- 1Private Practice, Brooklyn, NY, United States
- 2Department of Biological Sciences and Geology, Queensborough Community College, City University of New York, Bayside, NY, United States
- 3Department of Medicine, NYU School of Medicine, New York, NY, United States
Background: Recent studies identify a significant number of treated hypothyroid patients who express dissatisfaction with their therapy. At present there are sufficient measures of thyroid function to enable the clinician to establish a diagnosis of thyroid disease with a high degree of sensitivity and specificity. The purpose of this study was to quantitate the use of a new and novel assessment of clinically relevant hypothyroid symptoms in the management of patients with thyroid disease and to identify a tool that could help clinicians to assess adequacy of LT4 treatment.
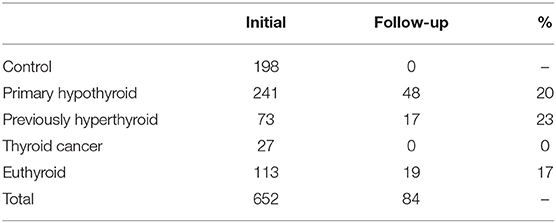
Methodology: Unselected outpatients of the Thyroid Clinic of the North Shore University Hospital at Manhasset completed a questionnaire asking them to rate their physical symptoms related to thyroid disease as part of their standard care. This questionnaire consisted of 10 signs and symptoms. The questionnaire was collected from 198 control subjects, 241 subjects with primary hypothyroidism (under treatment), 113 euthyroid subjects (benign nodular thyroid disease), 73 previously hyperthyroid subjects (previously treated), and 27 subjects with thyroid cancer. A repeat questionnaire was obtained from 48 subjects with primary hypothyroidism (20%), 19 euthyroid subjects (17%), and 17 subjects previously hyperthyroid (23%).
Data Analysis: The mean score for the sum of the signs and symptoms in the primary hypothyroid group with no medication change was 9.62 ± 1.29 for the initial questionnaire, and 10.04 ± 1.32 for the follow up questionnaire (not significant). For the primary hypothyroid patients requiring a medication change, at the time of the initial questionnaire the mean serum TSH was 12.86 ± 2.75 mcU/ml. Concurrently with the normalization of TSH, a statistically significant improvement in the sum of signs and symptoms mean score for this group was noted (16.32 ± 1.93 initial vs. 10.32 ± 1.46 after treatment to normalize TSH).
Conclusion: The proposed newly devised hypothyroid scale correctly identified subjects with TSH elevation and clinical/subclinical hypothyroidism based on their clinical signs and symptoms. In this particular subset of patients, the hypothyroid symptom scale showed a statistically significant improvement in the sum of the signs and symptoms with the normalization of the subjects' thyroid function.
Introduction
There are a number of thyroid function tests (TFTs), which enable the clinician to establish a diagnosis of thyroid disease with a high degree of sensitivity and specificity. The application of these tests, particularly the TSH, makes the diagnosis of overt thyroid dysfunction fairly straightforward in most cases. There are however notable exceptions in which these laboratory tests may not be a reliable measure of thyroid hormone action in individual patients (1). These conditions include, but are not limited to, secondary hyper and hypothyroidism, subclinical forms of hypothyroidism and hyperthyroidism, thyroid hormone resistance, alterations in thyroid hormone binding by a variety of serum carrier proteins, interactions with different medications such as lithium, amiodarone, and iodine, as well as nonthyroidal (low T3) illnesses (2–8). In the assessment of these patients, it would be useful to have an additional quantitative clinical measure that both directly and indirectly reflects the cellular action of thyroid hormone and could be used clinically to aid in diagnosis and treatment (1, 8–10). The recent interest in T4/T3 combination therapy in patients treated with L-T4 monotherapy calls out for a quantitative measure of well-recognized hypothyroid symptoms and the response of these symptoms to individualized treatments. Hypothyroid symptom scales have been previously developed and modified over the years to primarily aid in the diagnosis of hypothyroidism (11, 12) to target those patients who would likely be candidates for further testing, but none to date have been designed to assess the adequacy of treatment in patients currently on thyroid hormone replacement with persistent symptoms and to potentially guide novel therapeutic regimens.
Fifty years ago, before the development of adequate and reliable TFTs, Billewicz et al. (11) described a diagnostic index that assessed the presence or absence of various signs and symptoms of hypothyroidism for the purpose of establishing a diagnosis of hypothyroidism. With the development of newer, more reliable TFTs, in 1997 Zulewski et al. (12) revised the hypothyroid signs and symptoms clinical score for individual assessment of the severity of thyroid failure. It was designed based on signs and symptoms originally chosen by Billewicz and a scoring range was determined in patients with untreated overt hypothyroidism and compared to patients with normal thyroid function. Scores were based on the presence (1) or absence (0) of hypothyroid signs and symptoms. Ranges were determined based on average scores in patients and controls and the frequency of these symptoms in the same populations. In addition, a correction factor (+1) was added to a patient's score when his (her) age was <55 years. The diagnostic range for this clinical score was established as ≤ 2 = euthyroid; 3–5 = intermediate; >5 = overt hypothyroid. In overt hypothyroidism the average score was found to correlate with ankle reflex relaxation time, total cholesterol, and creatine kinase. However, while the score correlated with fT4 and fT3, it did not correlate with TSH, the gold standard for thyroid function testing. TSH and fT4 correlated with scores in the middle range. The study concluded that the classical signs and symptoms of hypothyroidism were only present in patients with severe overt hypothyroidism with low serum T3 levels, but were minimal or absent in patients with normal T3, despite low fT4 or in patients with subclinical hypothyroidism (12). Therefore, it seems that T3 levels were the most reliable predictor of hypothyroid signs and symptoms. Another important feature of both the Billewicz and Zulewski diagnostic tools is that the scoring is accomplished by physicians who function as examiners, and not by a questionnaire administered to the patient.
It has further been demonstrated that physical examination alone in the diagnosis of hypothyroidism cannot confirm or rule out hypothyroidism without including TFTs (13). To assess the significance of clinical vs. biochemical assessment of hypothyroidism in patients to optimize L-T4 dosing, the Billewicz scale was used and compared to biochemical measures (14). Of almost 400 subjects found to be biochemically hypothyroid, <1 fourth could be classified as hypothyroid based on the Billewicz score.
Contrary to the Billewicz and Zulewski scales, in 1997, Canaris et al. (15) examined a group of hypothyroid patients and compared them to matched controls by self -administered questionnaire. In this study, only newly diagnosed patients with a TSH above 20 μU/mL and decreased T4 were eligible to participate. The number and percentage of positive symptoms were determined for each patient. Symptoms were scored as present or absent (positive or negative). Using a scale of 1–5, only severe ratings of 4 or 5 were counted as present, ratings of 1–3 were considered absent. Patients were also asked whether the symptom or sign had changed within the past year. For hypothyroid patients, the number of conventional hypothyroid symptoms reported in that group was directly, albeit weakly, correlated to TSH, with a stronger association when more symptoms were reported. Likelihood ratios for the scoring ranges were determined to help assess whether to test for thyroid disease.
Each of the studies described was designed for the purpose of diagnosis of hypothyroidism to aid in deciding whether TFTs are warranted. We have devised a novel hypothyroid symptom scale to assess the adequacy of thyroid hormone replacement therapy. The symptoms were chosen based on those commonly reported to be associated with hypothyroid symptoms that could be self assessed by the patient (10–16). Using this tool, we have identified persistently symptomatic hypothyroid patients who subsequent to appropriate changes in levothyroxine dosages then showed improvement.
Methodology
Patient Acquisition
Four hundred fifty-four unselected outpatients attending the Thyroid Clinic of the North Shore University Hospital at Manhasset, and 198 control women undergoing routine outpatient mammography were randomly asked to complete a questionnaire asking them to rate their physical symptoms related to thyroid disease. Follow up questionnaires were randomly obtained at the standard 3–6 month return visit to the Thyroid Clinic.
Questionnaire
This questionnaire consisted of 10 signs and symptoms including dry skin, fatigue, weight gain, cold intolerance, constipation, muscle stiffness, puffiness, memory loss, feeling blue, and dizziness. The severity of each sign and symptom was rated on a scale from 0 to 4 points: absent (0), minimal (1), mild (2), moderate (3), and severe (4). The item scores were totaled to obtain the overall signs and symptoms score ranging from 0 to 40 points (Figure 1).
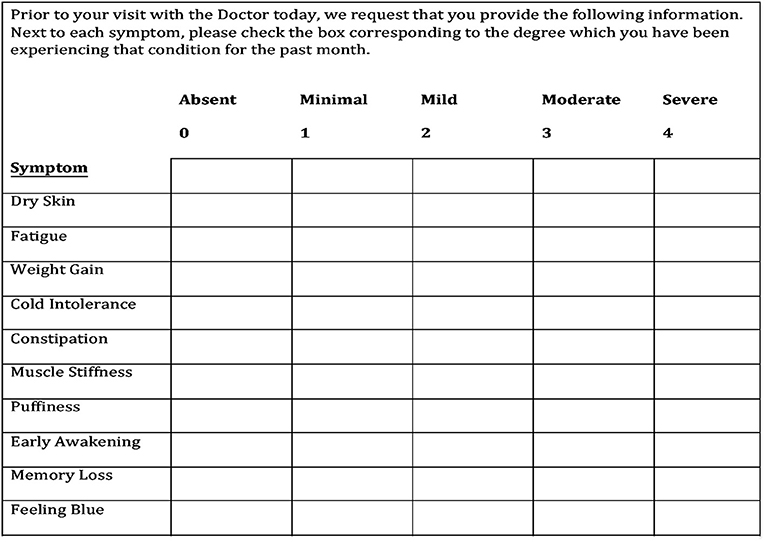
Figure 1. The hypothyroid signs and symptoms questionnaire. The final 10-symptom questionnaire is the result of refining an initial 12-symptom questionnaire after it was determined that two symptoms have no sensitivity or specificity for hypothyroidism.
Data Analysis
Data was collected from 652 patients from the department's patient population. The project was reviewed by the North Shore -LIJ IRB and found to be exempt. Patient and subject consents were not required. The data elements collected included patient age, patient ratings of physical symptoms and thyroid function measurements. For the controls, only age and physical symptoms were collected. Thyroid function measurements included TSH, T4, and T3 hormone levels. Patients were divided into groups based on their thyroid disease diagnosis for data analysis. The questionnaire was collected from 241 subjects with primary hypothyroidism (under treatment), 113 euthyroid subjects (benign nodular thyroid disease), 73 previously hyperthyroid subjects, and 27 subjects with thyroid cancer in addition to 198 control subjects with no known thyroid disease. Patients with hyperthyroidism were treated with either 131I or methimazole and thus became hypothyroid requiring replacement therapy. Thyroid cancer patients who have been thyroidectomized were also treated with thyroid hormone replacement therapy. A repeat questionnaire was obtained from 48 subjects with primary hypothyroidism (20%), 19 euthyroid subjects (17%), and 17 subjects who were previously hyperthyroid (23%) (Table 1). Data are expressed as the mean ± SE. Statistical analysis of the data obtained was done using SPSS 12.0.1 and Excel software.
Results
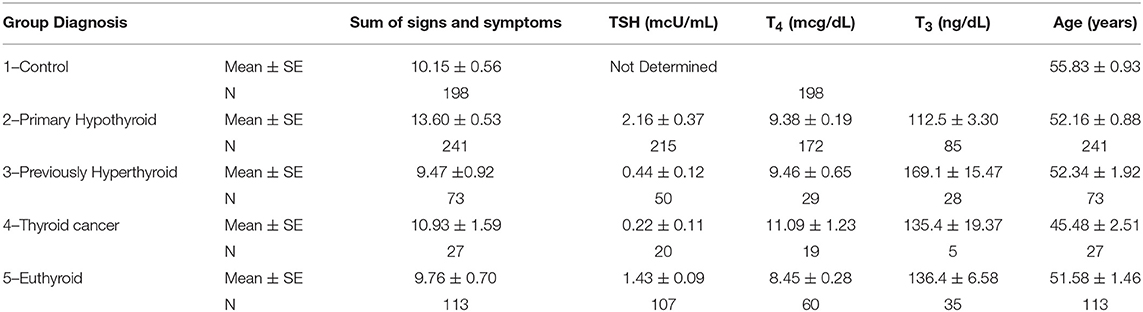
Table 2 summarizes our findings of all the study groups in this project for the sum of signs and symptoms, TSH, T4, T3, and the study subjects' age. The primary hypothyroid group had the highest ratings of symptoms as compared to other patients or controls despite treatment with levothyroxine sodium. Thyroid function tests (TSH, T4, and T3) also corresponded to the study groups' diagnoses. Thyroid function tests were not determined in the control study population. The mean age of all study participants was similar except for the thyroid cancer group. The mean of all symptoms in hypothyroid patients was significantly greater (13.6 ± 0.3) compared to all other groups (Table 2). The distribution of signs and symptoms among the study groups is shown in Figure 2. Significant differences (p ≤ 0.05) between hypothyroid and euthyroid subjects were found for fatigue, weight gain, cold weather intolerance, and puffiness. Significant differences (p ≤ 0.05) between hypothyroid and controls subjects were found for fatigue, weight gain, puffiness, and constipation.
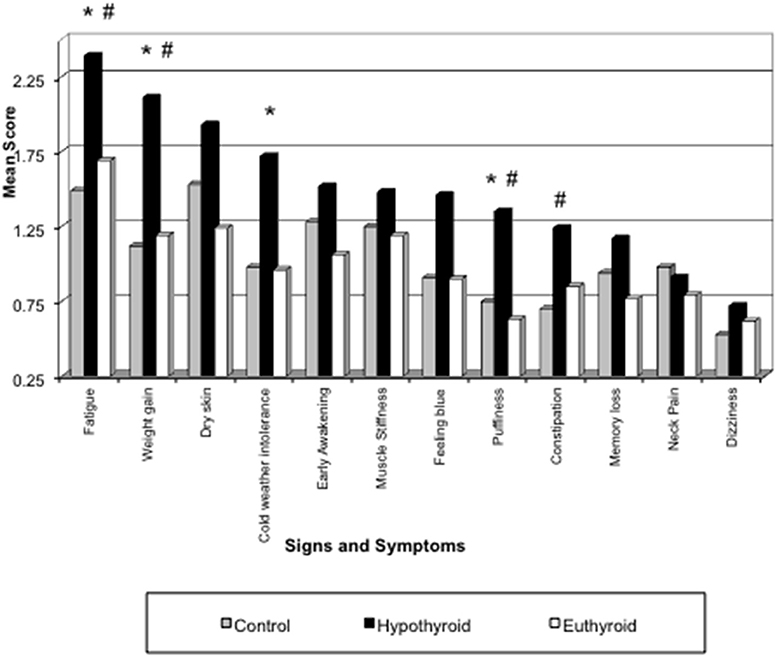
Figure 2. Distribution of signs and symptoms among the study groups. *p ≤ 0.05 for Hypothyroid vs. Euthyroid; #p ≤ 0.05 for Hypothyroid vs. Control.
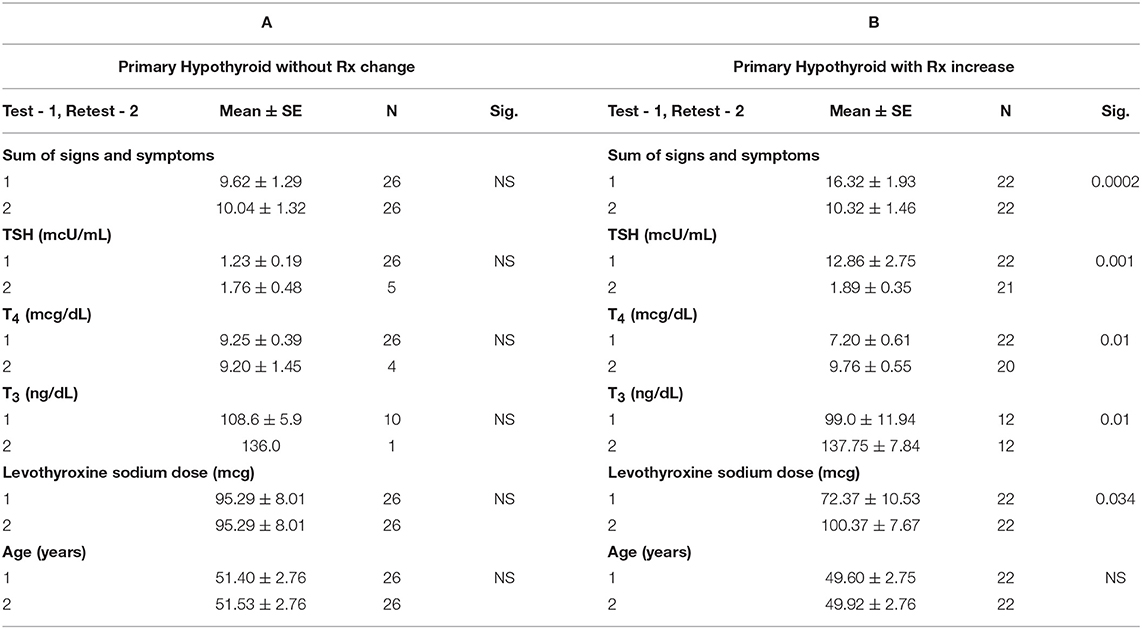
Within the primary hypothyroid group of subjects, 48 follow up questionnaires were obtained out of the original 241 study participants. The 48 follow up questionnaires included 22 study subjects who required increased thyroid medication during the study period and 26 study subjects who did not. The analyzed data also included serum TSH, T4, and T3 levels, which were obtained at the same time as the initial and follow up questionnaires.
The mean score for the sum of the signs and symptoms in the primary hypothyroid group with no medication change was 9.62 ± 1.29 for the initial questionnaire, and 10.04 ± 1.32 for the follow up questionnaire (p = 0.517) (Table 3).
For the primary hypothyroid patients requiring a medication change, at the time of the initial questionnaire the mean serum TSH was 12.86 ± 2.75 mcU/ml. With an average increase in levothyroxine from 72.37 ± 10.53 mcg to 100.37 ± 7.67 mcg between the initial and follow up points of the study, a statistically significant decrease of the mean TSH to 1.89 ± 0.35 was observed (p = 0.001). Concurrently with the normalization of TSH, a statistically significant improvement in the sum of signs and symptoms mean score for this group was noted (Table 3) (p = 0.0002). As expected, T4 and T3 levels increased, but remained within the normal range after the increase of levothyroxine sodium (p = 0.003 and 0.017, respectively).
Discussion
The results of our study demonstrated reproducibility and validity of this newly proposed hypothyroid signs and symptoms scale. There was no statistically significant change in the sum of the signs and symptoms in the primary hypothyroid group with no medication change between the initial and follow up visits, suggesting this novel scale's reproducibility. Also, the proposed hypothyroid signs and symptoms scale correctly identified patients with untreated or under treated primary hypothyroidism with the total mean score of 16.74 as compared to the euthyroid and control groups with the total mean scores of 11.31 and 12.40, respectively, suggesting this scale's validity.
Similarly to the results of Canaris et al. (15), our data showed that the symptoms associated with hypothyroidism were predictive of abnormal serum TSH, and the study participants with higher symptoms scores on the hypothyroid scale had significantly higher TSH values. Similarly to the results of Zulewski et al. (12), our hypothyroid scale showed a significantly higher signs and symptoms scores in the untreated or under treated hypothyroid group of patients as compared to the “baseline” score of the control, euthyroid, and adequately thyroxine—replaced hypothyroid study groups.
McAninch et al. (16) conducted a retrospective study that included 99 studies of hypothyroid patients treated with T4 monotherapy. Previous observations suggested that monotherapy with T4 may not be adequate because patients complained of persistent symptoms. Results demonstrated that in patients with T4 monotherapy and normalized serum TSH, not all systemic markers of thyroid hormone signaling were normalized, including serum LDL and total cholesterol. The failure to restore a euthyroid state with levothyroxine monotherapy may explain the ~15% dissatisfaction with hypothyroid patients treated with T4 alone. This is supported by Peterson et al. (17) who conducted an online survey of hypothyroid patients to determine their level of satisfaction with their current therapy or their physician. Higher satisfaction was reported by patients receiving desiccated thyroid extract followed by patients receiving combination T4 plus T3. The lowest satisfaction level was found in patients on T4 monotherapy. The study does not distinguish between physician and therapy dissatisfaction, but one can assume they are related.
A recent study reported on quality of life measures in hypothyroid patients using the Thyroid Patient-Reported Outcome (ThyPRO-39) questionnaire. Patients were on T4 monotherapy but experiencing persistent symptoms (18). Patients switched to combination LT4/LT3 combination therapy showed an improvement in quality of life measures, which was not associated with a change in TSH.
In preclinical studies, Escobar-Morreale et al. (19) demonstrated that the infusion of T4 alone to hypothyroid rats, at any dose, cannot normalize TSH, T4, and T3 in the blood, or in all tissues of the hypothyroid animal. In order to ensure normal T3 levels in all tissues supraphysiological plasma levels of T4 resulted. The minimal dose of T4 that resulted in normal plasma T4 and T3 was insufficient to normalize the concentration of T3 in most tissues analyzed including heart, lung, liver and kidney. Results also demonstrated differential uptake of circulating T4. This implies that in humans current replacement therapy with T4 alone would not be adequate to render all tissues euthyroid. In a second study by the same group (20) infusion of hypothyroid rats with T4 alone or T4 in combination with T3 (at three different doses) demonstrated that tissue euthyroidism is only possible when T4 is infused together with T3. In all treatment groups, plasma T4 was normalized but TSH and T3 in plasma and T3 in most tissues were only normalized with the combination of T4 plus T3.
In a clinical study by Celi et al. (21) 14 hypothyroid patients received either T4 monotherapy or T3 administered three times a day at doses that produced equivalent normalization of serum TSH. No difference was noted in TSH between groups. Results demonstrated that T3 treatment resulted in significant weight loss and a more favorable lipid profile (decreased total cholesterol and LDL cholesterol) when compared to T4 treatment, implying adequate tissue levels of serum T3 in these patients. T3 treatment produced no differences in cardiovascular function (heart rate, blood pressure, or exercise tolerance), HDLs, or in insulin sensitivity when compared to T4.
An easy to use hypothyroid symptom scale that identifies those patients with persistent symptoms is a critical component for addressing the needs of the 15% of patients who are not “satisfied” on their current therapy. In the case of suspected inadequate thyroid hormone replacement, the clinician may opt for LT4 dose increase, change in LT4 formulation or the addition of liothyronine (22). The proposed hypothyroid scale correctly identified the subjects with TSH elevation and clinical/subclinical hypothyroidism based on their clinical signs and symptoms. In this particular subset of patients, the hypothyroid symptom scale showed a statistically significant improvement in the sum of the signs and symptoms with the normalization of the subjects' thyroid function. At the same time, the sum of the signs and symptoms of patients with no change in medication remained unchanged, suggesting a reproducibility of this hypothyroid scale. This scale provides the clinician with an easily applied clinical tool to assess the adequacy of treatment in hypothyroid patients.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Reviewed by the North-Shore Hospital IRB in Manhasset, NY. Study was found to be EXEMPT as no identifying information was obtained.
Author Contributions
MB and IK: data collection, analysis, and manuscript preparation. SD: manuscript preparation and data analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Brent GA. The molecular basis of thyroid hormone action. N Engl J Med. (1994) 331:847–54. doi: 10.1056/NEJM199409293311306
2. Amico JA, Richardson V, Alpert B, Klein I. Clinical and chemical assessment of thyroid function during therapy with amiodarone. Arch Intern Med. (1984) 144:487–90. doi: 10.1001/archinte.144.3.487
3. Brucker-Davis F, Skarulis MC, Grace MB, Benichou J, Hauser P, Wiggs E, et al. Genetic and clinical features of 42 kindreds with resistance to thyroid hormone: The National Institutes of Health Prospective Study. Ann Intern Med. (1995) 123:572–83. doi: 10.7326/0003-4819-123-8-199510150-00002
4. DeGroot LJ. Dangerous dogmas in medicine: the non-thyroidal illness syndrome. J Clin Endocrinol Metab. (1999) 84:151–64. doi: 10.1210/jcem.84.6.5809-8
5. Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr Pract. (1999) 5:233–38. doi: 10.4158/EP.5.5.233
6. Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med. (1997) 126:63–9. doi: 10.7326/0003-4819-126-1-199701010-00009
7. Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Internal Med. (1994) 121:281–88. doi: 10.7326/0003-4819-121-4-199408150-00010
8. Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. (1993) 13:348–99. doi: 10.1210/edrv-14-3-348
9. Klein I, Ojamaa K. Thyroid (neuro) myopathy. Lancet. (2000) 356:614. doi: 10.1016/S0140-6736(00)02601-5
10. Trzepacz PT, Klein I, Roberts M, Greenhouse J, Levey GS. Graves' disease: an analysis of thyroid hormone levels and hyperthyroid signs and symptoms. Am J Med. (1989) 87:558–61. doi: 10.1016/0002-9343(89)90698-0
11. Billewicz WZ, Chapman RS, Crooks J, Day ME, Gossage J, Wayne E, et al. Statistical methods applied to the diagnosis of hypothyroidism. Q J Med. (1969) 38:255–66.
12. Zulewski H, Müller B, Exer P, Miserez AR, Staub JJ. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab. (1997) 82:771–76. doi: 10.1210/jc.82.3.771
13. Indra R, Patil SS, Joshi R, Pai M, Kalantri SP. Accuracy of physical examination in the diagnosis of hypothyroidism: a cross-sectional, double-blind study. J Postgrad Med. (2004) 50:7–10.
14. Bajaj S, Sharma GP, Kumar D, Mehrotra R. Dissociation of clinical and laboratory diagnosis in hypothyroidism. J Assoc Phys. (2005) 53:15–18.
15. Canaris G, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Intern Med. (1997) 12:544–50. doi: 10.1046/j.1525-1497.1997.07109.x
16. McAninch EA, Rajan KB, Miller CH, Bianco AC. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2018) 103:4533–42. doi: 10.1210/jc.2018-01361
17. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. (2018) 28:707–21. doi: 10.1089/thy.2017.0681
18. Michaelsson LF, la Cour JL, Medici BB, Watt T, Faber J, Nygaard B. Levothyroxine/liothyronine combination therapy and quality of life: is it all about weight loss? Eur Thyroid J. (2018) 7:243–50. doi: 10.1159/000490383
19. Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. (1995) 96:2828–38. doi: 10.1172/JCI118353
20. Escobar-Morreale HF, del Rey FE, Obregón MJ, de Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. (1996) 137:2490–502. doi: 10.1210/en.137.6.2490
21. Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, et al. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrin Metab. (2011) 96:3466–74. doi: 10.1210/jc.2011-1329
Keywords: symptom scale, T4, T3, treatment, diagnosis of hypothyroidism
Citation: Brokhin M, Danzi S and Klein I (2019) Assessment of the Adequacy of Thyroid Hormone Replacement Therapy in Hypothyroidism. Front. Endocrinol. 10:631. doi: 10.3389/fendo.2019.00631
Received: 25 April 2019; Accepted: 30 August 2019;
Published: 20 September 2019.
Edited by:
Jacqueline Jonklaas, Georgetown University, United StatesReviewed by:
Roberto Vita, University of Messina, ItalyGisah Amaral De Carvalho, Federal University of Paraná, Brazil
Copyright © 2019 Brokhin, Danzi and Klein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Danzi, c2FyYWRhbnppJiN4MDAwNDA7Z21haWwuY29t
 Matvey Brokhin1
Matvey Brokhin1 Sara Danzi
Sara Danzi Irwin Klein
Irwin Klein