
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 11 December 2018
Sec. Clinical Diabetes
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00755
This article is part of the Research Topic Diabetes During and Beyond Pregnancy: A Life Course Perspective View all 16 articles
 Song-Ying Shen1†
Song-Ying Shen1† Li-Fang Zhang1†
Li-Fang Zhang1† Jian-Rong He1
Jian-Rong He1 Jin-Hua Lu1
Jin-Hua Lu1 Nian-Nian Chen1
Nian-Nian Chen1 Wan-Qing Xiao1
Wan-Qing Xiao1 Ming-Yang Yuan1
Ming-Yang Yuan1 Hui-Min Xia1*
Hui-Min Xia1* Kin Bong Hubert Lam2
Kin Bong Hubert Lam2 Xiu Qiu1*
Xiu Qiu1*Objective: The overall impact of maternal hyperglycemia on maternal and birth outcomes is largely underestimated, therefore quantifying the true burden of hyperglycemia in a whole population it is a challenging task. This study aims at examining the association between blood glucose concentration during pregnancy and a composite score of adverse maternal-birth outcomes in a large-scale prospective cohort study in China.
Methods: Pregnant women within “the Born in Guangzhou Cohort Study” China who underwent a standard 75-g oral-glucose-tolerance-test (OGTT) between 22 and 28 gestational weeks were included. A composite score of stillbirth, duration of pregnancy, birth weight, preeclampsia, and cesarean section was developed based on a published maternal-fetal outcomes scale, weighed by the relative severity of the outcomes. Multiple linear regression models were used to assess the associations between OGTT glucose measurements and log composite score. Logistic regression models were used to assess relations with outcome as a categorical variable (0, 1– < 3, and ≥3).
Findings: Among 12,129 pregnancies, the composite score ranged from 0 to 100 with a median of 2.5 for non-zero values. Elevated fasting glucose level was associated with higher composite score (adjusted coefficients 0.03 [95% CI, 0.02–0.04] for 1-SD increase). For 1-SD increase in fasting glucose, the risk of having a composite score 1– < 3 and ≥3 rises by 13% (95% CI, 8–17%) and 15% (95% CI, 7–23%), respectively. Similar association and increase in risk was found for 1 and 2-h glucose.
Conclusion: Elevated fasting, 1 and 2-h glucose levels are associated with a range of adverse maternal-birth outcomes. The composite score model can be applied to the risk assessment for individual pregnant women and to evaluate the benefits for controlling glucose levels in the population.
Gestational diabetes mellitus (GDM) is one of the most prevalent major complications during pregnancy worldwide (1–3). Previous studies have linked maternal hyperglycemia to increased risks of various adverse perinatal outcomes, including macrosomia, large for gestational age (LGA), cesarean section, preterm birth, gestational hypertension and hyperbilirubinemia (3, 4). However, all such studies have only investigated the perinatal outcomes in isolation without taking into account the complex inter-relationship between maternal and fetal outcomes, and among the perinatal outcomes themselves; examples include macrosomia and cesarean section (5), gestational hypertension and preterm birth (6, 7). In addition, the varying strengths of association with these outcomes, which have different levels of severity and consequences, have not been fully appreciated (3, 4, 8). This has led to difficulties in assessing the influence of maternal hyperglycemia on both maternal and birth outcomes, and developing optimal management of maternal hyperglycemia, as well as evaluating the effectiveness of glucose-lowering interventions in the general pregnant women population. A comprehensive consideration of multiple adverse outcomes, both in pregnant women and in newborns (9), is therefore needed.
Indeed, some previous studies have attempted to assess multiple perinatal outcomes (8–12). For example, to evaluate the need of care during pregnancy and delivery, Novicoff et al. created an outcome score that sums the points of all maternal and fetal outcomes based on relative desirability and frequency of occurrence (10). Verma et al. developed a morbidity assessment index for newborns (called MAIN score), capturing the entire severity spectrum of morbidity at birth to estimate the effectiveness of obstetric interventions on neonatal morbidity (9, 12). However, none of these studies have evaluated the influence of maternal hyperglycemia on composite maternal and birth outcomes. A comprehensive measure focusing on capturing GDM-related morbidity in both mother and child should add an important dimension to current understanding of GDM risk and management.
The present study aims to examine the association between blood glucose concentrations during pregnancy, and risk-adjusted adverse maternal-birth outcomes represented by a composite score in a large-scale prospective cohort study in China.
The data used in the present study is part of the Born in Guangzhou Cohort Study (BIGCS), which is a birth cohort study conducted in the Guangzhou Women and Children's Medical Center (GWCMC), China. Study participants were recruited from pregnant women attending their first routine antenatal exam at GWCMC. Inclusion criteria were: residents in Guangzhou, <20 weeks gestation, intending to deliver at GWCMC, and planning to remain in Guangzhou with the child for at least 3 years after delivery. A detailed description of the BIGCS protocol has been published elsewhere (13). For this analysis, we excluded participants with pre-pregnancy diabetes or chronic hypertension, according to the self-reported medical history. Women with missing blood glucose and delivery data were also excluded (Figure 1).
At enrollment participants underwent an interviewer-administered questionnaire, which collected a wide range of information including socio-demographic data, exposures at home and at workplace, personal lifestyle, medical histories, and health status before pregnancy. The study protocol was approved by GWCMC Ethics Approval Board. Written informed consent was obtained from all participants.
Between 22 and 28 weeks of gestation, all pregnant women underwent a standard oral glucose tolerance test (OGTT), during which blood samples (2 mL) were collected at fasting, 1 and 2 h after a 75 g glucose load with NaF/EDTA tubes. Detailed procedures of the OGTT test were presented elsewhere (14). Women with GDM diagnosis [based on the International Association of Diabetes and Pregnancy Study Groups [IADPSG] criteria (15)] received routine consultation on diet and exercise. They were then asked to self-monitor their preprandial blood glucose levels after having dietary management for 3–5 days. Those with fasting blood glucose ≥5.3 mmol/L or 2 h postprandial glucose ≥6.7 mmol/L after dietary control were prescribed insulin in addition to a diet and exercise regime (16).
Information about pregnancy complications, mode of delivery, gestational age, birth weight, parity, and gender of newborns were obtained from GWCMC's electronic medical records. Gestational age was confirmed by ultrasound examination in the first- or second-trimester. Birth weight was measured by midwives immediately after delivery. Birthweight z scores were calculated using a local population-based birth weight reference (17). LGA was defined as a birthweight larger than the 90th percentile for gestational age by gender and small for gestational age (SGA) was defined as a birthweight lower than the 10th percentile for gestational age by gender, based on the same birth weight reference (17). Preterm birth was defined as birth before 37 weeks of gestation. Spontaneous preterm birth was the birth following spontaneous preterm labor and/or preterm premature rupture of the membranes before 37 weeks of gestation, irrespective of the mode of delivery (vaginal, cesarean section) (18); all this information was obtained from medical records, which was also independently confirmed by two pediatricians. Gestational hypertension was defined as blood pressure ≥140/90 mmHg on at least two occasions separated by at least 4 h after 20th week of gestation without the presence of protein in the urine and returned to normal within 12 weeks postpartum (19). Preeclampsia was defined as gestational hypertension combined with proteinuria [protein level in the urine ≥300 mg/24 h or ≥(+) by a dipstick test on at least two occasions separated by at least 6 h] (19). Stillbirth cases were identified from the medical records with the 10th revision of the International Classification of Diseases (ICD10) codes of O36.401 and P95.
We developed a composite outcome score based on the adverse maternal and infant outcomes related to GDM as suggested by the literature (3, 4); maternal-infant outcomes included stillbirth, duration of pregnancy, birth weight, preeclampsia, and cesarean section. A score was assigned to each outcome according to a published unified maternal-fetal outcome risk assessment model, ranging from 0 (perfect outcome) to 100 (maternal or infant death) (10). Each mother-newborn pair was scored based on the existence and severity of the five outcomes as shown in Table 1. A comprehensive outcome score was calculated by summing all above-mentioned outcomes scores for each mother-newborn pair. Higher scores indicated the presence of worse maternal and perinatal outcomes.
The following maternal risk factors were selected a priori as potential confounders: age, maternal education level, maternal income, pre-pregnancy body mass index (BMI), maternal smoking status, secondhand smoking (smoke exposure during pregnancy), use of assisted reproduction technology, parity, and gestational age at the time of OGTT. Except for gestational age at OGTT and parity, all information was derived from the self-reported questionnaire at enrollment.
Descriptive statistics of the characteristics and outcomes were reported for the whole sample and across categories of the composite scores. To assess the relationship between OGTT glucose measurements and maternal and fetal outcomes as represented by the composite score, we analyzed the data in two ways. First, the composite score was treated as a continuous variable and was log (base 10) transformed [log (score+1)]. Multiple linear regression models were used to assess the association between the OGTT glucose measurements and log composite score. Regression coefficients were calculated, representing the change in log composite score for 1-SD change in each fasting, 1 and 2 h glucose measurement. Second, we categorized the composite scores (without transformation) into three groups based on the median of non-zero scores (2.5): 0, low (including scores 1, 2, and 2.5) and high (≥3). Logistic regression models were used to evaluate the relationships of the OGTT glucose measurements with composite outcomes score categories, with “0” as reference. Odds ratios (ORs) were calculated for a 1-SD increase in each OGTT measurement. The linear and logistic regression models were adjusted for pre-specified potential confounders, including maternal age (continuous), maternal education (middle school or below, college, undergraduate or postgraduate), maternal income (< 1,500, 1,500–4,500, 4,501–9,000, ≥9,001 Yuan), maternal pre-pregnancy body mass index (BMI, continuous), parity (0, ≥1), smoking status (yes, no), second-hand smoking exposure during pregnancy (yes, no), use of assisted reproduction technology (yes, no), and gestational age when OGTT was performed (continuous).
All analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC, USA). P < 0.05 were considered to indicate statistical significance.
A total of 15,198 pregnant women in BIGCS delivered between February 2012 and January 2016. We excluded women with multiple births, those who dropped out before delivery or terminated their pregnancy, had pre-pregnancy diagnosed hypertension and diabetes, or whose delivery data and OGTT results were missing, resulting in 12,129 mothers and their singleton births in this analysis (Figure 1).
Characteristics of mothers and newborns, and by composite score groups are shown in Table 2. Pregnant women had a mean age of 29.1 years (SD 3.4). The majority of them had attained college or above qualifications and over half of them had a monthly income more than 4,500 Yuan. Few women smoked but 30% were exposed to secondhand smoke during pregnancy. Not unexpectedly, the vast majority (86.6%) of the pregnant women were primipara. Only 3.4% women undertook assisted reproduction technology. About 10% of the participants were overweight or obese before pregnancy BMI ≥24.0 kg/m2, according to the Chinese guidelines (20). The mean plasma glucose levels were 4.3 mmol/L (SD 0.4) at fasting, 7.7 mmol/L (SD 1.7) at 1-h, and 6.6 mmol/L (SD 1.3) at 2-h post 75 g glucose load, respectively. Using the IADPSG criteria, there were 1,662 (1,662/1,662, 100%) women diagnosed with GDM who received a subsequent diet and exercise advice. Ten (10/1,662, 0.6%) women went on to receive insulin therapy. Around 60% of the pregnancies (n = 7,296) resulted in optimal outcome (with a score of 0), one-third (n = 3,935) having scores between 1 and < 3, and 7% (n = 898) had a composite score ≥3 (20% being 3). There were statistically significant differences in most of the maternal characteristics measured across the three composite score groups.

Table 2. Characteristics of the 12,129 study participants and their newborns by different groups of composite score.
Overall, the prevalence of preeclampsia (0.7%) and stillbirth (0.1%) was low, although one-third of deliveries were by cesarean section. Infants were born weighing on average 3191.5 g (SD 423.9) after a mean gestational length of 38.8 weeks (SD 1.4), with 3.5% of the babies having spontaneous preterm birth (< 37 weeks).
Among those who had a non-zero composite score, 4,138 (84.2%) had one outcome, 620 (12.6%) had two, 155 (3.2%) had three or more. Cesarean delivery was the most common outcome, occurring in 4,161 (85.0%) of the women with non-zero score.
Table 3 summarizes selected pregnancy-related and birth outcomes by composite score groups. The prevalence of gestational hypertension, preeclampsia, cesarean delivery, preterm birth, spontaneous preterm birth, LGA, SGA, and stillbirth increased significantly with higher composite score categories.
Table 4 shows the associations between fasting, 1 and 2-h plasma glucose and composite perinatal outcomes score after adjusted for confounders. Elevated fasting glucose values was significantly associated with higher log composite score as a continuous variable (coefficient 0.03 [95% CI, 0.02–0.04] for 1-SD increase). For 1-SD increase in fasting glucose, the likelihoods of having composite outcomes score 1– < 3 and ≥3 increased by 13% (95% CI, 8–17%) and 15% (95% CI, 7–23%) in the logistic regression model, respectively. Similar associations were found for 1 and 2-h glucose values.
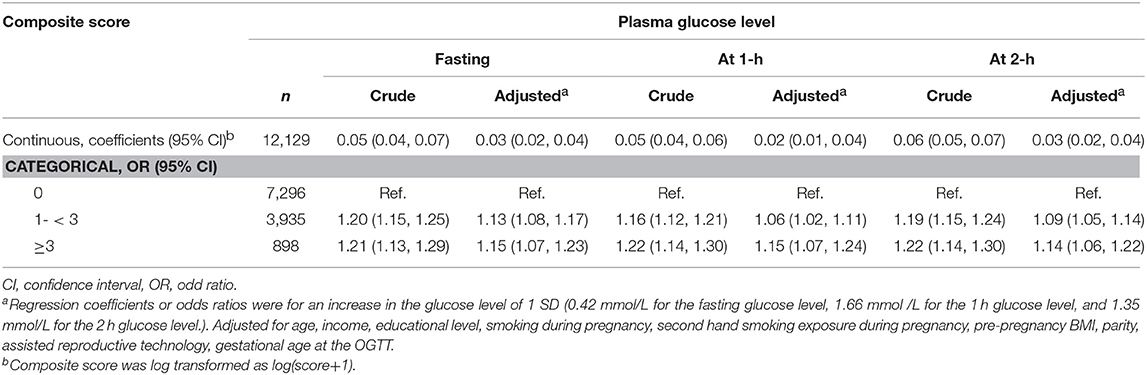
Table 4. Adjusted coefficients and odds ratios for associations between maternal glycemia and composite maternal-birth outcomes.
In the present study, a composite maternal-birth outcome scoring scale based on five perinatal outcomes that are most related to gestational diabetes was adapted from a risk model covering maternal and birth outcomes. Significant associations between increased fasting, 1 and 2-h post-load plasma glucose levels and increased composite maternal and birth outcomes score were found.
A number of studies have investigated the associations between maternal glucose levels and individual perinatal outcomes, such as preterm birth or LGA (3, 4). However, none has considered the multiple adverse maternal and birth outcomes holistically in the same study. Individual outcomes, e.g., LGA, cesarean delivery, preterm birth, preeclampsia, when considered separately, are unable to capture the overall impact of maternal hyperglycemia in mothers and their newborns, hampering the risk assessment of maternal hyperglycemia and the evaluation of effectiveness and benefit for glucose level management in the general pregnant women population. In the present study, the composite outcome score included birth weight and gestational age, both of which have been widely used as outcome measures of effectiveness of national health policies and interventions for pregnant women, especially in developing countries (17, 18), and have been reported to be strongly associated with GDM (via abnormal birthweight and preterm birth) (4, 21). We also included preeclampsia and cesarean section—both associated with elevated glucose concentrations during pregnancy (4, 21). Hence, the composite score combining complications in both mother and newborn is an interpretable measure of the actual morbidity that are most related to maternal hyperglycemia during pregnancy.
In the present analysis, we found that the risk of having a higher composite score (between 1 and < 3, ≥3) decreased for 1-SD lower in fasting, 1 and 2-h glucose levels. This may help to assess the risk of developing adverse maternal and birth outcomes for individual pregnant women and recognize specific needs for hyperglycemic women, thus facilitating resource-allocation and care optimization for the high-risk, high-cost patient encounter (8, 9). For example, among the women with hyperglycemia, only those who have high risk of overall adverse outcomes need more intensive care and intervention. On the other hand, the linear association between maternal glucose level and the comprehensive outcome score highlights the importance of controlling maternal plasma glucose at low levels, rather than aiming at the level just below the diagnostic cut-off. While there is evidence of substantial benefits from intervention for women with GDM and their newborns, previous intervention studies reported improvement in targeted morbidities separately (22, 23) rather than across a spectrum of major morbidities to assess overall health improvement. As a result, clinicians may opt to implement different treatment and management strategies depending on the particular outcome they focus on, which may not necessarily be the best option for the patients. The results also can be applied to evaluate the overall effectiveness and benefit of controlling glucose level in the whole pregnant women population. According to our findings, if the fasting glucose level increases by 1-SD (0.42 mmol/L) in pregnant women, the risk of having a composite adverse outcomes score 1- < 3 and ≥3 would increase by 13 and 15%, respectively. Consider China where there is a large number of pregnant women, ~18 million per year, an excess of 0.76 million (13% × 32.4% [prevalence of women with composite score 1– < 3] × 18 million) and 0.20 million (15% × 7.4% [prevalence of women with composite score ≥3] × 18 million) women per year would suffer from mild and relative severe adverse maternal and birth outcomes if fasting glucose would increase by 1-SD in the population. Adequate control of fasting glucose concentration is therefore necessary in both individual and population levels.
The strengths of our study include the use of high-quality outcome data extracted from medical records and that a wide range of potential confounders have been considered in the analysis. This study had some limitations though. First, we did not adjust for the dietary intake of the women during pregnancy, which could potentially affect fetal growth and other maternal-birth outcomes. Second, we recognize that the weights we applied for the five outcomes in the present study, adapted from the maternal and infant scoring system developed by Novicoff et al. (10), may not accurately reflect the relative severity of the outcomes. However, there has been no universally agreed standard values and ranking for the outcomes of interest. Once a more appropriate standardized scale for each outcome is developed, studies like ours will be required to confirm the findings. Third, neonatal hypoglycaemia is also an important outcome related to gestational diabetes. Unfortunately, the scale we used did not include hypoglycaemia as an outcome and we also did not collect data about this outcome.
In conclusion, using a composite scale that ranks the relative severity of multiple maternal and infant outcomes, we assessed the effects of elevated fasting, 1 and 2-h post-load plasma glucose and confirmed the potential benefits of adequate control of maternal glucose level in improving maternal-birth outcomes as a whole. This could be applied to the risk assessment for individual pregnant women as well as to the evaluation of effectiveness and benefit for controlling glucose level in the population.
XQ and H-MX designed the study and directed its implementation. S-YS, J-RH, J-HL, N-NC, W-QX, and M-YY were involved in study design, questionnaires development, data collection, and follow-up of participants. S-YS participated in the data analysis and drafted the manuscript. L-FZ carried out the data analysis, contributed to the writing of the paper. J-HL managed the data. KL and XQ revised the manuscript. All authors critically revised the manuscript, and approved the final version.
This study was supported by the Technology and Innovation Commission, Guangzhou, China (201508030037, 201807010086), National Natural Science Foundation of China (81673181, 81803251) and Guangdong Provincial Department of Science and Technology (2014A020213022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all the pregnant women who participated in the Born in Guangzhou Cohort Study participants and all staff in the cohort team for their contribution to this study, particularly the research nurses and midwives and other recruiting staff for their excellent work. We thank Professor Ben Willem J. Mol for the contribution of knowledge about the effects of maternal hyperglycemia and Dr. Justina Zurauskiene for help with language editing of the manuscript.
1. Kalter-Leibovici O, Freedman LS, Olmer L, Liebermann N, Heymann A, Tal O, et al. Screening and diagnosis of gestational diabetes mellitus: critical appraisal of the new international association of diabetes in pregnancy study group recommendations on a national level. Diabetes Care (2012) 35:1894–6. doi: 10.2337/dc12-0041
2. Hirst JE, Tran TS, Do MA, Morris JM, Jeffery HE. Consequences of gestational diabetes in an urban hospital in Viet Nam: a prospective cohort study. PLoS Med. (2012) 9:e1001272. doi: 10.1371/journal.pmed.1001272
3. Black MH, Sacks DA, Xiang AH, Lawrence JM. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care (2010) 33:2524–30. doi: 10.2337/dc10-1445
4. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
5. Liu Y, Li G, Chen Y, Wang X, Ruan Y, Zou L, et al. A descriptive analysis of the indications for caesarean section in mainland China. BMC Pregnan Childbirth (2014) 14:410. doi: 10.1186/s12884-014-0410-2
6. Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol. (2006) 30:16–9. doi: 10.1053/j.semperi.2006.01.008
7. Haedersdal S, Salvig JD, Aabye M, Thorball CW, Ruhwald M, Ladelund S, et al. Inflammatory markers in the second trimester prior to clinical onset of preeclampsia, intrauterine growth restriction, and spontaneous preterm birth. Inflammation (2013) 36:907–13. doi: 10.1007/s10753-013-9619-x
8. Roberts CL, Cameron CA, Bell JC, Algert CS, Morris JM. Measuring maternal morbidity in routinely collected health data: development and validation of a maternal morbidity outcome indicator. Med Care (2008) 46:786–94. doi: 10.1097/MLR.0b013e318178eae4
9. Verma A, Okun NB, Maguire TO, Mitchell BF. Morbidity assessment index for newborns: a composite tool for measuring newborn health. Am J Obstet Gynecol. (1999) 181:701–8.
10. Novicoff WM, Wagner DP, Knaus WA, Kane EK, Cecere F, Draper E, et al. Initial development of a system-wide maternal-fetal outcomes assessment program. Am J Obstet Gynecol. (2000) 183:291–300. doi: 10.1067/mob.2000.108087
11. Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. (2009) 170:173–80. doi: 10.1093/aje/kwp101
12. Verma A, Weir A, Drummond J, Mitchell BF. Performance profile of an outcome measure: morbidity assessment index for newborns. J Epidemiol Community Health (2005) 59:420–6. doi: 10.1136/jech.2003.019109
13. Qiu X, Lu JH, He JR, Lam KH, Shen SY, Guo Y, et al. The Born in Guangzhou Cohort Study (BIGCS). Eur J Epidemiol. (2017) 32:337–46. doi: 10.1007/s10654-017-0239-x
14. Shen S, Lu J, Zhang L, He J, Li W, Chen N, et al. Single fasting plasma glucose versus 75-g oral glucose-tolerance test in prediction of adverse perinatal outcomes: a cohort study. EBioMedicine (2017) 16:284–91. doi: 10.1016/j.ebiom.2017.01.025
15. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care (2010) 33:676–82. doi: 10.2337/dc09-1848
16. Obstetrics Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association, Group of Pregnancy with Diabetes Mellitus, Chinese Society of Perinatal Medicine, Chinese Medical Association. [Diagnosis and therapy guideline of pregnancy with diabetes mellitus]. Zhonghua Fu Chan Ke Za Zhi (2014) 49:561–9.
17. He JR, Xia HM, Liu Y, Xia XY, Mo WJ, Wang P, et al. A new birthweight reference in Guangzhou, southern China, and its comparison with the global reference. Arch Dis Child. (2014) 99:1091–7. doi: 10.1136/archdischild-2013-305923
18. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
19. Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS ONE (2014) 9:e100180. doi: 10.1371/journal.pone.0100180
20. Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. (2004) 17:1–36.
21. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. (2005) 192:989–97. doi: 10.1016/j.ajog.2004.11.039
22. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. (2009) 361:1339–48. doi: 10.1056/NEJMoa0902430
Keywords: hyperglycemia, pregnancy, stillbirth, birth weight, duration of pregnancy, preeclampsia, cesarean section, composite outcome
Citation: Shen S-Y, Zhang L-F, He J-R, Lu J-H, Chen N-N, Xiao W-Q, Yuan M-Y, Xia H-M, Lam KBH and Qiu X (2018) Association Between Maternal Hyperglycemia and Composite Maternal-Birth Outcomes. Front. Endocrinol. 9:755. doi: 10.3389/fendo.2018.00755
Received: 27 April 2018; Accepted: 28 November 2018;
Published: 11 December 2018.
Edited by:
Margarita de Veciana, Eastern Virginia Medical School, United StatesReviewed by:
Melissa Irene March, University Hospitals Cleveland Medical Center, United StatesCopyright © 2018 Shen, Zhang, He, Lu, Chen, Xiao, Yuan, Xia, Lam and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu Qiu, cXhpdTAxNjFAMTYzLmNvbQ==; eGl1LnFpdUBiaWdjcy5vcmc=
Hui-Min Xia, aHVpbWluLnhpYTg3NjAwMUBnbWFpbC5jb20=; aHVpbWluLnhpYUBiaWdjcy5vcmc=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.