
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 03 December 2018
Sec. Thyroid Endocrinology
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00737
This article is part of the Research Topic The Unusual Presentation of Thyroid Disorders View all 16 articles
 Virginia A. LiVolsi*
Virginia A. LiVolsi* Zubair W. Baloch
Zubair W. BalochThis article reviews those pathologic lesions which are associated with clinical and/or biochemical hyperthyroidism. Beginning with the descriptive pathology of classical Graves' disease and the less common toxic nodular goiter and hyper-functioning thyroid nodules, this paper describes the effects of non-thyroidal hormones, glandular function (including pituitary and hypothalamic lesions), ectopic production of thyroid stimulating proteins by non-thyroidal neoplasms, exogenous drug reactions causing hyper-function and finally conditions associated with a mechanic- destructive cause of hyperthyroidism.
Hyperthyroidism is a clinical syndrome characterized by hypermetabolic state due to the increased free serum thyroxine (T4) and/or free triiodothyronine (T3). There are many known factors and pathologies both inherent to the thyroid gland as well of non-thyroidal origin that lead to hyperthyroidism. It can result from hyperplasia and overstimulation of thyroid epithelium, acute destruction of thyroid follicles, and follicular epithelium due to various forms of thyroiditis or metastatic tumors. In addition, various drugs and antineoplastic agents can lead to thyroid dysfunction. In this review we provide a pathologist's perspective on various pathologic features that can be encountered in thyroids of patients with clinical hyperthyroidism.
This condition can be divided into diffuse and nodular types.
Most patients with classical hyperthyroidism caused by autoantibodies against the TSH receptors, stimulating thyroid follicular cell receptors show an enlarged hypervascular thyroid without obvious nodularity. This condition known in North America as Graves' disease and in Europe as Basedow disease is a disorder of young usually female patients who present with heat intolerance, tachycardia, tremors, weight loss and orbitopathy (1–6). This disease is characterized by thyroid enlargement with smooth capsule, non-nodular growth, and increased vascularity. Histologically one notes the presence of a diffuse papillary and follicular hyperplasia and varying degrees of lymphocytic infiltration into the thyroid stroma (7–9) (Figures 1, 2). In contrast to classic Hashimoto's disease, the lymphocytes do not infiltrate the follicular cells. The latter often are enlarged and can show cytoplasmic eosinophilia. The nuclei of these cells can also be enlarged and can in extreme cases mimic the nuclei of papillary thyroid carcinoma (10–12). However, the nuclei in Graves' disease tend to maintain a rounded shape and to have internal structure with minimal if any clearing (10–12).
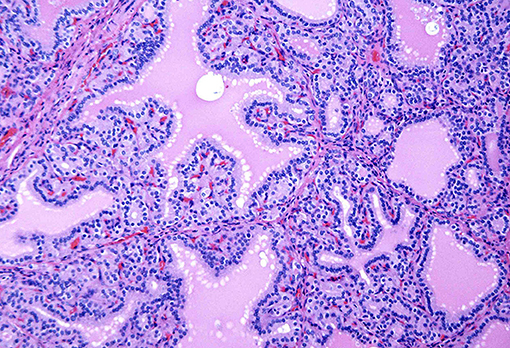
Figure 2. A case of Graves' disease on medium power showing cells with round nuclei and even chromatin pattern lining the papillae.
There has been controversy regarding whether the presence of Graves' disease can lead to the development of papillary carcinoma and if the two coexist, does the carcinoma behave more aggressively than similar tumors not arising in this background setting. Several studies and our own experience have shown that the data needs to be evaluated systematically (13–17). If a papillary microcarcinoma is discovered incidentally in a surgically removed hyperthyroid gland, the prognosis is excellent. If a clinically evident tumor is identified in a Graves' patient, then the pathological characteristics of that lesion (size, extent, mutlifocality) will determine the prognosis. It does not appear that the background gland influences the prognosis adversely (18).
As the name implies is an enlarged gland with multiple nodules of varying sizes. Usually the nodules show the papillary and follicular hyperplasia although the lymphocytic stromal infiltration may be within the nodules and in the non-nodular thyroid (9, 19). The correlation between the histology of the nodules and radioactive iodide scan results is fair to poor since nodules with histologic evidence of hyperfunction often are warm or cool on scan. Toxic nodular goiter tends to occur in older individuals and affects males as well as females. In some older patients, the clinical manifestations of the hyperthyroidism may not be related to the thyroid at all; many of these individuals show symptoms related to cardiac disease, frequently atrial fibrillation. This disorder, sometimes referred to as “apathetic hyperthyroidism” needs to be considered by treating clinicians and appropriate laboratory testing will lead to the correct diagnosis (20–22).
Most autonomously functioning thyroid tumors are benign that is follicular adenomas or hyperplastic nodules. These lesions are also designated as ‘autonomous nodules” and have been given the acronym “Plummer's disease” (23, 24).
Benign hyperfunctional adenomas (AKA Toxic Adenoma) are clonal, autonomously functioning follicular proliferations that produce supra-physiological amounts of thyroid hormones causing TSH suppression. These are more common in women and usually present at an older age. Usually, a radioisoptope scan confirms the preoperative diagnosis and most are not subjected to fine-needle aspiration (FNA). However, in rare cases a FNA is performed by a clinician or surgeon without the knowledge of thyroid function tests. In such cases, the FNA specimen is usually cellular and most likely will be diagnosed as a follicular neoplasm (Bethesda Category IV).
On surgical excision the toxic adenoma grossly shows a distinct capsule and may be centrally cystic. These lesions can also show a papillary pattern of growth without nuclear features of papillary carcinoma. The autonomously functioning nodule usually occurs in young females. This lesion also termed “papillary hyperplastic nodule” (25) (a term coined by the late Dr. Austin Vickery) is an encapsulated or at least circumscribed area in the thyroid composed of exuberant papillary structures often with some follicle formation in the cores of the papillae; the lesions are often centrally cystic and the papillae tend to point toward the center of the nodule. Importantly the nuclei lining these papillary structures are round, have internal structure and are often polarized within the cells (Figures 3, 4). Lymphocytes are rarely found within these lesions (11, 12). Most of these nodules are clonal proliferations and are therefore considered adenomas (26–29). (The term “papillary adenoma” would be an appropriate one for these lesions; however, this term is shunned since it has been used to described encapsulated papillary carcinomas in older literature) (30). Although the great majority of these hyperplastic nodules are not associated with clinical hyperthyroidism, about 15–20% of affected patients do have symptomatic hyperfunction and about another 30% have biochemical hyperthyroid indices (25, 31, 32).
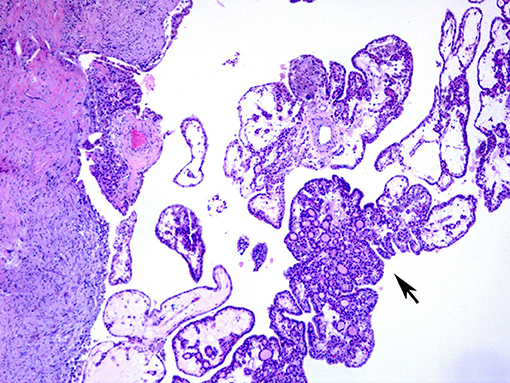
Figure 3. A case of papillary hyperplastic nodule on low power showing cystic nodule with papillary architecture (arrow).
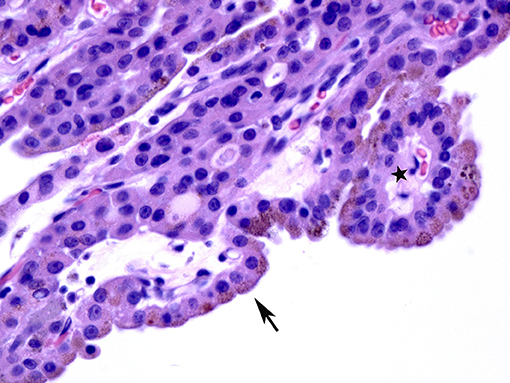
Figure 4. A case of papillary hyperplastic nodule on high power showing oncocytic cells lining the papillary structures (arrow).
Rarely, malignant tumors of the thyroid may be associated with hyperthyroidism. These are usually but not always follicular carcinomas; some are encapsulated follicular variants of papillary carcinoma (33–35). Although the tumors may lead to hyperfunction while still confined within the gland, many of the affected patients have metastatic disease. Some authors indicate that tumor burden correlates with the degree of hyperthyroidism (36–41).
Another interesting thyroid carcinoma that can present with hyperthyroidism is the rare diffuse follicular variant of papillary carcinoma (42). A tumor that is most often found in young females who present with goiter, the clinical picture resembles classic Graves' disease or toxic goiter. About 25% of these lesions will show hyperthyroidism and abnormal thyroid function tests. Treatment of the cancer will lead to resolution of the metabolic abnormality (40–44).
This term originally coined about 40 years ago by the Mayo Clinic group describes patients who present clinically with hyperthyroidism but whose glands show the histology of chronic lymphocytic thyroiditis including oxyphilia (Hürthle cell metaplasia) (45). This histologic presentation is also often seen in children and very young usually teenage patients who present with thyroid hyperfunction. Often these patients go through a phase of euthyroidism and subsequently hypothyroidism over a period of decades (46–48).
When hyperthyroidism is associated with lesions of the pituitary gland or the hypothalamus, it is considered secondary and tertiary hyperthyroidism respectively. In comparison to primary hyper thyroidism, these clinical conditions are extremely rare (< 1% of hyperthyroidism). The lesion in the pituitary gland is most frequently multifocal thyrotroph hyperplasia rather than a thyroid stimulating hormone (TSH) producing adenoma. Lesions of the hypothalamus producing thyrotropin releasing hormone (TRH) can stimulate the pituitary thyrotrophes to hyper secrete thyroid stimulating hormone (TSH) and subsequently to influence thyroid gland to produce excess thyroid hormone. Lesions of the hypothalamus responsible for this excess TRH include tumors, granulomatous disease (i.e., sarcoid) and other mass producing lesions (49–53).
The presence of thyroid tissue within the ovary is usually seen in benign cystic teratomas also known as dermoid cysts of the ovary (54). The thyroid in these lesions is often part of a multi-tissue proliferation, that is tissues from all three embryological germ layers are represented. When thyroid tissue is the only or majority of tissue (>50%) in a teratoma (often termed mono dermal teratoma) it is diagnosed as struma ovarii. In most cases the thyroid either appears normal or shows changes consistent with colloid goiter. In rare instances, the thyroid will appear hyperplastic or even show lymphocytic infiltration mimicking thyroiditis. Rarely neoplasms originating in the thyroid gland including papillary carcinoma, follicular carcinoma or even poorly differentiated carcinoma can arise in a background of struma ovarii. Most of these tumors do not involve the ovarian surface and do not spread (these tumors have been designated by some authors as “proliferating struma”) (55). Unusual situations have been described wherein the struma ovarii or tumors therein may hyper secrete thyroid hormone and lead to clinical hyperthyroidism (56–58).
Rare reported cases of non-endocrine malignant tumors secreting TSH or TRH have been reported. The most common histology is that of hepatocellular carcinoma. The tumor produces these stimulatory hormones and when tumor is entirely removed the levels of hormones drop and hyperthyroidism regresses (59, 60).
Gestational trophoblastic disease including hydatidiform mole and choriocarcinoma is associated with marked elevation of beta human chorionic gonadotropin (beta HCG). Because the beta subunit of HCG is identical in chemical structure to one of the subunits of TSH, the HCG elevation can mimic elevated TSH and stimulate the thyroid to produce excess thyroid hormone. Although it is rare to see tissue from the thyroid in these patients, it is expected that the gland would show a hyperplastic appearance with papillae and cellular enlargement. Lymphocytic infiltration would be absent. Treatment of the gestational trophoblastic disease by uterine evacuation followed by chemotherapy usually leads to resolution of the hyperthyroid state (61–64).
A variety of classes of pharmaceutical agents can cause thyroid dysfunction. It is beyond the purpose of this review to engage in a lengthy discussion of the clinical disorders caused by these drugs. Some of these interfere with metabolism of iodine, others with the production of thyroid hormone and its conversion to active moieties, and still others do not produce abnormalities in thyroid function but cause chemical interference with thyroid function test measurements. Many drugs can affect thyroid function (phenytoin and derivatives, therapies associated with interleukin administration usually in oncology settings) (65–67). The pathologic counterparts for these include lymphocytic infiltration of the gland with or without fibrosis (68).
Those drugs that cause hyperthyroidism are fewer and they usually exert their effect through interference with the metabolism of iodine. It is rare to see pathological specimens from these patients; the exceptions is the cardiac drug, amiodarone, interleukin containing regimens for chemotherapy and most recently PDL 1 or immune checkpoint inhibitors; these will be discussed below.
The literature notes that there are two types of thyroid lesions that are associated with amiodarone, an iodine containing compound used to treat cardiac arrhythmias. Amiodarone induced thyrotoxicosis (AIT) is classified as type I and type II, the former occurs in patients with underlying thyroid disease such as nodular goiter, autonomous nodular goiter or Graves' disease, whereas, Type II is caused by iodine-led destruction of the thyroid follicular epithelium in a normal thyroid gland. Because amiodarone is vital to control the cardiac problems, it is often not possible to wean the patient from the medication or to change to another drug. The first line of therapy in amiodarone induced thyrotoxicosis is treatment with Thionamides in AIT I and glucocorticoids in AIT II. Thyroid excision is undertaken in patients who do not respond to medical therapy in order to treat the hyperthyroidism which often worsens cardiac symptoms (69–72).
If the gland is already pathologically abnormal (nodular thyroid goiter, Graves' disease), the pathology of the resected gland shows follicular disruption with histiocytes infiltrating the follicular epithelium and colloid (Figures 5, 6). Rarely, inflammatory cells are noted within the thyroid parenchyma (Type I). On the other hand if the thyroid is histologically normal (Type II), the pathologic lesions show much milder follicular damage (73–75). These changes are similar to those seen in amiodarone induced pulmonary and liver toxicity (76, 77). Ultrastructural studies of both lung and thyroid tissues have shown lysosomal and mitochondrial inclusions in follicular cells consistent with follicle cell destruction (77). However, this simple explanation is not the only reason for the thyroid dysfunction. For example, co-cultures of amiodarone with human thyrocytes have shown the production of interleukin 6 and the drug also decreases the sodium-iodide symporter mRNA in the follicular cells (78).
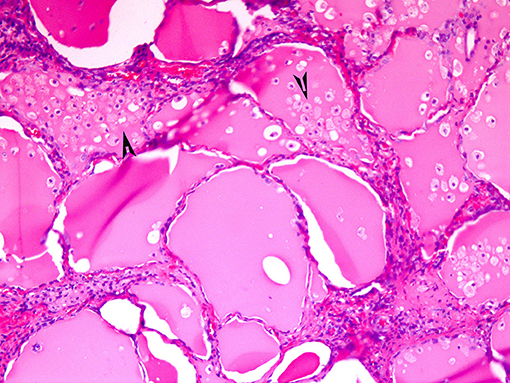
Figure 5. Amiodarone associated follicular cell damage. Low and high power showing large thyroid follicles filled with colloid and numerous histiocytes (arrow heads, 3A,B).
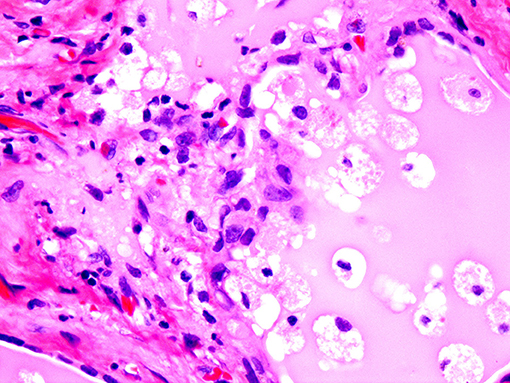
Figure 6. Same as Figure 5.
The removal of the thyroid in amiodarone induced hyperthyroidism results in resolution of the hyperfunction and reversion of the cardiac disorder to baseline (75, 79).
Thyroid dysfunction can occur in 20–50% patients receiving antineoplastic agents and targeted therapies. High dose IL-2 therapy can lead to hyperthyroidism in 7% of patients. In rare instances, interferon-alpha treatment can lead to classic Graves' disease and even Graves' opthalmopathy; and these condition can persist even after the cessation of therapy (67, 80). At present, several tyrosine kinase inhibitors (TK1) are being used to treat different types of malignant neoplasms. TKI can lead to various forms of toxicities including those related to endocrine organs. Transient hyperthyroidism can occur during TKI therapy and is often due to destructive thyroiditis (67, 80).
Immune check-point inhibitors with their antitumor activity have shown to improve the survival rates of non-small cell lung carcinoma, melanoma, bladder and renal carcinoma, and ovarian carcinoma. A small number of patients undergoing treatment with immune-checkpoint inhibitors such as anti PD-1/anti-PDL-1 can develop hyperthyroidism (81).
The term mechanico-destructive hyperthyroidism (non-hyperthyroid thyrotoxicosis) has been coined by us to describe those conditions in which relatively rapid destruction of thyroid tissue followed by release of stored thyroid hormone from colloid as follicles or destroyed produces hyperfunction. Both benign and malignant conditions can be associated with this type of hyperthyroidism. An important clinical clue to the possibility of one of these disorders is that in contrast to more common causes of hyperthyroidism, radionucleotide scans show uptakes in the range of 1% or less. This reflects the destruction of the thyroid gland by the inflammatory or neoplastic process; the follicular epithelium is destroyed and cannot take up the radioactive isotope.
This condition is believed to be associated with systemic and or thyroid infection usually viral in nature, is often a painful cause of hyperthyroidism. Patients with this disorder will often present with neck pain which may be referred to the jaw or the chest. In the initial phases of this disease symptoms of hyperthyroidism are often clinically evident. As the gland is replaced by the inflammatory granulomatous process, the follicular epithelium is destroyed, follicles of ruptured and stored thyroid hormone within the colloid is released into the circulation. Unlike usual Graves' disease however the thyroid cannot take up iodide and produce more hormone. Thus, a phase of hypothyroidism is noted until healing occurs (82–84).
Malignant neoplasms which are rapidly growing can be associated with this mechanic-destructive type of hyperthyroidism. The tumors most often identified are anaplastic thyroid carcinoma, malignant lymphoma usually primary in the thyroid and of large cell type and poorly differentiated metastatic cancers involving the thyroid (breast carcinoma and lung carcinoma most commonly). Histologically one sees the highly malignant tumor freely infiltrating the thyroid, destroying and replacing the tissue, with rupture of the follicles and release of thyroid hormone containing colloid. The rapidity of the process can lead to market elevation of thyroid hormone and a toxic state simulating thyroid storm (85–89).
In affected patients, there is often near complete destruction of the gland and the eventual development of hypothyroidism. Patients need to be supplemented with thyroid hormone to maintain a euthyroid metabolic state; if treatment of the tumor is successful, regeneration of thyroid follicles may occur from the residual thyroid tissue and as in subacute thyroiditis, normalization of thyroid function may occur (86–89).
This review has described the pathology and clinicopathologic correlations of unusual lesions of the thyroid and extrathyroidal tissues which can show clinical manifestations of hyperthyroidism. Although most of these conditions are rare especially when compared to Graves' disease or toxic nodular goiter, it is important for both the clinician and pathologist to be aware of them as diagnostic considerations.
VL and ZB have equally contributed to the literature review, drafting the manuscript and obtaining microscopic photographs. Both authors have reviewed the final version of this manuscript before submitting it to topic editors of the journal.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Carnell NE, Valente WA. Thyroid nodules in Graves' disease:classification, characterization, and response to treatment. Thyroid (1998) 8:571–6. doi: 10.1089/thy.1998.8.571
2. Gossage A, Munro D. The pathogenesis of Graves' disease. Clin Endocrinol Metab. (1985) 14:299–330. doi: 10.1016/S0300-595X(85)80036-0
3. Burman K, Baker J. Immune Mechanisms in Graves' disease. Endocr Rev. (1985) 6:183–223. doi: 10.1210/edrv-6-2-183
4. Karoutsou E, Polymeris A. Pathogenesis of Graves' disease focusing on Graves' ophthalmopathy. Endocr Regul. (2011) 45:209–20. doi: 10.4149/endo_2011_04_209
5. Leovey A, Bako G, Sztojka I, Szabo J, Kalman K, Szabo T. The common incidence of Basedow's-Graves' disease and chronic lymphocytic thyroiditis. Radiobiol Radiother. (1984) 25:769–74.
7. Hirota Y, Tamai H, Hayashi Y, Matsubayashi S, Matsuzuka F, Kuma K, et al. Thyroid function and histology in forty-five patients with hyperthyroid Graves' disease in clinical remission more than ten years after thionamide drug treatment. J Clin Endocrinol Metab. (1986) 62:165–9. doi: 10.1210/jcem-62-1-165
8. Mizukami Y, Matsubara F. [Clinicopathological study on the chronic thyroiditis and Graves' disease –relationship between histological classification and function (author's transl)]. Nihon Naika Gakkai Zasshi (1980) 69:321–9. doi: 10.2169/naika.69.321
9. Spjut H, Warren W, Ackerman L. Clinical-pathologic study of 76 cases of recurrent Graves' disease, toxic (nonexophthalmic) goiter, and nontoxic goiter. Am J Clin Pathol. (1957) 27:367–92. doi: 10.1093/ajcp/27.4.367
10. Albores-Saavedra J, Wu J. The many faces and mimics of papillary thyroid carcinoma. Endocrine Pathol. (2006) 17:1–18. doi: 10.1385/EP:17:1:1
11. Baloch ZW, LiVolsi VA. Cytologic and architectural mimics of papillary thyroid carcinoma. Diagnostic challenges in fine-needle aspiration and surgical pathology specimens. Am J Clin Pathol. (2006) 125(Suppl.):S135–44. doi: 10.1309/YY72M308WPEKL1YY
12. LiVolsi VA. Papillary neoplasms of the thyroid. Pathologic and prognostic features. Am J Clin Pathol. (1992) 97:426–34. doi: 10.1093/ajcp/97.3.426
13. Bitton RN, Sachmechi I, Tabriz MS, Murphy L, Wasserman P. Papillary carcinoma of the thyroid with manifestations resembling Graves' disease. Endocr Pract. (2001) 7:106–9. doi: 10.4158/EP.7.2.106
14. Braga M, Graf H, Ogata A, Batista J, Hakim NC. Aggressive behavior of papillary microcarcinoma in a patient with Graves' disease initially presenting as cystic neck mass. J Endocrinol Invest. (2002) 25:250–3. doi: 10.1007/BF03343999
15. Lucas Martin A, Sanmarti Sala A. [Association of Graves-Basedow disease with thyroid papillary carcinoma:a pathogenic relationship?]. Med Clin. (1987) 89:664–5.
16. Pujadas R, Fernandez F, Camacho L, Foz M. [Association of Graves-Basedow disease with thyroid papillary carcinoma:a pathogenic relationship?]. Med Clin. (1987) 88:786.
17. Valenti TM, Macchia E, Pisa R, Bucalo ML, Russo V, Colletti I, et al. Toxic adenoma and papillary thyroid carcinoma in a patient with Graves' disease. J Endocrinol Invest. (1999) 22:701–4. doi: 10.1007/BF03343633
18. Wei S, Baloch ZW, LiVolsi VA. Thyroid carcinoma in patients with Graves' disease:an institutional experience. Endocrine Pathol. (2015) 26:48–53. doi: 10.1007/s12022-014-9343-6
19. Studer H, Peter H, Gerber H. Toxic nodular goitre. Clin Endocrinol Metab. (1985) 14:351–72. doi: 10.1016/S0300-595X(85)80038-4
20. Thomas FB, Mazzaferri EL, Skillman TG. Apathetic thyrotoxicosis: A distinctive clinical and laboratory entity. Ann Intern Med. (1970) 72:679–85. doi: 10.7326/0003-4819-72-5-679
21. Johnson PC, Kahil ME. Apathetic hyperthyroidism. A type of masked thyrotoxicosis. Tex Med. (1967) 63:59–62.
22. Wu W, Sun Z, Yu J, Meng Q, Wang M, Miao J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology (2010) 77:46–51. doi: 10.1159/000272954
23. Miller JM. Plummer's disease. Med Clin North Am. (1975) 59:1203–16. doi: 10.1016/S0025-7125(16)31968-X
24. Messina G, Viceconti N, Trinti B. Diagnostic items and treatment of Plummer's disease:a study on 180 patients. La Clinica Terapeutica (1998) 149:191–5.
25. Vickery AL Jr. Thyroid papillary carcinoma. Pathological and philosophical controversies. Am J Surg Pathol. (1983) 7:797–807. doi: 10.1097/00000478-198307080-00009
26. Apel RL, Ezzat S, Bapat BV, Pan N, LiVolsi VA, Asa SL. Clonality of thyroid nodules in sporadic goiter. Diagn Mol Pathol. (1995) 4:113–21. doi: 10.1097/00019606-199506000-00007
27. Deleu S, Allory Y, Radulescu A, Pirson I, Carrasco N, Corvilain B, et al. Characterization of autonomous thyroid adenoma:metabolism, gene expression, and pathology. Thyroid (2000) 10:131–40. doi: 10.1089/thy.2000.10.131
28. Aeschimann S, Kopp PA, Kimura ET, Zbaeren J, Tobler A, Fey MF, et al. Morphological and functional polymorphism within clonal thyroid nodules. J Clin Endocrinol Metabol. (1993) 77:846–51.
29. Kopp P, Kimura ET, Aeschimann S, Oestreicher M, Tobler A, Fey MF, et al. Polyclonal and monoclonal thyroid nodules coexist within human multinodular goiters. J Clin Endocrinol Metabol. (1994) 79:134–9.
30. Meissner W, Warren S. Tumors of the Thyroid Gland. Washington, DC: Armed Forces Institute of Pathology (1969).
31. Khurana KK, Baloch ZW, LiVolsi VA. Aspiration cytology of pediatric solitary papillary hyperplastic thyroid nodule. Arch Pathol Lab Med. (2001) 125:1575–8. doi: 10.1043/0003-9985(2001)125<1575:ACOPSP>2.0.CO;2
32. Khayyata S, Barroeta JE, LiVolsi VA, Baloch ZW. Papillary hyperplastic nodule:pitfall in the cytopathologic diagnosis of papillary thyroid carcinoma. Endocr Pract. (2008) 14:863–8. doi: 10.4158/EP.14.7.863
33. Sasaki J, Odaka Y, Kato R, Tada T, Yagawa K, Kowata T, et al. [Hyperfunctioning follicular carcinoma of the thyroid. A case report]. Nippon Geka Gakkai Zasshi (1988) 89:286–91.
34. Michigishi T, Mizukami Y, Shuke N, Satake R, Noguchi M, Aburano T, et al. An autonomously functioning thyroid carcinoma associated with euthyroid Graves' disease. J Nucl Med. (1992) 33:2024–6.
35. Ardito G, Vincenzoni C, Cirielli C, Guidi ML, Corsello MS, Modugno P, et al. Papillary thyroid carcinoma mimicking an autonomous functioning nodule. Eur J Surg Oncol. (1997) 23:569. doi: 10.1016/S0748-7983(97)93397-7
36. Niepomniszcze H, Suarez H, Pitoia F, Pignatta A, Danilowicz K, Manavela M, et al. Follicular carcinoma presenting as autonomous functioning thyroid nodule and containing an activating mutation of the TSH receptor (T620I) and a mutation of the Ki-RAS (G12C) genes. Thyroid (2006) 16:497–503. doi: 10.1089/thy.2006.16.497
37. Siddiqui AR, Karanauskas S. Hurthle cell carcinoma in an autonomous thyroid nodule in an adolescent. Pediatr Radiol. (1995) 25:568–9. doi: 10.1007/BF02015798
38. Smith M, McHenry C, Jarosz H, Lawrence AM, Paloyan E. Carcinoma of the thyroid in patients with autonomous nodules. Am Surg. (1988) 54:448–9.
39. Tfayli HM, Teot LA, Indyk JA, Witchel SF. Papillary thyroid carcinoma in an autonomous hyperfunctioning thyroid nodule:case report and review of the literature. Thyroid (2010) 20:1029–32. doi: 10.1089/thy.2010.0144
40. Siddiqi IN, Friedman J, Barry-Holson KQ, Ma C, Thodima V, Kang I, et al. Characterization of a variant of t(14;18) negative nodal diffuse follicular lymphoma with CD23 expression, 1p36/TNFRSF14 abnormalities, and STAT6 mutations. Mod Pathol. (2016) 29:570–81. doi: 10.1038/modpathol.2016.51
41. Vinciguerra GL, Noccioli N, Bartolazzi A. Diffuse follicular variant of papillary thyroid carcinoma:a case report with a revision of literature. Rare Tumors (2016) 8:6536. doi: 10.4081/rt.2016.6536
42. Sobrinho-Simões M, Soares J, Carneiro F, Limbert E. Diffuse follicular variant of papillary carcinoma of the thyroid: report of eight cases of a distinct aggressive type of thyroid tumor. Surg Pathol. (1990) 3:189–203.
43. Cha YJ, Chang HS, Hong SW. Diffuse follicular variant of papillary thyroid carcinoma in a 69-year-old man with extensive extrathyroidal extension: a case report. J Korean Med Sci. (2013) 28:480–4. doi: 10.3346/jkms.2013.28.3.480
44. Mizukami Y, Nonomura A, Michigishi T, Ohmura K, Noguchi M, Ishizaki T. Diffuse follicular variant of papillary carcinoma of the thyroid. Histopathology (1995) 27:575–7. doi: 10.1111/j.1365-2559.1995.tb00331.x
45. Fatourechi V, McConahey WM, Woolner LB. Hyperthyroidism associated with histologic Hashimoto's thyroiditis. Mayo Clin Proc. (1971) 46:682–9.
46. Mori T. [Hashitoxicosis and Hashimoto's disease with the symptoms of thyrotoxicosis]. Nihon Rinsho (1980) 38:1677–83.
47. Nabhan ZM, Kreher NC, Eugster EA. Hashitoxicosis in children:clinical features and natural history. J Pediatr. (2005) 146:533–6. doi: 10.1016/j.jpeds.2004.10.070
48. Wasniewska M, Corrias A, Salerno M, Lombardo F, Aversa T, Mussa A, et al. Outcomes of children with hashitoxicosis. Horm Res Paediatr. (2012) 77:36–40. doi: 10.1159/000334640
49. Waldhausl W, Bratusch-Marrain P, Nowotny P, Buchler M, Forssmann WG, Lujf A, et al. Secondary hyperthyroidism due to thyrotropin hypersecretion:study of pituitary tumor morphology and thyrotropin chemistry and release. J Clin Endocrinol Metab. (1979) 49:879–87. doi: 10.1210/jcem-49-6-879
50. Gomez JB, Diaz MA, Jerez ML. [Tertiary hyperthyroidism. Criteria of evaluation]. Rev Med. (1973) 17:231–9.
51. Kourides I, Ridgway E, Weintraub B, Bigos S, Gershengorn M, Maloof F. Thyrotropin-induced hyperthyroidism:use of alpha and beta subunit levels to identify patients with pituitary tumors. J Clin Endocrinol Metab. (1977) 45:534–43. doi: 10.1210/jcem-45-3-534
52. Dorfman SG. Hyperthyroidism. Usual and unusual causes. Arch Internal Med. (1977) 137:995–6. doi: 10.1001/archinte.1977.03630200005005
53. Tolis G, Bird C, Bertrand G, McKenzie J, Ezrin C. Pituitary hyperthyroidism. Am J Med. (1978) 64:177–181. doi: 10.1016/0002-9343(78)90202-4
54. Prat J, Cao D, Carinelli SG, Nogales FF, Vang R, Zaloudek CJ. WHO Classification of Tumours of Female Reproductive Organs. Lyon: IARC (2014).
55. Devaney K, Snyder R, Norris HJ, Tavassoli FA. Proliferative and histologically malignant struma ovarii:a clinicopathologic study of 54 cases. Int J Gynecol Pathol. (1993) 12:333–43. doi: 10.1097/00004347-199310000-00008
56. Yang Q, Yang X, Liu ZZ, Jiang YX, Li JC, Su N, et al. Sonographic and pathologic features of struma ovarii. Zhongguo Yi Xue Ke Xue Yuan Xue Bao (2015) 37:309–14. doi: 10.3881/j.issn.1000-503X.2015.03.012
57. Yassa L, Sadow P, Marqusee E. Malignant struma ovarii. Nat Clin Pract Endocrinol Metabol. (2008) 4:469–72. doi: 10.1038/ncpendmet0887
58. Wei S, Baloch ZW, LiVolsi VA. Pathology of struma ovarii: a report of 96 Cases. Endocrine Pathol. (2015) 26:342–8. doi: 10.1007/s12022-015-9396-1
59. Helzberg JH, McPhee MS, Zarling EJ, Lukert BP. Hepatocellular carcinoma:an unusual course with hyperthyroidism and inappropriate thyroid-stimulating hormone production. Gastroenterology (1985) 88:181–4. doi: 10.1016/S0016-5085(85)80152-9
60. Carri J, Peral F, Surreco M, Lujan A, Leguizamon R, Martinez G, et al. [Fibrolamellar hepatocellular carcinoma:a clinical report with paraneoplastic hyperthyroidism (apropos of a case)]. Acta Gastroenterol Latinoam. (1989) 19:155–64.
61. Berdjis N, Baldauf A, Kittner T, Manseck A, Wirth M. [Paraneoplastic hyperthyroidism in a patient with metastasizing teratocarcinoma and excessively high HCG]. Aktuelle Urol. (2003) 34:407–9. doi: 10.1055/s-2003-43174
62. Kohler S, Tschopp O, Jacky E, Schmid C. Paraneoplastic hyperthyroidism. BMJ Case Rep. (2011) 2011. doi: 10.1136/bcr.04.2011.4163
63. Oosting SF, de Haas EC, Links TP, de Bruin D, Sluiter WJ, de Jong IJ, et al. Prevalence of paraneoplastic hyperthyroidism in patients with metastatic non-seminomatous germ-cell tumors. Ann Oncol. (2010) 21:104–8. doi: 10.1093/annonc/mdp265
64. Solomon CG, Dluhy RG. Paraneoplastic endocrine syndromes. Curr Ther Endocrinol Metab. (1994) 5:537–42.
65. Hein MD, Jackson IM Review:thyroid function in psychiatric illness. Gen Hosp Psychiatry (1990) 12:232–44. doi: 10.1016/0163-8343(90)90060-P
66. Visser WE, de Rijke YB, van Toor H, Visser TJ. Thyroid status in a large cohort of patients with mental retardation:the TOP-R (Thyroid Origin of Psychomotor Retardation) study. Clin Endocrinol. (2011) 75:395–401. doi: 10.1111/j.1365-2265.2011.04089.x
67. Krouse RS, Royal RE, Heywood G, Weintraub BD, White DE, Steinberg SM, et al. Thyroid dysfunction in 281 patients with metastatic melanoma or renal carcinoma treated with interleukin-2 alone. J Immunother Emphasis Tumor Immunol. (1995) 18:272–8. doi: 10.1097/00002371-199511000-00008
68. Rosenbaum A, Maruta T, Richelson E. Drugs that alter mood:lithium. Mayo Clin Proc. (1979) 54:401–7.
69. Amico J, Richardson V, Alpert B, Klein I. Clinical and chemical assessment of thyroid function during therapy with amiodarone. Arch Intern Med. (1984) 144:487–90. doi: 10.1001/archinte.1984.00350150071023
70. Alves L, Rose E, Cahill T. Amiodarone and the thyroid. Ann Intern Med. (1985) 102:412. doi: 10.7326/0003-4819-102-3-412_1
72. Bogazzi F, Bartalena L, Gasperi M, Braverman LE, Martino E. The various effects of amiodarone on thyroid function. Thyroid (2001) 11:511–9. doi: 10.1089/105072501300176471
73. Smyrk T, Goellner J, Brennan M, Carney J. Pathology of the thyroid in amiodarone assoicated thyrotoxicosis. Am J Surg Pathol. (1987) 11:197–204. doi: 10.1097/00000478-198703000-00004
74. Saad A, Falciglia M, Steward DL, Nikiforov YE. Amiodarone-induced thyrotoxicosis and thyroid cancer:clinical, immunohistochemical, and molecular genetic studies of a case and review of the literature. Arch Pathol Lab Med. (2004) 128:807–10. doi: 10.1043/1543-2165(2004)128<807:ATATCC>2.0.CO;2
75. Elnaggar MN, Jbeili K, Nik-Hussin N, Kozhippally M, Pappachan JM. Amiodarone-induced thyroid dysfunction:a clinical update. Exp Clin Endocrinol Diabetes (2018) 126:333–41. doi: 10.1055/a-0577-7574
76. Marchlinski F, Gansler T, Waxman H, Josephson M, Amiodarone pulmonary toxicity. Ann Intern Med. (1982) 97:839–45. doi: 10.7326/0003-4819-97-6-839
77. Dake M, Madison J, Montgomery C, Shellito JE, Hinchcliffe WA, Winkler ML, et al. Electron microscopic demonstration of lysosomal inclusion bodies in lung, liver, lymph nodes and blood leukocytes of patients with amiodarone pulmonary toxicity. Am J Med. (1985) 78:506–512. doi: 10.1016/0002-9343(85)90346-8
78. Yamazaki K, Mitsuhashi T, Yamada E, Yamada T, Kosaka S, Takano K, et al. Amiodarone reversibly decreases sodium-iodide symporter mRNA expression at therapeutic concentrations and induces antioxidant responses at supraphysiological concentrations in cultured human thyroid follicles. Thyroid (2007) 17:1189–200. doi: 10.1089/thy.2007.0215
79. Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M. 2018 European Thyroid Association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur Thyroid J. (2018) 7:55–66. doi: 10.1159/000486957
80. Hamnvik OP, Larsen PR, Marqusee E. Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst. (2011) 103:1572–87. doi: 10.1093/jnci/djr373
81. Guaraldi F, La Selva R, Sama MT, D'Angelo V, Gori D, Fava P, et al. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice:a long-term prospective study from a referral institution. J Endocrinol Invest. (2018) 41:549–56. doi: 10.1007/s40618-017-0772-1
82. Pichler R, Wolfl S, Bogner S, Sulzbacher H, Shamiyeh A, Maschek W. [Subacute thyroiditis with cell destruction and temporary hyperthyroidism in Graves'disease–case report]. Acta Medica Austriaca. (2002) 29:137–40. doi: 10.1046/j.1563-2571.2002.02008.x
83. Woolf PD. Painless thyroiditis as a cause of hyperthyroidism:subacute or chronic lymphocytic? Arch Internal Med. (1978) 138:26–7. doi: 10.1001/archinte.1978.03630250010006
84. Janssen OE. [Atypical presentation of subacute thyroiditis]. Dtsch Med Wochenschr. (2011) 136:519–22. doi: 10.1055/s-0031-1274535
85. De Ridder M, Sermeus AB, Urbain D, Storme GA. Metastases to the thyroid gland-a report of six cases. Eur J Intern Med. (2003) 14:377–9. doi: 10.1016/S0953-6205(03)90005-7
86. Shimaoka K. Thyrotoxicosis due to metastatic involvement of the thyroid. Arch Intern Med. (1980) 140:284–5. doi: 10.1001/archinte.1980.00330140142050
87. Shimaoka K. VanHerle AJ, Dindogru A, Thyrotoxicosis secondary to involvement of the thyroid with malignant lymphoma. J Clin Endocrinol Metab. (1976) 43:64–8. doi: 10.1210/jcem-43-1-64
88. Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid:a review of the literature from the last decade. Thyroid (2012) 22:258–68. doi: 10.1089/thy.2010.0154
Keywords: hyperthyroidism, thyrotoxicosis, non-hyperthyroid, Graves' disease, hyperfunctioning nodules, ectopic hyperthyroidism, drug reactions, mechanico-destructive
Citation: LiVolsi VA and Baloch ZW (2018) The Pathology of Hyperthyroidism. Front. Endocrinol. 9:737. doi: 10.3389/fendo.2018.00737
Received: 07 August 2018; Accepted: 20 November 2018;
Published: 03 December 2018.
Edited by:
Noriyuki Koibuchi, Gunma University, JapanReviewed by:
Cesidio Giuliani, Università degli Studi G. d'Annunzio Chieti e Pescara, ItalyCopyright © 2018 LiVolsi and Baloch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Virginia A. LiVolsi, bGludXNAcGVubm1lZGljaW5lLnVwZW5uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.