- 1Department of Clinical Laboratory, Xiamen Huli Guoyu Clinic, Co., Ltd., Xiamen, China
- 2Department of Anatomy, Physiology, and Pharmacology, College of Veterinary Medicine, Auburn University, Auburn, AL, United States
Adrenocorticotropic hormone (ACTH), and α-, β-, and γ-melanocyte-stimulating hormones (α-, β-, γ-MSH), collectively known as melanocortins, together with their receptors (melanocortin receptors), are components of an ancient modulatory system. The clinical use of ACTH in the treatment of rheumatoid arthritis started in 1949, originally thought that the anti-inflammatory action was through hypothalamus-pituitary-adrenal axis and glucocorticoid-dependent. Subsequent decades have witnessed extensive attempts in unraveling the physiology and pharmacology of the melanocortin system. It is now known that ACTH, together with α-, β-, and γ-MSHs, also possess glucocorticoid-independent anti-inflammatory and immunomodulatory effects by activating the melanocortin receptors expressed in the brain or peripheral immune cells. This review will briefly introduce the melanocortin system and highlight the action of melanocortins in the regulation of immune functions from in vitro, in vivo, preclinical, and clinical studies. The potential therapeutic use of melanocortins are also summarized.
Introduction
The melanocortin system consists of the melanocortins, two endogenous antagonists, the agouti-signaling protein (agouti) and agouti-related peptide (AgRP), and five receptors. The melanocortins, including adrenocorticotropic hormone (ACTH), and α-, β-, and γ-melanocyte-stimulating hormones (α-, β-, γ-MSHs), are derived from post-translational processing of a common precursor pro-opiomelanocortin (POMC). In addition to the well-documented adrenal responses stimulated by ACTH and pigmentary effects induced by α-MSH, other physiological functions, including energy homeostasis, sexual activity, exocrine secretion, as well as anti-inflammatory and immunomodulatory actions, exerted by these versatile neuropeptides upon the host, have also been reported extensively (1–3).
The first clinical experiment applying ACTH in the treatment of patients with severe rheumatoid arthritis, rheumatic fever, and certain other conditions, was performed by Hench et al. (4, 5), the Nobel Laureates in Physiology or Medicine in 1950 for their milestone discoveries on ACTH and the adrenal hormone cortisol (6). At that time, ACTH-induced improvement in clinical features in rheumatoid arthritis patients was thought to be via stimulation of hypothalamus-pituitary-adrenal gland axis and production of glucocorticoids.
Our understanding of melanocortin physiology and pharmacology has greatly improved with the recognition and cloning of melanocortin receptors (MCRs). Indeed, it was not until 2002 that local activation of melanocortin-3 receptor (MC3R) by ACTH, independent of glucocorticoid, was demonstrated to be also responsible for ACTH efficacy in gouty arthritis (7). This important study suggested that selective MC3R agonists might be utilized as novel anti-inflammatory agents for clinical management of chronic inflammatory conditions (7). In this review, we will briefly introduce the melanocortin system, including melanocortin peptides, MCRs, and intracellular signaling pathways. Then, the anti-inflammatory effects of melanocortins in vitro and in vivo, the underlying molecular mechanisms, as well as potential therapeutic use of melanocortins, will be elaborated.
The Melanocortin System
The Melanocortin Ligands
The melanocortins are derived from post-translational processing of a common precursor protein, POMC, first discovered in mouse pituitary tumor cells and later demonstrated in human nonpituitary tumor cells by several independent groups (8–10). Using recombinant DNA technology, Nakanishi et al. first reported the nucleotide sequence of the cDNA for bovine POMC (11). Subsequently, POMC sequences were reported in several mammals, amphibians, and teleosts (12–16). Sequence comparison revealed a recognizable POMC sequence in lamprey, the most ancient vertebrate, that shares structural similarity to those of higher vertebrates and teleosts, indicating the appearance of POMC might date back to 700 million or more years (17).
In addition to the pituitary and melanocytes and keratinocytes in skin where POMC were originally discovered, POMC mRNA has been found in hypothalamic arcuate nucleus, nucleus of solitary tract in the caudal brainstem, spinal cord, and dorsal root ganglion (18–21). POMC mRNA is also detected in peripheral immune cells such as lymphocytes and monocytes, suggesting a regulatory role of POMC-derived peptides in inflammation in these immune-related cells (22–26). Indeed, cytokine-, interferon-, or hormone-induced activation of signal transducers and activators of transcription signaling cascade enhances POMC expression and melanocortin synthesis at sites of infection or inflammation [reviewed in (27)].
The tissue-specific post-translational processing of POMC is performed by prohormone convertases (PCs), proteolytic enzymes belonging to the family of serine-type proteinases. PC1 cleaves POMC to produce ACTH and other peptides in the anterior pituitary gland, whereas in lower vertebrates and during fetal and infantile periods in humans, PC2 located in the pars intermedia accounts for the production of MSHs and β-endorphin. In addition, POMC is further processed into MSHs by PC2 in peripheral tissues such as skin and hair follicles and in central nervous system (CNS) (28–30).
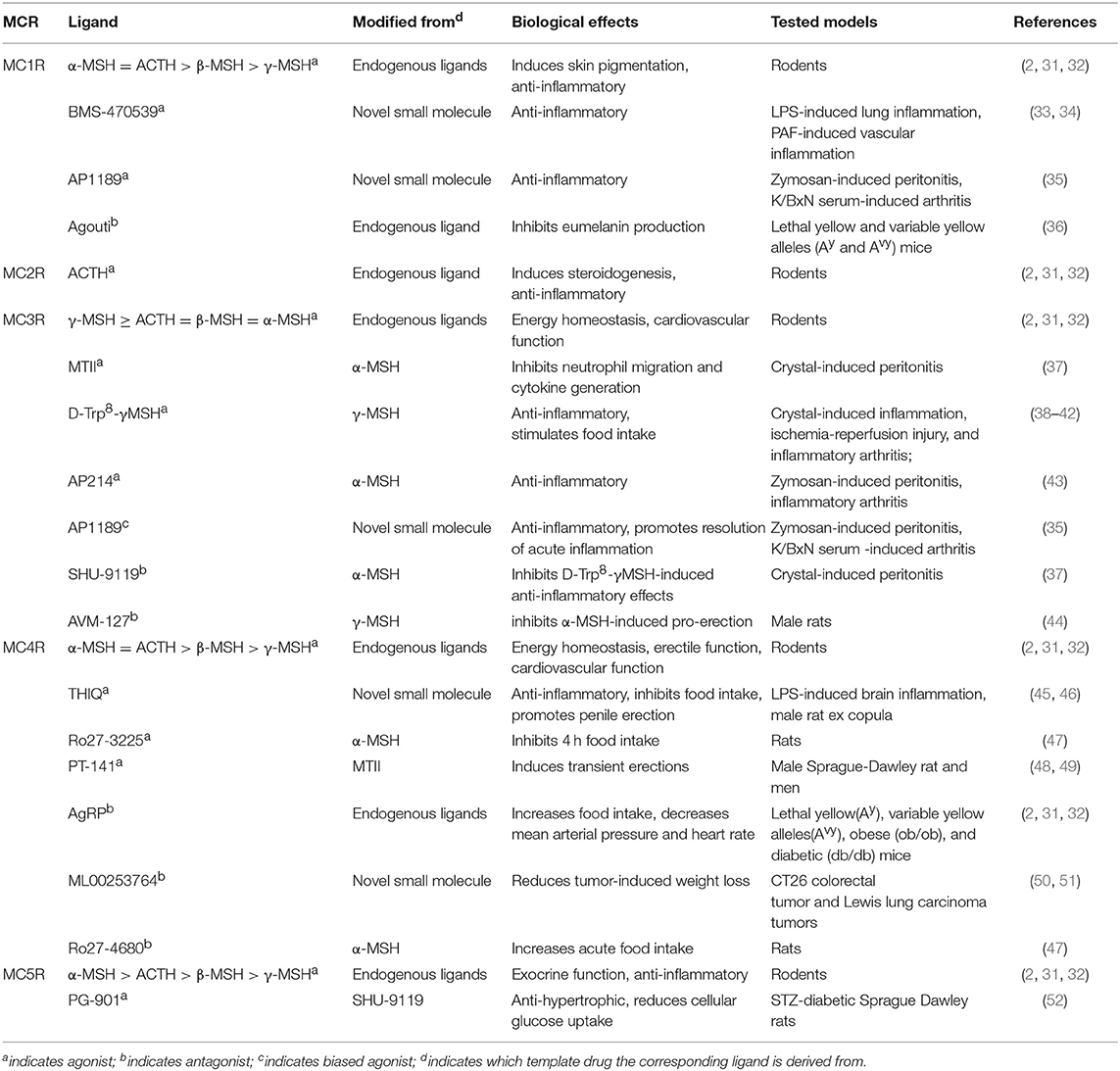
All melanocortins share the common amino acid motif HFRW, which is the minimum sequence required for receptor binding and activation. It should be noted that each melanocortin exerts different affinities for the specific receptor: ACTH is specific for the activation of MC2R, α-MSH exhibits the highest affinity for MC1R, MC4R, and MC5R, whereas γ-MSH displays the highest affinity for MC3R (as shown in Table 1). The melanocortin system is unique due to the existence of two natural endogenous antagonists, agouti, and AgRP, which act as inverse agonists to block the agonist binding at the MCRs and to decrease the constitutive activity (agouti for MC1R and AgRP for MC3R and MC4R). Recently, AgRP, through activating neural MC4R, was shown to induce Gi protein activation and potassium channel opening in a Gs-independent manner (53, 54). We also showed that AgRP decreases cAMP activity of constitutively active F347A hMC3R but stimulates ERK1/2 activation in both wild type and laboratory-generated mutant F347A hMC3Rs (55, 56). In the MC4R, AgRP is also an inverse agonist at the Gs-cAMP pathway but an agonist at the ERK1/2 pathway (57). These data demonstrated biased agonism of AgRP in neural MCRs, adding a novel layer of complexity to MCR signaling (58, 59).
The Melanocortin Receptors
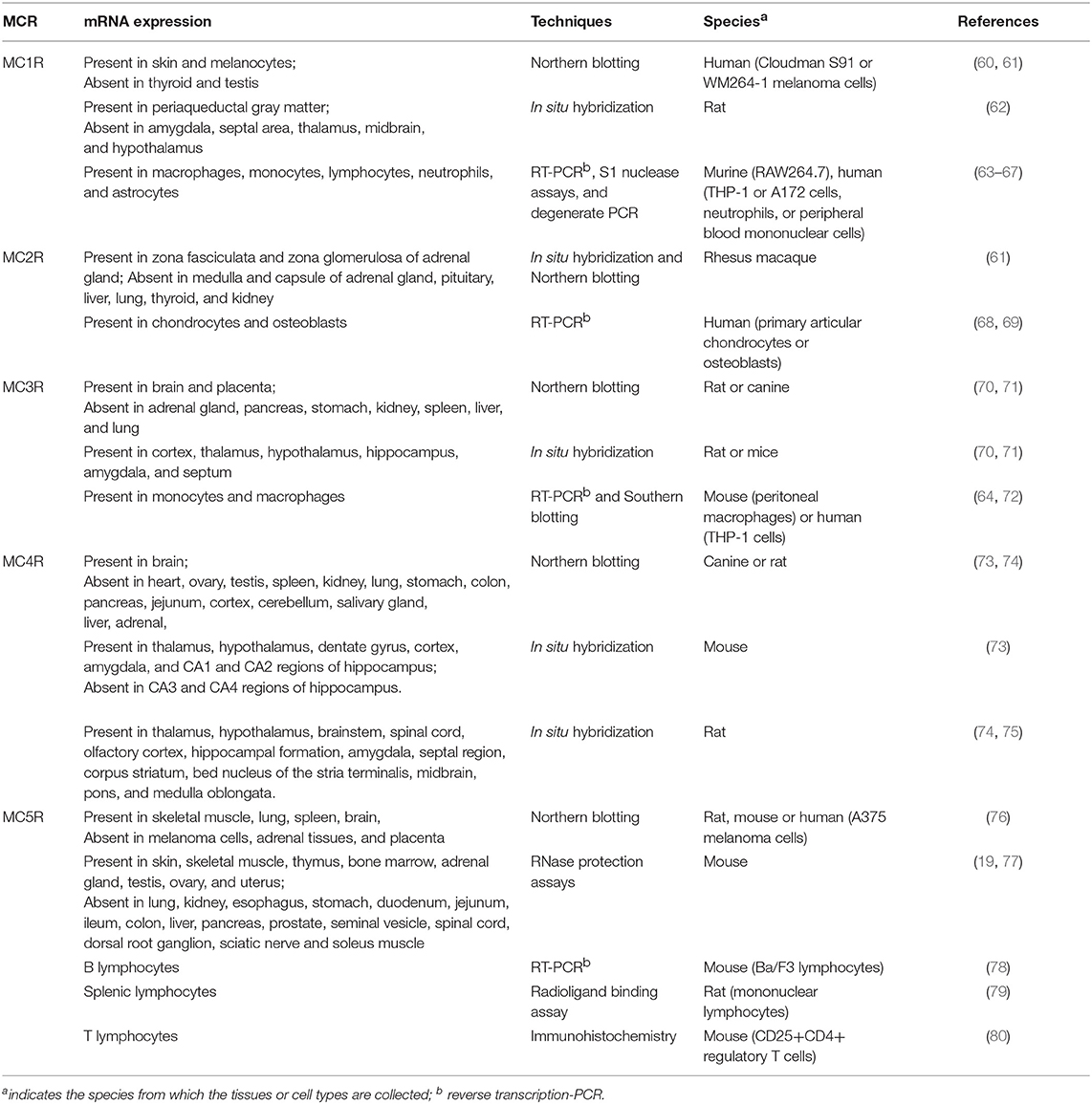
The versatile actions of melanocortin peptides are mediated by five MCRs, MC1R to MC5R, which are named according to the sequence of their cloning. As members of Family A G protein-coupled receptors, MCRs share the common hallmark structure consisting of seven α-helical transmembrane domains, an extracellular N terminus and intracellular C tail, connected by three alternating extracellular and three intracellular loops. Sequence comparison of human MCRs reveals high homologies, from 38% identity between MC2R and MC4R to 60% identity between MC4R and MC5R (27). As shown in Table 2, the MCR exerts diverse expression patterns, which might contribute to their multifaceted physiological functions. As challenging and popular drug targets, the MCRs have triggered intensive efforts to develop more potent and selective melanocortin agonists and antagonists. Table 1 includes some of the most commonly-used peptide and small molecule ligands for the MCRs.
The MC1R is the classical MSH receptor expressed in skin and hair follicles that regulates pigmentation. However, MC1R, with the highest affinity for α-MSH, is expressed in virtually all cell types responsive to the anti-inflammatory action of melanocortins. It was also shown that normal human monocytes and mouse brain contain a low number of MC1R binding sites which is upregulated by various stimuli such as lipopolysaccharide (LPS) or combinations of cytokines, and traumatic brain injury, respectively (81, 82). In addition, by measuring ionized calcium binding adaptor molecule-1 staining (Iba-1) in microglial cells, a known marker for cerebral inflammation, Schaible et al. showed that α-MSH (11–13), probably by targeting the MC1R, significantly attenuates cerebral inflammation, as evidenced by reduced activation of Iba-1 positive cells in the ipsilateral hemisphere (82). Two synthetic MC1R agonists, BMS-470539 and AP1189, were also reported to exhibit anti-inflammatory activities in various inflammation models (33–35).
The MC2R, the classical ACTH receptor, is expressed in the adrenal cortex that regulates adrenal steroidogenesis and cell proliferation (Table 2). MC2R is also expressed in chondrocytes and osteoblasts, where it might be involved in the regulation of local inflammation (68, 69).
As shown in Table 2, the neural MCRs, MC3R, and MC4R, are expressed primarily in the CNS and regulate energy homeostasis (83–85). However, the MC3R is also expressed in human placenta, heart, and gut as well as lymphocytes and macrophages, where it mediates potent anti-inflammatory effects of MC3R agonists such as MTII, D-Trp8-γMSH, AP214, and AP1189 (35, 37–39, 43, 70, 86, 87), whereas MC3R antagonists, SHU-9119 and AVM-217, prevents such protective effects (37, 44).
Extensive localization studies on rodent brains showed that the Mc4r mRNA is expressed in multiple brain regions, including cortex, brainstem, thalamus, hypothalamus, hippocampus, and spinal cord (73–75). The MC4R has been reported to mediate central anti-inflammatory effects of α-MSH. Schaible et al. showed that MC4R-mediated activation of anti-apoptotic pathways might at least in part explain the neuroprotective properties of α-MSH in a mouse model of traumatic brain injury (82), which is similar to the actions of MC4R ligands observed in animal models of cerebral ischemia (88–90).
The MC5R is widely expressed in the skin, adrenal glands, adipocytes, skeletal muscle, bone marrow, kidney, liver, lungs, spleen, thymus, gonads, uterus, and brain (76, 77, 91–93). Mc5r knockout mice display a phenotype with defective water repulsion and thermoregulation due to decreased sebogenesis, indicating a role for the receptor in sebum synthesis and thermoregulation (93). Additional studies suggested that the receptor also contributes to the immunomodulatory functions in B and T lymphocytes, and mast cells (78, 80).
Intracellular Signaling Pathways
It is accepted now that, once activated by the agonists, the MCRs at the cell surface undergo conformational changes and trigger a complex of intracellular network of pathways. The MCRs are primarily coupled to Gs proteins and result in activation of adenylyl cyclase, an enzyme that catalyzes the conversion of cytoplasmic ATP to cAMP. As a second messenger, cAMP activates protein kinase A, which further induces the phosphorylation of transcription factor, cAMP response element-binding protein, and affects the transcription of downstream genes.
In addition to this canonical cascade, MCRs can also signal through other pathways. For instance, all MCRs have been reported to induce ERK1/2 phosphorylation and intracellular Ca2+ mobilization, although the specific underlying mechanisms are not exactly the same with all five MCRs (94–99). The Gi protein was shown to be associated with MC3-5R activation and Gq was indicated to couple with MC4R (53, 96, 98, 100). In addition, MC1R and MC2R were shown to signal through p38 pathway in human HaCaT keratinocytes upon stimulation of ACTH (101). Phosphorylation of Janus kinase/signal transducer and activator of transcription in Ba/F3 cells and human cultured IM-9 lymphocytes expressing MC5R (78, 102) and c-Jun N-terminal kinase in HEK293 cells overexpressing MC4R were also reported (78, 102). However, some of these non-canonical signaling needs to be independently confirmed by different groups.
ACTH and Inflammation
ACTH Effects in vitro
The fact that ACTH is superior to corticosteroids in treating certain inflammatory diseases has raised a possibility that ACTH might have some therapeutic effects other than stimulating the levels of endogenous cortisol. In normal human keratinocytes, ACTH1−39 was shown to suppress NFκB activation stimulated by tumor necrosis factor-α (TNF-α), likely via enhancing nuclear translocation of the NFκB inhibitor IκBα to the nucleus (103). Further immunofluorescent labeling and western blot studies detected MC1R and MC2R in normal human keratinocytes, which might mediate the peptide-induced suppression on activation of NFκB (103).
In addition, the ability of ACTH to directly modulate local CNS inflammation, a factor in, and perhaps initiates, many CNS diseases, has been reported in several in vitro studies. Using rat brain cultures containing oligodendroglia, astrocytes, and microglia preincubated with cytotoxic agents, ACTH1−39 was shown to protect mature oligodendroglia and oligodendroglia progenitor cell (OPC) from death induced by staurosporine, AMPA, NMDA, kainate, quinolinic acid, or reactive oxygen species (ROS) (104, 105). In their studies, preincubation of the cytotoxic agents caused 50–75% death of mature oligodendroglia but little or no death of astrocytes or microglia (104). Since oligodendroglia and OPC uniquely provide metabolic support to neurons/axons, the protection of oligodendroglia and OPC from several excitotoxic and inflammation-related insults is of great importance in keeping the integrity of CNS (104).
Using rat glial cultures, the same group demonstrated that ACTH1−39 induces proliferation of OPC and accelerates differentiation of platelet-derived growth factor receptor-α (a phenotypic marker for OPC) positive OPC to a later stage characterized by greater expansion of oligodendroglia myelin-like sheets compared to untreated cells (105). Adenylyl cyclase was suggested to be involved in ACTH-mediated regulation of OPC proliferation, differentiation, and anti-inflammatory actions based on the findings that (1) OPC have delayed maturation and accelerated proliferation when adenylyl cyclase is activated in vitro (106, 107), (2) pituitary adenylyl cyclase-activating polypeptide-deficient mice have earlier onset of myelination, less time for axonal development, and synapse formation, as well as less neuronal plasticity (108), and (3) cAMP-inducing agents prevent oligodendroglial excitotoxicity and protect mature oligodendroglia from excitotoxic agents (109).
Furthermore, the same group showed that ACTH also protects rat forebrain neurons, the most vulnerable cells in CNS, from apoptotic, excitotoxic, and inflammation-related damage in a similar manner as mature oligodendroglia and OPC (110). Since excitotoxic damage to neurons is an important cause to several experimental and clinical CNS diseases, it is reasonable to explain the therapeutic benefits of ACTH in several inflammatory animal models of CNS disorders (110). However, the contribution from direct effects on oligodendroglia, OPC, or neurons within the brain and whether such beneficial actions can be observed in vivo in human patients remain to be investigated.
ACTH Effects in vivo
The efficacy of treatment with ACTH in human and animal rheumatoid arthritis and gouty have been demonstrated since 1940s. In a murine model with monosodium urate (MSU) crystal-induced gouty arthritis, Getting et al. demonstrated that ACTH4−10 (MEHFRWG), lacking any glucocorticoid stimulating action, inhibits macrophage activation as evidenced by reduced phagocytosis and keratinocyte-derived chemokine (KC) release, and neutrophil accumulation as evidenced by decreased IL-1β (a pro-inflammatory cytokine) release in the inflammatory exudates (72). Since only Mc3r mRNA is detected in mouse peritoneal macrophages by RT-PCR, they proposed that ACTH4−10 attenuates KC release and possibly synthesis of other cytokines, and subsequent reduction of the host inflammatory response by targeting the MC3R expressed in macrophages (72). Similarly, in a rat model of arthritis, the same group showed that local injection of ACTH1−39 have significant anti-inflammatory effects, with inhibition of 82, 88, and 75% on neutrophil influx, joint swelling, and arthritis score, respectively (7). Comparable degrees of attenuation in the synthesis and/or release of the cytokines IL-1β and IL-6 were also observed (7). Interestingly, the anti-inflammatory actions of ACTH1−39 retain in adrenalectomized rats (7). Together with the observations that MC3R/MC4R antagonist, SHU9119, blocks ACTH anti-inflammatory actions and a selective MC3R agonist, γ2-MSH, retains the anti-inflammatory activity, it is reasonable to propose that partial anti-inflammatory effects of ACTH might be achieved by targeting MC3R expressed in rat knee joint macrophages (7).
Furthermore, ACTH was also reported to reduce fever after peripheral administration (5). Subsequently, Kass et al. demonstrated that intramuscular injection of ACTH significantly reduces leukocytic pyrogen-induced fever in human and rabbits (111). Together with the evidence that intracerebroventricular administration of ACTH1−24 also produces antipyresis (112), it can be hypothesized that the antipyretic effects of ACTH might be achieved through altering the activity of hypothalamic heat-regulating centers. However, further findings that central and peripheral injections of ACTH1−24 reduce fever in adrenalectomized rabbits exclude the necessity of corticosteroid participation in ACTH-induced fever reduction. This is of great importance considering that ACTH-induced corticosteroids readily cross the blood-brain barrier and can reduce fever when administrated in intrahypothalamic manner (113, 114).
Mechanism of Anti-inflammatory Effects of ACTH
It is well-known that ACTH-mediated anti-inflammatory effects can be achieved through glucocorticoid-dependent and -independent manners. Regarding glucocorticoid-dependent action, ACTH is unique among melanocortins since it is the only peptide that activates MC2R. Generally, stimuli such as stress or inflammation trigger the synthesis and release of corticotropin-releasing hormone from the paraventricular nucleus of the hypothalamus, which further stimulates the release of ACTH from the pituitary. ACTH then circulates to the adrenal gland, where it activates MC2R located on the adrenal cortex and induces rapid synthesis of cortisol. Cortisol then activates glucocorticoid receptor and triggers downstream anti-inflammatory actions through genomic and non-genomic pathways, eventually resulting in decreased production of cytokines, chemokines, and inducible NO synthase, increased anti-inflammatory mediators, and phagocytosis of apoptotic neutrophils [reviewed in (6)]. It is also reported that glucocorticoids produced in the skin can also modulate local inflammation, although the underlying mechanism is not clear (115).
It is not until 2002, 50 years after the approval of ACTH for the treatment of many inflammatory conditions, that Getting et al. revealed the retention of anti-inflammatory effects of ACTH in adrenalectomized rats with knee gout, suggesting the existence of hypothalamus-pituitary-adrenal- and glucocorticoid-independent anti-inflammation mechanism (7). Until now, extensive in vitro and in vivo studies, as discussed above, have undoubtedly supported the notion that ACTH can exert its anti-inflammatory effects directly through activation of certain MCRs expressed in peripheral immune cells and hypothalamic neural cells.
Therapeutic Use of ACTH in Inflammatory Diseases
Currently, the practical therapeutic use of ACTH or its analogs are merely based on its ability to induce glucocorticoid synthesis and release. Two commercialized ACTH formulations are available in the market in the United States. One is the repository corticotropin injection (RCI), a highly purified porcine ACTH analog approved by the FDA for the treatment of many autoimmune and inflammatory diseases since 1952. Currently, RCI is considered first-line therapy for infantile spasms. For other autoimmune disorders such as rheumatoid arthritis, multiple sclerosis relapses, symptomatic sarcoidosis, systemic lupus erythematosus, proteinuria in nephrotic syndrome, and dermatomyositis/polymyositis, it is utilized primarily as later-line therapy in patients whose conditions exacerbate, or adjunctive option in patients fail to respond to or cannot tolerate the conventional therapies (116).
The initial idea of utilizing ACTH as a therapeutic drug in the treatment of inflammatory conditions such as rheumatoid arthritis was proposed by Hench et al. (4). However, the emerging of glucocorticoid therapy makes ACTH the second choice or an adjunctive therapy to combat the inflammation associated with rheumatoid arthritis. Indeed, in two independent prospective clinical trials attempting to assess the efficacy and safety of RCI as adjunctive therapy in rheumatoid arthritis patients, Gaylis et al. showed that eight of ten patients received methotrexate plus RCI have improved clinical symptoms as supported by increased Clinical Disease Activity Index scores. Two patients have disease remission and three patients show low disease activity (117). Similarly, Gillis et al. demonstrated that, after subcutaneous injection with RCI over 12 weeks, all six patients with refractory rheumatoid arthritis achieve significantly lower Disease Activity Score, decreased tender and swollen joint counts, and reduced physician global visual analog scale (118). In addition, significant improvement were also observed in terms of Health Assessment Questionnaire score (3/6), erythrocyte sedimentation rate (4/6), and C-reactive protein levels (4/6) (118).
In several clinical studies aiming to compare the efficacy of RCI and other conventional therapies for infantile spasms treatment, Baram et al. evaluated the efficacy of RCI and prednisone in 29 enrolled infants, and they showed that 86.6% of RCI-treated infants and 28.6% of prednisone-treated infants have cessation of spasms and elimination of hypsarrhythmia (119). Similar results were obtained by Knupp and colleagues, who performed a prospective, observational, multicenter study to compare the efficacy of RCI, prednisolone, vigabatrin, and other non-standard therapies in 230 enrolled infants diagnosed with infantile spasms. At 2 weeks of treatment, 68% of RCI-treated infants have evident response, compared with 49% for vigabatrin and 22% for non-standard medications (120). At 3 months of treatment, the response rate of RCI-treated infants is 55%, significantly higher than those treated with prednisolone (39%), vigabatrin (36%), and non-standard medications (9%) (120). Those results convincingly indicated that, RCI therapy is more effective than other standard therapies in the treatment of infantile spasms. Therefore, the American Academy of Neurology and Child Neurology Society recommended, in the 2004 infantile spasms guideline, that ACTH can be considered as short-term treatment of infantile spasms (level B evidence).
Several studies also pointed to the possibility of RCI therapy as an alternative option for the treatment of multiple sclerosis relapse. In a randomized, double-blind, placebo-controlled, multi-site trial consisting of 197 patients, the Disability Status Scale, known as the gold standard of outcome parameter in multiple sclerosis relapse trials, was monitored weekly for 4 weeks. Rose et al. showed that 65% RCI-treated patients have improved Disability Status Scale compared to 48% of those treated with placebo gel. The response rates to RCI therapy are 43 and 30% higher than those to placebo treatment at week 1 and week 3, respectively (121, 122). In a prospective, randomized, open-labeled pilot trial, Simsarian et al. showed that intramuscular and subcutaneous administration of RCI for 5 days causes comparable alleviation in symptoms (123).
In the past several decades, more than two dozen clinical and healthcare utilization studies of RCI in the treatment of autoimmune and inflammatory disorders have been performed, and the retrospective analyses convincedly suggested that initiation of RCI therapy in patients with autoimmune diseases reduces post-therapy healthcare utilization such as hospitalizations, hospital length of stay, outpatient visits, and emergency department visits, therefore improves patient quality of life and mitigates the economic burdens on families, healthcare systems, and society (116).
The second formulation, cosyntropin, a synthetic analog composed of the first 24 amino acids of ACTH (ACTH1−24), is as potent as the full-length peptide in terms of steroidogenic activity. However, cosyntropin used in the ACTH stimulation test is only intended for the diagnose of adrenal insufficiency in the USA. In the UK, ACTH1−24, labeled as Synacthen Depot®, in addition to diagnostic use, is utilized as an adjunctive and short-term option for the glucocorticoid therapy in conditions when patients fail to respond or cannot tolerate glucocorticoid treatment (6).
α-MSH and Inflammation
α-MSH Effects in vitro
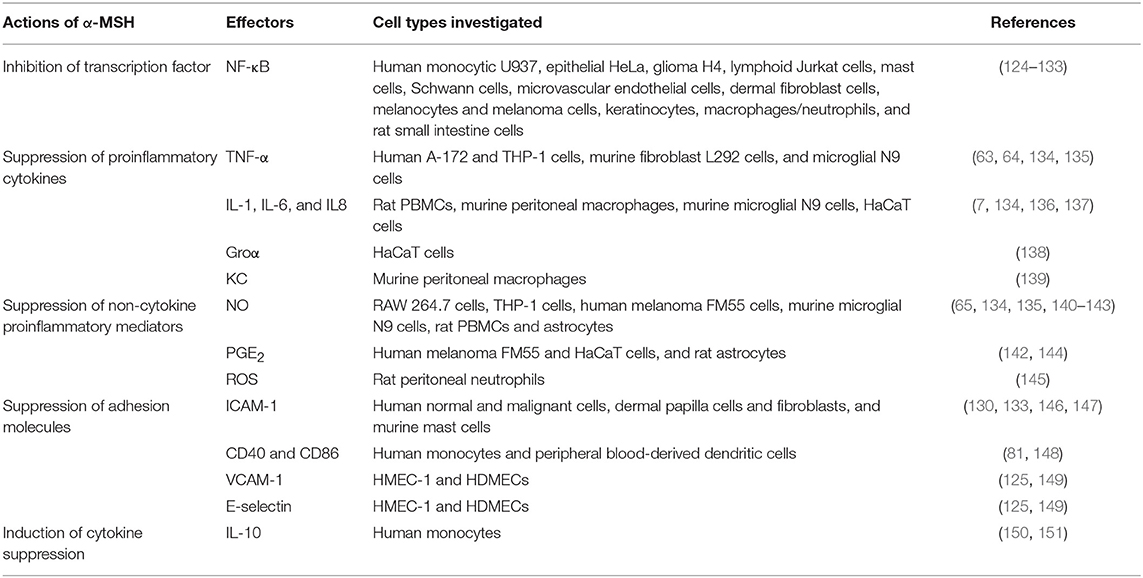
The molecular mechanisms of α-MSH-mediated anti-inflammatory functions have been studied extensively in vitro (summarized in Table 3). α-MSH-mediated inhibition of NF-κB activation, a well-known master regulator of inflammation that controls the expression of many cytokines, cytokine receptors, chemokines, growth factors, and adhesion molecules, was first discovered by Manna and Aggarwal, who demonstrated that α-MSH completely abolishes TNF-α-induced NF-κB phosphorylation in a dose- and time-dependent manner (124). It also blocks LPS-, okadaic acid-, and ceramide-mediated activation of NF-κB in human monocytic, epithelial, glioma, and lymphoid cells (124). Subsequently, similar effects exerted by α-MSH were reported in a variety of cell types including human microvascular endothelial cells (125), human melanocytes and melanoma cells (126), human glioma cells (127, 128), human pulmonary epithelial cells (129), human keratinocytes (103), mast cells (130), Schwann cells (131), human macrophages and neutrophils (132), human dermal fibroblast cells (133), and rat small intestine cells (152). Suppression of NF-κB translocation is achieved through generation of cAMP, activation of protein kinase A, and protection of IκBα from phosphorylation (124). Recently, α-MSH was reported to attenuate TNF-α-induced matrix metalloproteinase-13 expression by regulating activation of p38 and NF-κB in HTB-94 cells (a human chondrosarcoma cell line expressing MC1R), indicating that α-MSH might be used to prevent matrix metalloproteinase-13-mediated collagen degradation (153).
Extensive studies have demonstrated that α-MSH exerts the anti-inflammatory actions by suppressing the expression of proinflammatory cytokines such as TNF-α, interferon-γ (IFN-γ), IL-1, IL-6, IL-8, and KC. Lipton and colleagues showed that α-MSH, presumably by acting through MC1R, inhibits bacterial endotoxin-induced TNF-α production in human glioma cells (63). Similar inhibitory effects on TNF-α production exerted by α-MSH were observed in human monocyte/macrophages (64), melanocyte and melanoma cells (126), and human keratinocytes (103). Taylor et al. demonstrated that constitutive picomolar α-MSH detected in normal aqueous humor of human, rabbits, and mice is able to suppress the production of antigen-stimulated IFN-γ (154, 155). In addition, after preincubation of human peripheral blood mononuclear cells (PBMCs) with mitogen and different concentrations of α-MSH, Luger et al. showed that, in human PBMCs, the mitogen-induced transcription and biological activity of IFN-γ are significantly blocked by α-MSH in a dose-dependent manner, as evidenced by changes in IFN-γ mRNA expression and the major histocompatibility complex class I antigen expression, respectively (156). The production of other proinflammatory cytokines, including IL-1, IL-6, IL-8, Groα, and KC, when co-incubated with or without proinflammatory stimuli, are also inhibited by α-MSH (7, 136–138).
The ability of α-MSH in suppressing TNF-α-, IFN-γ-, or LPS-induced expression of intercellular adhesion molecule-1 (ICAM-1) have been reported in human normal and malignant cells, human dermal papilla cells and fibroblasts, and murine mast cells (130, 133, 146, 147). The inhibition of LPS-induced vascular cell adhesion molecule-1 (VCAM-1) and E-selectin expression were also reported in human microvascular endothelial cells (HMEC-1) and human dermal microvascular endothelial cells (HDMECs) (125, 149). In addition, α-MSH also regulates the expression of CD86 and CD40, cell surface molecules required for antigen presentation in monocytes and dendritic cells. Indeed, α-MSH was shown to, likely by acting at MC1R, down-regulate the surface expression of CD86 in both LPS-treated human monocyte and non-stimulated human peripheral blood-derived dendritic cells (81, 148).
Moreover, α-MSH was also reported to be a suppressor of proinflammatory non-cytokine regulators such as nitric oxide (a well-known common inflammatory mediator), prostaglandin E (PGE), and ROS. The ability of α-MSH in suppressing LPS- and cytokine-stimulated nitric oxide production and inducible nitric oxide synthase expression was first reported in murine macrophages expressing MC1R (65). Since then, similar findings were reported in cytotoxic agent-induced RAW 264.7 cells, THP-1 cells, human melanoma FM55 cells, murine microglial cells, rat PBMCs and astrocytes (134, 135, 140–143, 157, 158). It was demonstrated that α-MSH suppresses cytokine-stimulated PGE production in a cell-specific manner, with inhibitory effects observed in IL-1-induced fetal human lung fibroblasts and TNF-α-stimulated FM55 melanoma cells, but no effects observed in TNF-α-induced HaCaT keratinocytes (144, 159). In addition, α-MSH was recently reported to inhibit LPS- or phorbol ester-induced synthesis of superoxide radicals in rat neutrophils (145) and IL-8-stimulated oxidative burst in human monocytic cell line (132), suggesting an regulatory effect on the cellular redox balance and apoptotic pathways similar as that of a radical scavenger (160).
Other mechanisms that contribute to the anti-inflammatory actions of α-MSH have also been proposed. For instance, α-MSH was reported to induce the mRNA and protein levels of IL-10, a well-known potent cytokine suppressor, in human keratinocytes and monocytes, respectively (150, 161). In addition, the abilities of α-MSH in inducing CD25 and CD4 markers of regulatory T cells, suppressing the production IFN-γ of inflammatory T cells, and inhibiting bacterial antigen-induced proliferation of T lymphocytes were reported by several groups (161–163).
α-MSH Effects in vivo
Similar to ACTH, α-MSH exhibits anti-inflammatory effects in a wide variety of animal models of experimental inflammation disorders. For instance, Glyn and Lipton first showed that α-MSH is able to inhibit temperature increase in rabbits with leukocytic pyrogen-induced fever (112). Since then, the antipyretic properties of α-MSH was confirmed in several animal models (such as leukocytic pyrogen-induced rabbits and guinea pigs, and bacterial endotoxin-treated squirrel monkey) with the peptide administrated centrally, peripherally, or intragastrically (164–170).
Following the therapeutic use of ACTH in patients with rheumatoid arthritis, the anti-inflammatory actions of α-MSH in experimental rheumatoid arthritis have been also well-explored. In 1994, Ceriani et al. showed that α-MSH, injected intraperitoneally twice daily, markedly inhibits the clinical and histological signs of adjuvant-induced arthritis (171). It should be noted that although α-MSH exerts similar anti-inflammatory actions on joint inflammation as prednisolone, the effectiveness of α-MSH and prednisolone on the weight regulation of experimental rats are different, with no weight change observed between α-MSH-treated and untreated animals but a significant weight loss in prednisolone-treated animals compared to the control animals (171).
In addition, α-MSH has also been shown to be a potent inhibitor of systemic inflammation, including sepsis syndrome, septic shock, acute respiratory distress syndrome (ARDS), and several other conditions. Martin and Lipton showed that intravenous injection of α-MSH reduces endotoxin-induced acute phase response in rabbits (172). Subsequently, similar results were reported in endotoxemic mice when the peptide was administrated centrally or peripherally (173). In addition, Catania and colleagues demonstrated that systemic injection of α-MSH or gentamicin increases survival rate in a mouse model of peritonitis/endotoxemia/septic shock (174). Interestingly, it achieves even greater survival rates when α-MSH and gentamicin were given in combination, suggesting the anti-inflammatory effects of α-MSH and gentamicin are independent and additive to each other (174). In endotoxin-induced ARDS rats characterized by massive neutrophil influx into the lung and damage to lung epithelium, systemic administration of α-MSH reduces leukocyte concentration in the bronchoalveolar lavage fluid (175). Similar salutary effects were observed in acute bleomycin-induced lung injury in rats and α-MSH was also shown to regulate stress-, inflammation-, and fluid homeostasis-related genes, which are known to be involved in the development of acute lung injury stress response (175).
Mechanism of Anti-inflammatory Effects of α-MSH
With the aid of numerous cell culture systems, a variety of mechanisms as discussed above, including inhibition of nuclear transcription factor NF-κB activation, suppression of proinflammatory cytokine production and adhesion molecule expression, restraint of proinflammatory non-cytokine regulators, induction of cytokine suppressors, as well as modulation of lymphocyte function and proliferation, have been demonstrated to contribute to α-MSH-mediated anti-inflammatory effects, which are further validated in animal models of experimentally induced fever, rheumatoid arthritis, ARDS, inflammatory skin and bowel diseases, and brain inflammation (160).
Similar to ACTH, it is thought that the anti-inflammatory effects of α-MSH might be mediated through activation of specific MCRs expressed in peripheral immune cells and hypothalamic neurons. For instance, MC3R was shown to be required for ACTH- and α-MSH-mediated anti-inflammatory effects in rheumatoid arthritis (7). The α-MSH-induced inflammation-modulatory signaling pathways in the CNS are thought to be mediated by neural MC4R (176). MC1R and MC5R might be involved in α-MSH-mediated modulation of proinflammatory cytokines and collagens in human articular chondrocytes (68). Furthermore, protective effects of α-MSH in autoimmune uveoretinitis require a functional MC5R (177).
Therapeutic Potential of α-MSH in Inflammatory Diseases
Current studies attempting to explore the therapeutic potential of α-MSH for treatment of inflammation disorders mainly focus on the C-terminal tripeptide of α-MSH, KPV, and its derivative KdPT, which retain the anti-inflammatory effect but abandon its pigmentary action. Indeed, KPV and KdPT, similar to α-MSH, at molecular levels, were also shown to regulate a wide spectrum of signaling pathways involved in inflammation-related processes, including suppression of NF-κB activation, TNF-α synthesis, and induction of IL-10 (160).
Furthermore, in animal models of experimentally induced fever, brain inflammation, skin inflammation, rheumatoid arthritis, and systemic inflammation, KPV or KdPT was reported to reduce leukocytic pyrogen- or IL-1-induced hyperthermia (170, 178), LPS-induced activation of NF-κB (128), picrylic acid-, TNF-α-, IL-1β-, or IL-6-induced ear swelling (179–181), γ-carrageenan-induced hind paw edema (182), MSU crystal- or IL-1β-induced peritonitis and neutrophil accumulation (139), and dextran sodium sulfate-induced colitis with inflammatory cell infiltration and myeloperoxidase activity (183, 184), indicating therapeutic potential of α-MSH for treatment of inflammatory diseases.
It should be noted that intravenously administered full-length α-MSH and its tripeptides can only last in circulation for a few minutes due to the presence of several serum proteases (185). For instance, neutral endopeptidase 24.11, one of the serum proteases expressed on the cell membrane of a variety of cell types, can cleave α-MSH and thereby directly limit its biological activity (186). Although the pharmacokinetics of KPV and KdPT in circulation are still not clear, it may be speculated that D-enantiomers of KPV, compared to their stereochemical analogs, are more resistant to proteolysis of peptidases (160). Therefore, it is of great importance to identify a better route of administration of these peptides depending on the specific inflammatory disease.
In terms of the safety profiles of α-MSH and its tripeptides, very few data are currently available due to lack of toxicity studies. However, the super-potent analog of α-MSH, [Nle4-D-Phe7]-MSH, when administrated intravenously at doses of up to 0.6 mg/kg, results in occasional gastrointestinal upset and facial flushing without major detrimental effects (187, 188). In this scenario, α-MSH and its tripeptides should have better safety profiles compared to traditional immunosuppressive therapies and biologics, known to cause liver and kidney injury, bone marrow suppression, gastrointestinal upset, hypertension, and dyslipidemia.
Interestingly, recent studies demonstrated antimicrobial activity of α-MSH and KPV in eliminating two representative pathogens, Staphylococcus aureus and Candida albicans (189). This is opposite to established immunosuppressive and anti-inflammatory therapies that usually enhance the risk for infection during the prolonged process. These advantages over the conventional immunosuppressive therapies highlight α-MSH and related-tripeptide as attractive candidates for treatment of human inflammatory diseases. However, compared to α-MSH, KPV, and KdPT might confer more advantages to be utilized as clinical therapy for the treatment of immune-mediated inflammatory disorders since they are easier to synthesize due to smaller size and lower cost, theoretically with no pigmentary effect, more resistant to bacterial infection, and more easily to be administrated locally for several inflammatory diseases (160).
Other Melanocortins and Inflammation
Although β- and γ-MSHs share similar affinity for the immunomodulatory MC1R and MC3R as ACTH and α-MSH, the involvement of β- and γ-MSHs in the regulation of inflammation are less understood due to lack of experimental evidences.
The central antipyretic actions of γ2-MSH during the inflammatory response was reported by Bock et al., who showed that intraseptal or intraventricular infusion of γ2-MSH inhibits LPS-induced fever in guinea pig (190). Recently, the anti-inflammatory activity of γ2-MSH were further confirmed by Getting and colleagues, who demonstrated that in an in vivo murine model of peritonitis and in vitro cultured macrophages, γ2-MSH causes a dose-dependent attenuation of polymorphonuclear leukocyte migration (37). This inhibition was shown to be associated with a reduction in KC and IL-1β levels (37). In addition, SHU9119 blocks the ability of γ2-MSH to inhibit neutrophil migration, KC, and IL-1β release, suggesting effects of γ2-MSH are mediated by the MC3R or MC4R or both (37). However, only the mRNA and protein of MC3R, but not MC4R, were confirmed by RT-PCR and western blotting in murine and rat peritoneal macrophages (37). The MC3R is functional since it responds to γ2-MSH stimulation in initiating production of intracellular cAMP and this effect is blocked in the presence of SHU9119 (37). They concluded that γ2-MSH can inhibit the experimental inflammatory response by selectively activating MC3R on peritoneal macrophages.
Similar findings were observed in a recessive yellow mouse strain harboring a frameshift mutation in Mc1r gene that results in non-functional MC1R protein (191). Taking advantage of this mouse model and more selective melanocortin ligands, the same group showed that a functional MC1R is not necessary to elicit the anti-inflammatory actions of melanocortin peptides since (1) the recessive yellow mice does not display any signs of ongoing inflammatory response; (2) wild type and the recessive yellow mice showed no difference in MSU crystal-induced peritonitis as assessed by polymorphonuclear leukocyte migration and release of KC; (3) pre-administration of γ2-MSH reduces MSU crystal-induced polymorphonuclear leukocyte migration equally in wild-type and recessive yellow mice and this is associated with a reduction in IL-1β levels; (4) the selective MC1R agonist MS05 is inactive in inhibiting any of the inflammatory parameters in both wild type and recessive yellow mice (191).
Using male Sprague–Dawley rats, Xia et al. showed that intravenous infusion of γ2-MSH reverses LPS-induced hypotension and tachycardia, attenuates systemic inflammatory responses to endotoxin, prevents LPS-induced IL-1β gene expression in the brain and peripheral tissues, and inhibits LPS-induced increases in plasma nitric oxide levels, indicating a novel approach for modulating systemic inflammation through pharmacological manipulation of γ2-MSH-mediated autonomic activity (192). The specialized anti-inflammatory functions of β- and γ-MSHs within the brain were also reported by Muceniece and colleagues. In an acute mouse neuroinflammation model, four tested melanocortins were shown to reduce LPS-induced increases in brain nitric oxide as measured by electron paramagnetic resonance, with an order of effectiveness: β-MSH ≥ γ1-MSH = γ2-MSH > α-MSH (193).
Conclusions
Recent years have witnessed progressively increased scientific interests in melanocortins and its receptors due to their wide expression and functions. A variety of in vitro, in vivo, and clinical studies have demonstrated that the anti-inflammatory effects of melanocortins are achieved in glucocorticoid-dependent (unique for ACTH) and -independent manners. Recent improvement in understanding the mechanisms by which melanocortins regulate immune responses have yielded renewed interest in RCI as a therapeutic option for several inflammatory diseases, since it may improve disease control, quality of life, and reduce healthcare utilization. In addition, the advantages of α-MSH-related tripeptides over the traditional melanocortins indicate that they could be utilized as novel therapeutics for the treatment of inflammatory disorders.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
WW, D-YG, and Y-JL, Employees of Xiamen Huli Guoyu Clinic, Co., Ltd., Xiamen, China
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACTH, adrenocorticotropic hormone; AgRP, agouti-related peptide; ARDS, acute respiratory distress syndrome; CNS, central nervous system; HDMEC, human dermal microvascular endothelial cell; HMEC-1, human microvascular endothelial cell; ICAM-1, intercellular adhesion molecule-1; IFN-γ, interferon-γ; KC, keratinocyte-derived chemokine; MSH, melanocyte-stimulating hormone; OPC, oligodendroglia progenitor cell; PBMC, peripheral blood mononuclear cell; PC, prohormone convertase; PGE, prostaglandin E; POMC, pro-opiomelanocortin; MCR, melanocortin receptor; MSU, monosodium urate; RCI, repository corticotropin injection; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α.
References
1. Eberle AN. The Melanotropins. Chemistry, Physiology and Mechanism of Action. Basel: Karger (1988).
2. Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. (2006) 27:736–49. doi: 10.1210/er.2006-0034
3. Tao YX. Melanocortin receptors. Biochim Biophys Acta. (2017) 1863:2411–3. doi: 10.1016/j.bbadis.2017.08.001
4. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocortical hormone in arthritis: preliminary report. Ann Rheum Dis. (1949) 8:97–104. doi: 10.1136/ard.8.2.97
5. Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch Intern Med. (1950) 85:545–666. doi: 10.1001/archinte.1950.00230100002001
6. Montero-Melendez T. ACTH: The forgotten therapy. Semin Immunol. (2015) 27:216–26. doi: 10.1016/j.smim.2015.02.003
7. Getting SJ, Christian HC, Flower RJ, Perretti M. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum. (2002) 46:2765–75. doi: 10.1002/art.10526
8. Mains RE, Eipper BA, Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci USA. (1977) 74:3014–8. doi: 10.1073/pnas.74.7.3014
9. Roberts JL, Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci USA. (1977) 74:4826–30. doi: 10.1073/pnas.74.11.4826
10. Bertagna XY, Nicholson WE, Sorenson GD, Pettengill OS, Mount CD, Orth DN. Corticotropin, lipotropin, and beta-endorphin production by a human nonpituitary tumor in culture: evidence for a common precursor. Proc Natl Acad Sci USA. (1978) 75:5160–4. doi: 10.1073/pnas.75.10.5160
11. Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. (1979) 278:423–7. doi: 10.1038/278423a0
12. Douglass J, Civelli O, Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. (1984) 53:665–715. doi: 10.1146/annurev.bi.53.070184.003313
13. Soma GI, Kitahara N, Nishizawa T, Nanami H, Kotake C, Okazaki H, et al. Nucleotide sequence of a cloned cDNA for proopiomelanocortin precursor of chum salmon, Onchorynchus keta. Nucleic Acids Res. (1984) 12:8029–41. doi: 10.1093/nar/12.21.8029
14. Martens GJ, Civelli O, Herbert E. Nucleotide sequence of cloned cDNA for pro-opiomelanocortin in the amphibian Xenopus laevis. J Biol Chem. (1985) 260:13685–9.
15. Hilario E, Lihrmann I, Vaudry H. Characterization of the cDNA encoding proopiomelanocortin in the frog Rana ridibunda. Biochem Biophys Res Commun. (1990) 173:653–9. doi: 10.1016/S0006-291X(05)80085-3
16. Tollemer H, Vallarino M, Tonon MC, Vaudry H. Ontogeny of a novel decapeptide derived from POMC-A in the brain and pituitary of the rainbow trout. Brain Res Dev Brain Res. (2003) 143:83–97. doi: 10.1016/S0165-3806(03)00104-4
17. Heinig JA, Keeley FW, Robson P, Sower SA, Youson JH. The appearance of proopiomelanocortin early in vertebrate evolution: cloning and sequencing of POMC from a Lamprey pituitary cDNA library. Gen Comp Endocrinol. (1995) 99:137–44. doi: 10.1006/gcen.1995.1094
18. Plantinga LC, Verhaagen J, Edwards PM, Schrama LH, Burbach JP, Gispen WH. Expression of the pro-opiomelanocortin gene in dorsal root ganglia, spinal cord and sciatic nerve after sciatic nerve crush in the rat. Brain Res Mol Brain Res. (1992) 16:135–42. doi: 10.1016/0169-328X(92)90203-N
19. Van Der Kraan M, Tatro JB, Entwistle ML, Brakkee JH, Burbach JP, Adan RA, et al. Expression of melanocortin receptors and pro-opiomelanocortin in the rat spinal cord in relation to neurotrophic effects of melanocortins. Brain Res Mol Brain Res. (1999) 63:276–86. doi: 10.1016/S0169-328X(98)00291-5
20. Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. (2001) 25 (Suppl. 5):S63–7. doi: 10.1038/sj.ijo.0801913
21. Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. (2002) 172:411–21. doi: 10.1677/joe.0.1720411
22. Blalock JE. Proopiomelanocortin-derived peptides in the immune system. Clin Endocrinol. (1985) 22:823–7. doi: 10.1111/j.1365-2265.1985.tb00173.x
23. Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, et al. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. (1996) 1313:130–8. doi: 10.1016/0167-4889(96)00063-8
24. Blalock JE. Proopiomelanocortin and the immune-neuroendocrine connection. Ann N Y Acad Sci. (1999) 885:161–72. doi: 10.1111/j.1749-6632.1999.tb08673.x
25. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. (2000) 21:457–87. doi: 10.1210/er.21.5.457
26. Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. (2000) 80:979–1020. doi: 10.1152/physrev.2000.80.3.979
27. Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. (2004) 56:1–29. doi: 10.1124/pr.56.1.1
28. Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA. (1991) 88:3564–8. doi: 10.1073/pnas.88.9.3564
29. Day R, Schafer MK, Watson SJ, Chretien M, Seidah NG. Distribution and regulation of the prohormone convertases PC1 and PC2 in the rat pituitary. Mol Endocrinol. (1992) 6:485–97. doi: 10.1210/me.6.3.485
30. Marcinkiewicz M, Day R, Seidah NG, Chretien M. Ontogeny of the prohormone convertases PC1 and PC2 in the mouse hypophysis and their colocalization with corticotropin and alpha-melanotropin. Proc Natl Acad Sci USA. (1993) 90:4922–6. doi: 10.1073/pnas.90.11.4922
31. Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. (2003) 284:E468–74. doi: 10.1152/ajpendo.00434.2002
32. Irani BG, Haskell-Luevano C. Feeding effects of melanocortin ligands–a historical perspective. Peptides. (2005) 26:1788–99. doi: 10.1016/j.peptides.2004.11.038
33. Kang L, Mcintyre KW, Gillooly KM, Yang Y, Haycock J, Roberts S, et al. A selective small molecule agonist of the melanocortin-1 receptor inhibits lipopolysaccharide-induced cytokine accumulation and leukocyte infiltration in mice. J Leukoc Biol. (2006) 80:897–904. doi: 10.1189/jlb.1204748
34. Leoni G, Voisin MB, Carlson K, Getting S, Nourshargh S, Perretti M. The melanocortin MC(1) receptor agonist BMS-470539 inhibits leucocyte trafficking in the inflamed vasculature. Br J Pharmacol. (2010) 160:171–80. doi: 10.1111/j.1476-5381.2010.00688.x
35. Montero-Melendez T, Gobbetti T, Cooray SN, Jonassen TE, Perretti M. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J Immunol. (2015) 194:3381–8. doi: 10.4049/jimmunol.1402645
36. Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. (1994) 371:799–802. doi: 10.1038/371799a0
37. Getting SJ, Allcock GH, Flower R, Perretti M. Natural and synthetic agonists of the melanocortin receptor type 3 possess anti-inflammatory properties. J Leukoc Biol. (2001) 69:98–104. doi: 10.1189/jlb.69.1.98
38. Getting SJ, Riffo-Vasquez Y, Pitchford S, Kaneva M, Grieco P, Page CP, et al. A role for MC3R in modulating lung inflammation. Pulm Pharmacol Ther. (2008) 21:866–73. doi: 10.1016/j.pupt.2008.09.004
39. Leoni G, Patel HB, Sampaio AL, Gavins FN, Murray JF, Grieco P, et al. Inflamed phenotype of the mesenteric microcirculation of melanocortin type 3 receptor-null mice after ischemia-reperfusion. FASEB J. (2008) 22:4228–38. doi: 10.1096/fj.08-113886
40. Getting SJ, Lam CW, Chen AS, Grieco P, Perretti M. Melanocortin 3 receptors control crystal-induced inflammation. FASEB J. (2006) 20:2234–41. doi: 10.1096/fj.06-6339com
41. Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R). Peptides. (2006) 27:259–64. doi: 10.1016/j.peptides.2005.01.025
42. Patel HB, Bombardieri M, Sampaio AL, D'acquisto F, Gray M, Grieco P, et al. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J. (2010) 24:4835–43. doi: 10.1096/fj.10-167759
43. Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M. The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol. (2011) 179:259–69. doi: 10.1016/j.ajpath.2011.03.042
44. King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H. Melanocortin receptors, melanotropic peptides and penile erection. Curr Top Med Chem. (2007) 7:1098–106. doi: 10.2174/1568026610707011111
45. Martin WJ, Mcgowan E, Cashen DE, Gantert LT, Drisko JE, Hom GJ, et al. Activation of melanocortin MC(4) receptors increases erectile activity in rats ex copula. Eur J Pharmacol. (2002) 454:71–9. doi: 10.1016/S0014-2999(02)02479-2
46. Muceniece R, Zvejniece L, Vilskersts R, Liepinsh E, Baumane L, Kalvinsh I, et al. Functional evaluation of THIQ, a melanocortin 4 receptor agonist, in models of food intake and inflammation. Basic Clin Pharmacol Toxicol. (2007) 101:416–20. doi: 10.1111/j.1742-7843.2007.00133.x
47. Benoit SC, Schwartz MW, Lachey JL, Hagan MM, Rushing PA, Blake KA, et al. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. J Neurosci. (2000) 20:3442–8. doi: 10.1523/JNEUROSCI.20-09-03442.2000
48. Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. (2000) 56:641–6. doi: 10.1016/S0090-4295(00)00680-4
49. Molinoff PB, Shadiack AM, Earle D, Diamond LE, Quon CY. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Ann N Y Acad Sci. (2003) 994:96–102. doi: 10.1111/j.1749-6632.2003.tb03167.x
50. Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, et al. Identification of 2-[2-[2-(5-bromo-2- methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. (2004) 47:1602–4. doi: 10.1021/jm034244g
51. Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG. Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther. (2006) 317:771–7. doi: 10.1124/jpet.105.097725
52. Trotta MC, Maisto R, Alessio N, Hermenean A, D'amico M, Di Filippo C. The melanocortin MC5R as a new target for treatment of high glucose-induced hypertrophy of the cardiac H9c2 cells. Front Physiol. (2018) 9:1475. doi: 10.3389/fphys.2018.01475
53. Buch TR, Heling D, Damm E, Gudermann T, Breit A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. J Biol Chem. (2009) 284:26411–20. doi: 10.1074/jbc.M109.039339
54. Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. (2015) 520:94–8. doi: 10.1038/nature14051
55. Yang F, Huang H, Tao YX. Biased signaling in naturally occurring mutations in human melanocortin-3 receptor gene. Int J Biol Sci. (2015) 11:423–33. doi: 10.7150/ijbs.11032
56. Yang Z, Tao YX. Biased signaling initiated by agouti-related peptide through human melanocortin-3 and−4 receptors. Biochim Biophys Acta. (2016) 1862:1485–94. doi: 10.1016/j.bbadis.2016.05.008
57. Mo XL, Tao YX. Activation of MAPK by inverse agonists in six naturally occurring constitutively active mutant human melanocortin-4 receptors. Biochim Biophys Acta. (2013) 1832:1939–48. doi: 10.1016/j.bbadis.2013.06.006
58. Tao YX. Constitutive activity in melanocortin-4 receptor: biased signaling of inverse agonists. Ad Pharmacol. (2014) 70:135–54. doi: 10.1016/B978-0-12-417197-8.00005-5
59. Yang LK, Tao YX. Biased signaling at neural melanocortin receptors in regulation of energy homeostasis. Biochim Biophys Acta. (2017) 1863:2486–95. doi: 10.1016/j.bbadis.2017.04.010
60. Chhajlani V, Wikberg JE. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. (1992) 309:417–20. doi: 10.1016/0014-5793(92)80820-7
61. Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. (1992) 257:1248–51. doi: 10.1126/science.1325670
62. Xia Y, Wikberg JE, Chhajlani V. Expression of melanocortin 1 receptor in periaqueductal gray matter. Neuroreport. (1995) 6:2193–6. doi: 10.1097/00001756-199511000-00022
63. Wong KY, Rajora N, Boccoli G, Catania A, Lipton JM. A potential mechanism of local anti-inflammatory action of alpha-melanocyte-stimulating hormone within the brain: modulation of tumor necrosis factor-alpha production by human astrocytic cells. Neuroimmunomodulation. (1997) 4:37–41. doi: 10.1159/000097313
64. Taherzadeh S, Sharma S, Chhajlani V, Gantz I, Rajora N, Demitri MT, et al. alpha-MSH and its receptors in regulation of tumor necrosis factor-alpha production by human monocyte/macrophages. Am J Physiol. (1999) 276:R1289–94. doi: 10.1152/ajpregu.1999.276.5.R1289
65. Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci USA. (1995) 92:8016–20. doi: 10.1073/pnas.92.17.8016
66. Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. (1996) 17:675–9. doi: 10.1016/0196-9781(96)00037-X
67. Neumann Andersen G, Nagaeva O, Mandrika I, Petrovska R, Muceniece R, Mincheva-Nilsson L, et al. MC(1) receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin Exp Immunol. (2001) 126:441–6. doi: 10.1046/j.1365-2249.2001.01604.x
68. Grassel S, Opolka A, Anders S, Straub RH, Grifka J, Luger TA, et al. The melanocortin system in articular chondrocytes: melanocortin receptors, pro-opiomelanocortin, precursor proteases, and a regulatory effect of alpha-melanocyte-stimulating hormone on proinflammatory cytokines and extracellular matrix components. Arthritis Rheum. (2009) 60:3017–27. doi: 10.1002/art.24846
69. Bohm M, Grassel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocr Rev. (2012) 33:623–51. doi: 10.1210/er.2011-1016
70. Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, et al. Molecular cloning of a novel melanocortin receptor. J Biol Chem. (1993) 268:8246–50.
71. Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. (1993) 90:8856–60. doi: 10.1073/pnas.90.19.8856
72. Getting SJ, Gibbs L, Clark AJ, Flower RJ, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. (1999) 162:7446–53.
73. Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. (1993) 268:15174–9.
74. Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. (1994) 8:1298–308. doi: 10.1210/mend.8.10.7854347
75. Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. (2003) 457:213–35. doi: 10.1002/cne.10454
76. Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. (1994) 200:1214–20. doi: 10.1006/bbrc.1994.1580
77. Labbe O, Desarnaud F, Eggerickx D, Vassart G, Parmentier M. Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry. (1994) 33:4543–9. doi: 10.1021/bi00181a015
78. Buggy JJ. Binding of alpha-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem J. (1998) 331 (Pt 1):211–6. doi: 10.1042/bj3310211
79. Clarke BL. Binding and processing of (125)I-ACTH by isolated rat splenic lymphocytes. Biochem Biophys Res Commun. (1999) 266:542–6. doi: 10.1006/bbrc.1999.1848
80. Taylor A, Namba K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH). Immunol Cell Biol. (2001) 79:358–67. doi: 10.1046/j.1440-1711.2001.01022.x
81. Bhardwaj R, Becher E, Mahnke K, Hartmeyer M, Schwarz T, Scholzen T, et al. Evidence for the differential expression of the functional alpha-melanocyte-stimulating hormone receptor MC-1 on human monocytes. J Immunol. (1997) 158:3378–84.
82. Schaible EV, Steinstrasser A, Jahn-Eimermacher A, Luh C, Sebastiani A, Kornes F, et al. Single administration of tripeptide alpha-MSH(11-13) attenuates brain damage by reduced inflammation and apoptosis after experimental traumatic brain injury in mice. PLoS ONE. (2013) 8:e71056. doi: 10.1371/journal.pone.0071056
83. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. (2005) 8:571–8. doi: 10.1038/nn1455
84. Tao YX. Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol. (2005) 239:1–14. doi: 10.1016/j.mce.2005.04.012
85. Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. (2010) 31:506–43. doi: 10.1210/er.2009-0037
86. Getting SJ, Perretti M. MC3-R as a novel target for antiinflammatory therapy. Drug News Perspect. (2000) 13:19–27.
87. Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Sci World J. (2010) 10:1840–53. doi: 10.1100/tsw.2010.173
88. Giuliani D, Mioni C, Altavilla D, Leone S, Bazzani C, Minutoli L, et al. Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology. (2006) 147:1126–35. doi: 10.1210/en.2005-0692
89. Giuliani D, Zaffe D, Ottani A, Spaccapelo L, Galantucci M, Minutoli L, et al. Treatment of cerebral ischemia with melanocortins acting at MC4 receptors induces marked neurogenesis and long-lasting functional recovery. Acta Neuropathol. (2011) 122:443–53. doi: 10.1007/s00401-011-0873-4
90. Spaccapelo L, Bitto A, Galantucci M, Ottani A, Irrera N, Minutoli L, et al. Melanocortin MC(4) receptor agonists counteract late inflammatory and apoptotic responses and improve neuronal functionality after cerebral ischemia. Eur J Pharmacol. (2011) 670:479–86. doi: 10.1016/j.ejphar.2011.09.015
91. Fathi Z, Iben LG, Parker EM. Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype. Neurochem Res. (1995) 20:107–13. doi: 10.1007/BF00995160
92. Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int. (1996) 38:73–80.
93. Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. (1997) 91:789–98. doi: 10.1016/S0092-8674(00)80467-5
94. Le T, Schimmer BP. The regulation of MAPKs in Y1 mouse adrenocortical tumor cells. Endocrinology. (2001) 142:4282–7. doi: 10.1210/endo.142.10.8441
95. Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. (2006) 27:2846–57. doi: 10.1016/j.peptides.2006.05.005
96. Chai B, Li JY, Zhang W, Ammori JB, Mulholland MW. Melanocortin-3 receptor activates MAP kinase via PI3 kinase. Regul Pept. (2007) 139:115–21. doi: 10.1016/j.regpep.2006.11.003
97. Janes ME, Chu KM, Clark AJ, King PJ. Mechanisms of adrenocorticotropin-induced activation of extracellularly regulated kinase 1/2 mitogen-activated protein kinase in the human H295R adrenal cell line. Endocrinology. (2008) 149:1898–905. doi: 10.1210/en.2007-0949
98. Rodrigues AR, Pignatelli D, Almeida H, Gouveia AM. Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism. Mol Cell Endocrinol. (2009) 303:74–81. doi: 10.1016/j.mce.2009.01.014
99. Herraiz C, Journe F, Abdel-Malek Z, Ghanem G, Jimenez-Cervantes C, Garcia-Borron JC. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol Endocrinol. (2011) 25:138–56. doi: 10.1210/me.2010-0217
100. Newman EA, Chai BX, Zhang W, Li JY, Ammori JB, Mulholland MW. Activation of the melanocortin-4 receptor mobilizes intracellular free calcium in immortalized hypothalamic neurons. J Surg Res. (2006) 132:201–7. doi: 10.1016/j.jss.2006.02.003
101. Park HJ, Kim HJ, Lee JY, Cho BK, Gallo RL, Cho DH. Adrenocorticotropin hormone stimulates interleukin-18 expression in human HaCaT keratinocytes. J Invest Dermatol. (2007) 127:1210–6. doi: 10.1038/sj.jid.5700703
102. Chai B, Li JY, Zhang W, Wang H, Mulholland MW. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides. (2009) 30:1098–104. doi: 10.1016/j.peptides.2009.03.006
103. Moustafa M, Szabo M, Ghanem GE, Morandini R, Kemp EH, Macneil S, et al. Inhibition of tumor necrosis factor-alpha stimulated NFkappaB/p65 in human keratinocytes by alpha-melanocyte stimulating hormone and adrenocorticotropic hormone peptides. J Invest Dermatol. (2002) 119:1244–53. doi: 10.1046/j.1523-1747.2002.19602.x
104. Benjamins JA, Nedelkoska L, Bealmear B, Lisak RP. ACTH protects mature oligodendroglia from excitotoxic and inflammation-related damage in vitro. Glia. (2013) 61:1206–17. doi: 10.1002/glia.22504
105. Benjamins JA, Nedelkoska L, Lisak RP. Adrenocorticotropin hormone 1-39 promotes proliferation and differentiation of oligodendroglial progenitor cells and protects from excitotoxic and inflammation-related damage. J Neurosci Res. (2014) 92:1243–51. doi: 10.1002/jnr.23416
106. Lee M, Lelievre V, Zhao P, Torres M, Rodriguez W, Byun JY, et al. Pituitary adenylyl cyclase-activating polypeptide stimulates DNA synthesis but delays maturation of oligodendrocyte progenitors. J Neurosci. (2001) 21:3849–59. doi: 10.1523/JNEUROSCI.21-11-03849.2001
107. Lelievre V, Ghiani CA, Seksenyan A, Gressens P, De Vellis J, Waschek JA. Growth factor-dependent actions of PACAP on oligodendrocyte progenitor proliferation. Regul Pept. (2006) 137:58–66. doi: 10.1016/j.regpep.2006.04.024
108. Vincze A, Reglodi D, Helyes Z, Hashimoto H, Shintani N, Abraham H. Role of endogenous pituitary adenylate cyclase activating polypeptide (PACAP) in myelination of the rodent brain: lessons from PACAP-deficient mice. Int J Dev Neurosci. (2011) 29:923–35. doi: 10.1016/j.ijdevneu.2011.06.008
109. Yoshioka A, Shimizu Y, Hirose G, Kitasato H, Pleasure D. Cyclic AMP-elevating agents prevent oligodendroglial excitotoxicity. J Neurochem. (1998) 70:2416–23. doi: 10.1046/j.1471-4159.1998.70062416.x
110. Lisak RP, Nedelkoska L, Bealmear B, Benjamins JA. Melanocortin receptor agonist ACTH 1-39 protects rat forebrain neurons from apoptotic, excitotoxic and inflammation-related damage. Exp Neurol. (2015) 273:161–7. doi: 10.1016/j.expneurol.2015.08.012
111. Kass EH, Finland M. Effect of ACTH on induced fever. N Engl J Med. (1950) 243:693–5. doi: 10.1056/NEJM195011022431803
112. Glyn JR, Lipton JM. Hypothermic and antipyretic effects of centrally administered ACTH (1–24) and alpha-melanotropin. Peptides. (1981) 2:177–87. doi: 10.1016/S0196-9781(81)80032-0
113. Christy NP, Fishman RA. Studies of the blood-cerebrospinal fluid barrier to cortisol in the dog. J Clin Invest. (1961) 40:1997–2006. doi: 10.1172/JCI104426
114. Chowers I, Conforti N, Feldman S. Local effect of cortisol in the preoptic area on temperature regulation. Am J Physiol. (1968) 214:538–42. doi: 10.1152/ajplegacy.1968.214.3.538
115. Jozic I, Stojadinovic O, Kirsner RS, Tomic-Canic M. Stressing the steroids in skin: paradox or fine-tuning? J Invest Dermatol. (2014) 134:2869–72. doi: 10.1038/jid.2014.363
116. Philbin M, Niewoehner J, Wan GJ. Clinical and economic evaluation of repository corticotropin injection: a narrative literature review of treatment efficacy and healthcare resource utilization for seven key indications. Adv Ther. (2017) 34:1775–90. doi: 10.1007/s12325-017-0569-9
117. Gaylis N, Needell S, Sagliani J. The effect of adrenocorticotropin gel (HP Acthar Gel) in combination with MTX in newly diagnosed RA patients from a clinical and structural perspective. Ann Rheum Dis. (2015) 74(Suppl 2):1066–7.
118. Gillis T, Crane M, Hinkle C, Wei N. Repository corticotropin injection as adjunctive therapy in patients with rheumatoid arthritis who have failed previous therapies with at least three different modes of action. Open Access Rheumatol. (2017) 9:131–8. doi: 10.2147/OARRR.S131046
119. Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. (1996) 97:375–9.
120. Knupp KG, Coryell J, Nickels KC, Ryan N, Leister E, Loddenkemper T, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. (2016) 79:475–84. doi: 10.1002/ana.24594
121. Rose AS, Kuzma JW, Kurtzke JF, Sibley WA, Tourtellotte WW. Cooperative study in the evaluation of therapy in multiple sclerosis; ACTH vs placebo in acute exacerbations. Prelim Rep Neurol. (1968). 18 (Suppl.):1–10. doi: 10.1212/WNL.18.6_Part_2.1
122. Rose AS, Kuzma JW, Kurtzke JF, Namerow NS, Sibley WA, Tourtellotte WW. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs placebo–final report. Neurology. (1970) 20:1–59. doi: 10.1212/WNL.20.5_Part_2.1
123. Simsarian JP, Saunders C, Smith DM. Five-day regimen of intramuscular or subcutaneous self-administered adrenocorticotropic hormone gel for acute exacerbations of multiple sclerosis: a prospective, randomized, open-label pilot trial. Drug Des Devel Ther. (2011) 5:381–9. doi: 10.2147/DDDT.S19331
124. Manna SK, Aggarwal BB. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol. (1998) 161:2873–80.
125. Kalden DH, Scholzen T, Brzoska T, Luger TA. Mechanisms of the antiinflammatory effects of alpha-MSH. Role of transcription factor NF-kappa B and adhesion molecule expression. Ann N Y Acad Sci. (1999) 885:254–61. doi: 10.1111/j.1749-6632.1999.tb08682.x
126. Haycock JW, Wagner M, Morandini R, Ghanem G, Rennie IG, Mac Neil S. Alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation in human melanocytes and melanoma cells. J Invest Dermatol. (1999) 113:560–6. doi: 10.1046/j.1523-1747.1999.00739.x
127. Ichiyama T, Campbell IL, Furukawa S, Catania A, Lipton JM. Autocrine alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation in human glioma. J Neurosci Res. (1999) 58:684–9.
128. Ichiyama T, Zhao H, Catania A, Furukawa S, Lipton JM. alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation and IkappaBalpha degradation in human glioma cells and in experimental brain inflammation. Exp Neurol. (1999) 157:359–65. doi: 10.1006/exnr.1999.7064
129. Ichiyama T, Okada K, Campbell IL, Furukawa S, Lipton JM. NF-kappaB activation is inhibited in human pulmonary epithelial cells transfected with alpha-melanocyte-stimulating hormone vector. Peptides. (2000) 21:1473–7. doi: 10.1016/S0196-9781(00)00300-4
130. Sarkar A, Sreenivasan Y, Manna SK. alpha-Melanocyte-stimulating hormone induces cell death in mast cells: involvement of NF-kappaB. FEBS Lett. (2003) 549:87–93. doi: 10.1016/S0014-5793(03)00797-X
131. Teare KA, Pearson RG, Shakesheff KM, Haycock JW. Alpha-MSH inhibits inflammatory signalling in Schwann cells. Neuroreport. (2004) 15:493–8. doi: 10.1097/00001756-200403010-00022
132. Manna SK, Sarkar A, Sreenivasan Y. Alpha-melanocyte-stimulating hormone down-regulates CXC receptors through activation of neutrophil elastase. Eur J Immunol. (2006) 36:754–69. doi: 10.1002/eji.200535209
133. Hill RP, Macneil S, Haycock JW. Melanocyte stimulating hormone peptides inhibit TNF-alpha signaling in human dermal fibroblast cells. Peptides. (2006) 27:421–30. doi: 10.1016/j.peptides.2005.03.061
134. Delgado R, Carlin A, Airaghi L, Demitri MT, Meda L, Galimberti D, et al. Melanocortin peptides inhibit production of proinflammatory cytokines and nitric oxide by activated microglia. J Leukoc Biol. (1998) 63:740–5. doi: 10.1002/jlb.63.6.740
135. Galimberti D, Baron P, Meda L, Prat E, Scarpini E, Delgado R, et al. Alpha-MSH peptides inhibit production of nitric oxide and tumor necrosis factor-alpha by microglial cells activated with beta-amyloid and interferon gamma. Biochem Biophys Res Commun. (1999) 263:251–6. doi: 10.1006/bbrc.1999.1276
136. Bohm M, Schulte U, Kalden H, Luger TA. Alpha-melanocyte-stimulating hormone modulates activation of NF-kappa B and AP-1 and secretion of interleukin-8 in human dermal fibroblasts. Ann N Y Acad Sci. (1999) 885:277–86. doi: 10.1111/j.1749-6632.1999.tb08685.x
137. Bohm M, Schiller M, Stander S, Seltmann H, Li Z, Brzoska T, et al. Evidence for expression of melanocortin-1 receptor in human sebocytes in vitro and in situ. J Invest Dermatol. (2002) 118:533–9. doi: 10.1046/j.0022-202x.2001.01704.x
138. Brzoska T, Kalden DH, Scholzen T, Luger TA. Molecular basis of the alpha-MSH/IL-1 antagonism. Ann N Y Acad Sci. (1999) 885:230–8. doi: 10.1111/j.1749-6632.1999.tb08680.x
139. Getting SJ, Schioth HB, Perretti M. Dissection of the anti-inflammatory effect of the core and C-terminal (KPV) alpha-melanocyte-stimulating hormone peptides. J Pharmacol Exp Ther. (2003) 306:631–7. doi: 10.1124/jpet.103.051623
140. Rajora N, Ceriani G, Catania A, Star RA, Murphy MT, Lipton JM. alpha-MSH production, receptors, and influence on neopterin in a human monocyte/macrophage cell line. J Leukoc Biol. (1996) 59:248–53. doi: 10.1002/jlb.59.2.248
141. Tsatmali M, Graham A, Szatkowski D, Ancans J, Manning P, Mcneil CJ, et al. alpha-melanocyte-stimulating hormone modulates nitric oxide production in melanocytes. J Invest Dermatol. (2000) 114:520–6. doi: 10.1046/j.1523-1747.2000.00879.x
142. Caruso C, Durand D, Schioth HB, Rey R, Seilicovich A, Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology. (2007) 148:4918–26. doi: 10.1210/en.2007-0366
143. Jung EJ, Han DJ, Chang SH, Lim DG, Wee YM, Kim JH, et al. Protective effect of alpha-melanocyte-stimulating hormone on pancreas islet cell against peripheral blood mononuclear cell-mediated cytotoxicity in vitro. Transplant Proc. (2007) 39:1604–6. doi: 10.1016/j.transproceed.2006.11.011
144. Nicolaou A, Estdale SE, Tsatmali M, Herrero DP, Thody AJ. Prostaglandin production by melanocytic cells and the effect of alpha-melanocyte stimulating hormone. FEBS Lett. (2004) 570:223–6. doi: 10.1016/j.febslet.2004.06.041
145. Oktar BK, Yuksel M, Alican I. The role of cyclooxygenase inhibition in the effect of alpha-melanocyte-stimulating hormone on reactive oxygen species production by rat peritoneal neutrophils. Prostaglandins Leukot Essent Fatty Acids. (2004) 71:1–5. doi: 10.1016/j.plefa.2003.11.009
146. Morandini R, Boeynaems JM, Hedley SJ, Macneil S, Ghanem G. Modulation of ICAM-1 expression by alpha-MSH in human melanoma cells and melanocytes. J Cell Physiol. (1998) 175:276–282.
147. Bohm M, Eickelmann M, Li Z, Schneider SW, Oji V, Diederichs S, et al. Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of alpha-melanocyte-stimulating hormone in human dermal papilla cells. Endocrinology. (2005) 146:4635–46. doi: 10.1210/en.2005-0665
148. Becher E, Mahnke K, Brzoska T, Kalden DH, Grabbe S, Luger TA. Human peripheral blood-derived dendritic cells express functional melanocortin receptor MC-1R. Ann N Y Acad Sci. (1999) 885:188–95. doi: 10.1111/j.1749-6632.1999.tb08676.x
149. Scholzen TE, Sunderkotter C, Kalden DH, Brzoska T, Fastrich M, Fisbeck T, et al. Alpha-melanocyte stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression. Endocrinology. (2003) 144:360–70. doi: 10.1210/en.2002-220651
150. Redondo P, Garcia-Foncillas J, Okroujnov I, Bandres E. Alpha-MSH regulates interleukin-10 expression by human keratinocytes. Arch Dermatol Res. (1998) 290:425–8. doi: 10.1007/s004030050330
151. Bhardwaj RS, Schwarz A, Becher E, Mahnke K, Aragane Y, Schwarz T, et al. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J Immunol. (1996) 156:2517–21.
152. Zou L, Sato N, Kone BC. Alpha-melanocyte stimulating hormone protects against H2O2-induced inhibition of wound restitution in IEC-6 cells via a Syk kinase- and NF-kappabeta-dependent mechanism. Shock. (2004) 22:453–9. doi: 10.1097/01.shk.0000142255.15759.de
153. Yoon SW, Chun JS, Sung MH, Kim JY, Poo H. alpha-MSH inhibits TNF-alpha-induced matrix metalloproteinase-13 expression by modulating p38 kinase and nuclear factor kappaB signaling in human chondrosarcoma HTB-94 cells. Osteoarthritis Cartilage. (2008) 16:115–24. doi: 10.1016/j.joca.2007.05.026
154. Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. (1992) 11:1199–206. doi: 10.3109/02713689208999545
155. Taylor AW, Streilein JW, Cousins SW. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation. (1994) 1:188–94. doi: 10.1159/000097167
156. Luger TA, Schauer E, Trautinger F, Krutmann J, Ansel J, Schwarz A, et al. Production of immunosuppressing melanotropins by human keratinocytes. Ann N Y Acad Sci. (1993) 680:567–70. doi: 10.1111/j.1749-6632.1993.tb19741.x
157. Chiao H, Kohda Y, Mcleroy P, Craig L, Linas S, Star RA. Alpha-melanocyte-stimulating hormone inhibits renal injury in the absence of neutrophils. Kidney Int. (1998) 54:765–74. doi: 10.1046/j.1523-1755.1998.00075.x
158. Mandrika I, Muceniece R, Wikberg JE. Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced NF-kappaB DNA binding and nitric oxide production in macrophage-like RAW 264.7 cells: evidence for dual mechanisms of action. Biochem Pharmacol. (2001) 61:613–21. doi: 10.1016/S0006-2952(00)00583-9
159. Cannon JG, Tatro JB, Reichlin S, Dinarello CA. Alpha melanocyte stimulating hormone inhibits immunostimulatory and inflammatory actions of interleukin 1. J Immunol. (1986) 137:2232–6.
160. Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. (2008) 29:581–602. doi: 10.1210/er.2007-0027
161. Cooper A, Robinson SJ, Pickard C, Jackson CL, Friedmann PS, Healy E. Alpha-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J Immunol. (2005) 175:4806–13. doi: 10.4049/jimmunol.175.7.4806
162. Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH) Ann N Y Acad Sci. (2000) 917:239–47. doi: 10.1111/j.1749-6632.2000.tb05389.x
163. Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J Leukoc Biol. (2002) 72:946–52. doi: 10.1189/jlb.72.5.946
164. Murphy MT, Lipton JM. Peripheral administration of alpha-MSH reduces fever in older and younger rabbits. Peptides. (1982) 3:775–9. doi: 10.1016/0196-9781(82)90014-6
165. Glyn-Ballinger JR, Bernardini GL, Lipton JM. alpha-MSH injected into the septal region reduces fever in rabbits. Peptides. (1983) 4:199–203. doi: 10.1016/0196-9781(83)90114-6
166. Murphy MT, Richards DB, Lipton JM. Antipyretic potency of centrally administered alpha-melanocyte stimulating hormone. Science. (1983) 221:192–3. doi: 10.1126/science.6602381
167. Kandasamy SB, Williams BA. Hypothermic and antipyretic effects of ACTH (1-24) and alpha-melanotropin in guinea-pigs. Neuropharmacology. (1984) 23:49–53. doi: 10.1016/0028-3908(84)90216-8
168. Shih ST, Lipton JM. Intravenous alpha-MSH reduces fever in the squirrel monkey. Peptides. (1985) 6:685–7. doi: 10.1016/0196-9781(85)90172-X
169. Feng JD, Dao T, Lipton JM. Effects of preoptic microinjections of alpha-MSH on fever and normal temperature control in rabbits. Brain Res Bull. (1987) 18:473–7. doi: 10.1016/0361-9230(87)90111-0
170. Deeter LB, Martin LW, Lipton JM. Antipyretic effect of central alpha-MSH summates with that of acetaminophen or ibuprofen. Brain Res Bull. (1989) 23:573–5. doi: 10.1016/0361-9230(89)90203-7
171. Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation. (1994) 1:28–32. doi: 10.1159/000097087
172. Martin LW, Lipton JM. Acute phase response to endotoxin: rise in plasma alpha-MSH and effects of alpha-MSH injection. Am J Physiol. (1990) 259:R768–72. doi: 10.1152/ajpregu.1990.259.4.R768
173. Delgado Hernandez R, Demitri MT, Carlin A, Meazza C, Villa P, Ghezzi P, et al. Inhibition of systemic inflammation by central action of the neuropeptide alpha-melanocyte- stimulating hormone. Neuroimmunomodulation. (1999) 6:187–92. doi: 10.1159/000026381
174. Lipton JM, Ceriani G, Macaluso A, Mccoy D, Carnes K, Biltz J, et al. Antiinflammatory effects of the neuropeptide alpha-MSH in acute, chronic, and systemic inflammation. Ann N Y Acad Sci. (1994) 741:137–48. doi: 10.1111/j.1749-6632.1994.tb39654.x
175. Colombo G, Gatti S, Sordi A, Turcatti F, Carlin A, Rossi C, et al. Production and effects of alpha-melanocyte-stimulating hormone during acute lung injury. Shock. (2007) 27:326–33. doi: 10.1097/01.shk.0000239764.80033.7e
176. Lasaga M, Debeljuk L, Durand D, Scimonelli TN, Caruso C. Role of alpha-melanocyte stimulating hormone and melanocortin 4 receptor in brain inflammation. Peptides. (2008) 29:1825–35. doi: 10.1016/j.peptides.2008.06.009
177. Taylor AW, Kitaichi N, Biros D. Melanocortin 5 receptor and ocular immunity. Cell Mol Biol. (2006) 52:53–9.
178. Richards DB, Lipton JM. Effect of alpha-MSH 11-13 (lysine-proline-valine) on fever in the rabbit. Peptides. (1984) 5:815–7. doi: 10.1016/0196-9781(84)90027-5
179. Hiltz ME, Lipton JM. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide alpha-MSH. FASEB J. (1989) 3:2282–4. doi: 10.1096/fasebj.3.11.2550304
180. Hiltz ME, Catania A, Lipton JM. Anti-inflammatory activity of alpha-MSH(11-13) analogs: influences of alteration in stereochemistry. Peptides. (1991) 12:767–71. doi: 10.1016/0196-9781(91)90131-8
181. Hiltz ME, Catania A, Lipton JM. Alpha-MSH peptides inhibit acute inflammation induced in mice by rIL-1 beta, rIL-6, rTNF-alpha and endogenous pyrogen but not that caused by LTB4, PAF and rIL-8. Cytokine. (1992) 4:320–8. doi: 10.1016/1043-4666(92)90073-Z
182. Macaluso A, Mccoy D, Ceriani G, Watanabe T, Biltz J, Catania A, et al. Antiinflammatory influences of alpha-MSH molecules: central neurogenic and peripheral actions. J Neurosci. (1994) 14:2377–82. doi: 10.1523/JNEUROSCI.14-04-02377.1994
183. Maaser C, Kannengiesser K, Specht C, Lugering A, Brzoska T, Luger TA, et al. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. (2006) 55:1415–22. doi: 10.1136/gut.2005.083634
184. Kannengiesser K, Maaser C, Heidemann J, Luegering A, Ross M, Brzoska T, et al. Melanocortin-derived tripeptide KPV has anti-inflammatory potential in murine models of inflammatory bowel disease. Inflamm Bowel Dis. (2008) 14:324–31. doi: 10.1002/ibd.20334
185. Lipton JM. Modulation of host defense by the neuropeptide alpha-MSH. Yale J Biol Med. (1990) 63:173–82.
186. Deschodt-Lanckman M, Vanneste Y, Loir B, Michel A, Libert A, Ghanem G, et al. Degradation of alpha-melanocyte stimulating hormone (alpha-MSH) by CALLA/endopeptidase 24.11 expressed by human melanoma cells in culture. Int J Cancer. (1990) 46:1124–30. doi: 10.1002/ijc.2910460629
187. Ugwu SO, Blanchard J, Dorr RT, Levine N, Brooks C, Hadley ME, et al. Skin pigmentation and pharmacokinetics of melanotan-I in humans. Biopharm Drug Dispos. (1997) 18:259–69.
188. Dorr RT, Dvorakova K, Brooks C, Lines R, Levine N, Schram K, et al. Increased eumelanin expression and tanning is induced by a superpotent melanotropin [Nle4-D-Phe7]-alpha-MSH in humans. Photochem Photobiol. (2000) 72:526–32. doi: 10.1562/0031-8655(2000)0720526:IEEATI2.0.CO;2
189. Cutuli M, Cristiani S, Lipton JM, Catania A. Antimicrobial effects of alpha-MSH peptides. J Leukoc Biol. (2000) 67:233–9. doi: 10.1002/jlb.67.2.233
190. Bock M, Roth J, Kluger MJ, Zeisberger E. Antipyresis caused by stimulation of vasopressinergic neurons and intraseptal or systemic infusions of gamma-MSH. Am J Physiol. (1994) 266:R614–21. doi: 10.1152/ajpregu.1994.266.2.R614
191. Getting SJ, Christian HC, Lam CW, Gavins FN, Flower RJ, Schioth HB, et al. Redundancy of a functional melanocortin 1 receptor in the anti-inflammatory actions of melanocortin peptides: studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J Immunol. (2003) 170:3323–30. doi: 10.4049/jimmunol.170.6.3323
192. Xia Y, Wikberg JE, Krukoff TL. Gamma(2)-melanocyte-stimulating hormone suppression of systemic inflammatory responses to endotoxin is associated with modulation of central autonomic and neuroendocrine activities. J Neuroimmunol. (2001) 120:67–77. doi: 10.1016/S0165-5728(01)00408-8
Keywords: melanocortin, melanocortin receptor, immune modulation, signaling, therapeutics
Citation: Wang W, Guo D-Y, Lin Y-J and Tao Y-X (2019) Melanocortin Regulation of Inflammation. Front. Endocrinol. 10:683. doi: 10.3389/fendo.2019.00683
Received: 01 July 2019; Accepted: 19 September 2019;
Published: 09 October 2019.
Edited by:
Alain Couvineau, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Lourdes Mounien, Aix-Marseille Université, FranceLaurent Gautron, UT Southwestern Medical Center, United States
Copyright © 2019 Wang, Guo, Lin and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Yu Guo, eGlhbWVuaGFpamluJiN4MDAwNDA7MTYzLmNvbQ==; Ya-Xiong Tao, dGFveWF4aSYjeDAwMDQwO2F1YnVybi5lZHU=
 Wei Wang1
Wei Wang1 Ya-Xiong Tao
Ya-Xiong Tao