- 1AP-HP, Department of Reproductive Medicine & Fertility Preservation, Hôpital Antoine Béclère, Clamart, France
- 2AP-HP, Department of Reproductive Medicine & Fertility Preservation, Hôpital Jean Verdier, Bondy, France
- 3INSERM U1185, University Paris-Sud, Le Kremlin-Bicêtre, France
- 4INSERM U1133, University Paris Diderot, Paris, France
The management of low prognosis patients in ART represents a challenge for reproductive specialists. Different profiles and biologic characteristics have been identified among these patients. Indeed, while poor ovarian response can be seen in patients with impaired ovarian reserve, others, identified as hypo-responders, show unexpected poor or suboptimal response to controlled ovarian stimulation despite satisfying ovarian parameters. These hypo-responders are associated during FSH stimulation to slow initial responses in terms of estradiol levels and follicle growth, longer stimulations, and/or greater cumulative FSH doses. Hence, it appears that ovarian sensitivity to gonadotropins differs from a patient to another, and plays a determinant role on ovarian response to stimulation. Although precise mechanisms remain to be elucidated, increasing evidence suggests that ovarian sensitivity to FSH could be influenced by the presence of genetic mutations or single nucleotide polymorphisms of gonadotropins and their receptors. Evaluating ovarian sensitivity to FSH therefore appears as a key element to improve IVF success rates in these low prognosis patients and open new treatment perspectives. Since the traditional ovarian markers currently used are not sufficient to accurately reflect ovarian response to FSH, a tool to assess ovarian sensitivity to gonadotropin stimulation was required. The present review aims to present Follicular Output Rate (FORT) as an efficient quantitative and qualitative marker of ovarian responsiveness to gonadotropins, discuss the underlying mechanisms of impaired sensitivity to FSH and the possible FORT implications for Poseidon criteria.
Introduction
Mechanisms underlying poor ovarian response (POR) in assisted reproductive technology (ART) remain unclear. As no consensus on the management of poor responders exists, these low prognosis patients represent a challenge for reproductive specialists (1). The Bologna Criteria (2), established in 2011, defined poor responders to controlled ovarian stimulation (COS) by the presence of at least two of the following characteristics: advanced maternal age (≥40 years), a previous incident of POR (cycles canceled or ≤3 oocytes with a conventional ovarian stimulation protocol), and/or a low ovarian reserve tests [antral follicle count (AFC) <5–7 follicles or serum anti-Müllerian hormone (AMH) levels <0.5–1.1 ng/mL]. Although successful in reducing the variability of POR definitions (3), the Bologna criteria failed to reflect the very different profiles and significantly variable biologic characteristics of these patients (4, 5). Notably, whereas POR can be observed in patients with impaired ovarian reserve, others show an “unexpected” poor or suboptimal response to COS despite satisfying ovarian parameters.
Consequently, the Poseidon classification (6) (with as endpoint the number of oocytes required to obtain at least one euploid embryo), distinguishes patients of low prognosis despite an adequate ovarian reserve (Groups 1 and 2: AFC >5 and AMH >1.2 ng/mL) from those with poor ovarian features (Groups 3 and 4: AFC <5 and AMH <1.2 ng/mL) (7). Patients of Poseidon Groups 1 and 2 show an initial slow response to FSH stimulation in terms of estradiol levels and follicle growth, require longer stimulations, and/or greater cumulative FSH doses despite their correct ovarian parameters (8, 9). Hence, markers currently used (such as AFC and AMH) are not sufficient to predict ovarian response accurately, notably for these “hypo-responders” who raise the question of ovarian sensitivity to FSH (10–12). Other methods are needed to enable identification and optimal counseling for these patients.
The present review aims to present Follicular Output Rate (FORT) as a tool to assess ovarian responsiveness to gonadotropins, discuss the underlying mechanisms of impaired sensitivity to FSH and the possible FORT implications for Poseidon criteria.
Materials and Methods
A systematic search was led using the MEDLINE (PubMed), SCOPUS, EMBASE, and Cochrane Library databases, using the following keywords and MESH search terms: “follicular output rate,” “FORT,” “poor ovarian responder,” “poor ovarian response,” “POR,” “hypo-response,” “hypo-responder,” “ovarian sensitivity,” “Poseidon,” “assisted reproductive technology,” “ART,” “controlled ovarian stimulation,” “COS,” “COH,” “IVF,” “ICSI.” “FSH receptor,” “FSHR,” “polymorphism,” “LH receptor,” “LHR,” “pollution AND ovarian sensitivity.” All relevant studies (limited to human studies) published before October 2018 were considered, without language restrictions. The reference lists of relevant reviews and articles were also hand-searched.
Follicular Output Rate (FORT)
So far, the strength of ovarian response to ovarian stimulation had been analyzed by considering the number of pre-ovulatory follicles obtained at the end of COS (13–16). However, the number of pre-ovulatory follicles obtained does not reliably reflect antral follicle responsiveness to FSH since it is greatly dependent on the number of pre-treatment small antral follicles (17). Similarly, the quantitative relationship between AMH levels and the number of mature follicles and fertilizable eggs observed in certain studies (18–22) may merely result from the positive correlation between AMH levels and pre-treatment number of small antral follicles (23–27), and does not itself attest of the sensitivity to FSH treatment (28). Therefore, identifying an index that considered the number of small antral pre-treatment follicles appeared crucial.
Genro et al. (28) were the first to introduce the concept of FORT in a prospective study of 162 patients. FORT was defined as the ratio of pre-ovulatory follicle (16–22 mm in diameter) count (PFC) on hCG day × 100/small antral follicle (3–8 mm in diameter) count at baseline. Patients (mean age of 34.6 ± 0.3 years) were undergoing COS protocol with a single-dose of time-release GnRH agonist on cycle days 1–3 (3 mg, IM, Decapeptyl, Ipsen Pharma, Paris, France), followed after complete pituitary desensitization had been confirmed, by daily recombinant FSH injections (Gonal-F, Serono Pharmaceuticals, Lyon, France) at a dosage of 300 IU/day for at least 5 days, and continued until the day of hCG. At baseline, women had 14.8 ± 0.3 antral follicles. After treatment, the total number of pre-ovulatory follicles obtained was 6.9 ± 0.2, with a corresponding FORT of 47.5 ± 1.4%. A positive relationship between serum AMH levels and the number of small antral (p < 0.0001) and PFC (p < 0.04) was observed. PFC tended to be lower in the low-AMH group when compared to the other groups (p = 0.246). Interestingly, FORT was negatively and significantly correlated with serum AMH levels, both in univariate and after stepwise regression analysis (p < 0.001). FORT values significantly and progressively decreased from the low (AMH < 1.69 ng/mL; n = 41), average (AMH 1.69–3.20 ng/mL; n = 82), to high (>3.20 ng/mL; n = 39) AMH groups. Whereas, FORT was positively associated to total recombinant FSH dose (p < 0.006) and duration of COS (p < 0.001), it was not significantly associated to age, body mass index, nor to basal estradiol or FSH levels.
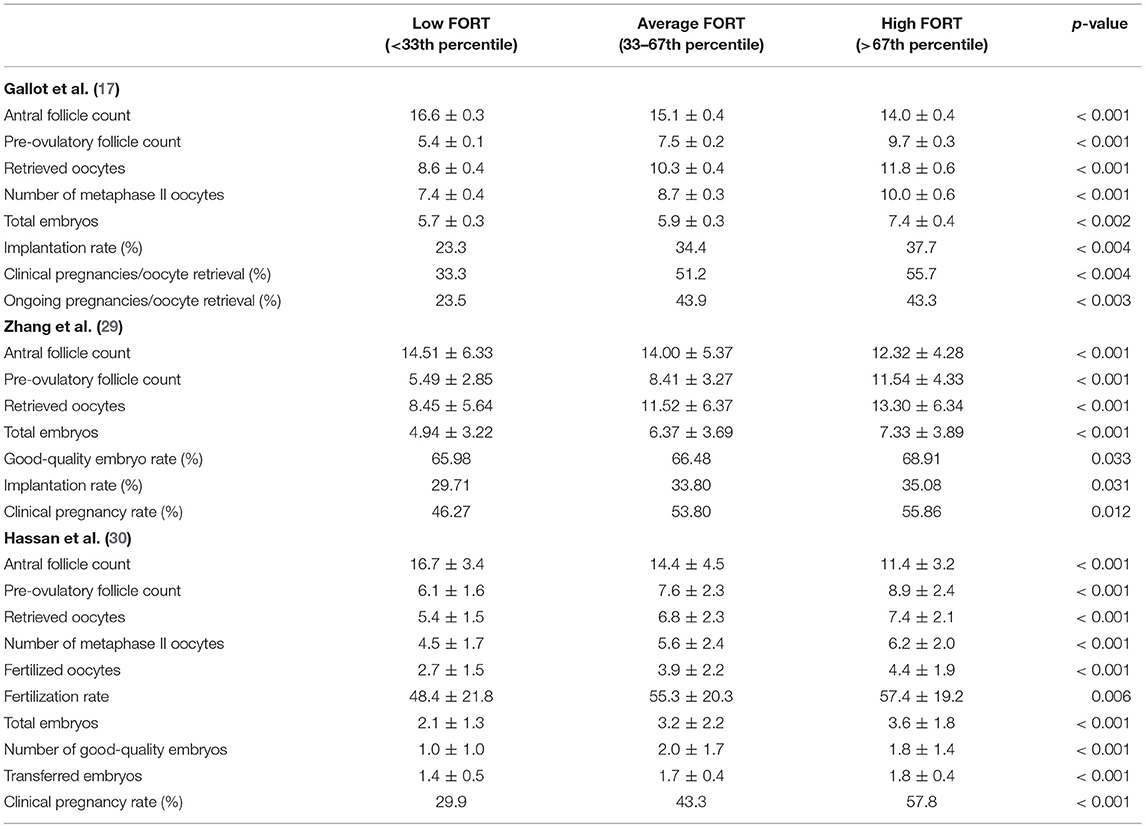
Since then, FORT has been confirmed as an efficient quantitative, as well as qualitative, marker of ovarian response during COS. Gallot et al. (17) prospectively analyzed 322 patients who underwent the same protocol as that of Genro et al. (28). Patients were classified into three distinct FORT groups, arbitrarily chosen according to whether FORT values were under the 33th percentile (<42%, low FORT group; n = 102), between the 33th and the 67th percentile (42–58%, average FORT group; n = 123), or above the 67th percentile (>58%, high FORT group; n = 97) of distribution. Similarly, sets of three different groups according to ages, AFC, and PFC values were formed. Initial AFC was of 15.2 ± 0.2 follicles. Overall, FORT was of 50.6% (range, 16.7–100.0%). Coherently with previous results regarding the negative association between AMH levels and FORT (28), most patients (41%) of the low AFC group had a high FORT, while a minority of patients of the high AFC group (20%) belonged to the high FORT group. The number of oocytes and embryos obtained increased progressively from the low to the high FORT groups (p < 0.001), irrespective of age and absolute pre-COS AFC and post-COS PFC (Table 1). Furthermore, FORT levels were significantly correlated with the percentage of top-morphology embryos (r = 0.14; p < 0.02). Patients with a larger proportion of FSH-responsive antral follicles had better outcomes after IVF-ET, supporting the hypothesis that scant responsiveness of antral follicles to exogenous FSH reveals some degree of follicle/oocyte dysfunction (31, 32).
Consistently, Zhang et al. (29) observed in a larger cohort of 1,503 non-PCOS patients that the number of retrieved oocytes and total number of embryos progressively increased from the low to high FORT groups (p < 0.001; Table 1). Mean FORT was of 65%. Moreover, Rehman et al. (33) found in a prospective study of 282 patients that an increase in FORT value by one unit was associated to increased mean numbers of oocytes retrieved (β coefficient: 0.135), metaphase II oocytes obtained (β coefficient: 0.128), and fertilized oocytes (β coefficient: 0.089). There was a positive relationship between FORT and clinical pregnancy rates (35.8%), and FORT values were higher in pregnant compared to non-pregnant patients (64.2 vs. 49.3%, respectively, p = 0.0001). Hassan et al. (30) reported similar results in a prospective study on 303 women undergoing IVF/ICSI for unexplained infertility. Patients were divided into three groups according to FORT: low FORT (n = 97), below the 33rd percentile, moderate FORT (n = 104) with values between the 33rd and the 67th percentiles, and high FORT (n = 102), above the 67th percentile. There was a progressive and significant increase from low to high FORT groups regarding number of retrieved oocytes (5.4 ± 1.5, 6.8 ± 2.8, and 7.4 ± 2.1, respectively; p < 0.001), clinical pregnancy rates (29.9, 43.3, and 57.8%, respectively; p < 0.001), and fertilization rates (48.4% ± 21.8 vs. 55.3% ± 20.3 and 57.4% ± 19.2, respectively; p = 0.006; Table 1). Multivariate logistic regression analysis revealed that the correlation between FORT and pregnancy was independent of potential confounding factors (p = 0.008).
Mechanisms of Hypo-Response
Although ovarian hypo-response in ART remains to be elucidated, increasing evidence suggests that the presence of genetic mutations or single nucleotide polymorphisms (SNPs) of gonadotropins and their receptors could influence ovarian sensitivity to gonadotropin stimulation (8, 34, 35).
FSH receptor (FSH-R) is a type of G-protein-coupled receptor that mediates FSH intracellular signals through cyclic adenosine monophosphate pathways (8). Two polymorphisms of FSH-R (Thr307/Asn680 and Ala307/Ser680) have been associated to a higher requirement of exogenous gonadotrophins during COS (36, 37). Perez et al. (36) showed that basal FSH levels were significantly different according to FSH genotype (6.4 ± 0.4 IU/L, 7.9 ± 0.3 IU/L, and 8.3 ± 0.6 IU/L for Asn/Asn, Asn/Ser, and Ser/Ser groups, respectively, p < 0.01). The number of FSH ampoules required for successful stimulation was also significantly different among the three groups (31.8 ± 2.4, 40.7 ± 2.3, and 46.8 ± 5.0 for the Asn/Asn, Asn/Ser, and Ser/Ser groups, respectively, p < 0.05).
To better illustrate the relationship between FSH-R and FSH doses, Alviggi et al. (8) conducted a retrospective randomized study in which 17 patients requiring a cumulative dose of recombinant FSH (rFSH) >2,500 UI were compared to 25 patients requiring <2,500 UI. Women requiring more than 2,500 UI of rFSH had significantly longer stimulations (p = 0.03), lower serum estradiol levels on hCG day (p = 0.001), a lower number of oocytes retrieved (p = 0.0005), and a lower number of transferred embryos (p = 0.001). Interestingly, the incidence of Ser/Ser genotype was higher in patients requiring greater doses of rFSH (p = 0.02). Also, patients with higher rFSH consumption and FSH-R Ser680 variant carriers had a longer infertility condition. Hence, FSH-R Ser680 may affect female fertility and delay pregnancy occurrence (8).
Moreover, the role of FSH polymorphisms on responsiveness to COS treatments was described in a meta-analysis of 33 studies lead by Alviggi et al. (38). Notably, the AA genotype of the FSH-R gene at position−29 has been reported to be associated with poor ovarian response. Achrekar et al. (39) showed in a retrospective analysis that subjects with AA genotype at the−29 position required higher amounts of exogenous FSH (p = 0.001), had significantly lower oestradiol concentrations before HCG day compared with the GA genotype (p = 0.015), and had a lower number of pre-ovulatory follicles (p = 0.001), and a lower number of retrieved oocytes (p = 0.003). Additionally, Desai et al. (40) observed that these AA genotype patients significantly expressed lower amounts of FSH-R protein, and that the relative mRNA expression of FSH-R was significantly decreased compared to GG genotype patients (p = 0.027). These results could be explained by the fact that DNA with the A allele might be less accessible for binding of transcription factors compared to the G allele.
However, polymorphisms of FSH-R do not seem to influence antral follicle responsiveness to strong FSH doses, as far as it is measurable by the FORT. Genro et al. (41) observed in 124 patients undergoing COS that FORT index were comparable between Thr307Ala and Asn680Ser carriers or non-carriers when stimulated with an initial dose of 300UI. Further studies using lower, yet more discriminating, FSH doses are required to determine whether this lack of difference is due to the intensity of the FSH signal or to a lack of functional relationship between these SNPs of FSHR and follicle reactivity to FSH.
The genotypic profile of LH receptors may also play a role in ovarian hypo-response. In the comparison of three groups undergoing a gonadotrophin-releasing hormone analog long protocol followed by stimulation with rFSH (Group A: 22 women requiring a cumulative dose of rFSH >3,500 IU; Group B: 15 patients requiring 2000–3500 IU; Group C: 23 women requiring <2,000 IU), Alviggi et al. (42) showed that Group A had significantly lower estradiol peaks (p < 0.05) and a lower number of oocytes retrieved (p < 0.05) (7.3 ± 1.5, 11.7 ± 2.4, and 14.7 ± 4.1 in the three groups, respectively). Seven carriers (31.8%) of v-betaLH were found in Group A, whereas only one variant (6.7%) was observed in Group B and no variant was detected in Group C.
These results were confirmed in a larger series of 220 patients stimulated by rFSH (34). V-betaLH was present in 11% of patients. The study population was divided into two groups according to their LH genotype (wt/wt, n = 196; v-betaLH, n = 24). Patients with v-betaLH received a significantly higher cumulative-dose of r-hFSH (p = 0.048). LH genotype had a statistically significant effect on the cumulative dose of rFSH (p < 0.01), showing a progressive increase from wt/wt to v-betaLH heterozygotic and homozygotic women.
As few data exist on the potential influence of pollution on ovarian sensitivity, the role of environmental factors on ovarian response to COS remains to be elucidated. A Chinese study exploring the influence of fluoride exposure on FSH-R gene polymorphism in 679 women suggested that fluoride exposure was associated to lower GnRH serum levels, but no correlation with the FSH-R polymorphism AA genotype at position−29 was observed (43).
Clinical Implications
Efficient as a quantitative and qualitative marker of ovarian sensitivity to FSH, FORT index should be used in everyday practice. Considering that only follicles between 16 and 22 mm on hCG day effectively respond to FSH may be a possible limitation of FORT. Smaller follicles might also present some degree of FSH responsiveness. However, as it is also possible that very small follicles, which could not be counted by ultrasound at baseline, may also have begun FSH-driven maturation after the start of COS and reached intermediate sizes on hCG day, the inclusion of average-sized follicles on hCG day into the calculation of FORT could confuse interpretation of the results. FORT is also limited by the technical impossibility to track the development of each follicle individually, and therefore cannot assess the possible differences in the FSH-driven growth of follicles (28).
Other tools such as Ovarian sensitivity index (OSI) (44) have also been suggested as a surrogate of AMH assay in predicting ovarian responsiveness to FSH in IVF. OSI corresponds to the total FSH dose administered divided by the number of retrieved oocytes. However, the interpretation of OSI is limited by the fact that it was obtained using a GnRH-agonist buserelin plus rFSH in a classical long protocol; hence, the correlation between OSI and AMH observed could differ in case of a different stimulation schedule or different drugs. More recently, authors introduced Follicle to Oocyte Index (FOI) (9), defined by the ratio between the total number of oocytes collected at the end of ovarian stimulation and the number of antral follicles available at the start of stimulation. FOI ≤ 50 was considered low. Trigger type and efficiency may influence FOI, and further studies are warranted to confirm the use of FOI as a marker of ovarian response.
Assessing ovarian sensitivity to FSH with FORT and understanding mechanisms behind hypo-response in ART opens new possibilities in the treatment of hypo-responders. Increasing FSH doses has been proposed, notably by Behre et al. (45), who evaluated its effect in patients with Ser680 polymorphism. Patients were randomly assigned to an FSH dose of 150 UI/day or 225 UI/day. The control group (Asn/Asn, n = 44) received a dose of 150 UI/day. Peak estradiol levels on hCG day were significantly lower for patients stimulated with 150 UI/day (p = 0.028). Increasing the FSH dose from 150 to 225 UI/day overcame the lower oestradiol response in women with Ser/Ser.
The benefit of adding LH in hypo-responders has also been explored (46, 47). Ferraretti et al. (48) randomized women showing a hyporesponsiveness to FSH into three groups, one receiving an increased dosage of FSH (n = 54), one receiving administered recombinant LH (rLH) in addition to the increased dose of FSH (n = 54), and another was given additional FSH and LH using hMG as a combined drug (n = 22). Addition of rLH significantly improved pregnancy, implantation, and live birth rates. Regarding LH doses, the randomization of 46 patients undergoing ovarian stimulation in two groups (supplementation with a daily rLH dose of 75 UI or 150 UI) showed a significant advantage for patients receiving 150 UI in terms of mean number of oocytes retrieved and percentage of mature oocytes, whereas these patients received a significantly lower mean number of rFSH vials (44.6 ± 7.4 vs. 36.1 ± 3.8) (49).
Moreover, older patients in ART may notably benefit from additional LH. On the one hand, the endocrine changes occurring with ovarian aging include an increase of serum FSH levels in the early follicular phase, which are not accompanied by an LH increase but by a progressive decrease of basal androgen levels. Follicular capacity to induce androstenedione synthesis after rFSH administration is reduced in older patients compared with younger reproductive-aged patients, whereas E2 secretion is preserved by increased aromatase function (50). In a study lead by Bosch et al. (50), whereas patients up to 35 years old (n = 380) did not appear to benefit from rLH, patients aged 36 to 39 years (n = 340) had significantly higher implantation rates (95%CI[1.04–2.33]) when rLH was added. Clinically higher although not significant ongoing pregnancy rates per started cycle (95% CI[0.93–2.38]) were observed. Consistently, Humaidan et al. (51) showed a significant benefit of exogenous LH supplementation for women aged above 35 years old in terms of implantation rates and significantly reduced total FSH consumption.
Conclusion
Considering the lack of efficient tool to accurately evaluate ovarian hypo-response, FORT proves to be a relevant and crucial quantitative, and qualitative index that should be used in everyday practice for the care and management of hypo-responders in ART. Impaired sensitivity to FSH revealed by FORT should be considered in the decision of treatment protocol, gonadotropin, and stimulation doses to be used for hypo-responders. Improving follicular responsiveness to FSH may also be a key to ameliorate prognosis of POSEIDON groups 3 and 4 “expected” poor responders. Reconsidering criteria for COH cancellation based on the output of follicle response to exogenous FSH rather than on the absolute counting of follicles recruited by treatment should be discussed. It is expected that a better understanding of low prognosis patients undergoing ART will help improve individualized ovarian stimulation management and identify more homogeneous populations for clinical trials, thereby, providing better tools with which to maximize IVF success rates.
Author Contributions
All authors listed have contributed to the work and approved the final version. JL and MG performed the literature research, wrote the paper and proofread it.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, Alviggi C. Defining low prognosis patients undergoing assisted reproductive technology: POSEIDON criteria—the why. Front Endocrinol. (2018) 9:461. doi: 10.3389/fendo.2018.00461
2. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. (2011) 26:1616–24. doi: 10.1093/humrep/der092
3. Polyzos NP Devroey PA systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. (2011) 96:1058–61.e7. doi: 10.1016/j.fertnstert.2011.09.048
4. Papathanasiou A. Implementing the ESHRE 'poor responder' criteria in research studies: methodological implications. Hum Reprod. (2014) 29:1835–8. doi: 10.1093/humrep/deu135
5. Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. (2016) 105:1452–3. doi: 10.1016/j.fertnstert.2016.02.005
6. Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of 'Low prognosis patients in Assisted Reproductive Technology' and its proposed marker of successful outcome. F1000Research. (2016) 5:2911. doi: 10.12688/f1000research.10382.1
7. Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. (2016) 31:370–6. doi: 10.1093/humrep/dev316
8. Alviggi C, Conforti A, Caprio F, Gizzo S, Noventa M, Strina I. In estimated good prognosis patients could unexpected 'hyporesponse' to controlled ovarian stimulation be related to genetic polymorphisms of FSH receptor? Reprod Sci. (2016) 23:1103–8. doi: 10.1177/1933719116630419
9. Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker-the follicle-to-oocyte (FOI) index. Front Endocrinol. (2018) 9:589. doi: 10.3389/fendo.2018.00589
10. Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod. (2013) 19:26–36. doi: 10.1093/humupd/dms041
11. How to Define Diagnose and Treat Poor Responders? Responses From a Worldwide Survey of IVF clinics. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/25892496 (accessed October 5, 2018).
12. Papathanasiou A, Searle BJ, King NM, Bhattacharya S. Trends in 'poor responder' research: lessons learned from RCTs in assisted conception. Hum Reprod. (2016) 22:306–19. doi: 10.1093/humupd/dmw001
13. Sharma V, Allgar V, Rajkhowa M. Factors influencing the cumulative conception rate and discontinuation of in vitro fertilization treatment for infertility. Fertil Steril. (2002) 78:40–6. doi: 10.1016/S0015-0282(02)03160-6
14. Carrera-Rotllan J, Estrada-García L, Sarquella-Ventura J. Prediction of pregnancy in IVF cycles on the fourth day of ovarian stimulation. J Assist Reprod Genet. (2007) 24:387–94. doi: 10.1007/s10815-007-9144-7
15. Ottosen LDM, Kesmodel U, Hindkjaer J, Ingerslev HJ. Pregnancy prediction models and eSET criteria for IVF patients–do we need more information? J Assist Reprod Genet. (2007) 24:29–36. doi: 10.1007/s10815-006-9082-9
16. Melo MA, Garrido N, Alvarez C, Bellver J, Meseguer M, Pellicer A. Antral follicle count (AFC) can be used in the prediction of ovarian response but cannot predict the oocyte/embryo quality or the in vitro fertilization outcome in an egg donation program. Fertil Steril. (2009) 91:148–56. doi: 10.1016/j.fertnstert.2007.11.042
17. Gallot V, Berwanger da Silva AL, Genro V, Grynberg M, Frydman N, Fanchin R. Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum Reprod. (2012) 27:1066–72. doi: 10.1093/humrep/der479
18. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. (2002) 77:468–71. doi: 10.1016/S0015-0282(01)03201-0
19. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimüllerian hormone/müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. (2004) 82:1323–9. doi: 10.1016/j.fertnstert.2004.03.061
20. La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. (2007) 22:766–71. doi: 10.1093/humrep/del421
21. Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. (2009) 91:705–14. doi: 10.1016/j.fertnstert.2007.12.013
22. Fanchin R, Louafi N, Méndez Lozano DH, Frydman N, Frydman R, Taieb J. Per-follicle measurements indicate that anti-müllerian hormone secretion is modulated by the extent of follicular development and luteinization and may reflect qualitatively the ovarian follicular status. Fertil Steril. (2005) 84:167–73. doi: 10.1016/j.fertnstert.2005.01.115
23. van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. (2002) 17:3065–71. doi: 10.1093/humrep/17.12.3065
24. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. (2002) 77:357–62. doi: 10.1016/S0015-0282(01)02993-4
25. Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. (2003) 88:5957–62. doi: 10.1210/jc.2003-030727
26. Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. (2005) 20:923–7. doi: 10.1093/humrep/deh688
27. Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. (2003) 18:323–7. doi: 10.1093/humrep/deg042
28. Genro VK, Grynberg M, Scheffer JB, Roux I, Frydman R, Fanchin R. Serum anti-Müllerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod. (2011) 26:671–7. doi: 10.1093/humrep/deq361
29. Zhang N, Hao CF, Zhuang LL, Liu XY, Gu HF, Liu S. Prediction of IVF/ICSI outcome based on the follicular output rate. Reprod Biomed Online. (2013) 27:147–53. doi: 10.1016/j.rbmo.2013.04.012
30. Hassan A, Kotb M, AwadAllah A, Wahba A, Shehata N. Follicular output rate can predict clinical pregnancy in women with unexplained infertility undergoing IVF/ICSI: a prospective cohort study. Reprod Biomed Online. (2017) 34:598–604. doi: 10.1016/j.rbmo.2017.03.004
31. Shima K, Kitayama S, Nakano R. Gonadotropin binding sites in human ovarian follicles and corpora lutea during the menstrual cycle. Obstet Gynecol. (1987) 69:800–6.
32. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. (1996) 17:121–55. doi: 10.1210/edrv-17-2-121
33. Rehman R, Mustafa R, Baig M, Arif S, Hashmi MF. Use of follicular output rate to predict intracytoplasmic sperm injection outcome. Int J Fertil Steril. (2016) 10:169–74. doi: 10.22074/ijfs.2016.4906
34. Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol. (2013) 11:51. doi: 10.1186/1477-7827-11-51
35. Alviggi C, Conforti A, Esteves SC. Impact of mutations and polymorphisms of gonadotrophins and their receptors on the outcome of controlled ovarian stimulation. In: Ghumman S, editor. Principles and Practice of Controlled Ovarian Stimulation in ART. New Delhi: Springer (2015). p. 147–56.
36. Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. (2000) 85:3365–9. doi: 10.1210/jc.85.9.3365
37. Yao Y, Ma CH, Tang HL, Hu YF. Influence of follicle-stimulating hormone receptor (FSHR) Ser680Asn polymorphism on ovarian function and in-vitro fertilization outcome: a meta-analysis. Mol Genet Metab. (2011) 103:388–93. doi: 10.1016/j.ymgme.2011.04.005
38. Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. Hum Reprod. (2018) 24:599–614. doi: 10.1093/humupd/dmy019
39. Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod Biomed Online. (2009) 18:509–15. doi: 10.1016/S1472-6483(10)60127-7
40. Desai SS, Achrekar SK, Pathak BR, Desai SK, Mangoli VS, Mangoli RV. Follicle-stimulating hormone receptor polymorphism (G−29A) is associated with altered level of receptor expression in granulosa cells. J Clin Endocrinol Metab. (2011) 96:2805–12. doi: 10.1210/jc.2011-1064
41. Genro VK, Matte U, De Conto E, Cunha-Filho JS, Fanchin R. Frequent polymorphisms of FSH receptor do not influence antral follicle responsiveness to follicle-stimulating hormone administration as assessed by the Follicular Output RaTe (FORT). J Assist Reprod Genet. (2012) 29:657–63. doi: 10.1007/s10815-012-9761-7
42. Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online. (2009) 18:9–14. doi: 10.1016/S1472-6483(10)60418-X
43. Zhao MX, Zhou GY, Zhu JY, Gong B, Hou JX, Zhou T. Fluoride exposure, follicle stimulating hormone receptor gene polymorphism and hypothalamus-pituitary-ovarian axis hormones in chinese women. Biomed Environ Sci. (2015) 28:696–700. doi: 10.3967/bes2015.099
44. Biasoni V, Patriarca A, Dalmasso P, Bertagna A, Manieri C, Benedetto C. Ovarian sensitivity index is strongly related to circulating AMH and may be used to predict ovarian response to exogenous gonadotropins in IVF. Reprod Biol Endocrinol. (2011) 9:112. doi: 10.1186/1477-7827-9-112
45. Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. (2005) 15:451–6. doi: 10.1097/01.fpc.0000167330.92786.5e
46. Alviggi C, Mollo A, Clarizia R, De Placido G. Exploiting LH in ovarian stimulation. Reprod Biomed Online. (2006) 12:221–33. doi: 10.1016/S1472-6483(10)60865-6
47. Gizzo S, Andrisani A, Noventa M, Manfè S, Oliva A, Gangemi M. Recombinant LH supplementation during IVF cycles with a GnRH-antagonist in estimated poor responders: a cross-matched pilot investigation of the optimal daily dose and timing. Mol Med Rep. (2015) 12:4219–29. doi: 10.3892/mmr.2015.3904
48. Ferraretti AP, Gianaroli L, Magli MC, D'angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril. (2004) 82:1521–6. doi: 10.1016/j.fertnstert.2004.06.041
49. De Placido G, Alviggi C, Mollo A, Strina I, Ranieri A, Alviggi E. Effects of recombinant LH (rLH) supplementation during controlled ovarian hyperstimulation (COH) in normogonadotrophic women with an initial inadequate response to recombinant FSH (rFSH) after pituitary downregulation. Clin Endocrinol. (2004) 60:637–43. doi: 10.1111/j.1365-2265.2004.02027.x
50. Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. Fertil Steril. (2011) 95:1031–6. doi: 10.1016/j.fertnstert.2010.10.021
51. Humaidan P, Bungum M, Bungum L, Yding Andersen C. Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down-regulation and stimulation with recombinant FSH: an opening study. Reprod Biomed Online. (2004) 8:635–43. doi: 10.1016/S1472-6483(10)61643-4
Keywords: follicular output rate, FORT, POSEIDON criteria, hypo-response, controlled ovarian stimulation, FSH receptor polymorphism
Citation: Grynberg M and Labrosse J (2019) Understanding Follicular Output Rate (FORT) and its Implications for POSEIDON Criteria. Front. Endocrinol. 10:246. doi: 10.3389/fendo.2019.00246
Received: 30 November 2018; Accepted: 29 March 2019;
Published: 16 April 2019.
Edited by:
Carlo Alviggi, University of Naples Federico II, ItalyReviewed by:
Alessandro Conforti, University of Naples Federico II, ItalyGiuliano Marchetti Bedoschi, University of São Paulo, Brazil
Copyright © 2019 Grynberg and Labrosse. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Grynberg, bWljaGFlbC5ncnluYmVyZ0BhcGhwLmZy
 Michael Grynberg
Michael Grynberg Julie Labrosse
Julie Labrosse