
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 12 July 2019
Sec. Clinical Diabetes
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00455
 Zhi Sheng1
Zhi Sheng1 Jia-Yu Cao1
Jia-Yu Cao1 Ying-Chang Pang1
Ying-Chang Pang1 Hang-Cheng Xu1
Hang-Cheng Xu1 Jing-Wen Chen1
Jing-Wen Chen1 Jun-Hua Yuan2
Jun-Hua Yuan2 Rui Wang2
Rui Wang2 Cai-Shun Zhang2
Cai-Shun Zhang2 Liu-Xin Wang2
Liu-Xin Wang2 Jing Dong2,3*
Jing Dong2,3*Background: Pre-diabetes is a risk factor for full-blown diabetes; it presents opportunities to prevent the actual diseases. It is therefore essential to identify effective preventive strategies, and to clarify the direction of future research.
Methods: PubMed, Embase and Cochrane Central Register of Controlled Trials were searched using key terms (Supplementary Table 1). We applied network meta-analysis to multiple comparisons among various diabetic preventive strategies, including lifestyle and pharmacological interventions; traditional meta-analysis for the synthesis of basal metabolic changes after interventions; and trial sequential analysis for determinations as to whether analysis conclusions meet expectations.
Results: We included 32 randomized controlled trials comprising 43,669 patients and 14 interventions in the meta-analysis. Both lifestyle modifications and anti-diabetic medications improved physical conditions, including weight loss, blood glucose, and blood pressure. Network meta-analysis suggested that the progression of diabetes could be delayed to varying degrees by lifestyle and pharmacological interventions, except for angiotensin-converting enzyme inhibitors, statins, sulfonylureas and vitamin D. The risk ratios (RR) [95% credible interval (CrI)] compared with control were: GLP-1RAs 0.28 (0.15, 0.50), Orlistat 0.33 (0.18, 0.55), TZM 0.33 (0.16, 0.63), TZD 0.39 (0.27, 0.53), LST 0.54 (0.32, 0.88), lifestyle 0.58 (0.49, 0.67), LSM 0.62 (0.45, 0.80), GI 0.66 (0.46, 0.88), SU 0.67 (0.40, 1.00), Vitamin D 0.91 (0.59, 1.40), ACEI 0.93 (0.62, 1.40), statins 1.20 (0.84, 1.60).
Conclusions: In adults with pre-diabetes, firm evidence supports the notion that lifestyle modifications and metformin reduces the incidence of diabetes with an average of 20% relative risk reduction, while statins increase the relative risk 20%. We found that lifestyle modifications, promising long-term strategies involving three factors (nutrition, exercise, and weight loss) contribute to health by reducing BMI, body weight, waist and hip circumference, systolic and diastolic pressure, fasting, and 2-h postprandial blood glucose, total cholesterol and by increasing HDL. We made this determination using TSA, avoiding further waste of experimental resources.
Type 2 diabetes mellitus (hereafter referred to as diabetes) is a major health problem associated with excessive morbidity and mortality, affecting approximately 5% of adults worldwide with rapidly rising prevalence (1, 2). Pre-diabetes, the precursor stage of diabetes, includes impaired fasting glucose (IFG), and impaired glucose tolerance (IGT), characterized by fasting plasma glucose (PFG) ≥6.1 and <7.0 or 2-h plasma glucose (2hPG) ≥7.8 and <11.1 (3). There were recently introduced as risk factors for both diabetes and cardiovascular disease by the American Diabetes Association (4). As many as 5–10% of individuals with pre-diabetes develop diabetes each year (5), and approximately 70% of these will progress to diabetes during their lifetime (6). Fortunately, prevention of diabetes in the pre-diabetes stage can restore normal blood glucose levels (FPG <6.1, 2hPG <7.8), making early intervention crucial (7).
Guidelines from the American Diabetes Association suggest that individuals with pre-diabetes should undertake lifestyle modification to prevent the onset of diabetes, with healthy meals, increased physical exercise and weight reduction (8). Prescription medication has also been considered for the prediabetic population. Evidence supports that not only classic anti-diabetes drugs such as metformin and acarbose, but also newer agents such as GLP-1 receptor agonists help prevent the development of diabetes. Several randomized controlled trials (RCTs) diabetes-prevention strategies (lifestyle and/or pharmacological interventions) have been conducted (9, 10) and the literature has reviewed current achievements (11). Nevertheless, modern clinical therapies demand complex analyses for decision-making processes (12), in spite of traditional meta-analyses. Therefore, to assess physical outcomes of pre-diabetes interventions and to interpret the contemporary state of pre-diabetes research, we performed a Bayesian network meta-analysis and trial sequential analysis.
The protocol of this review was registered in PROSPERO (ID: CRD 42018095121). Two review authors individually searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL) with database-appropriate terms and the text words (Supplementary Table 1). The reference lists of potentially relevant reviews were also screened. All references were eligible for inclusion regardless of language, published year and status.
(i) RCTs published in peer-reviewed journals between 1/1/1965 and 1/5/2018;
(ii) Patients were adults with pre-diabetes;
(iii) Group allocation was based on lifestyle or medication interventions;
(iv) Participants were randomly assigned;
(v) Cumulative duration of interventions and follow-ups had a minimum with 1 year;
(vi) Objective results were available, including the incidence of diabetes, regression of pre-diabetes, and physical condition changes.
(i) Medications that were forbidden in routine clinical practice, e.g., troglitazone (13) and phenformin (14) (Supplementary Table 3);
(ii) With the compliance of American Diabetes Association guidelines, lifestyle modification standardized to include both adjusted healthy meals and increased physical exercise (Supplementary Table 2) to ensure homogeneity, e.g., a study that only included health education (15);
(iii) Patients with a history of cardiovascular events and other diseases.
We extracted the incidence of diabetes, remission rate of pre-diabetes, and physical consequences with the principle of intention to treat analysis. Study quality was assessed using the Cochrane Collaboration's tool for risk bias (16).
Analyses were performed using Mantel-Haenszel and Bayesian random effects models using RevMan (version 3.4.3) and R software (version 3.4.4, www.r-project.org), respectively. The Cochran-Mantel-Haenszel test (CMH) was used in the analysis of stratified or matched categorical data, allowing an investigator to test the association between a binary predictor or treatment and a binary outcome. The Bayesian random effects model, a classical statistical methods of network meta-analysis, uses posterior probability to rank all the interventions involved in the comparisons and avoids the bias caused by repeated iteration in the estimation of parameters by frequency theory.
Consistent and simultaneous estimates of all interventions were obtained using Markov Chain Monte Carlo simulations using WinBugs software (version 1.4.3, http://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-winbugs/). The results were recorded with RR and 95% credible interval (CrI). Trial sequential analysis (TSA, version 0.9.5.10 Beta, http://www.ctu.dk/tsa/downloads.aspx) was managed to evaluate the cumulative evidence according to the information size achieved to date. When the same studies were repeatedly observed, the probability of a Type 1 error increased. Therefore, trial sequential analysis was intended to evaluate the overall Type 1 error rate assured at the desired level. Furthermore, with the trial sequential analysis, a conclusion may sometimes be reached at a much earlier stage once significant results are observed, at consequently lower financial and/or human cost. The cumulative ranking plot and the surface under the cumulative ranking (SUCRA) helps the researcher make decisions. The values of SUCRA are between 0 and 1 (0 ≤ SUCRA ≤ 1). When SUCRA is 1, the intervention is absolutely valid, and when it is 0, the intervention is absolutely invalid.
Results relating to identification and selection of eligible 32 RCTs with outcome data for the incidence of diabetes are summarized in Figure 1. These studies included 43,669 participants with a mean follow-up of 3.3 years ranging from 1 to 6 years.
The 32 RCTs included in the network meta-analysis are summarized in Table 1. In the network of available intervention comparisons, twelve trials focused solely on the effectiveness of lifestyle modification (all of these combined diet and exercise and health education was excluded), fourteen studies compared the effectiveness of only pharmacological interventions (nine anti-diabetic, two lipid lowering, one anti-obesity, one anti-hypertensive and one steroid), five studies combined lifestyle and pharmacological intervention groups and one combined the effect of the two medicines. No studies examined the effectiveness of surgical interventions.
Inclusion criteria of the valid 32 RCTs includes IGT, IFG and IGR. The diagnosis consists of World Health Organization (1980, 1985, 1994 and 1999); American Diabetes Association (1997, 2003, 2008, 2010). Population characteristics, including age, gender, BMI, weight and other data are displayed in Table 1.
As opposed to those of previous studies, in our review, diabetic prevention strategies were divided into 14 groups comprising the considerable baseline of all included studies: Control (standard care or placebo), GI (α-glucosidase inhibitor), GLP-1RAs (glucagon-like peptide 1 receptor agonists), Lifestyle (lifestyle modification), LSM (lifestyle modification plus metformin), LST (lifestyle modification plus thiazolidinedione), Metformin (metformin), Orlistat (orlistat), Statins (statins), SU (sulfonylureas), TZD (thiazolidinedione), TZM (thiazolidinedione plus metformin) Vitamin D (vitamin D) and ACEI (angiotensin converting enzyme inhibitors).
Network meta-analysis uses indirect comparison technology to comprehensively evaluate and rank all interventions in a body of evidence. The network of all direct and indirect comparisons of all commonly using anti-pre-diabetes strategies can be seen in Figure 2B.
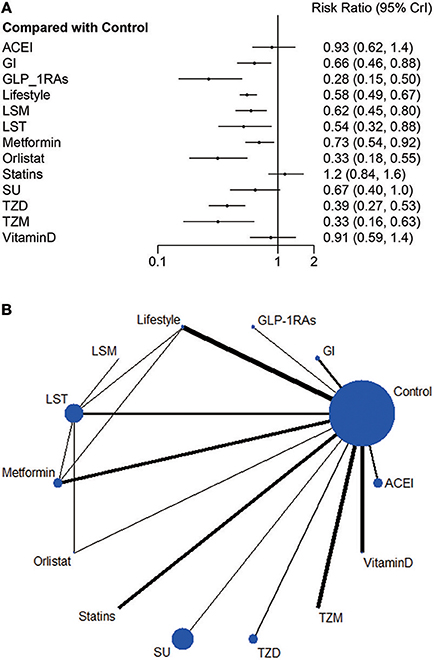
Figure 2. (A) Relative risk ratio and 95% credible interval of strategy interventions compared to control group in Bayesian random effect model of network meta-analysis. (B) Network plot: Weight the nodes according to the number of patients that have received each treatment; calculate the control group risk for studies including the control and weight the edges according to the mean control group risk for all comparisons vs. control.
Compared with the control group, current evidence suggests that nine strategies credibly reduced incidence of diabetes with RR ranging from 0.28 (0.15, 0.50) to 0.73 (0.54, 0.92), including GLP-1RAs, Orlistat, TZM, TZD, LST, Lifestyle, LSM, SU and Metformin. Nevertheless, SU, ACEI, statins and Vitamin D all intersect the ineffective line (Figure 2A).
After comparing several interventions, the investigator is informed of the optimal intervention. However, if optimal intervention is not available, or is difficult to implement or expensive, researchers need to consider interventions beyond the optimal intervention. The order of interventions can be ranked according to the size of the SUCRA value. Because this article explores the adverse event rates of diabetes, the greater the value, the less prioritized is the intervention (Figure 3).
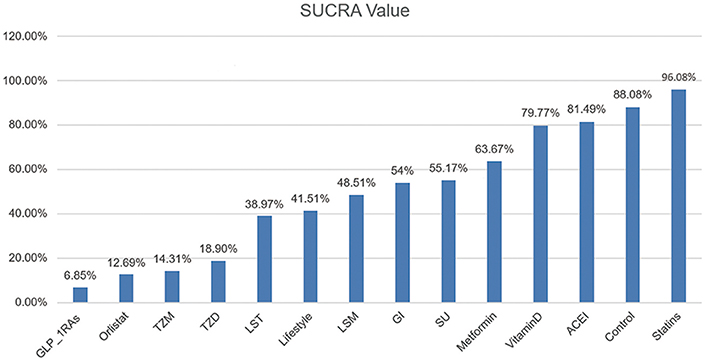
Figure 3. SUCRA Value of Diabetic Incidence. We ranked all fourteen intervention strategies based on their probabilities of prediabetes leading to diabetes and calculated SUCRA to obtain a more precise sorted consequence. The lower the SUCRA value, the more likely this measure is to prevent the progression of the diabetes process.
Consistent with the findings of previous studies (47), statins exposed participants to an incidence of diabetes greater than that of control and other intervention groups. The slight difference of LST, Lifestyle and LSM suggested a lack of evidence for supporting the superiority of lifestyle modification and these two pharmacological combination therapies.
The results of GI, LSM and SU (I2 = 72.5%, 48.2%, 70.6%) exhibited high heterogeneity vs. control (Supplementary Figure 1). We suspect that each of GI, SU, or LSM contains two or more medicine or lifestyle interventions, possibly explaining the higher heterogeneity.
Traditional meta-analysis supported the benefits of both lifestyle modification and anti-diabetic medication. Lifestyle modification with a duration of at least 1 year decreases body mass index (BMI), body weight, waist and hip circumference, systolic and diastolic blood pressure, 2-h postprandial blood glucose, and increases serum HDL (Figure 4). BMI, body weight, waist and hip circumference, systolic pressure and fasting blood glucose exhibited high heterogeneity (I2 value exceeded 50%, I2 = 95, 87, 84, 56, 73, and 84% respectively); however, all these individual trials supported physical improvements except for fasting blood glucose. Anti-diabetic medication (including GLP-1RAs and insulin-sensitizing agents) decreased BMI, systolic blood pressure, fasting blood glucose and 2-h postprandial blood glucose (Figure 5).
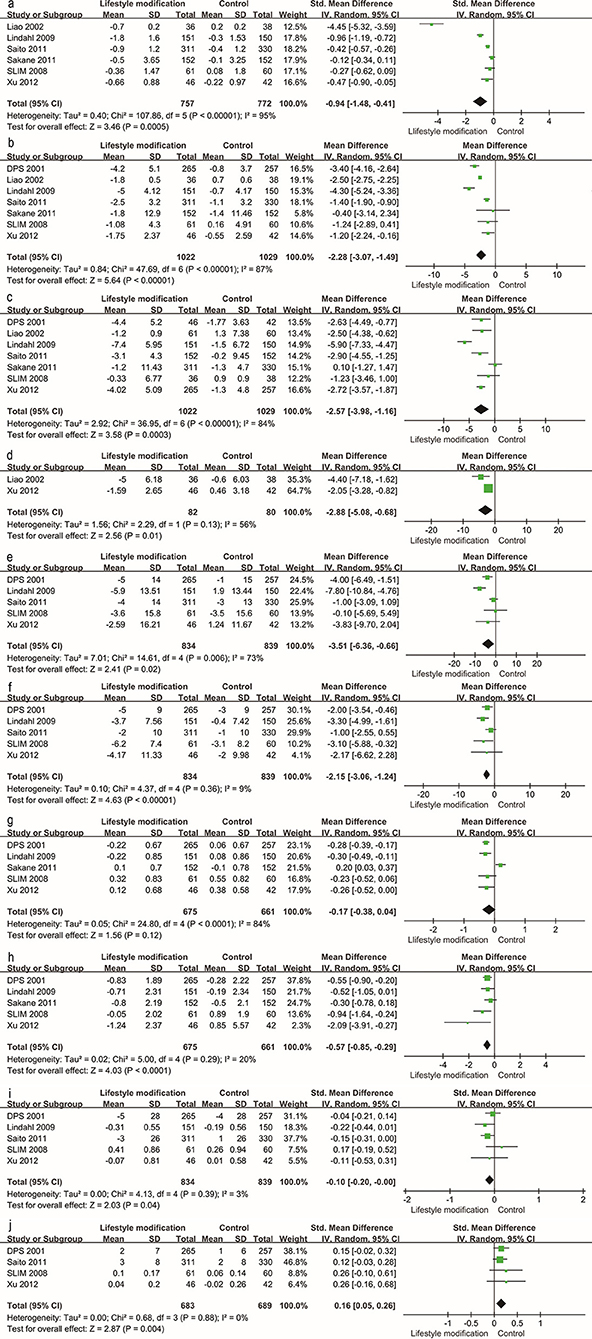
Figure 4. Traditional meta-analysis of the effect on physical conditions: Lifestyle modification vs. Control. (a–j) BMI (kg/m2), body weight (kg), waist circumference (cm), hip circumference (cm), systolic pressure (mmHg), diastolic pressure (mmHg), fasting blood glucose (mg/dL), 2 h postprandial blood glucose (mg/dL), total cholesterol (mmol/L, mg/dL), HDL (mmol/L, mg/dL).
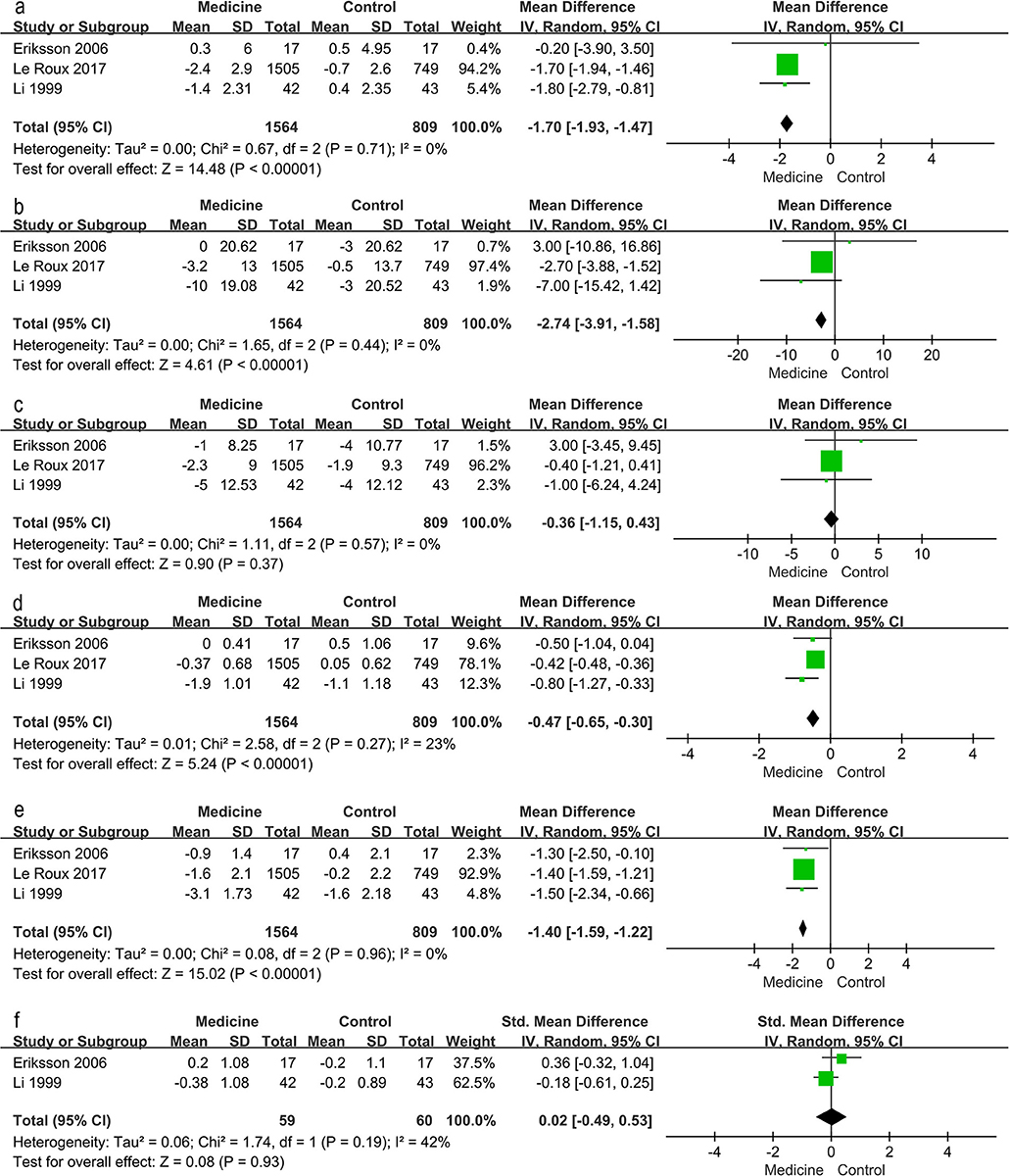
Figure 5. Traditional meta-analysis of the effect on physical conditions: Medicine vs. Control. (a–f) BMI (kg/m2), systolic pressure (mmHg), diastolic pressure (mmHg), fasting blood glucose (mg/dL), 2 h postprandial blood glucose (mg/dL), total cholesterol (mmol/L, mg/dL).
TSA was performed to evaluate random errors caused by limited data and repetitive testing of accumulating data. The cumulative z-curve crossed both the traditional boundary and the trial sequential monitoring boundary but not the futility boundary, suggesting firm evidence for an average of 20% relative risk reduction of diabetes with lifestyle modification (Figure 6). Similarly, TSA supported sufficient evidence for 20% relative increased risk of diabetes with statins and 20% relative risk reduction of diabetes with metformin (Supplementary Figure 2A). The lack of evidence for a 30%, 60% and 25% relative risk reduction in diabetes with GI, orlistat and sulfonylureas demands larger trials (Supplementary Figure 2B). Other intervention strategies failed to establish such an analysis for the limited information size.
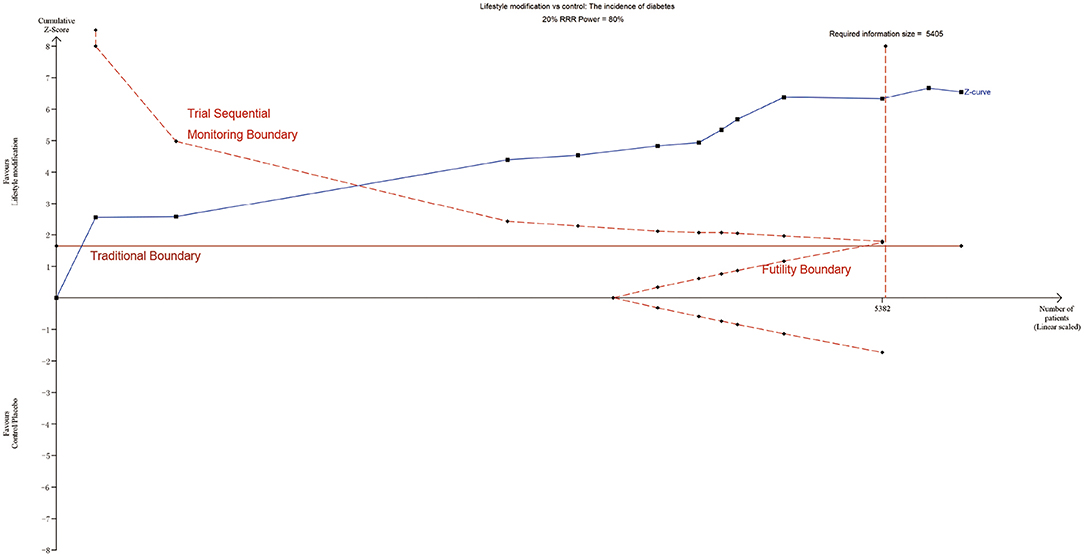
Figure 6. TSA of lifestyle modification. Effect of lifestyle modification vs. control on diabetes using a required information size of 5,405 participants in order to detect or reject a 20% RRR with a power of 80%.
We assessed several biases using Cochrane Collaboration's tool rating risk bias (Figures 7A,B). However, when trials assigned participants to undertake lifestyle modification, the potential allocation concealment were generated, increasing the likelihood of significant findings (48). Therefore, we should understand that the effects of lifestyle modification were at risk of exaggeration. Various definitions of the IFG, IGT, pre-diabetes and diabetes definitions in the trials may also interfere with the final results.
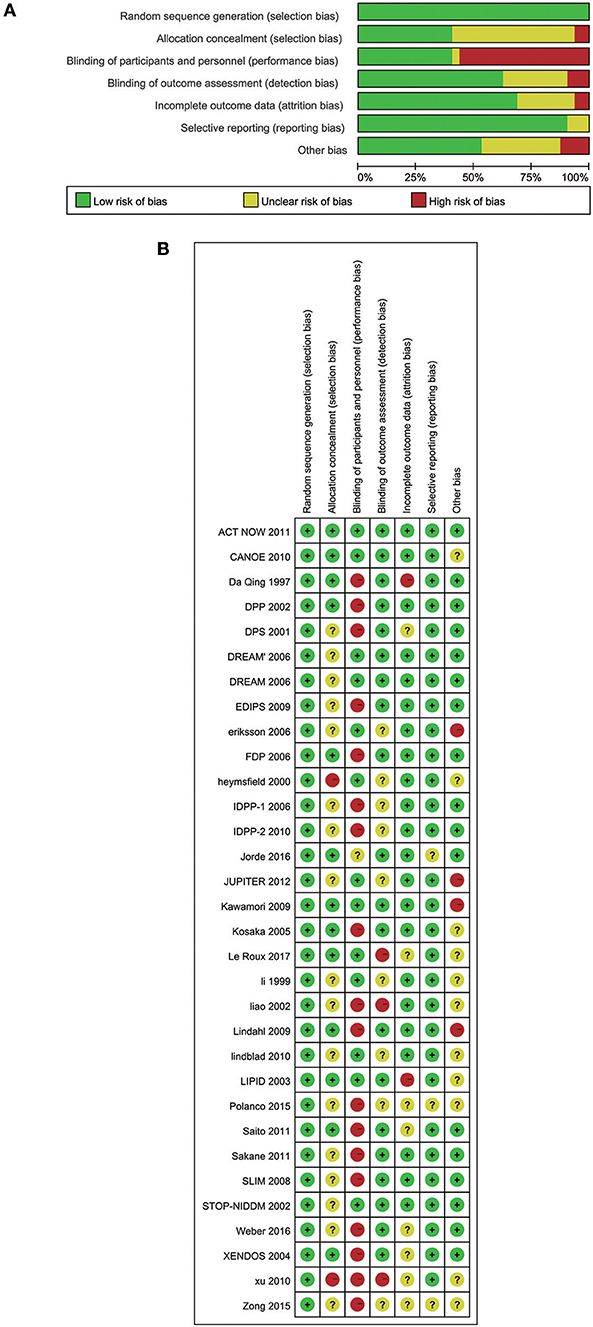
Figure 7. (A) Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included 32 studies. (B) Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
A total of 32 RCTs with available data contributed to this trial analysis, including traditional and network meta-analyses, TSA of the incidence of diabetes and a traditional meta-analysis of physical conditions.
Compared to placebo (Figure 2A), GLP-1RAs (0.28, [0.15, 0.50]), TZM (0.33, [0.16, 0.63]), and TZD (0.39, [0.27, 0.53]) significantly delayed the progression of diabetes; however, the limited sample size and the small quantity of studies caused instability of this inference. The data of both GLP-1RAs and orlistat were captured from severely obese people (mean BMI = 39 and 37 respectively), contributing to potential inconsistency. Metformin is less effective in people with lower baseline BMIs or lower FPG concentrations than in those with higher values for these variables; the drug works by inhibiting endogenous glucose production (49). It is not as flexible as lifestyle modifications that can be adjusted according to the specific physical conditions of the individual. Several studies reported that vitamin D supplementation reduced the incidence of diabetes in patients with both pre-diabetes and vitamin D deficiency (50); however, our review, similar to Angellotti and Pittas (51) showed the controversial result that vitamin D did not protect the prediabetic population without vitamin D deficiency from developing diabetes. There is evidence suggesting that obesity patients are susceptible to GLP-1RAs (32) and orlistat (25) on the progression of diabetic prevention. For population with prediabetes and other metabolic disturbances, including higher body weight or blood pressure or dyslipidemia, lifestyle modification should be a considerable intervening measure. Current researches support that patients are expected to benefit from GI (42) and statins for cardiovascular risk reduction (29, 37).
According to a review of collected trials, Haw et al. (11) suggested that lifestyle modification was a promising long-term diabetes prevention strategy; nevertheless, its sustained protective effects relied on maintenance interventions, even intermittent ones. This was consistent with prior results, to the effect that lifestyle interventions can somewhat prevent the conversion of pre-diabetes into diabetes. TSA can verify type I errors, thus avoiding more experiments to re-confirm this result, resulting in a waste of resources. Furthermore, reductions in BMI, body weight, waist and hip circumference induced by lifestyle intervention are expected to improve individual physical conditions, because weight loss appears to be the key factor associated with reduced diabetes progression (11). Their findings supported the use of pharmacological interventions (weight loss and insulin-sensitizing agents) to reduce diabetes incidence, and when the drug is eliminated from the body, its therapeutic effect will be weakened or even disappeared. It was suggested that the differences in insulin sensitivity and insulin secretion between IGT and IFG, and the greater severity of the abnormalities when both coexist might predict different rates of progression to diabetes, and different pharmacological agents might be needed to treat the pathophysiology. Recently, Pang et al. (52) reported multiple-treatment comparisons to discuss various diabetes preventing strategies in China, filling an investigative gap in traditional Chinese medicine. For the first time, we summarized the previous overview of pre-diabetes studies, and have found that medications and lifestyle interventions improve individual physical metabolism variously, permitting caregivers to individualize preventive care appropriate to individual clinical characteristics. The associated risk reduction of lifestyle modification, including healthy meals, increased physical exercise and weight loss is more pronounced than the effect of any single factor alone.
In order to distinguish unfinished and completed conclusions, avoid exceeding experimental waste of resources and therefore guiding the next step of clinical research, the collected studies were tested using TSA.
Despite the fact that this review performed trial sequential analysis of intervention strategies, the diabetes incidence was reported only once for ACEI, GLP-1RAs, LST, TZM and vitamin D, creating potential bias. Complications of diabetes increase patient suffering and mortality. Effective interventions may also delay or prevent complications, thereby significantly reducing the personal and public health burden of diabetes. Therefore, more relevant trials are needed to reinforce or further complement this review, especially for endpoints of clinical complications, such as cardiovascular events/death and data on cost-effectiveness.
In adults with pre-diabetes, firm evidence supports the notion that lifestyle modifications and metformin reduces the incidence of diabetes with an average of 20% relative risk reduction, while statins increase the relative risk 20%. We found that lifestyle modifications, promising long-term strategies involving three factors (nutrition, exercise and weight loss) contribute to health by reducing BMI, body weight, waist and hip circumference, systolic and diastolic pressure, fasting and 2-h postprandial blood glucose, total cholesterol and by increasing HDL. We made this determination using TSA, avoiding further waste of experimental resources.
The corresponding author had final responsibility for the decision to submit for publication.
ZS and J-YC contributed equally to this work, including the conception and design research, data extraction, data analysis, and drafted the composition. Y-CP, H-CX, and J-WC contributed to statistical analysis. J-HY, RW, C-SZ, and L-XW conducted the proofreading work. JD contributed to crucial revisal of the treatise for important intellectual content.
This work was financed by a fund (to JD) from the National Natural Science Foundation of China (No. 31872791 and No. 31371168).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thanks to Ting Zhai and Shi-Zhen Li in Qingdao University for helpful advice during the conceiving of the review.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00455/full#supplementary-material
Supplementary Figure 1. Heterogeneity test in network meta-analysis using I2 in pair-wise and network pooled comparison.
Supplementary Figure 2. (A) TSA of metformin and statins. Effect of metformin vs. control on diabetes using a required information size of 3,388 participants in order to detect or reject a 20% RRR with a power of 80%; effect of statins vs. control on diabetes using a required information size of 15,632 participants in order to detect or reject a 20% relative increased risk with a power of 80%. (B) TSA of GI, orlistat and sulphonylureas. Effect of GI vs. control on diabetes using a required information size of 11,993 participants in order to detect or reject a 30% RRR with a power of 80%; effect of orlistat vs. control on diabetes using a required information size of 368 participants in order to detect or reject a 60% RRR with a power of 80%; effect of sulphonylureas vs. control on diabetes using a required information size of 1,003 participants in order to detect or reject a 25% RRR with a power of 80%.
Supplementary Table 1. Database-appropriate terms and the text words.
Supplementary Table 2. Description of lifestyle modification.
Supplementary Table 3. Baseline characteristics of excluded trials.
ACEI, Angiotensin converting enzyme inhibitors; BMI, Body mass index; CENTRAL, Cochrane Central Register of Controlled Trials; CrI, Credible interval; GI, α-glycosidase inhibitors; GLP-1RAs, Glucagon-like peptide 1 receptor agonists; LSM, Lifestyle modification plus metformin; LST, Lifestyle modification plus thiazolidinedione; RCT, Randomized controlled trials; SUCRA, Surface under the cumulative ranking probabilities; TZD, Thiazolidinedione; TZM, Thiazolidinedione plus metformin.
1. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. (2011) 8:228–36. doi: 10.1038/nrendo.2011.183
2. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. (2015) 314:1021–9. doi: 10.1001/jama.2015.10029
3. WHO. Intermediate States of Hyperglycemia (1999). Available online at: https://www.who.int/diabetes/action_online/basics/en/index2.html
4. Classification and Diagnosis of Diabetes (2017). Diabetes Care 40(Suppl 1): S11–24. doi: 10.2337/dc17-S005
5. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. (2012) 379:2279–90. doi: 10.1016/S0140-6736(12)60283-9
6. Nathan DM, Davidson MB, Defronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance. Diabetes Care. (2007) 55:2355–9. doi: 10.2337/dc07-9920
7. Shehab E, Emara M, Shoker A. Prediabetes: a must to recognise disease state. Int J Clin Pract. (2010) 62:642–8. doi: 10.1111/j.1742-1241.2008.01705.x
8. American Diabetes Association(2017). Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin Diabetes. 35:5–26. doi: 10.2337/cd16-0067
9. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. (2001) 344:1343–50. doi: 10.1056/NEJM200105033441801
10. DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. (2011) 364:1104–15. doi: 10.1056/NEJMoa1010949
11. Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, et al. Long-term Sustainability of Diabetes Prevention Approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. (2017) 177:1808–17. doi: 10.1001/jamainternmed.2017.6040
12. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. (2013) 159:130–7. doi: 10.7326/0003-4819-159-2-201307160-00008
13. Durbin RJ. Thiazolidinedione therapy in the prevention/delay of type 2 diabetes in patients with impaired glucose tolerance and insulin resistance. Diabetes Obesity Metabol. (2004) 6:280–5. doi: 10.1111/j.1462-8902.2004.0348.x
14. Jarrett RJ, Keen H, Fuller JH, McCartney M. Worsening to diabetes in men with impaired glucose tolerance (borderline diabetes). Diabetologia. (1979) 16:25–30. doi: 10.1007/BF00423146
15. Davies MJ, Gray LJ, Ahrabian D, Carey M, Farooqi A, Gray A, et al. Programme grants for applied research. In: A Community-Based Primary Prevention Programme for Type 2 Diabetes Mellitus Integrating Identification and Lifestyle Intervention for Prevention: A Cluster Randomised Controlled Trial. Southampton (UK): NIHR Journals Library, (2017) 5:44–4.
16. Stewart LAF, Tierney J, Clarke M. Cochrane Handbook for Systematic Reviews of Intervention. (2011).
17. Zinman B, Harris SB, Neuman J, Gerstein HC, Retnakaran RR, Raboud J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet. (2010) 376:103–11. doi: 10.1016/S0140-6736(10)60746-5
18. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. Da Qing IGT Diabetes Study Diabetes Care. (1997) 20:537–44. doi: 10.2337/diacare.20.4.537
19. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
20. Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. (2006) 355:1551–62. doi: 10.1056/NEJMoa065061
21. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. (2006) 368:1096–105. doi: 10.1016/S0140-6736(06)69420-8
22. Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. (2009) 9:342. doi: 10.1186/1471-2458-9-342
23. Eriksson JG, Lehtovirta M, Ehrnström B, Salmela S, Groop L. Long-term beneficial effects of glipizide treatment on glucose tolerance in subjects with impaired glucose tolerance. J Inter Med. (2006) 259:553–60. doi: 10.1111/j.1365-2796.2006.01633.x
24. Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. (2006) 368:1673–9. doi: 10.1016/S0140-6736(06)69701-8
25. Heymsfield SB, Segal KR, Hauptman J, Lucas CP, Boldrin MN, Rissanen A, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Inter Med. (2000) 160:1321–6. doi: 10.1001/archinte.160.9.1321
26. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. (2006) 49:289–97. doi: 10.1007/s00125-005-0097-z
27. Ramachandran A, Arun N, Shetty AS, Snehalatha C. Efficacy of primary prevention interventions when fasting and postglucose dysglycemia coexist: analysis of the Indian Diabetes Prevention Programmes (IDPP-1 and IDPP-2). Diabetes Care. (2010) 33:2164–8. doi: 10.2337/dc09-1150
28. Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, et al. Vitamin D 20 000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metabol. (2016) 101:1647–55. doi: 10.1210/jc.2015-4013
29. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. J Vasc Surg. (2012) 380:565–71. doi: 10.1016/S0140-6736(12)61190-8
30. Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. (2009) 373:1607–14. doi: 10.1016/S0140-6736(09)60222-1
31. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. (2005) 67:152–62. doi: 10.1016/j.diabres.2004.06.010
32. le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. (2017) 389:1399–409. doi: 10.1016/S0140-6736(17)30069-7
33. Li CL, Pan CY, Lu JM, Zhu Y, Wang JH, Deng XX, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetic Med. (1999) 16:477–81. doi: 10.1046/j.1464-5491.1999.00090.x
34. Liao D, Asberry PJ, Shofer JB, Callahan H, Matthys C, Boyko EJ, et al. Improvement of BMI, body composition, and body fat distribution with lifestyle modification in Japanese Americans with impaired glucose tolerance. Diabetes Care. (2002) 25:1504–10. doi: 10.2337/diacare.25.9.1504
35. Lindahl B, Nilsson TK, Borch-Johnsen K, Røder ME, Söderberg S, Widman L, et al. A randomized lifestyle intervention with 5-year follow-up in subjects with impaired glucose tolerance: pronounced short-term impact but long-term adherence problems. Scand J Public Health. (2009) 37:434–42. doi: 10.1177/1403494808101373
36. Lindblad U, Lindberg G, Mansson NO, Ranstam J, Tyrberg M, Jansson S, et al. Can sulphonylurea addition to lifestyle changes help to delay diabetes development in subjects with impaired fasting glucose? Nepi Antidiabetes Study Diabetes Obesity Metabol. (2010) 13:185–8. doi: 10.1111/j.1463-1326.2010.01331.x
37. Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, et al. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care. (2003) 26:2713–21. doi: 10.2337/diacare.26.10.2713
38. Polanco MA, Barrientos R, Godinez SA, Sanchez S, Leanos R, Plascencia S. Preventing the development of type 2 diabetes in subjects at high risk in western Mexico, using metformin and changes in lifestyle, followed 10 years. Diabetes. (2015) 64:A687.
39. Saito T, Watanabe M, Nishida J, Izumi T, Omura M, Takagi T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Inter Med. (2011) 171:1352–60. doi: 10.1001/archinternmed.2011.275
40. Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tsuzaki K, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health. (2011) 11:40. doi: 10.1186/1471-2458-11-40
41. Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabetic Med. (2008) 25:597–605. doi: 10.1111/j.1464-5491.2008.02417.x
42. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. (2002) 359:2072–7. doi: 10.1016/S0140-6736(02)08905-5
43. Weber MB, Ranjani H, Staimez LR, Anjana RM, Ali MK, Narayan KM, et al. The stepwise approach to diabetes prevention: results From the D-CLIP Randomized Controlled Trial. Diabetes Care. (2016) 39:1760–7. doi: 10.2337/dc16-1241
44. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. (2004) 27:155–61. doi: 10.2337/diacare.27.1.155
45. Xu DF, Sun JQ, Chen M, Chen YQ, Xie H, Sun WJ, et al. Effects of lifestyle intervention and meal replacement on glycaemic and body-weight control in Chinese subjects with impaired glucose regulation: a 1-year randomised controlled trial. Br J Nutr. (2013) 109:487–92. doi: 10.1017/S0007114512001328
46. Zong Y, Duan P, Ding X, Si L, Liu J, Tu P. Effects of lifestyle and quantitative nutrition interventions on individuals with prediabetes. Natl Med J China. (2015) 95:3293–6. doi: 10.3760/cma.j.issn.0376-2491.2015.40.013
47. Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen SJ, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol. (2012) 60:1231–8. doi: 10.1016/j.jacc.2012.05.019
48. Schulz K, Chalmers FI, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA J Am Med Assoc. (1995) 273:408–12. doi: 10.1001/jama.1995.03520290060030
49. Defronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Inter Med. (2000) 133:73–4. doi: 10.7326/0003-4819-133-1-200007040-00016
50. Tsur A, Feldman BS, Feldhammer I, Hoshen MB, Leibowitz G, Balicer RD. Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetes. Diabetes Care. (2013) 36:1361–7. doi: 10.2337/dc12-1050
51. Angellotti E, Pittas AG. The role of vitamin D in the prevention of type 2 diabetes. To D or not to D? Endocrinology. 158:7. doi: 10.1210/en.2017-00265
Keywords: prediabetic state, drug therapy, healthy lifestyle, diabetes mellitus, type 2, randomized controlled trial, network meta-analysis, trial sequential analysis
Citation: Sheng Z, Cao J-Y, Pang Y-C, Xu H-C, Chen J-W, Yuan J-H, Wang R, Zhang C-S, Wang L-X and Dong J (2019) Effects of Lifestyle Modification and Anti-diabetic Medicine on Prediabetes Progress: A Systematic Review and Meta-Analysis. Front. Endocrinol. 10:455. doi: 10.3389/fendo.2019.00455
Received: 26 January 2019; Accepted: 24 June 2019;
Published: 12 July 2019.
Edited by:
Jan Polák, Charles University, CzechiaReviewed by:
Trudy R. Gaillard, FIU Nicole Wertheim College of Nursing and Health Sciences, Florida International University, United StatesCopyright © 2019 Sheng, Cao, Pang, Xu, Chen, Yuan, Wang, Zhang, Wang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Dong, ZG9uZ2ppbmc2QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.