- 1Laboratory on Thymus Research, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
- 2Laboratory of Inflammation, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
A substantial body of evidence supports that the gut microbiota plays a pivotal role in the regulation of metabolic, endocrine and immune functions. In recent years, there has been growing recognition of the involvement of the gut microbiota in the modulation of multiple neurochemical pathways through the highly interconnected gut-brain axis. Although amazing scientific breakthroughs over the last few years have expanded our knowledge on the communication between microbes and their hosts, the underpinnings of microbiota-gut-brain crosstalk remain to be determined. Short-chain fatty acids (SCFAs), the main metabolites produced in the colon by bacterial fermentation of dietary fibers and resistant starch, are speculated to play a key role in neuro-immunoendocrine regulation. However, the underlying mechanisms through which SCFAs might influence brain physiology and behavior have not been fully elucidated. In this review, we outline the current knowledge about the involvement of SCFAs in microbiota-gut-brain interactions. We also highlight how the development of future treatments for central nervous system (CNS) disorders can take advantage of the intimate and mutual interactions of the gut microbiota with the brain by exploring the role of SCFAs in the regulation of neuro-immunoendocrine function.
Introduction
The human body is inhabited by a wide variety of commensal microorganisms collectively called the microbiota. This host microbiota colonizes the skin and several mucosal cavities (nasal, oral, pulmonary, and vaginal); however, it is in the gastrointestinal (GI) tract that these organisms reach extraordinary densities since trillions of bacteria, fungi, and viruses coexist in symbiosis with the host for potential mutual benefit (1–3). Despite its significant influence on the state of human health and the development or progression of diseases, it is only in the last 20 years that our gut microbiota has become the focus of intense studies. Therefore, its pivotal roles in protecting against pathogens, regulating metabolic, endocrine, and immune functions and in influencing drug metabolism and absorption have started to be elucidated (4, 5). Further, it was recently unveiled that the influence of the microbiota is not restricted to the GI tract; it plays a major role in the bidirectional communication between the GI tract and the central nervous system (CNS). The growing body of evidence indicating that the gut microbiota exerts a profound influence on key brain processes has led to the development of the microbiota-gut-brain axis concept, which has attracted the interest of researchers worldwide (6–11).
Although the precise mechanisms involved in the crosstalk between the gut microbiota and brain remain to be fully determined, there are a number of potential pathways through which the gut microbiota can influence brain function (9). Microorganisms can influence CNS processes bidirectionally via the vagus nerve (12) and through modulation of the immune system (6), the hypothalamic-pituitary-adrenal (HPA) axis (13, 14), and tryptophan metabolism (15), along with their ability to synthetize a number of neurotransmitters (16–18) and produce metabolites, such as short-chain fatty acids (SCFAs), that possess neuroactive properties (17, 19–21).
The SCFAs acetate, propionate, and butyrate are the main metabolites produced in the colon by bacterial fermentation of dietary fibers and resistant starch (22). In addition to the long-known role of the colon in energy supply and trophic factors (22), as well as the regulation of T regulatory (Treg) cell colonies (23, 24), growing evidence supports the idea that SCFAs also exert crucial physiological effects on several organs, including the brain (17, 20, 21). This hypothesis is supported by studies in animals and humans showing that gut microbiota dysbiosis has been implicated in behavioral and neurologic pathologies, such as depression, Alzheimer's (AD) and Parkinson's (PD) diseases and autism spectrum disorder (ASD) (9, 21, 25–27). Furthermore, microbiota manipulation and SCFA administration have been proposed as treatment targets for such diseases (28).
In this review, we outline the current knowledge about the involvement of acetate, propionate, and butyrate in microbiota-gut-brain interactions. We also highlight how the development of future treatments for CNS disorders can take advantage of the intimate and mutual interactions of the gut microbiota with the brain by exploring the role of SCFAs in the regulation of neuro-immunoendocrine function.
The Microbiota-Gut-Brain Axis
The modulation of gut physiology by the CNS and its effects on gut function such as motility, secretion, blood flow, nociception, and immune function during neurological stressors are well-documented (17, 29, 30). Further, brain to gut signaling can directly affect the microbiota, either via immune system or gut functions such as motility, release of neurotransmitters and intestinal immune tone (12, 17, 21, 31). Comparatively, gut to CNS signaling has been studied for a short period, and the mechanisms underlying this crosstalk are starting to be understood (13, 32). It is noteworthy that several brain disorders have been linked to imbalances in the microbial composition of the gut (17, 19, 29, 33–37); however, whether these alterations in the microbiota are induced by brain signaling or whether brain dysfunction is driven by changes in the gut microbiota remains to be fully determined.
Although a more compelling causal relationship between altered gut microbial composition and brain dysfunction is still needed, it has been shown that disruption in the neuronal and microbial organization in prenatal and postnatal periods of mammalian development may lead to the onset of neurodevelopmental and other brain disorders later in life (9, 38–40). In a similar way, growing evidence has shown that alterations in maternal microbiome during pregnancy, such as use of antibiotics or probiotics (41, 42), variations in diet (43), immune activation (44, 45), and exposure to stress (46) can modulate the microbiome, neurodevelopment, and behavior of offspring in both rodents and humans (9, 29). Furthermore, delivery mode (47) and early-life occurrences such as feeding changes, infection, and antibiotics treatment (48, 49) have a huge effect on the gut microbiota composition with a long-term impact on brain and behavior (9, 29).
Under physiological conditions, activation of immune cells and production of cytokines can have a minor impact in the CNS. However, chronic systemic inflammation, mostly in the form of infections, has long been associated with behavioral alterations and cognitive dysfunction (50, 51). It is now widely known that peripheral insults that cause a systemic inflammatory response might affect ongoing inflammation in the CNS mainly by microglial activation, production of inflammatory molecules, as well as recruitment of peripheral immune cells into the brain, thus shaping a cerebral inflammatory milieu that may seriously affect neuronal function (50, 52, 53). Noteworthy, during gut pathologies with increased permeability of the intestinal barrier, the translocation of bacterial products can increase the production of cytokines and impact the blood-brain barrier (BBB), leading to more intense harmful effects (37). Further, it has already been shown that several bacterial strains can modify levels of neurotransmitter precursors in the gut lumen and even independently synthesize (or modulate the synthesis of) a number of neurotransmitters, including γ-aminobutyric acid (GABA), serotonin (5-HT), dopamine (DA), and noradrenaline (NA) (16–18). These neurotransmitters can potentially influence microglial activation and several cerebral functions (54). Additionally, the sympathetic branch of the autonomic nervous system is also involved in intestinal homeostasis and immune regulation (30). Conversely, the gut microbiota can interact with the CNS via gut modulation or directly via metabolites and endotoxin translocation from the lumen to the circulation (9, 17, 21). Possible signal transducers involved in the communication of the microbiota with the CNS include enterochromaffin cells, which can bind several microbial products and secrete serotonin into the lamina propria, increasing colonic and blood concentrations of 5-HT (55, 56). Gut-brain communication can also be achieved through vagus nerve signaling (57). Changes in enteric neuron activity perceived by the vagus nerve are essential for mediating satiety, stress, and mood (12, 58, 59). Given the close physical proximity, gut bacteria can interact with and activate the vagus nerve, thereby exerting effects upstream to the CNS. This notion is in full accordance with early studies showing that oral inoculation with pathogens or probiotics induces activation of the vagal sensory neurons that innervate the GI affecting the regulation of CNS functions, and this effect is absent in vagotomized mice (32, 58, 60). However, whether the vagus nerve is activated by physical interaction with bacteria or through soluble microbial components remain to be determined.
Finally, bacterial metabolic byproducts including SCFAs are often considered key candidate mediators of gut-brain communication, and altered SCFA production has been demonstrated in a variety of neuropathologies (19, 21, 33–35).
Metabolism and Peripheral Effects of SCFAs
SCFAs are small organic monocarboxylic acids with a chain length of up to six carbons atoms and are the main products of the anaerobic fermentation of indigestible polysaccharides such as dietary fiber and resistant starch produced by the microbiota in the large intestine (61, 62). Comprised mostly of acetate (C2), propionate (C3), and butyrate (C4) (63, 64) in an approximate molar rate of 60:20:20, respectively (65), approximately 500–600 mmol of SCFAs are produced in the gut per day depending on the fiber content in the diet, microbiota composition, and gut transit time (66, 67). Although anaerobic fermentation of fibers is the largest source of SCFAs, acetate, propionate, and butyrate can also be produced from amino acid metabolism (68). However, less than 1% of the large intestine microbiota uses these metabolic pathways to produce SCFAs (69, 70). Protein fermentation usually takes place in the distal large intestine where carbohydrates are already depleted and also leads to the production of potentially toxic metabolites, such as ammonia, phenols, and sulfides, as well as unique branched-chain fatty acids (BCFA) (69, 71). Further, acetate produced from acetyl-CoA derived from glycolysis can also be transformed into butyrate by the enzyme butyryl-CoA:acetyl-CoA transferase (72, 73), and bovine milk fats also provide a source of butyrate (74).
Following their production, SCFAs are absorbed by colonocytes, mainly via H+-dependent or sodium-dependent monocarboxylate transporters (MCTs and SMCTs, respectively) (75). MCTs show different subtypes and expression patterns in different tissues. SCFAs that are not metabolized in the colonocytes are transported into the portal circulation and are used as an energy substrate for hepatocytes (76), except for acetate that is not oxidized in the liver (76). Therefore, only a minor fraction of colon-derived acetate, propionate, and butyrate reaches the systemic circulation and other tissues (65). In this context, it is important to note that most of the recent works regarding microbial-derived SCFA, mainly in human studies, use fecal concentrations as a proxy of the production in the colon (17, 19, 29, 33–37). Although it represents a valid approach, there are many potential sources of bias, such as intestinal transit and permeability, metabolite transportation, and sample handling (77). Thus, these drawbacks must be taken into account when concluding the effects of administered SCFAs, given that some experiments might be conducted under non-physiological conditions.
SCFAs improve the gut health through a number of local effects, ranging from maintenance of intestinal barrier integrity, mucus production, and protection against inflammation to reduction of the risk of colorectal cancer (78–81). Although a thorough comprehension of signaling triggered by SCFAs is still lacking, it is already known that SCFAs bind to G protein-coupled receptors (GPCRs). The best-studied SCFA receptors are GPR43 and GPR41, later renamed free fatty acid receptor (FFAR2) and FFAR3, as well as GPR109a/HCAR2 (hydrocarboxylic acid receptor) and GPR164, which are expressed in a vast array of cells, from the gastrointestinal mucosa to the immune and nervous systems (82, 83). The effects of activation of these receptors differ greatly depending on the cell on which they are expressed. For instance, binding of SCFAs to their receptors on enteroendocrine cells results in stimulated secretion of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) (84), while signaling in β-pancreatic cells leads to increased insulin secretion (85).
Another mechanism by which SCFAs regulate systemic functions is through the inhibition of histone deacetylase (HDAC) activity, thus promoting the acetylation of lysine residues present in nucleosomal histones throughout various cell populations (20). This intracellular signaling mechanism has been found in both the gut and associated immune tissue (86), as well as in the peripheral nervous system and CNS (20).
Although only a minor fraction of colon-derived SCFAs reaches the systemic circulation and other tissues, their effects on different organ and systems have recently been widely outlined. One of the best-documented effects of SCFAs is on the immune system since butyrate is capable of inducing Treg differentiation and controlling inflammation (17, 23, 24, 87). Although fine-tuning of the gut immune response to the microbiota is still a matter of debate, microbiota metabolites are capable of alleviating or worsening gut conditions such as inflammatory bowel disease (88). Effects on brown adipose tissue activation (89), regulation of liver mitochondrial function (90), whole-body energy homeostasis (91), and control of appetite (89) and sleep (10) have been attributed to all SCFAs. Further, the influence of the microbiota and the effects of SCFAs on the CNS have been a matter of intense debate in the last few years.
SCFAs and the Brain
In addition to exerting local effects in the colon and in the peripheral tissues, SCFAs are speculated to play a pivotal role in microbiota-gut-brain crosstalk (Figure 1). The abundant expression of MCTs in endothelial cells (75, 92) might facilitate crossing of the BBB by SCFAs since brain uptake of SCFAs has previously been demonstrated in rats following injection of 14C-SCFAs into the carotid artery (93). Although studies on physiological concentrations of SCFAs in the brain are scarce, all three metabolites are detectable in the human cerebrospinal fluid (CSF), typically in the range of 0–171 μM for acetate, 0–6 μM for propionate, and 0–2.8 μM for butyrate (94). An average concentration of 17.0 pmol/mg of tissue for butyrate and 18.8 pmol/mg of tissue for propionate in the human brain was reported (95). Furthermore, the levels of butyrate in the brain of mice supplemented with live Clostridium butyricum reached a range from 0.4 to 0.7 μmol/g, which was about an order of magnitude higher than concentrations reported in peripheral blood (96, 97). In addition to crossing BBB, SCFAs seem to play an important role in maintaining its integrity, which is tightly associated with controlled passage of molecules and nutrients from the circulation to the brain, playing a central role in brain development and the preservation of CNS homeostasis. Supporting the notion that SCFAs regulate the BBB function, germ-free (GF) mice show reduced expression of tight junction proteins such as claudin and occludin, leading to increased permeability of the BBB from intrauterine life to adulthood (98). Furthermore, recolonization of these adult mice with a complex microbiota or monocolonization with SCFA-producing bacterial strains recovers the integrity of the BBB (98). Similarly, treatment of an in vitro model of cerebrovascular endothelial cells with propionate attenuates the permeabilizing effects of exposure to lipopolysaccharide (LPS) (99).
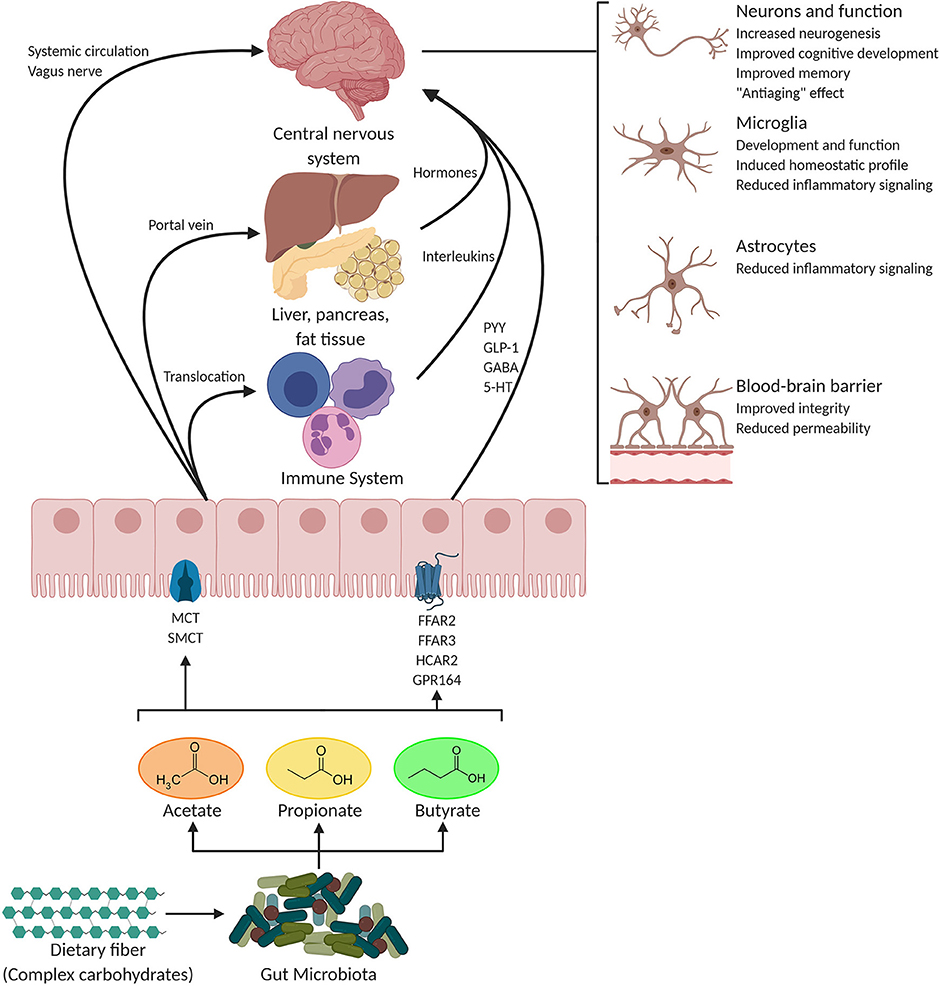
Figure 1. Potential pathways through which SCFAs influence gut-brain communication. Short-chain fatty acids (SCFAs) are the main metabolites produced by the microbiota in the large intestine through the anaerobic fermentation of indigestible polysaccharides such as dietary fiber and resistant starch. SCFAs might influence gut-brain communication and brain function directly or indirectly. Following their production, SCFAs are absorbed by colonocytes, mainly via H+-dependent monocarboxylate transporters (MCTs) or sodium-dependent monocarboxylate transporters (SMCTs). Through binding to G protein-coupled receptors (GPCRs) such as free fatty acid receptor 2 and 3 (FFAR2 and FFAR3), as well as GPR109a/HCAR2 (hydrocarboxylic acid receptor) and GPR164 or by inhibiting histone deacetylases, SCFAs influence intestinal mucosal immunity, and barrier integrity and function. SCFA interaction with their receptors on enteroendocrine cells promotes indirect signaling to the brain via the systemic circulation or vagal pathways by inducing the secretion of gut hormones such as glucagon-like peptide 1 (GLP1) and peptide YY (PYY), as well as γ-aminobutyric acid (GABA), and serotonin (5-HT). Colon-derived SCFAs reaches the systemic circulation and other tissues, leading to brown adipose tissue activation, regulation of liver mitochondrial function, increased insulin secretion by β-pancreatic cells, and whole-body energy homeostasis. Peripherally, SCFAs influence systemic inflammation mainly by inducing T regulatory cells (Treg) differentiation and by regulating the secretion of interleukins. SCFAs can cross the blood-brain barrier (BBB) via monocarboxylate transporters located on endothelial cells and influence BBB integrity by upregulating the expression of tight junction proteins. Finally, in the central nervous system (CNS) SCFAs also influence neuroinflammation by affecting glial cell morphology and function as well as by modulating the levels of neurotrophic factors, increasing neurogenesis, contributing to the biosynthesis of serotonin, and improving neuronal homeostasis and function. Together, the interaction of SCFAs with these gut-brain pathways can directly or indirectly affect emotion, cognition, and pathophysiology of brain disorders. Figure of this review was created with BioRender (https://biorender.com/).
Accumulating evidence suggests that SCFAs that cross into the CNS have neuroactive properties. Although the precise mechanisms involved in the action of SCFAs on the CNS remain largely unknown, a multitude of animal studies have shown that they exert widespread influence on key neurological and behavioral processes and may be involved in critical phases of neurodevelopmental and neurodegenerative disorders (17, 21, 29, 36, 100).
SCFAs and Microglia
The development of the nervous system is marked by the sculpting of the neuronal networks shaping the functional neural circuitry that is critical for normal cognitive, emotional, and social domains. In this context, glial cells, especially microglial cells, have been increasingly recognized to play a critical role in the elimination of excess or unnecessary synaptic connections, which is necessary for the maturation and refinement of circuits and connections in the nervous system (101, 102). Therefore, control of innate immune function in the CNS is critical for brain development, and the gut microbiota seems to play a pivotal role in the development and functionality of the immune system in the CNS. The results reported by Erny and collaborators shed light on how the microbiota might influence microglial maturation and function (6). While microglia from specific pathogen-free (SPF) mice shows normal maturation and function, non-colonized young GF mice exhibit stunted microglia under homeostatic conditions. It is noteworthy that the oral application of a mixture of the three major SCFAs acetate, propionate, and butyrate was sufficient to drive maturation of microglia in GF mice (6). Although the mechanisms involved in the control of maturation and function of microglia by SCFAs remain to be determined, the activation of FFAR2 could be conceivable since FFAR2-deficient mice displayed microglia reminiscent of those found in GF mice (103).
Neuroinflammation is also an important process shaping brain function. Similar to observations in GF mice, perturbations of the gut microbiota by antibiotics systemically produce altered immune responses in experimental models, notably toward a pro-inflammatory profile (6). This is also true in the CNS, which becomes more prone to extreme inflammatory responses when the microbiota is depleted by antibiotics early in life (104). It was shown that antibiotic-induced perturbations in gut microbial diversity influence neuroinflammation with altered microglial morphology (105–107). On the other hand, several studies have reported that sodium butyrate is capable of decreasing microglial activation and pro-inflammatory cytokines secretion (108–110). Also, butyrate treatment in vitro and in vivo induces morphological and functional changes in the microglia toward a homeostatic profile and inhibits LPS-induced pro-inflammatory modifications (109) and depression-like behavior (110). Likewise, acetate treatment of microglia primary culture has been shown to reduce inflammatory signaling through reduced IL-1β, IL-6, and TNF-α expression and p38 MAPK, JNK, and NF-κB phosphorylation (111). Similarly, acetate was also able to modulate inflammatory cytokines and signaling pathways in astrocyte primary culture (112). Although the precise signaling involved in the effects of SCFAs on microglia remain unveiled, inhibition of HDACs, which results in epigenetically regulated gene expression, has been considered the main effector mechanism triggered by SCFAs (113). In this way, histone acetylation seems to modulate glial cells in an anti-inflammatory and neuroprotective manner. Therefore, taking into account the role of microglia in shaping neuronal networks and the influence of the microbiota on this process, SCFAs might provide new methods to modulate the brain immunity disruption underlying neurodevelopmental and neurodegenerative disorders.
SCFAs and Neurons
Apart from providing the cells with energy and affecting microglia maturation, these microbial metabolites also seem to influence neuronal function. It was described that SCFAs may modulate the levels of neurotransmitters and neurotrophic factors. Acetate has previously been shown to alter the levels of the neurotransmitters glutamate, glutamine and GABA in the hypothalamus and increase anorexigenic neuropeptide expression (114). Propionate and butyrate exert an influence on the intracellular potassium level, which implies the involvement of SCFAs in the operation of cell signaling systems (115). In particular, these SCFAs regulate the expression levels of tryptophan 5-hydroxylase 1, the enzyme involved in synthesis of serotonin, and tyrosine hydroxylase, which is involved in a rate-limiting step in the biosynthesis of dopamine, noradrenaline and adrenaline; therefore, producing an effect on brain neurochemistry (21, 55, 56, 116, 117). Antibiotic depletion of the microbiota also results in hippocampal neurogenesis and memory impairments, which can be partially recovered by the reconstitution of specific SPF microbiota and completely recovered by probiotic treatment or exercise (118). This cognitive deficit might be associated with changes in the expression of cognition-relevant signaling molecules such as brain-derived neurotrophic factor (BDNF), N-methyl-D-aspartate receptor subunit 2B, serotonin transporter and neuropeptide Y system (119).
Neurotrophic factors, such as nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), and BDNF that regulate the growth, survival and differentiation of neurons and synapses in the CNS also play important parts in learning and memory and in a range of brain disorders have been also shown to be modulated by SCFAs (120–123). BDNF expression, neurogenesis, and neural proliferation in rodents (124–126), as well as facilitation of long-term memory consolidation, were stimulated by sodium butyrate (127). Further, physiological levels of all three SCFAs were shown to increase the growth rate of human neural progenitor cells and induce more cells to undergo mitosis (128), affording some hints of how SCFAs could regulate early neural system development. Further, SCFAs show effects on several neural functions, such as enhancing sleep (10), suppressing the activity of orexigenic neurons that express neuropeptide Y in the hypothalamus (89), and modulating the signaling triggered by the ghrelin receptor (129), contributing to circadian rhythm and appetite control. The seeking for mechanism involved in the modulation of neuronal function by SCFAs has unveiled that some of these effects are likely mediated by the activation of GPR41/GPR43 receptors. Other SCFA effects, especially of propionate and butyrate, are mediated through their HDAC inhibitory activity (108, 116).
Because of the similarity of SCFAs with the ketone bodies aceto-acetate and β-hydroxybutyrate (BHB), studies have been conducted to elucidate their role during fasting. Accordingly, fasting has been shown to sharply influence the gene regulation and protein expression of several MCTs, which alters the uptake of SCFAs in the gut and their transport to the brain (130). The regulation of the transporter is likely related to the direction of energy supplies to tissues during fasting. Moreover, Miletta and colleagues found that butyrate enhances growth hormone (GH) secretion in pituitary cells via GPR41/43 activation and intracellular accumulation of Ca2+ (131). This leads to the hypothesis that butyrate acts as a secondary mediator of metabolic adaptations of GH during fasting, which mainly include increased lipolysis and protein retention.
In summary, SCFAs might directly influence the brain by reinforcing BBB integrity, modulating neurotransmission, influencing levels of neurotrophic factors and promoting memory consolidation. However, further studies are needed to understand the precise mechanisms involved in these neuroactive effects.
SCFAs and Brain Disorders
The synthesis of new proteins is necessary for long-term changes in synaptic plasticity and learning (132–134). In this context, learning and long-term memory formation are improved by enhanced histone acetylation (135), which could be improved by HDAC inhibitors (HDACi). Given the HDAC inhibition property of SCFAs, several animal studies have focused mainly on the use of butyrate to elevate histone acetylation in the brain during a critical phase of memory formation. These studies have reported an enhancement of long-term potentiation (LTP) and contextual fear memory induced by HDAC inhibition (124, 127, 136, 137), pointing out enteric SCFAs as a promising learning and memory modulators. Therefore, the discovery that the microbiota can influence brain physiology has led to a plethora of experiments involving neurological disorders. The central hypothesis is supported by experimental and clinical evidence that the microbiota is altered in such diseases, which aggravates the condition, and/or its modulation might prevent or improve the development and progression of CNS pathologies (17, 19, 29, 33–37). Interestingly, several studies have found that the gut microbiome composition and, consequently, metabolome are altered in many brain disorders (138–142). Despite the knowledge that microbiota-gut-brain communication can theoretically occur through multiple systems (including the autonomic nervous system, enteric nervous system, neuroendocrine system, and immune system), increased evidence supports a potential key role of SCFAs in gut-brain axis signaling, and alterations in this signaling might underpin CNS disturbances ranging from neurodevelopmental disorders to neurodegenerative diseases.
SCFAs and Autism Spectrum Disorder
Characterized by behavioral symptoms including communication deficits, repetitive behaviors, and sensitivity to environmental changes, ASD comprises an array of neurodevelopmental disorders (143). Imbalances in the microbial composition of the gut are present in ASD. Support for this notion originates from animal studies and clinical evidence. However, the role of SCFAs in ASD is still controversial. Recently, Sharon and collaborators showed that microbiota transplantation from human ASD donors into mice could transfer ASD-relevant behavioral deficits (27). Although Sharon and coworkers did not evaluate the alteration in SCFAs, children with ASD have been previously reported to have both lower (144) and higher (33) fecal SCFA levels than controls. Interestingly, Wang and coworkers found similar proportions of specific SCFA and protein fermentation metabolites when comparing children with ASD with controls, even though the groups were controlled for gastrointestinal abnormalities, macronutrients intake and usage of probiotics, prebiotics, and antibiotics (33). However, neither of the previous studies performed a comprehensive evaluation of microbiota ecology.
In line with these findings, the microbiota has been suggested to affect the occurrence and severity of the disease through an increase in propionate-producing bacteria and a decrease in butyrate-producing bacteria (145, 146). The study conducted by Finegold and coworkers also found several pathobionts increased in the stool of ASD affected children such as Proteobacteria and hydrogen sulfide producing Desulfovibrio, raising a question for the causality of microbial metabolites unbalance (145, 146). Further, propionate-induced autism has become a validated animal model to study the disease. Administering high amounts of propionate through subcutaneous, intragastric, intraperitoneal, or intracerebroventricular routes to rodents has been suggested to induce high levels of microglia activation, neurotoxic cytokine production, genetic expression alterations, abnormal hippocampal histology, and abnormal neurobehaviors, such as repetitive actions and impaired social interaction (147). On the other hand, butyrate appears to have a beneficial effect on social and repetitive behavior in the BTBR mouse model, a strain-based ASD-like model (148). Epigenetic changes led to enhanced transcription of inhibitory neurotransmitter pathways in the frontal cortex, especially through HDAC inhibition (148). As described above, improvement of BBB impermeability by butyrate may be another mechanism through which butyrate can revert abnormalities in propionic acid-induced autism-like disorder (143). This evidence points to the importance of balance of a microbiota but also highlights the difficulty in drawing conclusions on the role of SCFAs in ASD and the need for more research in patients with ASD.
SCFAs and Mood Disorders
Despite the complex pathophysiology of mood disorders, several studies have indicated the participation of the gut microbiota in the severity of these diseases. Major depression is one of the most common mood disorders, seriously impairing the quality of life of patients and is one of the leading causes of social disability. Untreated depression is associated with an increased risk of morbidity and mortality, including suicide. Monoamine deficiency (149) and neurogenesis disruption (150) are two predominant theories underpinning depression. Furthermore, it has been shown that inflammation biomarkers are increased among patients with depression, and pro-inflammatory cytokines play an important role in the physiopathology of the disease (150). The importance of the microbiota in depression is supported by findings that the levels of SCFAs are decreased in a naturally occurring non-human primate model of depression (26). In line with these findings, clinical evidence has shown that fecal SCFA concentrations are lower in patients with depression than in controls (35, 151). Moreover, current knowledge shows that butyrate possesses an antidepressant-like effect that reverses behavioral alterations in mouse models, such as low energy (126, 152), anhedonia (153), and cognitive and sociability impairments (154). Therefore, taking into account the anti-inflammatory property of SCFAs, dysbiosis followed by decreased levels of these metabolites could play a role in the inflammation process related to the development of depression.
Studies on chronic psychosocial stress have also shown a possible application for prebiotics (154) and SCFAs (8) in reverting sociability impairment while also reducing stress-induced corticosterone release. Sodium butyrate has been shown to be capable of reversing behavioral hyperactivity (155) and depressive-like and manic-like behaviors in rats (156). There is also evidence for butyrate's antimanic effect on a rat model of bipolar disorder induced by intracerebroventricular administration of ouabain (157). Contrarily, a microbiome study in schizophrenic patients at risk of developing psychosis reported enriched Clostridiales, Prevotella, and Lactobacillus ruminis and predicted increased SCFA production (141). However, the study did not perform direct measurement of the metabolites and further research to confirm whether it is a case of SCFA overproduction or a specific metabolite unbalance is needed.
SCFAs and Alzheimer's Disease
Accumulating evidence has demonstrated that key neuropathological processes underlying AD might also be modulated by SCFAs (25, 34, 158, 159). Characterized by progressive cognitive impairment, AD is the most common form of dementia (160). Given that AD has a complex pathology and that therapies that effectively halt the disease progression are still lacking, recent studies have focused on environmental components and diet-based possible prevention strategies by using transgenic animal models (161, 162). In this context, several studies have established the benefits of a healthy microbiome on slowing AD and the correlation of dysbiosis with disease progression (7, 138, 163). Consistent with this notion, a study by Zhang and coworkers showed that the microbiota composition and diversity were perturbed and the level of SCFAs was reduced in AD mice, predicting alterations in more than 30 metabolic pathways, which may be associated with amyloid deposition and ultrastructural abnormalities in the APP/PS1 mouse model (25).
It is worth noting that SCFAs interfere with protein-protein interactions between amyloid-β peptides (Aβ), thereby disrupting their assembly into neurotoxic oligomers (34), the main toxins responsible for synapse dysfunction and cognitive deficits in AD (164). Given the close relation between gut dysbiosis and brain dysfunction, fecal microbiota transplantation (FMT) has been considered a promising therapeutic approach for the reestablishment of a healthy gut microbial community and has been shown to have beneficial effects on a plethora of diseases, including AD. Supporting this hypothesis, APP/PS1 mice exhibited significantly relieved cognitive deficits, Aβ accumulation, synaptic dysfunction, and neuroinflammation, mainly by the microglia, after FMT from healthy wild-type mice (165). These protective effects may be related to reversal of changes in the gut microbiota and SCFAs.
Oral bacteriotherapy through probiotic administration has become a potential treatment option for neurodegenerative diseases such as AD. Accordingly, the 3xTg mouse model of AD treated with probiotics in the early stage showed a promising reduction of inflammatory cytokines and decreased cognitive decline associated with reduced brain damage and Aβ aggregate accumulation (166). Moreover, other studies have shown beneficial effects of butyrate and probiotic treatment on cognition and memory in a D-galactose model of aging, a condition known to correlate with AD occurrence and progression (137, 167). The model consists of a long term administration of D-galactose, which can readily be metabolized but eventually leads to an overproduction of reactive oxygen species, thus causing genetic and cell damage impairing cognition (137, 168). Finally, through HDAC inhibition, butyrate administration recovered memory function and increased expression of genes implicated in associative learning in the APP/PS1 mouse model of AD (158).
SCFAs and Parkinson's Disease
SCFAs play a controversial role in Parkinson's disease (PD), a synucleinopathy and a multifactorial disorder with strong environmental influence characterized by tremors, muscle rigidity, bradykinesia, and impaired gait (169). Aggregation of the protein α-synuclein (αSyn) is thought to be the main pathogenic event in PD, which primarily affects dopaminergic neurons (169). Most PD patients also present gastrointestinal manifestations due to disturbances of the enteric nervous system. Hence, there has been great interest in the relationship between the gut microbiota and the development of the disease. Accordingly, sequencing of the microbiota of fecal samples from PD patients revealed reduced populations of Bacteroidetes and Prevotellaceae in contrast to increased Enterobacteriaceae and reduced production of SCFAs when compared to matched controls (139). However, the presence of gut microbes is necessary to elicit pathophysiological alterations in a mouse model of αSyn overexpression, because elimination of the gut microbiota with antibiotics ameliorated the condition (169). In contrast, FMT from PD patient donors worsens disease progression suggesting the presence of specific disease-promoting microbes (169). Accordingly, Li and colleagues confirmed that PD patients suffer alterations in the microbiota that correlate with disease progression, as there is a continuous decrease in fiber-degrading bacterial strains and an increase in pathobionts (170). This conversion probably leads to a decrease in SCFA production and an increase in endotoxin and neurotoxin production (170). Supporting this hypothesis, growing evidence has shown that FMT from healthy donors (171) as well as butyrate administration in animal models of PD improves motor impairment and dopamine deficiency (172–175).
SCFAs and Sclerosis
Multiple sclerosis (MS) is a neurodegenerative T-cell-mediated autoimmune disease of the CNS that mainly affects the myelin sheath around motor neurons. Among its etiological factors, the imbalance between pro and anti-inflammatory cells in the immune system seems to play an important role, which is highly affected by the gut microbiota composition and can be aggravated by dysbiosis (104, 176, 177). Given that SCFAs, mainly butyrate, are capable of inducing Treg polarization, modulation of the gut microbiota toward increased production of these metabolites could be an interesting therapeutic approach to MS. In fact, it is noteworthy that oral administration of SCFAs ameliorated the disease severity of experimental autoimmune encephalomyelitis (EAE), an animal model of MS (87, 178). Specifically, acetate supplementation is able to induce increased acetyl-CoA metabolism, which increases histone acetylation, resulting in preserved spinal cord lipid content and essentially preventing the onset of clinical symptoms of EAE (179). Furthermore, treatment with butyrate suppresses demyelination and enhances remyelination through oligodendrocyte maturation and differentiation (180).
Efforts to modify the course of amyotrophic lateral sclerosis (ALS) a disease that affects motor neurons but also involves a stronger genetic basis that leads to the premature death of those cells, has focused on the gut microbiota composition and its circulating metabolites (181). A comparative study conducted in human patients showed an elevated relative abundance of pathobionts compared to bacterial strains related to beneficial metabolism function (142). Another study found that transgenic ALS model mice had worse disease progression when raised under antibiotic treatment or GF conditions and identified several bacterial strains correlated with ameliorated or aggravated disease progression. A small assessment of the human microbiome/metabolite configuration was also conducted for comparison (181).
SCFAs and Metabolic Disorders
Much speculation currently surrounds the possible involvement of the gut microbiota in metabolic disorders such as type 2 diabetes and obesity. Compelling evidence have shown that the composition of the gut microbiota is altered in animal models of obesity and subjects with prediabetes or type 2 diabetes compared with controls (182–186). Despite differences in the identification of specific microbiome features responsible for these effects, a shift in the microbiome composition away from species able to produce butyrate was one consistent finding in type 2 diabetes subjects (187). Further, epidemiological and experimental studies have demonstrated that increased intake of dietary fiber reduces the risk for developing metabolic diseases (188–190), possibly by changing gut microbiome composition and diversity with increased production of SCFAs (187–189).
Animal studies suggest that SCFAs have an important role in the prevention and treatment of obesity-associated insulin resistance (89, 114, 191, 192). Mechanisms involved in the effects of SCFAs, mainly propionate and butyrate, in the brain responsible for controlling metabolic disorders include the activation of FFAR2 and FFAR3 receptors (91). It was shown that activation of these receptors leads to suppression of the activity of orexigenic neurons that express neuropeptide Y in the hypothalamus (89), and the modulation of the signaling mediate by the ghrelin receptor (129), contributing to circadian rhythm and appetite control. Studies in rodents show that the administration of prebiotics that influences a shift in the gut microbiome toward increased production of butyrate has beneficial effects associated with higher levels of GLP-1 (193–195), as well as hypothalamic expression of pro-opiomelanocortin (196), thereby influencing the hunger-satiety cycle. Although limited, some of these results were confirmed in human in vivo studies, as showed that acute rectal infusions of sodium acetate and SCFA mixtures increased circulating concentrations of PYY in individuals who were overweight (197–199).
Concluding Remarks
The gut microbiota has attracted considerable attention in recent years, putting it in the spotlight of biomedical research. Recent studies have suggested that an intestinal bacteria imbalance plays a role in the development of several disorders. The bidirectional communication that occurs between the microbiota and its mammalian host can be mediated through a variety of mechanisms, and it is clear that the biochemical messengers produced by the microbiota are an important facet of this crosstalk. Convincing evidence exists that SCFAs produced by the intestinal microbiota are involved in gastrointestinal physiology, immune function, host metabolism, and even in development and homeostasis of the CNS.
Although our understanding of microbiota-host interactions has considerably increased over recent years, there is still an unmet requirement for a deeper understanding of the complex microbiota-gut-brain communication. Furthermore, since most studies have been conducted in rodents, one must be cautious when translating the effects of SCFAs on humans. Given that SCFAs can regulate CNS processes through direct and indirect means and ultimately shape behavior and cognitive function, a thorough comprehension of how these metabolites participate in these complex gut-brain interactions may aid in developing novel therapeutic targets for treating CNS disorders. Further, through their effects on the development and maintenance of healthy brain function, these metabolites hold the potential for use as dietary interventions with a range of psychological functions.
Author Contributions
YS, AB, and RF planned, researched, and wrote the manuscript.
Funding
Work in the authors' laboratories has been supported by grants from Brazilian National Council for Development of Science and Technology (CNPq/Brazil), State of Rio de Janeiro Foundation for Funding Research (FAPERJ-JCNE-E-26/203.195/2016), Inova Fiocruz Program (Inova Fiocruz/VPPCB), National Institute of Science and Technology on Neuroimmunomodulation (INCT-NIM), and the Mercosur Program for Structural Convergence (FOCEM).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Aβ, amyloid-β peptide; AD, Alzheimer's disease; ASD, autism spectrum disorder; αSyn, α-synuclein; BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; BHB, β-hydroxybutyrate; CNS, central nervous system; DA, dopamine; EAE, experimental autoimmune encephalopathy; FFAR, free fatty acid receptor; FMT, fecal microbiota transplantation; GABA, γ-aminobutyric acid; GDNF, glial cell line-derived neurotrophic factor; GF, germ free; GH, growth hormone; GI, gastrointestinal (tract); GLP-1, glucagon-like peptide 1; GOS, galacto-oligosaccharides; GPCR, G protein-coupled receptors; HCAR2, hydrocarboxylic acid receptor; HDACs, histone deacetylases; HDACi, HDAC inhibitor; HPA, hypothalamus-pituitary-adrenal; LPS, lipopolysaccharide; MCT, H+-coupled monocarboxylate transporter; MS, multiple sclerosis; NA, noradrenaline; NGF, nerve growth factor; PD, Parkinson's disease; PKCδ, protein kinase Cδ; PYY, peptide YY; 5-HT, serotonin; SCFAs, short-chain fatty acids; SMCTs, sodium-coupled monocarboxylate transporters; SPF, specific pathogen-free; Tregs, T-regulatory lymphocytes.
References
1. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. (2012) 336:1268–73. doi: 10.1126/science.1223490
2. Erny D, Hrabe de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. (2017) 150:7–15. doi: 10.1111/imm.12645
3. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
4. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. (2012) 13:260–70. doi: 10.1038/nrg3182
5. Palm NW, Zoete MR De, Flavell RA, Haven N. Immune-microbiota interactions in health and disease. Clin Immunol. (2016) 159:122–7. doi: 10.1016/j.clim.2015.05.014
6. Erny D, De Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
7. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
8. van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O'Sullivan O, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J Physiol. (2018) 596:4923–44. doi: 10.1113/JP276431
9. Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. (2014) 20:509–18. doi: 10.1016/j.molmed.2014.05.002
10. Szentirmai É, Millican NS, Massie AR, Kapás L. Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci Rep. (2019) 9:7035. doi: 10.1038/s41598-019-43502-1
11. Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. (2019) 29:787–803. doi: 10.1038/s41422-019-0216-x
12. Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. (2014) 817:115–33. doi: 10.1007/978-1-4939-0897-4_5
13. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388
14. Mudd AT, Berding K, Wang M, Donovan SM, Dilger RN. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes. (2017) 8:589–600. doi: 10.1080/19490976.2017.1353849
15. O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. (2015) 277:32–48. doi: 10.1016/j.bbr.2014.07.027
16. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann NY Acad Sci. (2018) 1420:5–25. doi: 10.1111/nyas.13416
17. Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. (2017) 20:145–55. doi: 10.1038/nn.4476
18. Calvani R, Picca A, Lo Monaco MR, Landi F, Bernabei R, Marzetti E. Of microbes and minds: a narrative review on the second brain aging. Front Med. (2018) 5:53. doi: 10.3389/fmed.2018.00053
19. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. In: Advances in Immunology. Alt FW, editor. Cambridge, MA: Academic Press Inc. (2014). p. 91–119.
20. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
21. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
22. Pascale A, Marchesi N, Marelli C, Coppola A, Luzi L, Govoni S, et al. Microbiota and metabolic diseases. Endocrine. (2018) 61:357–71. doi: 10.1007/s12020-018-1605-5
23. Arpaia N, Campbell C, Fan X, Dikiy S, Van Der Veeken J, Deroos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
24. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science. (2013) 341:569–74. doi: 10.1126/science.1241165
25. Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, et al. Altered gut microbiota in a mouse model of Alzheimer's disease. J Alzheimer's Dis. (2017) 60:1241–57. doi: 10.3233/JAD-170020
26. Deng FL, Pan JX, Zheng P, Xia JJ, Yin BM, Liang WW, et al. Metabonomics reveals peripheral and central shortchain fatty acid and amino acid dysfunction in a naturally occurring depressive model of macaques. Neuropsychiatr Dis Treat. (2019) 15:1077–88. doi: 10.2147/NDT.S186071
27. Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. (2019) 177:1600–18.e17. doi: 10.1016/j.cell.2019.05.004
28. Dinan TG, Cryan JF. Microbes, immunity, and behavior: psychoneuroimmunology meets the microbiome. Neuropsychopharmacology. (2017) 42:178–92. doi: 10.1038/npp.2016.103
29. Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. (2016) 167:915–32. doi: 10.1016/j.cell.2016.10.027
30. Kim HL, Chang BJ, Nam SM, Nahm SS, Lee JH. Increased osteopontin expression and mitochondrial swelling in 3-nitropropionic acid-injured rat brains. Rom J Morphol Embryol. (2017) 58:1249–56.
31. Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. (2012) 30:313–35. doi: 10.1146/annurev-immunol-020711-075015
32. Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. (2002) 8:540–5. doi: 10.3748/wjg.v8.i3.540
33. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. (2012) 57:2096–102. doi: 10.1007/s10620-012-2167-7
34. Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer's disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. (2018) 18:83–90. doi: 10.1080/14737175.2018.1400909
35. Skonieczna-zydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A, et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients. (2018) 10:E1939. doi: 10.3390/nu10121939
36. Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. Cross talk: the microbiota and neurodevelopmental disorders. Front Neurosci. (2017) 11:490. doi: 10.3389/fnins.2017.00490
37. Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. (2017) 46:77–89. doi: 10.1016/j.gtc.2016.09.007
38. Ben-Ari Y. Neuropaediatric and neuroarchaeology: understanding development to correct brain disorders. Acta Paediatr Int J Paediatr. (2013) 102:331–4. doi: 10.1111/apa.12161
39. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. (2012) 17:1228–38. doi: 10.1038/mp.2012.23
40. Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. (2013) 18:666–73. doi: 10.1038/mp.2012.77
41. Russell SL, Gold MJ, Willing BP, Thorson L, McNagny KM, Finlay BB. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes. (2013) 4:158–64. doi: 10.4161/gmic.23567
42. Tochitani S, Ikeno T, Ito T, Sakurai A, Yamauchi T, Matsuzaki H. Administration of non-absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior. PLoS ONE. (2016) 11:e0138293. doi: 10.1371/journal.pone.0138293
43. Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. (2016) 165:1762–75. doi: 10.1016/j.cell.2016.06.001
44. Foley KA, Ossenkopp K-P, Kavaliers M, MacFabe DF. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS ONE. (2014) 9:e87072. doi: 10.1371/journal.pone.0087072
45. Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. (2016) 353:772–7. doi: 10.1126/science.aag3194
46. Jašarević E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol Stress. (2015) 1:81–8. doi: 10.1016/j.ynstr.2014.10.005
47. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. (2010) 107:11971–5. doi: 10.1073/pnas.1002601107
48. Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. (2015) 17:690–703. doi: 10.1016/j.chom.2015.04.004
49. Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. (2011) 108:4578–85. doi: 10.1073/pnas.1000081107
50. Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. (2003) 4:103–12. doi: 10.1038/nrn1032
51. Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. (2007) 7:161–7. doi: 10.1038/nri2015
52. Amor S, Peferoen LAN, Vogel DYS, Breur M, van der Valk P, Baker D, et al. Inflammation in neurodegenerative diseases - an update. Immunology. (2014) 142:151–66. doi: 10.1111/imm.12233
53. Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. (2018) 556:332–8. doi: 10.1038/s41586-018-0023-4
54. Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK. Microbiome–microglia connections via the gut–brain axis. J Exp Med. (2019) 216:41–59. doi: 10.1084/jem.20180794
55. Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. (2015) 29:1395–403. doi: 10.1096/fj.14-259598
56. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
57. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. (2009) 6:306–14. doi: 10.1038/nrgastro.2009.35
58. Goehler LE, Gaykema RPA, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. (2005) 19:334–44. doi: 10.1016/j.bbi.2004.09.002
59. Browning KN, Verheijden S, Boeckxstaens GE. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. (2017) 152:730–44. doi: 10.1053/j.gastro.2016.10.046
60. Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. (2011) 23:1132–9. doi: 10.1111/j.1365-2982.2011.01796.x
61. Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. (1996) 62:1589–92. doi: 10.1128/AEM.62.5.1589-1592.1996
62. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. (2009) 294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x
63. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. (2014) 4:e121. doi: 10.1038/nutd.2014.23
64. Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. (2019) 10:760. doi: 10.1038/s41467-019-08711-2
65. Cummings JH, Pomare EW, Branch HWJ, Naylor CPE, MacFarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. (1987) 28:1221–7. doi: 10.1136/gut.28.10.1221
66. Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. (1990) 70:567–90. doi: 10.1152/physrev.1990.70.2.567
67. Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. (2003) 62:67–72. doi: 10.1079/PNS2002207
68. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. (2017) 19:29–41. doi: 10.1111/1462-2920.13589
69. Smith EA, Macfarlane GT. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. (1997) 3:327–37. doi: 10.1006/anae.1997.0121
70. Smith E, Macfarlane G. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol Ecol. (1998) 25:355–68. doi: 10.1111/j.1574-6941.1998.tb00487.x
71. Windey K, de Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. (2012) 56:184–96. doi: 10.1002/mnfr.201100542
72. Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. (2002) 68:5186–90. doi: 10.1128/AEM.68.10.5186-5190.2002
73. Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. (2004) 91:915–23. doi: 10.1079/BJN20041150
74. Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B. (1987) 86:439–72. doi: 10.1016/0305-0491(87)90433-0
75. Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. (2014) 20:1487–98. doi: 10.2174/13816128113199990462
76. Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. (2016) 57:943–54. doi: 10.1194/jlr.R067629
77. Primec M, Mičetić-Turk D, Langerholc T. Analysis of short-chain fatty acids in human feces: a scoping review. Anal Biochem. (2017) 526:9–21. doi: 10.1016/j.ab.2017.03.007
78. Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. (2010) 16:1138–48. doi: 10.1002/ibd.21177
79. Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
80. Gaudier E, Rival M, Buisine M-P, Robineau I, Hoebler C, Hoebler C. Butyrate enemas upregulate muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res. (2009) 58:111–9.
81. O'Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. (2016) 13:691–706. doi: 10.1038/nrgastro.2016.165
82. Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, Faas M, et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. (2018) 57:1–14. doi: 10.1007/s00394-018-1703-4
83. Bolognini D, Tobin AB, Milligan G, Moss CE. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. (2016) 89:388–98. doi: 10.1124/mol.115.102301
84. Cherbut C, Ferrier L, Rozé C, Anini Y, Blottière H, Lecannu G, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. (1998) 275:G1415–22. doi: 10.1152/ajpgi.1998.275.6.G1415
85. Puddu A, Sanguineti R, Montecucco F, Viviani GL. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. (2014) 2014:162021. doi: 10.1155/2014/162021
86. Kien CL, Peltier CP, Mandal S, Davie JR, Blauwiekel R. Effects of the in vivo supply of butyrate on histone acetylation of cecum in piglets. J Parenter Enter Nutr. (2008) 32:51–6. doi: 10.1177/014860710803200151
87. Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. (2015) 43:817–29. doi: 10.1016/j.immuni.2015.09.007
88. Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol. (2013) 14:660–7. doi: 10.1038/ni.2611
89. Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. (2018) 67:1269–79. doi: 10.1136/gutjnl-2017-314050
90. Mollica MP, Raso GM, Cavaliere G, Trinchese G, De Filippo C, Aceto S, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes. 66:1405–18. doi: 10.2337/db16-0924
91. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. (2014) 156:84–96. doi: 10.1016/j.cell.2013.12.016
92. Kekuda R, Manoharan P, Baseler W, Sundaram U. Monocarboxylate 4 mediated butyrate transport in a rat intestinal epithelial cell line. Dig Dis Sci. (2013) 58:660–7. doi: 10.1007/s10620-012-2407-x
93. Oldendorf WH. Carrier mediated blood brain barrier transport of short chain monocarboxylic organic acids. Am J Physiol. (1973) 224:1450–3. doi: 10.1152/ajplegacy.1973.224.6.1450
94. Human Metabolome Database. Available online at: http://www.hmdb.ca/ (accessed December 27, 2019).
95. Bachmann C, Colombo JP, Berüter J. Short chain fatty acids in plasma and brain: quantitative determination by gas chromatography. Clin Chim Acta. (1979) 92:153–9. doi: 10.1016/0009-8981(79)90109-8
96. Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. (2015) 2015:1–12. doi: 10.1155/2015/412946
97. Sun J, Ling Z, Wang F, Chen W, Li H, Jin J, et al. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett. (2016) 613:30–5. doi: 10.1016/j.neulet.2015.12.047
98. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2015) 6:263ra158. doi: 10.1126/scitranslmed.3009759
99. Hoyles L, Snelling T, Umlai U-K, Nicholson JK, Carding SR, Glen RC, et al. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. (2018) 6:55. doi: 10.1186/s40168-018-0439-y
100. Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. (2017) 595:489–503. doi: 10.1113/JP273106
101. Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. (2016) 352:712–6. doi: 10.1126/science.aad8373
102. Wilton DK, Dissing-Olesen L, Stevens B. Neuron-glia signaling in synapse elimination. Annu Rev Neurosci. (2019) 42:107–27. doi: 10.1146/annurev-neuro-070918-050306
103. Gautiar EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. (2012) 13:1118–28. doi: 10.1038/ni.2419
104. Stanisavljević S, Cepić A, Bojić S, Veljović K, Mihajlović S, Ðedović N, et al. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in Dark Agouti rats. Sci Rep. (2019) 9:1–13. doi: 10.1038/s41598-018-37505-7
105. Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. (2016) 6:30028. doi: 10.1038/srep30028
106. Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APP SWE /PS1 ΔE9 murine model of Alzheimer's disease. Sci Rep. (2017) 7:10411. doi: 10.1038/s41598-017-11047-w
107. Jang H-M, Lee H-J, Jang S-E, Han MJ, Kim D-H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. (2018) 11:1386–97. doi: 10.1038/s41385-018-0042-3
108. Patnala R, Arumugam TV, Gupta N, Dheen ST. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol. (2017) 54:6391–411. doi: 10.1007/s12035-016-0149-z
109. Wang P, Zhang Y, Gong Y, Yang R, Chen Z, Hu W, et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol Dis. (2018) 111:12–25. doi: 10.1016/j.nbd.2017.12.006
110. Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K, et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. (2018) 1680:13–38. doi: 10.1016/j.brainres.2017.12.004
111. Soliman ML, Puig KL, Combs CK, Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J Neurochem. (2012) 123:555–67. doi: 10.1111/j.1471-4159.2012.07955.x
112. Soliman ML, Combs CK, Rosenberger TA. Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J Neuroimmune Pharmacol. (2013) 8:287–300. doi: 10.1007/s11481-012-9426-4
113. Reddy DS, Wu X, Golub VM, Dashwood WM, Dashwood RH. Measuring histone deacetylase inhibition in the brain. Curr Protoc Pharmacol. (2018) 81:e41. doi: 10.1002/cpph.41
114. Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. (2014) 5:3611. doi: 10.1038/ncomms4611
115. Oleskin AV, Shenderov BA. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb Ecol Heal Dis. (2016) 27:30971. doi: 10.3402/mehd.v27.30971
116. Nankova BB, Agarwal R, MacFabe DF, La Gamma EF. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells - possible relevance to autism spectrum disorders. PLoS ONE. (2014) 9:e103740. doi: 10.1371/journal.pone.0103740
117. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. (2014) 28:1221–38. doi: 10.1210/me.2014-1108
118. Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. (2016) 15:1945–56. doi: 10.1016/j.celrep.2016.04.074
119. Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav Immun. (2016) 56:140–55. doi: 10.1016/j.bbi.2016.02.020
120. Savignac HM, Corona G, Mills H, Chen L, Spencer JPE, Tzortzis G, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem Int. (2013) 63:756–64. doi: 10.1016/j.neuint.2013.10.006
121. Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, et al. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res. (2015) 61:114–21. doi: 10.1016/j.jpsychires.2014.11.003
122. Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. (2013) 38:2027–34. doi: 10.1038/npp.2013.104
123. Barichello T, Generoso JS, Simões LR, Faller CJ, Ceretta RA, Petronilho F, et al. Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol Neurobiol. (2015) 52:734–40. doi: 10.1007/s12035-014-8914-3
124. Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. (2009) 110:1226–40. doi: 10.1111/j.1471-4159.2009.06212.x
125. Yoo DY, Kim W, Nam SM, Kim DW, Chung JY, Choi SY, et al. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res. (2011) 36:1850–7. doi: 10.1007/s11064-011-0503-5
126. Wei Y Bin, Melas PA, Wegener G, Mathe AA, Lavebratt C. Antidepressant-like effect of sodium butyrate is associated with an increase in tet1 and in 5-hydroxymethylation levels in the BDNF gene. Int J Neuropsychopharmacol. (2015) 18:1–10. doi: 10.1093/ijnp/pyu032
127. Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. (2004) 279:40545–59. doi: 10.1074/jbc.M402229200
128. Yang LL, Millischer V, Rodin S, MacFabe DF, Villaescusa JC, Lavebratt C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J Neurochem. (2019) e14928. doi: 10.1111/jnc.14928. [Epub ahead of print].
129. Torres-Fuentes C, Golubeva AV, Zhdanov AV, Wallace S, Arboleya S, Papkovsky DB, et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. (2019) 33:13546–59. doi: 10.1096/fj.201901433R
130. Schutkowski A, Wege N, Stangl GI, Konig B. Tissue-specific expression of monocarboxylate transporters during fasting in mice. PLoS ONE. (2014) 9:e112118. doi: 10.1371/journal.pone.0112118
131. Miletta MC, Petkovic V, Eblé A, Ammann RA, Flück CE, Mullis PE. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PLoS ONE. (2014) 9:e107388. doi: 10.1371/journal.pone.0107388
132. Dale N, Kandel ER, Schacher S. Serotonin produces long-term changes in the excitability of aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci. (1987) 7:2232–8. doi: 10.1523/JNEUROSCI.07-07-02232.1987
133. Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. (1986) 234:1249–54. doi: 10.1126/science.3775383
134. Buffington SA, Huang W, Costa-Mattioli M. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci. (2014) 37:17–38. doi: 10.1146/annurev-neuro-071013-014100
135. Gräff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. (2013) 14:97–111. doi: 10.1038/nrn3427
136. Zhong T, Qing QJ, Yang Y, Zou WY, Ye Z, Yan JQ, et al. Repression of contexual fear memory induced by isoflurane is accompanied by reduction in histone acetylation and rescued by sodium butyrate. Br J Anaesth. (2014) 113:634–43. doi: 10.1093/bja/aeu184
137. Garcez ML, de Carvalho CA, Mina F, Bellettini-Santos T, Schiavo GL, da Silva S, et al. Sodium butyrate improves memory and modulates the activity of histone deacetylases in aged rats after the administration of D-galactose. Exp Gerontol. (2018) 113:209–17. doi: 10.1016/j.exger.2018.10.005
138. Hill JM, Bhattacharjee S, Pogue AI, Lukiw WJ. The gastrointestinal tract microbiome and potential link to Alzheimer's disease. Front Neurol. (2014) 5:43. doi: 10.3389/fneur.2014.00043
139. Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Park Relat Disord. (2016) 32:66–72. doi: 10.1016/j.parkreldis.2016.08.019
140. Li H, Sun J, Wang F, Ding G, Chen W, Fang R, et al. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. (2016) 1642:70–8. doi: 10.1016/j.brainres.2016.03.031
141. He Y, Kosciolek T, Tang J, Zhou Y, Li Z, Ma X, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry. (2018) 53:37–45. doi: 10.1016/j.eurpsy.2018.05.011
142. Zhai C-D, Zheng J-J, An B-C, Huang H-F, Tan Z-C. Intestinal microbiota composition in patients with amyotrophic lateral sclerosis. Chin Med J. (2019) 132:1815–22. doi: 10.1097/CM9.0000000000000351
143. Downs R, Perna J, Vitelli A, Cook D, Dhurjati P. Model-based hypothesis of gut microbe populations and gut/brain barrier permeabilities in the development of regressive autism. Med Hypotheses. (2014) 83:649–55. doi: 10.1016/j.mehy.2014.09.005
144. Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism - comparisons to typical children and correlation with autism severity. BMC Gastroenterol. (2011) 11:22. doi: 10.1186/1471-230X-11-22
145. Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. (2010) 16:444–53. doi: 10.1016/j.anaerobe.2010.06.008
146. Finegold SM. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses. (2011) 77:270–4. doi: 10.1016/j.mehy.2011.04.032
147. Choi J, Lee S, Won J, Jin Y, Hong Y, Hur T-Y, et al. Pathophysiological and neurobehavioral characteristics of a propionic acid-mediated autism-like rat model. PLoS ONE. (2018) 13:e0192925. doi: 10.1371/journal.pone.0192925
148. Kratsman N, Getselter D, Elliott E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology. (2016) 102:136–45. doi: 10.1016/j.neuropharm.2015.11.003
149. Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. (2000) 61:7–11.
150. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. doi: 10.1038/nri.2015.5
151. Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. (2016) 19:279–83. doi: 10.1179/1476830515Y.0000000007
152. Valvassori S, Resende W, Budni J, Dal-Pont G, Bavaresco D, Reus G, et al. Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Curr Neurovasc Res. (2015) 12:312–20. doi: 10.2174/1567202612666150728121121
153. Sun J, Wang F, Hong G, Pang M, Xu H, Li H, et al. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci Lett. (2016) 618:159–66. doi: 10.1016/j.neulet.2016.03.003
154. Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. (2017) 82:472–87. doi: 10.1016/j.biopsych.2016.12.031
155. Moretti M, Valvassori SS, Varela RB, Ferreira CL, Rochi N, Benedet J, et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav Pharmacol. (2011) 22:766–72. doi: 10.1097/FBP.0b013e32834d0f1b
156. Resende WR, Valvassori SS, Réus GZ, Varela RB, Arent CO, Ribeiro KF, et al. Effects of sodium butyrate in animal models of mania and depression: implications as a new mood stabilizer. Behav Pharmacol. (2013) 24:569–79. doi: 10.1097/FBP.0b013e32836546fc
157. Valvassori SS, Dal-Pont GC, Steckert AV, Varela RB, Lopes-Borges J, Mariot E, et al. Sodium butyrate has an antimanic effect and protects the brain against oxidative stress in an animal model of mania induced by ouabain. Psychiatry Res. (2016) 235:154–9. doi: 10.1016/j.psychres.2015.11.017
158. Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimer's Dis. (2011) 26:187–97. doi: 10.3233/JAD-2011-110080
159. Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. (2015) 14:957–70. doi: 10.1111/acel.12387
160. Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer Report 2016: Improving Healthcare for People Living With Dementia: Coverage, Quality and Costs Now and in the Future. (2016). Available online at: https://www.alz.co.uk/research/world-report-2016 (accessed December 28, 2019).
161. Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum Mol Genet. (2004) 13:159–70. doi: 10.1093/hmg/ddh019
162. Frozza RL, Lourenco MV, de Felice FG. Challenges for Alzheimer's disease therapy: insights from novel mechanisms beyond memory defects. Front Neurosci. (2018) 12:37. doi: 10.3389/fnins.2018.00037
163. Hoffman JD, Yanckello LM, Chlipala G, Hammond TC, McCulloch SD, Parikh I, et al. Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model. PLoS ONE. (2019) 14:e0221828. doi: 10.1371/journal.pone.0221828
164. Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer's disease. Front Cell Neurosci. (2015) 9:191. doi: 10.3389/fncel.2015.00191
165. Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, et al. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. (2019) 9:189. doi: 10.1038/s41398-019-0525-3
166. Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, et al. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. (2017) 7:1–21. doi: 10.1038/s41598-017-02587-2
167. Ho ST, Hsieh YT, Wang SY, Chen MJ. Improving effect of a probiotic mixture on memory and learning abilities in D-galactose–treated aging mice. J Dairy Sci. (2019) 102:1901–9. doi: 10.3168/jds.2018-15811
168. Hsieh HM, Wu WM, Hu ML. Genistein attenuates D-galactose-induced oxidative damage through decreased reactive oxygen species and NF-κB binding activity in neuronal PC12 cells. Life Sci. (2011) 88:82–8. doi: 10.1016/j.lfs.2010.10.021
169. Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. (2016) 167:1469–80.e12. doi: 10.1016/j.cell.2016.11.018
170. Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, et al. Structural changes of gut microbiota in Parkinson's disease and its correlation with clinical features. Sci China Life Sci. (2017) 60:1223–33. doi: 10.1007/s11427-016-9001-4
171. Sun MF, Zhu YL, Zhou ZL, Jia XB, Da Xu Y, Yang Q, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. (2018) 70:48–60. doi: 10.1016/j.bbi.2018.02.005
172. Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, et al. Sodium butyrate exerts protective effect against Parkinson's disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. (2017) 381:176–81. doi: 10.1016/j.jns.2017.08.3235
173. Paiva I, Pinho R, Pavlou MA, Hennion M, Wales P, Schütz AL, et al. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum Mol Genet. (2017) 26:2231–46. doi: 10.1093/hmg/ddx114
174. St. Laurent R, O'Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson's disease. Neuroscience. (2013) 246:382–90. doi: 10.1016/j.neuroscience.2013.04.037
175. Sharma S, Taliyan R, Singh S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: modulation of histone deacetylase activity. Behav Brain Res. (2015) 291:306–14. doi: 10.1016/j.bbr.2015.05.052
176. Jangi S, Gandhi R, Cox LM, Li N, Von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. (2016) 7:12015. doi: 10.1038/ncomms12015
177. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. (2016) 6:28484. doi: 10.1038/srep28484
178. Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS ONE. (2017) 12:e0173032. doi: 10.1371/journal.pone.0173032
179. Chevalier AC, Rosenberger TA. Increasing acetyl-CoA metabolism attenuates injury and alters spinal cord lipid content in mice subjected to experimental autoimmune encephalomyelitis. J Neurochem. (2017) 141:721–37. doi: 10.1111/jnc.14032
180. Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation. (2019) 16:165. doi: 10.1186/s12974-019-1552-y
181. Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. (2019) 572:474–80. doi: 10.1038/s41586-019-1443-5
182. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. (2009) 457:480–4. doi: 10.1038/nature07540
183. Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. (2010) 5:e9085. doi: 10.1371/journal.pone.0009085
184. Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. (2013) 498:99–103. doi: 10.1038/nature12198
185. Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. (2017) 26:611–9.e6. doi: 10.1016/j.cmet.2017.09.008
186. Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. (2013) 8:e71108. doi: 10.1371/journal.pone.0071108
187. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. (2019) 51:600–5. doi: 10.1038/s41588-019-0350-x
188. Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. (2012) 61:1058–66. doi: 10.1016/j.metabol.2012.01.017
189. Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, et al. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr. (2015) 6:198–205. doi: 10.3945/an.114.007419
190. Edwards CA, Xie C, Garcia AL. Dietary fibre and health in children and adolescents. Proc Nutr Soc. 74:292–302. doi: 10.1017/S0029665115002335
191. Den Besten G, Bleeker A, Gerding A, Van Eunen K, Havinga R, Van Dijk TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a pparg-dependent switch from lipogenesis to fat oxidation. Diabetes. (2015) 64:2398–408. doi: 10.2337/db14-1213
192. Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut microbiota. Sci Rep. (2016) 6:37589. doi: 10.1038/srep37589
193. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. (2012) 61:364–71. doi: 10.2337/db11-1019
194. Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. (2013) 288:25088–97. doi: 10.1074/jbc.M113.452516
195. Barrea L, Muscogiuri G, Annunziata G, Laudisio D, Pugliese G, Salzano C, et al. From gut microbiota dysfunction to obesity: could short-chain fatty acids stop this dangerous course? Hormones. (2019) 18:245–50. doi: 10.1007/s42000-019-00100-0
196. Ahmadi S, Nagpal R, Wang S, Gagliano J, Kitzman DW, Soleimanian-Zad S, et al. Prebiotics from acorn and sago prevent high-fat-diet-induced insulin resistance via microbiome–gut–brain axis modulation. J Nutr Biochem. (2019) 67:1–13. doi: 10.1016/j.jnutbio.2019.01.011
197. van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink SWMO, Holst JJ, et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci. (2016) 130:2073–82. doi: 10.1042/CS20160263
198. Freeland KR, Wolever TMS. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. Br J Nutr. (2010) 103:460–6. doi: 10.1017/S0007114509991863
Keywords: central nervous system, neuroinflammation, gut-brain axis, gut microbiota, short-chain fatty acids
Citation: Silva YP, Bernardi A and Frozza RL (2020) The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 11:25. doi: 10.3389/fendo.2020.00025
Received: 31 October 2019; Accepted: 14 January 2020;
Published: 31 January 2020.
Edited by:
Ana Rosa Pérez, National Council for Scientific and Technical Research (CONICET), ArgentinaReviewed by:
Claude Knauf, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceDouglas Morrison, University of Glasgow, United Kingdom
Norbert Sprenger, Nestle Institute of Health Sciences (NIHS), Switzerland
Copyright © 2020 Silva, Bernardi and Frozza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudimar Luiz Frozza, rudimar.frozza@ioc.fiocruz.br
 Ygor Parladore Silva
Ygor Parladore Silva Andressa Bernardi
Andressa Bernardi Rudimar Luiz Frozza
Rudimar Luiz Frozza