- Dipartimento di Medicina Clinica e Chirurgia, University “Federico II”, Naples, Italy
The pathogenesis of obesity and alterations in glucose profile have been linked to PRL excess, as it is reportedly associated with metabolic syndrome in thereabout one third of patients. In vitro exposure of pancreatic islet to PRL is known to stimulate insulin secretion and β-cell proliferation, and in turn overexpression of PRL in β-cells increases insulin release and β-cell replication. PRL excess has been found to worsen glucose profile because it reduces glucose tolerance and induces insulin resistance either in obese and non-obese patients. To note, pancreatic β-cells and adipocytes widely express dopamine receptors type 2, and dopamine has been hypothesized to play a key role as modulator of insulin and adipose functions. The dopamine agonists bromocriptine and cabergoline significantly improve abnormalities in glucose profile and reduce the prevalence of metabolic syndrome in a remarkable proportion of patients, regardless of whether body weight and PRL status may change. However, in men with hyperprolactinemia complicated by hypogonadism, testosterone replacement can ameliorate insulin resistance and abnormalities in glucose metabolism. Therefore, in patients with PRL-secreting pituitary adenomas control of PRL excess by dopamine agonists is mandatory to improve glucose and insulin abnormalities.
Introduction
PRL excess may promote the development of disorders in glucose and insulin metabolism (1–7). Indeed, hyperprolactinemia has been related to the pathogenesis of impaired glucose tolerance and hyperinsulinemia up to overt insulin resistance (8–14), as demonstrated by the evidence that high PRL levels promote the increase of the surrogate index of insulin resistance (HOMA-IR, 11–13) and the reduction of the surrogate index of insulin sensitivity (14), either in obese and lean patients. PRL excess induces the functional blockade of dopaminergic tone, which in turn may be accounted among factors implied in the pathogenesis of hyperphagia and weight gain seen in patients with hyperprolactinemia, so contributing to obesity (15–21). On the other hand, in vitro studies in rats and cell lines have clearly reported that insulin secretion and β-cell proliferation are enhanced by the exposure of isolated pancreatic islet to PRL (3, 6), and overexpression of PRL in β-cells has been demonstrated to inappropriately rise insulin secretion and release, also promoting β-cell replication (7). However, animal and human pancreatic β-cells (22) and human adipocytes (23) also express dopamine receptors type 2 (D2R), suggesting that, aside from PRL, also dopamine may modulate insulin and adipose functions at peripheral level.
The dopamine agonists bromocriptine and cabergoline nowadays represent the treatment of choice for patients with hyperprolactinemia (24, 25). Interestingly, both drugs have been demonstrated to significantly improve glucose profile in diabetic patients independently on PRL status (26–29, 29–35). Particularly, bromocriptine-Quick Release, a quick-release formulation of bromocriptine mesylate, has been found to reduce glucose levels in non-diabetic obese hyperinsulinemic women on a weight maintaining diet (29). Similarly, this drug has been reported to reduce body weight, glycated hemoglobin (HbA1c) and fasting plasma glucose in both diabetic and non-diabetic subjects (28, 32). Therefore, bromocriptine-Quick Release has been officially approved by FDA for patients with type 2 diabetes mellitus (33). It is recommended for patients with type 2 diabetes mellitus with mild hyperglycemia either as adjunctive treatment to metformin and sulfonylurea, or as monotherapy in patients with intolerance to such compounds (33). A similar efficacy on HbA1c has been recently demonstrated also for cabergoline in diabetic patients not adequately controlled by other anti-diabetic drugs (35).
In patients with prolactinomas, bromocriptine has been found to significantly improve glucose homeostasis and insulin resistance (17, 18, 36, 37), and to reduce body weight (17, 37). Likewise, in men and non-obese women with prolactinomas treated with cabergoline the percentage of body fat has been found significantly lower than in treatment-naïve patients (38, 39), suggesting that cabergoline may help in minimizing the risk of obesity (38, 39). The improvement in insulin resistance and glucose profile has been described also in patients with prolactinomas receiving therapy with cabergoline (40–43). Such metabolic gain appears to be independent on the degree of reduction in PRL levels, and may be directly attributable to cabergoline dose instead (42, 43). In men with prolactinomas and persistent hypogonadism, accounting for up to 50% of cases (44–46), proper testosterone treatment may enhance the effects of cabergoline by further reducing insulin resistance and metabolic syndrome (47).
This review focuses on the effects of PRL excess and its control by medical treatment with dopamine agonists on abnormalities in glucose profile.
Effects on Glucose Abnormalities
Several studies (2–7, 22, 47–70) have investigated the link between PRL levels and gluco-insulinemic metabolism. In rodents, PRL is responsible for peculiar changes in pancreatic β-cell mass and function during pregnancy (2–7). Indeed, the pregnancy-related physiological elevation in PRL levels results in increased β-cell mass and enhanced glucose-induced insulin secretion (2, 48, 49), and the expression of PRL receptors is known to grow on both insulin-secreting cell lines and β-cells (5). When isolated pancreatic islet are exposed to PRL in vitro, insulin release and β-cell proliferation rise (3, 6), and overexpression of PRL in β-cells promotes the increase in insulin concentrations and the replication of β-cells (7). In rats and humans, PRL enhances β-cell proliferation, insulin gene transcription and glucose-induced insulin secretion (50–60). In neonatal rat islets PRL treatment enhances islet insulin content and early insulin secretion (54–56), also increasing islet sensitivity to glucose (53). This effect may be partly explained by the increased β-cell and liver glucose transporter GLUT2 in membrane as seen in cultured neonatal rat islets (57, 58). In transgenic female mice with hyperprolactinemia, hyperandrogenism, and hyperprogesteronemia induced by overexpression of the human chorionic gonadotropin β-subunit, a 1-week treatment with cabergoline has been found to significantly reduce body weight and to improve dyslipidemia and insulin resistance up to complete normalization of triglycerides and insulin, besides PRL levels (61). Intraperitoneal glucose and insulin tolerance tests demonstrated cabergoline to significantly improve glucose levels and to prevent insulin resistance (61). These findings supported a key role for PRL as a modulator of insulin secretion and action, and suggested the potential use of medical therapy with dopamine agonists to treat metabolic alterations associated to hyperprolactinemia.
In patients with hyperprolactinemia, an increased response of insulin to glucose has been reported during the oral glucose tolerance test (62–64). This condition of hyperinsulinemia has been found not to parallel glucose uptake and utilization in skeletal muscle (64). Moreover, considering that the reduction of serum free fatty acid levels during the oral glucose load has been found smaller in hyperprolactinemic patients as compared to healthy controls, an impaired antilipolytic effect of insulin in patients with PRL excess has been hypothesized (64). However, such metabolic abnormalities may be independent on PRL levels. In a population of 27 men PRL levels have been found to inversely correlate with insulin and HOMA-IR levels and to directly correlate with adiponectin, regardless from patient BMI (65). In biopsies from visceral and subcutaneous adipose tissue of either lean, overweight and obese patients PRL levels resulted to positively correlate with the adipose tissue fitness markers PPARG, ADIPOQ, and GLUT4 (65), suggesting that PRL might act as a regulator of insulin sensitivity and metabolic homeostasis in adipose tissue in humans (65).
Pancreatic β-cells express also dopamine receptors, and their activation by treatment with dopamine agonists has been shown to suppress glucose-stimulated insulin secretion either in rodents (22) and in patients with Parkinson's disease (66–68). Moreover, in patients with Cushing's disease unsuccessfully treated by surgery chronic treatment with cabergoline has been reported to improve gluco-insulinemic profile and insulin resistance, also reducing the prevalence of diabetes mellitus and impaired fasting glucose, and requiring the withdrawal of treatment with anti-diabetic drugs in some cases (69). In diabetic normoprolactinemic patients, bromocriptine and cabergoline have been demonstrated to significantly improve glucose profile (28–35). Bromocriptine-Quick Release has been found to reduce glucose levels and to improve glucose tolerance in both diabetic and non-diabetic subjects (28, 29, 32). Particularly, in a 16-week double blind study in obese patients with type 2 diabetes mellitus (32) bromocriptine has been demonstrated to significantly reduce HbA1c, fasting plasma glucose and mean plasma glucose concentration during oral glucose tolerance test, independently on changes in body weight or body composition (32). During the insulin clamp, only patients treated bromocriptine, but not those receiving placebo, have been found to show a significant improvement in total and nonoxidative glucose disposal (32). Similarly, in diabetic normoprolactinemic patients with mild hyperglycemia, defined as a HbA1c level between 7 and 10%, the addition of cabergoline 0.5 mg/week to treatment with ≥2 oral glucose-lowering drugs has been demonstrated to significantly reduce HbA1c levels after 3 months as compared to placebo (35). Adequate metabolic control, defined as a HbA1c level < 7%, was achieved in 65% of patients and in 45% of subjects receiving placebo (35).
In patients with prolactinomas glucose profile and insulin resistance have been reported to improve following treatment with bromocriptine and cabergoline (39, 40, 42, 43, 47) (Table 1). Particularly, in patients treated with bromocriptine percent change in insulin sensitivity has been found correlated to percent decrease in PRL levels and to bromocriptine dose during the euglycemic hyperinsulinemic clamp (37). In patients treated with long-term cabergoline and receiving doses >0.5 mg/week a significant reduction in fasting insulin and HOMA-IR has been reported (42, 43), with no impact of changes in body weight and BMI (43). At the same time, insulin secretion and sensitivity, as well as fasting glucose levels, have been found also to significantly ameliorate after treatment with cabergoline (43), and in turn cabergoline dose has been demonstrated to predict the decrease in fasting insulin (43), supporting the hypothesis that cabergoline may directly modulate insulin secretion and sensitivity (43). Moreover, in men with prolactinomas and consequent hypogonadism, insulin profile significantly improved after long-term treatment with cabergoline, and an additional amelioration was demonstrated after complete testosterone normalization following androgen replacement (47). Interestingly, also in such patients cabergoline dose has been shown to significantly correlate with insulin levels (47), confirming the hypothesis that dopamine may play a key role as direct modulator of insulin secretion (43). Conversely, testosterone levels have been seen significantly correlated with peripheral insulin sensitivity (47).
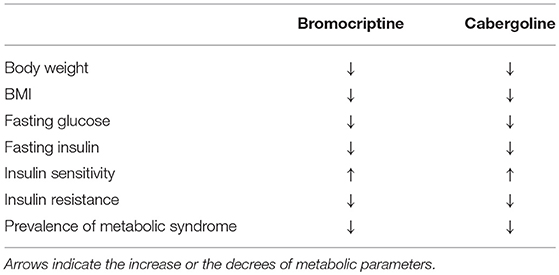
Table 1. Effects of dopamine agonists bromoscriptine and cabergoline on metabolic abnormalities in patients with hyperprolactinemia.
As a consequence of the effects of cabergoline on insulin secretion and peripheral sensitivity, prevalence of metabolic syndrome reduces from 32 to 10% (40, 42, 43, 47) of patients with hypeprolactinemia receiving long-term therapy.
Altogether, these findings have demonstrated the beneficial impact of the treatment with dopamine agonists on gluco-metabolic alterations in patients with prolactinomas, raising the question of whether these compounds might be proposed as valid alternative or adjunctive treatment in diabetic and non-diabetic patients who cannot achieve adequate metabolic control with standard therapies. Based on this evidence, the use of dopamine agonists might be recommended even independently on PRL status, mainly in hypogonadal premenopausal women with microadenomas with no pregnancy desire who may be theoretically treated only with oral contraceptives (71). In such patients, the treatment with dopamine agonists might induce a direct remarkable improvement of gluco-insulinemic profile. The amelioration of glucose homeostasis abnormalities and metabolic syndrome could be even more relevant in women in the postmenopausal period, when weight loss and improved insulin sensitivity might contribute to reduce cardiovascular risk (72). Therefore, the treatment with cabergoline might be maintained in menopausal women regardless from PRL levels in order to retain the beneficial action of dopamine agonists on metabolic profile.
Author Contributions
RA, DD, and RPir made substantial contributions to review of literature, acquisition of data, and interpretation of results. RA participated in drafting the article. RPiv and AC participated in revising critically the manuscript for important intellectual content. All authors provided critical feedback and helped shape the manuscript. AC gave final approval of the version to be submitted and any revised version.
Conflict of Interest Statement
AC has been principal investigator of research studies from Novartis, Ipsen, Pfizer, and Lilly, has received research grants from Ferring, Lilly, Ipsen, Merck-Serono, Novartis, Novo-Nordisk and Pfizer, has been occasional consultant for Novartis, Ipsen and Pfizer, and has received fees and honoraria from Ipsen, Novartis, and Pfizer. RPiv has been principal investigator of research studies from Novartis and HRA Pharma, has received research grants from Novartis, Ipsen, Pfizer, Viropharma and IBSA, has been occasional consultant for Novartis, Ipsen, Pfizer, Viropharma, Ferring and Italfarmaco, and received lecture fees and honoraria from Novartis, Pfizer, and Shire.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
References
1. Ben-Jonathan N, Hugo ER, Brandebourg TD, La Pensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. (2006) 17:110–6. doi: 10.1016/j.tem.2006.02.005
2. Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. (1997) 29:301–7. doi: 10.1055/s-2007-979040
3. Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorensonm RL. Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J Endocrinol. (2007) 193:367–81. doi: 10.1677/JOE-07-0043
4. Nagano M, Kelly PA. Tissue distribution and regulation of rat prolactin receptor gene expression. Quantitative analysis by polymerase chain reaction. J Biol Chem. (1994) 269:13337–45.
5. Moldrup A, Petersen ED, Nielsen JH. Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin-producing cells. Endocrinology. (1993) 133:1165–72. doi: 10.1210/endo.133.3.8365359
6. Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of Langerhans. Endocrinology. (2004) 145:4162–75. doi: 10.1210/en.2004-0201
7. Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, et al. Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem. (2000) 275:15399–406. doi: 10.1074/jbc.275.20.15399
8. Landgraf R, Landraf-Leurs MM, Weissmann A, Horl R, von Werder K, Scriba PC. Prolactin: a diabetogenic hormone. Diabetologia. (1977) 13:99–104. doi: 10.1007/BF00745135
9. Johnston DG, Alberti KG, Nattrass M, Burrin JM, Blesa-Malpica G, Hall K, et al. Hyperinsulinaemia in hyperprolactinaemic women. Clin Endocrinol. (1980) 13:361–8. doi: 10.1111/j.1365-2265.1980.tb03397.x
10. Schernthaner G, Prager R, Punzengruber C, Luger A. Severe hyperprolactinemia is associated with decreased insulin binding in vitro and insulin resistance in vivo. Diabetologia. (1985) 28:138–42.
11. Tuzcu A, Yalaki S, Arikan S, Gokalp D, Bahcec M, Tuzcu S. Evaluation of insulin sensitivity in hyperprolactinemic subjects by euglycemic hyperinsulinemic clamp technique. Pituitary. (2009) 12:330–3. doi: 10.1007/s11102-009-0183-1
12. Serri O, Li L, Mamputu JC, Beauchamp MC, Maingrette F, Renier G. The influences of hyperprolactinemia and obesity on cardiovascular risk markers: effects of cabergoline therapy. Clin Endocrinol. (2006) 64:366–70. doi: 10.1111/j.1365-2265.2006.02469.x
13. Tuzcu A, Bahceci M, Dursun M, Turgut C, Bahceci S. Insulin sensitivity and hyperprolactinemia. J Endocrinol Invest. (2003) 26:341–6 doi: 10.1007/BF03345182
14. Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gozu H, Sezgin O, et al. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. Eur J Endocrinol. (2003) 149:187–93. doi: 10.1530/eje.0.1490187
15. Baptista T, Lacruz A, de Mendoza S, Mendoza Guillen JM, Silvera R, Angeles F, et al. Body weight gain after administration of antipsychotic drugs: correlation with leptin, insulin and reproductive hormones. Pharmacopsychiatry. (2000) 33:81–8. doi: 10.1055/s-2000-8451
16. Doknic M, Pekic S, Zarkovic M, Medic-Stojanoska M, Dieguez C, Casanueva F, et al. Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur J Endocrinol. (2002) 147:77–84. doi: 10.1530/eje.0.1470077
17. Greenman Y, Tordjman K, Stern N. Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. ClinEndocrinol. (1998) 48:547–53. doi: 10.1046/j.1365-2265.1998.00403.x
18. Colao A, Sarno A, Cappabianca P, Briganti F, Pivonello R, Di Somma C, et al. Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur J Endocrinol. (2003) 148:325–31. doi: 10.1530/eje.0.1480325
19. Brandebourg T, Hugo E, Ben-Jonathan N. Adipocyte prolactin: regulation of release and putative functions. Diabetes Obes Metab. (2007) 9:464–76. doi: 10.1111/j.1463-1326.2006.00671.x
20. Strader AD, Buntin JD. Changes in agouti-related peptide during the ring dove breeding cycle in relation to prolactin and parental hyperphagia. J Neuroendocrinol. (2003) 15:1046–53. doi: 10.1046/j.1365-2826.2003.01092.x
21. Bina KG, Cincotta AH. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology. (2000) 71:68–78. doi: 10.1159/000054522
22. Rub í B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. (2005) 280:36824–32. doi: 10.1074/jbc.M505560200
23. Borcherding DC, Hugo ER, Idelman G, De Silva A, Richtand NW, Loftus J, et al. Dopamine receptors in human adipocytes: expression and functions. PLoS ONE. (2011) 6:e25537. doi: 10.1371/journal.pone.0025537
24. Biller BMK, Colao A, Petersenn S, Bonert VS, Boscaro M. Prolactinomas, Cushing's disease and acromegaly: debating the role of medical therapy for secretory pituitary adenomas. BMC Endocrine Disord. (2010) 10:10. doi: 10.1186/1472-6823-10-10
25. Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. (2006) 27:485–534. doi: 10.1210/er.2005-9998
26. Cincotta A, Schiller B, Meier A. Bromocriptine inhibits the seasonally occurring obesity, hyperinsulinemia, insulin resistance and impaired glucose tolerance in the Syrian hamster, Mesoricetusauratus. Metabolism. (1991) 40:639–44. doi: 10.1016/0026-0495(91)90057-4
27. Cincotta A, Mac Eachern T, Meierm A. Bromocriptine redirects metabolism and prevents seasonal onset of obese hyperinsulinemic state in Syrian hamsters. Am J Physiol. (1993) 264:E285–93. doi: 10.1152/ajpendo.1993.264.2.E285
28. Cincotta A, Meierm A. Bromocriptine (Ergoset). reduces body weight and improves glucose tolerance in obese subjects. Diabetes Care. (1996) 19:667–70. doi: 10.2337/diacare.19.6.667
29. Kamath V, Jones C, Yip J, Varasteh BB, Cincotta AH, Reaven GM, et al. Effects of a quick-release form of bromocriptine (Ergoset). on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese non diabetic hyperinsulinemic women. Diabetes Care. (1997) 20:1697–701. doi: 10.2337/diacare.20.11.1697
30. Cincotta AH, Meier AH, Taylor E, Hudson M. Bromocriptine (Ergoset). reduces body fat, hyperinsulinemia, and glucose intolerance in obese subjects. Diabetes. (1995) 44 (Suppl. 1):168A.
31. Galluzzi F, Salti R, Stagi S, La Cauza F, Chiarelli F. Reversible weight gain and prolactin levels—long-term follow-up in childhood. J Pediatr Endocrinol Metab. (2005) 18:921–4. doi: 10.1515/JPEM.2005.18.9.921
32. Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care. (2000) 23:1154–61. doi: 10.2337/diacare.23.8.1154
33. Mikhail N. Quick-release bromocriptine for treatment of type 2 diabetes. Curr Drug Deliv. (2011) 8:511–6. doi: 10.2174/156720111796642255
34. Holt RI, Barnett AH, Bailey CJ. Bromocriptine: old drug, new formulation and new indication. Diabetes Obes Metab. (2010) 12:1048–57. doi: 10.1111/j.1463-1326.2010.01304.x
35. Bahar A, Kashi Z, Daneshpour E, Akha O, Ala S. Effects of cabergoline on blood glucose levels in type 2 diabetic patients: a double-blind controlled clinical trial. Medicine. (2016) 95:e4818. doi: 10.1097/MD.0000000000004818
36. Pala NA, Laway BA, Misgar RA, Dar RA. Metabolic abnormalities in patients with prolactinoma: response to treatment with cabergoline. Diabetol Metab Syndr. (2015) 14:99. doi: 10.1186/s13098-015-0094-4
37. Berinder K, Nystrom T, Hoybye C, Hall K, Hulting AL. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary. (2011) 14:199–207. doi: 10.1007/s11102-010-0277-9
38. Naliato EC, Violante AH, Gaccione M, Caldasm D, Lamounier Filhom A, Loureirom CR, et al. Body fat in men with prolactinomas. J Endocrinol Invest. (2008) 31:985–90. doi: 10.1007/BF03345636
39. Naliato EC, Violante AH, Caldas D, Lamounier Filho A, Loureiro CR, Fontes R, et al. Body fat in nonobese women with prolactinoma treated with dopamine agonists. Clin Endocrinol. (2007) 67:845–52. doi: 10.1111/j.1365-2265.2007.02973.x
40. dos Santos Silva CM, Barbosa FR, Lima GA, Warszawski L, Fontes R, Domingues RC, et al. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity. (2011) 19:800–5. doi: 10.1038/oby.2010.150
41. Korner J, Lo J, Freda PU, Wardlaw SL. Treatment with cabergoline is associated with weight loss in patients with hyperprolactinemia. Obes Res. (2003) 11:311–2. doi: 10.1038/oby.2003.46
42. Ciresi A, Amato MC, Guarnotta V, Lo Castro F, Giordano C. Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clin Endocrinol. (2013) 79:845–52. doi: 10.1111/cen.12204
43. Auriemma RS, Granieri L, Galdiero M, Simeoli C, Perone Y, Vitale P, et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology. (2013) 98:299–310. doi: 10.1159/000357810
44. Bhansali A, Walia R, Dutta P, Khandelwal N, Sialy R, Bhadada S. Efficacy of cabergoline on rapid escalation of dose in men with macroprolactinomas. Indian J Med Res. (2010) 131:530–5.
45. De Rosa M, Ciccarelli A, Zarrilli S, Guerra E, Gaccione M, Di Sarno A, et al. The treatment with cabergoline for 24 month normalizes the quality of seminal fluid in hyperprolactinaemic males. Clin Endocrinol. (2006) 64:307–13. doi: 10.1111/j.1365-2265.2006.02461.x
46. Colao A, Vitale G, Cappabianca P, Briganti F, Ciccarelli A, De Rosa M, et al. Outcome of cabergoline treatment in men with prolactinoma: effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysis. J Clin Endocrinol Metab. (2004) 89:1704–11. doi: 10.1210/jc.2003-030979
47. Auriemma RS, Galdiero M, Vitale P, Granieri L, Lo Calzo F, Salzano C, et al. Effect of chronic cabergoline treatment and testosterone replacement on metabolism in male patients with prolactinomas. Neuroendocrinology. (2015) 101:66–81. doi: 10.1159/000371851
48. Amaral ME, Ueno M, Carvalheira JB, Carneiro EM, Velloso LA, Saad MJ, et al. Prolactin-signal transduction in neonatal rat pancreatic islets and interaction with the insulin-signaling pathway. Horm Metab Res. (2003) 35:282–9. doi: 10.1055/s-2003-41303
49. Amaral ME, Cunha DA, Anhê GF, Ueno M, Carneiro EM, Velloso LA, et al. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol. (2004) 183:469–76. doi: 10.1677/joe.1.05547
50. Balbach L, Wallaschofski H, Völzke H, Nauck M, Dörr M, Haring R. Serum prolactin concentrations as risk factor of metabolic syndrome or type 2 diabetes? BMC EndocrDisord. (2013) 13:12. doi: 10.1186/1472-6823-13-12
51. Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology. (1993) 133:2227–34. doi: 10.1210/en.133.5.2227
52. Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. (1993) 132:879–87. doi: 10.1210/endo.132.2.8425500
53. Boschero AC, Crepaldi SC, Carneiro EM, Delattre E, Atwater I. Prolactin induces maturation of glucose sensing mechanisms in cultured neonatal rat islets. Endocrinology. (1993) 133:515–20. doi: 10.1210/endo.133.2.8344197
54. de Mazancourt P, Carneiro EM, Atwater I, Boschero AC. Prolactin treatment increases GLUT2 but not the G protein subunit content in cell membranes from cultured neonatal rat islets. FEBS Lett. (1994) 343:137–40. doi: 10.1016/0014-5793(94)80305-6
55. Cunha DA, Amaral ME, Carvalho CP, Collares-Buzato CB, Carneiro EM, Boschero AC. Increased expression of SNARE proteins and synaptotagmin IV in islets from pregnant rats and in vitro prolactin-treated neonatal islets. Biol Res. (2006) 39:555–66. doi: 10.4067/S0716-97602006000300016
56. Cunha DA, Roma LP, Boschero AC. Prolactin modulates the association and phosphorylation of SNARE and kinesin/MAP-2 proteins in neonatal pancreatic rat islets. Mol Cell Endocrinol. (2007) 273:32–41. doi: 10.1016/j.mce.2007.05.001
57. Crepaldi-Alves SC, Carneiro EM, Bosqueiro JR, Boschero AC. Synergistic effect of glucose and prolactin on GLUT2 expression in cultured neonatal rat islets. Braz J Med Biol Res. (1997) 30:359–61. doi: 10.1590/S0100-879X1997000300008
58. Crepaldi SC, Carneiro EM, Boschero AC. Long-term effect of prolactin treatment on glucose-induced insulin secretion in cultured neonatal rat islets. Horm Metab Res. (1997) 29:220–4. doi: 10.1055/s-2007-979025
59. Petryk A, Fleenor D, Driscoll P, Freemark M. Prolactin induction of insulin gene expression: the roles of glucose and glucose transporter-2. J Endocrinol. (2000) 164:277–86. doi: 10.1677/joe.0.1640277
60. Fleenor DE, Freemark M. Prolactin induction of insulin gene transcription: roles of glucose and signal transducer and activator of transcription 5. Endocrinology. (2001) 142:2805–10. doi: 10.1210/endo.142.7.8267
61. Ratner LD, Stevens G, Bonaventura MM, Lux-Lantos VA, Poutanen M, Calandra RS, et al. Hyperprolactinemia induced by hCG leads to metabolic disturbances in female mice. J Endocrinol. (2016) 230:157–69. doi: 10.1530/JOE-15-0528
62. Kim SY, Sung YA, Ko KS, Cho BY, Lee HK, Koh CS, et al. Direct relationship between elevated free testosterone and insulin resistance in hyperprolactinemic women. Korean J Intern Med. (1993) 8:8–14. doi: 10.3904/kjim.1993.8.1.8
63. Pelkonen R, Nikkilä EA, Grahne B. Serum lipids, postheparin plasma lipase activities and glucose tolerance in patients with prolactinoma. ClinEndocrinol. (1982) 16:383–90. doi: 10.1111/j.1365-2265.1982.tb00731.x
64. Foss MC, Paula FJ, Paccola GM, Piccinato CE. Peripheral glucose metabolism in human hyperprolactinaemia. Clin Endocrinol. (1995) 43:721–6. doi: 10.1111/j.1365-2265.1995.tb00541.x
65. Ruiz-Herrera X, de Los Ríos EA, Díaz JM, Lerma-Alvarado RM, Martínez de la Escalera L, López-Barrera F, et al. Prolactin promotes adipose tissue fitness and insulin sensitivity in obese males. Endocrinology. (2017) 158:56–68. doi: 10.1210/en.2016-1444
66. Ericson LE, Håkanson R, Lundquist I. Accumulation of dopamine in mouse pancreatic B-cells following injection of L-DOPA. Localization to secretory granules and inhibition of insulin secretion. Diabetologia. (1977) 13:117–24. doi: 10.1007/BF00745138
67. Zern RT, Bird JL, Feldman JM. Effect of increased pancreatic islet norepinephrine, dopamine and serotonin concentration on insulin secretion in the golden hamster. Diabetologia. (1980) 18:341–6. doi: 10.1007/BF00251017
68. Rosati G, Maioli M, Aiello I, Farris A, Agnetti V. Effects of long-term L-dopa therapy on carbohydrate metabolism in patients with Parkinson's disease. Eur Neurol. (1976) 14:229–39. doi: 10.1159/000114744
69. Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, et al. The medical treatment of Cushing's disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. (2009) 94:223–30. doi: 10.1210/jc.2008-1533
70. Kok P, Roelfsema F, Frölich M, van Pelt J, Meinders AE, Pijl H. Short-term treatment with bromocriptine improves impaired circadian growth hormone secretion in obese premenopausal women. J Clin Endocrinol Metab. (2008) 93:3455–61. doi: 10.1210/jc.2008-0001
71. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte J, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:273–88. doi: 10.1210/jc.2010-1692
Keywords: hyperprolactinemia, pituitary tumor, dopamine agonists, bromocriptine, cabergoline, glucose metabolism, insulin metabolism, metabolic syndrome
Citation: Auriemma RS, De Alcubierre D, Pirchio R, Pivonello R and Colao A (2019) Glucose Abnormalities Associated to Prolactin Secreting Pituitary Adenomas. Front. Endocrinol. 10:327. doi: 10.3389/fendo.2019.00327
Received: 11 February 2019; Accepted: 07 May 2019;
Published: 22 May 2019.
Edited by:
Lucio Vilar, Federal University of Pernambuco, BrazilReviewed by:
Maria Lucia Bonfleur, Universidade Estadual do Oeste do Paraná, BrazilLeila Warszawski, Instituto Estadual de Diabetes e Endocrinologia Luiz Capriglione, Brazil
Copyright © 2019 Auriemma, De Alcubierre, Pirchio, Pivonello and Colao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annamaria Colao, Y29sYW8mI3gwMDA0MDt1bmluYS5pdA==
 Renata S. Auriemma
Renata S. Auriemma Dario De Alcubierre
Dario De Alcubierre Rosa Pirchio
Rosa Pirchio Rosario Pivonello
Rosario Pivonello Annamaria Colao
Annamaria Colao