
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 05 May 2020
Sec. Pediatric Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00278
This article is part of the Research Topic Debates in Clinical Management in Pediatric Endocrinology View all 9 articles
 Biagio Rapone1*
Biagio Rapone1* Massimo Corsalini2
Massimo Corsalini2 Ilaria Converti3
Ilaria Converti3 Maria Teresa Loverro2
Maria Teresa Loverro2 Antonio Gnoni1
Antonio Gnoni1 Paolo Trerotoli4†
Paolo Trerotoli4† Elisabetta Ferrara5†
Elisabetta Ferrara5†The emergence of link between periodontal disease and diabetes has created conditions for analyzing new interdisciplinary approach making toward tackling oral health and systemic issues. As periodontal disease is a readily modifiable risk factor this association has potential clinical implications. The aim of this paper was systematically review the extant literature related to analytics data in order to identify the association between type 1 diabetes (T1DM) in childhood and adolescence with periodontal inflammation. Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we conducted a database search between 2004 and 2019. A manual search of the literature was conducted as an additional phase of the search process, with the aim of identifying studies that were missed in the primary search. One hundred and thirty-nine records were screened and 10 fulfilled the inclusion criteria. Most studies were of moderate methodological quality. Outcomes included assessments of diabetes and periodontal status. In diabetic populations, compared to healthy subjects, interindividual differences in periodontal status are reflected in higher severity of periodontal inflammation. The most reported barriers to evidence uptake were the intrinsic limits of cross-sectional report data and relevant research, and lack of timely research output. Based on the evidence presented within the literature, the aforementioned biomarkers correlate with poor periodontal status in type 1 diabetic patients. Whilst the corpus of the evidence suggests that there may be an association between periodontal status and type 1 diabetes, study designs and methodological limitations hinder interpretation of the current research.
According to the literature, the most robust evidence among the association between periodontal disease and systemic disorders (1–7), concerns the biunivocal relationship between periodontal inflammation and diabetes, suggesting a consistent connection with the metabolic control (8–12). Periodontal disease (PD), i.e., plaque-induced inflammation of the periodontium, is an inflammatory disease bacteria-induced that results in the progressive destruction of the attachment apparatus of natural teeth, investing progressively the gingiva, periodontal ligament, and alveolar bone, which originates with the inflammation involving the region of the marginal gingiva above the crest of bone (13–15). Many large-scale prospective studies have shown that periodontitis is associated with elevated inflammatory markers in otherwise healthy populations, demonstrating that the C-reactive protein (CRP) is strongly correlated with PD severity (16–19). The study conducted by Poplawska-Kita et al. (20) underlined the difference between CRP, fibrinogen, and TNF-α levels in periodontics and patients with T1DM compared to subjects without periodontitis and increased levels of systemic inflammation have been reported in relation with poor periodontal status. However, in the same cohort of patients, a good metabolic control was related to lower CRP concentrations, demonstrating a mechanistic link between poor metabolic control and systemic inflammation. Although it was hypothesized that periodontal inflammation could promote the inflammation in systemic compartment, evidence from this study indicated no correlation between periodontitis and circulatory inflammatory markers in type 1 diabetes (T1DM). Indeed, several studies demonstrated that periodontal chronic inflammation is linked to persistent low-grade systemic inflammation (18, 21), concluding that could be involved as a risk factor in the pathogenesis of multiple systemic disorders, including obesity and diabetes mellitus (DM) (14, 15). Particularly, the pathogenic relevance of periodontal inflammation in type 2 diabetes has been amply confirmed in literature, while the nature of connection between T1DM and periodontitis have not yet found a clarification. It has been postulated that the peripheral inflammation might a stimulatory effect on blood glucose concentration. Periodontal treatment was shown to decrease blood glucose level in non-insuline dependent individuals by decreasing of systemic inflammation (22–24). However, despite a plethora of studies concerning the relationship between periodontitis and diabetes, the potential mechanism underlying the potential association between T1DM and periodontal inflammation in childhood remains unclear. Type 1 diabetes is an autoimmune disorder affecting the peripheral system, characterized by a chronic anti-self-inflammatory response (22, 23). The inflammation is a core feature of autoimmune disorder and periodontitis. Increased secretion of pro-inflammatory cytokines, including IL-1, IL-8, IL-6, and TNF-α, during periodontitis development, could exert its effect activating inflammatory pathways in patients with diabetes (25–27). This led to the general hypothesis that this chronic peripheral inflammation could play a key role in exacerbation of systemic inflammatory response that could be a risk factor for the dysregulation of metabolic control in T1DM (28–30). In consideration of the important role that inflammatory processes play in T1DM, and given that periodontitis is associated with a low-grade systemic inflammatory status and the increase of the inflammatory markers in the blood, such as CRP (31), recent works have been conducted to whether periodontal inflammation may influence the metabolic control of T1DM. With this background, the present meta-analysis was performed to analyze the existing evidence regarding the relationship between periodontal status and T1DM in childhood and in adolescence.
We carried out a meta-analysis of studies that investigated the association between periodontal inflammation with T1DM in children and adolescents. We followed the quality of reporting of meta-analysis guidelines [the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement] for performing and reporting the present meta-analysis. The articles reviewed in this section were systematically searched between 2004 and 2019 with MEDLINE database using the following medical subject headings (MeSH) terms and keywords: “DM, type 1” OR “type 1 DM” OR “type 1 diabetes” AND “periodontal” OR “periodontally” OR “periodontically” OR “periodontics” OR “periodontics” OR “periodontic” OR “periodontitis” OR “periodontitis” OR “periodontitides” AND “periodontal diseases” OR “periodontal” AND “diseases” OR “periodontal diseases” OR “periodontal” AND “disease” OR “periodontal disease” AND “child” OR “child” OR “children” OR “child's” OR “children's” OR “childrens” OR “childs” AND “adolescences” OR “adolescency” OR “adolescent” OR “adolescent” OR “adolescence” OR “adolescents” OR “adolescent's.” A manual search of the literature was conducted as an additional phase of the search process, with the aim of identifying studies that were missed in the primary search. The search was conducted without any restriction on setting or language to retrieve relevant studies.
A three-step procedure declined the selection and evaluation process of the eligible studies, as follows: (a) first, three reviewers scored the retrieved titles of references independently based on pre-defined criteria; (b) secondly, abstracts were scored by two reviewers independently; (c) subsequently, the full texts of all potentially relevant articles were then reviewed, and studies were included if they met the following criteria:
(1) defined diagnosis criteria for T1DM in children or adolescents;
(2) reported glycated hemoglobin value (HbA1C) level, age at diagnosis of diabetes, and periodontal inflammatory parameters used to assess and monitor the status of periodontal tissues, as follows:
- Plaque index (PI), used in order to record the level and rate of plaque formation on tooth surfaces (32),
- Gingival bleeding index which evaluates the soft periodontal tissues inflammation: Gingival Index, GI or Bleeding on Probing, BoP (33, 34),
- Probing Depth (PD), measuring the sulci of all teeth in order to evaluate the bone loss (35),
- Measurement of Clinical Attachment Level (CAL), indicator of the periodontal support around a tooth (36),
(3) inclusion of a non-exposed group in prospective studies or a control group in retrospective studies. Conference abstracts, reviews, and qualitative studies (i.e., interviews) were discarded, because of the low quality evidence that could possibly bias the research. Disagreements between reviewers will be resolved by a third reviewer.
The aim of using a meta-analysis procedure for the study was to evaluate findings of past studies that had examined the association between periodontal disease and T1DM. Standard mean difference (SMD) with 95% confidence interval were determined using Der Simonian and Laird random-effects modeling, when there was a significant heterogeneity between studies.
To compare periodontal status of diabetic and control patients, the measure of GI, CAL, PPD, PI e BOP were used. These variables, quantitative were analyzed with a method of meta-analysis for continuous outcome variables, the standardized difference in mean. The SMDs and 95% confidence intervals (CIs) from each study to evaluate the difference in the periodontal parameters between patients with type 1 diabetes and healthy control participants was pooled. A value of SMD > 0 means that the measure is higher in patients with diabetes respect to controls. Overall results were determined under the assumption of random effects model and were summarized SMD and 95% CI. Because the studies differed in terms of patients, outcome definitions, and study design, a test for heterogeneity of the effect on outcome between the included studies was assessed. The between-study statistical heterogeneity was detected measuring the Cochran's Q homogeneity test statistic and the I2 (inconsistency) index and its associated 95% confidence interval (37). Statistical analyses were performed using MedCalc software, version 19.1.5 (Ostend, Belgium; https://www.medcalc.org; 2020).
Overall, 105 references were initially identified. After the initial screening of titles and abstracts, a total of 63 articles were discarded, leaving 41 articles for retrieval. Full text assessment of these articles resulted in 10 eligible articles that met our inclusion criteria (38–46). Figure 1 displays the process of selection studies.
Table 1 shows the main characteristics of the included articles. The examined papers were quite heterogeneous. Seven were comparative-cross sectional studies (37–43) and three were observational case-control studies (40–42). The method of outcomes ascertainment varied across articles. The studies focused on the following indices of periodontal inflammation, including the afore mentioned Plaque Index (PI), Gingival Index (GI), Bleeding on Probing (BoP), Probing Pocket Depth (PPD), and Clinical attachment level (CAL).
Table 2 displays the results of the meta-analysis of the 10 articles. The Bleeding on Probing (BOP) was evaluated (Figure 2, Table 2) only in three studies, with a number of diabetic patients ranging from 32 to 95 and controls ranging from 32 to 61. Evidence from the study conducted by Ismail (10) suggested no statistically significant difference between group of patients with diabetes and controls (SMD = −016; −0.33 to 0.66; 95% CI). The other two studies, on the contrary, have shown a statistically significant difference between subjects with diabetes and control groups (11, 12). The Overall SMD (95% CI) was −0.65 (0.09–1.22), that suggests a higher BOP in diabetic respect to controls (t = 2.26, p = 0.024). The heterogeneity was statistically significant (Q = 10.48, p = 0.0053) and I2 was 80.9% (40.3–93.9%). The CAL index was evaluated in four studies (Figure 3, Table 3). The range of the sample size of the studies was 32–187 for group with diabetes and 32–178 in the control group. The SMD was 0.56 (0.13–0.79) in the study from Al-Khabbaz, that was the lower difference, while in the studies by Lalla and Ismail SMD was, respectively, 0.99 (0.76–1.21) and 0.98 (0.46–1.51). The overall value of the difference of CAL between individuals with diabetes and controls was 0.82 (0.59–1.04) and it was statistically significant (t = 7.09, p < 0.0001). The Cochran's Q was 7.2 (p = 0.0648) and I2 was 80.9% (0–86%). Seven studies (Figure 4, Table 4) allowed the evaluation of the GI. The study of Duque was the one with the low sample size, it had 24 patients with diabetes and 27 controls and the SMD was −0.23 with 95% CI: −0.79 to 0.32, results that suggest no statistically significant difference between the two groups. The study by Ismail resulted with no statistically significant difference between the two groups too. The SMD was −012 and its 95% CI was −0.62 to 0.37. The study of Babu, which included 160 patients, 80 individuals for each group, was inconclusive with a SMD = −0 and 95% CI: −0.31 to 0.31. Among the seven studies, only the study conducted by Al-Khabbaz, which compared 95 patients with diabetes vs. 61 controls, showed the higher difference. In this study, SMD was 1.58 (1.21–1.95). The overall SMD resulted 0.46 (0.09–0.84), revealing a statistically significant difference between diabetic and controls (t = 2.408, p = 0.016). Heterogeneity resulted statistically significant (q = 60.49, p < 0.0001) and I2 was 90.1% (82.1–94.5%). The evaluation of PI (Figure 5, Table 5) was made across seven studies. Only the study of Duque (24 diabetics and 27 controls) had a SMD −1.33 (−1.9 to −0.72), suggesting a statistically significant higher value of PI in controls. The other six studies had SMD that underlined a higher value of PI in group of patients with diabetes. The study of Da Cuna Coelho, 72 patients (36 for each group), had the highest value of SMD = 2.27 (1.67–2.87). The study of Orbaq, that involved 100 patients (50 by group) had a SMD of 1.13 (0.7–1.55). The lower value of SMD was that of Lalla: 0.28 (0.07–0.49). The overall SMD was 0.71 (0.19–1.22) and the difference showed a statistically significant higher value of PI in diabetic compared to controls (t = 2.71, p = 0.007). The heterogeneity was statistically significant (Q = 91.72, p < 0.0001) and I2 was 93.5% (88.9–96.1%). Only two studies evaluated the PPD index (Figure 6, Table 6): Duque's study has a 95% CI that suggest non-statistical significance (SMD = 0.14, 95% CI: −0.41 to 0.69); Dakovic's study had a SMD 0.39 (0.18–0.59). The overall SMD was 0.36 (0.16–0.55), showing a statistically significant difference between groups (t = 3.63, p < 0.001) and patients with diabetes with higher value of PPD. I2 was not estimable and heterogeneity resulted non-statistically significant (Q = 0.708, p = 0.4). In general, the association between periodontal disease and type 1 diabetes tended to be positive regardless of the severity of periodontitis and metabolic control degree.
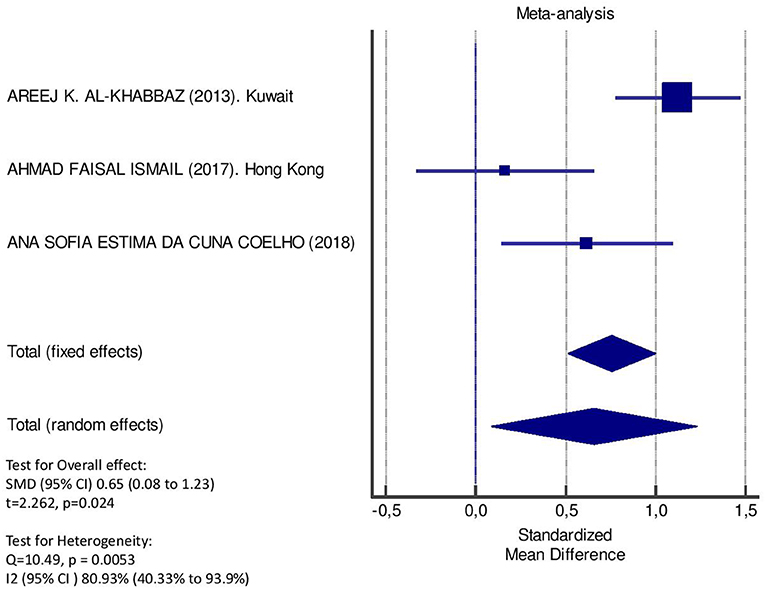
Figure 2. Forest plot of study effects for BOP. In the bottom, the overall effect for random effects model and heterogeneity test.
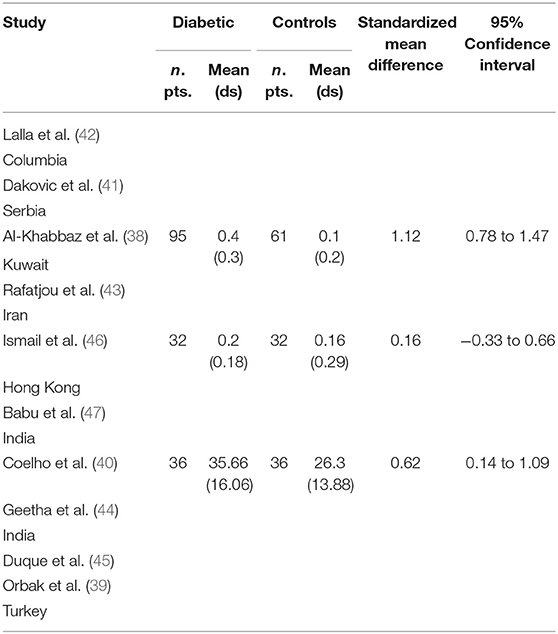
Table 2. Main results from the studies reporting BOP and evaluation of standardized mean difference for meta-analysis.
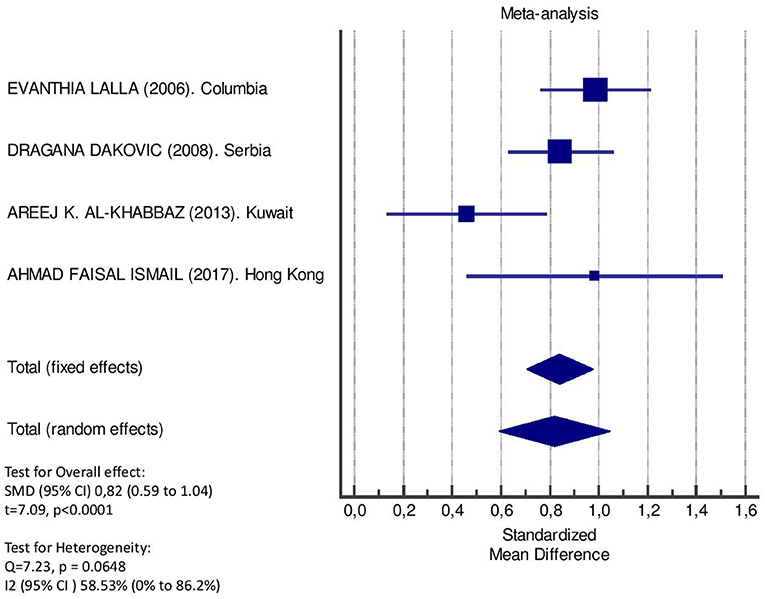
Figure 3. Forest plot of study effects for CAL. In the bottom, the overall effect for random effects model and heterogeneity test.
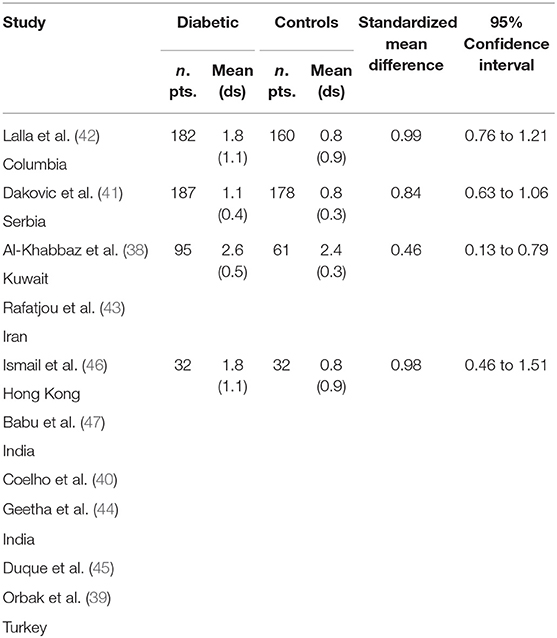
Table 3. Main results from the studies reporting CAL, and evaluation of standardized mean difference for meta-analysis.
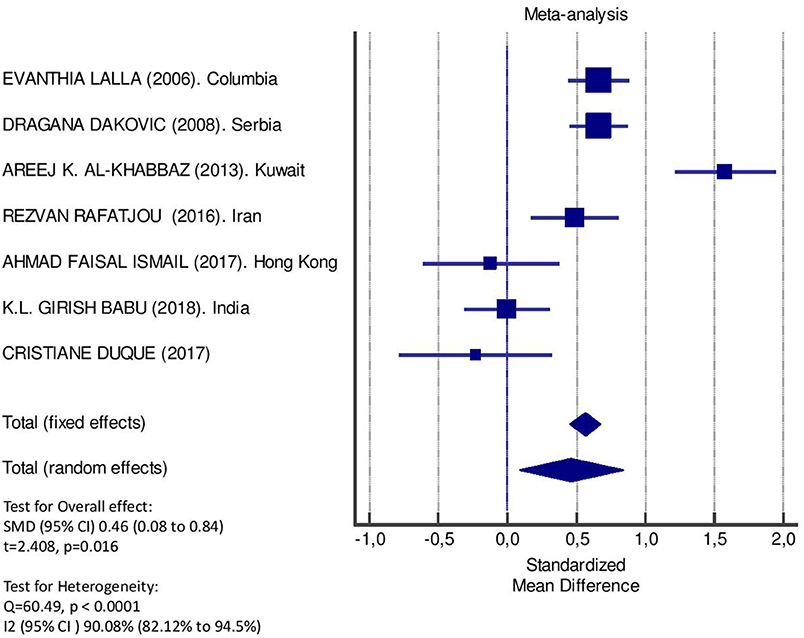
Figure 4. Forest plot of study effects for GI. In the bottom, the overall effect for random effects model and heterogeneity test.
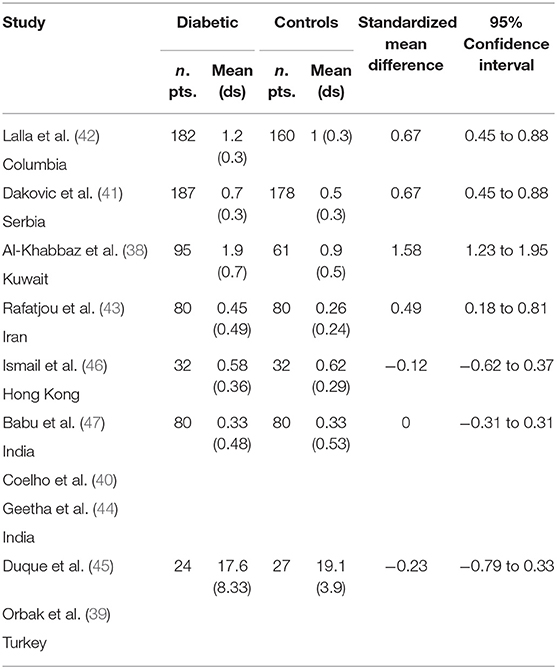
Table 4. Main results from the studies reporting GI, and evaluation of standardized mean difference for meta-analysis.
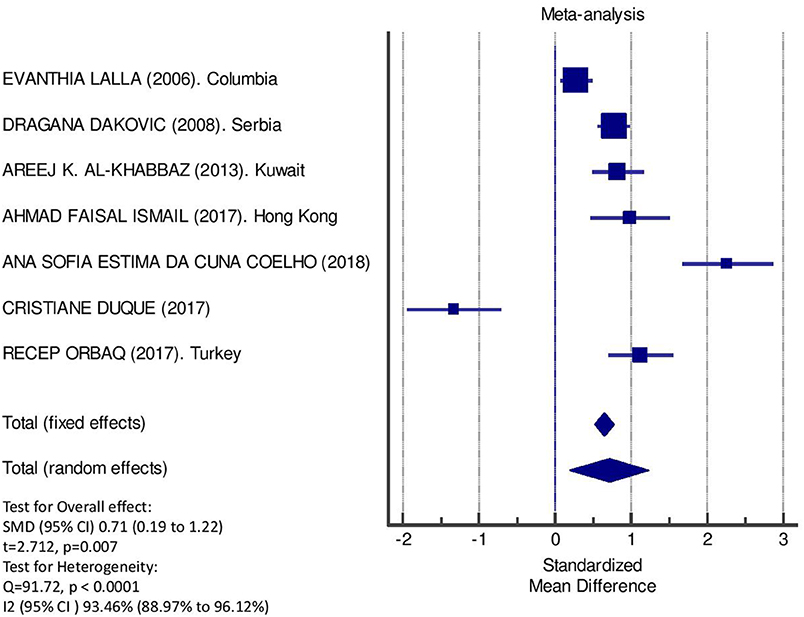
Figure 5. Forest plot of study effects for PI. In the bottom the overall effect for random effects model and heterogeneity test.
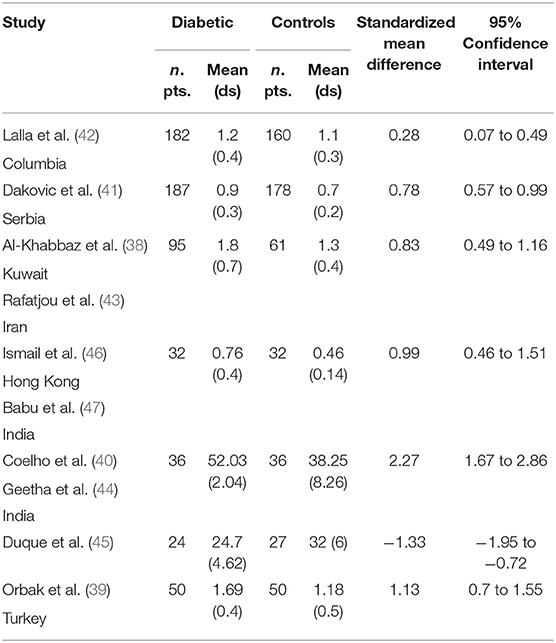
Table 5. Main results from the studies reporting PI, and evaluation of standardized mean difference for meta-analysis.
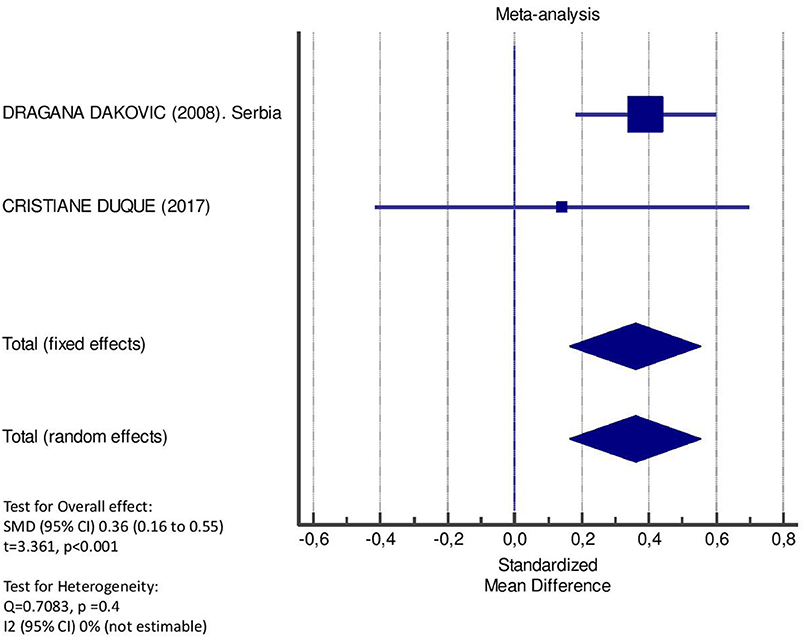
Figure 6. Forest plot of study effects for PPD. In the bottom, the overall effect for random effects model and heterogeneity test.
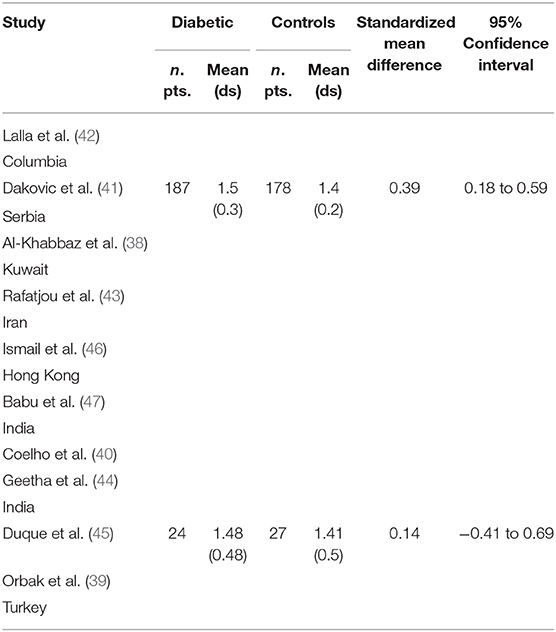
Table 6. Main results from the studies reporting PPD, and evaluation of standardized mean difference for meta-analysis.
Type 1 diabetes is one of the major autoimmune disease developing in childhood, with the incidence rate ranging between 3 and 5% every year (47–49). Periodontitis is a biological process leading to the disruption of the normal physiology of the alveolar bone homeostasis in response to the invasion of pathogenic microorganisms in the periodontium. PD is related to diabetes, and there is an increasing interest in research regarding the effects of periodontal inflammation on children and adolescents with T1DM. The literature demonstrates that inflammatory mediators released during periodontitis development could influence the metabolic control on patients with diabetes. Although PD has been well described in type 2 diabetes (T2DM) as the sixth complication of diabetes (50), there is a paucity of reports regarding the impact of periodontal inflammation on the pathogenesis and progression of T1DM in childhood and adolescence. The pathogenesis of T1DM and T2DM is quite different, probably leading to different risk of PD (51). Studies examining the association of poor periodontal status with glycemic control in subjects diagnosed with T1DM, emphasized on the prevalence of periodontitis in adults (21, 52). It has been observed that patients with diabetes exhibited greater susceptibility for PD. The relationship occurring at a cross-level with T1DM is not well-clarified as that occurring within the T2DM. Despite the quite differences between the pathogenic mechanism in T1DM and T2DM, periodontal implications share many similarities. In this regard, it has been demonstrated that despite the overlap that exists with respect the causes of the two, the increased inflammatory activity has been reported as a common denominator underlying hyperglycemia-induced insulin resistance and diabetic complications. The link between periodontal inflammation and diabetes is sufficiently strong to have prompted the suggestion that the presence of periodontitis ought to be viewed as a key risk factor for pathogenesis of diabetes. This involves promotion of proinflammatory cytokines release. Few evidences indicate that the periodontitis may be involved in the pathogenesis of T1DM, and the data available concerning the potential impact of periodontal inflammation on T1DM in childhood and adolescence is still limited. The data derived from human studies have revealed that the periodontitis chronicity elicits cytokine sensitivity, contributing to the vulnerability to long-term complications. Hyperglycemia is accompanied by an up-regulated expression of TNF-α, interleukin (IL)-1β, IL-6, and IL-18, which contribute to insulin resistance by both JNK and the IKKβ/NF-kβ pathway. It has been shown that periodontitis contributes to the development of low-grade systemic inflammation (53). Oxidative stress is one of the important examples of an essential host factor that can explain the prevalence and severity of periodontitis in patients with diabetes. The activity of the prooxidative enzyme myeloperoxidase in the gingival crevicular fluid of periodontal patients with diabetes is reduced compared to non-diabetics and non-periodontal patients with diabetes (54, 55). Parodontotic patients with diabetes shown an imbalance between the prooxidants and antioxidants in the body, thus promoting cellular injury and increasing the magnitude of inflammatory response effects. This leads to the question of how periodontal abnormalities and the related inflammatory changes are able to impact on the pathogenesis of T1DM in children and adolescents. Patients with diabetes are a greater risk for PD than healthy individuals but it didn't seem to be any impact for T1DM development. Our systematic analysis and meta-analysis persuasively explain that the severity of periodontal inflammation is increased in children and adolescents with T1DM compared to that in healthy controls. We systematically reviewed the published literature to evaluate the relationship between metabolic control in children and adolescents affected by T1DM and PD, but the great important set of studies into the impact of different kinds of systemic inflammation has been carried focusing on the adult patients or heterogeneous groups as the primary interest for consideration (49, 50). Our results are consistent with those reported by Löe (51), who conducted a systematic review and meta-analysis to investigate the association between T1DM and T2DM mellitus and PD. Interestingly, they have shown that the overall difference in the average gingival index between individuals with and without diabetes increased significantly. Roy et al. (52) also demonstrated that, when patients with diabetes were compared to non-diabetics in regard to clinical attachment loss, the pooled difference increased more in patients with T2DM (0.652) (95% CI: 0.465–0.840; Pb.0001) than in subjects with T1DM (0.691) (95% CI: 0.427–0.956; Pb.0001). The strong interindividual variability in oral health status depends on a number of factors, both constitutional and environmental, that determine the different clinical cases developmental. The research conducted by Listgarten et al. (35) revealed that the age impacted negatively on increased gingival inflammation, but also more accumulation of bacterial plaque and calculus deposits, the key pathogenic factors of periodontitis, were scored in children with diabetes than in the healthy groups. The causes of this process appear to be related to vascular changes which are part of the pathogenesis of diabetes complications, which may involve also the periodontal vasculature (52). The alterations of vascular components may result in increased gingival bleeding. Furthermore, the significant reduction of the salivary secretion rate it has been documented in children with T1DM when compared to healthy children (55). Similar findings have also been reported in another studies, which performed analysis for all periodontal parameters, showing that the gingival index, bleeding on probing, and higher dental plaque amount were significantly associated with T1DM in children (35–37, 39). A poor association was reported in patients with diabetes with periodontitis; however, this finding could not explain the association between diabetes and periodontitis in children and adolescents (52). A major finding of our review was that the periodontal status (PI and GI parameters) affects the impacts of uncontrolled diabetes (56), and this has practical implications for multidisciplinary management of primitive pathology. A similar conclusion was previously reached by a systematic review on the impact of periodontitis on T1DM (57). The emergence of close link between periodontal disease and diabetes has created conditions for analyzing a new interdisciplinary approach making toward tackling oral health and systemic issues.
The major limitation of the mentioned studies was the inexpensive nature of cross-sectional study design. Another restriction was the paucity and often low quality of primary data. The paucity of studies likely reflects the fact that periodontitis has not been identified as a serious issue in T1DM, because of the natural history of disease. However, there is no doubt that children with diabetes have poor oral hygiene. Another major limitation of the evidence base was the absence of primary research on the effectiveness of periodontal treatment to affect glycemic control in T1DM; this was particularly notable in comparison with the abundance of studies on T2DM. Due to this limitation, we were only able to analyze the overall correlation between periodontal parameters.
Study selection, data extraction, and quality assessment were performed independently by two authors according to predesigned criteria to minimize bias and transcription errors. Potential limitations of this meta-analysis arise from the unavailability of individual participant data from the included studies. Our meta-analysis was based on studies that varied in many ways (study design, population sample, adjustment for confounders, and different ascertainment methods for exposure and outcome), which may be considered another limitation. However, we adopted appropriate meta-analytic techniques with random-effect models, which enabled us to account for these differences. Second, we could not conduct a multivariable meta-regression, due to the small number of studies. Finally, the most reported barriers to evidence uptake were the intrinsic limits of cross-sectional report data and relevant research, and lack of timely research output. Based on the evidence presented within the literature, the poor metabolic control correlate with scarce periodontal status in patients with T1DM.
Whilst focusing emerging studies concerning the relationship between periodontal disease and type 2 diabetes in health sciences are being implemented in research, the studies of potential effects of periodontal inflammation on children and adolescents affected by T1DM are incomplete. Despite this recent exponential increase in the number of studies implicated in the association between periodontitis and diabetes, no general consensus has yet emerged of a causal effect of periodontal inflammation in T1DM. We have a mosaic of data from diverse results amenable for errors. Instead, we have a mosaic of data from diverse results amenable for errors, of disease interactions. The underlying mechanisms that support the biological connection between periodontitis and T1DM in childhood and adolescence can be discussed in a range of levels: the common implication of inflammation in the pathogenesis of both diseases, the transitory bacteremia originated by periodontal diseases, and the systemic immune inflammation as a response to chronic peripheral infection. In conclusion, this meta-analysis did not provide strong evidence that periodontitis is a significant risk factor for T1DM, and the link between PD and T1DM appears to be not solid as the connection with T2DM, although this association is less clarified. Given the high prevalence and incidence of T1DM in the general population, the observed relationship between PD and diabetes has clinical and public health importance. Further research to identify the link between T1DM and PD is warranted. Available reports evidently suggest a link between the mechanism of periodontal diseases and systemic/metabolic diseases where both conditions could aggravate each other. Despite this recent exponential increase in the number of studies implicated in relationship between periodontitis and T2DM, no general consensus has yet emerged of a causal effect of periodontal inflammation on T1DM. Longitudinal studies and additional research with larger sample sizes are needed to determine whether periodontitis is the result of T1DM or contributes to the worsening of metabolic control in children and adolescents with T1DM.
All datasets generated for this study are included in the article/supplementary material.
Conceptualization: BR; investigation: BR and EF; resources: IC, PT, and EF; data curation: BR, PT, and EF; validation: BR, EF, MC, IC, ML, and AG; writing-original draft preparation, writing-review, and editing: BR and EF; visualization: AG, IC, MC, and PT; supervision: EF and BR; project administration: BR.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. (2005) 76:2089–100. doi: 10.1902/jop.2005.76.11-S.2089
2. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. (2007) 13:3–10. doi: 10.1111/j.1469-0691.2007.01798.x
3. Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. (1996) 67:1103–13. doi: 10.1902/jop.1996.67.10.1103
4. Fentoglu O, Bozkurt FY. The bidirectional relationship between periodontal disease and hyperlipidemia. Eur J Dent. (2008) 2:142–46. doi: 10.1055/s-0039-1697370
5. Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. (2001) 6:99–112. doi: 10.1902/annals.2001.6.1.99
6. Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. (2008) 14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x
7. Andriankaja OM, Joshipura K. Potential association between prediabetic conditions and gingival and/or periodontal inflammation. J Diabetes Investig. (2014) 5:108–14. doi: 10.1111/jdi.12122
8. Perrino MA. Diabetes and periodontal disease: an example of an oral/systemic relationship. N Y State Dent J. (2007) 73:38–41.
9. Matthews DC. The relationship between diabetes and periodontal disease. J Can Dent Assoc. (2002) 68:161–4.
10. Cairo F, Rotundo R, Frazzingaro G, Muzzi L, Pini Prato GP. [Diabetes mellitus as a risk factor for periodontitis]. Minerva Stomatol. (2001) 50:321–30.
11. Grant-Theule DA. Periodontal disease, diabetes, and immune response: a review of current concepts. J West Soc Periodontol Periodontal Abstr. (1996) 44:69–77.
12. Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. (1998) 3:51–61. doi: 10.1902/annals.1998.3.1.51
13. Wolf DL, Lamster IB. Contemporary concepts in the diagnosis of periodontal disease. Dent Clin North Am. (2011) 55:47–61. doi: 10.1016/j.cden.2010.08.009
14. Armitage GC. Clinical evaluation of periodontal diseases. Periodontology (2000). (1995). 7:39–53. doi: 10.1111/j.1600-0757.1995.tb00035.x
15. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. (1999) 4:1–6. doi: 10.1902/annals.1999.4.1.1
16. Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. (2003) 74:1007–16. doi: 10.1902/jop.2003.74.7.1007
17. Adonogianaki E, Mooney J, Kinane DF. Detection of stable and active periodontitis sites by clinical assessment and gingival crevicular acute-phase protein levels. J Periodontal Res. (1996) 31:135–43. doi: 10.1111/j.1600-0765.1996.tb00475.x
18. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. (2001) 72:1221–7. doi: 10.1902/jop.2000.72.9.1221
19. D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. (2004) 39:236–41. doi: 10.1111/j.1600-0765.2004.00731.x
20. Popławska-Kita A, Siewko K, Szpak P, Król B, Telejko B, Klimiuk PA, et al. Association between type 1 diabetes and periodontal health. Adv Med Sci. (2014) 1:126–31 doi: 10.1016/j.advms.2014.01.002
21. Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. (2012) 55:21–31. doi: 10.1007/s00125-011-2342-y
22. Joseph R, Sasikumar M, Mammen J, Joseraj MG, Radhakrishnan C. Nonsurgical periodontal-therapy improves glycosylated hemoglobin levels in pre-diabetic patients with chronic periodontitis. World J Diabetes. (2017) 8:213–21. doi: 10.4239/wjd.v8.i5.213
23. Telgi RL, Tandon V, Tangade PS, Tirth A, Kumar S, Yadav V. Efficacy of nonsurgical periodontal therapy on glycaemic control in type II diabetic patients: a randomized controlled clinical trial. J Periodontal Implant Sci. (2013) 43:177–82. doi: 10.5051/jpis.2013.43.4.177
24. Perayil J, Suresh N, Fenol A, Vyloppillil R, Bhaskar A, Menon S. Comparison of glycated hemoglobin levels in individuals without diabetes and with and without periodontitis before and after non-surgical periodontal therapy. J Periodontol. (2014) 85:1658–66 doi: 10.1902/jop.2014.130661
25. Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. (2016) 92:63–9. doi: 10.1136/postgradmedj-2015-133281
26. Larger É, Diedisheim M, Donath X, Dehghani L, Sola-Gazagnes A. Circonstances diagnostiques et formes cliniques du diabète de type 1 [Diagnostic circumstances and clinical forms of type 1 diabetes]. Rev Prat. (2018) 68:614–8.
27. Tawfig N. Proinflammatory Cytokines and periodontal disease. J Dent Probl Solut. (2016) 3:012–7. doi: 10.17352/2394-8418.000026
28. Herrero ER, Fernandes S, Verspecht T, Ugarte-Berzal E, Boon N, Proost P, et al. Dysbiotic biofilms deregulate the periodontal inflammatory response. J Dent Res. (2018) 97:547–55. doi: 10.1177/0022034517752675
29. Noh MK, Jung M, Kim SH, Lee SR, Park KH, Kim DH, et al. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med. (2013) 6:847–51. doi: 10.3892/etm.2013.1222
30. Miyamoto M, Ishihara K, Okuda K. The Treponema denticola surface protease dentilisin degrades interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha. Infect Immun. (2006) 74:2462–7. doi: 10.1128/IAI.74.4.2462-2467.2006
31. Kim OS, Shin MH, Kweon SS, Lee YH, Kim OJ, Kim YJ, et al. The severity of periodontitis and metabolic syndrome in Korean population: the Dong-gu study. J Periodontal Res. (2018) 53:362–8. doi: 10.1111/jre.12521
32. Garcia D, Tarima S, Okunseri C. Periodontitis and glycemic control in diabetes: NHANES 2009 to 2012. J Periodontol. (2015) 86:499–506. doi: 10.1902/jop.2014.140364
33. Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. (1967) 38:610–6. doi: 10.1902/jop.1967.38.6.610
34. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. (1963) 21:533–51. doi: 10.3109/00016356309011240
35. Listgarten MA, Mao R, Robinson PJ. Periodontal probing and the relationship of the probe tip to periodontal tissues. J Periodontol. (1976) 47:511–3. doi: 10.1902/jop.1976.47.9.511
36. Preshaw PM, Kupp L, Hefti AF, Mariotti A. Measurement of clinical attachment levels using a constant-force periodontal probe modified to detect the cemento-enamel junction. J Clin Periodontol. (1999) 26:434–40. doi: 10.1034/j.1600-051X.1999.260704.x
37. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
38. Al-Khabbaz AK, Al-Shammari KF, Hasan A, Abdul-Rasoul M. Periodontal health of children with type 1 diabetes mellitus in Kuwait: a case-control study. Med Princ Pract. (2013) 22:144–9. doi: 10.1159/000342624
39. Orbak R, Simsek S, Orbak Z, Kavrut F, Colak M. The influence of type-1 diabetes mellitus on dentition and oral health in children and adolescents. Yonsei Med J. (2008) 49:357–65. doi: 10.3349/ymj.2008.49.3.357
40. Coelho ASEDC, Carneiro AS, Pereira VF, Paula AP, Macedo AP, Carrilho EVP. Oral health of portuguese children with type 1 diabetes: a multiparametric evaluation. J Clin Pediatr Dent. (2018) 42:231–5. doi: 10.17796/1053-4628-42.3.12
41. Dakovic D, Pavlovic MD. Periodontal disease in children and adolescents with type 1 diabetes in Serbia. J Periodontol. (2008) 79:987–92. doi: 10.1902/jop.2008.070549
42. Lalla E, Cheng B, Lal S, Tucker S, Greenberg E, Goland R, et al. Periodontal changes in children and adolescents with diabetes: a case-control study. Diabetes Care. (2006) 29:295–9. doi: 10.2337/diacare.29.02.06.dc05-1355
43. Rafatjou R, Razavi Z, Tayebi S, Khalili M, Farhadian M. Dental health status and hygiene in children and adolescents with type 1 diabetes mellitus. J Res Health Sci. (2016) 16:122–6.
44. Geetha S, Pramila M, Jain K, Suresh CM. Oral health status and knowledge among 10-15years old type 1 diabetes mellitus children and adolescents in Bengaluru. Indian J Dent Res. (2019) 30:80–6. doi: 10.4103/ijdr.IJDR_572_17
45. Duque C, João MF, Camargo GA, Teixeira GS, Machado TS, Azevedo RS, et al. Microbiological, lipid and immunological profiles in children with gingivitis and type 1 diabetes mellitus. J Appl Oral Sci. (2017) 25:217–26. doi: 10.1590/1678-77572016-0196
46. Ismail AF, McGrath CP, Yiu CKY. Oral health status of children with type 1 diabetes: a comparative study. J Pediatr Endocrinol Metab. (2017) 30:1155–9. doi: 10.1515/jpem-2017-0053
47. Babu KLG, Subramaniam P, Kaje K. Assessment of dental caries and gingival status among a group of type 1 diabetes mellitus and healthy children of South India - a comparative study. J Pediatr Endocrinol Metab. (2018) 31:1305–10. doi: 10.1515/jpem-2018-0335
48. Zhang P, Zhang X, Betz Brown J. Economic impact in diabetes. International Diabetes Federation Diabetes Atlas. Brussels (2009).
49. Soltesz G, Patterson CC, Dahlquist G. Worldwide childhood type 1 diabetes incidence-what can we learn from epidemiology? Pediatric Diabetes. (2007) 8:6–14. doi: 10.1111/j.1399-5448.2007.00280.x
50. EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet. (2000) 355:873–6. doi: 10.1016/S0140-6736(99)07125-1
51. Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. (1993) 16:329–34. doi: 10.2337/diacare.16.1.329
52. Roy M, Gastaldi G, Courvoisier DS, Mombelli A, Giannopoulou C. Periodontal health in a cohort of subjects with type 1 diabetes mellitus. Clin Exp Dent Res. (2019) 5:243–9. doi: 10.1002/cre2.178
53. Lindhe J, Niklaus P, Karring T. Clinical Periodontology and Implant Dentistry. 5th ed. Oxford: Blackwell Munksgaard (2008).
54. Borges I Jr, Moreira EA, Filho DW, de Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm. (2007) 2007:45794. doi: 10.1155/2007/45794
55. Mahalakshmi K, Arangannal P, Santoshkumari. Frequency of putative periodontal pathogens among type 1 diabetes mellitus: a case-control study. BMC Res Notes. (2019). 12:328. doi: 10.1186/s13104-019-4364-3
56. Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. (2006) 20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006
Keywords: type 1 diabetes, periodontitis, periodontal disease, children, adolescents
Citation: Rapone B, Corsalini M, Converti I, Loverro MT, Gnoni A, Trerotoli P and Ferrara E (2020) Does Periodontal Inflammation Affect Type 1 Diabetes in Childhood and Adolescence? A Meta-Analysis. Front. Endocrinol. 11:278. doi: 10.3389/fendo.2020.00278
Received: 01 February 2020; Accepted: 14 April 2020;
Published: 05 May 2020.
Edited by:
Barbara Predieri, University of Modena and Reggio Emilia, ItalyReviewed by:
Catherine Giannopoulou, Université de Genève, SwitzerlandCopyright © 2020 Rapone, Corsalini, Converti, Loverro, Gnoni, Trerotoli and Ferrara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biagio Rapone, YmlhZ2lvcmFwb25lNzlAZ21haWwuY29t
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.