- 1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Tabuk, Tabuk, Saudi Arabia
- 2Department of Biochemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
- 3Pharmacology Department, Faculty of Medicine, University of Tabuk, Tabuk, Saudi Arabia
- 4Department of Pharmacology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
- 5Department of Physiology, Faculty of Medicine, Taibah University, Medina, Saudi Arabia
- 6Department of Physiology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
- 7Department of Biochemistry, Faculty of Pharmacy, Suez Canal University, Ismailia, Egypt
- 8Department of Pathology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
- 9Clinical Pathology Department, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
- 10Department of Internal Medicine, Gastroenterology Division, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
- 11Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Tabuk, Tabuk, Saudi Arabia
- 12Department of Pharmacology and Toxicology, Faculty of Pharmacy, Suez Canal University, Ismailia, Egypt
Obesity is a public health burden disturbing all body functions and reproductive hormones. As obesity increases among females, there will be a rising challenge to physicians in care from fertility problems. Evening primrose oil (EPR oil) contains essential fatty acids including omega-6 linoleic acid with strong anti-inflammatory activity. Since EPR oil has utility in alleviating dysmenorrhea, this study aimed to ascertain its modulatory effect on systemic inflammation, reproductive hormones and estrus cycle irregularity in female obese rats. Thirty-two female rats were distributed to 4 groups: (i) normal, (ii) dietary obese-control female rats, and (iii and iv) dietary obese female rats treated with EPR oil (5 or 10 g/kg). Rats were examined for estrus regularity by taking vaginal smears daily during the last 2 weeks of the experiment. Serum level of insulin, leptin, adiponectin, and inflammatory cytokines was measured. In addition, serum lipid profile, and liver enzyme activities were estimated. Adipose tissues were taken for histopathologic examination as well as determination of gene expression for leptin, leptin receptors, adiponectin, and visfatin. Obese rats exhibited significant weight gain (90.69 ± 8.9), irregular prolonged estrus cycles (83.33%), increased serum levels of insulin, leptin, prolactin and testosterone and decreased gonadotropin levels. EPR oil exhibited a curative effect on obesity-related irregularity in estrus cycle and ovarian pathology. The underlying molecular mechanism may be related to reduction of systemic inflammation, alleviating insulin resistance and modulation of adipokine expression. EPR oil may be considered as a promising therapeutic intervention against obesity-related female hormonal disturbances and estrus irregularity.
Introduction
Clinical observations have indicated a solid association between obesity and infertility in females (1). Obese women suffer from insulin and leptin resistance, inducing hyperandrogenemia, modulation of steroidogenesis and polycystic ovaries syndrome (PCOS) (2–5). Obesity increases systemic inflammation and disturbs the reproductive functions via negative effects on the ovaries (2). The levels of insulin, estrone, luteinizing hormone (LH), and androstenedione are known to increase. These hormonal changes deteriorates hypothalamic-pituitary-gonadal axis leading to different gynecological consequences (6).
Adipose tissue secretes a variety of molecules known as adipokines which regulate many functions. Some of the major adipokines released from adipose tissue are leptin, adiponectin, and visfatin. Adipokines are considered key players in regulating the reproductive function (7).
Leptin is a 16 kD protein consisting of 146 amino acids which is principally secreted by adipose tissue (8) that functions to inhibit feeding (9). Furthermore, it has various effects on the endocrine systems, tissue remodeling, and fertility (10). Leptin influences gonadotropin secretion and ovarian steroidogenesis in human (11) and rat (12). A lack of leptin or leptin receptors has been linked to infertility and delayed puberty development in humans and rodents (13).
On the other hand, adiponectin increases insulin sensitivity and its level declines with obesity and increases with weight loss (14, 15). Adiponectin receptors are widely located in female reproductive tissues, including ovaries, endometrium and placenta (16). It is reported to be beneficial to reproductive function (17). Visfatin is an insulin-mimetic adipokine that promotes the synthesis and storage of fat, regulates glucose and lipid metabolism in vivo, Visfatin controls energy metabolism balance and is linked to the reproductive function (18). It was reported to directly correlates with PCOS (19).
Evening primrose oil (EPR oil), derived from Oenothera biennis L plant seeds, contains high percentage of essential fatty acids for body health comprising omega-6 linoleic acid (LA) and γ-LA (20). It possesses direct and indirect anti-inflammatory action due to its sterols content (21) or since its γ-LA content acts as a precursor of prostaglandin E1 (22).
In the field of gynecology, many midwives widely suggest EPR oil to accelerate ripening of the cervix in order to reduce the time of labor and the incidence of delayed pregnancies (23). Interestingly, γ-LA has been reported to alleviate premenstrual syndrome and dysmenorrhea (24). Therefore, EPR oil is perhaps a good treatment for these conditions. Previous clinical studies suggested the usefulness of EPR oil in rheumatoid arthritis (25), ulcerative colitis (26) as well as insulin resistance when used in combination with vitamin D (27).
In an attempt to determine the utility of EPR oil in dietary obese female rats, its effect was assessed in terms of controlling systemic insulin resistance and inflammation in addition to modulation of adipokine and gonadotropin levels. The impact of EPR oil on obesity-induced ovarian pathology and estrus cyclicity was also determined. Positive results may suggest EPR oil as a candidate for obesity related PCOS.
Materials and Methods
Diet and Treatments
The high fat diet (HFD) is often utilized for induction of obesity in female rats. It was prepared by mixing the standard chow diet (87.7% w/w) with the following fatty components: 10% w/w pork fat, 2% w/w cholesterol and 0.3% w/w bile salts (28, 29). Each gelatin capsule of Primaleve (GlaxoSmithKline) contained 1,000 mg of EPR oil (including 9–10% γ-LA). The oily content of the capsule was withdrawn with a sharp needle of a 3-ml syringe.
Design of the Experiment
All procedures for animal handling were in accord with the Guideline for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 80-23, revised in 1978). All experimental procedures were approved by the Institutional Research Ethics Committee at Faculty of Pharmacy, Suez Canal University [approval number 201605RA2].
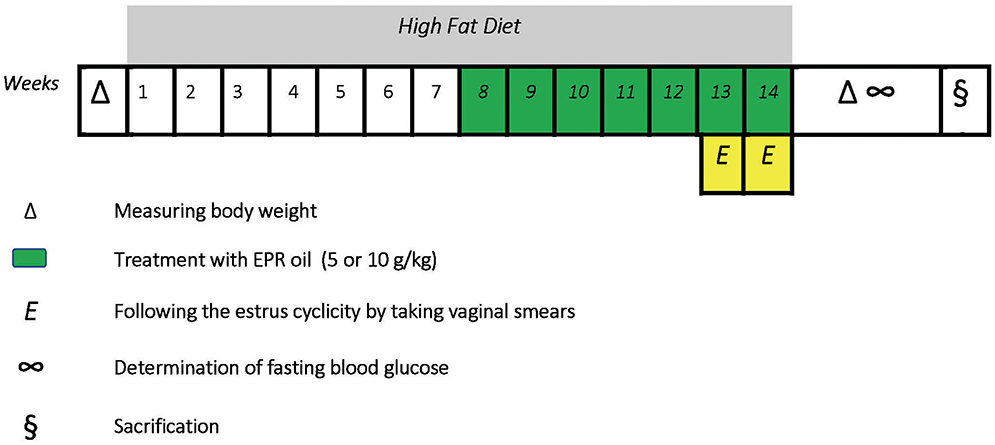
Twenty four female albino Wistar rats were purchased from Moustafa Rashed Company in Saqqarah (Giza, Egypt). The rats aged 8–9 weeks at the beginning of the experiment. Female rats were kept in plastic cages at room temperature (27± 5°C) and normal light-dark phases with unhindered access to food and water. Rats had initial body weights equal 110–142 g. Six rats received standard diet for 14 weeks (WKs), received oral doses of distilled water (10 ml/kg, weeks 8–14) and categorized as a normal group; other rats received HFD for 14 weeks to begin a model of dietary obesity. Rats receiving HFD were divided equally into three groups. The first one received oral doses of distilled water (10 ml/kg, weeks 8–14) and considered the obese control group while the other two groups received oral treatment with EPR oil (5 or 10 g/kg/day, p.o.) (30, 31). Treatment with distilled water or EPR oil started from the beginning of week 8 and continued along with HFD until the end of week 14 (a 7-week therapeutic period). The time-course of the study is shown in Figure 1.
Detection of Disturbances in Estrus Cyclicity
The percentage of animals with irregular cycles was determined by vaginal smears. The cytology samples were collected daily in the early morning (8:30–9:30 a.m.) for minimum 2 successive cycles (32) by applying a water-moistened cotton bud to be inserted 1 cm intra-vaginally to ensure loading sufficient number of cells on the swab. The removed aqueous fluids were then spread on slides to prepare smears and left at room temperature until completely dried. The prepared smears were immediately immersed in a jar containing 70% alcohol for 1 or 2 min for fixation followed by rapid staining with 0.5% Loba chemie methylene blue solution (Mumbai, India) for 3 min. This was followed by washing with tap water and examined by a light microscope (33, 34). The distinguishing phases of the cycle can be classified in the following as estrus (E), metestrus (M), diestrus (D), and proestrus (P). A typical regular cycle lasts for 4 days. If the of estrus cycle is <4 or >5 days hence, the cycle is marked as not regular (35).
Blood Glucose Measurement and Collection of Samples
At the end of week 14, body weights of the animals were measured and body weight gain % was calculated relative to the baseline value. After an overnight, fasting blood sugar (FBS) levels were determined using blood samples attained from the tail tip using a blood glucometer. Then, rats were anesthetized and sacrificed by dislocation at the cervical vertebra. Blood samples were taken from the orbital sinus, kept for 20 min at room temperature and centrifuged at 1,600 × g for 8 min. Then, sera were separated and stored at −20°C till the time of analyses. Moreover, the total abdominal white adipose tissues were collected from mesenteric, parametrial, and retroperitoneal tissues and weighed. After that, the adipose tissue index was determined as the percentage of the ratio between adipose tissue weight (g) and whole body weight (g) × 100.
Determination of Serum Hormone and Cytokine Levels
The insulin level was measured by the SunRed Bio rat insulin ELISA kit (Shanghai, China). Rat estrogen(E) ELISA kit (MBS703614) employs the competitive inhibition enzyme immunoassay technique, a quantitative sandwich rat follicle stimulating hormone (FSH) ELISA kit (MBS017508), a sandwich ELISA kit for rat LH (MBS 2509833), sandwich ELISA for rat prolactin (PRL) (MBS727546) and rat testosterone ELISA kit (MBS262661) were obtained from MyBioSource (San Diego, California, USA). Rat progesterone ELISA kit (CSB-E07282r) employed the competitive enzyme immunoassay technique obtained from CUSABIO (Hubei, China). Serum levels of adiponectin, leptin, interlukin1β (IL1β) and tumor necrosis factor-α (TNF-α) were estimated by CUSABIO rat ELISA kits (8400 Baltimore Avenue, MD, USA) in accordance to the manufacturer's instructions.
Calculation of the Insulin Resistance Index
For calculation of the homeostasis model assessment of insulin resistance [HOMA-IR] index, the next formula was applied: HOMA-IR index = [FBS mM per L x fasting insulin μU per mL]/22.5 (36).
Colorimetric Assays for Liver Enzymes and Lipid Profile
Serum level of liver enzyme activities including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assayed colorimetrically. Furthermore, serum lipid profile fractions comprising triglycerides (TG), total cholesterol (TC) in addition to low- and high-density lipoprotein–cholesterol (LDL-C, HDL-C) were determined using assay kits from Biodiagnostic (Cairo, Egypt) and an ultraviolet-visible spectrophotometer UNICO 7200 Spectrophotometer (New Jersey, USA).
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction for Adipokines mRNAs in Adipose Tissue
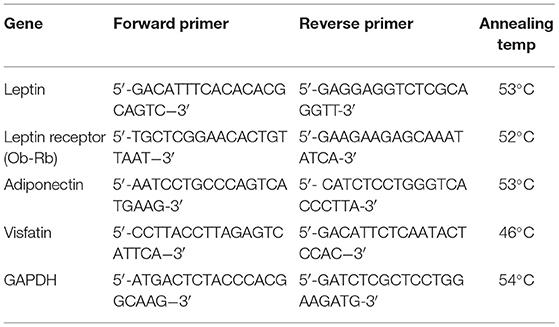
White adipose tissue expression of leptin, leptin receptor, adiponectin, and visfatin was determined by one step quantitative real-time PCR as described previously (37). GAPDH was measured as a housekeeping gene. Table 1 demonstrates the specific primers and annealing temperatures used for each determined gene. Relative quantification of the gene in each sample was estimated using LIVAK method (38).
Histopathology
Retroperitoneal fats were fixed for 48 h in formalin-alcohol, inserted in paraffin, cut into 3-μm thick specimens and finally hematoxylin and eosin (HE) staining was done. Sections were examined by a CX21 microscope (Olympus, Tokyo, Japan) and images were captured at 20 magnification. Adipocyte space diameters were measured in two different microscopic fields and the average was then calculated.
Furthermore, samples were collected from ovarian tissues and fixation was done in 10% paraformaldehyde for 1 day. After that, sections were dipped in water followed by serial dilutions of alcohol. Sections were then inserted in paraffin wax and were sectioned at 4-μ. The cut tissue specimens were stained by HE or Masson's trichrome and inspected by a light microscope (39) to detect pathological changes. A calibrated digital microscope camera (Tucsen ISH1000, Yuscen Photonics Co. Ltd., China) using Olympus® CX21 microscope, with resolution of 10 megapixels (3,656 × 2,740 pixel for every image). All Masson's trichrome stained sections were captured at original magnification 400x (Objective 40x), UIS optical system (Universal Infinity System, Olympus, Tokyo, Japan).
Statistics
SPSS 21 software was utilized to explore the obtained results. Quantitative data were represented as means ± SEM. Assessment was completed by one-way ANOVA followed by Bonferroni's test. Chi square test was applied for qualitative data for % of estrus cycle irregularity. All reported data were two-tailed and P < 0.05 was set as the level of statistical significance.
Results
Dietary Obesity Markers
Rats fed with a HFD for 14 weeks showed approximately 91% rise in body weight vs. 51% in the normal group. Treatment with the large dose of EPR oil produced a significant reduction in the body weight gain vs. the obese control group (Figure 2A). Furthermore, there was a 3-fold increase in the calculated adipose tissue index in the obese control female rats vs. the normal females. Therapeutic doses of EPR oil (5 or 10 g/kg) in obese female rats significantly reduced the adipose tissue index vs. the obese female group (Figure 2B).
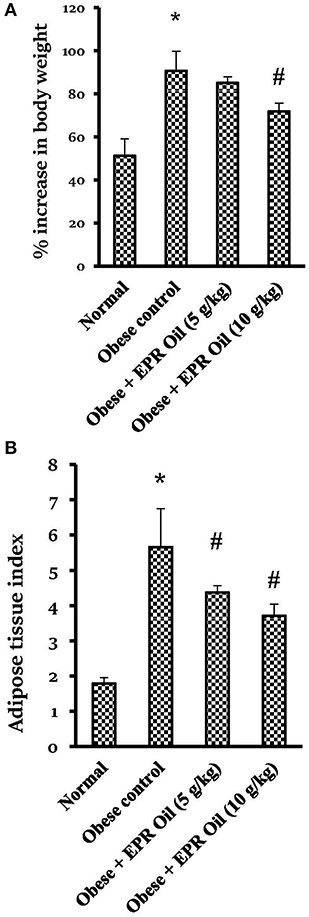
Figure 2. Percent change in body weight and adipose tissue index in the experimental groups. (A) % increase in body weight and (B) Adipose tissue index. Data are mean ± S.E.M. Bonferroni's test was used to determine differences among individual groups. *Different from normal group, #different from obese control group at P < 0.05.
Serum insulin, FBS and hence HOMA-IR index levels in obese control female rats were greater than the levels reported in normal female rats. EPR oil created dose-dependent improvements in fasting insulin and HOMA-IR index vs. the obese control group (Table 2). Further, the serum levels of the adipokines, leptin, and the inflammatory indicators, TNF-α, and IL1β, were elevated in obese female controls compared to the normal females. Treatment with EPR oil significantly decreased these three markers compared to the obese female group. Conversely, serum adiponectin level was significantly lower in obese rats, with a marked increase upon treatment with EPR oil (10 g/kg) (Table 2).
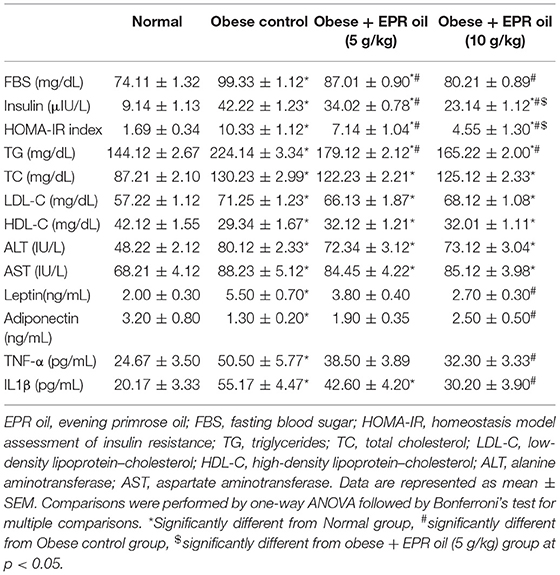
Table 2. Effect of evening primrose oil (5 or 10 g/kg) on serum biochemical parameters in obese female rats.
Table 2 demonstrates that obese control group showed greater serum TG, TC, LDL-C, and lower HDL-C vs. the normal group. EPR oil (5 or 10 g/kg) equally and significantly lessened the high serum TG level without effect on the other parameters. Serum ALT and AST increased in obese female group vs. the normal female group. EPR oil did not produce decreases in serum levels of these enzymes (Table 2).
Adipose tissue expression of leptin and leptin receptors was increased in obese control rats (≈3.5- and 4-fold increases, respectively) vs. the expression levels in the normal group. A similar increase (≈3.1-fold) was detected in mRNA expression of visfatin; however, a decrease was detected in the expression of adiponectin. Treatment with the large dose of EPR oil ameliorated the aforementioned changes vs. the obese control group. The low dose of EPR oil failed to produce a similar effect (Figure 3).
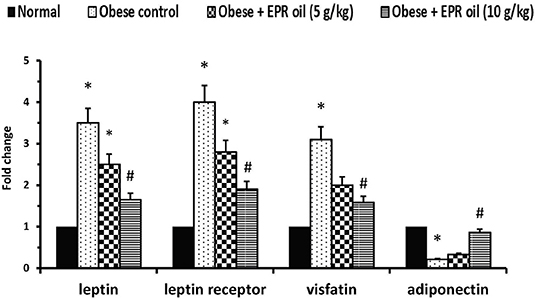
Figure 3. Polymerase chain reaction for adipokines in the experimental groups. Data are mean ± S.E.M. Bonferroni's test was used to determine differences among individual groups. *Different from normal group, #different from obese control group at P < 0.05.
Figure 4 shows the adipocyte space diameters in retroperitoneal adipose tissue section which was significantly larger in obese female rats vs. the measured diameters in normal rats. EPR oil (5 or 10 g/kg) equally and significantly reduced the adipocyte diameters.
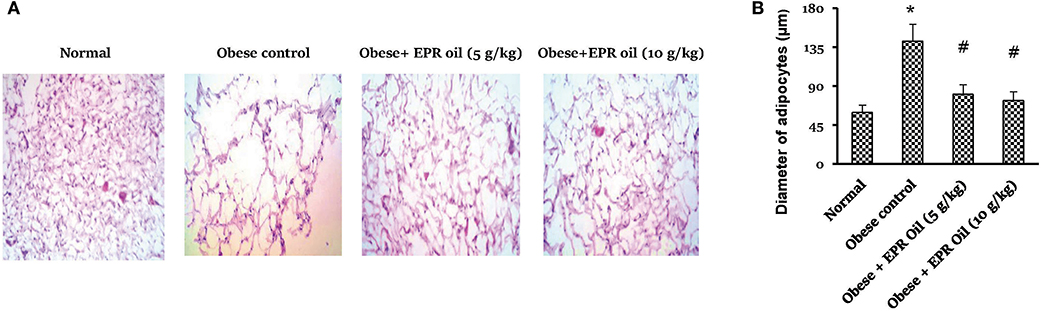
Figure 4. Diameter of adipocyte spaces in white adipose tissue. (A) Photographs for adipose tissue specimens stained with hematoxylin and eosin showing different adipocyte spaces in the study group. (B) Column chart showing mean value for adipocyte space diameters. Data are mean ± S.E.M. Bonferroni's test was used to determine differences among individual groups. *Different from normal group, #different from obese control group at P < 0.05.
Estrus Cycles and Sex Hormone Levels
In the vaginal smear, each phase can be primarily identified according to the percentage of types of 3 primary cells present. These types include nucleated epithelial cells, cornified squamous epithelial cells, and leukocytes (Figures 5A,B). Numerous cornified cells were generally observed as clumps and sheets in estrus stage. On the other hand, metestrus stage consists completely of great number of leukocytes and few big non-nucleated epithelial cells. Diestrus stage contains chiefly leukocytes along with large numbers of epithelial cells. Furthermore, proestrus stage is characterized by nucleated epithelial cells. Importantly, obese female rats showed high percentage of estrus irregularity (Figure 5C). A considerable fraction of female rats have 6–7 days cycles. The prolonged cycles were detected in form of existence of a 2nd or 3rd day of a couple of the 4 stages. The commonly prolonged stages were the diestrus and estrus.
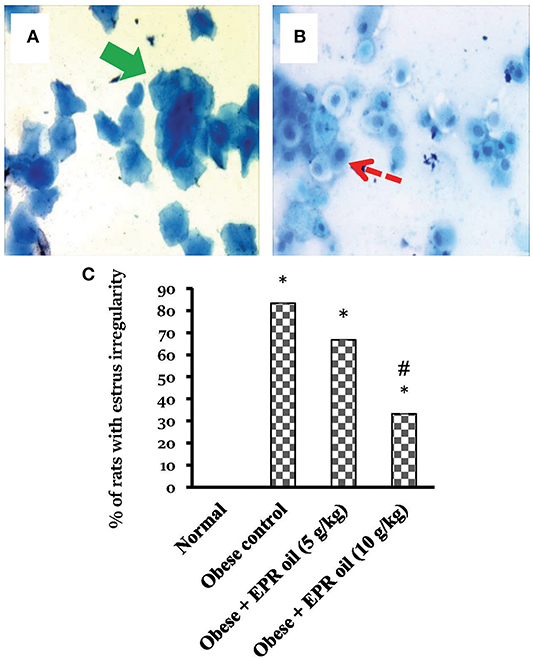
Figure 5. Cell types in vaginal smears and percentages of rats with estrus cycle irregularities. Photos represent the squamous cornified epithelial cells (Thick green arrow) (A), the nucleated epithelial cells (dashed red arrow) (B). The presence or absence of specific cell types indicates the estrus stage estrus, metestrus, diestrus, and proestrus cycle. Methylene blue ×40. (C) Percentage of rats with irregular cycles in the experimental groups. Irregularity of estrus cycle in female rats was tested using vaginal smears for a period of 2 weeks. Data are presented as % of irregular cycles (out of 6 female rats). Chi square test was applied at P < 0.05. EPR oil: evening primrose oil. *Different from normal rats, #different from obese control rats.
Moreover, obese control female rats showed hyperprolactinemia and greater serum testosterone and estrogen levels compared to normal female rats. In contrast, serum progesterone, FSH and LH levels were lower compared to the normal female rats. Treatment with both doses of EPR oil (5 or 10 g/kg) significantly ameliorated the serum levels of these hormones vs. the obese control females (Figure 6).
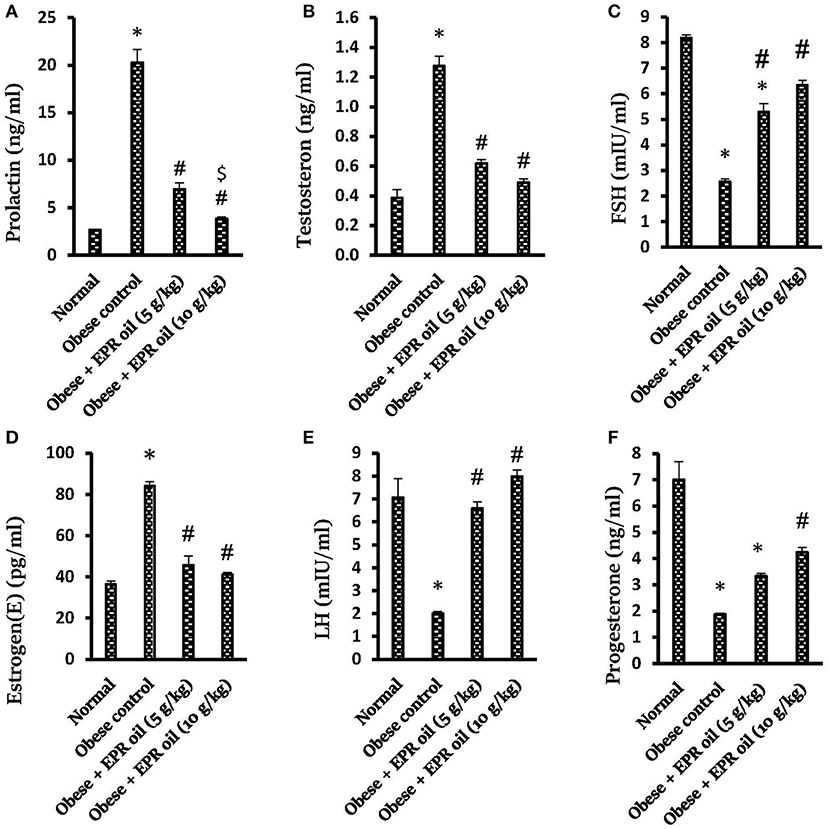
Figure 6. Effect of evening primrose oil (5 or 10 g/kg) on serum hormone levels in obese female rats. (A) Prolactin, (B) Testosterone, (C) FSH, (D) Estrogen(E), (E) LH and (F) Progesterone levels. Data are mean ± S.E.M. Bonferroni's test was used to determine differences among individual groups. *Different from normal group, #significantly different from obese control group, $different from obese + EPR oil (5 g/kg) group at P < 0.05. EPR oil, evening primrose oil; LH, luteinizing hormone; FSH, follicle stimulating hormone; PRL, prolactin.
Figure 7 shows histopathologic features of HE stained ovarian sections. Ovarian cortices from the normal group contained many mature Graafian follicles with many primordial follicles, stroma in-between formed of spindle cells and vessels. Mature Graafian follicles contained ova in the center surrounded by granulosa cells, antral space, and a nearby primordial follicle (Figures 7a,b). Ovarian cortices from the obese control group showed cysts, atretic follicles and congested dilated vessels. Marked stroma edema with cystification was prominent at high power fields (Figures 7c,d). Ovarian cortices from all obese rats treated with EPR oil (5 g/kg) showed marked improvement, no cysts, multiple numerous primordial follicles detected and secondary follicles. Secondary follicles, primordial follicles and residual edema were shown (Figures 7e,f). Ovarian cortices from obese rats treated with EPR oil (10 g/kg) showed mild to moderate improvement, residual congested vessels, secondary follicles and scattered atretic follicles. Some primordial follicles were shown with residual minimal edema and mild congestion (Figures 7g,h).
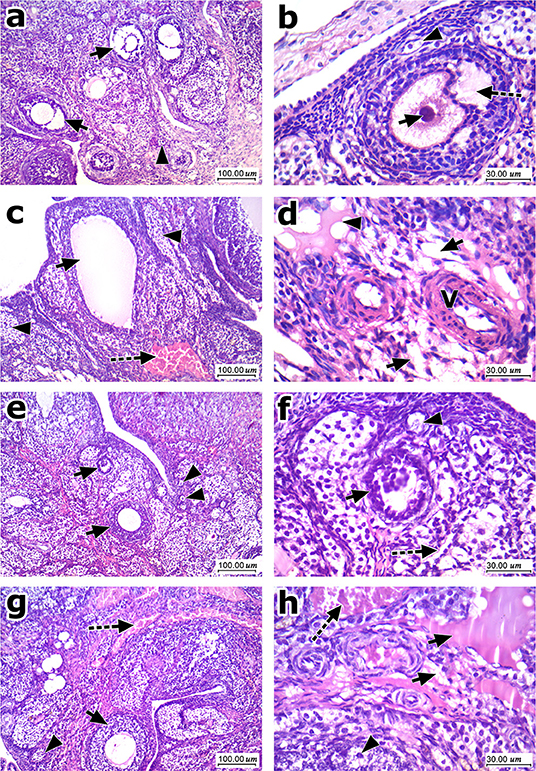
Figure 7. Histopathologic features of ovarian sections stained with hematoxylin and eosin. (a) Low magnification of ovarian cortex of normal group showed many mature Graafian follicles (arrow) with many primordial follicles (arrowhead), stroma in-between formed of spindle cells and vessels. (b) High magnification showed maturing follicle (Antral) with ovum (arrow) in the center surrounded by granulosa cells with antral space (dashed arrow), and a nearby primordial follicle (arrowhead). (c) Low magnification of ovarian cortex of control obese group showed one of the cysts (arrow), atretic follicles (arrowhead), and marked congestion (dashed arrow). (d) High magnification showed marked stromal edema (arrow) with cystification (arrowhead) and scattered vessels showing slight thickening of their walls (V). (e) Low magnification of ovarian cortex of EPR oil (5 g/kg) treated group showed marked improvement, no cysts presented, multiple numerous primordial follicles (arrowhead) detected, and secondary follicles (arrow). (f) High magnification showed secondary follicles (arrow), primordial follicles (arrowhead) and residual mild stroma edema (dashed arrow). (g) Low magnification of ovarian cortex of EPR oil (10 g/kg) treated group showed moderate improvement, residual congested vessels (dashed arrow) secondary follicles (arrow), and scattered atretic follicles (arrowhead). (h) High magnification showed primordial follicles (arrowhead) with residual stromal edema (arrow) and mild congestion (dashed arrow), H&E, 100x, and 400x.
Figure 8 shows Masson's trichrome stained ovarian sections. High degree of fibrosis was shown in ovaries from obese control rats (Figures 8a,b). Treatment with EPR oil (5 or 10 g/kg) decreased the amount of fibrotic tissues shown in ovaries (Figures 8c,d).
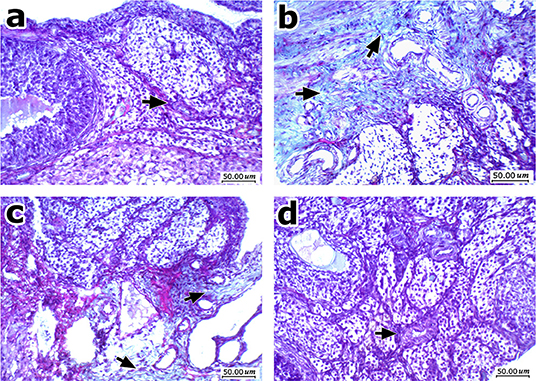
Figure 8. Histopathologic features of ovarian sections stained with Masson's trichrome stain. (a) Normal ovary shows ovarian cellular stroma with scattered small thin vessels (arrow) and no fibrosis. (b) An image from obese control group showing moderate fibrosis and deposition of thick collagen fibers stained green, with more evident perivascular arrangement. (c) An image from obese rats treated with EPR oil (5 g/kg) showing improvement with mild residual fibrosis mainly perivascular. (d) An image from obese rats treated with EPR oil (10 g/kg) showing improvement with cellular ovarian stroma without perivascular fibrosis, with scattered small vessels (arrow), Masson's trichrome stain, 200x.
Discussion
This study explored -for the first time- the effect of EPR oil on obesity related estrus irregularity, hormonal disturbances, and pathologic changes affecting the genital system. Since EPR oil is already invited to be used in the field of gynecology, it would be acceptable to suggest such remedy for future investigations on obese females with fertility problems.
In the current study, feeding female rats with a HFD led to significant weight gain, adiposity, and increased adipocyte diameters. Serologic investigations indicated hyperglycemia, hyperinsulinemia, insulin resistance in addition to increased lipids and inflammatory cytokines. Comparable results were found previously in rats fed the same diet for 14–16 weeks (31, 40). One human study conveyed a relationship between weight increases and resistance of tissue to insulin (41).
It is well-documented that excess adipose tissue leads to reproductive dysfunction. In females, obesity is linked to menstrual disorder, infertility, and obstetric compilations (17) as well as increased adipokine secretion and modulation of reproduction (42). One of the most common obesity-associated female reproductive disorders is PCOS which is characterized by overweight, amenorrhea and anovulation (43).
In the present study, female rats fed with a HFD showed hyperleptinemia and upregulation of the adipose tissue expression of leptin and the long isoform of leptin receptors. Similar results were obtained earlier by many research groups (28, 44). Leptin is in a direct relation with obesity and insulin resistance while adiponectin level is in an inverse correlation (45). High caloric intakes increase leptin which by turn suppresses appetite and improves thermogenesis in a central mechanism (46).
In relation to fertility, leptin was reported to enhance gonadotropin releasing hormone (GnRH) production via kisspeptin neurons located in arcuate nucleus of hypothalamus (47), thus plays an important role in initiation of puberty (48). Mice deficient in leptin or leptin receptors display low LH level and partially developed reproductive organs. In agreement with the previous findings, exogenous administration of leptin to ob/ob mice stimulates pubertal development and reproductive organ maturation, increases secretion of LH and restores fertility. Consequently, this explains the role of leptin signaling in female reproductive function (13). Moreover, mice and humans missing leptin receptor were reported to acquire hypothalamic hypogonadism, which delays puberty and results in infertility. In addition to the female circumstance, testicular atrophy and compromised spermatogenesis were found in male mice deficient in leptin signaling (49). However, in most dietary obesity rodent models, hyperleptinemia was documented and explained by occurrence of systemic leptin resistance and reduced physiologic action of leptin (50–52); this may help to describe the affected LH and gonadotropin level in our rat model.
The relationship between hyperleptinemia and female infertility was highlighted in some studies. One study documented that leptin-transgenic mice with 10-fold higher peripheral concentrations of leptin than controls begin to gain excessive weight and develop hypothalamic hypogonadism when their age reaches 20 week (53). Another study on HFD female DBA/2J mice proposed that obesity-linked hyperleptinemia, slowly prompted central leptin resistance, amplified hypothalamic neuropeptide Y transmission and eventually led to hypothalamic hypogonadism (1). Our study demonstrated that treatment with EPR oil mitigated hyperleptinemia and corrected the serum LH and gonadotropin levels.
In the present experiment, female rats fed with a HFD showed lower serum adiponectin level and downregulation of the expression of adiponectin gene in adipose tissues. Adiponectin has been shown to suppress LH and GnRH release (54, 55), representing its putative action in the regulation of hypothalamo-pituitary-gonadal axis (56). Increments in adiponectin serum level were observed in females treated with human chorionic gonadotropin during an in vitro fertilization (57); this highlights the action of adiponectin in regulation of the central reproductive endocrine axis (56). Consistently, women with PCOS show decreased high molecular weight adiponectin independent of their body mass index (58). These previous data agree with our findings as EPR oil enhanced serum adiponectin level and boosted serum gonadotropin level.
Additionally, female obese rats in the current study showed high circulating testosterone, estrogen, and PRL levels, with significant decrease of progesterone, FSH and LH levels. Clinical data showed that obesity was considered as a major cause of estrogen/progesterone imbalance (59) and low pituitary production of LH and FSH (60). A smart in vitro study in granulosa cells from an ovum from a polycystic ovary highlighted that insulin encourages formation of estradiol in cultured human granulosa cells (61).
In support to the aforementioned evidences, obese women usually suffer from hyperleptinemia, hyperandrogenemia, amplified the aromatization of androgens to estrogens in peripheral tissues, changed secretion of gonadotrophin, declined sex hormone binding globulin, and changed neuroregulation of the hypothalamic-pituitary-gonadal axis.
Moreover, high levels of circulating androgen are mutual characters in obese women, if amenorrhea is present (5, 62). Androgens were thought to produce arrest of follicular maturation.
Regarding PRL, it was found high in women suffering from obesity (63) and high serum PRL levels were linked to insulin resistance and increased HOMA-IR index in both men and women (64, 65). PRL by turn looks to impact ovarian function and long-term hyperprolactinemia is known to hinder the hypothalamus-pituitary axis and endorse anovulatory cycles (26). Collectively, these have been assumed to contribute to the subsequent events leading to discrepancies in the ovulatory process (66).
Indeed, clinical data highlighted a direct relation between circulating visfatin level and visceral adiposity (67). A different research indicated that visfatin is in a direct correlation with inflammation (68). Our findings agree with the aforementioned examples from the literature; obese rats showed upregulated expression of visfatin in adipose tissues and greater serum inflammatory cytokines. Rats treated with EPR oil showed less visfatin expression and low serum level of the inflammatory cytokines.
In the current experiment, obesity was associated with ovarian histopathologic changes like marked stromal edema, cystification, congestion, and thickening of blood vessels. Follicle formation is a highly coordinated process that is influenced by many factors. Studies highlighted many hormones, growth factor and cytokines in the ovary playing crucial roles in the follicular development. The follicles grow and develop orderly under the control of endocrine hormone (69, 70), growth factor precisely produced at different time points (71, 72) and the cytokines associated with the development of follicles include TNF-α, IL1β, and interferon-γ (73–75). The impact of adiposity has been involved in troubles of female reproduction (1). Histological assessment of ovaries from female rats nourished with a cafeteria diet revealed low amount of preantral follicles compared to lean females (76). Moreover, obesity and insulin resistance were linked to ovarian dysfunction in female experimental animals (77). Studies indicate that ovarian high leptin levels may impede the progress of dominant follicles and oocyte maturation (78) and leptin appears to antagonize the ovarian cell stimulation (11). Hence, hyperleptinemia in obese female rats was documented to play a crucial effect in regulating fertility (79).
Beside the ovarian changes, our obese female rats showed disturbances in estrus cyclicity in form of prolongation of cycle days. In agreement with the current results, experimental studies indicated that gestational, and/or post-natal feeding of adult female rodents fed on HFD were reported to enhance pubertal maturity onset and induce estrus cycle irregularity (80, 81). Studies indicated that obesity also reduce implantation (82) and pregnancy rates, induce irregular menses and increase complications with PCOS (83). Interestingly, data from clinical studies provided similar conclusions. For instance, overweight women exhibited greater prevalence of anovulation and menstrual dysfunction (84) and increased risk for poor reproductive function, infertility, conception and pregnancy complications (85). Conversely, weight loss improved reproductive outcomes in these women (86).
EPR oil reduced weight gain and adiposity and favorably improved the elevated insulin resistance seen in female rats fed with HFD. This was accompanied with favorable effects on serum leptin, adiponectin and TG in addition to lessening of serum inflammatory markers, TNF-α, and IL1β. Additionally, EPR oil regulated the estrus cyclicity and improved the histopathologic features seen in the ovaries.
Overall, EPR oil has a strong record of anti-inflammatory activity that suggested its use in many inflammatory conditions including dysmenorrhea. Montserrat-de la Paz et al. (87) considered EPR oil as a source of a new nutraceutical value since it is sometimes used for treating diabetic neuropathy, arthritic and rheumatic conditions and premenstrual/menopausal syndrome (88). The same authors published another article and concluded that long-chain fatty alcohols from EPR oil prevent inflammation in macrophages (87). EPR oil was also suggested to abrogate the prothrombotic adverse effects of celecoxib (31, 89).
The favorable effects of EPR oil are assumed to be linked to the contented γ-LA, since ω-6 fatty acids are used in the 1-series anti-inflammatory eicosanoids synthesis and employ a suppressor effect on leukotriene synthesis (90). The lipid profile from EPR oil showed 7% oleic, 74% LA, and 9% γ-LA and its beneficial properties are related to the direct effect of its content of essential fatty acids (91). Supplementation with EPR oil increases plasma level of γ-LA and its metabolite dihomo-γ-LA. Detailed mechanism is summarized in that this compound is oxidized by 5-lipoxygenase to 15-hydroxyeicosatrienoic acid (15-HETrE) or under the influence of cyclooxygenase, dihomo-γ-LA is converted to series 1 prostaglandins; these molecules possess anti-inflammatory characters. Furthermore, 15-HETrE is able to block arachidonic acid conversion to leukotriene A4 by an inhibitory action on 5-lipoxygenase (92). Overall, since the structure of EPR oil is well-known and all contented acids are known for their anti-inflammatory properties and no previous documentation for any possible anorexic effect was highlighted before. Remarkably, the low dose of EPR oil did not produce any significant effect on body weight but improved insulin resistance and reduced IL1β level beside amelioration of serum PRL, testosterone and gonadotropins. Therefore, we suppose the current action of EPR oil in alleviating obesity related complications is dependent on its anti-inflammatory properties rather than any anticipated effect on food intake.
Additionally, EPR oil was considered to reduce the severity of cyclical mastalgia, a state that is caused mainly by hormonal imbalance in premenopausal women (93). Oral administration of EPR oil was also reported to have an effect against menopausal hot flashes (94). In addition, one study demonstrated that dietary-EPR oil modifies the nociceptive reaction and symptoms accompanying intermittent cold stress and reduces inflammation (88). In animals fed hyperlipeidimic diet, dietary supplementation with O. biennis was mentioned to improve the endothelial antithrombotic ability, decrease sub-endothelial thrombogenicity and reduce vascular wall lesions (95). O. biennis was found to be effective in the treatment of inflammatory bowel disease (96).
Conclusions
In the current study, EPR oil enhanced most of the metabolic and endocrine impact of feeding with HFD in female rats. The most genuine effect was the improving of estrus cyclicity and eliminating the changes in ovarian pathology. Importantly, EPR oil should be tested in other models of PCOS to ensure a direct improving effect on ovarian structures and to ensure data obtained in the currents study are not simply due to improving insulin resistance. Future studies are warranted to test the protective effect of EPR oil on other models of chemically induced PCOS to ensure whether the protective effect is based on anti-inflammatory properties and to firmly exclude any anticipated anorexic effect in the current study.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Research ethics committee at Faculty of Pharmacy, Suez Canal University, Ismailia, Egypt.
Author Contributions
HA, SA, NE-S, NF, AY, and SZ: conceived and designed research. HA, SZ, and EM: conducted animal experiments and analyzed data. EM and GI: performed blood tests. EM and HA: PCR analysis and biochemical assays. RE: histopathological examination. All authors: contributed to reagents or analytical tools, wrote, read, and approved the manuscript.
Funding
This work was supported by the Deanship of Scientific Research (DSR) at the University of Tabuk, Tabuk, Saudi Arabia [Grant Number S-0140-1439].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELISA, enzyme-linked immunosorbent assay; EPR oil, evening-primrose oil; FSH, follicle stimulating hormone; HDL, high-density lipoprotein–cholesterol; HFD, high fat diet; IL1β, interlukin1β; MOMA-IR index, the homeostasis model assessment of insulin resistance index; LA, linoleic acid; γ-LA, γ-Linoleic acid; LDL-C, low-density lipoprotein–cholesterol; LH, luteinizing hormone; PCOS, polycystic ovaries syndrome; PRL, prolactin; WK, week; TG, triglycerides; TC, total cholesterol; TNF-α, tumor necrosis factor-α.
References
1. Tortoriello DV, McMinn J, Chua SC. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. (2004) 145:1238–47. doi: 10.1210/en.2003-1406
2. Bellver J, Melo MAB, Bosch E, Serra V, Remohí J, Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. (2007) 88:446–51. doi: 10.1016/j.fertnstert.2006.11.162
3. Cirik DA, Dilbaz B. What do we know about metabolic syndrome in adolescents with PCOS? J Turk Ger Gynecol Assoc. (2014) 15:49–55. doi: 10.5152/jtgga.2014.95776
4. Linné Y. Effects of obesity on women's reproduction and complications during pregnancy. Obes Rev. (2004) 5:137–43. doi: 10.1111/j.1467-789X.2004.00147.x
5. Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. (2003) 9:359–72. doi: 10.1093/humupd/dmg024
6. Parihar M. Obesity and infertility. Rev Gynaecol Pract. (2003) 3:120–6. doi: 10.1016/S1471-7697(03)00061-3
7. Chen X, Jia X, Qiao J, Guan Y, Kang J. Adipokines in reproductive function: a link between obesity and polycystic ovary syndrome. J Mol Endocrinol. (2013) 50:R21–37. doi: 10.1530/JME-12-0247
8. Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. (2002) 26:1407–33. doi: 10.1038/sj.ijo.0802142
9. Jéquier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. (2002) 967:379–88. doi: 10.1111/j.1749-6632.2002.tb04293.x
10. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. (2010) 152:93–100. doi: 10.7326/0003-4819-152-2-201001190-00008
11. Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab. (1999) 84:1072–6. doi: 10.1210/jc.84.3.1072
12. Dagklis T, Kouvelas D, Kallaras K, Papazisis G, Petousis S, Margioula-Siarkou C, et al. Leptin increases luteinizing hormone secretion of fasting female rats. Clin Exp Obstet Gynecol. (2015) 42:18–21. doi: 10.12891/ceogl786.2015
13. Donato J, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. (2011) 121:355–68. doi: 10.1172/JCI45106
14. Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. (2003) 88:2838–43. doi: 10.1210/jc.2002-021721
15. Abo-Elmatty DM, Zaitone SA. Topiramate induces weight loss and improves insulin sensitivity in dietary obese rats: comparison to sibutramine. Eur Rev Med Pharmacol Sci. (2011) 15:1187–95. Retrieved from https://www.europeanreview.org/wp/wp-content/uploads/1053.pdf
16. Michalakis KG, Segars JH. The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertil Steril. (2010) 94:1949–57. doi: 10.1016/j.fertnstert.2010.05.010
17. Campos DB, Palin M-F, Bordignon V, Murphy BD. The “beneficial” adipokines in reproduction and fertility. Int J Obes. (2008) 32:223–31. doi: 10.1038/sj.ijo.0803719
18. Kowalska I, Straczkowski M, Nikolajuk A, Adamska A, Karczewska-Kupczewska M, Otziomek E, et al. Serum visfatin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Hum Reprod. (2007) 22:1824–9. doi: 10.1093/humrep/dem118
19. Rafraf M, Mohammadi E, Asghari-Jafarabadi M, Farzadi L. Omega-3 fatty acids improve glucose metabolism without effects on obesity values and serum visfatin levels in women with polycystic ovary syndrome. J Am Coll Nutr. (2012) 31:361–8. doi: 10.1080/07315724.2012.10720443
20. Bayles B, Usatine R. Evening primrose oil. Am Fam Physician. (2009) 80:1405–8. Retrieved from https://www.aafp.org/afp/2009/1215/p1405.html
21. Montserrat-de la Paz S, Fernández-Arche A, Angel-Martín M, García-Giménez MD. The sterols isolated from evening primrose oil modulate the release of proinflammatory mediators. Phytomedicine. (2012) 19:1072–6. doi: 10.1016/j.phymed.2012.06.008
22. Namal Senanayake SPJ, Shahidi F. Incorporation of docosahexaenoic acid (DHA) into evening primrose (Oenothera biennis L.) oil via lipase-catalyzed transesterification. Food Chem. (2004) 85:489–96. doi: 10.1016/S0308-8146(02)00412-0
23. Dove D, Johnson P. Oral evening primrose oil: its effect on length of pregnancy and selected intrapartum outcomes in low-risk nulliparous women. J Nurse Midwifery. (1999) 44:320–4. doi: 10.1016/S0091-2182(99)00055-5
24. Watanabe S, Sakurada M, Tsuji H, Matsumoto S, Kondo K. Efficacy of γ-linolenic acid for treatment of premenstrual syndrome, as assessed by a prospective daily rating system. J Oleo Sci. (2005) 54:217–24. doi: 10.5650/jos.54.217
25. Brzeski M, Madhok R, Capell HA. Evening primrose oil in patients with rheumatoid arthritis and side-effects of non-steroidal anti-inflammatory drugs. Br J Rheumatol. (1991) 30:370–2. doi: 10.1093/rheumatology/30.5.370
26. Greenfield SM, Green AT, Teare JP, Jenkins AP, Punchard NA, Ainley CC, Thompson RP. A randomized controlled study of evening primrose oil and fish oil in ulcerative colitis. Aliment Pharmacol Ther. (1993) 7:159–66. doi: 10.1111/j.1365-2036.1993.tb00085.x
27. Jamilian M, Karamali M, Taghizadeh M, Sharifi N, Jafari Z, Memarzadeh MR, et al. Erratum to: vitamin D and evening primrose oil administration improve glycemia and lipid profiles in women with gestational diabetes. Lipids. (2016) 51:357. doi: 10.1007/s11745-016-4138-9
28. Abdel-Sattar E, Mehanna ET, El-Ghaiesh SH, Mohammad HMF, Elgendy HA, Zaitone SA. Pharmacological action of a pregnane glycoside, Russelioside B, in dietary obese rats: impact on weight gain and energy expenditure. Front Pharmacol. (2018) 9:990. doi: 10.3389/fphar.2018.00990
29. Pan M, Song YL, Xu JM, Gan HZ. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J Pineal Res. (2006) 41:79–84. doi: 10.1111/j.1600-079X.2006.00346.x
30. Abo-Gresha NM, Abel-Aziz EZ, Greish SM. Evening primrose oil ameliorates platelet aggregation and improves cardiac recovery in myocardial-infarct hypercholesterolemic rats. Int J Physiol Pathophysiol Pharmacol. (2014) 6:23–36. Retrieved from http://www.ijppp.org/files/ijppp1312004.pdf
31. Zaitone SA, Moustafa YM, Mosaad SM, El-Orabi NF. Effect of evening primrose oil and ω-3 polyunsaturated fatty acids on the cardiovascular risk of celecoxib in rats. J Cardiovasc Pharmacol. (2011) 58:72–9. doi: 10.1097/FJC.0b013e31821c8353
32. Akamine EH, Marçal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, et al. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. (2010) 206:65–74. doi: 10.1677/JOE-09-0461
33. Abdel-Latif RT, Zaitone SA, Abdel-Mottaleb Y, El-Maraghy NN. The anorectic agent, lorcaserin, disturbs estrous cyclicity and produces endometrial hyperplasia without affecting ovarian population in female rats. Life Sci. (2017) 183:69–77. doi: 10.1016/j.lfs.2017.06.006
34. Omar SA, Abdel EL, Samad A. Modified vaginal smear cytology for the determination of the rat estrous cycle phases, versus ordinary Papanicolaou technique, verified by light and scanning electron microscopic examination of the endometrium. Egypt J Histol. (2007) 30:397–408. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?ID=425505
35. Organisation for the Economic Co-operation and Development (OECD). “Part 5: Preparation, Reading and Reporting of Vaginal Smears. Available at: http://www.oecd.org/chemicalsafety/testing/40581357.pdf
36. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
37. Mehanna ET, Barakat BM, ElSayed MH, Tawfik MK. An optimized dose of raspberry ketones controls hyperlipidemia and insulin resistance in male obese rats: effect on adipose tissue expression of adipocytokines and Aquaporin 7. Eur J Pharmacol. (2018) 832:81–9. doi: 10.1016/j.ejphar.2018.05.028
38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
39. Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 6th ed. Edinburgh : Churchill Livingstone (2008). Available online at: https://trove.nla.gov.au/work/10963990 (accessed February 10, 2019).
40. Zaitone S, Hassan N, El-Orabi N, El-Awady E-S. Pentoxifylline and melatonin in combination with pioglitazone ameliorate experimental non-alcoholic fatty liver disease. Eur J Pharmacol. (2011) 662:70–7. doi: 10.1016/j.ejphar.2011.04.049
41. Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. (1990) 132:501–3. doi: 10.1093/oxfordjournals.aje.a115686
42. Palin MF, Bordignon VV, Murphy BD. Adiponectin and the control of female reproductive functions. Vitam Horm. (2012) 90:239–87. doi: 10.1016/B978-0-12-398313-8.00010-5
43. Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. (2006) 113:1148–59. doi: 10.1111/j.1471-0528.2006.00990.x
44. Zaitone SA, Essawy S. Addition of a low dose of rimonabant to orlistat therapy decreases weight gain and reduces adiposity in dietary obese rats. Clin Exp Pharmacol Physiol. (2012) 39:551–9. doi: 10.1111/j.1440-1681.2012.05717.x
45. Koleva DI, Orbetzova MM, Atanassova PK. Adipose tissue hormones and appetite and body weight regulators in insulin resistance. Folia Med. (2013) 55:25–32. doi: 10.2478/folmed-2013-0002
46. Alcalá M, Calderon-Dominguez M, Bustos E, Ramos P, Casals N, Serra D, et al. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep. (2017) 7:16082. doi: 10.1038/s41598-017-16463-6
47. Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. (2010) 151:2233–43. doi: 10.1210/en.2009-1190
48. Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. (2015) 38:73–88. doi: 10.1016/j.yfrne.2015.04.002
49. Smith MS, True C, Grove KL. The neuroendocrine basis of lactation-induced suppression of GnRH: role of kisspeptin and leptin. Brain Res. (2010) 1364:139–52. doi: 10.1016/j.brainres.2010.08.038
50. Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity. (2006) 14(Suppl. 5):254–8S. doi: 10.1038/oby.2006.319
51. Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, et al. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. (2006) 55:716–24. doi: 10.2337/diabetes.55.03.06.db05-0917
52. Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metab Clin Exp. (2015) 64:35–46. doi: 10.1016/j.metabol.2014.10.015
53. Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, et al. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest. (2000) 105:749–55. doi: 10.1172/JCI8353
54. Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJG. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. (2008) 22:760–71. doi: 10.1210/me.2007-0330
55. Wen JP, Lv WS, Yang J, Nie AF, Cheng XB, Yang Y, et al. Globular adiponectin inhibits GnRH secretion from GT1-7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun. (2008) 371:756–61. doi: 10.1016/j.bbrc.2008.04.146
56. Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. (2009) 89:38–47. doi: 10.1159/000151396
57. Liu Y, Tsai EM, Chen YL, Chen HS, Chen YC, Wu LC, et al. Serum adiponectin levels increase after human chorionic gonadotropin treatment during in vitro fertilization. Gynecol Obstet Invest. (2006) 62:61–5. doi: 10.1159/000092260
58. O'Connor A, Phelan N, Tun TK, Boran G, Gibney J, Roche HM. High-molecular-weight adiponectin is selectively reduced in women with polycystic ovary syndrome independent of body mass index and severity of insulin resistance. J Clin Endocrinol Metab. (2010) 95:1378–85. doi: 10.1210/jc.2009-1557
59. Carlson MJ, Thiel KW, Yang S, Leslie KK. Catch it before it kills: progesterone, obesity, and the prevention of endometrial cancer. Discov Med. (2012) 14:215–22.
60. De Pergola G, Maldera S, Tartagni M, Pannacciulli N, Loverro G, Giorgino R. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity. (2006) 14:1954–60. doi: 10.1038/oby.2006.228
61. Franks S, Robinson S, Willis DS. Nutrition, insulin and polycystic ovary syndrome. Rev Reprod. (1996) 1:47–53. doi: 10.1530/ror.0.0010047
62. Lefebvre P, Bringer J, Renard E, Boulet F, Clouet S, Jaffiol C. Influences of weight, body fat patterning and nutrition on the management of PCOS. Hum Reprod. (1997) 12(Suppl. 1):72–81. doi: 10.1093/humrep/12.suppl_1.72
63. Kok P, Roelfsema F, Frölich M, Meinders AE, Pijl H. Prolactin release is enhanced in proportion to excess visceral fat in obese women. J Clin Endocrinol Metab. (2004) 89:4445–9. doi: 10.1210/jc.2003-032184
64. Daimon M, Kamba A, Murakami H, Mizushiri S, Osonoi S, Yamaichi M, et al. Association between serum prolactin levels and insulin resistance in non-diabetic men. PLoS ONE. (2017) 12:e0175204. doi: 10.1371/journal.pone.0175204
65. Tuzcu A, Bahceci M, Dursun M, Turgut C, Bahceci S. Insulin sensitivity and hyperprolactinemia. J Endocrinol Invest. (2003) 26:341–6. doi: 10.1007/BF03345182
66. Diamanti-Kandarakis E, Bergiele A. The influence of obesity on hyperandrogenism and infertility in the female. Obes Rev. (2001) 2:231–8. doi: 10.1046/j.1467-789X.2001.00041.x
67. Berndt J, Klöting N, Kralisch S, Kovacs P, Fasshauer M, Schön MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. (2005) 54:2911–6. doi: 10.2337/diabetes.54.10.2911
68. Auguet T, Terra X, Porras JA, Orellana-Gavaldà JM, Martinez S, Aguilar C, et al. Plasma visfatin levels and gene expression in morbidly obese women with associated fatty liver disease. Clin Biochem. (2013) 46:202–8. doi: 10.1016/j.clinbiochem.2012.11.006
69. Qin Q, Sun A, Guo R, Lei M, Ying S, Shi Z. The characteristics of oviposition and hormonal and gene regulation of ovarian follicle development in Magang geese. Reprod Biol Endocrinol. (2013) 11:65. doi: 10.1186/1477-7827-11-65
70. Szafarowska M, Jerzak M. [Ovarian aging and infertility]. Ginekol Pol. (2013) 84:298–304. doi: 10.17772/gp/1580
71. Liu L, Ge W. Growth differentiation factor 9 and its spatiotemporal expression and regulation in the zebrafish ovary. Biol Reprod. (2007) 76:294–302. doi: 10.1095/biolreprod.106.054668
72. Wang J, Roy SK. Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod. (2004) 70:577–85. doi: 10.1095/biolreprod.103.023234
73. Zolti M, Ben-Rafael Z, Meirom R, Shemesh M, Bider D, Mashiach S, et al. Cytokine involvement in oocytes and early embryos. Fertil Steril. (1991) 56:265–72. doi: 10.1016/S0015-0282(16)54483-5
74. Jackson LR, Farin CE, Whisnant S. Tumor necrosis factor alpha inhibits in vitro bovine embryo development through a prostaglandin mediated mechanism. J Anim Sci Biotechnol. (2012) 3:7. doi: 10.1186/2049-1891-3-7
75. Dobrzanski MJ, Rewers-Felkins KA, Samad KA, Quinlin IS, Phillips CA, Robinson W, et al. Immunotherapy with IL-10- and IFN-γ-producing CD4 effector cells modulate “Natural” and “Inducible” CD4 TReg cell subpopulation levels: observations in four cases of patients with ovarian cancer. Cancer Immunol Immunother. (2012) 61:839–54. doi: 10.1007/s00262-011-1128-x
76. Zenardini B, Silveira MAD, Sagae SC. Cafeteria diet and obesity: mutagenicity in wistar rats and consequences on female offspring. Int J Develop Res. (2017) 7:16016–9. Available online at: http://www.journalijdr.com/sites/default/files/issue-pdf/10584.pdf
77. Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Heras V, Castellano JM, et al. Metabolic and gonadotropic impact of sequential obesogenic insults in the female: influence of the loss of ovarian secretion. Endocrinology. (2015) 156:2984–98. doi: 10.1210/en.2014-1951
78. Duggal PS, Van Der Hoek KH, Milner CR, Ryan NK, Armstrong DT, Magoffin DA, et al. The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology. (2000) 141:1971–6. doi: 10.1210/endo.141.6.7509
79. Tong Q, Xu Y. Central leptin regulation of obesity and fertility. Curr Obes Rep. (2012) 1:236–44. doi: 10.1007/s13679-012-0025-8
80. Boukouvalas G, Gerozissis K, Kitraki E. Adult consequences of post-weaning high fat feeding on the limbic-HPA axis of female rats. Cell Mol Neurobiol. (2010) 30:521–30. doi: 10.1007/s10571-009-9476-1
81. Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS ONE. (2009) 4:e6744. doi: 10.1371/journal.pone.0006744
82. Hall LF, Neubert AG. Obesity and pregnancy. Obstet Gynecol Surv. (2005) 60:253–60. doi: 10.1097/01.ogx.0000158509.04154.9e
83. Douchi T, Kuwahata R, Yamamoto S, Oki T, Yamasaki H, Nagata Y. Relationship of upper body obesity to menstrual disorders. Acta Obstet Gynecol Scand. (2002) 81:147–50. doi: 10.1034/j.1600-0412.2002.810210.x
84. Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. (1994) 5:247–50. doi: 10.1097/00001648-199403000-00016
85. Bolúmar F, Olsen J, Rebagliato M, Sáez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. (2000) 151:1072–9. doi: 10.1093/oxfordjournals.aje.a010150
86. Dag ZÖ, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. (2015) 16:111–7. doi: 10.5152/jtgga.2015.15232
87. Montserrat-de la Paz S, García-Giménez MD, Ángel-Martín M, Pérez-Camino MC, Fernández Arche A. Long-chain fatty alcohols from evening primrose oil inhibit the inflammatory response in murine peritoneal macrophages. J Ethnopharmacol. (2014) 151:131–6. doi: 10.1016/j.jep.2013.10.012
88. Montserrat-de la Paz S, García-Giménez MD, Ángel-Martín M, Marín-Aguilar F, Fernández-Arche A. Dietary supplementation evening primrose oil improve symptoms of fibromyalgia syndrome. J Funct Foods. (2013) 5:1279–87. doi: 10.1016/j.jff.2013.04.012
89. Mosaad SM, Zaitone SA, Ahmed AAM, Abo-Elmatty DM, El-Baz AA, Moustafa YM. Evening primrose oil or forskolin ameliorates celecoxib-enhanced upregulation of tissue factor expression in mice subjected to lipopolysaccharide-induced endotoxemia. Naunyn Schmiedebergs Arch Pharmacol. (2017) 390:483–92. doi: 10.1007/s00210-017-1342-y
90. Belch JJ, Hill A. Evening primrose oil and borage oil in rheumatologic conditions. Am J Clin Nutr. (2000) 71:352S–6S. doi: 10.1093/ajcn/71.1.352s
91. Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr. (1998) 128:1411–4. doi: 10.1093/jn/128.9.1411
92. Timoszuk M, Bielawska K, Skrzydlewska E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants. (2018) 7:E108. doi: 10.3390/antiox7080108
93. Pruthi S, Wahner-Roedler DL, Torkelson CJ, Cha SS, Thicke LS, Hazelton JH, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. (2010) 15:59–67. Retrieved from http://archive.foundationalmedicinereview.com/publications/15/1/59.pdf
94. Farzaneh F, Fatehi S, Sohrabi M-R, Alizadeh K. The effect of oral evening primrose oil on menopausal hot flashes: a randomized clinical trial. Arch Gynecol Obstet. (2013) 288:1075–9. doi: 10.1007/s00404-013-2852-6
95. Villalobos MA, De La Cruz JP, Martín-Romero M, Carmona JA, Smith-Agreda JM, Sánchez de la Cuesta F. Effect of dietary supplementation with evening primrose oil on vascular thrombogenesis in hyperlipemic rabbits. Thromb Haemost. (1998) 80:696–701.
96. Sałaga M, Lewandowska U, Sosnowska D, Zakrzewski PK, Cygankiewicz AI, Piechota-Polanczyk A, et al. Polyphenol extract from evening primrose pomace alleviates experimental colitis after intracolonic and oral administration in mice. Naunyn Schmiedebergs Arch Pharmacol. (2014) 387:1069–78. doi: 10.1007/s00210-014-1025-x
Keywords: dietary obese female rats, estrus cyclicity, evening primrose oil, hyperleptinemia, reproductive hormone disturbances, insulin resistance
Citation: Atteia HH, Alzahrani S, El-Sherbeeny NA, Youssef AM, Farag NE, Mehanna ET, Elhawary R, Ibrahim GA, Elmistekawy A and Zaitone SA (2020) Evening Primrose Oil Ameliorates Hyperleptinemia and Reproductive Hormone Disturbances in Obese Female Rats: Impact on Estrus Cyclicity. Front. Endocrinol. 10:942. doi: 10.3389/fendo.2019.00942
Received: 01 October 2019; Accepted: 31 December 2019;
Published: 30 January 2020.
Edited by:
Oliana Carnevali, Marche Polytechnic University, ItalyReviewed by:
Taisen Iguchi, National Institute for Basic Biology, JapanLila Oyama, Federal University of São Paulo, Brazil
Copyright © 2020 Atteia, Alzahrani, El-Sherbeeny, Youssef, Farag, Mehanna, Elhawary, Ibrahim, Elmistekawy and Zaitone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sawsan A. Zaitone, c3phaXRvbmVAdXQuZWR1LnNh; c2F3c2FuX3pheXRvb25AcGhhcm0uc3Vlei5lZHUuZWc=
†ORCID: Sawsan A. Zaitone orcid.org/0000-0002-9688-7683
‡Present address: Sawsan A. Zaitone, Faculty of Pharmacy, University of Tabuk, Tabuk, Saudi Arabia
 Hebatallah H. Atteia
Hebatallah H. Atteia Sharifa Alzahrani3
Sharifa Alzahrani3 Nagla A. El-Sherbeeny
Nagla A. El-Sherbeeny Noha E. Farag
Noha E. Farag Eman T. Mehanna
Eman T. Mehanna Reda Elhawary
Reda Elhawary Amr Elmistekawy
Amr Elmistekawy Sawsan A. Zaitone
Sawsan A. Zaitone