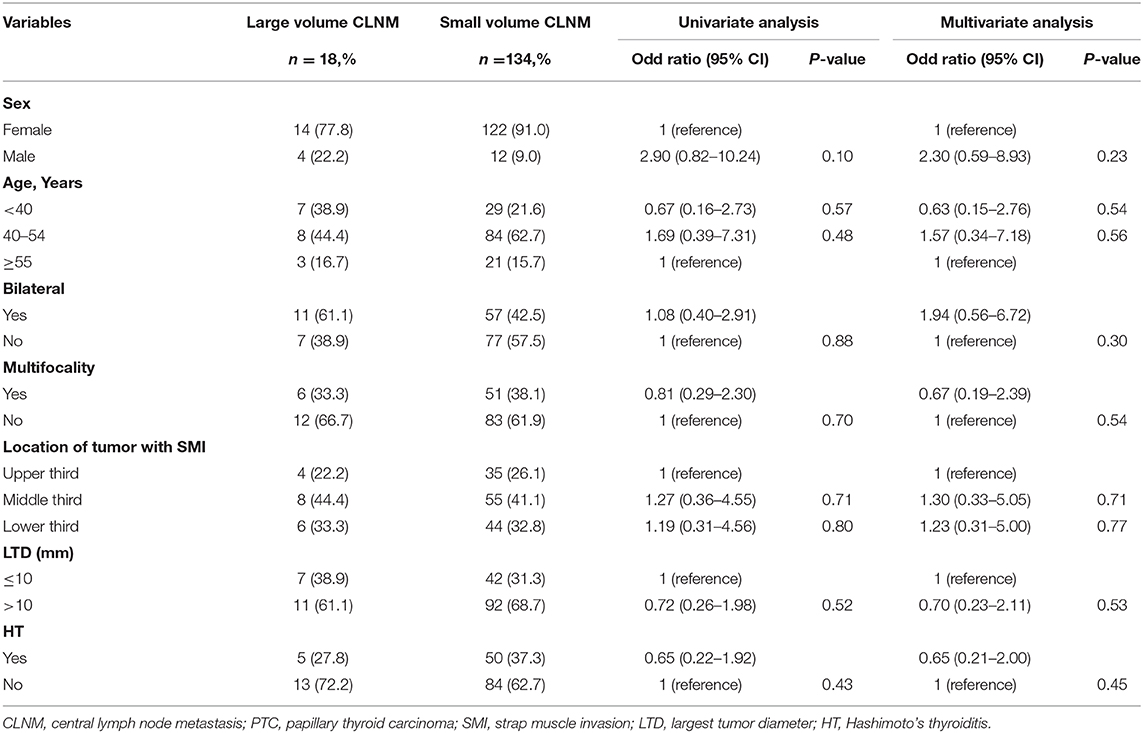
Table 3. Univariate and multivariate analysis of clinicopathological characteristics for large volume CLNM in cN0 PTCs with SMI.
To the best of our knowledge, this is the first paper to determine the risk factors for CLNM in cN0 PTCs with SMI. In our 152 PTC patients with SMI, only 77 of the 152 patients (50.7%) presented with microscopic CLNM. Moreover, only 18 patients (11.8%) were reported to have large-volume CLNM (≥5-mm metastatic central lymph nodes). Furthermore, male sex and patients aged <40 years were identified as the risk factors for CLNM in cN0 PTCs with SMI.
It has been well-known that prognosis is worse in patients with gross ETE than in those with microscopic local invasion (11). However, SMI has little effect on the outcome of PTC patients, which has already been confirmed by a substantial amount of evidence (12, 16). Moran et al. reported that non-metastatic differentiated thyroid cancer patients with SMI shared similar locoregional recurrence-free rates with those with microscopic ETE (20). Compared with patients with gross ETE in the trachea, esophagus, and recurrent laryngeal nerve, patients with anterior SMI had a relatively better outcome because the invaded strap muscle can be resected easily with negative margins (17, 21, 22). Accordingly, some researchers recommended that the actual effects of SMI should be reevaluated and revised in future staging systems.
Whether routine prophylactic CLND should only be performed in PTCs with only SMI has remained controversial. The potential benefits of performing routine prophylactic CLND, such as better risk stratification of recurrence according to micrometastatic central lymph nodes and lower thyroglobulin levels after operation, should be balanced by the potential risks, such as permanent hypoparathyroidism (23, 24). Hence, a number of researchers believed that prophylactic CLND was an optimal treatment for cN0 PTCs with risk factors (25). Additionally, we previously summarized a total of 1,555 cN0 PTC patients and identified that male sex and younger age were considered as the risk factors for CLNM (25). Andrew MT also reported that younger age and male sex were the strongest predictive factors for CLNM in PTC cases, which is consistent with our results (26). Regarding papillary thyroid microcarcinoma (PTMC), multiple studies have also found that male sex and younger age were associated with CLNM (27, 28). Moreover, large-volume CLNM was more frequently observed in younger and male PTMC patients than in older and female PTMC patients (29). Considering the significant number of debates on prophylactic CLND, it is recommended only in PTC patients with some risk factors. In our study, only 50.7% of cN0 PTC patients with SMI presented with microscopic CLNM. Furthermore, the CLNM positive rate of male patients aged <40 years was 87.5%. Accordingly, prophylactic CLND may be more often considered for younger male PTC patients with SMI.
This study has several limitations. First, this study was a retrospective single-center study, which may limit the generalization of the findings on a broader scale because of selection bias. Hence, prospective studies with a randomized controlled selection might be required. Second, considering the small number of cN0 PTC with SMI, predictive factors may be generated using a multivariate logistic regression model with some biases. Finally, the difference of disease outcome between SMI patients with and without CLNM was not reported in our study because of the lack of follow-up data. Despite these limitations, our study was the first study to analyze the risk factors of CLNM in cN0 PTC with SMI. Furthermore, sex and age can be easily identified preoperatively. It may have potential significant implications for prophylactic CLND in cN0 PTCs with SMI.
In conclusion, nearly half of PTC patients with SMI did not clinically present with lymph node metastasis. Male sex and patients aged <40 years were identified as the predictive factors of CLNM in cN0 PTCs with SMI. The results of this single-center study suggest the possibility that prophylactic CLND may be more often considered for younger male PTC patients with SMI.
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to Guang Chen, amlkYXlpeXVhbmp6eEBzaW5hLmNvbQ==.
The studies involving human participants were reviewed and approved by The Institutional Review Board of the 1st Hospital of the Jilin University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This study was supported by the Natural Science Foundation of Jilin Province (20180101138JC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
2. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
3. Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. (2005) 90:5723–9. doi: 10.1210/jc.2005-0285
4. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. (2012) 22:1144–52. doi: 10.1089/thy.2012.0043
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
6. Kim E, Park JS, Son K-R, Kim J-H, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. (2008) 18:411–8. doi: 10.1089/thy.2007.0269
7. Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. (2008) 32:1552–8. doi: 10.1007/s00268-008-9588-7
8. Zhang L, Wei W-j, Ji Q-h, Zhu Y-x, Wang Z-y, Wang Y, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1,066 patients. J Clin Endocrinol Metab. (2012) 97:1250–7. doi: 10.1210/jc.2011-1546
9. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. (2010) 148:526–31. doi: 10.1016/j.surg.2010.01.003
10. Barczynski M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. (2013) 100:410–8. doi: 10.1002/bjs.8985
11. Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid. (2014) 24:241–4. doi: 10.1089/thy.2012.0567
12. Song E, Lee YM, Oh HS, Jeon MJ, Song DE, Kim TY, et al. A relook at the T stage of differentiated thyroid carcinoma with a focus on gross extrathyroidal extension. Thyroid. (2019) 29:202–8. doi: 10.1089/thy.2018.0300
13. Tam S, Amit M, Boonsripitayanon M, Busaidy NL, Cabanillas ME, Waguespack SG, et al. Effect of tumor size and minimal extrathyroidal extension in patients with differentiated thyroid cancer. Thyroid. (2018) 28:982–90. doi: 10.1089/thy.2017.0513
14. Park SY, Kim HI, Kim JH, Kim JS, Oh YL, Kim SW, et al. Prognostic significance of gross extrathyroidal extension invading only strap muscles in differentiated thyroid carcinoma. Br J Surg. (2018) 105:1155–62. doi: 10.1002/bjs.10830
15. Jung SP, Kim M, Choe JH, Kim JS, Nam SJ, Kim JH. Clinical implication of cancer adhesion in papillary thyroid carcinoma: clinicopathologic characteristics and prognosis analyzed with degree of extrathyroidal extension. World J Surg. (2013) 37:1606–13. doi: 10.1007/s00268-013-2034-5
16. Shaha AR. Extrathyroidal extension-what does it mean. Oral Oncol. (2017) 68:50–2. doi: 10.1016/j.oraloncology.2017.03.008
17. Wang LY, Nixon IJ, Patel SG, Palmer FL, Tuttle RM, Shaha A, et al. Operative management of locally advanced, differentiated thyroid cancer. Surgery. (2016) 160:738–46. doi: 10.1016/j.surg.2016.04.027
18. Xue S, Wang P, Zhang Q, Yin Y, Guo L, Wang M, et al. Routine lateral level V dissection may not be necessary for papillary thyroid microcarcinoma with lateral lymph node metastasis: a retrospective study of 252 cases. Front Endocrinol. (2019) 10:558. doi: 10.3389/fendo.2019.00558
19. Xue S, Zhang L, Wang P, Liu J, Yin Y, Jin M, et al. Predictive factors of recurrence for multifocal papillary thyroid microcarcinoma with Braf(v600e) mutation: a single center study of 1,207 Chinese patients. Front Endocrinol. (2019) 10:407. doi: 10.3389/fendo.2019.00407
20. Amit M, Boonsripitayanon M, Zafereo ME. ASO author reflections: strap muscle invasion does not influence recurrence and survival in patients with differentiated thyroid cancer. Ann Surg Oncol. (2018) 25:892–3. doi: 10.1245/s10434-018-6816-8
21. Price DL, Wong RJ, Randolph GW. Invasive thyroid cancer: management of the trachea and esophagus. Otolaryngol Clin North Am. (2008) 41:1155–68. doi: 10.1016/j.otc.2008.08.002
22. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. (2018) 68:55–63. doi: 10.3322/caac.21439
23. Henry JF, Gramatica L, Denizot A, Kvachenyuk A, Puccini M, Defechereux T. Morbidity of prophylactic lymph node dissection in the central neck area in patients with papillary thyroid carcinoma. Langenbeck's Archiv Surg. (1998) 383:167–9. doi: 10.1007/s004230050111
24. Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. (2010) 148:1100–6. doi: 10.1016/j.surg.2010.09.019
25. Xue S, Wang P, Liu J, Li R, Zhang L, Chen G. Prophylactic central lymph node dissection in cN0 patients with papillary thyroid carcinoma: a retrospective study in China. Asian J Surg. (2016) 39:131–6. doi: 10.1016/j.asjsur.2015.03.015
26. Thompson AM, Turner RM, Hayen A, Aniss A, Jalaty S, Learoyd DL, et al. A preoperative nomogram for the prediction of ipsilateral central compartment lymph node metastases in papillary thyroid cancer. Thyroid. (2014) 24:675–82. doi: 10.1089/thy.2013.0224
27. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. (2016) 26:807–15. doi: 10.1089/thy.2015.0429
28. Kim JY, Jung EJ, Park T, Jeong SH, Jeong CY, Ju YT, et al. Impact of tumor size on subclinical central lymph node metastasis in papillary thyroid microcarcinoma depends on age. World J Surg Oncol. (2015) 13:88. doi: 10.1186/s12957-015-0478-9
Keywords: predictive factors, central-compartment lymph node metastasis, clinical N0, papillary thyroid carcinoma, strap muscle invasion
Citation: Xue S, Zhang L, Pang R, Wang P, Jin M, Guo L, Zhou Y, Dong B and Chen G (2020) Predictive Factors of Central-Compartment Lymph Node Metastasis for Clinical N0 Papillary Thyroid Carcinoma With Strap Muscle Invasion. Front. Endocrinol. 11:511. doi: 10.3389/fendo.2020.00511
Received: 14 December 2019; Accepted: 25 June 2020;
Published: 08 September 2020.
Edited by:
Christoph Reiners, University Hospital Würzburg, GermanyReviewed by:
Yuri Nikiforov, University of Pittsburgh, United StatesCopyright © 2020 Xue, Zhang, Pang, Wang, Jin, Guo, Zhou, Dong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingfei Dong, ZG9uZ2JpbmdmZWlAaG90bWFpbC5jb20=; Guang Chen, amlkYXlpeXVhbmp6eEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.