- 1Research Division, Department of Biochemistry and Molecular Biology, Dasman Diabetes Institute, Kuwait City, Kuwait
- 2Research Division, Department of Genetics and Bioinformatics, Dasman Diabetes Institute, Kuwait City, Kuwait
- 3Department of Human Genetics, McGill University, Montreal, QC, Canada
- 4King Hussein Cancer Center, Amman, Jordan
Melanocortin 4 receptor (MC4R), a notable component of the melanocortin system, regulates appetite, body weight, and energy homeostasis. Genome-wide association studies have identified several MC4R variants associated with adiposity; of these, rs17782313, which is associated with increased body mass index (BMI) and overeating behavior, is of particular interest. Another gene associated with increased adiposity in global genome-wide association studies is DNAJC27, a heat shock protein known to be elevated in obesity. The detailed mechanisms underlying the role of MC4R variants in the biological pathways underlying metabolic disorders are not well-understood. To address this, we assessed variations of rs17782313 in a cohort of 282 Arab individuals from Kuwait, who are deeply phenotyped for anthropometric and metabolic traits and various biomarkers, including DNAJC27. Association tests showed that the rs17782313_C allele was associated with BMI and DNAJC27 levels. Increased levels of DNAJC27 reduced the MC4R-mediated formation of cAMP in MC4R ACTOne stable cells. In conclusion, this study demonstrated an association between the rs17782313 variant near MC4R and increased BMI and DNAJC27 levels and established a link between increased DNAJC27 levels and lower cAMP levels. We propose that regulation of MC4R activity by DNAJC27 enhances appetite through its effect on cAMP, thereby regulating obesity.
Introduction
Obesity has become a global epidemic and is increasing at an alarming rate worldwide, especially in the Arabian Peninsula region. Obesity can dramatically affect an individual's quality of life and is associated with increased risk of developing metabolic diseases, including diabetes, hypertension, and cardiovascular complications.
The leptin–melanocortin pathway is commonly dysregulated in obesity. The melanocortin system integrates neural, hormonal, and metabolic signals (1). Its action is mediated by melanocortins, a group of peptide hormones that include adrenocorticotropic hormone (ACTH), melanocyte-stimulating hormone (MSH), and Agouti-related peptide (AgRP) (2–4). These hormones can bind to and activate a group of melanocortin receptors (MCRs) that belong to the family of G protein-coupled receptors (GPCRs). To date, five melanocortin receptors (MC1R through MC5R) have been cloned and characterized and shown to have distinct distribution patterns and physiological functions (5). MC3R and MC4R, which are mainly expressed in the central nervous system, are often referred to as neural MCRs. Both play a key role in regulating energy homeostasis, as has been demonstrated using both agonists and antagonists for the receptors, especially for MC4R, confirming its physiological role. In addition, MC4R-knockout mice display obesity, hyperphagia, hyperinsulinemia, and hyperglycemia, providing further evidence that MC4R has a role in the regulation of energy homeostasis (2, 6, 7). The action of MC4R is mediated through two main populations of neurons that regulate feeding behavior: the anorexigenic pro-opiomelanocortin (POMC)/CART neurons and the orexigenic AgRP/neuropeptide Y (NPY) neurons. These neurons are expressed in the arcuate nucleus of the hypothalamus and function in opposition to each other. POMC is the prohormone for MSH and ACTH. POMC neurons project into the paraventricular hypothalamus (PVH) and release MSH, which in turn binds to and activates MC4R on the PVH neurons. This activation results in blocking the appetite and reducing food intake. Conversely, AgRP neurons project into the PVH releasing AgRP, which blocks MC4R. This results in the activation of appetite and an increase in food intake (1, 3, 8).
MC4R signaling is coupled to the three main heterotrimeric G proteins: Gs (stimuli), Gi (inhibition), and Gq (2, 4, 9, 10). When MC4R couples to Gs, this activates a cyclic adenosine monophosphate (cAMP)-dependent pathway (11), which results in the activation of adenylyl cyclase (AC) and the subsequent release of cAMP and the activation of protein kinase A (PKA). Conversely, when MC4R couples to Gi, this inhibits AC, whereas coupling to Gq results in the stimulation of phospholipase C (PLC), leading to the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). IP3 results in the release of Ca2+ from intracellular stores while DAG activates PKC. MC4R signaling via G protein-independent pathways has also been demonstrated, including via the mitogen-activated protein kinase (MAPK) pathway to activate ERK1/2 and JNK phosphorylation, and through coupling to the inwardly rectifying potassium channel, Kir7.1 (12–14).
Multiple variants within and near the MC4R gene have been shown to be associated with metabolic diseases; indeed, these have been reported to be the most frequent genetic cause of obesity in humans, with more than 150 variants reported in patients with various ethnic origins (2, 10). Variants in MC4R have been reported in 3%−5% of early onset or severe adult obesity cases (15). MC4R has often been used as a target for obesity treatment because variants that result in decreased MC4R activity are associated with obesity (6, 16, 17). In addition, MC4R loss-of-function variants have been shown to affect an individual's ability to maintain weight reduction after exercise (18). A study on newborns showed that the variant rs17782313 is associated with changes in BMI over the first 2 weeks of life, and with body weight and BMI at the age of 2 weeks (19). Other genetic studies have suggested that some single nucleotide polymorphisms (SNPs) of MC4R influence the success of bariatric surgery (10, 20, 21). Furthermore, a recent study based on data from 0.5 million individuals in the UK Biobank demonstrated that gain-of-function MC4R variants were associated with protection against obesity (22). The MC4R rs17782313 variant is located 188 kb downstream of the gene and has been shown to have the strongest association with body mass index and obesity risk in a number of populations (23). Even though this variant is downstream of the MC4R gene by 188 kb, disruption of transcriptional control of the MC4R has been proposed as the likely functional mechanism for the variant (23). The GTeX database reports this variant as upregulating the expression of MC4R in tissues including testis, ovary, and brain (basal ganglia). The MC4R rs17782313 variant has also been shown to be associated with an increased risk of type 2 diabetes mellitus (T2D) in a meta-analysis that included more than 100,000 individuals; remarkably, this association was independent of BMI (24). There has been some inconsistency in the previous studies regarding this variant's role in diabetes and this could be attributed to discrepancy in study design, sample size and the population under investigation. It's also not very clear how it could be increasing the risk for developing diabetes. One possibility is that the MC4R gene is involved in regulating insulin resistance since animal studies revealed that MC4R knockout mice have 2-fold higher plasma insulin than their controls (25). However, further studies are still needed to understand this effect.
Additionally, data from the GWAS catalog showed that MC4R rs17782313-T was associated with height in Europeans (26) and East Asians (27) and MC4R rs17782313-C was associated with increased BMI (in Europeans) (23, 28), obesity (in Europeans, both children and adults) (29), physical activity measurement, and BMI (in African Americans and Europeans) (30).
The gene DNAJC27/RBJ has also been shown by GWAS to be associated with increased BMI (31). This gene is located near the adenylyl cyclase 3 (ADCY3) gene, which has been shown to interact with MC4R (32). DNAJC27/RBJ, a member of the heat shock protein (HSP) 40 family involved in the heat shock response pathway, plays an essential role in the pathology of insulin resistance and T2D. We recently demonstrated that DNAJC27 levels are elevated in PBMCs, plasma, and adipose tissue of individuals with obesity and T2D and that it is downregulated in obese participants after exercise (33, 34). We also reported that DNAJC27 was positively associated with obesity biomarkers such as leptin and resistin (34).
Little is known about the impact of MC4R variants on the biological pathways implicated in metabolic diseases or about the involvement of DNAJC27 protein, which could constitute an important target for understanding the functional role of MC4R in metabolic diseases. Given the important role of rs17782313 in regulating MC4R activity, the aim of this study was to evaluate the prevalence of this variant in a Kuwaiti cohort and to determine its role in regulating novel obesity biomarkers, especially DNAJC27.
Materials and Methods
Study Population
The study population comprised 282 participants resident in Kuwait. Their age, sex, comorbidities (such as diabetes and cardiovascular complications), baseline characteristics including height, weight, and waist circumference (WC) were recorded at enrolment. Participants with BMI > 30 kg/m2 were classified as obese. Diabetes and hypertension status were self-reported by participants. The study protocol was reviewed and approved by the Ethical Review Committee of Dasman Diabetes Institute and was conducted in accordance with the guidelines of the Declaration of Helsinki and the US Federal Policy for the Protection of Human Subjects. All participants signed an informed consent form before participating in the study.
Sample Processing
The collection of blood samples was performed in accordance with established institutional guidelines. Participants were requested to fast for 8 h overnight prior to blood sample collection and samples were collected in the morning between 8 and 11 am. DNA was extracted using a Gentra Puregene kit (Qiagen, Valencia, CA, USA) and quantified using Quant-iT PicoGreen dsDNA Assay Kits (Life Technologies, Grand Island, NY, USA) and an Epoch Microplate Spectrophotometer (BioTek Instruments). Absorbance values at 260–280 nm were checked for adherence to an optical density range of 1.8–2.1.
Anthropometric Measurements and Blood Biochemistry
The BMI of each participant was calculated as the ratio of their weight (kg) to height (m) squared. Fasting blood glucose (FBG) and lipid profiles, including triglyceride (TGL), low density lipoprotein (LDL), high density lipoprotein (HDL), and total cholesterol, were measured using a Siemens Dimension RXL integrated chemistry analyzer (Diamond Diagnostics, Holliston, MA, USA). HbA1c levels were determined using a Variant testing system (Bio-Rad, Hercules, CA, USA). C-peptide, leptin, adiponectin, ghrelin, and visfatin were measured using bead-based multiplexing technology on a Bio-Plex system (Bio-Rad, Hercules, CA, USA). We used the Human Diabetes 10-Plex kit (for C-peptide, leptin, ghrelin, and visfatin) and the 2-Plex Pro Human Diabetes kit (for adiponectin and adipsin) (Bio-Rad, Hercules, CA, USA). The average intra-assay coefficient of variation for all analytes was 4.25% with a range of 3.0–6.0%, and the average inter-assay coefficient of variation for all analytes was 3.75% with a range of 2.0–6.0%. HsCRP secreted levels were measured using an ELISA kit (Biovender, USA). The intra-assay coefficient of variation was 4.0–7.0%, and the inter-assay coefficient of variation was 5.5–6.3%.
Genotyping
The TaqMan Genotyping Assay on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) was used to perform the candidate SNP genotyping. Each polymerase chain reaction sample comprised 10 ng of DNA, 5× FIREPol Master Mix (Solis BioDyne, Estonia), and 1 μl of 20× TaqMan SNP Genotyping Assay, with the thermal cycling conditions set at 60°C for 1 min and 95°C for 15 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The genotypes determined by these techniques were confirmed for selected cases of homozygotes and heterozygotes by Sanger sequencing using a BigDye Terminator v3.1 Cycle Sequencing Kit on an 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
Measurement of Plasma Levels of DNAJC27 Using ELISA
Plasma levels of DNAJC27 were measured using an ELISA kit (Wuhan EIAab Science Co., Wuhan, China). The plasma samples were thawed on ice and then centrifuged for 5 min at 10,000 × g at 4°C to remove any remaining cells or platelets. The samples were then diluted by a factor of four with sample diluent. ELISA was performed in accordance to kit instructions. Briefly, the samples and standards were loaded onto the assay plate and incubated for 2 h at 37°C, then washed and incubated for 1 h at 37°C, successively followed by the addition of the conjugated antibody and then streptavidin. Finally, the plate was incubated with TMB substrate for 30 min at 37°C, the reaction was stopped using acidic stop solution, and the absorbance was measured using a Synergy H4 plate reader at a wavelength of 450 nm. All the reagents used were provided in the kit. The intra-assay coefficient of variation was 3.0–5.0%, and the inter-assay coefficient of variation was 3.5–6.0%. According to the manufacturer's manual, recovery was determined by spiking various levels of DNAJC27 into serum and plasma and they reported 92% recovery in serum and 94% recovery in plasma.
Cell Culture
MC4R ACTOne stable cells (e.Enzyme, Gaithersburg, Maryland, USA) which are a HEK-293-CNG cell line expressing a recombinant human MC4R and a modified cyclic nucleotide gated channel that opens in response to elevated intracellular cAMP levels were used in all in vitro assays. They were grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 250 μg/ml G418, and 1 μg/ml puromycin. The cells were plated on 10-cm dishes 24 h prior to transfection. All experiments were performed on 75–80% confluent plates. Transient transfections were performed using Lipofectamine 2000, following the manufacturer's instructions, with 3 μg of each construct. An empty pCMV vector was used as a control. pCMV and DNAJC27 were purchased from Origene (Herford, Germany). The expression vector for DNAJC27 is pCMV. At 18 h post-transfection, 70,000 cells were re-seeded into 96-well plates coated with poly-D-lysine and left to attach overnight. A membrane potential assay was performed the following day.
cAMP Formation
cAMP formation was measured using a membrane potential assay kit (e.Enzyme, Gaithersburg, Maryland, USA), following the manufacturer's instructions. The cells were starved in Dulbecco's phosphate-buffered saline for 1 h at 37°C and then incubated with 1X dye-loading solution for 2 h at room temperature in the dark. The cells were then stimulated with increasing concentrations of NDP-α-MSH (Sigma, Taufkirchen, Germany) or setmelanotide (MedChemExpress, Sollentuna, Sweden) for 10 min at 37°C. Setmelanotide was used because of its specificity to MC4R. The fluorescence was measured with a Synergy H4 plate reader with excitation at 530/20 nm and emission at 590/20 nm. The percentage of cAMP formation was calculated after correcting for the non-stimulated cells and normalizing to the maximum response to the highest agonist concentration (3 μM).
Quality Assessment of SNP
The PLINK genome association analysis toolset version 1.9 was used to assess the quality and statistical association of the rs17782313 SNP. Quality assessments including minor allele frequency (MAF) and consistency with the Hardy–Weinberg equilibrium were performed for the MC4R variant. Any quantitative trait values <Q1–1.5 × the interquartile range (IQR) or >Q3+1.5 × IQR were considered to be outliers and excluded from the statistical analysis.
Statistical Analysis
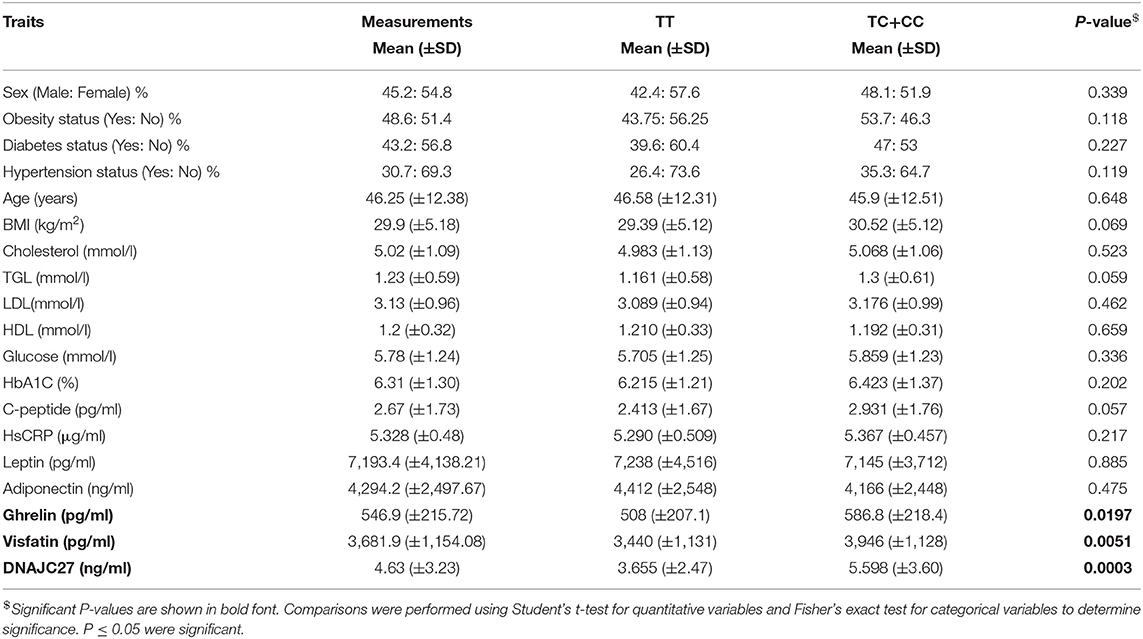
Data are expressed as mean ± standard deviation (SD) and comparisons were performed using Student's t-test for quantitative variables and Fisher's exact test for categorical variables to determine significance. P ≤ 0.05 were significant. Allele-based associations between the rs17782313 variant and the three binary traits of disease status (obesity, diabetes, and hypertension) were evaluated using a logistic regression model adjusted for age and sex. In this model, the logarithm of the odds of each disease was used as response variable, with additive combinations of the explanatory variables such as alleles and the covariates of age and sex in the models as its predictors to estimate probability of minor allele being risk for disease. The risk of disease status due to the effect allele was assessed by the odds ratio and standard error of the odds ratio. Further, allele-based associations between the rs17782313 variant and each of the quantitative traits were evaluated using a linear regression model adjusted for age and sex. In this model, quantitative trait was used as response variable, with additive combinations of the explanatory variables such as alleles and any covariates (age, sex) in the model as its predictors. The change in the mean of phenotype measurement was assessed by regression coefficient (Beta), where a positive regression coefficient means that the minor allele increases risk effect. Associations were considered statistically significant when the P ≤ 0.05.
Results
Characteristics of the Study Participants and Genotyping Data
The rs17782313 variant near MC4R has previously been reported to be associated with obesity in various populations, but not yet in a Kuwaiti population. We therefore investigated the presence of this polymorphism in a population of 282 people living in Kuwait. Table 1 presents an overview of the study population and their clinical and anthropometric parameters. The minor allele frequency (MAF) for the MC4R loci in our sample for rs17782313 was 28%. The average rate of successful genotyping across this SNP was >99%, and all the markers were in Hardy–Weinberg equilibrium. Among the 282 samples that were genotyped, 22 (7.8%) were homozygote for C, 146 (51.77%) were homozygote for T, and 114 (40.43%) were heterozygote (TC).
The population was classified into two groups based on their genotyping profile: SNP non-carriers (TT) and SNP carriers (TC+CC). The mean BMI was marginally higher in the participants that harbored the C allele than in the non-carriers (30.52 ± 5.12 kg/m2 vs. 29.39 ± 5.19 kg/m2; P = 0.0691) (Figure 1A). Table 2 summarizes the results of allele-based association tests that assessed the risk for obesity in individuals carrying the effect allele (C allele). This showed that the C allele was significantly associated with the obesity status of the participants with an odds ratio of 1.5 and a P-value of 0.05. Furthermore, the C allele was seen associated with the hypertension status of the participants with an odds ratio of 1.6 and a P-value of 0.04.
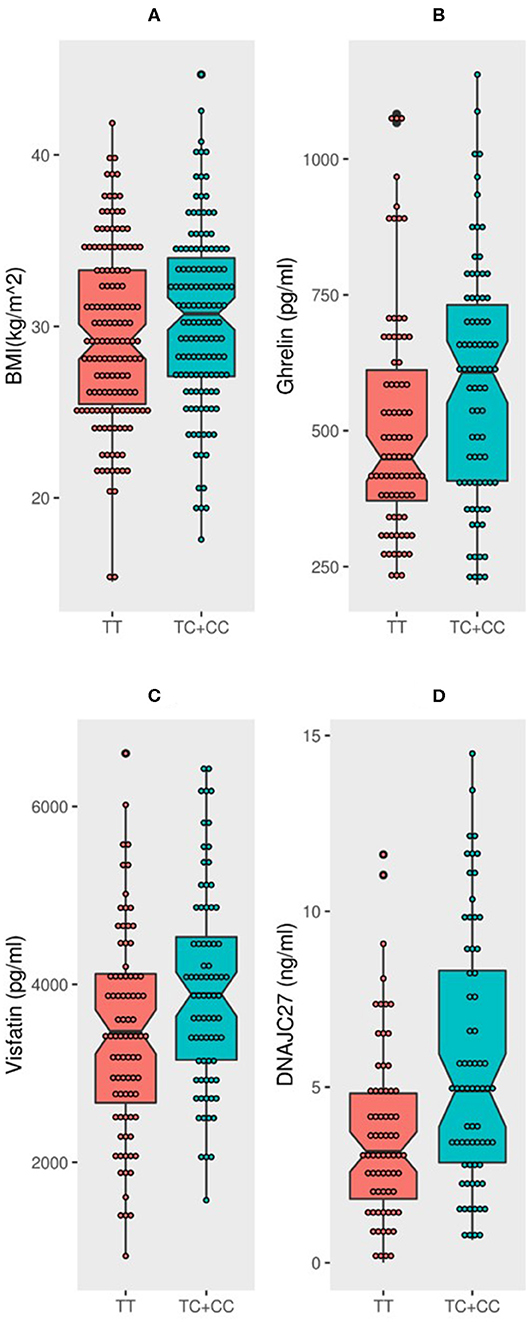
Figure 1. MC4R rs17782313 carriers have significantly higher BMI, DNAJC27, ghrelin, and visfatin than non-carriers: Boxplot analysis of (A) BMI, (B) Ghrelin, (C) Visfatin, and (D) DNAJC27 plasma levels in participants based on their genotype.
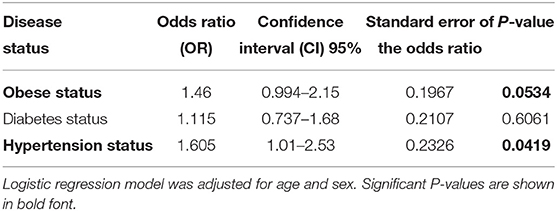
Table 2. Allele-based association tests to assess risk for disease status in individuals carrying the effect allele (C allele).
The participants with the carrier genotypes had significantly higher levels of some obesity markers. Ghrelin levels were significantly higher in those with the TC or CC genotypes compared to those with the TT genotype (586.8 ± 218 pg/ml and 508 ± 207 pg/ml, respectively; P-value = 0.0197) (Figure 1B). Similarly, the SNP carriers (TC+CC) had higher levels of visfatin than the TT carriers (3,946 ± 1,128 pg/ml vs. 3,440 ± 1,131 pg/ml; P-value = 0.0051) (Figure 1C). Interestingly, we observed a highly significant increase in plasma DNAJC27 levels in the rs17782313 carriers compared to the non-carriers (5.598 ± 3.602 ng/ml vs. 3.655 ± 2.473 ng/ml; P-value = 0.0003) (Figure 1D). The levels of leptin and adiponectin were comparable between the rs17782313 carriers and non-carriers (Table 1) and no association was observed between the SNP and the two hormones (Table 3).
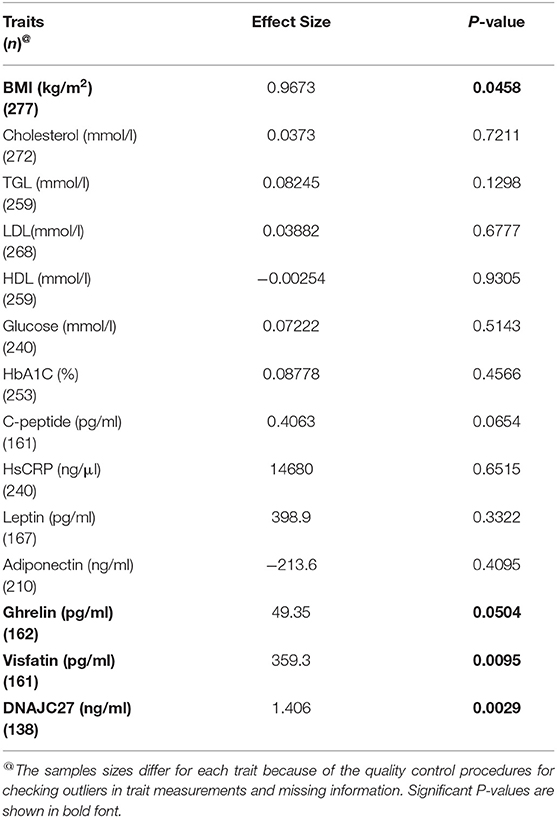
Table 3. Results of association tests between the rs17782313 and the quantitative phenotype traits. Linear regression model was adjusted for age and sex.
Association Between rs17782313 Near MC4R and Obesity-Related Markers
The association tests for the variant and traits or markers showed that four traits were significantly associated with the MC4R rs17782313 variant (Table 3): BMI (P-value = 0.046), DNAJC27 (P-value = 0.003), ghrelin (P-value = 0.05), and visfatin (P-value = 0.009).
Effect of DNAJC27 Expression on MC4R-Mediated cAMP Formation
To elucidate the association between DNAJC27 and MC4R and the underlying mechanism, we investigated the effect of the overexpression of DNAJC27 on MC4R signaling. We performed membrane potential assay to measure the formation of cAMP in the stable HEK-MC4R cell line transiently transfected with either pCMV (as an empty vector control) or DNAJC27 and treated for 10 min with increasing concentrations of NDP-α-MSH. When DNAJC27 was overexpressed, there was a significant reduction in the efficacy of NDP-α-MSH-stimulated cAMP signaling shown by a rightwards shift in the dose–response curve (Figure 2A) and higher EC50 value of 6.06 nM in the DNAJC27-transfected cells compared to 4.08 nM in the control pCMV-transfected cells (Figure 2C). Similar results were observed when setmelanotide (another selective MC4R agonist, also known as BIM-493 and RM-493), was used (Figures 2B,D); in this case, the EC50 value for the DNAJC27-transfected cells was 26.72 nM compared to 18.62 nM for the control pCMV-transfected cells.
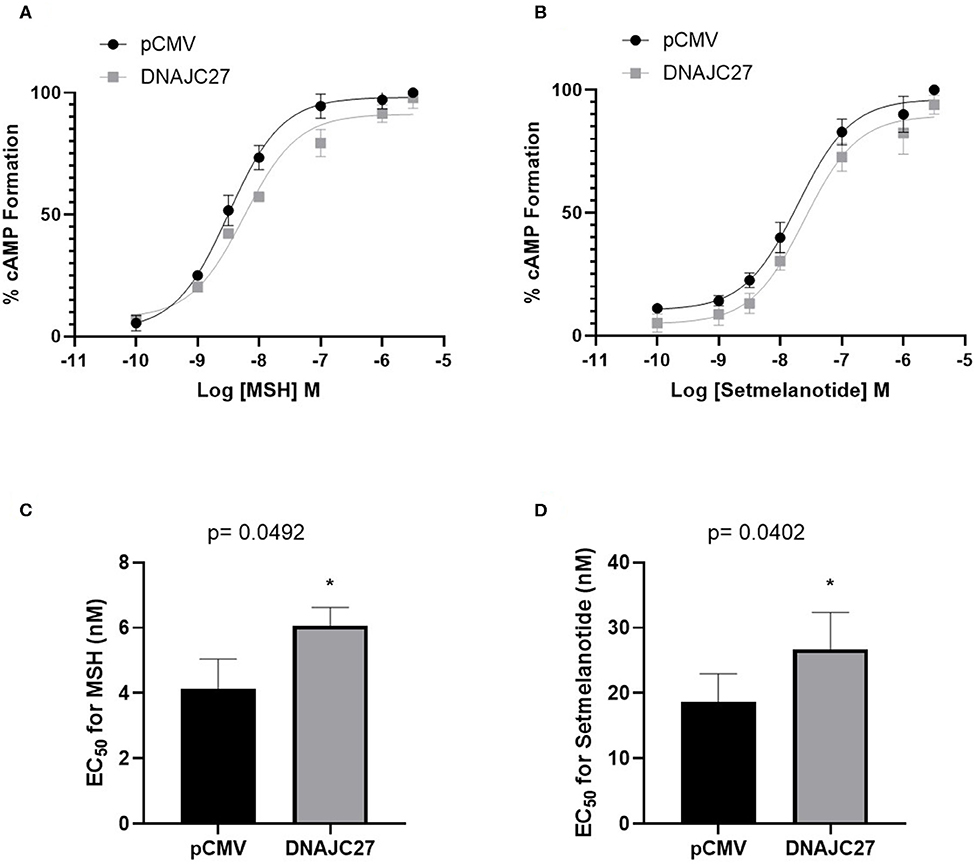
Figure 2. Overexpression of DNAJC27 can reduce MC4R-mediated cAMP formation: Dose response for MC4R-stimulated cAMP formation assessed using membrane potential assay. (A) MSH and (B) Setmelanotide-mediated cAMP formation in either pCMV or DNAJC27- transfected HEK-MC4R cells. EC50 values from stimulation with (C) MSH or (D) Setmelanotide. Data are representative of three independent experiments. *P ≤ 0.05 vs. empty vector control.
Discussion
The results of this study showed that the rs17782313 variant near MC4R was associated with hypertension, obesity, and high plasma levels of ghrelin and visfatin in a cohort of Arabic participants. The variant was also associated with elevated plasma DNAJC27/RBJ levels. In an independent experiment using cell lines, we have tested the impact of increased DNAJC27 on MC4R. In this cell line model, we showed that the overexpression of DNAJC27 resulted in decreased cAMP production by the in vitro activation of wild-type MC4R.
Several studies have reported the association of MC4R variants with obesity in various ethnic groups (23, 28–30). Recent association studies have begun to investigate MC4R polymorphisms in Arabs. A study in 2019 showed that MC4R rs17782313 was associated with obese polycystic ovary syndrome in the Western region of Saudi Arabia (35), and another study from Saudi Arabia showed rs17782313 to be associated with moderate obesity (36). The mechanism for such associations is usually discussed in terms of inducing appetite (37); it has also been proposed that appetite-determining genes might have a greater effect in Arab individuals (38). The present study showed a modest increase in BMI in individuals with carrier genotypes of the rs17782313 variant near MC4R, although the allele-based association tests showed a significant risk for obesity, with an odds ratio of 1.5. The MC4R rs17782313 variant was also significantly associated with BMI. These observations are notable considering that most of the study participants were classified as obese (the mean BMI of the participants was 29.9 ± 5.18 kg/m2). Our findings support the results of the previous studies on MC4R variants and reveal a novel association between DNAJC27 levels and the MC4R variant.
In the 1000 Genomes Project populations, the minor allele frequency of the rs17782313_T>C variant was 0.24. Data from the PAGE study (39) showed a variation in frequency across different populations, with the highest value of 0.36 observed in South Asians and the lowest value of 0.109 observed in Central Americans. The allele frequency of the variant among the participants in the present study was 0.2801, which is comparable to that previously reported for Africans (0.278) and African Americans (0.2795). In our cohort, TGL levels were higher in the participants with the carrier genotypes (TC+CC) compared to those with the TT genotype at close to statistical significance with borderline P-value of 0.0586. It would be interesting to investigate further the association between MC4R and lipid profile in a larger population, especially because previous studies have demonstrated that the MC4R rs17782313 variant was associated with visceral and subcutaneous fat distribution in a Chinese population (40) and a Polish population (41). Furthermore, a mechanistic study that explored the effect of lipid stress on MC4R trafficking in hypothalamic neuronal cells showed that treatment with palmitate inhibited the clathrin-mediated endocytosis of MC4R and impaired its desensitization (42). The present study also showed strong associations between the MC4R rs17782313 variant and the obesity-related proteins ghrelin and visfatin. The participants who carried the MC4R genotypes (TC+CC) had significantly higher levels of ghrelin and visfatin compared to those carrying the TT genotype. The increase in ghrelin levels was expected since this variant is associated with obesity and probably affects the functionality of MC4R which is known to regulate appetite. In fact, the GTeX database reports the rs17782313 variant as upregulating the expression of MC4R in tissues including testis, ovary, and brain. Since both ghrelin and visfatin are also obesity-related hormones, it is not surprising to see them at higher circulating levels in individuals with the carrier genotypes. Several studies suggest that MC4R regulates glucose homeostasis and insulin resistance (2, 43), therefore, visfatin might be involved in this regulation but further studies are still required to understand the mechanism governing those MC4R functions.
Taken together, our results support a potential role of the MC4R rs17782313 variant in hypertension and obesity. The role of ghrelin in the hypothalamic control of appetite is well-established since ghrelin decreases the activity of POMC neurons and increases the activity of AgRP neurons (3, 44). However, and to the best of our knowledge, no previous studies have investigated the effect of MC4R function on the secretion of ghrelin or visfatin. There are also no reports of an association between MC4R rs17782313 and ghrelin or visfatin levels. Only few studies have evaluated the impact of carrier genotypes on the levels of the markers examined in our study. For example, Brodowski et al. (45) reported significantly higher triglyceride levels in MC4R polymorphism carriers (C/X genotype) compared to those with the TT genotype in a population of non-morbid premenopausal women with obesity. Arrizabalaga et al. (46) reported no difference in serum ghrelin levels between those who carried the MC4R polymorphism (TC+CC) compared to those with the TT genotype. These discrepancies with our results may be due to ethnic differences as well as study design and population. For example, both previous studies limited the participants to females and while Brodowski et al. investigated postmenopausal women, Arrizabalaga et al. looked at premenopausal women. However, further studies with larger cohorts are needed for a more conclusive outcome.
In our previous studies of DNAJC27 in the context of obesity and T2D (33, 34), we demonstrated a positive association between DNAJC27 and the common obesity biomarkers leptin and resistin, and also that DNAJC27 levels were elevated in PBMCs, plasma, and adipose tissue from individuals with obesity and T2D. In the present study, the participants carrying the MC4R rs17782313_C allele had significantly higher plasma levels of DNAJC27 than the other participants. Because of the novelty of this finding, as well as the location of the DNAJC27 gene in close proximity to ADCY3, a known regulator of the MC4R response, we investigated the effect of DNAJC27 on MC4R activity, measured by cAMP formation. The overexpression of DNAJC27 in HEK-MC4R cells reduced the efficacy of the MC4R-mediated formation of cAMP by two MC4R agonists, NDP-α-MSH and setmelanotide. Previous studies have reported that the activity of MC4R is a key modulator of food intake; stimulating MC4R induced satiety whereas blocking MC4R increased the appetite (1, 3, 8). Furthermore, loss-of-function genetic variants of MC4R are associated with obesity whereas gain-of-function genetic variants are protective against obesity (22). Our findings suggest that higher levels of DNAJC27 can reduce wild-type MC4R signaling in vitro, however, we still cannot make a direct link between the circulating levels of DNAJC27 and MC4R signaling in the hypothalamus and further studies are now required to understand how DNAJC27 could affect the appetite and the eventual development of obesity.
DNAJC27/RBJ has previously been shown to have a general effect in regulating the MAPK pathway through the accumulation of active MEK1/2 in the nucleus, which results in the activation of ERK1/2 (47). MC4R can also signal through the MAPK pathway, leading to ERK1/2 phosphorylation. This highlights the importance of studying the effect of DNAJC27 on the multiple signaling pathways activated by MC4R. These pathways eventually determine the physiological effects of the receptor and the functions it mediates in different tissues and disease contexts. For example, coupling to the G protein Gs and cAMP formation controls energy homeostasis, whereas ERK1/2 activation mediates insulin signaling and food intake.
Our findings showed that cAMP formation decreased when DNAJC27 was overexpressed, but the underlying mechanism for this remains unclear. Given that DNAJC27 belongs to the heat shock protein (HSP) family, and that HSPs are known to function as molecular chaperones in regulating many of the essential aspects in the life cycle of GPCRs (48, 49), we can speculate that DNAJC27 may bind to MC4R. This is supported by the overlapping tissue distribution of MC4R and DNAJC27, especially in the brain. One possibility is that DNAJC27 regulates the folding of MC4R. This in turn affects the subcellular trafficking of the receptor; misfolded receptors are polyubiquitinated and targeted for degradation by the proteasome while correctly folded receptors are transported to the Golgi apparatus for post-translational modifications and eventually fused into the cell membrane. DNAJC27 might also be enhancing the internalization of the receptor. Generally, the various functions of molecular chaperones can have a critical effect on the surface expression of GPCRs and hence mediate their downstream signaling cascades. Interestingly, a recent study in mice explored the effect of glucose-regulated protein 78 (GRP78) on the regulation of MC4R trafficking and signaling, showing an interaction between MC4R and GRP78 in hypothalamic protein extracts (50). The study also showed that knocking down endogenous GRP78 in the paraventricular nucleus (PVN) of the hypothalamus in a diet-induced obesity mouse model resulted in a significant increase in body weight. Another study investigating the role of Hsc70 and HSP90 in regulating MC4R expression reported that overexpression of these HSPs released the retained mutant forms of the receptor to the cell surface (51). Therefore, further studies using animal models are needed to determine the exact mechanism of crosstalk between MC4R and DNAJC27 and whether DNAJC27 functions as a molecular chaperone for the receptor.
It is important to point out here that although heat shock proteins are mainly thought of as intracellular proteins, many studies are showing members of the family to be detected in human serum and plasma. Moreover, HSP40 proteins are known to interact with HSP70 which has been well-documented to be expressed in plasma. The studies are also associating the differential expression of these circulating proteins with various diseases. For example, serum levels of DNAJB9 were shown to be elevated in fibrillary glomerulonephritis patients (52). HSP70 together with HSP60 were shown to be associated with cardiovascular diseases and inflammation. Specifically, while circulating HSP70 levels are elevated in patients after acute myocardial infarction (53) and were shown to be predictive of acute coronary syndrome (54), HSP60 levels are involved in hypertension and atherosclerosis (55). Several studies also investigated the effect of exercise on modulating the circulating levels of HSPs (HSP27, HSP70, and HSP72) and generally found them to be increased (56, 57).
One of the limitations of this study is the cross-sectional design as well as lack of data on the levels of DNAJC27 in the hypothalamus of individuals who carry the C-allele. However, this study demonstrated the strong association between the C allele at the rs17782313 polymorphic locus of the MC4R gene and obesity, hypertension, and elevated plasma levels of DNAJC27. Taken together, the findings of the study suggest that overexpressed DNAJC27 reduces cAMP formation by wild-type MC4R, and this provides a potential mechanism of action for the effect of elevated circulating DNAJC27 on appetite and eventually the development of obesity.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Review Committee of Dasman Diabetes Institute. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JA, TT, and FA-M designed, supervised, helped in writing, and reviewed the manuscript. MH, MA-F, and PH wrote the manuscript and supervised the experiments. IA, MM, FA, and PC performed the experiments. OA analyzed the data and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Kuwait Foundation for the Advancement of Sciences (KFAS), research projects RA HM-2018-023 and RA-2015-043.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the National Dasman Diabetes Biobank Core Facility at DDI for their contribution in sample processing. We are also indebted to Kuwait Foundation for the Advancement of Sciences (KFAS) for financial support of this research project (RA HM-2018-023 and RA-2015-043). The funding agency was not involved in data collection, analysis, or interpretation; trial design; patient recruitment; or any aspect pertinent to the study.
References
1. Dietrich MO, Horvath TL. Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nat Rev Drug Discov. (2012) 11:675. doi: 10.1038/nrd3739
2. Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. (2010) 31:506. doi: 10.1210/er.2009-0037
3. Girardet C, Butler AA. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim Biophys Acta. (2014) 1842:482. doi: 10.1016/j.bbadis.2013.05.004
4. Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci. (2016) 19:206. doi: 10.1038/nn.4202
5. Dib L, San-Jose LM, Ducrest AL, Salamin N, Roulin A. Selection on the major color gene melanocortin-1-receptor shaped the evolution of the melanocortin system genes. Int J Mol Sci. (2017) 18:2618. doi: 10.3390/ijms18122618
6. Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. (2005) 6:221. doi: 10.1038/nrg1556
7. Kumar KG, Sutton GM, Dong JZ, Roubert P, Plas P, Halem HA, et al. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides. (2009) 30:1892–900. doi: 10.1016/j.peptides.2009.07.012
8. Kouidhi S, Clerget-Froidevaux MS. Integrating thyroid hormone signaling in hypothalamic control of metabolism: crosstalk between nuclear receptors. Int J Mol Sci. (2018) 19:2017. doi: 10.3390/ijms19072017
9. Goncalves JPL, Palmer D, Meldal M. MC4R agonists: structural overview on antiobesity therapeutics. Trends Pharmacol Sci. (2018) 39:402. doi: 10.1016/j.tips.2018.01.004
10. Kuhnen P, Krude H, Biebermann H. Melanocortin-4 receptor signalling: importance for weight regulation and obesity treatment. Trends Mol Med. (2019) 25:136–48. doi: 10.1016/j.molmed.2018.12.002
11. Podyma B, Sun H, Wilson EA, Carlson B, Pritikin E, Gavrilova O, et al. The stimulatory G protein Gsalpha is required in melanocortin 4 receptor-expressing cells for normal energy balance, thermogenesis, and glucose metabolism. J Biol Chem. (2018) 293:10993–1005. doi: 10.1074/jbc.RA118.003450
12. Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. (2015) 520:94–98. doi: 10.1038/nature14051
13. Yang Z, Tao YX. Biased signaling initiated by agouti-related peptide through human melanocortin-3 and −4 receptors. Biochim Biophys Acta. (2016) 1862:1485–94. doi: 10.1016/j.bbadis.2016.05.008
14. Yang LK, Tao YX. Biased signaling at neural melanocortin receptors in regulation of energy homeostasis. Biochim Biophys Acta Mol Basis Dis. (2017) 1863(Pt. A):2486–95. doi: 10.1016/j.bbadis.2017.04.010
15. Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P, et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. (2000) 106:253–62. doi: 10.1172/JCI9238
16. Govaerts C, Srinivasan S, Shapiro A, Zhang S, Picard F, Clement K, et al. Obesity-associated mutations in the melanocortin 4 receptor provide novel insights into its function. Peptides. (2005) 26:1909. doi: 10.1016/j.peptides.2004.11.042
17. Fani L, Bak S, Delhanty P, van Rossum EF, van den Akker EL. The melanocortin-4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options. Int J Obes. (2014) 38:163. doi: 10.1038/ijo.2013.80
18. Reinehr T, Hebebrand J, Friedel S, Toschke AM, Brumm H, Biebermann H, et al. Lifestyle intervention in obese children with variations in the melanocortin 4 receptor gene. Obesity. (2009) 17:382. doi: 10.1038/oby.2008.422
19. Petry CJ, López-Bermejo A, Díaz M, Sebastiani G, Ong KK, de Zegher F, et al. Association between a common variant near MC4R and change in body mass index develops by two weeks of age. Horm Res Paediatr. (2010) 73:275–80. doi: 10.1159/000284392
20. Bandstein M, Voisin S, Nilsson EK, Schultes B, Ernst B, Thurnheer M, et al. A genetic risk score is associated with weight loss following roux-en y gastric bypass surgery. Obes Surg. (2016) 26:2183–9. doi: 10.1007/s11695-016-2072-9
21. Resende C, Durso D, Borges K, Pereira R, Rodrigues G, Rodrigues K, et al. The polymorphism rs17782313 near MC4R gene is related with anthropometric changes of bariatric surgery over 60 months of follow up in women. Clin Nutr. (2017) 37:1286–92. doi: 10.1016/j.clnu.2017.05.018
22. Lotta LA, Mokrosinski J, Mendes de Oliveira E, Li C, Sharp SJ, Luan J, et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. (2019) 177:597. doi: 10.1016/j.cell.2019.03.044
23. Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. (2008) 40:768–75. doi: 10.1038/ng.140
24. Xi B, Takeuchi F, Chandak GR, Kato N, Pan HW, Consortium A-TD, et al. Common polymorphism near the MC4R gene is associated with type 2 diabetes: data from a meta-analysis of 123,373 individuals. Diabetologia. (2012) 55:2660. doi: 10.1007/s00125-012-2655-5
25. Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. (2006) 48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9
26. Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. (2010) 467:832–8. doi: 10.1038/nature09410
27. He M, Xu M, Zhang B, Liang J, Chen P, Lee JY, et al. Meta-analysis of genome-wide association studies of adult height in East Asians identifies 17 novel loci. Hum Mol Genet. (2015) 24:1791–800. doi: 10.1093/hmg/ddu583
28. Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. (2009) 41:25–34. doi: 10.1038/ng.287
29. Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. (2009) 41:157–9. doi: 10.1038/ng.301
30. Graff M, Scott RA, Justice AE, Young KL, Feitosa MF, Barata L, et al. Genome-wide physical activity interactions in adiposity - a meta-analysis of 200,452 adults. PLoS Genet. (2017) 13:e1006528. doi: 10.1371/journal.pgen.1006528
31. Wen W, Cho YS, Zheng W, Dorajoo R, Kato N, Qi L, et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet. (2012) 44:307. doi: 10.1038/ng.1087
32. Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet. (2018) 50:180–5. doi: 10.1038/s41588-017-0020-9
33. Abu-Farha M, Tiss A, Abubaker J, Khadir A, Al-Ghimlas F, Al-Khairi I, et al. Proteomics analysis of human obesity reveals the epigenetic factor HDAC4 as a potential target for obesity. PLoS ONE. (2013) 8:e75342. doi: 10.1371/journal.pone.0075342
34. Cherian PT, Al-Khairi I, Sriraman D, Al-Enezi A, Al-Sultan D, AlOtaibi M, et al. Increased circulation and adipose tissue levels of DNAJC27/RBJ in obesity and type 2-diabetes. Front Endocrinol. (2018) 9:423. doi: 10.3389/fendo.2018.00423
35. Batarfi AA, Filimban N, Bajouh OS, Dallol A, Chaudhary AG, Bakhashab S, et al. MC4R variants rs12970134 and rs17782313 are associated with obese polycystic ovary syndrome patients in the Western region of Saudi Arabia. BMC Med Genet. (2019) 20:144. doi: 10.1186/s12881-019-0876-x
36. Cyrus C, Ismail MH, Chathoth S, Vatte C, Hasen M, Al Ali A. Analysis of the impact of common polymorphisms of the FTO and MC4R genes with the risk of severe obesity in Saudi Arabian population. Genet Test Mol Biomark. (2018) 22:170–7. doi: 10.1089/gtmb.2017.0218
37. Yilmaz Z, Davis C, Loxton NJ, Kaplan AS, Levitan RD, Carter JC, et al. Association between MC4R rs17782313 polymorphism and overeating behaviors. Int J Obes. (2015) 39:114–20. doi: 10.1038/ijo.2014.79
38. Alharbi KK, Richardson TG, Khan IA, Syed R, Mohammed AK, Boustred CR, et al. Influence of adiposity-related genetic markers in a population of Saudi Arabians where other variables influencing obesity may be reduced. Dis Mark. (2014) 2014:758232. doi: 10.1155/2014/758232
39. Fesinmeyer MD, North KE, Ritchie MD, Lim U, Franceschini N, Wilkens LR, et al. Genetic risk factors for BMI and obesity in an ethnically diverse population: results from the population architecture using genomics and epidemiology (PAGE) study. Obesity. (2013) 21:835–46. doi: 10.1002/oby.20268
40. Wang T, Ma X, Peng D, Zhang R, Sun X, Chen M, et al. Effects of obesity related genetic variations on visceral and subcutaneous fat distribution in a Chinese population. Sci Rep. (2016) 6:20691. doi: 10.1038/srep20691
41. Adamska-Patruno E, Goscik J, Czajkowski P, Maliszewska K, Ciborowski M, Golonko A, et al. The MC4R genetic variants are associated with lower visceral fat accumulation and higher postprandial relative increase in carbohydrate utilization in humans. Eur J Nutr. (2019) 58:2929–41. doi: 10.1007/s00394-019-01955-0
42. Cooney KA, Molden BM, Kowalczyk NS, Russell S, Baldini G. Lipid stress inhibits endocytosis of melanocortin-4 receptor from modified clathrin-enriched sites and impairs receptor desensitization. J Biol Chem. (2017) 292:17731–45. doi: 10.1074/jbc.M117.785758
43. Morgan DA, McDaniel LN, Yin T, Khan M, Jiang J, Acevedo MR, et al. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes. (2015) 64:1976–87. doi: 10.2337/db14-1257
44. Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. (2013) 36:65–73. doi: 10.1016/j.tins.2012.12.005
45. Brodowski J, Szkup M, Jurczak A, Wieder-Huszla S, Brodowska A, Laszczynska M, et al. Searching for the relationship between the parameters of metabolic syndrome and the rs17782313 (T>C) polymorphism of the MC4R gene in postmenopausal women. Clin Interv Aging. (2017) 12:549–55. doi: 10.2147/CIA.S129874
46. Arrizabalaga M, Larrarte E, Margareto J, Maldonado-Martin S, Barrenechea L, Labayen I, et al. Preliminary findings on the influence of FTO rs9939609 and MC4R rs17782313 polymorphisms on resting energy expenditure, leptin and thyrotropin levels in obese non-morbid premenopausal women. J Physiol Biochem. (2013) 70:255–62. doi: 10.1007/s13105-013-0300-5
47. Chen T, Yang M, Yu Z, Tang S, Wang C, Zhu X, et al. Small GTPase RBJ mediates nuclear entrapment of MEK1/MEK2 in tumor progression. Cancer Cell. (2014) 25:682–96. doi: 10.1016/j.ccr.2014.03.009
48. Dupre DJ, Hammad MM, Holland P, Wertman J. Role of chaperones in G protein coupled receptor signaling complex assembly. Subcell Biochem. (2012) 63:23. doi: 10.1007/978-94-007-4765-4_2
49. Tao YX, Conn PM. Chaperoning G protein-coupled receptors: from cell biology to therapeutics. Endocr Rev. (2014) 35:602–47. doi: 10.1210/er.2013-1121
50. Yoon YR, Lee TG, Choi MH, Shin SW, Ko YG, Rhyu IJ, et al. Glucose-regulated protein 78 binds to and regulates the melanocortin-4 receptor. Exp Mol Med. (2018) 50:120. doi: 10.1038/s12276-018-0144-8
51. Meimaridou E, Gooljar SB, Ramnarace N, Anthonypillai L, Clark AJ, Chapple JP, et al. The cytosolic chaperone Hsc70 promotes traffic to the cell surface of intracellular retained melanocortin-4 receptor mutants. Mol Endocrinol. (2011) 25:1650–60. doi: 10.1210/me.2011-1020
52. Nasr SH, Dasari S, Lieske JC, Benson LM, Vanderboom PM, Holtz-Heppelmann CJ, et al. Serum levels of DNAJB9 are elevated in fibrillary glomerulonephritis patients. Kidney Int. (2019) 95:1269–72. doi: 10.1016/j.kint.2019.01.024
53. Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M, et al. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail. (2006) 8:810–5. doi: 10.1016/j.ejheart.2006.03.004
54. Zhang X, Xu Z, Zhou L, Chen Y, He M, Cheng L, et al. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. (2010) 15:675–86. doi: 10.1007/s12192-010-0180-3
55. Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J, et al. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. (2000) 36:303–7. doi: 10.1161/01.HYP.36.2.303
56. Banfi G, Dolci A, Verna R, Corsi MM. Exercise raises serum heat-shock protein 70 (Hsp70) levels. Clin Chem Lab Med. (2004) 42:1445–6. doi: 10.1515/CCLM.2004.268
Keywords: cAMP, DNAJC27, ghrelin, MC4R, obesity, visfatin
Citation: Hammad MM, Abu-Farha M, Hebbar P, Cherian P, Al Khairi I, Melhem M, Alkayal F, Alsmadi O, Thanaraj TA, Al-Mulla F and Abubaker J (2020) MC4R Variant rs17782313 Associates With Increased Levels of DNAJC27, Ghrelin, and Visfatin and Correlates With Obesity and Hypertension in a Kuwaiti Cohort. Front. Endocrinol. 11:437. doi: 10.3389/fendo.2020.00437
Received: 16 February 2020; Accepted: 03 June 2020;
Published: 07 July 2020.
Edited by:
Pierrette Gaudreau, Université de Montréal, CanadaReviewed by:
Ya-Xiong Tao, Auburn University, United StatesLi Chan, Queen Mary University of London, United Kingdom
Copyright © 2020 Hammad, Abu-Farha, Hebbar, Cherian, Al Khairi, Melhem, Alkayal, Alsmadi, Thanaraj, Al-Mulla and Abubaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thangavel Alphonse Thanaraj, YWxwaG9uc2UudGhhbmdhdmVsJiN4MDAwNDA7ZGFzbWFuaW5zdGl0dXRlLm9yZw==; Fahd Al-Mulla, ZmFoZC5hbG11bGxhJiN4MDAwNDA7ZGFzbWFuaW5zdGl0dXRlLm9yZw==; Jehad Abubaker, amVoYWQuYWJ1YmFrciYjeDAwMDQwO2Rhc21hbmluc3RpdHV0ZS5vcmc=
†These authors have contributed equally to this work and share first authorship
 Maha M. Hammad
Maha M. Hammad Mohamed Abu-Farha
Mohamed Abu-Farha Prashantha Hebbar
Prashantha Hebbar Preethi Cherian
Preethi Cherian Irina Al Khairi
Irina Al Khairi Motasem Melhem
Motasem Melhem Fadi Alkayal
Fadi Alkayal Osama Alsmadi
Osama Alsmadi Thangavel Alphonse Thanaraj
Thangavel Alphonse Thanaraj Fahd Al-Mulla
Fahd Al-Mulla Jehad Abubaker
Jehad Abubaker