
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 11 September 2019
Sec. Cancer Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00619
This article is part of the Research Topic Testicular Cancer: New Insights on the Origin, Genetics, Treatment, Fertility, General Health, Quality of Life and Sexual Function View all 18 articles
 Giuseppe Grande1,2
Giuseppe Grande1,2 Domenico Milardi1,2*
Domenico Milardi1,2* Maurizio Martini3
Maurizio Martini3 Tonia Cenci3
Tonia Cenci3 Gaetano Gulino4
Gaetano Gulino4 Francesca Mancini2
Francesca Mancini2 Antonio Bianchi1
Antonio Bianchi1 Alfredo Pontecorvi1,2
Alfredo Pontecorvi1,2 Francesco Pierconti3
Francesco Pierconti3Seminomas are the most frequent kind of testicular germ cell tumors (TGCTs), accounting for 50% of tumor diagnosis in young men, whereas non-seminomas account for 40% and mixed forms for 10% of cases. It is currently supposed that TGCTs evolve from a pre-invasive stage of carcinoma in situ (CIS). Octamer-binding transcription factor 4 (OCT4) is essential for self-renewal of stem cells. It is considered as a major regulator of cell pluripotency. Prior studies have shown that seminoma expresses OCT4. Transcription factor Krüppel-like factor 4 (KLF4) has moreover associated with embryonic stem cell maintenance. Finally, we previously demonstrated the expression of PTTG1 in CIS and seminomas. In this pilot study, we compared the combined expression of PTTG1 with KLF4 and OCT4 in seminoma, in order to validate our hypotesis that PTTG1 marks a specific population of stem cells in neoplastic tissue, strictly related with tumor. Formalin-fixed and paraffin-embedded testicular tissues by 5 patients who underwent an orchidectomy for seminoma have been collected and immunofluorescence analysis was performed using antibody rabbit monoclonal PTTG-1 and mouse monoclonal OCT4 or mouse monoclonal KLF4 antibody. In seminoma we observed that tumor cells strongly express OCT-4 in all seminomas and in the intratubular areas of seminoma. Expression of KLF-4 was observed in many tumor cells. PTTG1 marks some specific OCT4- and KLF4-positive tumor cells, mainly localized at the periphery of the neoplasm. In the intertubular infiltration areas nests of cells expressing both OCT4/KLF4 and PTTG1 have been observed. This is the first identification of a cell population in seminoma characterized for being OCT4, KLF4, and PTTG1 positive cells in seminoma, associated with cancer invasiveness. Further investigation is needed to elucidate if a functional abrogation of PTTG1 might be used in order to offer new therapeutic approaches in the clinical workout of seminoma.
Seminomas are the most frequent type of testicular germ cell tumors (TGCTs), accounting for 50% of cases in young men, whereas non-seminomas account for 40% and mixed forms for 10% of cases (1). Despite the high prevalence of TGCTs, the molecular mechanisms associated with their development have not been still completely clarified.
It is currently supposed that TGCTs evolve from a pre-invasive stage of carcinoma in situ (CIS) (2).
CIS are macroscopically distinct cells that are located on the basement membrane of the seminiferous tubules in the testis and have specific morphological features more similar to embryonic germ cells than spermatogonial stem cells (3). CIS are considered the precursors of seminomas since they both histologically resemble primordial germ cells (PGCs) and gonocytes and have a positive staining for c-kit and PLAP.
For instance, the oncogene c-kit, which encodes for a transmembrane tyrosine kinase receptor, is highly expressed in TGCTs. C-kit has as its specific ligand the stem cell factors and it is required for normal development of germ cells (4, 5). c-kit is highly expressed in seminomas and teratomas (6).
Placental alkaline phosphatase (PLAP) is moreover considered a widely used marker for TGCTs (7).
Apart from the well-known markers (i.e., PLAP and c-kit), previous studies have been carried out to identify new molecular markers for TGCTs.
Octamer-binding transcription factor 4 (OCT4) is a homeobox transcription factor that is essential for self-renewal of stem cells. It is considered as a major regulator of cell pluripotency (8). Importantly, it has been implicated in tumorigenesis of primordial germ cells. Prior studies demonstrated the expression of OCT4 in seminoma (9).
Transcription factor Krüppel-like factor 4 (KLF4) is strongly expressed in postmeiotic spermatids and in Leydig cells, but has been not reported in spermatogonia (10). KLF4 is involved in embryonic stem (ES) cell maintenance (11, 12). Simultaneous depletion of Klf4, Klf2, and Klf5 lead to ES cell differentiation, confirming the critical role of KLF4 in the maintenance of ES cell pluripotency and selfrenewal. Moreover, KLF4 was used, associated with other transcriptional factors, to induce pluripotency in differentiated cells (13). Finally, KLF4 was expressed in mouse spermatogonial stem cells shortly after withdrawal from the stem cell niche (14) in addition to pluripotent cells derived from human testis.
Previous data reported that altered levels of Pituitary-tumor-transforming-gene 1 (PTTG1) are expressed in pre-cancer lesions, suggesting that PTTG1 has a role in human tumorigenesis (15). We previously examined firstly the expression of PTTG1 in CIS and seminomas (16). In CIS, only isolated cells express PTTG1. Furthermore, in the peripheral area of seminoma, PTTG1 was mostly detected as localized in the nucleus, whereas in the central nucleus of seminoma, PTTG1 was mainly expressed in cytoplasm. Moreover, in the zones of seminoma infiltration we demonstrated the presence of clusters of PTTG1-positive cells. We hypotyzed that PTTG1 marks a population of neoplastic cells, both in CIS and in seminoma, so linking CIS to seminoma carcinogenesis. Interestingly, no differences have been observed in the expression of PTTG1 in foci and micronodules of seminoma, so that we hypothesized that when the tumor has a small size, in the early stage of the carcinogenesis, PTTG-1 expression is homogeneously distributed. On the contrary, with the increasing tumor size, this subgroup of nuclear PTTG1-positive cells move from the center to the periphery of the tumor, and it might be associated with neoplastic infiltration of surrounding tissue. PTTG1 in fact is known to play an important role in tumor infiltration and neoplastic angiogenesis. PTTG1 expression in neoplastic cells on the tumor infiltration area and in the intertubular spaces may reflect this property important for tumor cells in invading surrounding tissues and inducing neoplastic angiogenesis.
In this pilot study, we compared the combined expression of PTTG1 with KLF4 and OCT4 in seminoma, in order to validate our hypothesis that PTTG1 could mark a specific subset of neoplastic stem cells, strictly related with tumor.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from each patient.
Formalin-fixed and paraffin-embedded testicular tissues by 5 patients who underwent an orchidectomy for seminoma were collected at the Department of Surgical Pathology Fondazione Policlinico “A. Gemelli” from 2014 to 2017. The age of the patients ranged between 25 and 70 years with a median of 36.
After deparaffinization tissues slides were rehydrated using a graded alcohol solution. Antigen retrieval was performed in 10 mM citrate buffer at pH 6.0 for 10 min in microwave oven. After allowing to cool room temperature, slides were washed twice in distilled water for 2 min and sequentially rinsed once in PBS for 5 min.
To confirm the diagnosis of seminoma we evaluated the co-expression of PLAP and c-kit. For detection of PLAP the monoclonal antibody 8A9 (Dako, Hamburg, Germany; dilution 1:50) was chosen because staining with this antibody is more sensitive compared with other PLAP antibodies.c-KIT was detected by rabbit polyclonal antibody (c-KIT antibody from Dako; dilution 1:100). All primary antibodies were incubated overnight at 4°C. Immunoreactions were visualized by means of the avidin–biotin–complex (ABC method) using AEC (3-amino-9-ethylcarbazol) as chromogen on an immunostainer (Techmate 500; Dako).
Sections have been then incubated a room temperature with primary antibody rabbit monoclonal PTTG-1 (Securin, clone EPR3240, abcam, Cambrige, UK, 1:500 for 1 h). The PTTG-1 were visualized using the highly cross-adsorbed, Alexa Fluor 488-conjugated goat anti-Rabbit IgG secondary antibody (TermoFisher Scientific, USA, 1:1000 for 1 h). After the slides were rinsed in PBS and subsequently were incubated with mouse monoclonal OCT4 (clone OTI9B7, Novus Biological, UK 1:50 for 30 min) or with mouse monoclonal KLF4 (clone CL5785, Novus Biological, 1:1000 for 30 min). This antibodies were visualized using the highly cross-adsorbed, Alexa Flour 594-conjugated goat anti-Mouse IgG secondary antibody (TermoFisher Scientific, 1:1000 for 1 h). Slides are rinsed in PBS, mounted in Vectashield (H-1000, Vector Laboratories, Peterborough, UK) and double immunofluorescence slides were visually examined under immunofluorescence microscopy using an Olympus BX41 fluorescence microscope (Olympus, Although Center, Valley;PA, USA) and digital images were captured using and attached Olympus DP71 digital camera with the x40 objective.
Clinical and ultrasonographic informations are reported in Table 1.
All seminomas demonstrated the co-expression of PLAP and c-kit.
We moreover observed that tumor cells strongly express OCT-4 in all seminoma cells and in the areas of intratubular seminoma (Figure 1A). Expression of KLF-4 was observed in many tumor cells (Figure 1B). PTTG1 marks some specific OCT4-(Figure 1A) and KLF4-positive (Figure 1B) tumor cells, mainly localized at the periphery of the neoplasm.
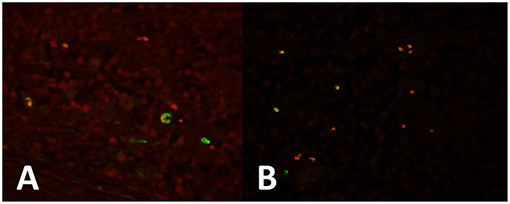
Figure 1. (A) Seminoma expresses strong OCT4 expression in all cells (red). PTTG1 marks some specific OCT4-positive (green) tumor cells, mainly localized at the periphery of the neoplasm. (B) KLF-4 is expressed in some tumor cells (red). PTTG1 marks some specific KLF4-positive tumor cells (green).
In the intertubular infiltration areas nests of cells expressing both OCT4/KLF4 and PTTG1 (Figures 2A,B, respectively) have been observed.
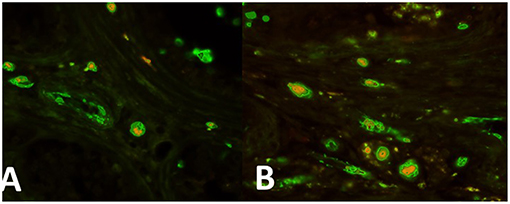
Figure 2. (A) Nests of cells expressing both OCT4 (green) and PTTG1 (yellow) in the intertubular infiltration infiltration area. (B) Areas nests of cells expressing both KLF4 (green) and PTTG1 (yellow) in the intertubular infiltration infiltration area.
PTTG1 is a securin protein involved in facilitating sister-chromatid separation in metaphase (17) and exerts a crucial role during the mitosis by defending the chromosomal stability (18). In normal testicular tubules PTTG1 has been identified in normal spermatocytes and spermatids, suggesting that PTTG-1 may play a pivotal function in spermatogenesis in testis, and specifically in differentiation and survival of germ cells (16). PTTG1 in moreover an oncogene since its overexpression induces aneuploidy (19) and stimulates tumor formation, as previously reported in pituitary, thyroid, breast, uterine, ovarian, lung and colon tumors (20–24). In malignant tumors, previous data demonstrated the association between PTTG1 levels, tumor angiogenesis and metastasis (24). Moreover, high expression of PTTG1 is associated with greatly aggressive tumor and with the onset of metastasis (25).
PTTG1 has been demonstrated to contribute to cell migration, invasion and angiogenesis by induction of MMP-2 secretion and expression (26). In lung cancer has been demonstrated the concomitant overexpression of PTTG1 or MMP9. Xu et al. demonstrated that the co-expression of PTTG1 and MMP9 is associated with tumor cell migration and proliferation (27). PTTG1 might also play a pivotal role in inducing tumor angiogenesis in seminoma mainly through the regulation of the expression of many angiogenic factors, including bFGF, VEGF and IL-8 (28–30).
In this study we confirmed that some cells in seminoma express PTTG1 and demonstrated that it is a sub-population of tumor stem cells OCT4- and KLF4- positive cells.
Germ cells, in the early phases of embryonic development, are reserved as primordial germ cells, in order to escape the signals involved in the differentiation of the somatic cells of the embryo (31). Primordial germ cells preserve so their undifferentiated state, as demonstrated by the expression of a lot of stem cell markers, including OCT4, NANOG, stage-specific embryonic antigens and tumor rejection antigens (2, 32). The spermatogonial stem cells (SSC) can moreover be reprogrammed in cell culture to recover a pluripotent state. These findings demonstrate that the SSC are in a relatively primitive developmental state, permitting the reconversion into an early embryonic, pluripotent state without any genetic modification. Molecular studies demonstrated that SSC occasionally functionally go away from the control of their niche, which naturally restricts their developmental potency to normal spermatogenesis and also regulates proliferation. If the stem cell niche fails to control the proliferation of gonocytes or SSC, a transformation of germline stem cells is supposed to occur, thus resulting in a CIS (33, 34). This early neoplasia can subsequently produce a seminoma or an embryonal carcinoma (35).
Furthermore, we have reported in intertubular infiltration areas the presence of nests of cells expressing both OCT4/KLF4 and PTTG1. Malik reported that PTTG1 is associated with cell angiogenesis, migration and invasion. PTTG1 in fact induces expression and secretion of MMP-2 (26). The functional blocking of PTTG1 may induce suppression of tumor growth and metastasis development, by the down-regulation of MMP-2 expression. Moreover, it is known that PTTG1 over-expression is associated with the secretion and expression of MMP-2. Previous data in HUVEC cells have been reported demonstrating that MMP-2 regulates cell migration, invasion, and endothelial tubule formation. All these data may bring to the conclusion that PTTG1 serves as one of the principal controller of MMP-2 and that some of the oncogenic effects of PTTG1 are mediated through the regulation of expression of MMP-2 (29). In neoplastic cells the expression of PTTG1 localized in the intertubular spaces, associated with OCT4 and KLF4 expression, suggest us that PTTG1 mark a specific subpopulation of SSC, characterized by high invasivity. The expression in seminoma stem cells of PTTG1 may permit them to invade surrounding tissues and to lead to neoplastic angiogenesis.
This study presents some limitations, which are represented by the limited number of clinical cases investigated and the absence of control cases (i.e., non-germ cell tumors).
This pilot study provides the first identification of a cell population in seminoma characterized for being OCT4, KLF4, and PTTG1 positive cells in seminoma, associated with cancer invasiveness. Further investigation are needed to extend the number of clinical cases investigated, to analyze the co-localization of PTTG1 with MMP2, MMP9, VEGF and to clarify if a functional abrogation of PTTG1 might represent a novel therapeutic approaches in the clinical management of seminoma.
All datasets for this study are included in the manuscript/supplementary files.
Institutional Scientific Board of International Scientific Institute Paul VI approved the study. This study was conducted in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from each patient.
DM and FP: conceptualization. DM and GGr: literature analysis. MM, TC, and FM performed immunofluorescence analysis. DM and GGr: writing—original draft preparation. GGu and AB: writing—review and editing. AP: supervision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Esther Mahoney (Cardiff, UK) for her kind and careful English editing.
1. Vasdev N, Moon A, Thorpe AC. Classification, epidemiology and therapies for testicular germ cell tumours. Int J Dev Biol. (2013) 57:133–9. doi: 10.1387/ijdb.130031nv
2. Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. (2006) 12:303–23. doi: 10.1093/humupd/dmk006
3. van Echten J, van Gurp RJ, Stoepker M, Looijenga LH, de Jong J, Oosterhuis W. Cytogenetic evidence that carcinoma in situ is the precursor lesion for invasive testicular germ cell tumors. Cancer Genet Cytogenet. (1995) 85:133–7.
4. Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. (1990) 63:213–24.
5. Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, et al. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice–evidence for an impaired c-kit kinase in mutant mice. Genes Dev. (1989) 3:816–26. doi: 10.1101/gad.3.6.816
6. Nakai Y, Nonomura N, Oka D, Shiba M, Arai Y, Nakayama M, et al. KIT (c-kit oncogene product) pathway is constitutively activated in human testicular germ cell tumors. Biochem Biophys Res Commun. (2005) 337:289–96. doi: 10.1016/j.bbrc.2005.09.042
7. Rajpert-De Meyts E, Nielsen JE, Skakkebaek NE, Almstrup K. Diagnostic markers for germ cell neoplasms: from placental-like alkaline phosphatase to micro-RNAs. Folia Histochem Cytobiol. (2015) 53:177–88. doi: 10.5603/FHC.a2015.0020
8. Jerabek S, Merino F, Schöler HR, Cojocaru V. OCT4: Dynamic DNA binding pioneers stem cell pluripotency. Biochim Biophys Acta - Gene Regul Mech. (2014) 1839:138–54. doi: 10.1016/j.bbagrm.2013.10.001
9. Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. (2004) 28:935–40. doi: 10.1097/00000478-200407000-00014
10. Behr R, Deller C, Godmann M, Muller T, Bergmann M, Ivell R, et al. Kruppel-like factor 4 expression in normal and pathological human testes. Mol Hum Reprod. (2007) 13:815–820. doi: 10.1093/molehr/gam064
11. Li Y, McClintick J, Zhong L, Edenberg HJ, Yoder MC, Chan RJ. Murine embryonic stem cell differentiation is promoted by SOCS-3 and inhibited by the zinc finger transcription factor Klf4. Blood. (2005) 105:635–637. doi: 10.1182/blood-2004-07-2681
12. Jiang J, Chan YS, Loh YH, Cai J, Tong G-Q, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. (2008) 10:353–360. doi: 10.1038/ncb1698
13. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. (2007) 448:313–17. doi: 10.1038/nature05934
14. Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, Toyokuni S, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. (2008) 78:681–7. doi: 10.1095/biolreprod.107.066068
15. Vlotides G, Eigler T, Melmed S. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev. (2007) 28:165–86. doi: 10.1210/er.2006-0042
16. Pierconti F, Milardi D, Martini M, Grande G, Cenci T, Gulino G, et al. Pituitary-tumour-transforming-gene 1 expression in testicular cancer. Andrologia. (2015) 47:427–32. doi: 10.1111/and.12283
17. Jallepalli PV, Waizenegger IC, Bunz F, Langer S, Speicher MR, Peters JM, et al. Securin is required for chromosomal stability in human cells. Cell. (2001) 105:445–57. doi: 10.1016/s0092-8674(01)00340-3
18. Wang Z, Yu R, Melmed S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol. (2001) 15:1870–9. doi: 10.1210/mend.15.11.0729
19. Kakar SS. Molecular cloning, genomic organization, and identification of the promoter for the human pituitary tumor transforming gene (PTTG). Gene. (1999) 240:317–24.
20. Zhang X, Horwitz GA, Heaney AP, Nakashima M, Prezant TR, Bronstein MD, et al. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. (1999) 84:761–767. doi: 10.1210/jcem.84.2.5432
21. Watkins RJ, Read ML, Smith VE, Sharma N, Reynolds GM, Buckley L, et al. Pituitary tumor transforming gene binding factor: a new gene in breast cancer. Cancer Res. (2010) 70:3739–49. doi: 10.1158/0008-5472.CAN-09-3531
22. Hsueh C, Lin JD, Chang YS, Hsueh S, Chao TC, Yu JS, et al. Prognostic significance of pituitary tumour-transforming gene-binding factor (PBF) expression in papillary thyroid carcinoma. Clin Endocrinol. (2013) 78:303–309. doi: 10.1111/cen.12007
23. Li H, Yin C, Zhang B, Sun Y, Shi L, Liu N, et al. PTTG1 promotes migration and invasion of human non-small cell lung cancer cells and is modulated by miR-186. Carcinogenesis. (2013) 34:2145–55. doi: 10.1093/carcin/bgt158
24. Zhou C, Tong Y, Wawrowsky K, Melmed S. PTTG acts as a STAT3 target gene for colorectal cancer cell growth and motility. Oncogene. (2014) 33:851–61. doi: 10.1038/onc.2013.16
25. Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. (2003) 33:49–54. doi: 10.1038/ng1060
26. Malik MT, Kakar SS. Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2). Mol Cancer. (2006) 5:61. doi: 10.1186/1476-4598-5-61
27. Xu X, Cao L, Zhang Y, Yin Y, Hu X, Cui Y. Network analysis of DEGs and verification experiments reveal the notable roles of PTTG1 and MMP9 in lung cancer. Oncol Lett. (2018) 15:257–263. doi: 10.3892/ol.2017.7329
28. McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, et al. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab. (2002) 87:4238–44. doi: 10.1210/jc.2002-020309
29. Kim DS, Franklyn JA, Boelaert K, Eggo MC, Watkinson JC, McCabe CJ. Pituitary tumor transforming gene (PTTG) stimulates thyroid cell proliferation via a vascular endothelial growth factor/kinase insert domain receptor/inhibitor of DNA binding-3 autocrine pathway. J Clin Endocrinol Metab. (2006) 91:4603–4611. doi: 10.1210/jc.2006-1291
30. Kim DS, Franklyn JA, Stratford AL, Boelaert K, Watkinson JC, Eggo MC, et al. Pituitary tumor-transforming gene regulates multiple downstream angiogenic genes in thyroid cancer. J Clin Endocrinol Metab. (2006) 91:1119–1128. doi: 10.1210/jc.2005-1826
31. McLaren A. Primordial germ cells in the mouse. Dev Biol. (2003) 262:1–15. doi: 10.1016/s0012-1606(03)00214-8
32. Kerr CL, Hill CM, Blumenthal PD, Gearhart JD. Expression of pluripotent stem cell markers in the human fetal testis. Stem Cells. (2008) 26:412–421. doi: 10.1634/stemcells.2007-0605
33. Bahrami A, Ro JY, Ayala AG. An overview of testicular germ cell tumors. Arch Pathol Lab Med. (2007) 131:1267–80. doi: 10.1043/1543-2165(2007)131<1267:AOOTGC>2.0.CO;2
34. Clark AT. The stem cell identity of testicular cancer. Stem Cell Rev. (2007) 3:49–59. doi: 10.1007/s12015-007-0002-x
Keywords: testis cancer, seminoma, PTTG-1, OCT-4, KLF-4, infiltration
Citation: Grande G, Milardi D, Martini M, Cenci T, Gulino G, Mancini F, Bianchi A, Pontecorvi A and Pierconti F (2019) Protein Expression of PTTG-1, OCT-4, and KLF-4 in Seminoma: A Pilot Study. Front. Endocrinol. 10:619. doi: 10.3389/fendo.2019.00619
Received: 28 February 2019; Accepted: 27 August 2019;
Published: 11 September 2019.
Edited by:
Donatella Paoli, Sapienza University of Rome, ItalyReviewed by:
Andrea M. Isidori, Sapienza University of Rome, ItalyCopyright © 2019 Grande, Milardi, Martini, Cenci, Gulino, Mancini, Bianchi, Pontecorvi and Pierconti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico Milardi, bWlsYXJkaWRAeWFob28uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.