
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 27 February 2020
Sec. Thyroid Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00090
Purpose: To investigate the association between lymph node (N) stage and clinical outcome in thyroid cancer patients with initial distant metastasis.
Methods: A total of 3,198 cases (1,435 males and 1,763 females) between 2004 and 2015 with initial distant metastasis were obtained from the surveillance, epidemiology, and end results (SEER) database. Patients with a median follow up time of 13 months and a median age of 66 years were analyzed. A total of 1,407 cases had detailed information regarding the four most common metastatic organs after the year 2010. Kaplan-Meier (KM) analyses, log-rank tests, Cox regression, and logistic regression analyses were used.
Results: Among the whole cohort, 33.4% (1,069/3,198), 14.5% (464/3,198), 10.1% (322/3,198), 34.2% (1,094/3,198), and 7.8% (249/3,198) of the patients were at the stage of N0, NX, N1a, N1b, and N1NOS (referring to metastasis to regional lymph nodes but not otherwise specified), respectively. The KM curves demonstrated that the patients at the NX stage had the worst survival. The NX and N1b groups had the highest hazard ratios (HRs) of 1.83 (95%CI 1.46-2.31) and 1.78 (95%CI 1.52-2.10) after adjusting age, race, gender, and tumor size (p < 0.001) compared with N0 group. The lung was the most common metastatic site, with a rate of 51.2% (720/1,407). Compared with the N0 group, N1 patients had higher odds (OR 1.63, 95%CI 1.31-2.01, p < 0.001) for lung metastasis. Similar results were obtained in papillary thyroid cancer (PTC) sub-cohort.
Conclusions: Overall, the TC patients at the NX stage had the highest mortality risk, followed by N1b, N1a, and N0 groups. Compared with N0 patients, N1 patients were more likely to have lung metastasis. The poor prognosis for TC patients with the NX stage may make more aggressive treatment reasonable.
The incidence of thyroid cancer (TC) has continued to rise in the United States over the last four decades (1), and TC was reported to be the fifth most common malignant tumor in women in 2018 (2). Based on the recent data, the American Cancer Society has predicted that the number of new TC cases and deaths due to TC will become the sixth most common cause of malignant tumors for women in 2019 (3). Among several variants of TC, papillary thyroid cancer (PTC) takes up 85-90% of all cases (4).
PTC and follicular thyroid cancer (FTC) are classified as differentiated TCs that both arise from thyroid follicular cells and generally have excellent prognoses, while the poorly differentiated thyroid cancers have a poorer prognosis which probably arises from either PTCs or FTCs. Medullary thyroid cancer (MTC) has been derived from the parafollicular C cells, accounting for about 1-2% of TCs (5). Anaplastic thyroid cancer (ATC) originates from follicular cells and is an aggressive, undifferentiated, and rapidly fatal tumor (6).
The American Joint Committee on Cancer (AJCC) Staging Manual 6th Edition (7) sets out the lymph node (N) stages of cancer as follows: no metastatic nodes (N0); nodes not assessed at surgery (NX); metastases to level VI [pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes] (N1a); metastasis to unilateral, bilateral, or contralateral cervical or superior mediastinal mode metastases (N1b). In the 8th edition, lymph node stage of N0 were divided into 2 groups with more detailed information, which referred to N0a (One or more cytologic or histologically confirmed benign lymph node) and N0b (no radiologic or clinical evidence of local regional lymph node metastasis). However, the N1a stage in the 8th edition, which can be unilateral or bilateral disease, covers not only the lymph node VI but also the lymph node VII (the upper mediastinal lymph nodes). Additionally, in the 8th edition, the N1b stage referred to metastasis to levels I, II, III, IV, or V (unilateral, bilateral, or contralateral lateral neck lymph nodes) or retropharyngeal lymph nodes, which include all disease in the lateral neck (8). Compared with 6th edition, the other N stages of 8th edition remain unchanged. In the United States, it has been reported that 5% of TC patients have distant metastasis (9), while the most commonly observed metastatic sites have been reported as lung and bone, followed by brain and liver (10).
Although previous studies have shown that TC patients have favorable survival outcomes, with a mortality rate of ~10% and a propensity to have lymph node metastases (11, 12), certain lymph node involvement was reported to have an adverse impact on the prognosis for TC (13). Moreover, the prognosis for NX patients and the association between N stage and distant metastasis remain unknown. In addition, most of these studies were based on data derived from a small sample size population and were limited due to the types of statistical analyses undertaken.
The objective of this study is to investigate the prognosis of TC patients at the NX stage and to determine the association between N stages and distant metastasis in TC based on the surveillance, epidemiology, and end results (SEER) database.
The purpose of this retrospective study is to investigate the association between N stage and distant metastasis, and disease-specific mortality of TC and its common variants, including PTC, FTC, ATC, and MTC. Data on TC patients were retrieved from the SEER database (https://seer.cancer.gov/), which is maintained by the National Cancer Institute. The SEER project is a United States population-based cancer registry that began in 1973 and includes ~34.6% of the US population.
Patients with TC were classified by the 3rd Edition of the International Classification of Diseases for Oncology (ICD-O-3). Lymph node stages were classified into five groups according to the 6th AJCC Staging Manual (7) and SEER as following: N0, NX, N1a, N1b, and N1NOS, in which N1NOS referred to metastasis to regional lymph nodes but not otherwise specified. These groups were analyzed to determine the association between the N stage and the specific mortality of TC and its common variants. In addition, patients with detailed information on the four most common organ metastases after the year 2010 were identified to determine the association between N stage and distant metastasis.
Survival probabilities for TC and its common variants were assessed using Kaplan-Meier (KM) analyses and log-rank tests to compare the differences between Kaplan-Meier curves of different lymph node groups. Cox regression was performed to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) to determine the effects of different N stages on disease-specific mortality in TC and its common variants. The association between N stages and distant organ metastases were analyzed by logistic regression. A two-tailed P < 0.05 was considered to be statistically significant. The Statistical Package for Social Science version 25.0 (SPSS, Inc., New York, NY, USA) was used for data analyses.
A total of 3,198 TC patients (1,435 males and 1,763 females) with initial distant metastasis between 2004 and 2015 were investigated. The median follow-up time was 13 months (interquartile range [IQR], 2-46 months) and the median age was 66 years (IQR, 55-76 years). In addition, 1,407 patients with detailed information concerning the four most common organ metastases after the year 2010 were identified. The clinical characteristics of the whole cohort were shown in Table 1.
Table 2 shows that N1b was the most common N stage for patients with TC in the whole cohort. The incident rate was 33.4% (1,069/3,198), 14.5% (464/3,198), 10.1% (322/3,198), 34.2% (1,094/3,198), and 7.8% (249/3,198) for N0, NX, N1a, N1b, and N1NOS stage, respectively. The lung metastatic rate was 51.2% (720/1,407), and the bone metastatic rate was 19.3% (271/1,407). Among all variants of TC, PTC was the most common type (n = 1,475 patients), and the incidence rate was 31.9% (471/1,475), 8.8% (130/1,475), 13.1% (193/1,475), 38.4% (566/1,475), and 7.8% (115/1,475) for PTC patients at N0, NX, N1a, N1b, and N1NOS stage, respectively. The metastatic rates for bone and lung among patients were 61.8% (379/613) and 19.1% (117/613), respectively. Also, the incidence rate of each N stage and the metastatic rates for patients with other common variants of TC, including FTC, ATC, and MTC were shown in Table 2.
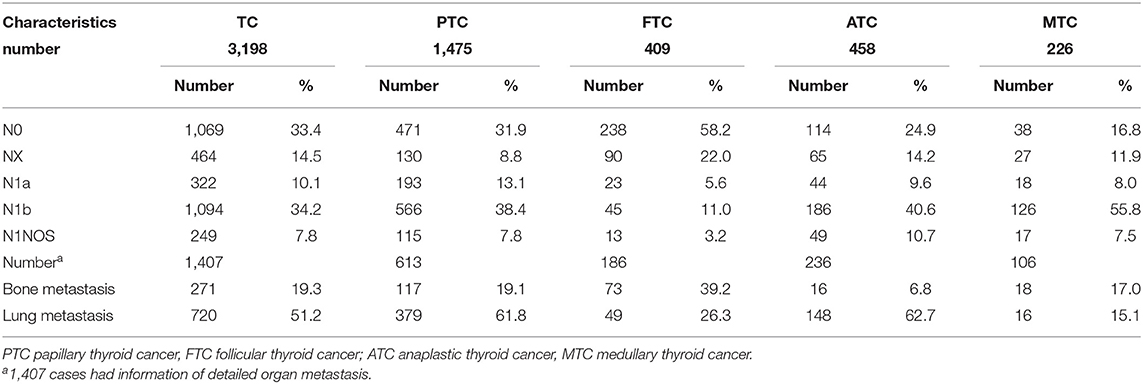
Table 2. The general information of lymph node stage and distant metastasis in thyroid cancer and its common variants.
The mortality rate was 46.7% (388/831), 61.6% (223/362), 46.3% (124/268), 53.0% (480/906), and 53.7% (115/214) for N0, NX, N1a, N1b, and N1NOS, respectively. Compared with N0 patients, the crude hazard ratio (HR) of disease-specific mortality was 1.71 (95% CI 1.45-2.02, P < 0.001), 0.98 (95%CI 0.80-1.19, P = 0.808), 0.74 (95%CI 0.65-0.85, P < 0.001), and 0.81 (95%CI 0.65-1.00, P = 0.045) for NX, N1a, N1b, and N1NOS patients, respectively. After adjusting age, race, sex, and tumor size, the adjusted HRs of NX, N1b, and N1NOS groups remained significant, was 1.83 (95% CI 1.46-2.31, P < 0.001), 1.78 (95% CI 1.52-2.10, P < 0.001), and 1.46 (95% CI 1.14-1.86, P = 0.003), respectively. In PTC, the mortality rate was 31.4% (113/360), 45.4% (44/97), 28.5% (45/158), 31.8% (149/468), and 27.0% (27/100) for N0, NX, N1a, N1b, and N1NOS group, respectively. Compared with N0 PTC group, the crude HR was 1.68 (95% CI 1.19-2.38, P = 0.004) and 1.02 (95% CI 0.80-1.31, P = 0.857), and the adjusted HR was 1.83 (95% CI 1.10–3.02, P = 0.019) and 1.70 (95% CI 1.27-2.29, P < 0.001) for NX and N1b group, respectively. In FTC, N1NOS group has the highest mortality rate of 70.0% (7/10), while NX group has the lowest mortality rate of 36.8% (28/76). Compared with N0 FTC patients, the hazards of NX and N1NOS groups did not show any significance. N1b group had a higher crude HR but the significance was lost after adjustment. N1a group had a crude HR of 2.06 (95% CI 1.09–3.89, P = 0.026) and an adjusted HR of 2.33 (95% CI 1.17–4.65, P = 0.016). In ATC, the mortality rates were all over 80.0% for all N stages. NX ATC group had the highest mortality rate of 96.0% (48/50), followed by 93.3% (83/89) for N0 group, 88.1% (37/42) for N1NOS group, 86% (135/157) for N1b group and 85.0% (34/40) for N1a group (Table 3).
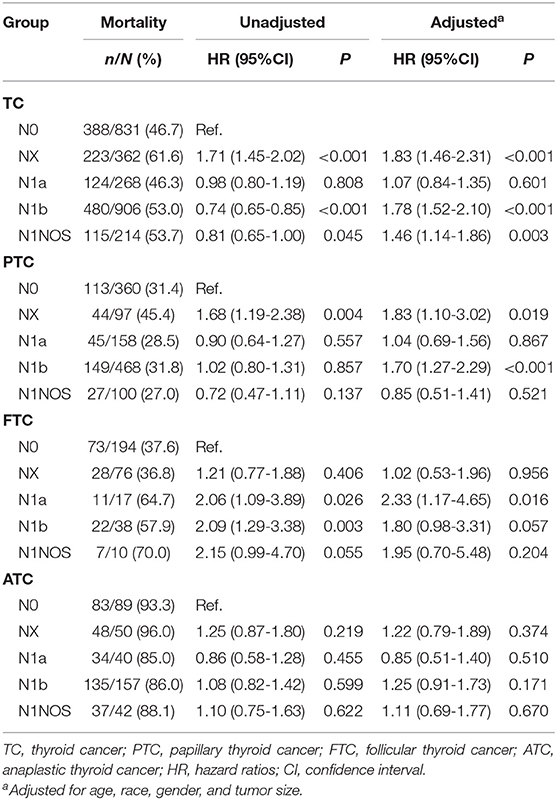
Table 3. The Association between lymph node stage and thyroid cancer specific mortality. (SEER database years of 2004-2015).
In TC, the metastatic rate of bone was 25.9% (151/584), 21.9% (39/178), and 8.9% (81/910) for N0, NX, and N1 group, respectively. Compared with the N0 group, the NX group did not show any difference in the odds of bone metastasis. However, the crude OR of the N1 group was 0.46 (95%CI 0.37-0.57, P < 0.001) and remained significant after adjustment. The metastatic rate of the lung was 37.5% (219/584), 36.0% (64/178), and 48.0% (437/910) for N0, NX, and N1 group, respectively. Compared with the N0 group, N1 patients had higher odds of lung metastasis, with crude OR of 1.63 (95% CI 1.31-2.01, P < 0.001) and an adjusted OR of 1.99 (95% CI 1.55-2.55, P < 0.001). NX group did not show significant OR because of the small sample size (Table 4).
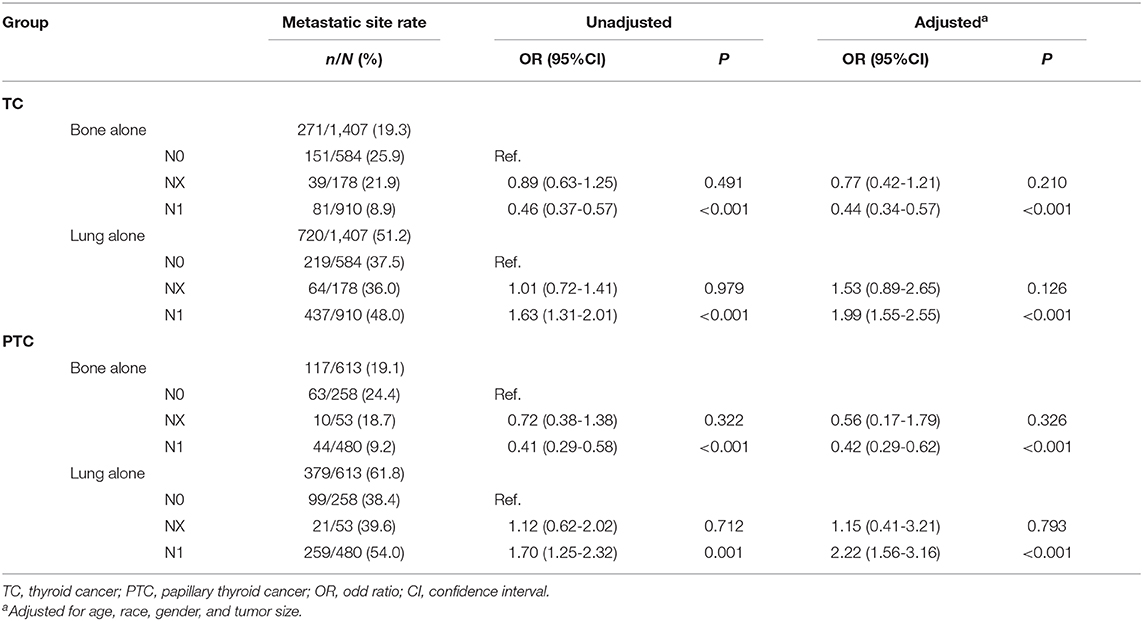
Table 4. The association between lymph node stage and distant metastasis in thyroid cancer (SEER database years of 2010-2015).
Similar results could be obtained in PTC. The metastatic rate of bone was 24.2% (63/258), 18.7% (10/53), and 9.2% (44/480) for N0, NX, and N1, respectively. Compared with the N0 group, the N1 group showed significant OR of 0.41 (95%CI 0.29-0.58, P < 0.001) and remained significant after adjustment. The metastatic rate of the lung was 38.4% (99/258), 39.6% (21/53), and 54.0% (259/480) for N0, NX, and N1, respectively. Compared with the N0 group, N1 patients had significant crude and adjusted OR of 1.70 (95%CI 1.25-2.32, P = 0.001) and 2.22 (95%CI 1.56-3.16, P < 0.001) (Table 4).
On the analysis of disease-specific survival of all TC patients, NX was associated with a statistically significant decrease in survival, followed by N1NOS and N1b groups, while N1a and N0 showed better survival (log-rank P < 0.001) (Figure 1). In PTC, The NX group had the poorest survival with a sharp decrease, followed by N1b, N1a, and N0 groups, while N1NOS groups had better survival (log-rank P = 0.006) (Figure 2). In FTC, the worst survival was obtained in the N1NOS group, followed by N1a and N1b groups with a sharp decrease, while N0 and NX groups had better survival (log-rank P = 0.006) (Figure 3). There was no statistically significant decrease in MTC (Figure 4). In ATC, the N stage had no significant effect on disease-specific survival (log Rank P = 0.313) (Figure 5).
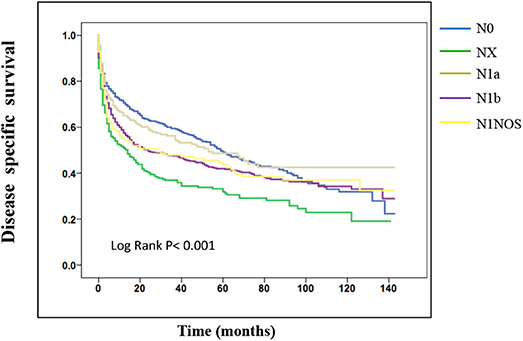
Figure 1. Disease specific survival of TC patients stratified by N stage using Kaplan-Meier analysis.
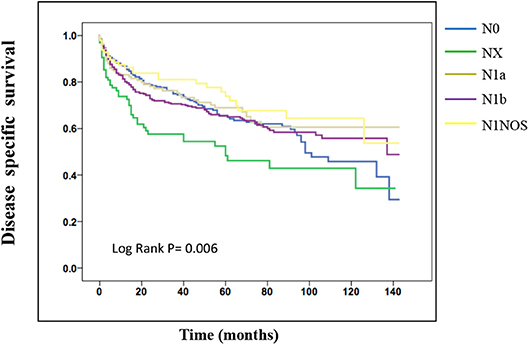
Figure 2. Disease specific survival of PTC patients stratified by N stage using Kaplan-Meier analysis.
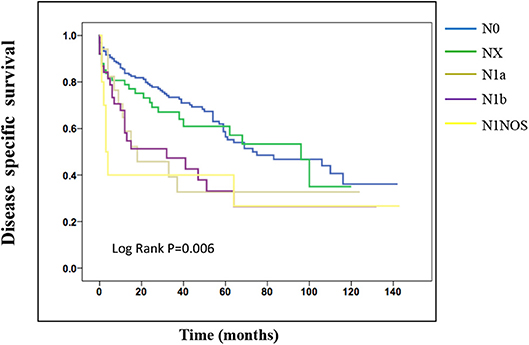
Figure 3. Disease specific survival of FTC patients stratified by N stage using Kaplan-Meier analysis.
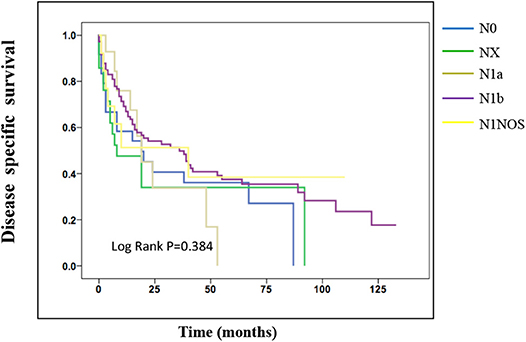
Figure 4. Disease specific survival of MTC patients stratified by N stage using Kaplan-Meier analysis.
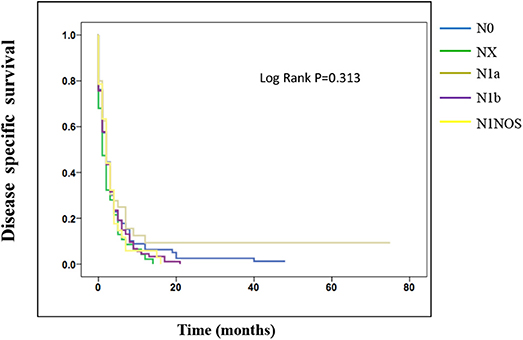
Figure 5. Disease specific survival of ATC patients stratified by N stage using Kaplan-Meier analysis.
In this study, we have investigated the association between the N stage and disease-specific mortality in TC patients with initial distant metastasis. Our data indicated that TC patients with NX had the highest mortality risk, while the N0 group had the lowest mortality risk. In addition to this, our results also suggest that the N1 group had a higher probability of lung metastasis.
The TNM stage system was designed empirically to predict the survival of most TC patients and to guide therapeutic methods and clinical follow-up setup. An interesting retrospective study by Adam et al. found that the cervical lymph node metastases were not in correlation with a favorable but compromised prognosis in PTC patients younger than 45 years (14). Compared with the 7th AJCC Staging Manual, the 8th AJCC Staging Manual has a remarkable change in the definition of N1a and N1b, which may result in better accuracy of central and lateral neck disease (8). However, there is no special attention paid to the NX stage. Previous studies have investigated the risk factors and clinical outcomes of the NX stage in TC and showed its association with poor prognosis (15–17). A study by Baek et al. retrospectively analyzed data concerning 189 PTC patients who had undergone surgery and confirmed that lymph node metastasis was a factor for increasing the risk of recurrence (18, 19). Although it has been reported that lymph node involvement has prognostic significance for TC patients (14, 17, 20), the significance of different N stages still remains controversial. In a study conducted in 1961, TC patients whose disease extended beyond the capsule of the thyroid gland to the regional lymph nodes, surrounding organs or tissues, etc., had a much higher survival rate than that of other groups at 20 years after surgery (21). On the contrary, recent study results suggest that the lymph node involvement have a bad effect on the prognosis of TC (22, 23). Whereas in such a situation, there is still not much attention paid to the investigation on the survival outcomes of patients with different lymph node stages, especially for the NX stage. Liu et al. reported that differentiated TC patients with the NX stage had an unexpectedly poor prognosis via evaluating the survival outcomes (16). They have found that either cancer-specific mortality or all-cause mortality for NX stage patients was higher than N1 and N0 stage patients. Meanwhile, they ascertained that the prognosis for NX stage patients was worse compared to N0 patients. After propensity score matching for relevant confounding factors, patients with N1 stage sustained similar results, which may result from insufficient use of thyroidectomy. In our study, we found that the NX patients had the highest mortality followed by N1b, N1NOS, and N1a groups in PTC, which was consistent with the results of Liu et al. However, the NX did not show any prognostic value when analyzing FTC population alone. To our knowledge, previous studies have noted the risk factors of prognosis for TC patients, such as BRAF mutation (24) and multifocality (25), and categorized the disease stages using the N stage of N0-N1 to assess the clinical outcomes of TC (8). However, the studies focused on the prognosis of NX stage TC patients with distant organ metastasis at diagnosis are scarce. Our study filled the research gap by investigating the prognosis of NX in TC patients, which may provide new clinical-relevant information for improving the treatment strategies. The incidence of TC is increasing rapidly with its diagnosis becoming more and more convenient with the development of images, computed tomography, magnetic resonance imaging, and other medical auxiliary inspection technologies. Even a small percentage change in the mortality rate is likely to result in a big difference in disease-specific mortality. More attention should be given to the NX patients in terms of clinical diagnosis and treatment because of its poor prognosis compared with other N stages. Similar results could be obtained in PTC patients, which may be the consequence of the fact that PTC accounts for ~85-90% of all TC cases (4). Conversely, in the FTC group, our study did not show results similar to the whole TC group and PTC group, which indicates that the N1a was a risk factor for FTC-specific death instead of the NX and N1b. A study by Witte et al. reported that the lymph node metastasis is an important factor affecting FTC patients' survival, while no detail information such as the lymph node location was provided despite the N stage (26). Thus, it still needs further investigation on the correlation between the N stage and the prognosis of patients with FTC.
A high potential survival risk has been reported for patients with TC and distant organ metastasis (27). The four most common distant metastasis organs identified in the 2010 AJCC Staging Manual were bone, brain, liver, and lung. The previous study has shown that lungs and bones were more likely to be metastatic sites than brains and livers for TC patients (28). Additionally, organ metastases were reported to have a correlation with worse disease-specific survival in TC (29). We have investigated the association between the N stage and the two organ metastases, lung, and bone. The metastatic rate for lung is 51.2% (720/1,407), which is consistent with the results of a study by Hirsch et al., the study reported that the lung was the most common distant metastatic organ with an incidence rate of 85.5% (118/138) (28). According to the previous reports the most common cause of death for TC patients with lung metastasis to be respiratory failure due to the progression of lung metastasis (30, 31). Therefore, chest computed tomography should be used to diagnose TC patients, especially PTC patients with N1 stage. Bone is regarded as a common metastatic site. Though Slook et al. reported that bone metastasis is linked to high mortality risk as a risk factor having an impact on the survival of differentiated TC patients (32), there was no previous study focused on the association between N stage and bone metastasis. Our study indicated that N1 patients were more likely to have lung metastasis than bone metastasis. TC patients with regional lymph node metastasis should carefully undergo ultrasonography or biopsy of lymph nodes must be performed to potentially identify clinical lung metastasis rather than bone metastasis, even though bone metastasis has been reported to lead to high morbidity rates for skeletal-related events such as pathologic fractures, spinal cord compression, the requirement for bone irradiation, or bone surgery (7). In terms of treatments, even though radioactive iodine therapy has great significance in TC patients with lung metastasis, more than half of the cases showed progressive metastasis (33). For TC patients with radioactive iodine ablation-refractory metastasis, Moneke et al. (34) reported the effectiveness of surgical resection. More aggressive treatment could be arranged for individuals to balance the clinical benefits and harms and reach the optimal results.
This study had some limitations. Though the SEER database is a well-validated dataset, this was a retrospective study. Moreover, the SEER database does not provide information which may be required for further prospective studies, such as gene mutations and types of therapy. Besides, our study only obtained the data between 2004 and 2015 from the SEER database to investigate the association between N stage and distant organ metastases of lungs and bones. Future studies on the other two common distant metastases sites, brains and livers, and multi-organ metastases are being conducted.
TC Patients with NX stage had the poorest survival, and the highest disease-specific mortality risk, followed by patients with N1b, N1a, and N0 lymph node stages. The lung was found to be the most common metastatic site. Among different lymph node stages, N1 patients were more prone to lung metastasis. Therefore, we recommend chest computed tomography screening during follow-ups for TC patients, particularly PTC patients with N1 stage. Our study provided new information regarding the treatment of patients with NX stage differentiated TC.
Data on TC patients were retrieved from the SEER database (https://seer.cancer.gov/), which is maintained by the National Cancer Institute. The datasets generated for this study are available on request to the corresponding author.
JZ, XC, LS, and SQ: conception and design. All authors contributed to acquisition, statistical analysis or interpretation of the data, drafting of the manuscript. All authors reviewed and approved the final version of the manuscript.
This work was supported by National Key R&D Program of China (Nos. 2018YFC1314100 and 2016YFC1305600), Shanghai Municipality: Shanghai Outstanding Academic Leaders Plan (049), National Natural Science Foundation of China (81970677), Shanghai Committee of Science and Technology, China (18411951803 and 17DZ1910603), New Exploration of Blood Glucose Management Mode in Patients with Diabetes Mellitus in Chongming Area of Shanghai (CKY2018-19), Newly Stratified Enhancement and Follow-up System for the Diagnosis of Type 2 Diabetes (2018YFC1314100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all the staff of the National Cancer Institute for their efforts toward the SEER program and to thank Muthukumaran Jayachandran for English language polishing.
1. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. (2017) 317:1338¬48. doi: 10.1001/jama.2017.2719
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7¬30. doi: 10.3322/caac.21442
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7-34. doi: 10.3322/caac.21551
4. Huang Y, Qu S, Zhu G, Wang F, Liu R, Shen X, et al. BRAF V600E mutation-assisted risk stratification of solitary intrathyroidal papillary thyroid cancer for precision treatment. J Natl Cancer Inst. (2018) 110:362¬70. doi: 10.1093/jnci/djx227
5. Rottenburger C, Nicolas GP, McDougall L, Kaul F, Cachovan M, Vija AH, et al. Cholecystokinin-2 receptor agonist. (177)Lu-PP-F11N for radionuclide therapy of medullary thyroid carcinoma - results of the Lumed Phase 0a study. J Nucl Med. (2019). doi: 10.1530/endoabs.63.GP226. [Epub ahead of print].
6. Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin. (2013) 63:374¬94. doi: 10.3322/caac.21195
7. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. (2006) 16:109¬42. doi: 10.1089/thy.2006.16.109
8. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. (2018) 68:55¬63. doi: 10.3322/caac.21439
9. Choksi P, Papaleontiou M, Guo C, Worden F, Banerjee M, Haymart M. Skeletal complications and mortality in thyroid cancer: a population-based study. J Clin Endocrinol Metab. (2017) 102:1254¬60. doi: 10.1210/jc.2016-3906
10. Muresan MM, Olivier P, Leclere J, Sirveaux F, Brunaud L, Klein M, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. (2008) 15:37¬49. doi: 10.1677/ERC-07-0229
11. Lee YK, Hong N, Park SH, Shin DY, Lee CR, Kang SW, et al. The relationship of comorbidities to mortality and cause of death in patients with differentiated thyroid carcinoma. Sci Rep. (2019) 9:11435. doi: 10.1038/s41598-019-47898-8
12. Al Afif A, Williams BA, Rigby MH, Bullock MJ, Taylor SM, Trites J, et al. Multifocal papillary thyroid cancer increases the risk of central lymph node metastasis. Thyroid. (2015) 25:1008¬12. doi: 10.1089/thy.2015.0130
13. Chen L, Zhu Y, Zheng K, Zhang H, Guo H, Zhang L, et al. The presence of cancerous nodules in lymph nodes is a novel indicator of distant metastasis and poor survival in patients with papillary thyroid carcinoma. J Cancer Res Clin Oncol. (2017) 143:1035¬42. doi: 10.1007/s00432-017-2345-2
14. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. (2015) 33:2370¬5. doi: 10.1200/JCO.2014.59.8391
15. Sung TY, Yoon JH, Song DE, Lee YM, Kim TY, Chung KW, et al. Prognostic value of the number of retrieved lymph nodes in pathological Nx or N0 classical papillary thyroid carcinoma. World J Surg. (2016) 40:2043¬50. doi: 10.1007/s00268-016-3490-5
16. Liu Z, Zeng W, Zhao Q, Huang L, Zhou W, Wei W, et al. The prognosis of NX stage differentiated thyroid cancer based on propensity score matching and SEER data. Am J Transl Res. (2018) 10:3782¬9.
17. Nixon IJ, Wang LY, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery. (2014) 156:137¬46. doi: 10.1016/j.surg.2014.03.027
18. Baek SK, Jung KY, Kang SM, Kwon SY, Woo JS, Cho SH, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. (2010) 20:147¬52. doi: 10.1089/thy.2008.0243
19. Dai C, Xie H, Kadeer X, Su H, Xie D, Ren Y, et al. Relationship of lymph node micrometastasis and micropapillary component and their joint influence on prognosis of patients with stage I lung adenocarcinoma. Am J Surg Pathol. (2017) 41:1212¬20. doi: 10.1097/PAS.0000000000000901
20. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. (2008) 144:1070¬7; discussion 7¬8. doi: 10.1016/j.surg.2008.08.034
21. Hirabayashi RN, Lindsays. Carcinoma of the thyroid gland: a statistical study of 390 patients. J Clin Endocrinol Metab. (1961) 21:1596¬610. doi: 10.1210/jcem-21-12-1596
22. Xue S, Wang P, Zhang Q, Yin Y, Guo L, Wang M, et al. Routine lateral level V dissection may not be necessary for papillary thyroid microcarcinoma with lateral lymph node metastasis: a retrospective study of 252 cases. Front Endocrinol. (2019) 10:558. doi: 10.3389/fendo.2019.00558
23. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Predictive factors for lymph node metastasis in papillary thyroid microcarcinoma. Ann Surg Oncol. (2016) 23:2866¬73. doi: 10.1245/s10434-016-5225-0
24. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Role of BRAF V600E mutation as an indicator of the extent of thyroidectomy and lymph node dissection in conventional papillary thyroid carcinoma. Surgery. (2015) 158:1500¬11. doi: 10.1016/j.surg.2015.05.016
25. Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol. (2015) 22:125¬31. doi: 10.1245/s10434-014-3899-8
26. Witte J, Goretzki PE, Dieken J, Simon D, Roher HD. Importance of lymph node metastases in follicular thyroid cancer. World J Surg. (2002) 26:1017¬22. doi: 10.1007/s00268-002-6668-y
27. Chopra S, Garg A, Ballal S, Bal CS. Lung metastases from differentiated thyroid carcinoma: prognostic factors related to remission and disease-free survival. Clin Endocrinol. (2015) 82:445¬52. doi: 10.1111/cen.12558
28. Hirsch D, Levy S, Tsvetov G, Gorshtein A, Slutzky-Shraga I, Akirov A, et al. Long-term outcomes and prognostic factors in patients with differentiated thyroid cancer and distant metastases. Endocr Pract. (2017) 23:1193¬200. doi: 10.4158/EP171924.OR
29. Wang LY, Palmer FL, Nixon IJ, Thomas D, Patel SG, Shaha AR, et al. Multi-organ distant metastases confer worse disease-specific survival in differentiated thyroid cancer. Thyroid. (2014) 24:1594¬9. doi: 10.1089/thy.2014.0173
30. Leite AK, Kulcsar MA, de Godoi Cavalheiro B, de Mello ES, Alves VA, Cernea CR, et al. Death related to pulmonary metastasis in patients with differentiated thyroid cancer. Endocr Pract. (2017) 23:72¬8. doi: 10.4158/EP161431.OR
31. Song HJ, Qiu ZL, Shen CT, Wei WJ, Luo QY. Pulmonary metastases in differentiated thyroid cancer: efficacy of radioiodine therapy and prognostic factors. Eur J Endocrinol. (2015) 173:399¬408. doi: 10.1530/EJE-15-0296
32. Slook O, Levy S, Slutzky-Shraga I, Tsvetov G, Robenshtok E, Shimon I, et al. Long-term outcomes and prognostic factors in patients with differentiated thyroid carcinoma and bone metastases. Endocr Pract. (2019) 25:427¬37. doi: 10.4158/EP-2018-0465
33. Sabra MM, Dominguez JM, Grewal RK, Larson SM, Ghossein RA, Tuttle RM, et al. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab. (2013) 98:E829¬36. doi: 10.1210/jc.2012-3933
Keywords: thyroid cancer, lymph nodes, NX, initial distant metastasis, SEER
Citation: Zhang J, Cheng X, Shen L, Wang X, Wang L, Sun X and Qu S (2020) The Association Between Lymph Node Stage and Clinical Prognosis in Thyroid Cancer. Front. Endocrinol. 11:90. doi: 10.3389/fendo.2020.00090
Received: 07 December 2019; Accepted: 13 February 2020;
Published: 27 February 2020.
Edited by:
Alessandro Antonelli, University of Pisa, ItalyReviewed by:
Roberto Vita, University of Messina, ItalyCopyright © 2020 Zhang, Cheng, Shen, Wang, Wang, Sun and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shen Qu, cXVzaGVuY25AaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.