
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 04 October 2019
Sec. Molecular and Structural Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00666
This article is part of the Research Topic Endocrine Forms of Hypertension: Clinical and Emerging Molecular Aspects View all 7 articles
 Syahirah Kaja Mohideen1
Syahirah Kaja Mohideen1 Muaatamarulain Mustangin2
Muaatamarulain Mustangin2 Nor Azmi Kamaruddin1
Nor Azmi Kamaruddin1 Rohaizak Muhammad3
Rohaizak Muhammad3 A. Rahman A. Jamal4
A. Rahman A. Jamal4 Norlela Sukor1
Norlela Sukor1 Geok Chin Tan2
Geok Chin Tan2 Elena Aisha Azizan1*
Elena Aisha Azizan1*Studies on excised adrenals from primary aldosteronism patients have found that somatic mutations in KCNJ5 frequently cause excess aldosterone production in the culprit aldosterone-producing adenoma (APA). KCNJ5 mutant APAs were reported to be peculiarly overrepresented among young females and in Oriental cohorts, compared to their older male, or Caucasian counterparts. These larger APAs were also reported to have similarities with the zona fasciculata (ZF) in the adrenal both from the steroid production profile and the morphology of the cell. We therefore aimed to corroborate these findings by characterizing the APAs from a multi-ethnic Malaysian cohort. The prevalence of KCNJ5 mutations was estimated through targeted DNA sequencing of KCNJ5 in 54 APAs. Confirmation of APA sample acquisition was performed by CYP11B2 immunohistochemistry (IHC) staining. The ZF steroid production profile was based on the ZF enzyme CYP17A1 IHC staining, and ZF cell morphology was based on a high cytoplasm to nucleus ratio. Seventeen (31.5%) APAs studied, harbored a KCNJ5 mutation. No female over-representation was seen in this cohort though females were found to have a higher expression of CYP11B2 than males (p = 0.009; Mann-Whitney U test). Age at adrenalectomy correlated negatively with the percentage of ZF-like cells in the APA (p = 0.01; Spearman's rho) but not with the KCNJ5 genotype. KCNJ5 mutant APAs had a high percentage of ZF-like cells (and high CYP17A1 expression) but so did the wild-type APAs. In summary, prevalence of KCNJ5 mutant APAs in this cohort was similar to other Caucasian cohorts, however, over-representation of females did not occur, which is similar to some studies in Oriental cohorts.
Primary aldosteronism (PA) is the most common cause of secondary hypertension, with an estimated prevalence of ~10% among hypertensive patients and ~20% among resistance hypertension (1–6). The presence of an autonomous aldosterone-producing lesion causes uncontrolled production of aldosterone which under normal circumstances is regulated by the renin-angiotensin-aldosterone system. Unilateral aldosterone-producing adenomas (APA) in the adrenals and bilateral adrenal hyperplasia (BAH) are major causes of the occurrence of PA, accounting for ~95% of all PA patients (7). Patients diagnosed with a unilateral APA can be surgically cured of their PA by adrenalectomy which can also clinically cure 33–77% of hypertension in these patient cases (8–10).
Studies performed in excised APA tissues found functional somatic mutations in KCNJ5, ATP1A1, ATP2B3, CACNA1D, and CTNNB1 cause PA (11–15). These aldosterone-driver mutations have also been found in aldosterone-producing cell clusters (APCC) in normal adrenal glands (16) and in micronodular lesions (17). Surprisingly, the gene most frequently mutated in APAs, KCNJ5, is frequently of the wild-type in APCCs and micronodular lesions (16–20). The KCNJ5 gene encodes for the G-protein-activated inward rectifier K+ channel 4, GIRK4. The two most common somatic mutations in KCNJ5 are the G151R and L168R mutations located in or near the selectivity filter of this K+ channel (13). Presence of these mutations causes loss of the K+ channel selectivity leading to increased Na+ conductance, cell depolarization, and thus autonomous aldosterone production.
Peculiarly, most studies documenting the prevalence of causal somatic mutations in PA patients found KCNJ5-mutated APA commonly occurs in females which were also more frequently on the large side of the APA spectrum and had a zona fasciculata (ZF)-like steroid production profile and cell morphology (18–25). To note, even aldosterone- and cortisol-co-secreting adrenal adenomas have been found to have KCNJ5 mutations (26). Of further interest, a higher prevalence of KCNJ5 mutation were found in Oriental cohorts when compared to Caucasian cohorts. More than 50% of the APAs studied in cohorts from China, Japan, Korea, Taiwan, and Thailand were reported to have a KCNJ5 somatic mutation (23, 27–33). A meta-analysis study performed on available studies at the time, estimated that the prevalence of the KCNJ5 mutation in APAs from Oriental cohorts were almost twice than that in Caucasian cohorts (63 vs. 35%) (34).
In this study, we therefore aim to interrogate the prevalence and histopathological characteristics of KCNJ5 mutant APAs, from a multi-ethnic Malaysian cohort in a single tertiary center.
The medical records of patients who had undergone adrenalectomy at the National University of Malaysia Medical Center between 2000 and 2015 were taken from either the CT scan report, adrenal vein sampling report, or histopathology report. Patients who had undergone adrenalectomy due to PA and who had archived FFPE adrenal samples were consecutively recruited for the study. Fifty-four confirmed APAs had sufficiently good quality material for immunohistochemistry and genetic analyses. Follow-up clinical data was available for 29 patients. The protocol used in this study has been approved by the local research ethics committee of the National University of Malaysia Medical Center.
All IHC staining was performed on 4 μm formalin-fixed paraffin embedded sections of adrenals. CYP11B2, CYP17A1, KCNJ5, and active caspase 3 staining was performed as detailed in the Supplementary Methods. Positive control tissues were used to optimize the IHC protocol (optimized results shown in Supplementary Figure 1). Negative controls where the primary antibodies are omitted were performed for all IHC experiments. IHC staining for CYP11B2 was performed on all FFPE blocks that were available of the excised adrenals, to confirm sampling of an aldosterone-producing lesion. Sections that had a positive nodule with CYP11B2 were then stained with CYP17A1, KCNJ5, and active caspase 3 and scored. Percentage of cells in the APAs with ZF-like cell morphology (high cytoplasm: nucleus ratio), percentage of atypical cells, and counts of spironolactone bodies were determined using hematoxylin and eosin (H&E) stained sections. All analyses of IHC and H&E staining were performed by a histopathologist blinded to the genotype results. Primary antibodies and parameters used for IHC staining are described in Supplementary Table 1, and the scoring table used for the IHC staining is detailed in Supplementary Table 2. Representative images of the IHC staining scores are shown in Figure 1. Examples of histopathology morphology based on H&E staining are shown in Figure 2.
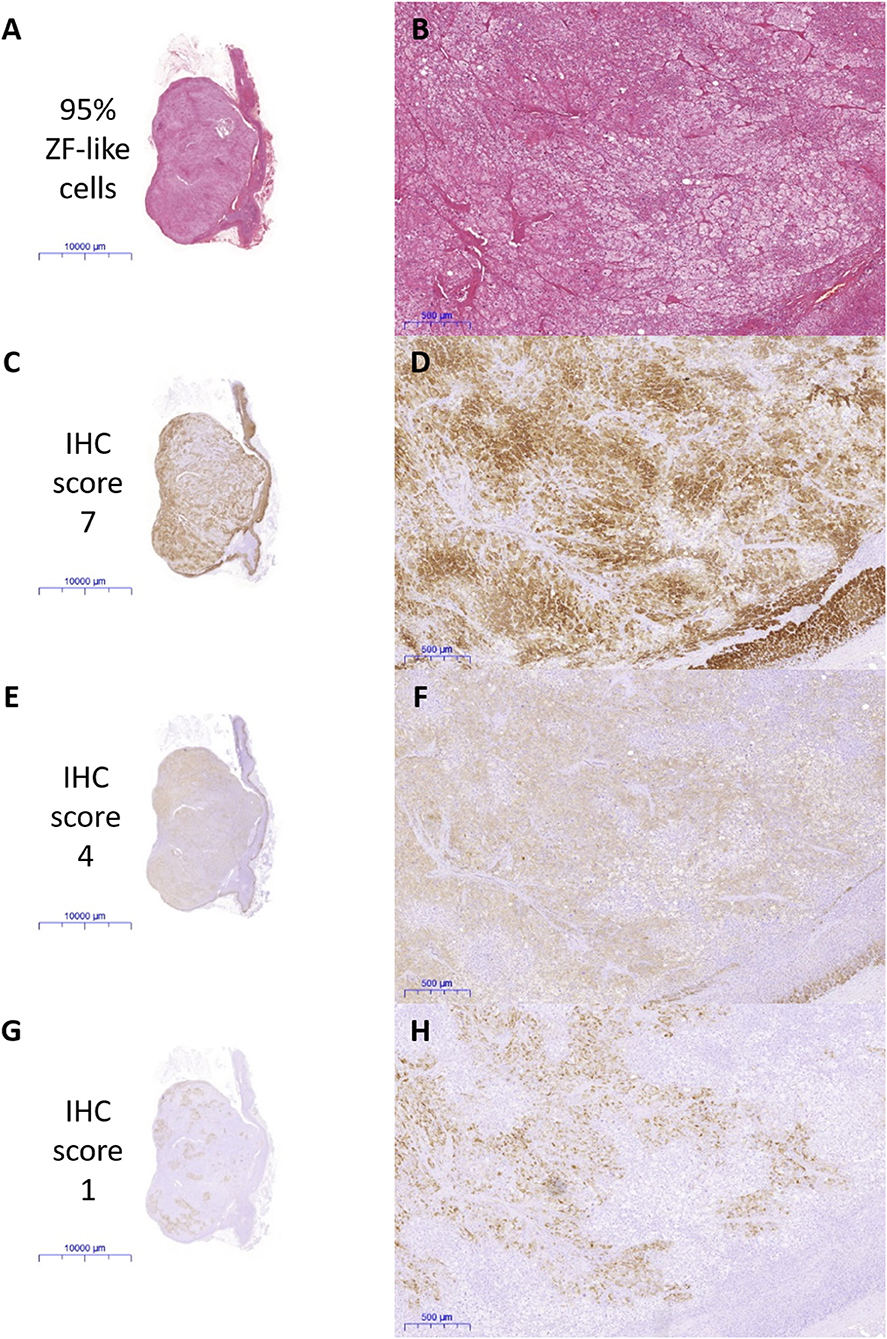
Figure 1. Representative scan and microscope images of the immunohistochemical (IHC) staining scored by a blinded histopathologist. (A,B) Percentage of ZF-like cells, (C,D) IHC staining of CYP17A1, (E,F) IHC staining of KCNJ5, and (G,H) IHC staining of CYP11B2.
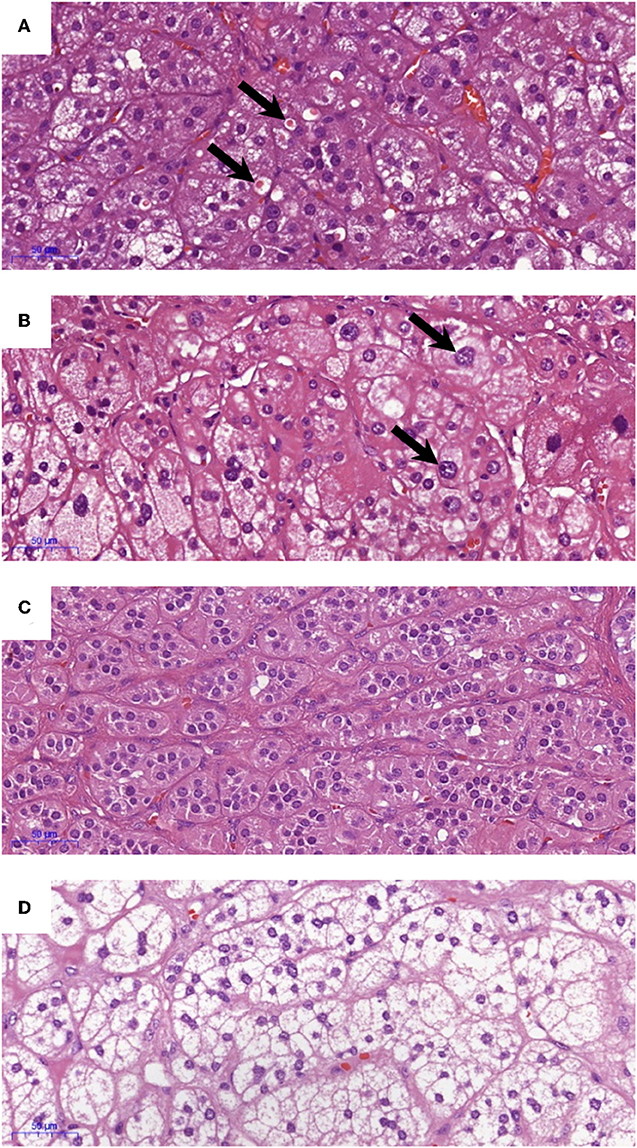
Figure 2. Examples of histopathology morphology based on hematoxylin and eosin (H&E) staining. (A) Spironolactone bodies, (B) atypical cells, (C) ZG-like cells, and (D) ZF-like cells.
DNA samples of APAs were extracted from FFPE tissue blocks or FFPE sectioned slides using the commercially available kit ReliaPrep FFPE gDNA Miniprep System (Promega, USA) according to the manufacturer's instructions. The DNA sequence encoding the selectivity filter region in KCNJ5 was amplified using the AmpliTaq Gold™ Fast PCR Master Mix (ThermoFisher Scientific, USA) according to the manufacturer's instructions. The primers and parameters used are listed in Supplementary Tables 3 and 4. PCR products were then Sanger Sequenced commercially (Apical Scientific Sdn Bhd, Malaysia) and any mutations were confirmed through sequencing of a replicated PCR product in the opposite direction.
Results are expressed as mean ± standard deviation (SD) unless specified otherwise. Normally distributed datasets were compared using the two-tailed t-test, whereas non-normally distributed datasets were compared using the Mann-Whitney U test. Dichotomous categorical datasets were compared using Fisher's Exact test. Relationships between variables were tested either using the Pearson's Correlation for normally distributed datasets or using Spearman's rho for non-normally distributed datasets. Normal distribution of datasets was tested using the Shapiro-Wilk test and the homogeneity of variances was tested using the Levene's test. A p < 0.05 was considered statistically significant. Statistical analyses and graphs were performed using IBM SPSS Statistics (version 25).
Genetic analysis identified 17 patients with a KCNJ5 mutant APA (Table 1). Fourteen patients had a G151R KCNJ5 mutation, while three had a L168R KCNJ5 mutation. There was no overrepresentation of females with KCNJ5 mutant APAs and there were no significant differences between the number of Malay patients and the number of Chinese patients that had a KCNJ5 mutant APA (Table 1). There were also no significant differences in age at adrenalectomy or tumor size between patients that harbored a KCNJ5 mutant APA, compared to patients that harbored a KCNJ5 wild-type APA (47 ± 12.6 vs. 46 ± 11.0 years old; 13.6 ± 4.47 vs. 13.0 ± 4.39 mm), though male patients in general tended to have adrenalectomy at an older age than females (49 ± 12.0 vs. 43 ± 10.2, p = 0.056; Table 2). Clinical attributes of pre-adrenalectomy and post-adrenalectomy were also not significantly different between patients with or without a KCNJ5 mutant APA; though when compared by gender, pre-adrenalectomy and post-adrenalectomy serum creatinine levels, and post-adrenalectomy diastolic blood pressure were significantly lower in females (Table 2).
There were no significant differences between CYP11B2, CYP17A1, KCNJ5, or active caspase 3 protein expression between KCNJ5 mutant APAs compared to the wild-type. When compared by gender, APAs from a female patient had higher CYP11B2 expression (U = 220, p = 0.01, Figure 3A, Table 2) whereas APAs from a male patient had a trend to have higher active caspase 3 expression than APAs from a female patient (U = 280, p = 0.08, Figure 3B, Table 2). Similarly, there were no significant differences between the percentage of cells with ZF-like cell morphology (high cytoplasm: nucleus ratio), the percentage of atypical cells, and counts of spironolactone bodies in KCNJ5 mutant APAs compared to the wild-type. However, of all histological parameters interrogated, only the percentage of cells with ZF-like cell morphology in the KCNJ5 mutant and wild-type APAs had unequal variances [F (1, 52) = 5.932, p = 0.02]. This is mainly driven by the bimodal distribution of the percentage of the ZF-like cell morphology in KCNJ5 wild-type APAs caused by the large variances in wild-type APAs from male patients (Figure 3C and Supplementary Figure 2).
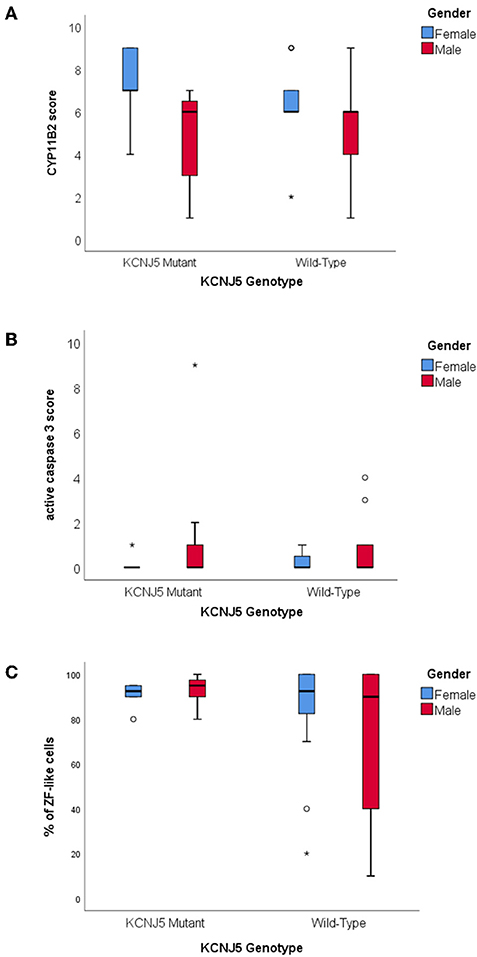
Figure 3. Histological characteristics of KCNJ5 mutant APAs. (A) CYP11B2 protein expression, (B) active caspase 3 protein expression, and (C) percentage of ZF-like cells (i.e., cells with high cytoplasm: nucleus ratio), in KCNJ5 mutant APAs grouped by gender.
The age of adrenalectomy correlated negatively with tumor size and the percentage of cells with ZF-like cell morphology in APA (Figure 4A, rs = −0.475, p = 0.0003; and Figure 4B, rs = −0.350, p = 0.01), and positively with active caspase 3 expression in APA (Figure 4C, rs = 0.329, p = 0.015). The majority of tumors with a diameter <12.5 mm were adrenalectomized when the patients were above the mean age of adrenalectomy, whereas the majority of tumors with a diameter >20.0 mm were adrenalectomized when the patients were below the mean age of adrenalectomy. Interestingly, tumor size also weakly correlated positively with CYP17A1 expression in APA (Supplementary Figure 3A, rs = 0.297, p = 0.03) and there was a trend of a correlation between tumor size and the percentage of cells with a ZF-like cell morphology in APA (rs = 0.242, p = 0.08). The majority of tumors with <30% of ZF-like cells in APA were therefore adrenalectomized when the patients were above the mean age of adrenalectomy, whereas the majority of tumors with 100% ZF-like cells in APA were adrenalectomized when the patients were below the mean age of adrenalectomy. Between histological parameters, spironolactone body count correlated with CYP11B2 expression (Supplementary Figure 3B, rs = 0.389, p = 0.004). To note, six of the 11 APAs with spironolactone bodies had <80% ZF-like cells, while APAs with >20 spironolactone body counts had <20% ZF-like cells.
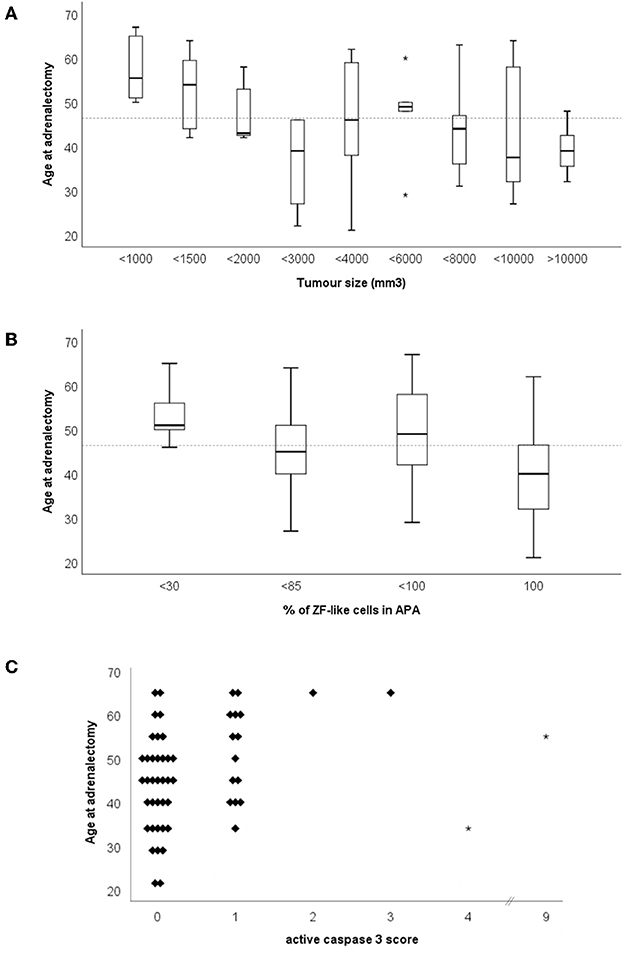
Figure 4. Age of adrenalectomy correlations with (A) tumor size, (B) percentage of ZF-like cells in APA (i.e., cells with high cytoplasm: nucleus ratio), and (C) active caspase three score. *Dotted line represents the mean age of adrenalectomy (46.4 years old).
The recommended treatment for a unilateral aldosterone-producing lesion that causes primary aldosteronism associated hypertension is adrenalectomy of the affected adrenal (35). Numerous studies performed on the excised tissues have found that a high prevalence of the KCNJ5 mutation to be the cause of this pathology (18–20, 34). Although this study similarly showed KCNJ5 mutant APAs to be common among Malaysian PA patients, the estimated prevalence of 37% is much lower than other Oriental cohorts that documented a prevalence of >50% (23, 27–33). The current population of Malaysia is comprised of 69.1% Bumiputera (of which the majority are Malays), 23.0% Chinese, 6.9% Indians and 1% other ethnicities (36). In this study, a similar number of Malay PA patients and Chinese PA patients were recruited, yet there were no significant differences between the number of Malay patients and the number of Chinese patients that had a KCNJ5 mutant APA (31 vs. 33%).
IHC staining of tumor tissues has become an important tool to further characterize APAs (37). As such, four protein staining—CYP11B2, CYP17A1, active caspase 3, and KCNJ5, were used to characterize the KCNJ5 mutant APAs. CYP11B2, also known as aldosterone synthase, is an enzyme that is essential for aldosterone synthesis as it is the sole enzyme responsible for the conversion of 11-deoxycorticosterone to corticosterone, to 18-hydroxycorticosterone, and finally to aldosterone. CYP17A1 has 17-alpha-hydroxylase and 17, 20 lyase activity which is needed to form the pre-cursors of cortisol, the main steroid produced physiologically in the ZF of the adrenal. Active caspase 3 is a protein that has been reported to play a role in cell apoptosis to the extent that alteration of the caspase 3 gene, CASP3, is reported to promote human tumorigenesis (38). KCNJ5 protein expression is not only of interest, as somatic mutations in the gene is common in APAs, but also as it is more highly expressed in the ZG, the physiological zone in the adrenal that produces aldosterone, than the ZF (13).
In this study, there were no significant differences in the expression of CYP11B2, CYP17A1, active caspase 3, or KCNJ5 between the KCNJ5 mutant and wild-type APAs. Similarly, the percentage of cells with a ZF-like cell morphology (high cytoplasm: nucleus ratio), the percentage of atypical cells, and counts of spironolactone bodies in KCNJ5 mutant APAs compared to the wild-type, showed no significant difference. Some studies in the Caucasian cohorts had found KCNJ5 mutant APAs to have a significantly lower expression of the KCNJ5 protein and a ZF-like cell profile (25, 37, 39, 40) which is in agreement with the infrequent KCNJ5 mutations observed in the ZG-like APCCs and nodular lesions (16, 17). The lack of the KCNJ5 genotype correlation with the ZF-like cell profile in this study is arguably more likely due to a difference in the Malaysian “KCNJ5 wild-type” APA cohort rather than due to a true difference between Malaysian KCNJ5 mutant APAs and Caucasian KCNJ5 mutant APAs. In this Malaysian cohort only five APAs had a diameter <10 mm3, likely due to our center's low success rate in adrenal vein sampling (<25%) preventing adenoma's not seen by CT-scan from undergoing adrenalectomy. Thus, our “KCNJ5 wild-type” APA cohort may contain fewer CACNA1D mutant APAs as these APAs have been reported to be smaller in size and angiotensin II-responsive, making them harder to be diagnosed (18–20, 41). These CACNA1D mutant APAs have been reported to be more common among males and to have a non-ZF-like (i.e., ZG-like) cell profile (11, 19, 20). Therefore, if we were to assume that our “KCNJ5 wild-type” cohort is missing smaller ZG-like CACNA1D mutant APAs, the results from this Malaysian cohort is in agreement with previous reports of KCNJ5 mutant APAs with a ZF-like cell profile, as almost all the cells in KCNJ5 mutant APAs were ZF-like, whereas the percentage of ZF-like cells in KCNJ5 wild-type APAs varied especially in males (Figure 1C and Supplementary Figure 2).
The majority of studies documenting the KCNJ5 genotype of APAs reported that patients harboring a KCNJ5 mutant APA were more commonly females, adrenalectomized at a younger age, with a larger tumor size than those harboring a KCNJ5 wild-type APA (18–25, 34, 41). Though, some studies in Oriental cohorts had found no gender bias, as male and female APAs had similar KCNJ5 mutation rates (23, 29–31, 33). In this study, males more commonly harbored a KCNJ5 mutant APA compared to females (39% vs. 23%) and there were no significant differences in age at adrenalectomy or tumor size between patients with a KCNJ5 mutant APA compared to patients with a KCNJ5 wild-type APA. However, compared to female patients, male patients in general tended to have adrenalectomy at an older age (49 + 12.0 vs. 43 + 10.2; p = 0.056) and tended to have a higher expression of the apoptosis marker active caspase 3 (U = 280, p = 0.08). Altogether the findings suggest that perhaps undergoing adrenalectomy at a younger age and having a larger tumor size is reflective of patients' gender rather than the KCNJ5 genotype of the APA. The difference in adrenalectomy age would also explain the significantly lower post-adrenalectomy diastolic blood pressure in females. It is worth noting that the primary female hormone, estrogen, is well-known to play a role in the development and malignant progression of multiple cancers, and that estrogen receptors located in both the nucleus and the cytoplasm of tumor cells regulates genes involved in cell survival and proliferation (42–44). We had previously noted that the estrogen related receptor beta gene, ESRRB, was 3-fold up-regulated in APAs compared to their adjacent normal adrenals, and in this study, APAs from female patients had more CYP11B2 expression than APAs from male patients [Figure 3A; (25)]. Concurringly, during murine embryogenesis the ESRRB protein is expressed in the adrenal primordium, supporting the role of estrogen in the development and proliferation of adrenal cells (45).
Correlation of histological parameters in this study showed that age of adrenalectomy negatively correlated with tumor size and the percentage of ZF-like cells in APA. There was also a weak positive correlation between the ZF enzyme CYP17A1 expression in APA and tumor size, and a trend for a positive correlation between the percentage of ZF-like cells in APA and tumor size. Conjointly our results suggest that APAs with a ZF-like profile (either based on steroid enzyme expression or cell morphology) tends to be larger and thus probably diagnosed faster resulting in the treatment, adrenalectomy, to occur in the patient at a younger age. Interestingly, we did not find spironolactone bodies to be common in ZF-like APAs, as APAs with >20 spironolactone body counts had <20% ZF-like cells. Association between spironolactone bodies and ZG or ZG-like APAs has previously been documented (46, 47). Perhaps this finding is due to ZG-like APAs being harder to diagnose and therefore the patient's hypertension is treated with spironolactone for longer, compared to patients with a ZF-like APA.
As this was a retrospective study, the mutation analysis was limited to patients for whom APA tissues were available and that were of good quality. Therefore, whether this cohort truly represents the prevalence of KCNJ5 mutant APAs in a Malaysian population is still questionable as there would be more usable tissues for larger APAs. Moreover, due to reliance on CT-scan findings for a decision of adrenalectomy, when adrenal vein sampling is unsuccessful, the findings from this study could simply be a case of a comparison between “low-hanging fruits”—i.e., the comparison of KCNJ5 mutant APAs among large APAs. Nevertheless, despite these limitations, our findings do speculatively suggest that the phenotype previously connected with KCNJ5 mutant APAs may actually be the phenotype of APAs adrenalectomized from female PA patients. Further investigation through a multicenter prospective study, recruiting a larger number of Malaysian patients, is warranted.
This manuscript contains previously unpublished data. The data that support the findings of this study are available from the corresponding author, EA, upon reasonable request.
This study was carried out in accordance with the recommendations of the local research ethics committee of the National University of Malaysia Medical Center. As only somatic mutations in the hotspot of KCNJ5 was interrogated in archived tissue, and results were not presented for individuals, individual informed consent of subjects were not taken. The protocol was approved by the local research ethics committee of the National University of Malaysia Medical Center under the project code FF-2016-161.
EA, NK, RM, AJ, and NS contributed to the conception and design of the research. SM, MM, and GT contributed to the acquisition of the work. EA and SM contributed to the analysis and interpretation of data for the work.
This research was supported by the Malaysian Ministry of Higher Education grant (FRGS/1/2015/SKK08/UKM/02/3) and The National University of Malaysia (UKM) Medical Center Fundamental Grant (FF-2016-161).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Prof. Celso Gomez-Sanchez for gifting his highly specific and selective CYP11B2 and CYP17A1 antibodies. We thank Ms. Afifah Binti Azam and Ms. Long Kha Chin for helping with the processing of samples and setting up of the targeted sequencing protocol.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00666/full#supplementary-material
1. Fardella CE, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, et al. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab. (2000) 85:1863–7. doi: 10.1210/jc.85.5.1863
2. Lim PO, Dow E, Brennan G, Jung RT, MacDonald TM. High prevalence of primary aldosteronism in the Tayside hypertension clinic population. J Hum Hypertens. (2000) 14:311–5. doi: 10.1038/sj.jhh.1001013
3. Michalak R, Jagodzinska A, Zieleniewski W. The prevalence of primary aldosteronism (PA) in a group of 350 hypertensive patients. Arter Hypertens. (2015) 19:9–12. doi: 10.5603/AH.2015.0002
4. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. (2017) 69:1811–20. doi: 10.1016/j.jacc.2017.01.052
5. Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. (2003) 17:349–52. doi: 10.1038/sj.jhh.1001554
6. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. (2006) 48:2293–300. doi: 10.1016/j.jacc.2006.07.059
7. Amar L, Plouin PF, Steichen O. Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism. Orphanet J Rare Dis. (2010) 5:9. doi: 10.1186/1750-1172-5-9
8. Sawka AM, Young WF, Thompson GB, Grant CS, Farley DR, Leibson C, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. (2001) 135:258–61. doi: 10.7326/0003-4819-135-4-200108210-00010
9. Letavernier E, Peyard S, Amar L, Zinzindohoué F, Fiquet B, Plouin P-F. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens. (2008) 26:1816–23. doi: 10.1097/HJH.0b013e3283060f0c
10. Zhou Y, Zhang M, Ke S, Liu L. Hypertension outcomes of adrenalectomy in patients with primary aldosteronism : a systematic review and meta-analysis. BMC Endocr Disord. (2017) 17:61. doi: 10.1186/s12902-017-0209-z
11. Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. (2013) 45:1055–60. doi: 10.1038/ng.2716
12. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. (2013) 45:440–2. doi: 10.1038/ng.2550
13. Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. (2011) 331:768–72. doi: 10.1126/science.1198785
14. Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. (2013) 45:1050–4. doi: 10.1038/ng.2695
15. Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med. (2015) 373:1429–36. doi: 10.1056/NEJMoa1504869
16. Omata K, Anand SK, Hovelson DH, Liu CJ, Yamazaki Y, Nakamura Y, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. (2017) 12:787–99. doi: 10.1210/js.2017-00134
17. Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. (2017) 102:1182–92. doi: 10.1210/jc.2016-2986
18. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. (2014) 64:354–61. doi: 10.1161/HYPERTENSIONAHA.114.03419
19. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol. (2015) 83:779–89. doi: 10.1111/cen.12873
20. Åkerström T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. (2015) 22:735–44. doi: 10.1530/ERC-15-0321
21. Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. (2012) 59:592–8. doi: 10.1161/HYPERTENSIONAHA.111.186478
22. Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. (2014) 63:188–95. doi: 10.1161/HYPERTENSIONAHA.113.01733
23. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. (2015) 22:191–200. doi: 10.5551/jat.24455
24. Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. (2016) 67:139–45. doi: 10.1161/HYPERTENSIONAHA.115.06186
25. Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. (2012) 97:819–29. doi: 10.1210/jc.2011-2965
26. Yamada M, Nakajima Y, Taguchi R, Okamura T, Ishii S, Tomaru T, et al. KCNJ5 mutations in aldosterone- and cortisol-co-secreting adrenal adenomas. Endocr, J. (2012) 59:735–41. doi: 10.1507/endocrj.EJ12-0247
27. Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab. (2012) 97:1311–9. doi: 10.1210/jc.2011-2885
28. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. (2015) 65:622–8. doi: 10.1161/HYPERTENSIONAHA.114.03346
29. Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine. (2015) 94:e708. doi: 10.1097/MD.0000000000000708
30. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Nat Publ Gr. (2015) 5:1–10. doi: 10.1038/srep11396
31. Okamura T, Nakajima Y, Katano-Toki A, Horiguchi K, Matsumoto S, Yoshino S, et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr J. (2017) 64:39–47. doi: 10.1507/endocrj.EJ16-0243
32. Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong MW, et al. Genetics of aldosterone-producing adenoma in Korean patients. PLoS ONE. (2016) 11:e0147590. doi: 10.1371/journal.pone.0147590
33. Warachit W, Atikankul T, Houngngam N, Sunthornyothin S. Prevalence of somatic KCNJ5 mutations in Thai patients with aldosterone-producing adrenal adenomas. J Endocr Soc. (2018) 2:1137–46. doi: 10.1210/js.2018-00097
34. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K+ channel mutations in 1,636 patients with an aldosterone- producing adenoma. J Clin Endocrinol Metab. (2015) 100:1089–95. doi: 10.1210/jc.2015-2149
35. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:1889–916. doi: 10.1210/jc.2015-4061
36. Department of Statistics Malaysia. Current Population Estimates, Malaysia, 2017–2018 Putrajaya (2018).
37. Fernandes-Rosa FL, Amar L, Tissier F, Bertherat J, Meatchi T, Zennaro MC, et al. Functional histological markers of aldosterone producing adenoma and somatic KCNJ5 mutations. Mol Cell Endocrinol. (2015) 408:220–6. doi: 10.1016/j.mce.2015.01.020
38. McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. (2015) 7:a026716. doi: 10.1101/cshperspect.a026716
39. Boulkroun S, Golib Dzib JF, Samson-Couterie B, Rosa FL, Rickard AJ, Meatchi T, et al. KCNJ5 mutations in aldosterone producing adenoma and relationship with adrenal cortex remodeling. Mol Cell Endocrinol. (2013) 371:221–7. doi: 10.1016/j.mce.2013.01.018
40. Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. (2015) 411:146–54. doi: 10.1016/j.mce.2015.04.022
41. Azizan EA, Murthy M, Stowasser M, Gordon R, Kowalski B, Xu S, et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension. (2012) 59:587–91. doi: 10.1161/HYPERTENSIONAHA.111.186239
42. Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. (2003) 144:4562–74. doi: 10.1210/en.2003-0567
43. Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M. Regulation of endogenous gene expression in human non– small cell lung cancer cells by estrogen receptor ligands. Am Assoc Cancer Res. (2005) 65:1598–606. doi: 10.1158/0008-5472.CAN-04-2694
44. Rothenberger NJ, Somasundaram A, Stabile LP. The role of the estrogen pathway in the tumor microenvironment. Int J Mol Sci. (2018) 19:1–16. doi: 10.3390/ijms19020611
45. Collin RW, Kalay E, Tariq M, Peters T, van der Zwaag B, Venselaar H, et al. Mutations of ESRRB encoding estrogen-related receptor beta cause autosomal-recessive non-syndromic hearing impairment DFNB35. Am J Hum Genet. (2008) 82:125–38. doi: 10.1016/j.ajhg.2007.09.008
46. Tan GC, Negro G, Pinggera A, Tizen Laim NMS, Mohamed Rose I, Ceral J, et al. Aldosterone-producing adenomas: histopathology-genotype correlation and identification of a novel CACNA1D mutation. Hypertension. (2017) 70:129–36. doi: 10.1161/HYPERTENSIONAHA.117.09057
Keywords: aldosterone-producing adenomas, KCNJ5, primary aldosteronism, Malaysia, Asia
Citation: Mohideen SK, Mustangin M, Kamaruddin NA, Muhammad R, Jamal ARA, Sukor N, Tan GC and Azizan EA (2019) Prevalence and Histopathological Characteristics of KCNJ5 Mutant Aldosterone-Producing Adenomas in a Multi-Ethnic Malaysian Cohort. Front. Endocrinol. 10:666. doi: 10.3389/fendo.2019.00666
Received: 06 May 2019; Accepted: 13 September 2019;
Published: 04 October 2019.
Edited by:
Teresa Seccia, University of Padova, ItalyReviewed by:
Saulo J. A. Felizola, Tohoku University, JapanCopyright © 2019 Mohideen, Mustangin, Kamaruddin, Muhammad, Jamal, Sukor, Tan and Azizan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Aisha Azizan, ZWxlbmEuYXppemFuQHVrbS5lZHUubXk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.