- 1Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Reproductive Medicine Center of the First Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
- 3Qilu Children's Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
Background: The aim of this retrospective study was to analyze the association between prolactin (PRL) and metabolic parameters in infertile patients with polycystic ovary syndrome (PCOS).
Methods: A total of 2,052 patients with PCOS and 9,696 patients with tubal infertility (non-PCOS) undergoing in vitro fertilization and embryo transfer (IVF-ET) at the reproductive medicine center of the first affiliated hospital of Wenzhou Medical University from January 2007 to July 2017 were enrolled in this study. Serum PRL, basic endocrine hormones, fasting plasma lipid, fasting plasma glucose (FPG), liver function, thyroid hormone and other parameters were measured and analyzed.
Result: PRL levels were significantly lower in PCOS patients than controls over all age groups (p < 0.05). In the PCOS patients, serum PRL was significantly and positively correlated with FPG, serum TSH and serum FT4, and significantly and negatively correlated with LH, LH/FSH, TC, TG, LDL-C, AST, ALT, γ-GGT, FT3, and FT3/FT4 (p < 0.05 or 0.01). After adjusted for age and body mass index (BMI), serum PRL was positively correlated with FPG, TSH, and FT4, and negatively correlated with LH and LH/FSH.
Conclusion: Low serum PRL may be an important cause of metabolic risk in infertile patients with PCOS.
Background
Studies have demonstrated that prolactin (PRL) has more than 300 other functions in addition to regulating the development of breast and lactation (1–5). Among them, the regulatory roles of PRL in metabolism has been a focus of research in recent years.
PRL may be secreted by macrophages in the adipose tissue in response to inflammation and hyperglycemia (6), promoting the formation of adipose and inhibiting the decomposition of lipid in the adipose tissue (7). The role of PRL in promoting insulin secretion in islets is well-demonstrated (8). PRL stimulates the proliferation and survival of islet β cells, improves the quality of β cells and enhances the secretion of insulin and sensitizes the liver to insulin (9–11). Study has shown that PRL can affect metabolism homeostasis, glucose and lipid storage by regulating key enzymes and transporters related to glucose and lipid metabolism in the target organs (12). Therefore, PRL has been suggested to be closely associated with insulin resistance, hypertension, thrombembolia, stroke and coronary syndrome (13–17).
Serum PRL is closely related to metabolism. It has been demonstrated that there is a significant association between hyperprolactinemia and all-cause mortality and specific cardiovascular disease mortality (18, 19). However, the associations between PRL and metabolic risk factors are different when serum PRL is within and outside the normal range. Low serum PRL within the physiological range has a negative correlation with insulin sensitivity and plasma glucose level (20–22), and is often associated with poor metabolic outcomes of metabolic syndrome and type 2 diabetes (23–25). Muldon et al. confirmed that person meeting either the National Cholesterol Education Program or International Diabetes Federation criteria for the metabolic syndrome has lower mean PRL response (26). Ruiz-Herrera et al. and a large cohort study in 2013 also confirmed that lower level of serum PRL disrupts the metabolic balance (21, 27). PRL levels in men and women with impaired glucose tolerance, type 2 diabetes and insulin resistance and children with metabolic syndrome and obesity are lower, and might increase after lifestyle intervention in obese children (23). In other words, the high serum PRL within normal physiological range gives rise to better insulin sensitivity, better distribution of adipose tissue and ultimately improved metabolic disorders. However, if the level is outside the normal physiological range, the effect would reverse (19).
Despite of numerous studies, little is known about the correlation between serum PRL and metabolism in infertile females. PCOS patients are a very important subset of infertile women. PCOS is closely associated with hyper-inflammation, insulin resistance, impaired glucose tolerance, metabolic syndrome, hyperlipidemia, hypertension, and increased incidence of cardiovascular disease (28–33). Glintborg et al. found that serum PRL in hirsutism and/or PCOS patients are significantly lower than in healthy controls, and are negatively correlated with LDL-C (34). This study aimed to analyze the serum PRL level in a large number of infertile PCOS patients and its correlation with various types of metabolic indicators to better understand the relationship between PRL and metabolism.
Methods
Patients
This was a retrospective study including a total of 2,052 PCOS patients and 9,696 patients with tubal infertility (non-PCOS) who underwent in vitro fertilization and embryo transfer (IVF-ET) from January 2007 to July 2017 at the reproductive medicine center of the first affiliated hospital of Wenzhou Medical University. PCOS patients were enrolled according to the diagnostic criteria recommended by ESHRE/ASRM in 2004 (35). All patients were Chinese women and were included if they were treated with IVF-ET (fresh cycle) for the first time and had the normal range of serum PRL levels. Because the legal marriage age is 20 years and up and only legally married women are eligible for IVF-ET, and the incidence of metabolic disorders such as endocrine dysfunction, hypertension, hyperlipidemia and diabetes will increase after 40 years old, the inclusive age of the patients in the study was set at 20–40 years to minimize the impact of other healthy conditions of patients on the results of the study.
Patients were excluded if she smoked, treated with any medication in 3 months, had hypertension, diabetes, hyperlipemia, cardiovascular disease, hyperthyreosis, or hypothyroidism, positive HBsAg reaction, C hepatitis, abnormal liver function, chronic nephritis, renal dysfunction or pituitary microadenoma. Patients were also excluded if she had unexplained infertility, recurrent abortion, congenital abnormalities, congenital adrenal hyperplasia, Cushing syndrome or androgen secreting tumors. Informed consent was obtained from every patient and the study protocols were approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Sampling and Patient Data Collection
Patient information about smoking, diseases, past-operation and medical therapy were questioned and recorded. Fasting blood samples were collected between 9 and 11 am in the morning at least 2 h after wake-up and 8 h after fasting on day 2–5 of a menstrual cycle. The height (m) and weight (kg) were measured to calculate body mass index [BMI, BMI = weight (kg)/height (m2)]. Blood pressure was taken twice in an interval of 2 min after at least 10 min rest using a mercury sphygmomanometer.
Laboratory Tests
PRL, FSH, LH, T, E2 TSH, FT3, and FT4 levels in blood samples were measured using chemiluminescence assay on UniCel® DxI 800 Immunoassay System (Beckman Coulter, USA) with commercial kits (Access Prolactin, Access hFSH, Access hLH, Access Testosterone, Access Estradiol, Access TSH, Access Free T3 and Access Free T4, Beckman Coulter, USA) according to manufacturer's and supplier's instructions. FPG, TC, TG, LDL-C, HDL-C, uric acid and liver function were measured using Cobas 8000 modular analyzer and kits [Glucose HK, Gen.3 (GLUC3), Triglycerides (TRIGL), Cholesterol Gen.2 (CHOL2), Aspartate Aminotransferase (ASTL), Alanine aminotransferase acc. to IFCC (ALTL), r-Glutamyltransferase ver. 2(GGT-2) and Alkaline Phosphatase acc. to IFCC Gen.2 (ALP2), Diagnostics Gmb, Germany; Cholestest LDL, Cholestest HDL, SEKISUI MEDICAL CO., LTD. Japan] according to manufacturer's instructions. The detection limit for PRL was 20 mIu/L. If serum PRL is over >25 μg/L (or 530 mIu/L), it is considered to be hyperprolactinemia (36, 37). However, due to variation in detection method and kit used, the normal range of PRL varies slightly among hospitals. Based on the method and kit used at our hospital and published consensus on diagnosis and treatment of hyperprolactinemia, the normal range was set between 70.81 and 566.46 mIu/L (38).
Statistical Analysis
The normal distribution of continuous data was tested by Shapiro-Wilk test. Parameters were not normally distributed and were therefore described using medians and quartiles. The Mann-Whitney test was used to compare differences between PCOS patients and controls. The Kruskal-Wallis test was used to compare differences among the four PCOS patient groups according to PRL level. The Chi-square test was used to compare the differences in frequencies between groups. Regression analyses were used to adjust for differences in BMI between PCOS patients and controls. The correlation among variables was analyzed using the Spearman correlation analysis. Multivariate linear regression was used to analyze the effect of variables on PRL. All statistical analyses were performed with SPSS 22. p < 0.05 were considered significant.
Results
Effect of Age-Groups on PRL, Endocrine, and Other Metabolic Parameters
After adjusted for BMI, the levels of PRL and FSH were significantly lower, while systolic blood pressure (SBP), diastolic blood pressure (DBP), LH, LH/FSH, testosterone (T), TG, TC, LDL-C, AST, ALT, γ -GGT, ALP, and uric acid were significantly higher in PCOS patients than in non- PCOS patients group (Table 1; p < 0.05 or p < 0.01) in all age groups. PCOS patients older than 31 years had significantly lower HDL-C level and significantly higher FT3/FT4 than control (Table 1; p < 0.05 or p < 0.01 and Supplementary Table 1).
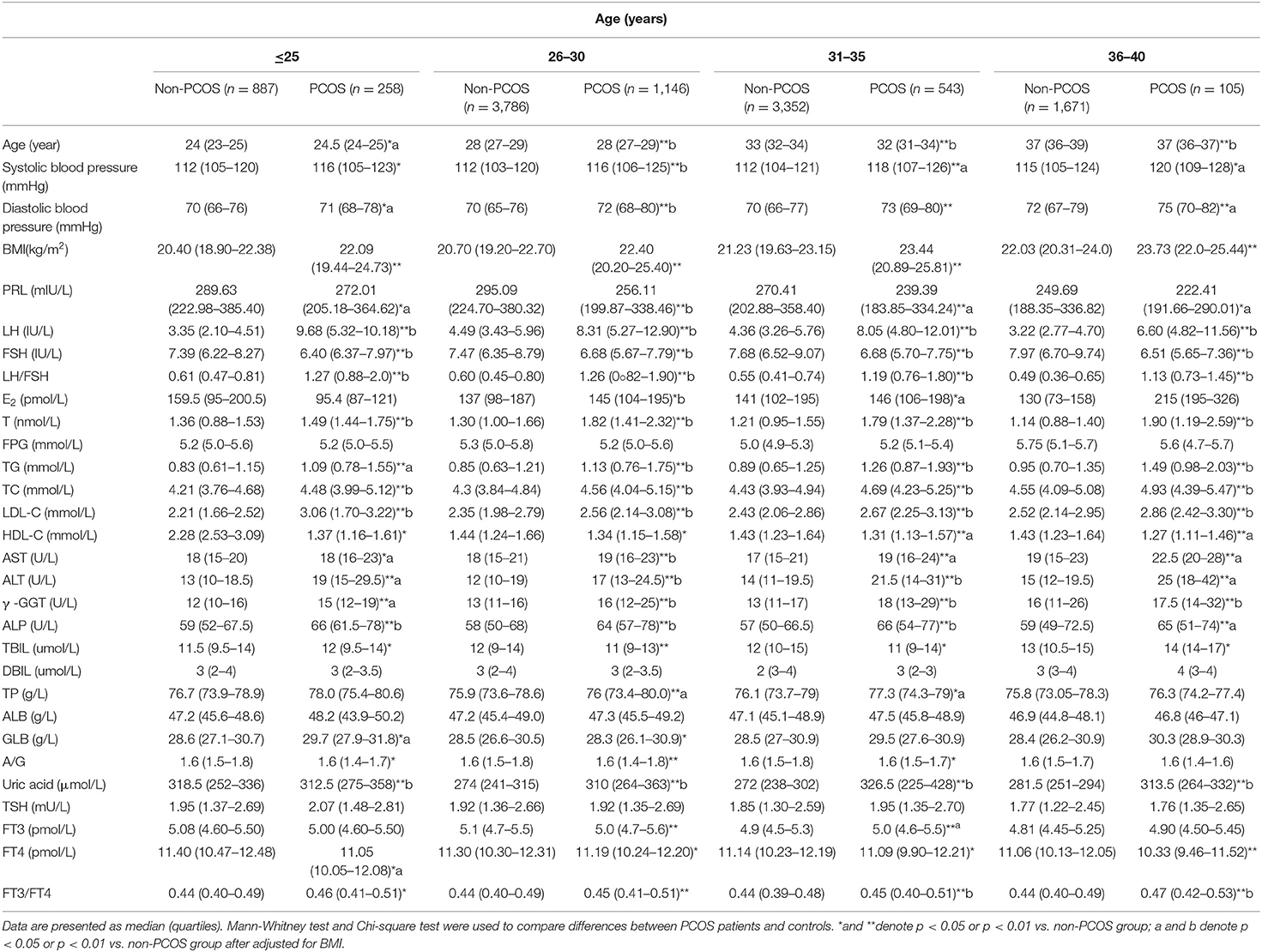
Table 1. Comparison of PRL, endocrine, and other metabolic parameters in PCOS and controls of different age groups.
Association Between PRL and Endocrine Metabolic Parameters
To analyze the association between PRL and metabolic parameters in PCOS patients, we grouped the patients according to the quartile PRL levels and analyzed their differences (Table 2). The results showed that in lower PRL quartile, the age, BMI, LH, LH/FSH, TG, TC, LDL-C, AST, ALT, y-GGT, FT3, and FT3/FT4 were higher, while FPG, TSH, and FT4 were lower (p < 0.05 or p < 0.01).
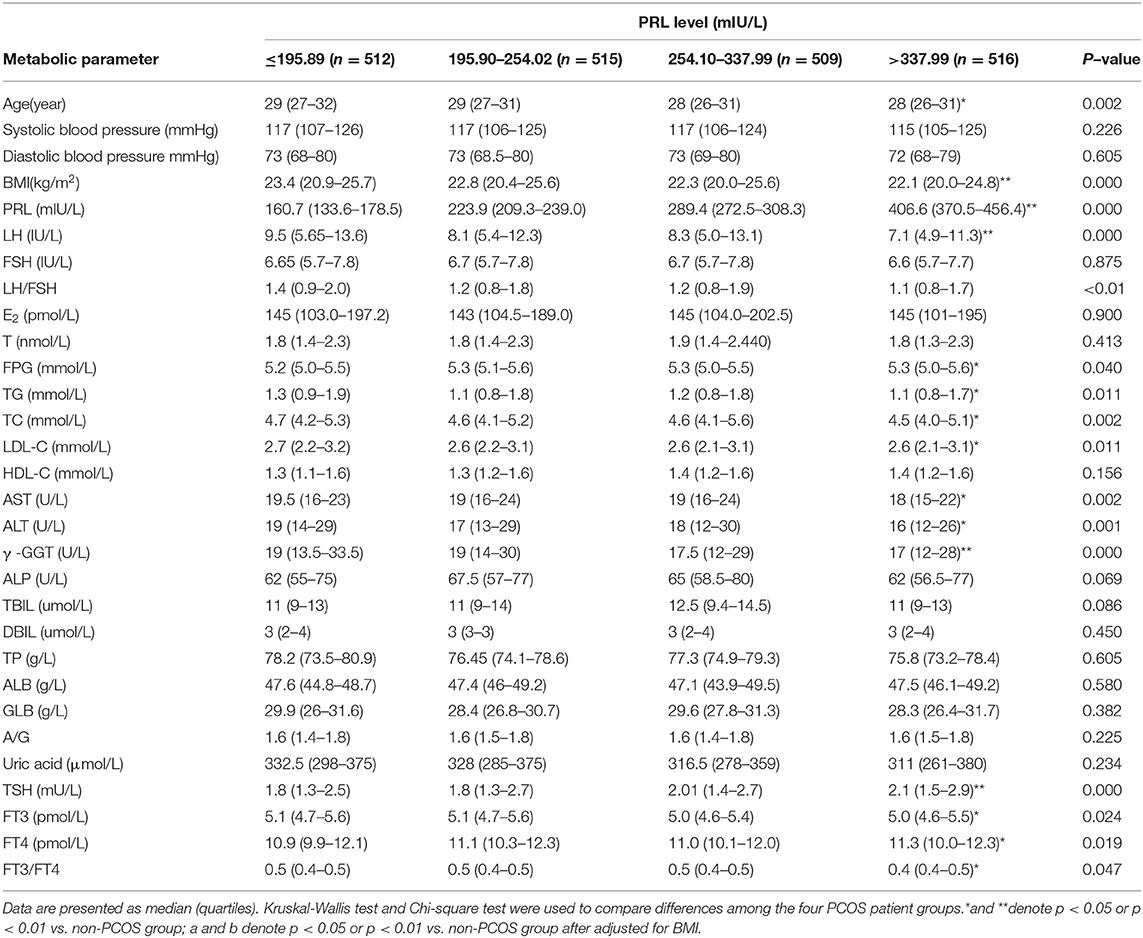
Table 2. Comparison of endocrine and other metabolic parameters in PCOS patients with different PRL levels.
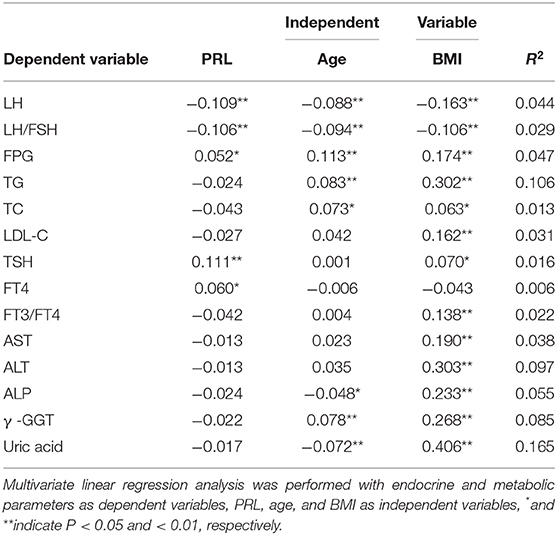
Multivariate Linear Regressions Between PRL and Metabolic Parameters
To further analyze the association between PRL and metabolic parameters, we perform Spearman correlation analysis in the PCOS patients. The results showed that PRL was significantly and positively correlated to FPG (R =0.005, p < 0.05), TSH (R = 0.118, p < 0.01), FT4 (R = 0.048, p < 0.05), and negatively correlated to age (R = −0.093, p < 0.01), BMI (R = −0.115, p < 0.01), LH (R = −0.100, p < 0.01), FH/LSH (R = −0.100, p < 0.01), TG (R = −0.067, p < 0.01), TC (R = −0.089, p < 0.01), LDL-C (R = −0.074, p < 0.01), AST (R = −0.060, p < 0.05), ALT (R = −0.089, p < 0.01), γ -GGT (R = −0.110, p < 0.01), FT3/FT4 (R = −0.070, p < 0.01), and uric acid (R = −0.049, p < 0.05). To adjust the effect of age and BMI, multiple linear regression was used to analyze the relationship between PRL and metabolic parameters. PRL was found to be positively correlated to FPG, TSH, and FT4, and negatively correlated to LH and LH/FSH (Table 3; p < 0.05 or p < 0.01).
Discussion
The secretion of PRL is influenced by many factors, such as age and smoking status (39). To exclude the influence of age, we divided the patients into different age groups. The results showed that PRL is significantly lower in PCOS patients than controls in each age group. After adjusted for BMI effect, the difference is still significant. This is consistent with previous study (34). Smoking is shown to reduce serum PRL (40). Glintborg et al. suggested that smoking should be taken into consideration when analyzing the relationship between PRL and metabolic risks (34). However, the percentage of smoking women is only 2.7% for women aged over 15 years in 2019 according to a report from Chinese Center for Disease Control and Prevention. Therefore, smokers were excluded and smoking was not analyzed in our study.
E2 can effectively stimulate PRL secretion through pituitary and hypothalamus (41, 42). Dombrowicz et al. suggested that PRL could directly stimulate T production (43). However, we did not find any difference in E2 and T levels among the groups. We found significant negative correlation between serum PRL and LH and LH/FSH. Under certain physiological and pathological conditions, high serum PRL above the normal range can directly inhibit the synthesis and release of gonadotropin releasing hormone (GnRH) from the hypothalamus (44) and reduce the secretion of GnRH to the portal vein (45), and high serum PRL also can suppress kisspeptin neurons in the arcuate nucleus (46), resulting in the suppressing the frequency and amplitude of FSH and LH pulses (47, 48). Whether the significant negative relationship between serum PRL and LH or LH/FSH found in our study is an indication that serum PRL in normal range would suppress the secretion of gonadotropin is still not clear. PCOS patients tend to have low quality of life and low mood, which may lead to increased secretion of dopamine and reduced serum PRL levels (18, 49, 50). These may partially explain the negative correlations among PRL, LH and LH/FSH found in this study.
Current studies suggest that serum PRL interacts with glucose metabolism (51). On one side, serum PRL at different levels has different effects on glucose metabolism. Hyperprolactinemia patients were more likely to develop impaired glucose tolerance and insulin resistance (18, 52, 53). These changes can be reduced by using dopamine receptor agonists that reduce serum PRL levels. However, when serum PRL is in normal physiological range, its relationship with glucose metabolism is reversed (22, 27, 54). Low serum PRL may indicate a higher risk of developing metabolic syndrome and type 2 diabetes mellitus (23, 24). On the other side, PRL secretion is affected by blood glucose level. When the blood glucose decreases, the glucose receptor in the hypothalamic neurons will transmit the information to related neurons, stimulating TRH cells and finally regulating PRL secretion (55). However, our study did not find any difference in FPG between PCOS and non-PCOS patients, although serum PRL and FPG is positively correlated in the PCOS patients. These results are inconsistent with previous studies. We speculate that infertile PCOS patients with normal blood glucose may be a special group, whose glucose metabolism is somewhat different from healthy population, although the specific reasons and mechanism are unclear.
Our study found that the BMI of PCOS patients in all age groups is significantly higher than that of non-PCOS patient and the lower serum PRL the higher the BMI. This is consistent with earlier study (56). PRL is produced and released by fat cells and glands in normal human breasts (57), as well as by macrophages in the adipose tissue in response to inflammation and hyperglycemia (6). It promotes the formation of adipose and inhibits the breakdown of lipid in the adipose tissue (7). PRL released from subcutaneous adipose tissue grafted from obese patients is significantly lower than that from non-obese patients (58) and serum PRL of obese children is lower than that of non-obese children (23). Although PRL release is affected by obesity, the mechanism and consequences of decreased PRL release due to obesity are still largely unknown. But, some study showed that PRL is not related to BMI in obese and non-obese patients, and basic serum PRL not related to BMI (59). Our results support the view that obesity decreases PRL release and lows serum PRL. In addition, we showed that even after removing the effects of BMI, the levels of TG, TC and LDL-C in PCOS patients are still significantly higher than in non-PCOS patients and PRL is negatively correlated with TG, TC and LDL-C in the PCOS patients. This is basically consistent with the previous results (60). It can be concluded that lipid metabolism in PCOS patients is abnormal. Since serum PRL is closely related to lipid metabolism, low PRL in physiological range would lead to more serious disorder of lipid metabolism.
Prolactin receptor (PRLR) is highly expressed in the liver, which is the key organ to maintain the homeostasis of metabolism. However, little is known about the role of serum PRL in the liver. Zhang et al. evaluated the expression of PRLR and signaling molecules involved in lipid metabolism in human liver and HepG2 cells, and found that the serum PRL in patients with non-alcoholic fatty liver is lower than that of control (61). They believed that there is a new link between the central nervous system and the liver, and PRL improves steatosis of the liver through PRLR and fat/CD36. In our PCOS patients, the serum PRL level was within normal range and patients with hepatitis and abnormal liver function were excluded The analysis showed that PRL is negatively correlated with AST, ALT, γ –GGT, and ALP, suggesting that lower serum PRL may damage liver cells. Even after removing the effects of BMI, the levels of AST, ALT γ -GGT, ALP, and uric acid are significantly higher in PCOS and in non-PCOS patients. Moreover, our results showed that the lower the serum PRL, the higher the serum TG, TC, and LDL. Therefore, it is necessary to further study whether there are other mechanisms related to hepatic microcirculatory dysfunction caused by lipid metabolism disorder under low PRL condition. Since PRL regulates enzymes and transporters related to glucose and lipids in other target organs, future studies should be performed to investigate the pathophysiological effects on liver tissue.
Thyroid gland and gonad are controlled by pituitary gland. The secretion of PRL and TSH is controlled by the neuroendocrine regulation mechanism of triiodothyronine (T3), thyroxine (T4), thyrotropin releasing hormone (TRH), and dopamine. TRH can not only promote the release of TSH, but also promote the release of PRL (62, 63). TSH is confirmed to be closely associated with metabolic syndrome (64). On the one hand, thyroid hormone acts on tissues and organs such as the liver and participate in the synthesis and decomposition of TG and TC through multiple pathways (65). On the other hand, TSH receptor is also expressed in tissues other than thyroid, such as liver and adipocytes, where TSH interacts with the receptors to play a number of biological functions (61, 66, 67). For example, TSH can promote the synthesis of cholesterol in hepatocytes in a dose-time-dependent manner after binding to the receptor on the membrane of hepatocytes, and can also reduce the activity of cholesterol 7 α-hydroxylase to participate in cholesterol transformation (66). Human adipocytes and preadipocytes also have TSH receptors. TSH can induce preadipocytes to differentiate into adipocytes by binding to the receptors, thus promoting adipogenesis (68). TSH can also facilitate the decomposition of lipid by phosphorylating lipid body coating proteins and hormone- sensitive lipase (69). Most of the previous studies showed that the level of TSH is positively correlated with TG, TC and LDL-C in either hypothyroidism, subclinical hypothyroidism or normal population (70–72). We found that PRL is positively related to TSH. However, in low PRL patients, TSH is lower but TG, TC, and LDL-C are higher, which is contrary to previous studies. This difference might be attributed to the differences in metabolic mechanisms between the PCOS infertile patients and other populations. Well-designed prospective studies are needed to have a better understand of involvement and pathophysiological significance of PRL in lipid metabolism via TSH, and would help elucidate the differences.
In addition, in healthy young men, the less favorable body compositions (or higher fat and lower muscle mass, with higher leptin concentration) are shown to be correlated with insulin resistance and FT3 levels in normal thyroid function (73) and in healthy middle-aged men and women with normal thyroid function, TSH, FT3, and FT3/FT4 are positively correlated with BMI, waist circumference and components of metabolic syndrome (such as TG, SBP, DBP, and FPG), and negatively correlated with FT4 (74). We found that SBP and DBP in different age groups and FT3/FT4 in patients older than 31 years are significantly higher in PCOS and non-PCOS patients, while FT4 is significantly lower. In our cohort, PRL is positively related to FT4, and negatively to FT3/FT4 and FT3 (although not statistically significant) and to TG, TC, and LDL-C. These results are consistent with previous studies. Therefore, we concluded that the low PRL in infertile PCOS patients is an effective marker of poor metabolic spectrum and higher cardiovascular risk.
As a retrospective analysis, there were a number of limitations in this study. For instance, PRL secretion occurs as pulse and is better measured in repeated samples from venous catheter to ensure the accuracy. In addition, the ratio of waist to hip is closely related to metabolism, but the relevant data was not available. For thyroid function assessment, information on thyroid-associated antibodies was absent. Patients with fatty liver were unable to be excluded due to the lack of ultrasound examination. Furthermore, only fasting blood glucose content, no fasting insulin data and glucose tolerance data were available.
In conclusion, our study suggested that low serum PRL may be an important cause of metabolic risk in infertile PCOS patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital, Wenzhou Medical University, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HY and JD: project conceptualization, investigation, and data analysis. JD, JP, and RY: data collection, analysis, and methodology development. RY and YT: data collection, analysis, and methodology development. ZC and XD: investigation and methodology development. XD: project conceptualization and manuscript writing. HY, JD, and XD: project conceptualization, investigation, and manuscript writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00263/full#supplementary-material
Abbreviations
PRL, Prolactin; PCOS, Polycystic ovary syndrome; IVE-ET, In vitro fertilization and embryo transfer; FPG, Fasting plasma glucose; BMI, Body mass index; TSH, Thyroid stimulating hormone; FT4, Free thyroxine; FT3, Free triiodothyronine; LH, Luteinizing hormone; FSH, Follicle stimulating hormone; LDL-C, Low density lipoprotein cholesterol; HDL-C, High density lipoprotein cholesterol; TC, Total cholesterol; TG, Triglyceride; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; Y-GGT, Glutaminyl transferasel; ALP, Alkaline phosphatase; T, Testosterone; E2, Estradiol; TBIL, Total bilirubin; DBil, Direct bilirubin; TP, Total protein; ALB, Albumin; SBP, systolic blood pressure; DBP, diastolic blood pressure.
References
1. Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol. (2002) 64:47–67. doi: 10.1146/annurev.physiol.64.081501.131049
2. Patil M, Belugin S, Mecklenburg J, Wangzhou A, Paige C, Barba-Escobedo PA, et al. Prolactin regulates pain responses via a female-selective nociceptor-specific mechanism. iScience. (2019) 20:449–65. doi: 10.1016/j.isci.2019.09.039
3. Arnold E, Thebault S, Arona RM, Martinez de la Escalera G, Clapp C. Prolactin mitigates deficiencies of retinal function associated with aging. Neurobiol Aging. (2020) 85:38–48. doi: 10.1016/j.neurobiolaging.2019.10.002
4. Jiao P, Yuan Y, Zhang M, Sun Y, Wei C, Xie X, et al. PRL/microRNA-183/IRS1 pathway regulates milk fat metabolism in cow mammary epithelial cells. Genes. (2020) 11:196. doi: 10.3390/genes11020196
5. Rahbar A, AlKharusi A, Costa H, Pantalone MR, Kostopoulou ON, Cui HL, et al. Human cytomegalovirus infection induces high expression of prolactin and prolactin receptors in ovarian cancer. Biology. (2020) 9:E44. doi: 10.3390/biology9030044
6. Bouckenooghe T, Sisino G, Aurientis S, Chinetti-Gbaguidi G, Kerr-Conte J, Staels B, et al. Adipose tissue macrophages (ATM) of obese patients are releasing increased levels of prolactin during an inflammatory challenge: a role for prolactin in diabesity? Biochim Biophys Acta. (2014) 1842:584–93. doi: 10.1016/j.bbadis.2013.12.005
7. Carre N, Binart N. Prolactin and adipose tissue. Biochimie. (2014) 97:16–21. doi: 10.1016/j.biochi.2013.09.023
8. Grattan DR, Kokay IC. Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol. (2008) 20:752–63. doi: 10.1111/j.1365-2826.2008.01736.x
9. Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. (2002) 143:1378–85. doi: 10.1210/endo.143.4.8722
10. Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology. (2009) 150:1618–26. doi: 10.1210/en.2008-1003
11. Yu J, Xiao F, Zhang Q, Liu B, Guo Y, Lv Z, et al. PRLR regulates hepatic insulin sensitivity in mice via STAT5. Diabetes. (2013) 62:3103–13. doi: 10.2337/db13-0182
12. Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. (2006) 17:110–6. doi: 10.1016/j.tem.2006.02.005
13. Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gozu H, Sezgin O, et al. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. Eur J Endocrinol. (2003) 149:187–93. doi: 10.1530/eje.0.1490187
14. Raaz D, Wallaschofski H, Stumpf C, Yilmaz A, Cicha I, Klinghammer L, et al. Increased prolactin in acute coronary syndromes as putative Co-activator of ADP-stimulated P-selectin expression. Horm Metab Res. (2006) 38:767–72. doi: 10.1055/s-2006-955090
15. Muldoon MF, Mackey RH, Sutton-Tyrrell K, Flory JD, Pollock BG, Manuck SB. Lower central serotonergic responsivity is associated with preclinical carotid artery atherosclerosis. Stroke. (2007) 38:2228–33. doi: 10.1161/STROKEAHA.106.477638
16. Georgiopoulos GA, Stamatelopoulos KS, Lambrinoudaki I, Lykka M, Kyrkou K, Rizos D, et al. Prolactin and preclinical atherosclerosis in menopausal women with cardiovascular risk factors. Hypertension. (2009) 54:98–105. doi: 10.1161/HYPERTENSIONAHA.109.132100
17. van Zaane B, Squizzato A, Reuwer AQ, van Zanten AP, Twickler MT, Dekkers OM, et al. Prolactin and venous thrombosis: indications for a novel risk factor? Arterioscler Thromb Vasc Biol. (2011) 31:672–7. doi: 10.1161/ATVBAHA.110.209569
18. Serri O, Li L, Mamputu JC, Beauchamp MC, Maingrette F, Renier G. The influences of hyperprolactinemia and obesity on cardiovascular risk markers: effects of cabergoline therapy. Clin Endocrinol. (2006) 64:366–70. doi: 10.1111/j.1365-2265.2006.02469.x
19. Haring R, Friedrich N, Volzke H, Vasan RS, Felix SB, Dorr M, et al. Positive association of serum prolactin concentrations with all-cause and cardiovascular mortality. Eur Heart J. (2014) 35:1215–21. doi: 10.1093/eurheartj/ehs233
20. Friedrich N, Rosskopf D, Brabant G, Volzke H, Nauck M, Wallaschofski H. Associations of anthropometric parameters with serum TSH, prolactin, IGF-I, and testosterone levels: results of the study of health in Pomerania (SHIP). Exp Clin Endocrinol Diabetes. (2010) 118:266–73. doi: 10.1055/s-0029-1225616
21. Balbach L, Wallaschofski H, Volzke H, Nauck M, Dorr M, Haring R. Serum prolactin concentrations as risk factor of metabolic syndrome or type 2 diabetes? BMC Endocr Disord. (2013) 13:12. doi: 10.1186/1472-6823-13-12
22. Wagner R, Heni M, Linder K, Ketterer C, Peter A, Bohm A, et al. Age-dependent association of serum prolactin with glycaemia and insulin sensitivity in humans. Acta Diabetol. (2014) 51:71–8. doi: 10.1007/s00592-013-0493-7
23. Chirico V, Cannavo S, Lacquaniti A, Salpietro V, Mandolfino M, Romeo PD, et al. Prolactin in obese children: a bridge between inflammation and metabolic-endocrine dysfunction. Clin Endocrinol. (2013) 79:537–44. doi: 10.1111/cen.12183
24. Wang T, Lu J, Xu Y, Li M, Sun J, Zhang J, et al. Circulating prolactin associates with diabetes and impaired glucose regulation: a population-based study. Diabetes Care. (2013) 36:1974–80. doi: 10.2337/dc12-1893
25. Corona G, Wu FC, Rastrelli G, Lee DM, Forti G, O'Connor DB, et al. Low prolactin is associated with sexual dysfunction and psychological or metabolic disturbances in middle-aged and elderly men: the European male aging study (EMAS). J Sex Med. (2014) 11:240–53. doi: 10.1111/jsm.12327
26. Muldoon MF, Mackey RH, Korytkowski MT, Flory JD, Pollock BG, Manuck SB. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. J Clin Endocrinol Metab. (2006) 91:718–21. doi: 10.1210/jc.2005-1654
27. Ruiz-Herrera X, de Los Rios EA, Diaz JM, Lerma-Alvarado RM, Martinez de la Escalera L, Lopez-Barrera F, et al. Prolactin promotes adipose tissue fitness and insulin sensitivity in obese males. Endocrinology. (2017) 158:56–68. doi: 10.1210/en.2016-1444
28. Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecol Endocrinol. (2010) 26:281–96. doi: 10.3109/09513590903247873
29. Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, et al. Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin Endocrinol. (2013) 78:926–34. doi: 10.1111/cen.12068
30. Alviggi C, Cariati F, Conforti A, De Rosa P, Vallone R, Strina I, et al. The effect of FT500 Plus[(R)] on ovarian stimulation in PCOS women. Reprod Toxicol. (2016) 59:40–4. doi: 10.1016/j.reprotox.2015.10.014
31. Alviggi C, Conforti A, De Rosa P, Strina I, Palomba S, Vallone R, et al. The Distribution of stroma and antral follicles differs between insulin-resistance and hyperandrogenism-related polycystic ovarian syndrome. Front Endocrinol. (2017) 8:117. doi: 10.3389/fendo.2017.00117
32. Barber TM, Hanson P, Weickert MO, Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health. (2019) 13:1179558119874042. doi: 10.1177/1179558119874042
33. Rimmer M, Tan BK, Teede H, Thangaratinam SBHAW. Metabolic inflexibility in women with polycystic ovary syndrome: a systematic review. Gynecol Endocrinol. (2019) 3:1–7. doi: 10.1080/09513590.2019.1698025
34. Glintborg D, Altinok M, Mumm H, Buch K, Ravn P, Andersen M. Prolactin is associated with metabolic risk and cortisol in 1007 women with polycystic ovary syndrome. Hum Reprod. (2014) 29:1773–9. doi: 10.1093/humrep/deu133
35. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
36. Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Guidelines of the pituitary society for the diagnosis and management of prolactinomas. Clin Endocrinol. (2006) 65:265–73. doi: 10.1111/j.1365-2265.2006.02562.x
37. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:273–88. doi: 10.1210/jc.2010-1692
38. Neurosurgery Branch of Chinese Medical Association., Branch of Obstetrics and Gynecology, CMA., Association EBOCM. Consensus on diagnosis and treatment of hyperprolactinemia. Natl Med J China. (2011) 91:147–55.
39. Glintborg D, Mumm H, Hougaard DM, Ravn P, Andersen M. Smoking is associated with increased adrenal responsiveness, decreased prolactin levels and a more adverse lipid profile in 650 white patients with polycystic ovary syndrome. Gynecol Endocrinol. (2012) 28:170–4. doi: 10.3109/09513590.2011.589926
40. Xirofotos D, Trakakis E, Peppa M, Chrelias C, Panagopoulos P, Christodoulaki C, et al. The amount and duration of smoking is associated with aggravation of hormone and biochemical profile in women with PCOS. Gynecol Endocrinol. (2016) 32:143–6. doi: 10.3109/09513590.2015.1101440
41. Molitch ME. Pharmacologic resistance in prolactinoma patients. Pituitary. (2005) 8:43–52. doi: 10.1007/s11102-005-5085-2
42. Roelfsema F, Pijl H, Keenan DM, Veldhuis JD. Prolactin secretion in healthy adults is determined by gender, age and body mass index. PLoS ONE. (2012) 7:e31305. doi: 10.1371/journal.pone.0031305
43. Dombrowicz D, Sente B, Closset J, Hennen G. Dose-dependent effects of human prolactin on the immature hypophysectomized rat testis. Endocrinology. (1992) 130:695–700. doi: 10.1210/endo.130.2.1733717
44. Tay CC, Glasier AF, McNeilly AS. The 24 h pattern of pulsatile luteinizing hormone, follicle stimulating hormone and prolactin release during the first 8 weeks of lactational amenorrhoea in breastfeeding women. Hum Reprod. (1992) 7:951–8. doi: 10.1093/oxfordjournals.humrep.a137777
45. Sarkar DK, Frautschy SA, Mitsugi N. Pituitary portal plasma levels of oxytocin during the estrous cycle, lactation, and hyperprolactinemia. Ann N Y Acad Sci. (1992) 652:397–410. doi: 10.1111/j.1749-6632.1992.tb34370.x
46. Araujo-Lopes R, Crampton JR, Aquino NS, Miranda RM, Kokay IC, Reis AM, et al. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. (2014) 155:1010–20. doi: 10.1210/en.2013-1889
47. Grattan DR, Selmanoff M. Prolactin- and testosterone-induced inhibition of LH secretion after orchidectomy: role of preoptic and tuberoinfundibular gamma-aminobutyric acidergic neurones. J Endocrinol. (1994) 143:165–74. doi: 10.1677/joe.0.1430165
48. Silva JF, Henriques PC, Campideli-Santana AC, Araujo-Lopes R, Aquino NSS, Hipolito LTM, et al. Estradiol potentiates but is not essential for prolactin-induced suppression of luteinizing hormone pulses in female rats. Endocrinology. (2020) 161:bqaa022. doi: 10.1210/endocr/bqaa022
49. Hahn S, Janssen OE, Tan S, Pleger K, Mann K, Schedlowski M, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol. (2005) 153:853–60. doi: 10.1530/eje.1.02024
50. Altinok ML, Glintborg D, Depont Christensen R, Hallas J, Andersen M. Prescription of antidepressants is increased in danish patients with polycystic ovary syndrome and is associated with hyperandrogenism. A population-based cohort study. Clin Endocrinol. (2014) 80:884–9. doi: 10.1111/cen.12365
51. Al-Nami MS, Al-Kuraishy HM, Al-Gareeb AI, Al-Mamoori F. Metabolic profile and prolactin serum levels in men with type 2 diabetes mellitus: old-new rubric. Int J Crit Illn Inj Sci. (2019) 9:120–6. doi: 10.4103/IJCIIS.IJCIIS_40_19
52. Pala NA, Laway BA, Misgar RA, Dar RA. Metabolic abnormalities in patients with prolactinoma: response to treatment with cabergoline. Diabetol Metab Syndr. (2015) 7:99. doi: 10.1186/s13098-015-0094-4
53. Auriemma RS, De Alcubierre D, Pirchio R, Pivonello R, Colao A. Glucose abnormalities associated to prolactin secreting pituitary adenomas. Front Endocrinol. (2019) 10:327. doi: 10.3389/fendo.2019.00327
54. Wang T, Xu Y, Xu M, Ning G, Lu J, Dai M, et al. Circulating prolactin and risk of type 2 diabetes: a prospective study. Am J Epidemiol. (2016) 184:295–301. doi: 10.1093/aje/kwv326
55. Macotela Y, Triebel J, Clapp C. Time for a new perspective on prolactin in metabolism. Trends Endocrinol. Metab. (2020) 31:276–86. doi: 10.1016/j.tem.2020.01.004
56. Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab. (2004) 89:266–71. doi: 10.1210/jc.2003-031295
57. Zinger M, McFarland M, Ben-Jonathan N. Prolactin expression and secretion by human breast glandular and adipose tissue explants. J Clin Endocrinol Metab. (2003) 88:689–96. doi: 10.1210/jc.2002-021255
58. Hugo ER, Borcherding DC, Gersin KS, Loftus J, Ben-Jonathan N. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J Clin Endocrinol Metab. (2008) 93:4006–12. doi: 10.1210/jc.2008-1172
59. Ernst B, Thurnheer M, Schultes B. Basal serum prolactin levels in obesity–unrelated to parameters of the metabolic syndrome and unchanged after massive weight loss. Obes Surg. (2009) 19:1159–62. doi: 10.1007/s11695-009-9856-0
60. Ponce AJ, Galvan-Salas T, Lerma-Alvarado RM, Ruiz-Herrera X, Hernandez-Cortes T, Valencia-Jimenez R, et al. Low prolactin levels are associated with visceral adipocyte hypertrophy and insulin resistance in humans. Endocrine. (2020) 67:331–43. doi: 10.1007/s12020-019-02170-x
61. Zhang P, Ge Z, Wang H, Feng W, Sun X, Chu X, et al. Prolactin improves hepatic steatosis via CD36 pathway. J Hepatol. (2018) 68:1247–55. doi: 10.1016/j.jhep.2018.01.035
62. Beck-Peccoz P, Persani L. Variable biological activity of thyroid-stimulating hormone. Eur J Endocrinol. (1994) 131:331–40. doi: 10.1530/eje.0.1310331
63. Galas L, Raoult E, Tonon MC, Okada R, Jenks BG, Castano JP, et al. TRH acts as a multifunctional hypophysiotropic factor in vertebrates. Gen Comp Endocrinol. (2009) 164:40–50. doi: 10.1016/j.ygcen.2009.05.003
64. De Pergola G, Giorgino F, Benigno R, Guida P, Giorgino R. Independent influence of insulin, catecholamines, and thyroid hormones on metabolic syndrome. Obesity. (2008) 16:2405–11. doi: 10.1038/oby.2008.382
65. Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. (2012) 97:326–33. doi: 10.1210/jc.2011-2532
66. Zhang W, Tian LM, Han Y, Ma HY, Wang LC, Guo J, et al. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med. (2009) 13:4636–42. doi: 10.1111/j.1582-4934.2008.00670.x
67. Williams GR. Extrathyroidal expression of TSH receptor. Ann Endocrinol. (2011) 72:68–73. doi: 10.1016/j.ando.2011.03.006
68. Bastemir M, Akin F, Alkis E, Kaptanoglu B. Obesity is associated with increased serum TSH level, independent of thyroid function. Swiss Med Wkly. (2007) 137:431–4.
69. Gagnon A, Antunes TT, Ly T, Pongsuwan P, Gavin C, Lochnan HA, et al. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metab Clin Exp. (2010) 59:547–53. doi: 10.1016/j.metabol.2009.08.018
70. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. (2000) 160:526–34. doi: 10.1001/archinte.160.4.526
71. Bell RJ, Rivera-Woll L, Davison SL, Topliss DJ, Donath S, Davis SR. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease - a community-based study. Clin Endocrinol. (2007) 66:548–56. doi: 10.1111/j.1365-2265.2007.02771.x
72. Asvold BO, Bjoro T, Vatten LJ. Associations of TSH levels within the reference range with future blood pressure and lipid concentrations: 11-year follow-up of the HUNT study. Eur J Endocrinol. (2013) 169:73–82. doi: 10.1530/EJE-13-0087
73. Roef G, Lapauw B, Goemaere S, Zmierczak HG, Toye K, Kaufman JM, et al. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol. (2012) 167:719–26. doi: 10.1530/EJE-12-0447
74. Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. (2014) 24:223–31. doi: 10.1089/thy.2013.0314
Keywords: PRL, polycystic ovary syndrome, metabolism, IVF-ET, infertility
Citation: Yang H, Di J, Pan J, Yu R, Teng Y, Cai Z and Deng X (2020) The Association Between Prolactin and Metabolic Parameters in PCOS Women: A Retrospective Analysis. Front. Endocrinol. 11:263. doi: 10.3389/fendo.2020.00263
Received: 14 December 2019; Accepted: 09 April 2020;
Published: 12 May 2020.
Edited by:
Tom Kelsey, University of St Andrews, United KingdomReviewed by:
Giulia Rastrelli, University of Florence, ItalyAlessandro Conforti, University of Naples Federico II, Italy
Copyright © 2020 Yang, Di, Pan, Yu, Teng, Cai and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Deng, dxiaohui2018@163.com
†These authors have contributed equally to this work
 Haiyan Yang1,2†
Haiyan Yang1,2† Xiaohui Deng
Xiaohui Deng