- 1Endocrinology Department, Renmin Hospital of Wuhan University, Wuhan, China
- 2Diabetes Research Unit, Sheffield Teaching Hospitals, Royal Hallamshire Hospital, Sheffield, United Kingdom
Diabetic peripheral neuropathy (DPN) is a common chronic complication of diabetes mellitus. It leads to distressing and expensive clinical sequelae such as foot ulceration, leg amputation, and neuropathic pain (painful-DPN). Unfortunately, DPN is often diagnosed late when irreversible nerve injury has occurred and its first presentation may be with a diabetic foot ulcer. Several novel diagnostic techniques are available which may supplement clinical assessment and aid the early detection of DPN. Moreover, treatments for DPN and painful-DPN are limited. Only tight glucose control in type 1 diabetes has robust evidence in reducing the risk of developing DPN. However, neither glucose control nor pathogenetic treatments are effective in painful-DPN and symptomatic treatments are often inadequate. It has recently been hypothesized that using various patient characteristics it may be possible to stratify individuals and assign them targeted therapies to produce better pain relief. We review the diagnostic techniques which may aid the early detection of DPN in the clinical and research environment, and recent advances in precision medicine techniques for the treatment of painful-DPN.
Introduction
Neuropathic syndromes are common complications of diabetes mellitus. By far the most prevalent is chronic diabetic peripheral sensorimotor neuropathy (DPN), affecting up to 50% of people with diabetes (1, 2). DPN is associated with increased mortality and leads to morbidity, principally as a result of its two major clinical consequences, diabetic foot ulceration, and neuropathic pain (3–5). Diabetic foot ulceration occurs as a result of a complex interaction of risk factors and patient behaviors, but sensory loss secondary to DPN is most often the primary cause (6). Lower-limb complications of diabetes are expensive and a substantial burden for patients, potentially leading to devastating outcomes such as lower limb amputation and death (3, 4, 6). Furthermore, up to half of patients with DPN suffer with painful neuropathic symptoms (painful-DPN) (7). These painful symptoms are commonly severe and often lead to depression, anxiety and sleep disorders, and reduced quality of life (8, 9).
Unfortunately, our understanding of the pathophysiology of DPN remains incomplete. Consequently, we do not have any effective disease modifying pharmacotherapies with which to treat the condition. The mainstay of modern management is to control risk factors for DPN, and prevent and manage its complications (10). Similarly, although a number of differences have been discovered between painless- and painful-DPN, the specific mechanisms causing the condition are unknown (11, 12). Disease modifying treatments are not widely used for painful-DPN and the treatment remains largely symptomatic (11, 12). Unfortunately, the available treatments for neuropathic pain are often ineffective and poorly tolerated (13). It has recently been hypothesized that by using certain patient characteristics (e.g., clinical features, quantitative sensory testing [QST], genetics and cerebral imaging) it may be possible to stratify individuals and assign them targeted therapies to produce better pain outcomes (14).
The prevalence of diabetes, DPN and foot amputations continue to increase at an alarming rate. It is essential that the condition is diagnosed early and accurately so that measures may be implemented to reduce the risk of diabetic foot complications. We review the recent advances in the diagnosis of DPN, which may supplement clinical assessment and could aid the early detection of DPN in the clinical and research environments, and precision medicine techniques, which may be used to improve the treatment of painful-DPN in the future.
The Classification and Definition of Diabetic Neuropathies
Diabetic neuropathies are heterogenous in their clinical presentation, risk factors and pathophysiology. The neuropathic syndromes may be classified according to the nerve type affected (sensory vs. motor vs. autonomic), site of nerve injury (focal vs. multi-focal vs. generalized), and disease time course (acute vs. chronic) (2, 10, 15). The neuropathic syndromes may broadly be divided into typical DPN and atypical diabetic neuropathies, the latter of which are outside the scope of this review (16). The American Diabetes Association has recently developed a simplified classification schema for diabetic neuropathies, reproduced in Table 1 (10). Typical DPN is by far the most prevalent form of neuropathy in diabetes and characteristically affects both sensory and motor nerves in a peripheral distribution (1). However, the relative impact on small and large sensory fibers, and motor fibers varies among individuals. The Toronto Diabetic Neuropathy Expert Group defined DPN as “a symmetrical, length dependent sensorimotor polyneuropathy attributable to metabolic and microvessel alterations as a result of chronic hyperglycemia exposure (DM) and cardiovascular risk covariates (16).”
Pathological Changes of Diabetic Neuropathy and Mechanisms
DPN leads to degenerative and atrophic changes throughout the peripheral and central nervous system (7, 17). The peripheral end terminals of nociceptors, intra-epidermal nerve fibers, are depleted in a distal symmetrical manner in DPN (7, 18). More proximally, peripheral nerve changes have been well-described and include; demyelination of myelinated nerve fibers, axonal degeneration and necrosis, Schwannopathy, and microangiopathy (19). Furthermore, autopsy and more recent advanced imaging studies have found spinal cord and cerebral atrophy associated with DPN (20–23).
A precise understanding of the pathophysiology of DPN remains elusive (24). A number of molecular pathways correlate with functional nerve impairment and pathological neuronal changes (Figure 1), including, but not limited to: polyol pathway activation, oxidative stress, protein kinase C activation, and advanced glycation end product formation (24, 25). However, the exact causal links between hyperglycemia and clinical DPN is uncertain. Our current understanding is that hyperglycemia, as well as vascular risk factors, activate detrimental pathways ultimately leading to downstream injury to the microvessel endothelium, nerve support cells, and nerve axons (25). Recent advances suggest that the cumulative effect of these injurious events may lead to neuronal death via reactive oxygen species generation and mitochondrial dysfunction. Furthermore, mechanistic and pathological findings do not discriminate between painful- and painless-DPN (12).
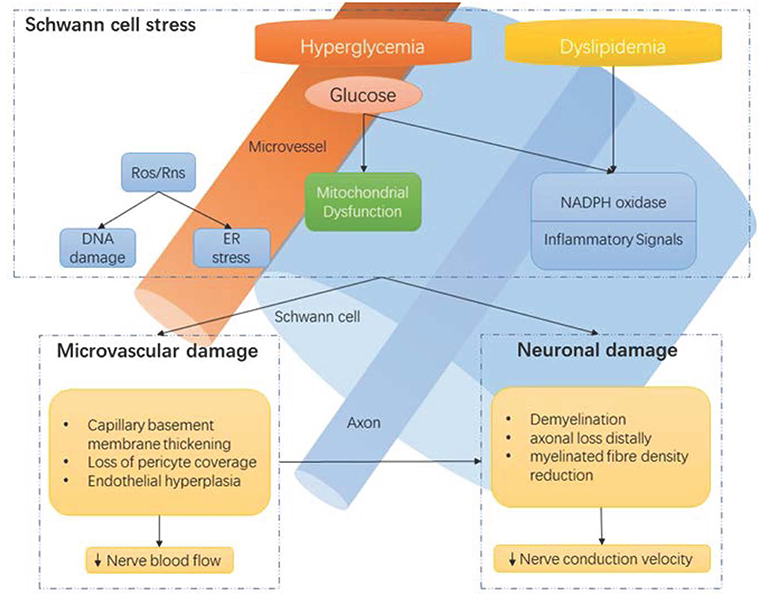
Figure 1. Hyperglycaemia-driven Schwann cell stress and neuronal damage. Hyperglycaemia and dyslipidemia lead to reduction of neuronal support from Schwann cells and microvessels. Disruption of neuronal support by Schwann cells and the vascular system contributes to neuropathy, in conjunction with the direct effects of hyperglycaemia on neurons. ER, endoplasmic reticulum; NADPH, Nicotinamide adenine dinucleotide phosphate; Ros, reactive oxygen species; Rns, reactive nitrogen species. Reproduced and permission gained from Sloan et al. (7).
Epidemiology of DPN
The prevalence of DPN, with or without pain, varies from study to study and is heavily dependent on the population selected, type of diabetes and case definition criteria used (26). Dyck et al. found that two thirds of patients with diabetes had objective evidence of some form of neuropathy (1). The most common was DPN, affecting ~50%. The duration of diabetes and glycemic control are the most significant risk factors for DPN (27). Other risk factors for cardiovascular disease are also associated with DPN, including: obesity, hypertension, smoking, and dyslipidemia (27–29).
Approximately 50% of people with DPN suffer with peripheral neuropathic pain (5, 29). Many risk factors for painful-DPN have been postulated such as the severity of neuropathy, hyperglycemic burden, and obesity (12). However, recent studies have demonstrated strong evidence that female sex is a risk factor for painful-DPN (12, 30). Idiopathic neuropathy is more prevalent in pre-diabetic states such as impaired glucose tolerance (IGT) (31). This lends further weight to the importance of vascular risk factors, such as the features of the metabolic syndrome other than hyperglycemia, playing an important role in the pathogenesis of peripheral neuropathy.
Clinical Features of Diabetic Neuropathy
DPN may present with a wide range of clinical symptoms and signs. Some people may be entirely asymptomatic, where a foot ulcer can be the first presentation. However, other patients may experience one or a number of different symptoms such as paresthesia (tingling/pins and needles), numbness and neuropathic pain (often described as burning, lancinating, shooting, or aching) which can range from mildly troublesome to intractable, causing great suffering (32). These symptoms may be sporadic or constant, and their natural history varies among patients. Sensory symptoms may be present for only a short period of time before they disappear entirely, or they may become chronic. Sensory symptoms and clinical examination signs begin in the toes/distal foot symmetrically. On physical examination, light touch and pin-prick of the distal foot is commonly impaired first, followed by more advanced sensory (i.e., vibration and proprioception loss) and motor (i.e., weakness, clawing of the toes, ankle reflex loss, and loss of muscle bulk) abnormalities. As the disease progresses, it spreads proximally up the leg before impacting the finger tips and upper limbs. The physical examination for patients with painful-DPN is generally indistinct from those without neuropathic pain. However, some patients may have a pure small fiber neuropathy which results in a loss of small fiber modalities (i.e., pin-prick and temperature sensory loss) with normal large fiber function (16). Additionally, a small sub-set of patients have the so called “irritable nociceptor” phenotype with “positive” sensory signs such as allodynia and hyperalgesia (33, 34).
Diagnosis of DPN
The diagnosis of DPN is often made during diabetic foot screening. Type 2 diabetes is often diagnosed after it has been present for some time; therefore, patients with type 2 diabetes should be screened for DPN from diagnosis (10). However, the risk of DPN is low at diagnosis of type 1 diabetes, so foot screening should commence 5 years after diagnosis. Subsequently, all patients should be assessed on an annual basis for lower limb sensory and vascular deficits (10). Once there is a clinical suspicion of DPN, a thorough clinical assessment must exclude other causes of neuropathy, and should involve a comprehensive history and examination including: temperature/pinprick sensation testing to assess small-fiber function; vibration sensation testing with 128-Hz tuning fork and assessment of ankle reflexes to assess large fiber function; and 10-g monofilament for the assessment of protective sensation. Clinical scoring systems may also be used to aid in diagnosing DPN e.g., Toronto Clinical Scoring System (35). Routine biochemical assay should be performed to determine the quality of glycemic and cardiovascular risk factor control and rule out other causes of peripheral neuropathy (e.g., coeliac disease, vitamin B12 deficiency, hypothyroidism, infectious/inflammatory disease). When the clinical features are atypical or the diagnosis is unclear then patients should be referred for specialist assessment. Nerve conduction studies remain the gold standard measure of large fiber function, but QST and skin biopsy may be used for diagnosing small fiber neuropathy (16).
Unfortunately, by the time clinical DPN is diagnosed irreversible nerve injury has already taken place. More advanced diagnostic techniques may be able to diagnose the condition at an early stage. Additionally, these methods may play an important role in clinical research as they may be more sensitive to changes in nerve function than current clinical measures and could be used as endpoints to assess the efficacy of pathogenetic treatments in clinical trials.
Skin Biopsy and Quantification of Intra-epidermal Nerve Fiber Density
Skin biopsy of the distal leg with quantification of intra-epidermal nerve fiber density (IENFD) is the gold standard technique to diagnose small fiber neuropathy (SFN) and it is also recommended for diagnosing DPN (16, 36). The procedure involves infiltration of subcutaneous local anesthetic and removal of a small skin sample using a punch biopsy tool. The sample must be immediately fixed, prepared and then epidermal innervation is quantified using either immunofluorescent or immunohistochemistry microscopy. The biopsy itself is quick and easy to perform but it is necessary to have suitable laboratory equipment and expertise to analyse. The technique is minimally invasive with a low complication rate, infection occurs in ~1 in 1,000.
IENFD correlates with other measures of neuronal function and has a sensitivity of 61–90% and specificity 64–82.8% for diagnosing DPN (37–43). The natural rate of epidermal innervation depletion is accelerated in DPN and IENFD may act as an early marker for DPN (44, 45). Despite being a measure of nociceptor density in the epidermis, IENFD is not related to the presence or intensity of neuropathic pain (7, 12). However, recent studies indicate that IENF regeneration and dermal vasculature differentiate painless- from painful-DPN (7, 45–47). Due to its invasive nature, skin biopsy with IENFD quantification is unlikely to be an appropriate screening tool for DPN. However, it has utility in clinical and research environments as a diagnostic tool. Moreover, IENFD has also been used as a clinical endpoint, Smith et al. found that diet and exercise counseling of patients with pre-diabetic neuropathy could lead to improvements in IENFD which corresponded with improvement in neuropathic pain (48). Further validation is required before IENFD can be used as a suitable biomarker for clinical trials in DPN (49).
Corneal and Retinal Innervation
A number of different ophthalmic measures of neuronal integrity have been proposed as surrogate measures of DPN and other neurological diseases, including corneal confocal microscopy (CCM), retinal nerve fiber layer thickness, and pupil responsiveness (50–52). CCM is a rapid and non-invasive modality for the study of corneal innervation and has emerged as a technique for diagnosing DPN (53). It has a high sensitivity (68–92%) and a specificity of 40–64% to diagnose DPN (54–56). Furthermore, CCM measures correlate with IENFD on skin biopsy (38). Pritchard et al. demonstrated that a reduced corneal nerve fiber length was predictive of incident DPN (57). Moreover, Dehghani et al. found that corneal nerve parameters rapidly declined prior to the development of foot complications (58).
Optical coherence tomography (OCT) has been used to identify the loss of retinal nerve fibers in a number of neurological disease, including DPN (50). Retinal nerve fiber layer (RNFL) loss is observed in patients with diabetes and correlates with the stage of diabetic retinopathy (59, 60). However, reports have shown that RNFL loss in patients with diabetes without diabetic retinopathy (61, 62). Indeed, two recent studies have found that measures of RNFL loss are associated with DPN (60, 63). OCT and CCM measures hold promise as a reliable and repeatable non-invasive measure which may be used to detect early DPN in the clinical and research setting. However, they are not currently widely available as they require specialist expertise and expensive equipment to perform (50).
Neurometer
The Neurometer is a non-invasive neurodiagnostic, QST device to measure current perception threshold (CPT) (64). It selectively determines the functional status of three nerve types, large myelinated (Aβ) fibers, medium-size myelinated (Aδ) fibers, and unmyelinated (C) fibers by measuring CPT at 2,000, 250, and 5 Hz, respectively (65). The device is quick, painless and can detect hypo- and hyper-aesthesia (64). Studies have found that measurement of the CPT using the Neurometer detects milder DPN more sensitively than vibration perception threshold (VPT) (66, 67) and Monofilament testing (66, 68). A recent study enrolled 202 patients with type 2 diabetes mellitus and compared clinical phenotyping using the CPT against a clinical scoring system (Michigan Neuropathy Screening Instrument; MNSI) and nerve conduction studies (NCS) (69). NCS variables differed across CPT clinical phenotypes. However, the study found that NCS detected more cases of subclinical DPN than the Neurometer. Furthermore, Matsutomo et al. found that the neurometer identified dysfunction of myelinated, but not unmyelinated fibers, in the diagnosis of DPN (65). Additionally, Koo et al. found that although the CPT correlated with neuropathic symptoms and signs it provides little additional information compared with conventional testing (70). As with other QST techniques, CPT abnormalities are not specific to DPN, and the test may be influenced by other psychological factors.
DPN-Check
DPN-Check is a handheld point-of-care device which provides the sural nerve amplitude and conduction velocity without the need for neuroelectrophysiologist expertise or expensive equipment. It is user-friendly and requires only basic training to use. The device stimulates the sural nerve orthodromically with distal probes, as opposed to antidromically as in standard NCS protocols, and records using a biosensor covering a wide area of the lower limb proximally. It has a sensitivity of 95 and 71% specificity to diagnose DPN (71). Additionally, it demonstrates inter-rater and intra-rater reliability and performs well in comparison to clinical examination and laser-doppler “FLARE” imaging (71, 72). The DPN-Check sural nerve amplitude measurements demonstrate strong agreement with standard NCS; however, DPN-Check over-estimates sural nerve conduction velocity (71). Additionally, any sural nerve amplitudes below 1.5 μV are adjusted to zero. Although further work to determine the generalizability to the clinical and research setting is required, this simple technique has potential to accurately measure sensory nerve function quickly and cheaply (73). A recent study by Binns-Hall et al. demonstrated that the DPN-check was effectively used to detect early DPN during combined eye, foot and retinal screening visits (74).
Sudomotor Testing
The foot sweat glands are innervated by sudomotor, unmyelinated cholinergic nerve fibers which may become impaired in DPN (16). Sudomotor dysfunction leads to foot skin dryness which is associated with an elevated risk of foot ulceration (75). There are several methods to determine sudomotor function in DPN, including: quantitative sudomotor axon reflex test, thermoregulatory sweat test and the quantitative direct and indirect reflex test (16). Neuropad is a sudomotor functional index test. It is a simple patch applied to the skin whose color changes from blue to pink through chemical reaction to evaluate sudomotor function (76). The presence of neuropathy is determined by color change after the patch has been adhered to the skin for 10 min. It has a sensitivity ranging from 86 to 95% but a specificity of only 45–69.8% for diagnosing DPN (77–80). A more recent study found that automated quantification of Neuropad improves the diagnostic ability of the test, especially for peripheral small fiber neuropathy (81). Neuropad is easy to use and provides a non-subjective result but its relatively poor specificity limits its applicability.
A more recent sudomotor testing device is Sudoscan, which is a non-invasive, FDA-approved device for the diagnosis of DPN. It measures the electrochemical skin conductance (ESC) of the hands and feet by reverse iontophoresis to objectively measure sudomotor function (82). It is quick and easy to perform and has a sensitivity ranging between 70 and 87.5%, and specificity 76.2–92%, to detect DPN (82–84). A recent large cross sectional study found ESC as measured by Sudoscan to be the most sensitive measure (Area under receiver-operator characteristic curve plot 0.88) for the early detection of DPN in comparison to VPT and clinical assessment (85). Additionally, it has a similar diagnostic utility as skin biopsy with IENFD measurement and it correlates with other measures such as clinical neuropathy scoring systems, QST, autonomic function testing and NCS parameters (82–86). However, a recent systematic review concluded that there was insufficient evidence to support that Sudoscan as a measure of sensory nerve fiber function, listing conflicts of interests, inconsistent normative values and insufficient sensitivity and specificity from pooled data-sets (87). Further validation is required to determine the value of sudomotor testing in predicting clinically relevant outcomes such as foot ulceration to recommend as a suitable diagnostic test for DPN.
Treatment of Diabetic Neuropathy
Prevention
There is a lack of treatments which reverse the underlying nerve damage causing DPN. Therefore, prevention of DPN is a key component of diabetes care (10). The ADA recommend achieving optimal glucose control in type 1 and type 2 diabetes to prevent or slow the progression of DPN. However, the evidence for enhancing glycemic control in the prevention of DPN is much greater for type 1 than type 2 diabetes (88). Meta-analyses of large, well-conducted randomized controlled trials have identified a clear benefit for optimizing glucose control in type 1 diabetes. For example, the Diabetes Control of Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study found that intensive therapy significantly reduced the risk of DPN (89). However, the benefits for both glucose and multifactorial risk factor control on DPN are inconclusive in type 2 diabetes (88, 89). Large studies such as the ADDITION-Denmark, UKPDS, Steno-2, and ACCORD trial found intensive glucose and multifactorial treatment had little effect on the incidence of DPN (90–94). However, the presence of multiple comorbidities and risk factors may contribute to the inconsistent findings in these studies (89). Additionally, the types of glucose lowering treatment used may also impact on the results in these studies. Pop-Busui et al. recently found that patients with type 2 diabetes treated with insulin sensitizing therapies had a significantly reduced incidence of DPN compared with insulin providing treatments (10, 95). A meta-analyses of eight randomized studies concluded that there was a trend toward intensive therapy reducing the incidence of DPN in type 2 diabetes, but this did not quite reach statistical significance (p = 0.06) (88).
Pathogenetic Treatments
Pathogenetic treatments of DPN target the underlying disease mechanisms to improve neuronal function. Pathogenetic therapies have shown efficacy in some randomized controlled trials, but the results of pre-clinical studies have largely not translated into clinically meaningful results (96–100). Some of these agents, α-lipoic acid, benfotiamine, actovegin, and epalrestat, are used in some countries (101). However, further robust evidence from clinical trials is necessary before these therapeutic agents can be recommended worldwide (100, 101).
Symptomatic Treatment of Painful-DPN
The mainstay of neuropathic pain treatment in DPN is symptomatic treatment. Unfortunately, pathogenetic treatments and good glycemic control have not been shown to improve neuropathic pain (11). Duloxetine and Pregabalin are the only treatments which have received regulatory FDA approval for the treatment of painful-DPN (10). Whereas, the United Kingdom National Institute of Clinical Excellence recommend Amitriptyline, Duloxetine, Pregabalin, and Gabapentin as first line therapies for neuropathic pain (102). A treatment algorithm is shown in Figure 2 (103).
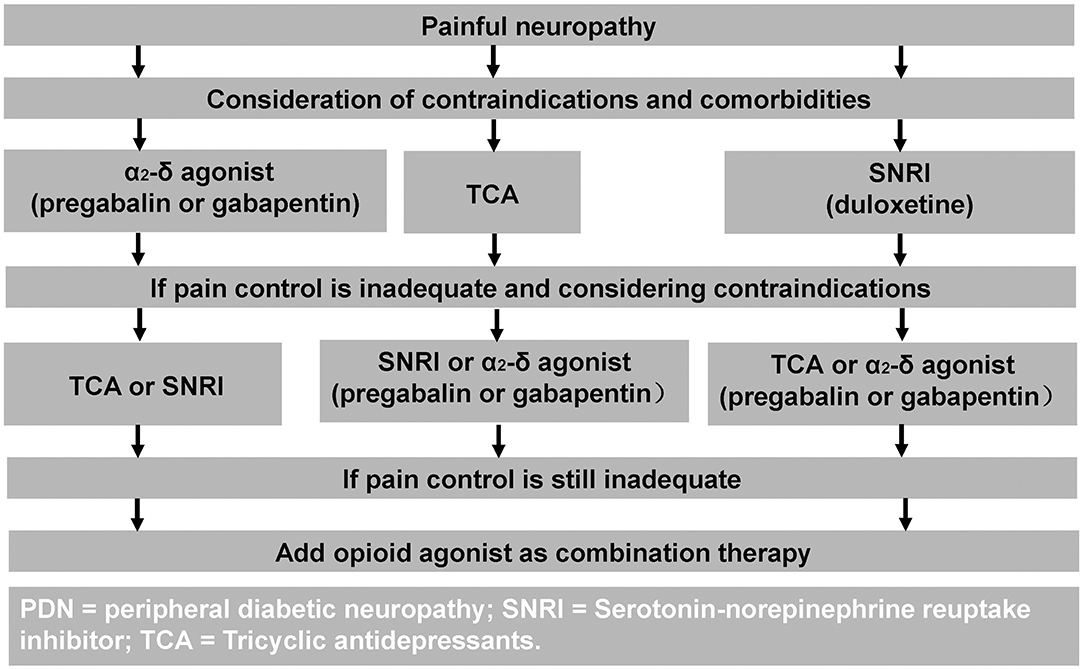
Figure 2. Treatment algorithm for painful-DPN. Reproduced and permission gained from Tesfaye et al. (103).
The α2δ agonists, i.e., gabapentin and pregabalin, are widely recommended, and prescribed agents for painful-DPN. These agents enact their analgesic effect through modulation of the α2δ-1 and α2δ-2 subunits of voltage-sensitive calcium channels (104). Gabapentin is efficacious for the treatment of pain and sleep interference in painful-DPN but has a high rate of side effects, most commonly dizziness, and somnolence (105, 106). The reported number needed to treat to achieve pain relief of at least 50%, is 5.9 (4.6–8.3) (106). Moreover, a network meta-analysis found gabapentin to be the most efficacious and safe therapy for painful-DPN (107).
Pregabalin has linear pharmacokinetics, in contrast to gabapentin, and may be titrated over a short period of time (10, 11). It is the most studied drug for painful-DPN and is recommended as a first line agent by all the major treatment guidelines. It is effective for neuropathic pain and has a side effect profile similar to gabapentin, i.e., dizziness, somnolence, and peripheral oedema (108). In view of the risk of weight gain, and therefore theoretical risk of worsening of metabolic control, Parsons et al. reviewed glycemic/lipid parameters of 11 randomized controlled trials and found no deterioration associated with pregabalin (109). Recent statistics within England and Wales have found an increased number of deaths linked to pregabalin and gabapentin drug misuse prompting a reclassification in the controlling of these medications (110). However, at recommended doses the risk of addiction and dependence for these medications is low in comparison to benzodiazepines, alcohol, and opioids (111, 112). The evidence for other anti-convulsant therapies (e.g., carbamazepine, oxcarbazepine, phenytoin, lamotrigine, and lacosamide) in the treatment of painful-DPN remains limited, but may be effective in some individuals (103).
The other first line pharmacotherapeutic agents for painful-DPN are commonly prescribed anti-depressants, selective serotonin noradrenalin reuptake inhibitors (SNRI) and tricyclic antidepressants (TCA). SNRIs increase the synaptic availability of 5-hydroxytryptamine and noradrenaline increasing the activity of descending pain inhibition pathways (11). Duloxetine is the most widely used agent in this drug class. A Cochrane Collaboration review concluded that at doses of 60 and 120 mg duloxetine is effective in treating painful-DPN, with rare serious side effects (113, 114). The most common side effects include nausea, somnolence, dizziness, constipation, dry mouth, and reduced appetite, although these are commonly mild and transient (104). One of the few comparator drug studies in painful-DPN, the “COMBO-DN” study, found that duloxetine had better efficacy than pregabalin at standard doses (Pregabalin 300 mg/day vs. Duloxetine 60 mg /day) (114).
TCAs have a multimodal analgesic action, including blocking of serotonin and noradrenaline reuptake from synaptic clefts and varying degrees of anticholinergic receptor inhibition (115). Amitriptyline is the most commonly used class of TCA and has been used for neuropathic pain for decades. However, a recent Cochrane Collaboration review and meta-analysis concluded that there is limited evidence for neuropathic pain relief and a poor side effect profile (98, 116). Side effects include dry mouth, constipation, postural hypotension and somnolence, and should be used with caution in elderly patients and those with cardiac disease. Despite its caveats, amitriptyline has been reported to be more effective than placebo in a meta-analysis and remains recommended as a first or second line treatment in all the current guidelines (117).
There are several other treatments which have been studied and are prescribed for painful-DPN with inconclusive evidence. Opioid class medications are an effective means for the treatment of painful-DPN; however, the risk of addiction, side effects and psychosocial complications should limit their use (10). Topical treatments have a theoretical benefit as there is a lower risk of systemic side effects. Agents such as lidocaine patches, capsaicin cream and topical vasodilators however have limited evidence to suggest efficacy (50). For refractory cases of painful-DPN, small studies have found intravenous lidocaine infusions to provide relatively long lasting analgesia (118); however, patients require cardiac monitoring and the treatment is not efficacious in all cases. Open label studies have found vitamin D supplementation to improve neuropathic pain in DPN in patients with vitamin D deficiency (119, 120). Furthermore, non-pharmacological treatments may be considered to complement drug treatments, such as acupuncture, or used as a last resort in resistant cases, such as electrical spinal cord stimulator insertion (103, 121, 122).
Precision Medicine for Painful-DPN
Unfortunately, the current pharmacotherapeutic agents available for neuropathic pain, including painful-DPN, remain inadequate with the best agents offering only modest improvements in pain which is often offset with significant side effects (13). Traditional neuropathic pain treatments have been prescribed according to disease etiology. However, the clinical features, and perhaps underlying disease mechanisms, of neuropathic pain may vary greatly from individual to individual (123). Recent studies have explored whether stratification according to patient characteristics can identify patients more likely to respond to a particular treatment. The ultimate end goal is “personalized medicine” which is currently only possible in rare cases of neuropathic pain secondary to gene mutations. Over recent years, the stratification methods employed for neuropathic pain treatments include: detailed clinical assessment, sensory profiling, psychology/co-morbidities, physiological changes (e.g., electrophysiology/neuroimaging) and molecular profiling (e.g., genotyping) (14).
Clinical Phenotype
Somatosensory phenotyping using detailed symptom based questionnaires, such as the Neuropathic Pain Symptom Inquiry (NPSI), or QST may be used to identify patient subgroups reflecting underlying unique nerve mechanistic changes (124). QST is a psychophysical testing method to assess the function of a range of somatosensory modalities. Older techniques such as VPT and thermal thresholds measure large and small fiber function, respectively. However, more recent studies have employed the German Research Network on Neuropathic Pain (DFNS) protocol which quantifies 13 measures of small and large fiber sensory loss and gain abnormalities against normative datasets (125). Using this QST protocol three clusters of somatosensory profiles have been found in neuropathies of varying etiologies (126). Large studies using this QST protocol in DPN have demonstrated sensory loss, particularly thermal hyposensitivity, in painful- compared with painless-DPN (33, 34). However, there is limited data as to whether patient stratification into somatosensory profile clusters predicts response to neuropathic pain treatments. One phenotype-stratified study found that patients with peripheral neuropathic pain and the “irritable nociceptor” phenotype (reserved thermal sensation and gain of sensory function) responded better to oxcarbazepine than those with a non-irritable nociceptor phenotype (127). Moreover, cluster analysis of patient subgroups from the COMBO-DN study using the NPSI found that the addition of pregabalin to duloxetine was effective in patients with pressing and evoked pain, but high dose duloxetine monotherapy was more beneficial for relief of para/dys-aesthesias (128). Additionally, one study showed that conditional pain modulation, a bedside measure of inhibition of experimental pain, predicted duloxetine efficacy in painful-DPN (129). Clinical phenotyping for neuropathic pain, especially using DFNS QST, is receiving enormous attention but further evidence such as positive clinical trials with patient stratification at baseline are required before such phenotyping can be integrated into clinical practice (124).
Magnetic Resonance Neuroimaging
Central nervous system changes have been well-described in chronic DPN using advanced MR techniques (17). Selvarajah et al. demonstrated that patients with DPN, even those with subclinical DPN, have a lower spinal cord cross-sectional area compared to healthy volunteers and patients with diabetes without peripheral neuropathy (22). Moreover, DPN is associated with peripheral brain gray matter volume loss localized to the primary somatosensory cortex, supramarginal gyrus, and cingulate cortex (23). Quantification of cerebral metabolites using proton MR spectroscopy (1H-MRS) has demonstrated reduced N-acetyl aspartate:creatine ratio suggesting neuronal dysfunction within the thalamus in DPN (130). Additionally, an imbalance of the cerebral neurotransmitters glutamate/glutamine and gamma-aminobutyric acid has been found in the posterior insula in DPN (131).
Neuroimaging has identified a number of neurochemical, structural, neurovascular, and functional alterations secondary to chronic pain diseases. In painful-DPN, studies have shown increased thalamic microvascularity (132), impaired spinal inhibitory function (133), and altered functional connectivity between brain regions involved in pain processing (134). Moreover, MR alterations are related to different clinical phenotypes in painful-DPN (135). A recent study found that patients with insensate painful-DPN, compared to groups with painless-DPN and sensate painful-DPN, had lower somatosensory cortical thickness and expansion of the homuncular area representing pain. Limited studies have also demonstrated that cerebral alterations may be predictive of response to pain treatments (136). Watanabe et al. assessed the cerebral blood flow of patients with and without painful-DPN and longitudinally assessed flow changes after treatment with duloxetine (137). They found that greater baseline cerebral blood flow within the anterior cingulate cortex was associated with better pain relief. However, a recent study found that neurometabolites measured using 1H-MRS in painful-DPN were not significantly altered between placebo and pregabalin, but small differences were observed between pregabalin doses (138). Although, cerebral alterations have been described in painful-DPN, further study of biomarkers as clinical endpoints is required (17, 49).
Genetic
The increased efficiency and availability in gene sequencing technology has led to the exploration of potential genetic factors predisposing to a number of chronic diseases. A meta-analysis found that variants in several genes, e.g., HLA, COMT, OPRM1, TNFA, IL-6, and GCH1, were associated to neuropathic pain in at least one study (139). Moreover, genetic variants have been associated with DPN and neuropathic pain in diabetes (12). Genome-wide association studies have found a number of gene polymorphisms related to painful-DPN (140, 141). Moreover, rare voltage-gated sodium channel Nav 1.7 genetic variants have been shown to be associated with small fiber neuropathy and painful-DPN (142, 143). With the development of the voltage-gated sodium channel Nav 1.7 research, more mutation sites related to painful-DPN have been found, but the mutation perhaps needs further validation (144). Interestingly, Blesneac et al. found 10 out of 111 patients with painful-DPN harbored rare Nav 1.7 variants and these patients reported more severe pain and increased sensitivity to pressure stimuli on QST (145). These findings indicate a link between clinical phenotype and genetic variants which may predict response to treatment. Furthermore, a recent study found that patients with Nav 1.7 mutations and small fiber neuropathy treated with the anticonvulsant lacosamide had significantly improved pain compared with placebo (146).
Conclusions
The prevalence of diabetes mellitus is rising to epidemic proportions. Consequently, there will be a dramatic rise in the numbers of patients suffering with its chronic complications, including DPN. The current management strategy for DPN is focused upon early detection of the condition and prevention of diabetic foot syndromes. New diagnostic techniques may aid the clinical assessment in detecting clinical and subclinical DPN, but further research is required to determine whether clinical outcomes such as foot ulceration, amputation and cardiovascular disease can be prevented with their routine use and whether they may be used as surrogate end points for DPN.
Up to half of patients with DPN suffer with neuropathic pain. Our understanding of why some patients develop painful neuropathic symptoms is unclear. Our current treatments aim to alleviated symptoms, but at best reduce pain scores by 30–50% in about a third of cases. However, the failure of drug trials may be as a result of the empirical use of treatments whereas a more individualized approach by using patient characteristics (e.g., clinical phenotypes, cerebral biomarkers or genotype, etc.) to stratify patients may be more effective (14). However, further validation is required before any of these factors can be considered for stratification in clinical practice but there is potential that it may improve patient outcomes in painful-DPN.
Author Contributions
HY: prepared and wrote up the draft. GS: prepared and majorly revised the draft. YY: prepared the paragraphs on DPN precision medicine. SW: prepared the paragraphs on DPN treatment. BD: prepared the reference list. ST: revised the draft. LG: prepared, wrote up, and revised the manuscript.
Funding
This study was supported by Natural Science Foundation of China (Project # 81170767 and # 81571376, to LG), Diabetes Study Fund from Chinese Medical Association (Project # 13060906481 to LG).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special thanks to ST, whose teachings and lectures inspired this work.
References
1. Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. (1993) 43:817–24. doi: 10.1212/WNL.43.4.817
2. Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. (2014) 14:473. doi: 10.1007/s11910-014-0473-5
3. Dietrich I, Braga GA, de Melo FG, da Costa Silva Silva ACC. The diabetic foot as a proxy for cardiovascular events and mortality review. Curr Atheroscler Rep. (2017) 19:44. doi: 10.1007/s11883-017-0680-z
4. Vadiveloo T, Jeffcoate W, Donnan PT, Colhoun HC, McGurnaghan S, Wild S, et al. Amputation-free survival in 17,353 people at high risk for foot ulceration in diabetes: a national observational study. Diabetologia. (2018) 61:2590–7. doi: 10.1007/s00125-018-4723-y
5. Alleman CJ, Westerhout KY, Hensen M, Chambers C, Stoker M, Long S, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diab Res Clin Practice. (2015) 109:215–25. doi: 10.1016/j.diabres.2015.04.031
6. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
7. Sloan G, Shillo P, Selvarajah D, Wu J, Wilkinson ID, Tracey I, et al. A new look at painful diabetic neuropathy. Diab Res Clin Pract. (2018) 144:177–91. doi: 10.1016/j.diabres.2018.08.020
8. Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. (2015) 29:212–7. doi: 10.1016/j.jdiacomp.2014.10.013
9. Kioskli K, Scott W, Winkley K, Kylakos S, McCracken LM. Psychosocial factors in painful diabetic neuropathy: a systematic review of treatment trials and survey studies. Pain Med. (2019) 20:1756–73. doi: 10.1093/pm/pnz071
10. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the american diabetes association. Diabetes Care. (2017) 40:136–54. doi: 10.2337/dc16-2042
11. Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. (2013) 36:2456–65. doi: 10.2337/dc12-1964
12. Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, Elliott J, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diabetes Rep. (2019) 19:32. doi: 10.1007/s11892-019-1150-5
13. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14:162–73. doi: 10.1016/S1474-4422(14)70251-0
14. Themistocleous AC, Crombez G, Baskozos G, Bennett DL. Using stratified medicine to understand, diagnose, and treat neuropathic pain. Pain. (2018) 159 (Suppl. 1):S31–42. doi: 10.1097/j.pain.0000000000001301
15. Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes. (1997) 46 (Suppl. 2):S54–7. doi: 10.2337/diab.46.2.S54
16. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. (2010) 33:2285–93. doi: 10.2337/dc10-1303
17. Tesfaye S, Selvarajah D, Gandhi R, Greig M, Shillo P, Fang F, et al. Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain. (2016) 157 (Suppl. 1):S72–80. doi: 10.1097/j.pain.0000000000000465
18. Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. (1996) 47:1042–8. doi: 10.1212/WNL.47.4.1042
19. Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol. (1996) 55:1181–93. doi: 10.1097/00005072-199612000-00001
20. Reske-Nielsen E, Lundbaek K, Rafaelsen OJ. Pathological changes in the central and peripheral nervous system of young long-term diabetics: I. Diabetic encephalopathy. Diabetologia. (1966) 1:233–41. doi: 10.1007/BF01257917
21. Reske-Nielsen E, Lundbaek K. Pathological changes in the central and peripheral nervous system of young long-term diabetics. II. The spinal cord and peripheral nerves. Diabetologia. (1968) 4:34–43. doi: 10.1007/BF01241031
22. Selvarajah D, Wilkinson ID, Emery CJ, Harris ND, Shaw PJ, Witte DR, et al. Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care. (2006) 29:2664–9. doi: 10.2337/dc06-0650
23. Selvarajah D, Wilkinson ID, Maxwell M, Davies J, Sankar A, Boland E, et al. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care. (2014) 37:1681–8. doi: 10.2337/dc13-2610
24. Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. (2001) 44:1973–88. doi: 10.1007/s001250100001
25. Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. (2017) 93:1296–313. doi: 10.1016/j.neuron.2017.02.005
26. Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. (2014) 126:3–22. doi: 10.1016/B978-0-444-53480-4.00001-1
27. Callaghan BC, Gao L, Li Y, Zhou X, Reynolds E, Banerjee M, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. (2018) 5:397–405. doi: 10.1002/acn3.531
28. Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-denmark. Diabetes Care. (2018) 41:1068–75. doi: 10.2337/dc17-2062
29. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. (2011) 34:2220–4. doi: 10.2337/dc11-1108
30. Truini A, Spallone V, Morganti R, Tamburin S, Zanette G, Schenone A, et al. A cross-sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. Pain. (2018) 159:2658–66. doi: 10.1097/j.pain.0000000000001378
31. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. (2009) 10:393–400. doi: 10.1111/j.1526-4637.2008.00555.x
32. Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. (2000) 47:123–8. doi: 10.1016/S0168-8227(99)00112-6
33. Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, et al. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. (2016) 157:1132–45. doi: 10.1097/j.pain.0000000000000491
34. Raputova J, Srotova I, Vlckova E, Sommer C, Uceyler N, Birklein F, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain. (2017) 158:2340–53. doi: 10.1097/j.pain.0000000000001034
35. Bril V, Perkins BA. Validation of the toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care. (2002) 25:2048–52. doi: 10.2337/diacare.25.11.2048
36. Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. (2010) 17:903–12; e44–9. doi: 10.1111/j.1468-1331.2010.03023.x
37. Vlckova-Moravcova E, Bednarik J, Dusek L, Toyka KV, Sommer C. Diagnostic validity of epidermal nerve fiber densities in painful sensory neuropathies. Muscle Nerve. (2008) 37:50–60. doi: 10.1002/mus.20889
38. Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. (2007) 56:2148–54. doi: 10.2337/db07-0285
39. Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care. (2004) 27:1974–9. doi: 10.2337/diacare.27.8.1974
40. Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. (2004) 127:1593–605. doi: 10.1093/brain/awh180
41. Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. (2008) 131:1912–25. doi: 10.1093/brain/awn093
42. Nebuchennykh M, Loseth S, Lindal S, Mellgren SI. The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J Neurol. (2009) 256:1067–75. doi: 10.1007/s00415-009-5065-y
43. Alam U, Jeziorska M, Petropoulos IN, Asghar O, Fadavi H, Ponirakis G, Marshall A, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS ONE. (2017) 12:e0180175. doi: 10.1371/journal.pone.0180175
44. Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve. (2007) 35:591–8. doi: 10.1002/mus.20732
45. Bonhof GJ, Strom A, Puttgen S, Ringel B, Bruggemann J, Bodis K, et al. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia. (2017) 60:2495–503. doi: 10.1007/s00125-017-4438-5
46. Shillo P. Nerve and vascular biomarkers in skin biopsies differentiate painful from painless advanced diabetic peripheral neuropathy. EASD. (2017) 2017:31–3.
47. Cheng HT, Dauch JR, Porzio MT, Yanik BM, Hsieh W, Smith AG, et al. Increased axonal regeneration and swellings in intraepidermal nerve fibers characterize painful phenotypes of diabetic neuropathy. J Pain. (2013) 14:941–7. doi: 10.1016/j.jpain.2013.03.005
48. Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. (2006) 29:1294–9. doi: 10.2337/dc06-0224
49. Smith SM, Dworkin RH, Turk DC, Baron R, Polydefkis M, Tracey I, et al. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain. (2017) 18:757–77. doi: 10.1016/j.jpain.2017.02.429
50. Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. (2018) 40:828–49. doi: 10.1016/j.clinthera.2018.04.001
51. Ferrari GL, Marques JL, Gandhi RA, Emery CJ, Tesfaye S, Heller SR, et al. An approach to the assessment of diabetic neuropathy based on dynamic pupillometry. Conf Proc IEEE Eng Med Biol Soc. (2007) 2007:557–60. doi: 10.1109/IEMBS.2007.4352351
52. Ferrari GL, Marques JL, Gandhi RA, Heller SR, Schneider FK, Tesfaye S, et al. Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: a pilot study. Biomed Eng Online. (2010) 9:26. doi: 10.1186/1475-925X-9-26
53. Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. (2003) 46:683–8. doi: 10.1007/s00125-003-1086-8
54. Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. (2012) 35:821–8. doi: 10.2337/dc11-1396
55. Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. (2010) 33:1792–7. doi: 10.2337/dc10-0253
56. Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. (2015) 52:363–70. doi: 10.1002/mus.24553
57. Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. (2015) 38:671–5. doi: 10.2337/dc14-2114
58. Dehghani C, Russell AW, Perkins BA, Malik RA, Pritchard N, Edwards K, et al. A rapid decline in corneal small fibers and occurrence of foot ulceration and Charcot foot. J Diabetes Complications. (2016) 30:1437–9. doi: 10.1016/j.jdiacomp.2016.07.004
59. van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. (2009) 50:3404–9. doi: 10.1167/iovs.08-3143
60. Park HY, Shin J, Lee JH, Park CK. Retinal nerve fiber layer loss in patients with type 2 diabetes and diabetic neuropathy. Diabetes Care. (2016) 39:e69–70. doi: 10.2337/dc15-2675
61. Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. (2006) 55:2401–11. doi: 10.2337/db05-1635
62. Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, et al. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem. (1996) 44:1469–79. doi: 10.1177/44.12.8985139
63. Dehghani C, Srinivasan S, Edwards K, Pritchard N, Russell AW, Malik RA, et al. Presence of peripheral neuropathy is associated with progressive thinning of retinal nerve fiber layer in type 1 diabetes. Invest Ophthalmol Vis Sci. (2017) 58:Bio234–9. doi: 10.1167/iovs.17-21801
64. Inceu GV, Veresiu IA. Measurement of current perception thresholds using the Neurometer((R)) - applicability in diabetic neuropathy. Clujul Med. (2015) 88:449–52. doi: 10.15386/cjmed-491
65. Matsutomo R, Takebayashi K, Aso Y. Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. J Int Med Res. (2005) 33:442–53. doi: 10.1177/147323000503300410
66. Cheng WY, Jiang YD, Chuang LM, Huang CN, Heng LT, Wu HP, et al. Quantitative sensory testing and risk factors of diabetic sensory neuropathy. J Neurol. (1999) 246:394–8. doi: 10.1007/s004150050370
67. Bril V, Perkins BA. Comparison of vibration perception thresholds obtained with the Neurothesiometer and the CASE IV and relationship to nerve conduction studies. Diabetic Med. (2002) 19:661–6. doi: 10.1046/j.1464-5491.2002.00759.x
68. Nather A, Neo SH, Chionh SB, Liew SC, Sim EY, Chew JL. Assessment of sensory neuropathy in diabetic patients without diabetic foot problems. J Diabetes Complications. (2008) 22:126–31. doi: 10.1016/j.jdiacomp.2006.10.007
69. Park JH, Won JC. Patterns of nerve conduction abnormalities in patients with type 2 diabetes mellitus according to the clinical phenotype determined by the current perception threshold. Diabetes Metab J. (2018) 42:519–28. doi: 10.4093/dmj.2018.0068
70. Koo BK, Ohn JH, Kwak SH, Moon MK. Assessment of diabetic polyneuropathy and autonomic neuropathy using current perception threshold in korean patients with diabetes mellitus. Diabetes Metab J. (2014) 38:285–93. doi: 10.4093/dmj.2014.38.4.285
71. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy. PLoS ONE. (2014) 9:e86515. doi: 10.1371/journal.pone.0086515
72. Chatzikosma G, Pafili K, Demetriou M, Vadikolias K, Maltezos E, Papanas N. Evaluation of sural nerve automated nerve conduction study in the diagnosis of peripheral neuropathy in patients with type 2 diabetes mellitus. Arch Med Sci. (2016) 12:390–3. doi: 10.5114/aoms.2016.59265
73. Sharma S, Vas PR, Rayman G. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol. (2015) 9:123–31. doi: 10.1177/1932296814551044
74. Binns-Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S. One-stop microvascular screening service: an effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabetic Med. (2018) 35:887–94. doi: 10.1111/dme.13630
75. Tentolouris N, Marinou K, Kokotis P, Karanti A, Diakoumopoulou E, Katsilambros N. Sudomotor dysfunction is associated with foot ulceration in diabetes. Diabetic Med. (2009) 26:302–5. doi: 10.1111/j.1464-5491.2009.02677.x
76. Papanas N, Ziegler D. New diagnostic tests for diabetic distal symmetric polyneuropathy. J Diabetes Complications. (2011) 25:44–51. doi: 10.1016/j.jdiacomp.2009.09.006
77. Papanas N, Papatheodorou K, Christakidis D, Papazoglou D, Giassakis G, Piperidou H, et al. Evaluation of a new indicator test for sudomotor function (Neuropad) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. (2005) 113:195–8. doi: 10.1055/s-2005-837735
78. Liatis S, Marinou K, Tentolouris N, Pagoni S, Katsilambros N. Usefulness of a new indicator test for the diagnosis of peripheral and autonomic neuropathy in patients with diabetes mellitus. Diabetic Med. (2007) 24:1375–80. doi: 10.1111/j.1464-5491.2007.02280.x
79. Papanas N, Papatheodorou K, Papazoglou D, Monastiriotis C, Christakidis D, Maltezos E. A comparison of the new indicator test for sudomotor function (Neuropad) with the vibration perception threshold and the clinical examination in the diagnosis of peripheral neuropathy in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. (2008) 116:135–8. doi: 10.1055/s-2007-984455
80. Quattrini C, Jeziorska M, Tavakoli M, Begum P, Boulton AJ, Malik RA. The Neuropad test: a visual indicator test for human diabetic neuropathy. Diabetologia. (2008) 51:1046–50. doi: 10.1007/s00125-008-0987-y
81. Ponirakis G, Fadavi H, Petropoulos IN, Azmi S, Ferdousi M, Dabbah MA, et al. Automated quantification of neuropad improves its diagnostic ability in patients with diabetic neuropathy. J Diabetes Res. (2015) 2015:847854. doi: 10.1155/2015/847854
82. Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. (2013) 15:948–53. doi: 10.1089/dia.2013.0129
83. Krieger SM, Reimann M, Haase R, Henkel E, Hanefeld M, Ziemssen T. Sudomotor testing of diabetes polyneuropathy. Front Neurol. (2018) 9:803. doi: 10.3389/fneur.2018.00803
84. Selvarajah D, Cash T, Davies J, Sankar A, Rao G, Grieg M, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS ONE. (2015) 10:e0138224. doi: 10.1371/journal.pone.0138224
85. Goel A, Shivaprasad C, Kolly A, Sarathi HAV, Atluri S. Comparison of electrochemical skin conductance and vibration perception threshold measurement in the detection of early diabetic neuropathy. PLoS ONE. (2017) 12:e0183973. doi: 10.1371/journal.pone.0183973
86. Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications. (2014) 28:511–6. doi: 10.1016/j.jdiacomp.2014.02.013
87. Rajan S, Campagnolo M, Callaghan B, Gibbons CH. Sudomotor function testing by electrochemical skin conductance: does it really measure sudomotor function? Clin Auton Res. (2019) 29:31–9. doi: 10.1007/s10286-018-0540-0
88. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012:Cd007543. doi: 10.1002/14651858.CD007543.pub2
89. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diabetes Rep. (2014) 14:528. doi: 10.1007/s11892-014-0528-7
90. Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, et al. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. (2011) 34:2244–9. doi: 10.2337/dc11-0903
91. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. (1998) 352:837–53. doi: 10.1016/S0140-6736(98)07019-6
92. Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. (2010) 33:721–7. doi: 10.2337/dc09-1471
93. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. (2010) 376:419–30. doi: 10.1016/S0140-6736(10)60576-4
94. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. (2003) 348:383–93. doi: 10.1056/NEJMoa021778
95. Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. (2013) 36:3208–15. doi: 10.2337/dc13-0012
96. Malik RA. Why are there no good treatments for diabetic neuropathy? Lancet Diabetes Endocrinol. (2014) 2:607–9. doi: 10.1016/S2213-8587(14)70067-1
97. Agathos E, Tentolouris A, Eleftheriadou I, Katsaouni P, Nemtzas I, Petrou A, et al. Effect of alpha-lipoic acid on symptoms and quality of life in patients with painful diabetic neuropathy. J Int Med Res. (2018) 46:1779–90. doi: 10.1177/0300060518756540
98. Hotta N, Kawamori R, Fukuda M, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabetic Med. (2012) 29:1529–33. doi: 10.1111/j.1464-5491.2012.03684.x
99. Calabek B, Callaghan B, Feldman EL. Therapy for diabetic neuropathy: an overview. Handb Clin Neurol. (2014) 126:317–33. doi: 10.1016/B978-0-444-53480-4.00022-9
100. Boulton AJ, Kempler P, Ametov A, Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. (2013) 29:327–33. doi: 10.1002/dmrr.2397
101. Bonhof GJ, Herder C, Strom A, Papanas N, Roden M, Ziegler D. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr Rev. (2019) 40:153–92. doi: 10.1210/er.2018-00107
102. Centre for Clinical Practice at N. National Institute for Health and Care Excellence: Clinical Guidelines Neuropathic Pain: The Pharmacological Management of Neuropathic Pain in Adults in Non-specialist Settings. London: National Institute for Health and Care Excellence; National Institute for Health and Care Excellence (2013).
103. Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. (2011) 27:629–38. doi: 10.1002/dmrr.1225
104. Javed S, Alam U, Malik RA. Mirogabalin and emerging therapies for diabetic neuropathy. J Pain Res. (2018) 11:1559–66. doi: 10.2147/JPR.S145999
105. Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. (1998) 280:1831–6. doi: 10.1001/jama.280.21.1831
106. Wiffen PJ, Derry S, Bell RF, Rice AS, Tolle TR, Phillips T, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. (2017) 6:Cd007938. doi: 10.1002/14651858.CD007938.pub4
107. Rudroju N, Bansal D, Talakokkula ST, Gudala K, Hota D, Bhansali A, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. (2013) 16:E705–14.
108. Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. (2008) 31:1448–54. doi: 10.2337/dc07-2105
109. Parsons B, Emir B. Glycemic and serum lipid control in patients with painful diabetic peripheral neuropathy treated with pregabalin. J Diabetes Complications. (2017) 31:489–93. doi: 10.1016/j.jdiacomp.2016.03.019
110. Statistics. OfN. Deaths Related To Drug Poisoning in England and Wales Statistical Bulletins. (2018).
111. Azmi S, ElHadd KT, Nelson A, Chapman A, Bowling FL, Perumbalath A, et al. Pregabalin in the management of painful diabetic neuropathy: a narrative review. Diabetes Ther. (2019) 10:35–56. doi: 10.1007/s13300-018-0550-x
112. Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs. (2014) 28:491–6. doi: 10.1007/s40263-014-0164-4
113. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. (2014) 2014:Cd007115. doi: 10.1002/14651858.CD007115.pub3
114. Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tolle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”–a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. (2013) 154:2616–25. doi: 10.1016/j.pain.2013.05.043
115. Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Therap Adv Chronic Dis. (2015) 6:15–28. doi: 10.1177/2040622314552071
116. Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. (2015) 2015:Cd008242. doi: 10.1002/14651858.CD008242.pub3
117. Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. (2014) 161:639–49. doi: 10.7326/M14-0511
118. Viola V, Newnham HH, Simpson RW. Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J Diabetes Complications. (2006) 20:34–9. doi: 10.1016/j.jdiacomp.2005.05.007
119. Basit A, Basit KA, Fawwad A, Shaheen F, Fatima N, Petropoulos IN, et al. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care. (2016) 4:e000148. doi: 10.1136/bmjdrc-2015-000148
120. Ghadiri-Anari A, Mozafari Z, Gholami S, Khodaei SA, Aboutorabi-Zarchi M, Sepehri F, et al. Dose vitamin D supplementations improve peripheral diabetic neuropathy? A before-after clinical trial. Diabetes Metab Syndr. (2019) 13:890–3. doi: 10.1016/j.dsx.2018.12.014
121. Thakral G, Kim PJ, LaFontaine J, Menzies R, Najafi B, Lavery LA. Electrical stimulation as an adjunctive treatment of painful and sensory diabetic neuropathy. J Diabetes Sci Technol. (2013) 7:1202–9. doi: 10.1177/193229681300700510
122. Chen W, Yang GY, Liu B, Manheimer E, Liu JP. Manual acupuncture for treatment of diabetic peripheral neuropathy: a systematic review of randomized controlled trials. PLoS ONE. (2013) 8:e73764. doi: 10.1371/journal.pone.0073764
123. von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. (2012) 73:638–52. doi: 10.1016/j.neuron.2012.02.008
124. Forstenpointner J, Otto J, Baron R. Individualized neuropathic pain therapy based on phenotyping: are we there yet? Pain. (2018) 159:569–75. doi: 10.1097/j.pain.0000000000001088
125. Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. (2006) 123:231–43. doi: 10.1016/j.pain.2006.01.041
126. Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. (2017) 158:261–72. doi: 10.1097/j.pain.0000000000000753
127. Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. (2014) 155:2263–73. doi: 10.1016/j.pain.2014.08.014
128. Bouhassira D, Wilhelm S, Schacht A, Perrot S, Kosek E, Cruccu G, et al. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: data from the randomized, double-blind, COMBO-DN study. Pain. (2014) 155:2171–9. doi: 10.1016/j.pain.2014.08.020
129. Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. (2012) 153:1193–8. doi: 10.1016/j.pain.2012.02.021
130. Selvarajah D, Wilkinson ID, Emery CJ, Shaw PJ, Griffiths PD, Gandhi R, et al. Thalamic neuronal dysfunction and chronic sensorimotor distal symmetrical polyneuropathy in patients with type 1 diabetes mellitus. Diabetologia. (2008) 51:2088–92. doi: 10.1007/s00125-008-1139-0
131. Petrou M, Pop-Busui R, Foerster BR, Edden RA, Callaghan BC, Harte SE, et al. Altered excitation-inhibition balance in the brain of patients with diabetic neuropathy. Acad Radiol. (2012) 19:607–12. doi: 10.1016/j.acra.2012.02.004
132. Selvarajah D, Wilkinson ID, Gandhi R, Griffiths PD, Tesfaye S. Microvascular perfusion abnormalities of the Thalamus in painful but not painless diabetic polyneuropathy: a clue to the pathogenesis of pain in type 1 diabetes. Diabetes Care. (2011) 34:718–20. doi: 10.2337/dc10-1550
133. Marshall AG, Lee-Kubli C, Azmi S, Zhang M, Ferdousi M, Mixcoatl-Zecuatl T, et al. Spinal disinhibition in experimental and clinical painful diabetic neuropathy. Diabetes. (2017) 66:1380–90. doi: 10.2337/db16-1181
134. Cauda F, Sacco K, D'Agata F, Duca S, Cocito D, Geminiani G, et al. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. (2009) 10:138. doi: 10.1186/1471-2202-10-138
135. Selvarajah D, Wilkinson ID, Fang F, Sankar A, Davies J, Boland E, et al. Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal MRI study. Diabetes. (2019) 68:796–806. doi: 10.2337/db18-0509
136. Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. (2013) 119:1453–64. doi: 10.1097/ALN.0000000000000017
137. Watanabe K, Hirano S, Kojima K, Nagashima K, Mukai H, Sato T, et al. Altered cerebral blood flow in the anterior cingulate cortex is associated with neuropathic pain. J Neurol Neurosurg Psychiatry. (2018) 89:1082–7. doi: 10.1136/jnnp-2017-316601
138. De Jaeger M, Goudman L, Van Schuerbeek P, De Mey J, Keymeulen B, Brouns R, et al. Cerebral biochemical effect of pregabalin in patients with painful diabetic neuropathy: a randomized controlled trial. Diabetes Ther. (2018) 9:1591–604. doi: 10.1007/s13300-018-0460-y
139. Veluchamy A, Hebert HL, Meng W, Palmer CNA, Smith BH. Systematic review and meta-analysis of genetic risk factors for neuropathic pain. Pain. (2018) 159:825–48. doi: 10.1097/j.pain.0000000000001164
140. Meng W, Deshmukh HA, Donnelly LA, Torrance N, Colhoun HM, Palmer CN, et al. A genome-wide association study provides evidence of sex-specific involvement of Chr1p35.1 (ZSCAN20-TLR12P) and Chr8p23.1 (HMGB1P46) with diabetic neuropathic pain. EBioMedicine. (2015) 2:1386–93. doi: 10.1016/j.ebiom.2015.08.001
141. Meng W, Deshmukh HA, van Zuydam NR, Liu Y, Donnelly LA, Zhou K, et al. A genome-wide association study suggests an association of Chr8p21.3 (GFRA2) with diabetic neuropathic pain. Eur J Pain. (2015) 19:392–9. doi: 10.1002/ejp.560
142. Li QS, Cheng P, Favis R, Wickenden A, Romano G, Wang H. SCN9A variants may be implicated in neuropathic pain associated with diabetic peripheral neuropathy and pain severity. Clin J Pain. (2015) 31:976–82. doi: 10.1097/AJP.0000000000000205
143. Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. (2012) 71:26–39. doi: 10.1002/ana.22485
144. Alsaloum M, Estacion M, Almomani R, Gerrits MM, Bonhof GJ, Ziegler D, et al. A gain-of-function sodium channel beta2-subunit mutation in painful diabetic neuropathy. Mol Pain. (2019) 15:1744806919849802. doi: 10.1177/1744806919849802
145. Blesneac I, Themistocleous AC, Fratter C, Conrad LJ, Ramirez JD, Cox JJ, et al. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain. (2018) 159:469–80. doi: 10.1097/j.pain.0000000000001116
Keywords: diabetic neuropathy, painful diabetic neuropathy, stratified medicine, diagnosis diabetic neuropathy, painful diabetic neuropathy treatment
Citation: Yang H, Sloan G, Ye Y, Wang S, Duan B, Tesfaye S and Gao L (2020) New Perspective in Diabetic Neuropathy: From the Periphery to the Brain, a Call for Early Detection, and Precision Medicine. Front. Endocrinol. 10:929. doi: 10.3389/fendo.2019.00929
Received: 09 October 2019; Accepted: 19 December 2019;
Published: 17 January 2020.
Edited by:
Yoshimi Nakagawa, University of Tsukuba, JapanReviewed by:
Andrew Marshall, University of Liverpool, United KingdomAkihiko Ando, Nishio Hospital, Japan
Copyright © 2020 Yang, Sloan, Ye, Wang, Duan, Tesfaye and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Gao, bGluZy5nYW9Ad2h1LmVkdS5jbg==
†These authors have contributed equally to this work
 Heng Yang
Heng Yang Gordon Sloan
Gordon Sloan Yingchun Ye1
Yingchun Ye1 Shuo Wang
Shuo Wang Solomon Tesfaye
Solomon Tesfaye Ling Gao
Ling Gao