- 1Department of Internal Medicine and Clinical Nutrition, Centre for Bone and Arthritis Research, Institute of Medicine, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
- 2Department of Drug Treatment, Sahlgrenska University Hospital, Gothenburg, Sweden
The 2019 International Skeletal Dysplasia Society nosology update lists 441 genes for which mutations result in rare human skeletal disorders. These genes code for enzymes (33%), scaffolding proteins (18%), signal transduction proteins (16%), transcription factors (14%), cilia proteins (8%), extracellular matrix proteins (5%), and membrane transporters (4%). Skeletal disorders include aggrecanopathies, channelopathies, ciliopathies, cohesinopathies, laminopathies, linkeropathies, lysosomal storage diseases, protein-folding and RNA splicing defects, and ribosomopathies. With the goal of evaluating the ability of mouse models to mimic these human genetic skeletal disorders, a PubMed literature search identified 260 genes for which mutant mice were examined for skeletal phenotypes. These mouse models included spontaneous and ENU-induced mutants, global and conditional gene knockouts, and transgenic mice with gene over-expression or specific base-pair substitutions. The human X-linked gene ARSE and small nuclear RNA U4ATAC, a component of the minor spliceosome, do not have mouse homologs. Mouse skeletal phenotypes mimicking human skeletal disorders were observed in 249 of the 260 genes (96%) for which comparisons are possible. A supplemental table in spreadsheet format provides PubMed weblinks to representative publications of mutant mouse skeletal phenotypes. Mutations in 11 mouse genes (Ccn6, Cyp2r1, Flna, Galns, Gna13, Lemd3, Manba, Mnx1, Nsd1, Plod1, Smarcal1) do not result in similar skeletal phenotypes observed with mutations of the homologous human genes. These discrepancies can result from failure of mouse models to mimic the exact human gene mutations. There are no obvious commonalities among these 11 genes. Body BMD and/or radiologic dysmorphology phenotypes were successfully identified for 28 genes by the International Mouse Phenotyping Consortium (IMPC). Forward genetics using ENU mouse mutagenesis successfully identified 37 nosology gene phenotypes. Since many human genetic disorders involve hypomorphic, gain-of-function, dominant-negative and intronic mutations, future studies will undoubtedly utilize CRISPR/Cas9 technology to examine transgenic mice having genes modified to exactly mimic variant human sequences. Mutant mice will increasingly be employed for drug development studies designed to treat human genetic skeletal disorders.
Significance
Great progress is being made identifying mutant genes responsible for human rare genetic skeletal disorders and mouse models for genes affecting bone mass, architecture, mineralization and strength. This review organizes data for 441 human genetic bone disorders with regard to heredity, gene function, molecular pathways, and fidelity of relevant mouse models to mimic the human skeletal disorders. PubMed weblinks to citations of 249 successful mouse models are provided.
Introduction
Rare human genetic diseases cumulatively affect about 1 in 200 individuals and involve an estimated 7,000 genes. Major research efforts are underway to identify these mutant genes and characterize their disease phenotypes. Knowledge gained can guide therapies and provide hypotheses to develop future treatments. As recently summarized (1), “Genome sequencing has revolutionized the diagnosis of genetic diseases. Close collaborations between basic scientists and clinical genomicists are now needed to link genetic variants with disease causation. To facilitate such collaborations, we recommend prioritizing clinically relevant genes for functional studies, developing reference variant-phenotype databases, adopting phenotype description standards, and promoting data sharing.”
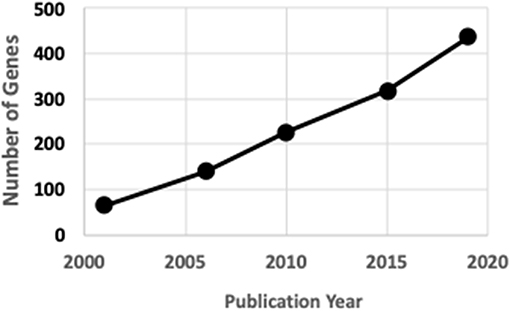
Rare human genetic skeletal dysplasias affect about 1 in 5,000 individuals (2) and account for 5% of all birth defects (3). The International Skeletal Dysplasia Society (ISDS, https://www.isds.ch), promotes scientific progress in the field of skeletal dysplasias and dysostoses, meets every second year, and published skeletal nosology summaries during 2001 (4), 2006 (5), 2010 (6), 2015 (7), and 2019 (8). There are presently 441 skeletal nosology genes, with an average of 20 new genes identified yearly (Figure 1). The classification aims to (i) identify metabolic pathways active in cartilage and bone, and their regulatory mechanisms; (ii) identify cellular signaling networks and gene expression sequences implicated in skeletal development; (iii) identify candidate genes for genetic disorders; (iv) facilitate integration of data coming from spontaneous and genetically engineered mouse mutants; (v) help in developing diagnostic strategies; (vi) stimulate the design and exploration of new therapeutic possibilities; and (vii) provide a knowledge framework accessible to physicians as well as to basic scientists and thus to facilitate communication between clinical genetics and pediatrics and the basic sciences (4).
The objectives of the present review include further characterizations of these 441 skeletal nosology genes and evaluating the reliability of mutant mouse models to mimic these human skeletal disorders.
Historical Highlights
Short stature and other visually obvious skeletal dysplasias were apparent throughout human history (9). The discovery of X-rays by Wilhelm Röntgen (10) was quickly followed by the description of osteopetrosis by Albers-Schönberg (11) and many skeletal dysplasias during the following decades (12). Dual-energy X-ray absorptiometry (DXA) technology, developed during the 1980s (13), permitting quantitation of bone mineral density (BMD), and continued advances in computed tomography (CT), providing 3 dimensional images, lead to increasing sophisticated understanding of bone dysmorphology. The first nosology gene identified was CA2 (carbonic anhydrase 2, osteopetrosis), initially in 1983 using electrophoretic, enzymatic and immunologic techniques on red blood cell extracts (14), and subsequently by genetic mutation analysis in 1991 (15). The first genetic mutation for any human disease to be identified by WES was DHODH (dihydroorotate dehydrogenase), responsible for postaxial acrofacial dysostosis, in 2010 (16).
Nosology
Nosology is the classification of diseases, which in its simplest form involves symptoms and pathogenic mechanisms. No classification system is perfect and there are often multiple ways to classify a given disorder. At the extremes, “lumpers” and “splitters” prefer few and many categories, respectively (17). Heredity can be X-linked, autosomal dominant, or autosomal recessive. Skeletal dysplasias can affect the skeleton only, or be part of pleiotropic syndromes affecting multiple organs. Mutations of various genes within a molecular pathway can each produce similar phenotypes. Loss-of function (LoF) mutations completely disrupt the activities of their encoded proteins but hypomorphic mutations allowing reduced protein activities occur. Gain-of-function (GoF) mutations increase the activities of enzymes and receptors and produce different phenotypes than LoF mutations. Dominant-negative mutations adversely affect functions of wild-type proteins. Mutations can occur within the protein-coding region of the genome (exome), within introns, or between gene coding regions. Mutations include deletions, duplications, and inversions.
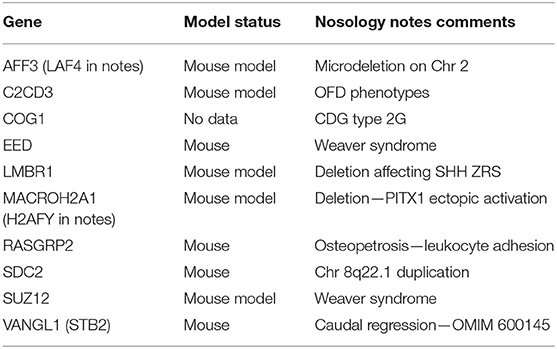
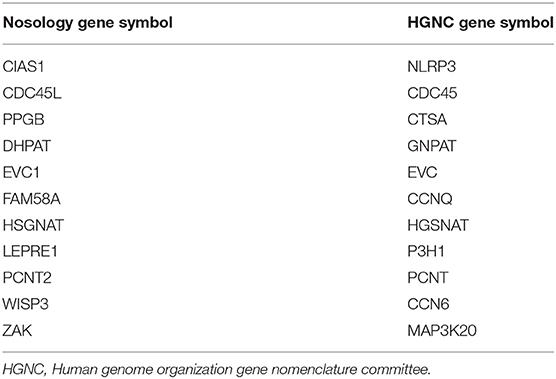
The 2019 edition of the ISDS Nosology and Classification of Skeletal Disorders database organizes mutant human skeletal phenotypes into 42 groups, based on clinical observations and known gene/phenotype relationships (8). A total of 461 disorders and 441 genes are provided, when all 10 genes listed within the Notes sections of the tables (Table 1) are included. Updated HGNU gene symbols for 11 genes (Table 2) are employed. Supplemental Table 1 provides an alphabetical list in spreadsheet format of all 441 genes, with information on heredity, gene function and mouse model status. Genetic disorders are not listed, as mutations in many genes result in multiple phenotypes. Inheritance patterns are 242 autosomal recessive, 135 autosomal dominant, 34 autosomal recessive or autosomal dominant depending upon the exact mutation in the gene, 21 X-linked and 11 non-inherited, somatic mutations. Three genes can have either germline or somatic mutations.
RMRP encodes an RNA regulating DNA transcription, RNU4ATAC encodes an RNA that is a component of an enzyme complex, and MIR140 is a microRNA. Proteins (and the 3 RNAs) function as enzymes (146, 33%), scaffold components (79, 18%), ligand/receptor signaling molecules (72, 16%), transcription factors (62, 14%), cilia components (36, 8%), matrix proteins (23, 5%), membrane transporters (19, 4%), and cohesionopathy proteins (4, 1%). These eight gene function categories are informative but arbitrary, and other categories can be envisioned. For example, 23 enzymes are involved in the synthesis, processing, and degradation of protein and glycosaminoglycan matrix components. Skeletal disorders include malfunctions of lysosomal function. Signaling genes can be assigned to BMP, FGF, WNT, and other pathways.
There are no orthologous mouse genes for human ARSE (arylsulfatase E) and RNU4ATAC (RNA, U4atac small nuclear, U12-dependent splicing). Supplemental Table 1 summarizes published data on the availability and fidelity of mouse models for the 439 human rare bone disease genes. Mutant mice with bone phenotypic data exist for 260 of the 439 genes (59%) with similar bone phenotypes observed for 249 (96%) genes. Supplemental Table 2 contains PubMed hyperlinks to publications for all 249 genes provided in Supplement Table 1 having mutant mouse bone phenotypes. These two supplemental tables should provide a major resource for the bone research community.
Mutant mouse bone data are inconsistent with human skeletal phenotypes for 11 genes (Ccn6, Cyp2r1, Flna, Galns, Gna13, Lemd3, Manba, Mnx1, Nsd1, Plod1, Smarcal1). There are no obvious explanations for or commonalities among these human-mouse phenotype inconsistencies. For 97 genes (22%) mutant mice have been generated and examined, but no skeletal data were reported. Mutant mice do not appear to have been examined for 82 genes (19%) and 36 (8%) of these genes belong to the understudied Ignorome/Dark Genome (18–20). Individual laboratories and/or consortia are encouraged to examine these genes, now known to contribute to poorly understood human rare bone diseases.
The number of bone nosology genes continues to increase as novel genes affecting skeletal metabolism are identified in human subjects. The genes described in this report form an arbitrary “snapshot” taken during August 2019 and will undoubtedly increase. Skeletal disorders for which mutant genes have not been identified include CDAGS syndrome (OMIM 603116), cherubism with gingival fibromatosis (OMIM 266270), chondrodysplasia punctata tibial-metacarpal type (OMIM 118651), dysplasia epiphysealis hemimelica (OMIM 127800), femur fibula ulna syndrome (OMIM 228200), hemifacial microsomia (OMIM 1642100, genochondromtosis (OMIM 1373600, Moreno–Nishimura–Schmidt syndrome (OMIM 608811), pachydermoperiostosis (OMIM 167100), and thoracolaryngopelvic dysplasia (OMIM 187760).
Formation of a normal skeleton involves BMP, FGF, and WNT signaling pathways and mutations in multiple genes within these pathways often produce skeletal dysplasias. Bone cells respond to parathyroid hormone, the active vitamin D metabolite calcitriol, and circulating FGF23 as part of the calcium-phosphate homeostatic system and disruptions in these hormones produce skeletal endocrinopathies. Skeletal disorders involving aggrecanopathies (13), channelopathies (21), ciliopathies (22, 23), cohesinopathies (24), lamiopathies (25), linkeropathies (26), protein-folding defects (27), ribosomopathies (28), spliceosomopathies (29), and transcription factors (30) show the importance of pathways not often thought to be involved in bone development.
Skeletal Disorder Vignettes
This section briefly summarizes selected skeletal disorders resulting from various mutations, highlighting the wide range of transcription and translation events that can be disrupted.
• Mutations can be benign with healthy nutrition but produce disease when key nutrients are lacking. All humans have an inactivating mutation in GULO, encoding an enzyme involved in the synthesis of ascorbic acid, and develop scurvy without sufficient dietary intake of vitamin C. The ascorbate synthetic pathway, involving aldehyde and aldose reductases, was only fully characterized in 2010 (31). Ascorbic acid is a required cofactor for the hydroxylation of proline and lysine residues in collagen and disruption of the mouse gulonolactone oxidase gene results in spontaneous bone fractures (32). Similarly, human and mouse HAAO and KYNU genes are involved in the synthesis of the enzymatic cofactor NAD and inactivating mutations in these human and mouse genes can result in congenital malformations (33).
• X-linked human mutations comprise 6% of the total skeletal disorders. X-inactivation of one of the two X chromosomes in women by long non-coding RNA specific transcript XIST occurs, but about 20% of X chromosome genes escape this inactivation (34). AMER1 and PORCN are X-linked genes that code for components of the WNT signaling pathway, with dominant mutations in women causing osteopathia striata with cranial sclerosis and focal dermal hypoplasia (including osteopathia striata), respectively. Due to developmental lethality male patients are extremely rare, but a few males having post-zygotic mosaic mutations have been identified (35, 36). Amer1 mutations in mice disrupt bone architecture (37) and treating adult mice with inhibitors of the PORCN enzyme reduces bone mass (38).
• Somatic gene mutations in 11 genes (AKT1, FLBN, GNAS, GREM1, HRAS, IDH1, IDH2, MAP2K1, NOTCH2, NRAS, PIK3CA) arise in the developing zygote and are not transmitted genetically. Loeys-Dietz syndrome includes several skeletal dysplasias and can result from mutations in SMAD2, SMAD3, TGFB2, TGFB3, TGFBR1, or TGFBR2 and 75% of affected subjects have somatic mutations (39). Melorheostotic, dense hyperostotic bone lesions are caused by somatic mosaic mutations in KRAS (40) and MAP2K1 (41). MAP2K1 mutations are thought to arise after the formation of dorso-ventral plane (42). KRAS and MAP2K1 are not included among the 441 Nosology disorders. Mutations in COL11A1, EZH2, and MET can be either germline or somatic.
• Deleterious mutations can occur at multiple sites within genes. For example, there are 1053 COL1A1 DNA variants in the Osteogenesis Imperfecta Variant Database as of September 2019 (https://oi.gene.le.ac.uk/home.php?select_db=COL1A1, accessed 13 December, 2019).
• Splicing mutations that disrupt normal exon transcription within the spliceosome are estimated to contribute to 15% of human genetic diseases (43, 44). Acrofacial and mandibulofacial dysostosis often involve spliceosome defects and mutations in EFTUD2, EIF4A3, and SF3B4 genes each result in distinct craniofacial phenotypes. Splice site mutations in AIFM1 (45), SERPINF1 (46), and TRAPPC2 (47) result in skeletal dysplasias.
• MicroRNAs are non-protein coding single-stranded RNAs (48) that regulate gene expression in bone and other tissues. Mouse studies show that microRNA-140 is involved in growth plate development (49, 50). A gain-of function mutation in microRNA-140 results in human skeletal dysplasia (51).
• Subjects with intragenic duplications of IFT81 (tandem duplication of exons 9 and 10) and MATN3 (tandem duplication of exons 2–5), detected by WGS, have skeletal dysplasias similar to subjects with LoF mutations in these genes (52).
• Autosomal-dominant syndactyly, synpolydactyly, and brachydactyly types D and E can result from dominant-negative mutations in the homeobox gene HOXD13. Duplications of the HOXD gene cluster locus produce mesomelic dysplasia with shortened limbs (53, 54). Similar Hoxd locus GoF alterations in ulnaless mutant mice, generated by X-irradiation, produce similar bone phenotypes (55, 56).
• ISDS nosology includes skeletal disorders resulting from disruptions of calcium-phosphate homeostasis, including various endocrinopathies. Regulation of calcium and phosphorus homeostasis involves ALPL, CASR, DMP1, ENPP1, FAM20C, FGF23, GALANT3, HRAS, KL, NRAS and TRPV6 genes. Parathyroid hormone synthesis and action involve CDC73, FAM111A, GCM and PTH1R. Vitamin D synthesis and actions involve CYP2R1, CYP27B1 and VDR. Normal Ca and P homeostasis occurs in humans (57) and mice (58) with deletions of the GC gene and thereby lacking the circulating vitamin D-binding protein (DBP) that binds serum 25-OH-D. Multiple neonatal bone fractures were observed due to maternal hypoparathyroidism and vitamin D deficiency (59).
Heredity of Bone Mass Without Skeletal Dysplasia
Osteoporosis is a common skeletal disease in which reduced amounts of otherwise normal bone lead to fragility and fractures. Adult bone mass, even within the normal range, has a strong heredity influence (60, 61) and identifying the genes involved in bone mass accumulation during growth and loss during aging has received great interest within the context of the etiology and treatment of osteoporosis. GWAS studies over the past decade described an increasing number of genes affecting BMD, with 518 loci identified in the 2019 UK Biobank analysis (62). Juvenile osteoporosis, although not a true dysplasia as bone architecture is normal, usually has genetic causes (63, 64). There are healthy subjects with unexplained high bone mass (65, 66) and attempts are underway to identify the genes responsible. Recent discoveries of such genes include LRP6 (67) and SMAD9 (68).
Mouse Models
All models are wrong, but some are less imperfect than others, and many are useful - George Box
Mouse models make important contributions to understanding and treating human diseases (69–72), including skeletal disorders (73, 74). Mutant mice that model human phenotypes also model successful drugs (75), help identify genes responsible for human genetic disorders and can provide insights for osteoporosis drug development (76). Bone mass and architecture vary in healthy humans and among laboratory mouse strains, with the most commonly studied C57BL/6 mouse strain an outlier having limb bones with high diameters and low cortical thickness (77–81).
The majority of mouse data summarized in this review involve individual investigator-initiated studies examining possible skeletal phenotypes in transgenic mice with specific alterations in genes chosen by the investigator. This approach, known as reverse genetics, utilizes the expertise of the laboratories involved.
In contrast, human studies involve forward genetics, with genes responsible for known skeletal phenotypes identified. Forward genetics is also employed in mouse studies, as genes responsible for spontaneous and mutagen-induced skeletal malformations are identified. The Jackson Laboratories (JAX), with a long history of studying mouse strains, recently employed WES to identify 14 genes having spontaneous mutations causing bone phenotypes (82, 83). Several laboratories employed N-ethyl-N-nitrosourea (ENU) in chemical mutagenesis campaigns to produce mouse lines having a wide-range of phenotypes. This approach yielded 41 genes having mutations causing bone phenotypes similar to the corresponding human skeletal disorders. These 41 genes with relevant citations are provided in Supplemental Table 3.
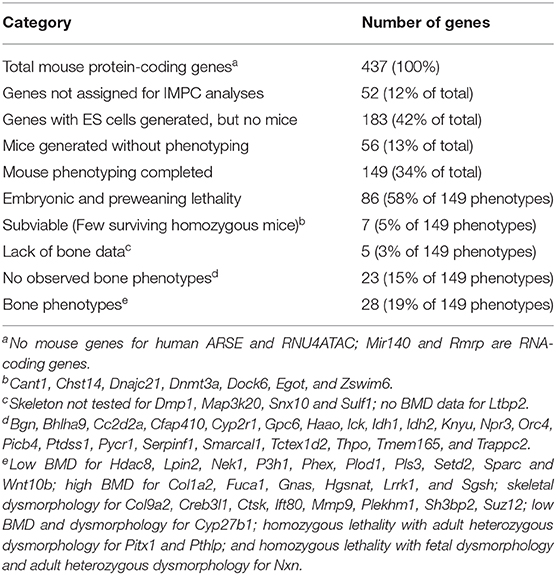
Two high-throughput mouse reverse genetics gene knockout phenotyping campaigns have been undertaken (84). The International Mouse Phenotyping Consortium (IMPC, www.mousephenotype.org) aims to characterize knockout mouse phenotypes for all 20,000 genes (74, 85). Lexicon Pharmaceuticals' Genome5000™ effort examining the druggable genome confirmed known bone phenotypes for 23 genes and identified 11 genes, including Notum (86), for which bone phenotypes were not previously characterized (87). Importantly, skeletal phenotypes were described for Fam20c (non-lethal Raine syndrome), Lrrk1 (osteosclerotic metaphyseal dysplasia), Pappa2 (short stature), Sfrp4 (Pyle's disease), and Slc10a7 (skeletal dysplasia) prior to knowledge of the human skeletal dysplasias when mutated in humans (84). For the 439 mouse genes discussed in this review, 149 genes have been examined by the IMPC, yielding 63 viable adult homozygous mouse mutants. Skeletal phenotypes (either body BMD or radiological dysmorphology) were observed for 28 genes. Results from the IMPC phenotyping campaign are summarized in Table 3.
Mouse models of human genetic disorders are employed to evaluate potentially beneficial skeletal actions of therapies approved for other disease indications. Teriparatide treatment increases bone mass in Lrp5 KO mice mimicking humans with osteoporosis pseudoglioma syndrome from loss of function LRP5 mutations (88, 89). Similarly, anti-sclerostin antibody treatment increases bone mass in mutant mouse models with low bone mass from gene disruptions (90) of Col1a1 (91, 92), Col1a2 (93, 94), Crtap (95), Dmp1 (96), Lrp5 (97), and Zmpste24 (98). Mechanistic hypotheses can be tested, such as periostin treatment retarding skull suture fusion in heterozygous Twist1 mice with craniosynostosis (99).
Mouse Study Precautions
Several experimental pitfalls should be avoided when performing mouse studies (100).
• Knockout of individual genes can disrupt the functions of neighboring genes (101). Examples include the presence of orofacial defects resulting from a hypomorphic Pax9 allele during knockout of the neighboring Slc25a21 gene (102) and glycosaminoglycan accumulation resulting from reduced expression of the Naglu gene during knockout of the neighboring Hsd17b1 gene (103).
• Transgenic Cre mouse lines are invaluable for conditionally activating or inactivating genes of interest. Several reporter genes are available for visualizing bone cells at different stages of development (104). But not all Cre lines are as specific as originally believed (105–107). Understanding these limitations is critical for experimental design and interpretation.
• Quantitative PCR methods are often not optimized and MIQE (Minimum Information for the publication of qPCR Experiments) guidelines have been established (108, 109). Selection of the appropriate reference gene(s) is important (110–112).
• Many antibodies suffer from a lack of specificity resulting from cross-reactivity to similar epitopes present on multiple proteins. Clifford Saper in 2005, as Editor-in-Chief of The Journal of Comparative Neurology, repeatedly received “… distressed communications from authors … to withdraw papers because an antibody against a novel marker was found to stain tissue in knockout animals …” (113). Excellent reviews (not cited here) provide guidelines for successful antibody validation and the purposeful joviality in their titles (“Antibody Can Get It Right … Antibody Anarchy … Antibody Crimes … A Guide to the Perplexed … Garbage In, Garbage Out … Hitchhiker Antigens … Not for the Faint-Hearted … The Dark Side of the Immunohistochemical Moon … The Good, Bad, and Really Ugly”) emphasizes the seriousness of the problem. Antibodies claimed to be specific for particular proteins should not react against tissues from KO mice missing the gene of interest and validation of antibody specificity using tissues from KO cells or mice is strongly encouraged.
• Established cell lines employed in conjunction with mouse studies can become contaminated and replaced by more robust, faster growing cells (114). Cell line authentication methods exist and should be employed (115, 116). MC3T3-E1 cell subclones vary as models of osteoblast biology (117).
Large Animal and Zebrafish Models
Large animals can have advantages over rodents for understanding human genetic disease and drug development. Hypophosphatasia occurs in sheep (118) and dogs (119) having mutations in ALPL. Canine genetic skeletal disorders include mutations in ADAMTSL2—geleophysic dysplasia (120), COL1A2—osteogenesis imperfecta (121), DVL2—Robinow syndrome (122), HES7—spondylocostal dysostosis (123), and SERPINH1—osteogenesis imperfecta (124). Spontaneous mutations in chicken KIAA0586 (125) and LMBR1 (126) genes result in the expected bone phenotypes.
Zebrafish are increasing contributing to our knowledge of skeletal genomics (127, 128) and advantages over mouse models include acquiring data more rapidly. Zebrafish mutants have been described for several of the 441 genes in this review. One complication of zebrafish studies is that zebrafish underwent a teleost-specific whole genome duplication and have more than 26,000 protein-coding genes (129). There is a one-to-one relationship between 47% of human genes and a zebrafish ortholog. There are multiple zebrafish genes associated to a single human gene, and vice versa.
Drug Development
Exciting advances are being made in developing drug treatments for patients with genetic skeletal disorders (130, 131) and mouse models invariably contribute to this progress. These advances are best reviewed by the laboratories involved, but three examples are illustrative. An antibody to NOTCH2 reverses osteopenia in a mouse model of Hajdu-Cheney syndrome (132). Cinacalcet corrects hypercalcemia in a mouse model of familial hypercalcemia type 2 (133). ENPP1 enzyme replacement therapy improves blood pressure and cardiovascular function in a mouse model of generalized arterial calcification of infancy (134).
Understanding genetic skeletal disorders provides key knowledge for developing osteoporosis therapies (76, 135). Disruptions in genes coding for proteins in the RANK—RANKL—osteoprotegerin signaling pathway involved in osteoclast generation cause human skeletal disorders. The RANKL neutralizing antibody denosumab is a successful osteoporosis therapy. The recently approved anabolic osteoporosis treatment romosozumab, a sclerostin neutralizing antibody, was developed with knowledge gained from subjects with osteosclerosis resulting from SOST gene mutations. Subjects with pinocytosis have mutations in the cathepsin K coding gene CTSK. Treatment with odanacatib, an inhibitor of cathepsin K in osteoclasts, reduced bone fractures in postmenopausal women but cardiovascular side effects precluded regulatory approval.
Future Directions
Since many human disorders involve hypomorphic, gain-of-function, dominant-negative and intronic mutations, future studies will undoubtedly utilize CRISPR/Cas9 technology and other evolving techniques to examine transgenic mice having genes modified to exactly mimic variant human sequences (72, 136). RNA sequencing will increasingly be employed for diagnosis and mechanistic understanding of genetic diseases (137–141).
The IFMRS (International Federation of Musculoskeletal Research Societies), in collaboration with the Broad Institute, is establishing a Musculoskeletal Genomics Knowledge Portal (MGKP) to integrate, interpret and present human data linked to musculoskeletal traits and diseases (http://www.kp4cd.org/about/bone).
Author Contributions
RB performed the literature search, data analyses, and prepared the manuscript. CO provided helpful suggestions and reviewed the manuscript.
Funding
This study was supported by funding from the Swedish Research Council (Grant 2016-01001), ALF/LUA research grant from the Sahlgrenska University Hospital, Lundberg Foundation, the Torsten Söderberg Foundation, The Knut and Alice Wallenberg's Foundation and the Novo Nordisk Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Matt Warman (Boston Children's Hospital) encouraged this project. Scott Youlten (Garvan Institute of Medical Research) independently extracted gene IDs from the 2019 ISDS Nosology paper and provided Ensembl gene IDs.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00934/full#supplementary-material
References
1. Manolio TA, Fowler DM, Starita LM, Haendel MA, MacArthur DG, Biesecker LG, et al. Bedside back to bench: building bridges between basic and clinical genomic research. Cell. (2017) 169:6–12. doi: 10.1016/j.cell.2017.03.005
2. Geister KA, Camper SA. Advances in skeletal dysplasia genetics. Annu Rev Genom Hum Genet. (2015) 16:199–227. doi: 10.1146/annurev-genom-090314-045904
3. Tosi LL, Warman ML. Mechanistic and therapeutic insights gained from studying rare skeletal diseases. Bone. (2015) 76:67–75. doi: 10.1016/j.bone.2015.03.016
4. Superti-Furga A, Bonafé L, Rimoin DL. Molecular-pathogenetic classification of genetic disorders of the skeleton. Am J Med Genet. (2001) 106:282–93. doi: 10.1002/ajmg.10233
5. Superti-Furga A, Unger S. Nosology and classification of genetic skeletal disorders: 2006 revision. Am J Med Genet A. (2007) 143A:1–18. doi: 10.1002/ajmg.a.31483
6. Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, LeMerrer M, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A. (2011) 155A:943–68. doi: 10.1002/ajmg.a.33909
7. Bonafé L, Cormier-Daire V, Hall C, Lachman R, Mortier G, Mundlos S, et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am J Med Genet A. (2015) 167A:2869–92. doi: 10.1002/ajmg.a.37365
8. Mortier GR, Cohn DH, Cormier-Daire V, Hall C, Krakow D, Mundlos S, et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am J Med Genet A. (2019) 179:2393–419. doi: 10.1002/ajmg.a.61366
9. Kozma C. Skeletal dysplasia in ancient Egypt. Am J Med Genet A. (2008) 146A:3104–12. doi: 10.1002/ajmg.a.32501
11. Albers-Schönberg HE. Röntgenbilder einer seltenen Knochenerkrankung. Münchener Medizinische Wochenschrift. (1904) 51:365–76.
12. Superti-Furga A, Unger S. Genetic disorders of bone - An historical perspective. Bone. (2017) 102:1–4. doi: 10.1016/j.bone.2017.07.025
13. Lewiecki EM, Binkley N. DXA: 30 years and counting: Introduction to the 30th anniversary issue. Bone. (2017) 104:1–3. doi: 10.1016/j.bone.2016.12.013
14. Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci USA. (1983) 80:2752–6. doi: 10.1073/pnas.80.9.2752
15. Venta PJ, Welty RJ, Johnson TM, Sly WS, Tashian RE. Carbonic anhydrase II deficiency syndrome in a Belgian family is caused by a point mutation at an invariant histidine residue (107 His-Tyr): complete structure of the normal human CA II gene. Am J Hum Genet. (1991) 49:1082–90.
16. Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, Huff CD, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. (2010) 42:30–5. doi: 10.1038/ng.499
17. McKusick VA. On lumpers and splitters, or the nosology of genetic disease. Perspect Biol Med. (1969) 12:298–312. doi: 10.1353/pbm.1969.0039
18. Pandey AK, Lu L, Wang X, Homayouni R, Williams RW. Functionally enigmatic genes: a case study of the brain ignorome. PLoS ONE. 9:e88889. doi: 10.1371/journal.pone.0088889
19. Oprea TI, Bologa CG, Brunak S, Campbell A, Gan GN, Gaulton A, et al. Unexplored therapeutic opportunities in the human genome. Nat Rev Drug Discov. (2018) 17:317–32. doi: 10.1038/nrd.2018.14
20. Stoeger T, Gerlach M, Morimoto RI, Nunes Amaral LA. Large-scale investigation of the reasons why potentially important genes are ignored. PLoS Biol. (2018) 16:e2006643. doi: 10.1371/journal.pbio.2006643
21. Gibson BG, Briggs MD. The aggrecanopathies; an evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet J Rare Dis. (2016) 11:86. doi: 10.1186/s13023-016-0459-2
22. Huber C, Cormier-Daire V. Ciliary disorder of the skeleton. Am J Med Genet C Semin Med Genet. (2012) 160C:165–74. doi: 10.1002/ajmg.c.31336
23. Yuan X, Serra RA, Yang S. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann NY Acad Sci. (2015) 1335:78–99. doi: 10.1111/nyas.12463
24. Cohen-Zinder M, Karasik D, Onn I. Structural maintenance of chromosome complexes and bone development: the beginning of a wonderful relationship? Bonekey Rep. (2013) 2:388. doi: 10.1038/bonekey.2013.122
25. Zhang H, Kieckhaefer JE, Cao K. Mouse models of laminopathies. Aging Cell. (2013) 12:2–10. doi: 10.1111/acel.12021
26. Ritelli M, Cinquina V, Giacopuzzi E, Venturini M, Chiarelli N, Colombi M. Further defining the phenotypic spectrum of B3GAT3 mutations and literature review on linkeropathy syndromes. Genes. (2019) 10:E631. doi: 10.3390/genes10090631
27. Makareeva E, Aviles NA, Leikin S. Chaperoning osteogenesis: new protein-folding disease paradigms. Trends Cell Biol. (2011) 21:168–76. doi: 10.1016/j.tcb.2010.11.007
28. Trainor PA, Merrill AE. Ribosome biogenesis in skeletal development and the pathogenesis of skeletal disorders. Biochim Biophys Acta. (2014) 1842:769–78. doi: 10.1016/j.bbadis.2013.11.010
29. Lehalle D, Wieczorek D, Zechi-Ceide RM, Passos-Bueno MR, Lyonnet S, Amiel J, Gordon CT. A review of craniofacial disorders caused by spliceosomal defects. Clin Genet. (2015) 88:405–15. doi: 10.1111/cge.12596
30. Chatterjee S, Sivakamasundari V, Lee WJ, Chan HY, Lufkin T. Making no bones about it: transcription factors in vertebrate skeletogenesis and disease. Trends Dev Biol. (2012) 6:45–52.
31. Gabbay KH, Bohren KM, Morello R, Bertin T, Liu J, Vogel P. Ascorbate synthesis pathway: dual role of ascorbate in bone homeostasis. J Biol Chem. (2010) 285:19510–20. doi: 10.1074/jbc.M110.110247
32. Aghajanian P, Hall S, Wongworawat MD, Mohan S. The roles and mechanisms of actions of vitamin C in bone: new developments. J Bone Miner Res. (2015) 30:1945–55. doi: 10.1002/jbmr.2709
33. Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, et al. NAD deficiency, congenital malformations, and niacin upplementation. N Engl J Med. (2017) 377:544–52. doi: 10.1056/NEJMoa1616361
34. Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, et al. Landscape of X chromosome inactivation across human tissues. Nature. (2017) 550:244–8. doi: 10.1038/nature24265
35. Joseph DJ, Ichikawa S, Econs MJ. Mosaicism in osteopathia striata with cranial sclerosis. J Clin Endocrinol Metab. (2010) 95:1506–7. doi: 10.1210/jc.2009-2343
36. Vreeburg M, van Geel M, van den Heuij LG, Steijlen PM, van Steensel MA. Focal dermal hypoplasia in a male patient due to mosaicism for a novel PORCN single nucleotide deletion. J Eur Acad Dermatol Venereol. (2011) 25:592–5. doi: 10.1111/j.1468-3083.2010.03782.x
37. Comai G, Boutet A, Tanneberger K, Massa F, Rocha AS, Charlet A, Panzolini C, et al. Genetic and molecular insights into genotype-phenotype relationships in Osteopathia Striata with Cranial Sclerosis (OSCS) through the analysis of novel mouse Wtx mutant alleles. J Bone Miner Res. (2018) 33:875–87. doi: 10.1002/jbmr.3387
38. Funck-Brentano T, Nilsson KH, Brommage R, Henning P, Lerner UH, Koskela A, et al. Porcupine inhibitors impair trabecular and cortical bone mass and strength in mice. J Endocrinol. (2018) 238:13–23. doi: 10.1530/JOE-18-0153
39. Loeys BL, Dietz HC. Loeys-Dietz Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews®. Seattle, WA: University of Washington (1993–2019).
40. Whyte MP, Griffith M, Trani L, Mumm S, Gottesman GS, McAlister WH, et al. Melorheostosis: exome sequencing of an associated dermatosis implicates postzygotic mosaicism of mutated KRAS. Bone. (2017) 101:145–55. doi: 10.1016/j.bone.2017.04.010
41. Fratzl-Zelman N, Roschger P, Kang H, Jha S, Roschger A, Blouin S, et al. Melorheostotic bone lesions caused by somatic mutations in MAP2K1 have deteriorated microarchitecture and periosteal reaction. J Bone Miner Res. (2019) 34:883–95. doi: 10.1002/jbmr.3656
42. Jha S, Laucis N, Kim L, Malayeri A, Dasgupta A, Papadakis GZ, et al. CT analysis of anatomical distribution of melorheostosis challenges the sclerotome hypothesis. Bone. (2018) 117:31–6. doi: 10.1016/j.bone.2018.09.005
43. Park E, Pan Z, Zhang Z, Lin L, Xing Y. The expanding landscape of alternative splicing variation in human populations. Am J Hum Genet. (2018) 102:11–26. doi: 10.1016/j.ajhg.2017.11.002
44. Scotti MM, Swanson MS. RNA mis-splicing in disease. Nat Rev Genet. (2016) 17:19–32. doi: 10.1038/nrg.2015.3
45. Miyake N, Wolf NI, Cayami FK, Crawford J, Bley A, Bulas D, et al. Neurogenetics X-linked hypomyelination with spondylometaphyseal dysplasia (H-SMD) associated with mutations in AIFM1. Neurogenetics. (2017) 18:185–94. doi: 10.1007/s10048-017-0520-x
46. Jin Z, Burrage LC, Jiang MM, Lee YC, Bertin T, Chen Y, et al. Whole-exome sequencing identifies an intronic cryptic splice site in SERPINF1 causing osteogenesis imperfecta Type VI. JBMR Plus. (2018) 2:235–9. doi: 10.1002/jbm4.10044
47. Fukuma M, Takagi M, Shimazu T, Imamura H, Yagi H, Nishimura G, et al. A familial case of spondyloepiphyseal dysplasia tarda caused by a novel splice site mutation in TRAPPC2. Clin Pediatr Endocrinol. (2018) 27:193–6. doi: 10.1297/cpe.27.193
48. Cheng VK, Au PC, Tan KC, Cheung CL. MicroRNA and human bone health. JBMR Plus. (2018) 3:2–13. doi: 10.1002/jbm4.10115
49. Nakamura Y, Inloes JB, Katagiri T, Kobayashi T. Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol Cell Biol. (2011) 31:3019–28. doi: 10.1128/MCB.05178-11
50. Papaioannou G, Mirzamohammadi F, Lisse TS, Nishimori S, Wein MN, Kobayashi T. MicroRNA-140 provides robustness to the regulation of hypertrophic chondrocyte differentiation by the PTHrP-HDAC4 pathway. J Bone Miner Res. (2015) 30:1044–52. doi: 10.1002/jbmr.2438
51. Grigelioniene G, Suzuki HI, Taylan F, Mirzamohammadi F, Borochowitz ZU, Ayturk UM, et al. Gain-of-function mutation of microRNA-140 in human skeletal dysplasia. Nat Med. (2019) 25:583–90. doi: 10.1038/s41591-019-0353-2
52. Pettersson M, Vaz R, Hammarsjö A, Eisfeldt J, Carvalho CMB, Hofmeister W, et al. Alu-Alu mediated intragenic duplications in IFT81 and MATN3 are associated with skeletal dysplasias. Hum Mutat. (2018) 39:1456–67. doi: 10.1002/humu.23605
53. Kantaputra PN, Klopocki E, Hennig BP, Praphanphoj V, Le Caignec C, Isidor B, et al. Mesomelic dysplasia Kantaputra type is associated with duplications of the HOXD locus on chromosome 2q. Eur J Hum Genet. (2010) 18:1310–14. doi: 10.1038/ejhg.2010.116
54. Le Caignec C, Pichon O, Briand A, de Courtivron B, Bonnard C, Lindenbaum P, et al. Fryns type mesomelic dysplasia of the upper limbs caused by inverted duplications of the HOXD gene cluster. Eur J Hum Genet. (2019). doi: 10.1038/s41431-019-0522-2. [Epub ahead of print].
55. Peichel CL, Prabhakaran B, Vogt TF. The mouse Ulnaless mutation deregulates posterior HoxD gene expression and alters appendicular patterning. Development. (1997) 124:3481–92.
56. Hérault Y, Fraudeau N, Zákány J, Duboule D. Ulnaless (Ul), a regulatory mutation inducing both loss-of-function and gain-of-function of posterior Hoxd genes. Development. (1997) 124:3493–500.
57. Henderson CM, Fink SL, Bassyouni H, Argiropoulos B, Brown L, Laha TJ, et al. Vitamin D-binding protein deficiency and homozygous deletion of the GC gene. N Engl J Med. (2019) 380:1150–7. doi: 10.1056/NEJMoa1807841
58. Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. (1999) 103:239–51. doi: 10.1172/JCI5244
59. Alikasifoglu A, Gonc EN, Yalcin E, Dogru D, Yordam N. Neonatal hyperparathyroidism due to maternal hypoparathyroidism and vitamin D deficiency: a cause of multiple bone fractures. Clin Pediatr. (2005) 44:267–9. doi: 10.1177/000992280504400312
60. Bjørnerem Å, Bui M, Wang X, Ghasem-Zadeh A, Hopper JL, Zebaze R, et al. Genetic and environmental variances of bone microarchitecture and bone remodeling markers: a twin study. J Bone Miner Res. (2015) 30:519–27. doi: 10.1002/jbmr.2365
61. Karasik D, Demissie S, Zhou Y, Lu D, Broe KE, Bouxsein ML, et al. Heritability and genetic correlations for bone microarchitecture: the framingham study families. J Bone Miner Res. (2017) 32:106–14. doi: 10.1002/jbmr.2915
62. Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. (2019) 51:258–66. doi: 10.1038/s41588-018-0302-x
63. Collet C, Ostertag A, Ricquebourg M, Delecourt M, Tueur G, Isidor B, et al. Primary osteoporosis in young adults: Genetic basis and identification of novel variants in causal genes. JBMR Plus. (2017) 2:12–21. doi: 10.1002/jbm4.10020
64. Mäkitie RE, Costantini A, Kämpe A, Alm JJ, Mäkitie O. New insights into monogenic causes of osteoporosis. Front Endocrinol. (2019) 10:70. doi: 10.3389/fendo.2019.00070
65. Gregson CL, Wheeler L, Hardcastle SA, Appleton LH, Addison KA, Brugmans M, et al. Mutations in known monogenic high bone mass loci only explain a small proportion of high bone mass cases. J Bone Miner Res. (2016) 31:640–9. doi: 10.1002/jbmr.2706
66. Gregson CL, Newell F, Leo PJ, Clark GR, Paternoster L, Marshall M, et al. Genome-wide association study of extreme high bone mass: contribution of common genetic variation to extreme BMD phenotypes and potential novel BMD-associated genes. Bone. (2018) 114:62–71. doi: 10.1016/j.bone.2018.06.001
67. Whyte MP, McAlister WH, Zhang F, Bijanki VN, Nenninger A, Gottesman GS, et al. New explanation for autosomal dominant high bone mass: mutation of low-density lipoprotein receptor-related protein 6. Bone. (2019) 127:228–43. doi: 10.1016/j.bone.2019.05.003
68. Gregson CL, Bergen DJM, Leo P, Sessions RB, Wheeler L, Hartley A, et al. A rare mutation in SMAD9 associated with high bone mass identifies the SMAD-dependent BMP signaling pathway as a potential anabolic target for osteoporosis. J Bone Miner Res. (2019). doi: 10.1002/jbmr.3875. [Epub ahead of print].
69. Gurumurthy CB, Lloyd KCK. Generating mouse models for biomedical research: technological advances. Dis Model Mech. (2019) 12:1. doi: 10.1242/dmm.029462
70. Hmeljak J, Justice MJ. From gene to treatment: supporting rare disease translational research through model systems. Dis Model Mech. (2019) 12:dmm039271. doi: 10.1242/dmm.039271
71. Spector JM, Harrison RS, Fishman MC. Fundamental science behind today's important medicines. Sci Transl Med. (2018) 10:438. doi: 10.1126/scitranslmed.aaq1787
72. Wangler MF, Yamamoto S, Chao HT, Posey JE, Westerfield M, Postlethwait J, et al. Model organisms facilitate rare disease diagnosis and therapeutic research. Genetics. (2017) 207:9–27. doi: 10.1534/genetics.117.203067
73. Kimmel DB. Animal models in bone research. In: Smith SY, Varela A, Samadfam R, editors. Bone Toxicology. Cham: Springer Nature (2017). p. 129–171. doi: 10.1007/978-3-319-56192-9_4
74. Maynard RD, Ackert-Bicknell CL. Mouse models and online resources for functional analysis of osteoporosis genome-wide association studies. Front Endocrinol. (2019) 10:277. doi: 10.3389/fendo.2019.00277
75. Zambrowicz BP, Sands AT. Knockouts model the 100 best-selling drugs–will they model the next 100? Nat Rev Drug Discov. (2003) 2:38–51. doi: 10.1038/nrd987
76. Brommage R. Genetic approaches to identifying novel osteoporosis drug targets. J Cell Biochem. (2015) 116:2139–45. doi: 10.1002/jcb.25179
77. Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res. (2005) 20:1085–92. doi: 10.1359/JBMR.050307
78. Li X, Mohan S, Gu W, Wergedal J, Baylink DJ. Quantitative assessment of forearm muscle size, forelimb grip strength, forearm bone mineral density, and forearm bone size in determining humerus breaking strength in 10 inbred strains of mice. Calcif Tissue Int. (2001) 68:365–9. doi: 10.1007/s00223-001-0004-7
79. Lodberg A, Vegger JB, Jensen MV, Larsen CM, Thomsen JS, Brüel A. Immobilization induced osteopenia is strain specific in mice. Bone Rep. (2015) 2:59–67. doi: 10.1016/j.bonr.2015.04.001
80. Lovell DP, Johnson FM, Willis DB. Quantitative genetic variation in the skeleton of the mouse: II. Description of variation within and between inbred strains. Am J Anat. (1986) 176:287–303. doi: 10.1002/aja.1001760304
81. Sabsovich I, Clark JD, Liao G, Peltz G, Lindsey DP, Jacobs CR, et al. Bone microstructure and its associated genetic variability in 12 inbred mouse strains: microCT study and in silico genome scan. Bone. (2008) 42:439–51. doi: 10.1016/j.bone.2007.09.041
82. Fairfield H, Srivastava A, Ananda G, Liu R, Kircher M, Lakshminarayana A, et al. Exome sequencing reveals pathogenic mutations in 91 strains of mice with Mendelian disorders. Genome Res. (2015) 25:948–57. doi: 10.1101/gr.186882.114
83. Palmer K, Fairfield H, Borgeia S, Curtain M, Hassan MG, Dionne L, Yong Karst S, et al. Discovery and characterization of spontaneous mouse models of craniofacial dysmorphology. Dev Biol. (2016) 415:216–27. doi: 10.1016/j.ydbio.2015.07.023
84. Brommage R, Powell DR, Vogel P. Predicting human disease mutations and identifying drug targets from mouse gene knockout phenotyping campaigns. Dis Model Mech. (2019) 12:5. doi: 10.1242/dmm.038224
85. Cacheiro P, Haendel MA, Smedley D, International Mouse Phenotyping Consortium and the Monarch Initiative. New models for human disease from the international mouse phenotyping consortium. Mamm Genome. (2019) 30:143–50. doi: 10.1007/s00335-019-09804-5
86. Brommage R, Liu J, Vogel P, Mseeh F, Thompson AY, Potter DG, et al. NOTUM inhibition increases endocortical bone formation and bone strength. Bone Res. (2019) 7:2. doi: 10.1038/s41413-018-0038-3
87. Brommage R, Liu J, Hansen GM, Kirkpatrick LL, Potter DG, Sands AT, et al. High-throughput screening of mouse gene knockouts identifies established and novel skeletal phenotypes. Bone Res. (2014) 2:14034. doi: 10.1038/boneres.2014.34
88. Iwaniec UT, Wronski TJ, Liu J, Rivera MF, Arzaga RR, Hansen G, et al. PTH stimulates bone formation in mice deficient in Lrp5. J Bone Miner Res. (2007) 22:394–402. doi: 10.1359/jbmr.061118
89. Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. (2006) 281:23698–711. doi: 10.1074/jbc.M601000200
90. Jacobsen CM. Application of anti-sclerostin therapy in non-osteoporosis disease models. Bone. (2017) 96:18–23. doi: 10.1016/j.bone.2016.10.018
91. Roschger A, Roschger P, Keplingter P, Klaushofer K, Abdullah S, Kneissel M, et al. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone. (2014) 66:182–8. doi: 10.1016/j.bone.2014.06.015
92. Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. (2013) 28:73–80. doi: 10.1002/jbmr.1717
93. Jacobsen CM, Barber LA, Ayturk UM, Roberts HJ, Deal LE, Schwartz MA, et al. Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J Bone Miner Res. (2014) 29:2297–306. doi: 10.1002/jbmr.2198
94. Little DG, Peacock L, Mikulec K, Kneissel M, Kramer I, Cheng TL, et al. Combination sclerostin antibody and zoledronic acid treatment outperforms either treatment alone in a mouse model of osteogenesis imperfecta. Bone. (2017) 101:96–103. doi: 10.1016/j.bone.2017.04.016
95. Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, et al. Sclerostin antibody treatment improves the bone phenotype of Crtap(-/-) mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res. (2016) 31:1030–40. doi: 10.1002/jbmr.2776
96. Ren Y, Han X, Jing Y, Yuan B, Ke H, Liu M, et al. Sclerostin antibody (Scl-Ab) improves osteomalacia phenotype in dentin matrix protein 1 (Dmp1) knockout mice with little impact on serum levels of phosphorus and FGF23. Matrix Biol. (2016) 52–4:151–61. doi: 10.1016/j.matbio.2015.12.009
97. Kedlaya R, Veera S, Horan DJ, Moss RE, Ayturk UM, Jacobsen CM, et al. Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci Transl Med. (2013) 5:211ra158. doi: 10.1126/scitranslmed.3006627
98. Choi JY, Lai JK, Xiong ZM, Ren M, Moorer MC, Stains JP, et al. Diminished canonical β-catenin signaling during osteoblast differentiation contributes to osteopenia in progeria. J Bone Miner Res. (2018) 33:2059–70. doi: 10.1002/jbmr.3549
99. Bai S, Li D, Xu L, Duan H, Yuan J, Wei M. Recombinant mouse periostin ameliorates coronal sutures fusion in Twist1+/- mice. J Transl Med. (2018) 16:103. doi: 10.1186/s12967-018-1454-2
100. He Y, Yuan C, Chen L, Liu Y, Zhou H, Xu N, et al. While it is not deliberate, much of today's biomedical research contains logical and technical flaws, showing a need for corrective action. Int J Med Sci. (2018) 15:309–22. doi: 10.7150/ijms.23215
101. West DB, Engelhard EK, Adkisson M, Nava AJ, Kirov JV, Cipollone A, et al. Transcriptome analysis of targeted mouse mutations reveals the topography of local changes in gene expression. PLoS Genet. (2016) 12:e1005691. doi: 10.1371/journal.pgen.1005691
102. Maguire S, Estabel J, Ingham N, Pearson S, Ryder E, Carragher DM, et al. Targeting of Slc25a21 is associated with orofacial defects and otitis media due to disrupted expression of a neighbouring gene. PLoS ONE. (2014) 9:e91807. doi: 10.1371/journal.pone.0091807
103. Jokela H, Hakkarainen J, Kätkänaho L, Pakarinen P, Ruohonen ST, Tena-Sempere M, et al. Deleting the mouse Hsd17b1 gene results in a hypomorphic Naglu allele and a phenotype mimicking a lysosomal storage disease. Sci Rep. (2017) 7:16406. doi: 10.1038/s41598-017-16618-5
104. Roeder E, Matthews BG, Kalajzic I. Visual reporters for study of the osteoblast lineage. Bone. (2016) 92:189–95. doi: 10.1016/j.bone.2016.09.004
105. Dallas SL, Xie Y, Shiflett LA, Ueki Y. Mouse Cre models for the study of bone diseases. Curr Osteoporos Rep. (2018) 16:466–77. doi: 10.1007/s11914-018-0455-7
106. Heffner CS, Herbert Pratt C, Babiuk RP, Sharma Y, Rockwood SF, Donahue LR, et al. Supporting conditional mouse mutagenesis with a comprehensive Cre characterization resource. Nat Commun. (2012) 3:1218. doi: 10.1038/ncomms2186
107. Huang W, Olsen BR. Skeletal defects in Osterix-Cre transgenic mice. Transgenic Res. (2015) 24:167–72. doi: 10.1007/s11248-014-9828-6
108. Bustin SA, Wittwer CT. MIQE: a step toward more robust and reproducible quantitative PCR. Clin Chem. (2017) 63:1537–8. doi: 10.1373/clinchem.2016.268953
109. Bustin S, Nolan T. Talking the talk, but not walking the walk: RT-qPCR as a paradigm for the lack of reproducibility in molecular research. Eur J Clin Invest. (2017) 47:756–74. doi: 10.1111/eci.12801
110. Abuna RPF, Oliveira FS, Ramos JIR, Lopes HB, Freitas GP, Souza ATP, et al. Selection of reference genes for quantitative real-time polymerase chain reaction studies in rat osteoblasts. J Cell Physiol. (2018) 234:749–56. doi: 10.1002/jcp.26886
111. Yang X, Hatfield JT, Hinze SJ, Mu X, Anderson PJ, Powell BC. Bone to pick: the importance of evaluating reference genes for RT-qPCR quantification of gene expression in craniosynostosis and bone-related tissues and cells. BMC Res Notes. (2012) 5:222. doi: 10.1186/1756-0500-5-222
112. Couasnay G, Frey C, Elefteriou F. Promoter cre-specific genotyping assays for authentication of Cre-driver mouse lines. JBMR Plus. (2019) 3:e10128. doi: 10.1002/jbm4.10128
113. Saper CB. Editorial: an open letter to our readers on the use of antibodies. J Comp Neurol. (2005) 493:477–8. doi: 10.1002/cne.20839
115. Almeida JL, Cole KD, Plant AL. Standards for cell line authentication and beyond. PLoS Biol. (2016) 14:e1002476. doi: 10.1371/journal.pbio.1002476
116. Bairoch A. The cellosaurus, a cell-line knowledge resource. J Biomol Tech. (2018) 29:25–38. doi: 10.7171/jbt.18-2902-002
117. Hwang PW, Horton JA. Variable osteogenic performance of MC3T3-E1 subclones impacts their utility as models of osteoblast biology. Sci Rep. (2019) 9:8299. doi: 10.1038/s41598-019-44575-8
118. Williams DK, Pinzón C, Huggins S, Pryor JH, Falck A, Herman F, et al. Genetic engineering a large animal model of human hypophosphatasia in sheep. Sci Rep. (2018) 8:16945. doi: 10.1038/s41598-018-35079-y
119. Kyöstilä K, Syrjä P, Lappalainen AK, Arumilli M, Hundi S, Karkamo V, et al. A homozygous missense variant in the alkaline phosphatase gene ALPL is associated with a severe form of canine hypophosphatasia. Sci Rep. (2019) 9:973. doi: 10.1038/s41598-018-37801-2
120. Packer RA, Logan MA, Guo LT, Apte SS, Bader H, O'Brien DP, et al. Clinical phenotype of Musladin-Lueke syndrome in 2 beagles. J Vet Intern Med. (2017) 31:532–8. doi: 10.1111/jvim.14654
121. Quist EM, Doan R, Pool RR, Porter BF, Bannasch DL, Dindot SV. Identification of a candidate mutation in the COL1A2 gene of a chow with osteogenesis imperfecta. J Hered. (2018) 109:308–14. doi: 10.1093/jhered/esx074
122. Mansour TA, Lucot K, Konopelski SE, Dickinson PJ, Sturges BK, Vernau KL, et al. Whole genome variant association across 100 dogs identifies a frame shift mutation in DISHEVELLED 2 which contributes to Robinow-like syndrome in Bulldogs and related screw tail dog breeds. PLoS Genet. (2018) 14:e1007850. doi: 10.1371/journal.pgen.1007850
123. Willet CE, Makara M, Reppas G, Tsoukalas G, Malik R, Haase B, et al. Canine disorder mirrors human disease: exonic deletion in HES7 causes autosomal recessive spondylocostal dysostosis in miniature Schnauzer dogs. PLoS ONE. (2015) 10:e0117055. doi: 10.1371/journal.pone.0117055
124. Lindert U, Weis MA, Rai J, Seeliger F, Hausser I, Leeb T, et al. Molecular consequences of the SERPINH1/HSP47 mutation in the dachshund natural model of osteogenesis imperfecta. J Biol Chem. (2015) 290:17679–89. doi: 10.1074/jbc.M115.661025
125. Yin Y, Bangs F, Paton IR, Prescott A, James J, Davey MG, et al. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. (2009) 136:655–64. doi: 10.1242/dev.028464
126. Maas SA, Suzuki T, Fallon JF. Identification of spontaneous mutations within the long-range limb-specific Sonic hedgehog enhancer (ZRS) that alter Sonic hedgehog expression in the chicken limb mutants oligozeugodactyly and silkie breed. Dev Dyn. (2011) 240:1212–22. doi: 10.1002/dvdy.22634
127. Kwon RY, Watson CJ, Karasik D. Using zebrafish to study skeletal genomics. Bone. (2019) 126:37–50. doi: 10.1016/j.bone.2019.02.009
128. Lleras-Forero L, Winkler C, Schulte-Merker S. Zebrafish and medaka as models for biomedical research of bone diseases. Dev Biol. (2019). doi: 10.1016/j.ydbio.2019.07.009. [Epub ahead of print].
129. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. (2013) 496:498–503. doi: 10.1038/nature12111
130. Briggs MD, Bell PA, Wright MJ, Pirog KA. New therapeutic targets in rare genetic skeletal diseases. Exp Opin Orphan Drugs. (2015) 3:1137–1154. doi: 10.1517/21678707.2015.1083853
131. Semler O, Rehberg M, Mehdiani N, Jackels M, Hoyer-Kuhn H. Current and emerging therapeutic options for the management of rare skeletal diseases. Paediatr Drugs. (2019) 21:95–106. doi: 10.1007/s40272-019-00330-0
132. Canalis E, Sanjay A, Yu J, Zanotti S. An antibody to Notch2 reverses the osteopenic phenotype of Hajdu-Cheney mutant male mice. Endocrinology. (2017) 158:730–42. doi: 10.1210/en.2016-1787
133. Howles SA, Hannan FM, Gorvin CM, Piret SE, Paudyal A, Stewart M, et al. Cinacalcet corrects hypercalcemia in mice with an inactivating Gα11 mutation. JCI Insight. (2017) 2:20. doi: 10.1172/jci.insight.96540
134. Khan T, Sinkevicius KW, Vong S, Avakian A, Leavitt MC, Malanson H, et al. ENPP1 enzyme replacement therapy improves blood pressure and cardiovascular function in a mouse model of generalized arterial calcification of infancy. Dis Model Mech. (2018) 11:dmm035691. doi: 10.1242/dmm.035691
135. Brommage R. New targets and emergent therapies for osteoporosis. Handb Exp Pharmacol. (2019). doi: 10.1007/164_2019_329. [Epub ahead of print].
136. Wu N, Liu B, Du H, Zhao S, Li Y, Cheng X, et al. The progress of CRISPR/Cas9-mediated gene editing in generating mouse/zebrafish models of human skeletal diseases. Comput Struct Biotechnol J. (2019) 17:954–62. doi: 10.1016/j.csbj.2019.06.006
137. Ayturk U. RNA-seq in skeletal biology. Curr Osteoporos Rep. (2019) 17:178–85. doi: 10.1007/s11914-019-00517-x
138. Gonorazky HD, Naumenko S, Ramani AK, Nelakuditi V, Mashouri P, Wang P, et al. Expanding the boundaries of RNA sequencing as a diagnostic tool for rare Mendelian disease. Am J Hum Genet. (2019) 104:466–83. doi: 10.1016/j.ajhg.2019.01.012
139. Khayal LA, Grünhagen J, Provazník I, Mundlos S, Kornak U, Robinson PN, et al. Transcriptional profiling of murine osteoblast differentiation based on RNA-seq expression analyses. Bone. (2018) 113:29–40. doi: 10.1016/j.bone.2018.04.006
140. Sebastian A, Hum NR, Morfin C, Murugesh DK, Loots GG. Global gene expression analysis identifies Mef2c as a potential player in Wnt16-mediated transcriptional regulation. Gene. (2018) 675:312–21. doi: 10.1016/j.gene.2018.06.079
Keywords: skeletal dysplasia, skeletome, mouse models, genetic disease, nosology
Citation: Brommage R and Ohlsson C (2020) High Fidelity of Mouse Models Mimicking Human Genetic Skeletal Disorders. Front. Endocrinol. 10:934. doi: 10.3389/fendo.2019.00934
Received: 02 September 2019; Accepted: 23 December 2019;
Published: 04 February 2020.
Edited by:
Elisabeth Marelise W. Eekhoff, VU University Medical Center, NetherlandsReviewed by:
David M. Findlay, University of Adelaide, AustraliaJawed Akhtar Siddiqui, University of Nebraska Medical Center, United States
Copyright © 2020 Brommage and Ohlsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert Brommage, YnJvbW1hZ2UmI3gwMDA0MDtvdXRsb29rLmNvbQ==
 Robert Brommage
Robert Brommage Claes Ohlsson
Claes Ohlsson