
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 30 January 2020
Sec. Obesity
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00022
This article is part of the Research TopicUnderstanding Sarcopenic Obesity: From Definition to Health Consequences and ManagementView all 5 articles
Objectives: Understanding the condition that describes the coexistence of obesity and sarcopenia, termed sarcopenic obesity (SO), is becoming a scientific and clinical priority. In this study, we aimed to assess the prevalence of SO in treatment-seeking adults with obesity and investigate any potential association between SO and a sedentary lifestyle, expressed in terms of daily steps.
Methods: In this cross-sectional, prospective observational study, body composition and daily steps measurements were obtained using a segmental body composition analyser (Tanita BC-418) and an Omron HJ-320 pedometer, respectively, in 111 adults of both genders with obesity (body mass index; BMI ≥ 30 kg/m2), referred to the Outpatient Clinic in the Department of Nutrition and Dietetics at Beirut Arab University (BAU) in Lebanon. The participants were then categorized according to the presence of absence of SO, defined as an appendicular lean mass divided by body weight (ALM/weight) × 100%) of less than 23.40 and 29.60 in females and males, respectively.
Results: Fifty-five of the 111 participants with obesity, with a mean age of 39.62 ± 16.55 years and a mean BMI of 38.05 ± 5.33 kg/m2 met the criteria for SO and displayed a significantly higher prevalence of inactivity (<5,000 daily steps), i.e., nearly double (54.5% vs. 32.1%; p = 0.017) and they had a lower mean number of daily steps than those in the group without SO (5,279 ± 2,641 vs. 6,732 ± 2,989; p = 0.008). Linear regression analysis showed that SO is associated with a lower number of daily steps by 1,421 (β = −1421.4; −2508.9, −333.9; p = 0.011) after adjusting for age, gender employment and the presence of cardiometabolic disease.
Conclusion: Sarcopenic obesity affects nearly 50% of treatment-seeking adults with obesity. Moreover, it seems to be associated with a lower number of daily steps and a sedentary lifestyle. Future studies are needed to clarify whether this may influence clinical outcomes. If this is shown to be the case, weight management programmes should incorporate additional physical activity strategies in this population.
A new phenotype has been identified, which occurs in the presence of both sarcopenia and obesity, and is termed sarcopenic obesity (SO), describing the coexistence of increased body-fat mass deposition and a reduction in lean mass as well as muscle strength (1, 2). Understanding of this condition is now becoming a scientific and clinical priority (1). Indeed, several studies have demonstrated that regardless of gender, individuals with SO have worse profiles in terms of cardio-metabolic conditions (i.e., hyperglycaemia, hypertension, dyslipidaemia, insulin resistance, and type 2 diabetes) (3–6). However, the underlying mechanism is still unclear, but it seems there is a bi-directional interaction between “obesity” and “sarcopenia” through a further enhancement of chronic inflammation levels (i.e., common denominator seen in both conditions) (7, 8), that appears to exacerbate the cardiometabolic comorbidities (9) in individuals affected by SO, rather than obesity alone.
In the same direction, interestingly, recent studies have found a strong association between SO and impairment of physical performance (10). However, to the best of our knowledge, it is still unclear whether individuals with SO are likely to have a more sedentary lifestyle than those with obesity alone, however, if this is true, it may have significant clinical implications, especially among those seeking treatment and in the identification of SO in patients with obesity, for whom it becomes critically important to target interventions (11).
Definitions of SO based only on lean mass and physical fitness without accounting for body mass (i.e., body weight, BMI, etc.) may be strongly skewed for at least two reasons (6, 12–14). Firstly, patients with obesity tend to have a relatively large lean mass (15). Hence, sarcopenia criteria may not be met in these individuals, and the prevalence of sarcopenia may be underestimated (16). Second, low physical fitness is more closely associated with obesity than sarcopenia (17).
Based on these considerations, we aimed to assess the prevalence of sarcopenia in treatment-seeking adults of both genders with obesity, using a definition that, in addition to appendicular lean mass (ALM), also includes body weight (18), namely the definition proposed by Oh and colleagues from previous studies, which has been determined to be more clinically useful than other definitions (3, 19). Moreover, we also sought to examine any association between SO and a sedentary lifestyle—expressed as a reduction in the number of daily steps—when compared with those without SO.
A priori calculation of the sample size showed that a sample of 92 patients is need for a power of 80% with medium effect size and 5 predictors. However, 111 participants of both genders seeking weight-loss treatment and with obesity were recruited consecutively, following referral by general practitioners to the Nutritional and Weight Management Outpatient Clinic in the Department of Nutrition and Dietetics at Beirut Arab University (BAU) in Lebanon during the period May 2017 to July 2019. The eligibility criteria were assessed by a medical doctor involved in the study and comprised: age ≥ 18 years, with a BMI ≥ 30.0 kg/m2, indicative of obesity status and considered a criterion for an absolute indication for weight loss (20). There were no specific exclusion criteria, except pregnancy or lactation, medication known to influence body weight or composition, or any clinical condition that does not indicate weight loss. The study was approved by the Institutional Review Board of BAU (No. 2017H-0034-HS-R-0241), and all participants gave informed, written consent for the use of their anonymized personal data. A questionnaire was administered to participants and controls in the test to elicit information regarding medical history, lifestyle, demographic and social conditions.
Body weight was measured by trained dieticians using an electronic weighing scale (SECA 2730-ASTRA, Germany). Height was measured using a stadiometer. BMI was then calculated according to the standard formula.
Body composition was measured in the morning in our clinics by trained dieticians using a segmental body composition analyser (BC-418, Tanita Corp., Tokyo, Japan). Participants wore their own clothes and weight adjustment for clothing was applied. This method allows bioelectrical impedance measurement of the whole body and each part (right leg, left leg, right arm and left arm) at a single frequency. Gender, age and height information was then entered into the device and participants were asked to stand in a stable position with bare feet. Their toes and heels were placed in contact with the anterior and posterior electrodes of the weighing platform, respectively. The device provides separate body mass readings for different segments of the body and uses an algorithm incorporating impedance, age and height to estimate the total and regional body fat and fat-free mass. Total fat, lean mass percentages and the ALM were calculated using standard formulas (21). Sarcopenic obesity was defined based on the definition of Oh and colleagues ((ALM/weight) × 100%), which is less than 23.40 and 29.60 in females and males, respectively (18).
Daily steps were measured by means of a validated commercial grade pedometer (Omron HJ-320; Omron Healthcare Co., Ltd., Kyoto, Japan), which is considered accurate within ±5% of the criterion measure and was used to record the total number of steps taken each day, with the device automatically resetting itself at the end of the day and possessing a 7-day memory (22). The pedometer was placed either in participants' trouser pocket or attached to their waistband. Participants were encouraged to wear the pedometer all day for 7 days once awake and to remove the device only when sleeping or bathing and they were advised to continue with their usual lifestyle. A step-defined sedentary lifestyle index is <5,000 steps/day (23).
Cardiometabolic disease in this study is defined as the presence of any diseases such as type 2 diabetes, cardiovascular diseases (coronary heart disease, stroke, transient ischaemic attack, and peripheral arterial disease) and dyslipidaemia (a decreased concentration of high-density lipoprotein cholesterol and an increased concentration of high-density lipoprotein cholesterol and triglycerides) based on self-reported diagnosis, either simultaneously or separately.
Descriptive statistics were calculated as means and standard deviations, frequencies and proportions. A χ2 test and student's t-test were used to compare proportions and means, respectively, between participants with and without SO in the clinical sample. Simple and linear regression analysis was performed to calculate changes in the total daily steps among individuals with SO. A multiple linear regression model was adjusted for age, sex, employment, and the presence of cardiometabolic disease. All analyses were performed using SPSS version 25.0 (IBM Corp.; IBM, Armonk, NY, USA). Statistical significance was considered to be p < 0.05.
Table 1 describes socio-demographic characteristics of the study population, which included 111 participants with obesity with a mean age of 37.12 ± 15.58 years [32 males (28.8%) and 79 females (71.2%)]. Application of the chosen definition of SO (18) indicated that a total of 55 patients (49.5%) had SO, of whom 17 were male (53.1%) and 38 were female (48.1%). On the other hand, 56 (50.5%) did not have SO, of whom 15 were male (46.9%) and 41 were female (51.9%) (Table 1).
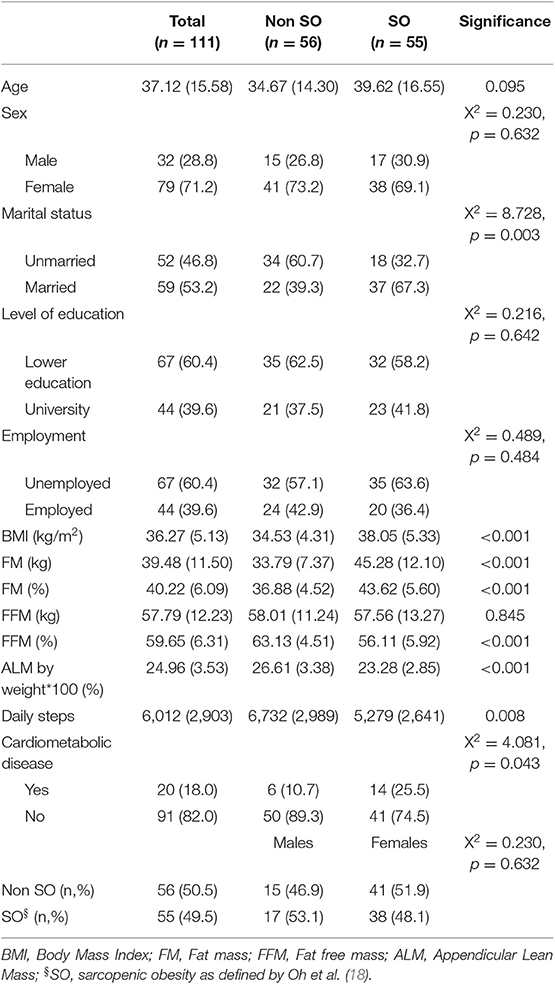
Table 1. Socio-demographic and anthropometric body composition characteristics of the study population (n = 111).
The group with SO, when compared with the group without SO, had a significantly higher BMI, total body-fat percentage and lower fat-free mass percentage as well as ALM/weight × 100 and a higher prevalence of cardiometabolic disease (25.5% vs. 10.7%) (Table 1). Moreover, the SO group had a lower mean number of daily steps than those in the group without SO (5,279 ± 2,641 vs. 6,732 ± 2,989; p = 0.008) (Table 1; Figure 1) and a significantly higher prevalence of inactive individuals (i.e., <5,000 daily steps) than those in the group without SO (54.5% vs. 32.1%; p = 0.017) (Figure 2). Linear regression analysis showed that having SO is associated with a lower number of daily steps by 1,421 (β = −1421.4; −2508.9, −333.9; p = 0.011) after adjusting for age, gender, employment and the presence of cardiometabolic diseases.
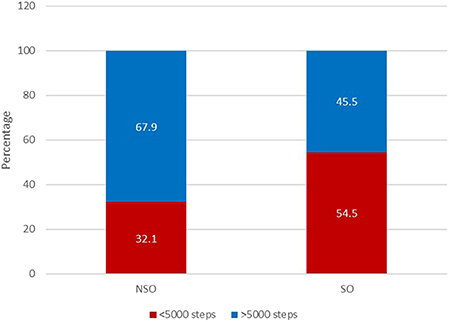
Figure 2. Proportion of patients with SO considered inactive, performing less than 5,000 steps daily (n = 111).
Our study aimed to provide data on the prevalence of sarcopenia in treatment-seeking adults with obesity and to assess any association between SO and a more sedentary lifestyle, expressed as measured routine daily steps. In turn, two major findings were revealed.
Firstly, our assessment of the prevalence of sarcopenia among patients with obesity seeking treatment, using criteria accounting for body weight, revealed an SO prevalence of 49.5% across the entire sample (53.1% in males and 48.1% in females). This falls within the large prevalence range of 0–100% reported for males and 0–85% reported for females, a range that may depend on the SO definition applied (24), in which a greater prevalence may be likely to be reported in studies accounting for body weight or BMI, whereas a smaller prevalence will be reported in those that do not (16). However, specifically, the prevalence of sarcopenia in our sample (53.1% in males and 48.1% in females) highly exceeded that found by Oh and colleagues in their original sample (19.6% in males and 31.3% in females) (18). Several factors may underlie this discrepancy, the sample in Oh and colleagues studies was composed of normal and overweight as well as obese individuals from the general population (18), unlike the present study, which included patients exclusively with obesity (BMI ≥ 30 kg/m2) in a clinical setting and affected by obesity-related (i.e., cardiometabolic) diseases. We speculate that obesity as well as cardiometabolic disease may act synergistically to increase the inflammatory status (25), when the latter is known to have a role in sarcopenia pathogenesis (8). In other words, concomitant cardiometabolic complications could increase the prevalence of sarcopenia in obesity and this can partially explain such a high prevalence of sarcopenia in our young population. However, we cannot exclude the existence of other factors (i.e., ethnicity) that may explain the higher prevalence of SO in our sample.
Second, 54.5% of participants with SO were considered inactive (i.e., <5,000 daily steps), with both conditions strongly associated; in fact, the presence of SO decreased the number of daily steps by 1,421 after adjusting for age, gender and employment. However, the cross-sectional design, at best, reveals only simple associations between SO and reduced mean daily steps but cannot provide firm information on any causal relationships between the two conditions (1, 26), in other words, whether SO causes a sedentary lifestyle or whether a tendency to be physically inactive over time causes SO.
Some clinical implications can be derived from our findings given the need to raise awareness among clinicians (and patients) of the presence of sarcopenia in those seeking treatment for obesity. Second, our results reveal the importance of screening for SO in treatment-seeking patients with obesity, since this condition seems to be associated with a more sedentary lifestyle and may have an impact on the effectiveness of weight management programmes based on lifestyle modification work, focusing on enabling patients to cope with sedentary behavior and helping them to develop a more active lifestyle (27–37).
Our study has certain strengths. Firstly, to the best of our knowledge, it is the first study to examine the association between SO and a sedentary lifestyle (i.e., daily steps), based on physical activity measurements using an objective, validated and accurate tool suitable for assessing lifestyle (22) rather than self-reported data, which has never been previously investigated. Second, it is one of the few to assess SO in the MENA region and one of the few studies that takes into account not only ALM but also body mass (18). Second, our results are based on physical activity measurements using an objective, validated and accurate tool suitable for assessing lifestyle (22) rather than self-reported data.
However, the study has certain limitations. Firstly, our results need to be interpreted with caution because they may not applicable to patients treated in other settings (i.e., inpatients or bariatric surgery). Second, we assessed body composition using an impedance analyser; despite being validated, this has not yet been accepted as a gold-standard technique for patients with obesity (38). Third, the cross-sectional design, small sample size and the absence of macronutrient nutritional and biochemical assessments in our study, which permit a better understanding of the mechanisms underlying the high prevalence of sarcopenia in individuals with obesity, in addition, the use of a definition for SO that was initially established in an Asian population, that was based only on reduction in LBM, not taking into account low muscle strength or low physical function, should be considered further limitations. Finally, in the diagnosis of cardiometabolic disease, we relied on self-reported data.
Our findings provide preliminary evidence that nearly 50% of adults with obesity, who are seeking weight-loss treatment, have sarcopenia. This condition seems to be strongly associated with a more sedentary lifestyle. It would, therefore, be clinically useful to screen SO in this population. However, future longitudinal studies are needed to clarify whether this may influence clinical outcomes, namely weight loss and maintenance as well as dropout rates. If this is confirmed, weight management programmes should take into consideration additional physical activity strategies in this population to promote the adoption of more active lifestyles.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board—Beirut Arab University. The patients/participants provided their written informed consent to participate in this study.
All authors claim authorship, and have approved and made substantial contributions to the conception, drafting and final version of the paper. This study was designed by ME, while LI conducted the statistical analysis. DK, DE, and HT collected data. ME and LI co-wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. El Ghoch M, Calugi S, Dalle Grave R. Sarcopenic obesity: definition, health consequences and clinical management. Open Nutr. J. (2018) 12:70–3. doi: 10.2174/1874288201812010070
2. El Ghoch M, Fakhoury R. Challenges and new directions in obesity management: lifestyle modification programs, pharmacotherapy and Bariatric surgery. J Populat Therapeut Clin Pharmacol. (2019) 26:1–4. doi: 10.15586/jptcp.v26i2.599
3. Khazem S, Itani L, Kreidieh D, El Masri D, Tannir H, Citarella R, et al. Reduced lean body mass and cardiometabolic diseases in adult males with overweight and obesity: a pilot study. Int J Environ Res Public Health. (2018) 15:E2754. doi: 10.3390/ijerph15122754
4. Khadra D, Itani L, Tannir H, Kreidieh D, El Masri D, El Ghoch M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: a systematic review and meta-analysis. World J Diab. (2019) 10:311–23. doi: 10.4239/wjd.v10.i5.311
5. Kreidieh D, Itani L, El Masri D, Tannir H, Citarella R, El Ghoch M. Association between sarcopenic obesity, type 2 diabetes, and hypertension in overweight and obese treatment-seeking adult women. J Cardiovasc Dev Dis. (2018) 5:E51. doi: 10.3390/jcdd5040051
6. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. (2008) 11:693–700. doi: 10.1097/MCO.0b013e328312c37d
7. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediat Inflamm. (2010) 2010:289645. doi: 10.1155/2010/289645
8. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. (2017) 96:10–5. doi: 10.1016/j.maturitas.2016.11.006
9. Esser N, Paquot N, Scheen AJ. Inflammatory markers and cardiometabolic diseases. Acta Clin Belg. (2015) 70:193–9. doi: 10.1179/2295333715Y.0000000004
10. El Ghoch M, Rossi AP, Calugi S, Rubele S, Soave F, Zamboni M, et al. Physical performance measures in screening for reduced lean body mass in adult females with obesity. Nutr Metab Cardiovasc Dis. (2018) 28:917–21. doi: 10.1016/j.numecd.2018.06.008
11. Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. (2013) 20:412–9. doi: 10.1097/01.med.0000433071.11466.7f
12. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
13. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
14. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
16. Johnson Stoklossa CA, Sharma AM, Forhan M, Siervo M, Padwal RS, Prado CM. Prevalence of sarcopenic obesity in adults with class II/III obesity using different diagnostic criteria. J Nutr Metab. (2017) 2017:7307618. doi: 10.1155/2017/7307618
17. Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal study. Obesity (Silver Spring). (2009) 17:2082–8. doi: 10.1038/oby.2009.109
18. Oh C, Jho S, No JK, Kim HS. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr Res. (2015) 35:1–6. doi: 10.1016/j.nutres.2014.07.018
19. Tannir H, Kreidieh D, Itani L, El Masri D, El Ghoch M. Reduction of resting energy expenditure and sarcopenic obesity in adults with overweight and obesity: a brief report. Curr Diabetes Rev. (2019). doi: 10.2174/1573399815666191030092138. [Epub ahead of print].
20. Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
21. Bredella MA, Ghomi RH, Thomas BJ, Torriani M, Brick DJ, Gerweck AV, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring). (2010) 18:2227–33. doi: 10.1038/oby.2010.5
22. Park W, Lee V, Ku B, Tanaka H. Effect of walking speed and placement position interactions in determining the accuracy of various newer pedometers. J. Exerc. Sci. Fitness. (2014) 12:31–7. doi: 10.1016/j.jesf.2014.01.003
23. Tudor-Locke C, Craig CL, Thyfault JP, Spence JC. A step-defined sedentary lifestyle index: <5000 steps/day. Appl Physiol Nutr Metab. (2013) 38:100–14. doi: 10.1139/apnm-2012-0235
24. Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring). (2009) 17:1281–6. doi: 10.1038/oby.2009.14
25. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. (2017) 13:851–63. doi: 10.5114/aoms.2016.58928
26. Solem RC. Limitation of a cross-sectional study. Am J Orthod Dentofacial Orthop. (2015) 148:205. doi: 10.1016/j.ajodo.2015.05.006
27. Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. (2016) 9:37–46. doi: 10.2147/DMSO.S89836
28. Dalle Grave R, Calugi S, Centis E, El Ghoch M, Marchesini G. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. J Obes. (2011) 2011:348293. doi: 10.1155/2011/348293
29. Dalle Grave R, Calugi S, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Lifestyle modification in the management of the metabolic syndrome: achievements and challenges. Diabetes Metab Syndr Obes. (2010) 3:373–85. doi: 10.2147/DMSOTT.S13860
30. Dalle Grave R, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Major factors for facilitating change in behavioral strategies to reduce obesity. Psychol Res Behav Manag. (2013) 6:101–10. doi: 10.2147/PRBM.S40460
31. Dalle Grave R, Calugi S, El Ghoch M. Lifestyle modification in the management of obesity: achievements and challenges. Eat Weight Disord. (2013) 18:339–49. doi: 10.1007/s40519-013-0049-4
32. Dalle Grave R, Sartirana M, El Ghoch M, Calugi S. Personalized multistep cognitive behavioral therapy for obesity. Diabetes Metab Syndr Obes. (2017) 10:195–206. doi: 10.2147/DMSO.S139496
33. Kreidieh D, Itani L, El Kassas G, El Masri D, Calugi S, Grave RD, et al. Long-term lifestyle-modification programs for overweight and obesity management in the arab states: systematic review and meta-analysis. Curr Diabetes Rev. (2018) 14:550–8. doi: 10.2174/1573399813666170619085756
34. El Ghoch M, Bazzani PV, Calugi S, Dalle Grave R. Weight-loss cognitive-behavioural treatment and essential amino acid supplementation in a patient with spinal muscular atrophy and obesity. Case Rep Med. (2018) 2018:4058429. doi: 10.1155/2018/4058429
35. Dalle Grave R, Sartirana M, El Ghoch M, Calugi S. Module 3: developing an active lifestyle. In: Treating Obesity with Personalized Cognitive Behavioral Therapy. Cham: Springer (2018). P. 75–88. doi: 10.1007/978-3-319-91497-8_6
36. El Masri D, Kreidieh D, Tannir H, Itani L, El Ghoch M. Long-term weight-loss lifestyle modification programme in a patient with severe lumbar intervertebral disc degeneration and obesity: a case report. Reports. (2018) 1:1–5. doi: 10.3390/reports1030021
37. Dalle Grave R, Calugi S, El Ghoch M, Sartirana M. Treating Obesity With Personalised Cognitive Behavioural Therapy. Basel: Springer International Publishing AG (2018). doi: 10.1007/978-3-319-91497-8
Keywords: physical activity, body composition, obesity, sarcopenic obesity, daily steps, sedentary lifestyle
Citation: Kreidieh D, Itani L, El Masri D, Tannir H and El Ghoch M (2020) Association Between Reduced Daily Steps and Sarcopenic Obesity in Treatment-Seeking Adults With Obesity. Front. Endocrinol. 11:22. doi: 10.3389/fendo.2020.00022
Received: 20 August 2019; Accepted: 13 January 2020;
Published: 30 January 2020.
Edited by:
Paolo Marzullo, Università degli Studi del Piemonte Orientale, ItalyReviewed by:
Valeria Guglielmi, University of Rome Tor Vergata, ItalyCopyright © 2020 Kreidieh, Itani, El Masri, Tannir and El Ghoch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marwan El Ghoch, bS5naG9jaEBiYXUuZWR1Lmxi
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.