- 1Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Institute of Clinical Pharmacy, Central South University, Changsha, China
- 3Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha, China
- 4Department of Family Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada
Background: The association between paraoxonase 2 (PON2) gene polymorphisms and type 2 diabetes mellitus (T2DM) has been extensively investigated in the Chinese population with conflicting results. In this study, we systematically evaluated the association between PON2 Ser311Cys and Ala148Gly polymorphisms and T2DM risk by pooling all relevant studies.
Methods: We searched PubMed, Embase, CNKI, and Wanfang databases for the studies. The strength of association was determined by the allelic, homozygous, heterozygous, recessive, and dominant genetic models and measured as odds ratio (OR) and 95% confidence interval (CI), under fixed- or random-effect models.
Results: There was no significant association between PON2 Ser311Cys polymorphism and T2DM under any of the genetic models: allelic (OR = 1.06, 95% CI = 0.77–1.45; P = 0.721), heterozygous (OR = 1.13, 95% CI = 0.87–1.45; P = 0.362), dominant (OR = 1.10, 95% CI = 0.80–1.51; P = 0.562), recessive (OR = 0.87, 95% CI = 0.48–1.58; P = 0.648), homozygous (OR = 0.94, 95% CI = 0.47–1.89; P = 0.865). Similarly, no significant association was found in PON2 Arg148Gly polymorphism under any of the models: allelic (OR = 1.17, 95% CI = 0.91–1.50; P = 0.218), heterozygous (OR = 1.28, 95% CI = 0.94–1.74; P = 0.117), dominant (OR = 1.25, 95% CI = 0.93–1.67; P = 0.142), recessive (OR = 0.99, 95% CI = 0.52–1.88; P = 0.973), homozygous (OR = 1.08, 95% CI = 0.57–2.07; P = 0.808).
Conclusions: The PON2 Ser311Cys and Ala148Gly polymorphisms were not associated with the risk of developing T2DM in the Chinese population.
Introduction
Diabetes is a major cause of mortality and morbidity worldwide, and it has become an important public health problem in China (1, 2). National surveys indicate that the prevalence of diabetes increased dramatically among Chinese adults during the past three decades. The rising prevalence of diabetes highlights the urgent need for aggressive strategies aimed at the prevention and control of diabetes (3, 4). Type 2 diabetes mellitus (T2DM) is the most prevalent type of diabetes around the world. Substantial evidence demonstrates that T2DM is a complex metabolic disease triggered by lifestyle, environmental and genetic factors (5–8).
The paraoxonase 2 (PON2) gene encodes a member of the PON multigene family, which includes two other known members sharing approximately 65% sequence similarity at the amino acid level, PON1 and PON3, located adjacent to each other on the chromosome 7q21.3–22.1 in humans (9). PON1 and PON3 are primarily expressed in the liver while PON2 is ubiquitously expressed in many different mammalian tissues (10). The PON2 gene contains eight introns and nine exons. It is polymorphic and several common single nucleotide polymorphisms (SNP) have been identified thus far. Currently, genetic variations in PON2 gene may be associated with a number of disorders, such as cardiovascular disease and T2DM (11–14).
In the previous genetic epidemiologic studies, the association between the Ser311Cys (rs6954345/rs7493) and Ala148Gly (rs11545942/rs12026) polymorphisms in PON2 gene and the risk in developing T2DM has increased the focus on the Chinese population. In Qu's study, PON2 Ser311Cys gene polymorphism was found to be significantly associated with an increased risk of T2DM in a northern Chinese population (15). In 2014, Xu and Dai also revealed that PON2 311Cys allele could increase the T2DM risk in the Qinghai population (16). In contrast, Sun reported an opposite result that the PON2 311Ser contributed to the development of T2DM in another northern Chinese population, with the frequency of PON2 311Ser allele being significantly higher in T2DM patients than the control groups (17). In addition, Xu et al. (18) failed to find an association between PON2 Ser311Cys gene variation and T2DM in an Anhui population in 2007 and Sun et al. (19) also observed a similar effect in another northern population.
It is crucial to address this inconsistency among the currently published studies. In the present study, a comprehensive meta-analysis was conducted by pooling all qualified individual data from case-control studies to make a precise conclusion on the association between PON2 Ser311Cys and Ala148Gly gene polymorphisms and the risk of developing T2DM in the Chinese population.
Methods
This study was performed in accordance with the guidelines of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement (20).
Publication Search and Inclusion Criteria
The following electronic databases PubMed, Embase, Wanfang Data, and China National Knowledge Infrastructure (CNKI) were searched for all case-control studies published up to August 2017 on the association of PON2 Ser311Cys and Ala148Gly gene polymorphisms and the risk of developing T2DM in the Chinese population. The search was performed in the databases in English and Chinese with proper keywords: paraoxonase 2 gene (“paraoxonase 2,” “PON2”) or variations (e.g., “Single Nucleotide Polymorphism,” “SNP,” “polymorphism,” “Mutation,” “variation,” “variant”) in combination with T2DM (e.g., “Type 2 Diabetes Mellitus,” “Diabetes Mellitus, Type 2,” “Noninsulin Dependent Diabetes Mellitus,” “Diabetes Mellitus, Noninsulin Dependent”). In addition, the references from relevant reviews or primary studies were hand searched to identify additional studies.
Two investigators independently reviewed all studies for eligibility. The inclusion criteria were as follows: (a) study investigating the associations between Ser311Cys (rs6954345/rs7493) and Ala148Gly (rs11545942/rs12026) in PON2 gene and the risk of developing T2DM. (b) case-control studies, regardless of sample size. (c) studies providing the numbers of PON2 genotypes or alleles in case and control subjects. (d) the distribution of genotypes in the control groups met the Hardy-Weinberg equilibrium (HWE, P > 0.05). Disagreement between two investigators regarding the eligibility of any study was resolved by the consensus of a third reviewer.
Data Extraction
Data extraction was independently performed by two reviewers. A consensus on all items of data extraction was reached by both reviewers. The following items were extracted from each study: the first author's name, publication year, region of the study, mean age and gender distribution of the participants, number of genotypes, distribution of alleles, sample size in case, and control groups.
Statistical Analysis
In our meta-analysis, 5 genetic models as the allelic (C vs. S of PON2 Ser311Cys gene polymorphism; G vs. A of PON2 Ala148Gly gene polymorphism), homozygous (CC vs. SS of PON2 Ser311Cys gene polymorphism; GG vs. AA of PON2 Ala148Gly gene polymorphism), dominant (CC+SC vs. SS of PON2 Ser311Cys gene polymorphism; GG+AG vs. AA of PON2 Ala148Gly gene polymorphism), heterozygous (SC vs. SS of PON2 Ser311Cys gene polymorphism; AG vs. AA of PON2 Ala148Gly gene polymorphism) and recessive (CC vs. SS+SC of PON2 Ser311Cys gene polymorphism; GG vs. AA+AG of PON2 Ala148Gly gene polymorphism) were performed.
The odds ratio (OR) and their corresponding 95% confidence interval (CI) were employed to assess the association of PON2 Ser311Cys and Ala148Gly gene polymorphisms with the risk of T2DM. The pooled OR was evaluated by Z-test with significance set at P-value < 0.05. The test for heterogeneity between studies was performed using the Chi-square based Q-test and Higgins I2 index (ranging from 0 to 100%) (21). If there was significant heterogeneity between studies, the random-effect model using DerSimonian and Laird method was used. Otherwise, the fixed-effect model (Mantel–Haenszel method) was adopted for the meta-analysis. Galbraith plot was used to explore sources of between-study heterogeneity. The potential publication bias was evaluated by Begg's funnel plot and Egger's linear regression test (22, 23). The statistical analyses were performed by using Stata 12.0 software (StataCorp, College Station, TX, USA).
Results
Summary of Included Studies
In the initial screening, 208 articles were identified and 60 articles were excluded due to duplicate publication. Based on the inclusion criteria for meta-analysis the association of PON2 Ser311Cys and Arg148Gly polymorphisms with T2DM, 89 articles were excluded after screening the abstract and title, and 44 articles were excluded after screening the full-texts. Finally, 15 articles were included. One article investigated the two polymorphisms in the same population. As a result, a total of 12 eligible studies were included for meta-analysis of PON2 Ser311Cys polymorphism (15–19, 24–30), and 4 studies for meta-analysis of PON2 Arg148Gly polymorphism (17, 31–33). Figure 1 illustrated the selection process. Characteristics of the 16 studies on PON2 Ser311Cys and Arg148Gly polymorphisms and risk of developing T2DM susceptibility were summarized in Table 1. In addition, the HWE-P value in the control group in all studies was also listed in Table 1.

Figure 1. Flow diagram of the selection process of research studies The terms “n” in the boxes represent the number of corresponding studies.
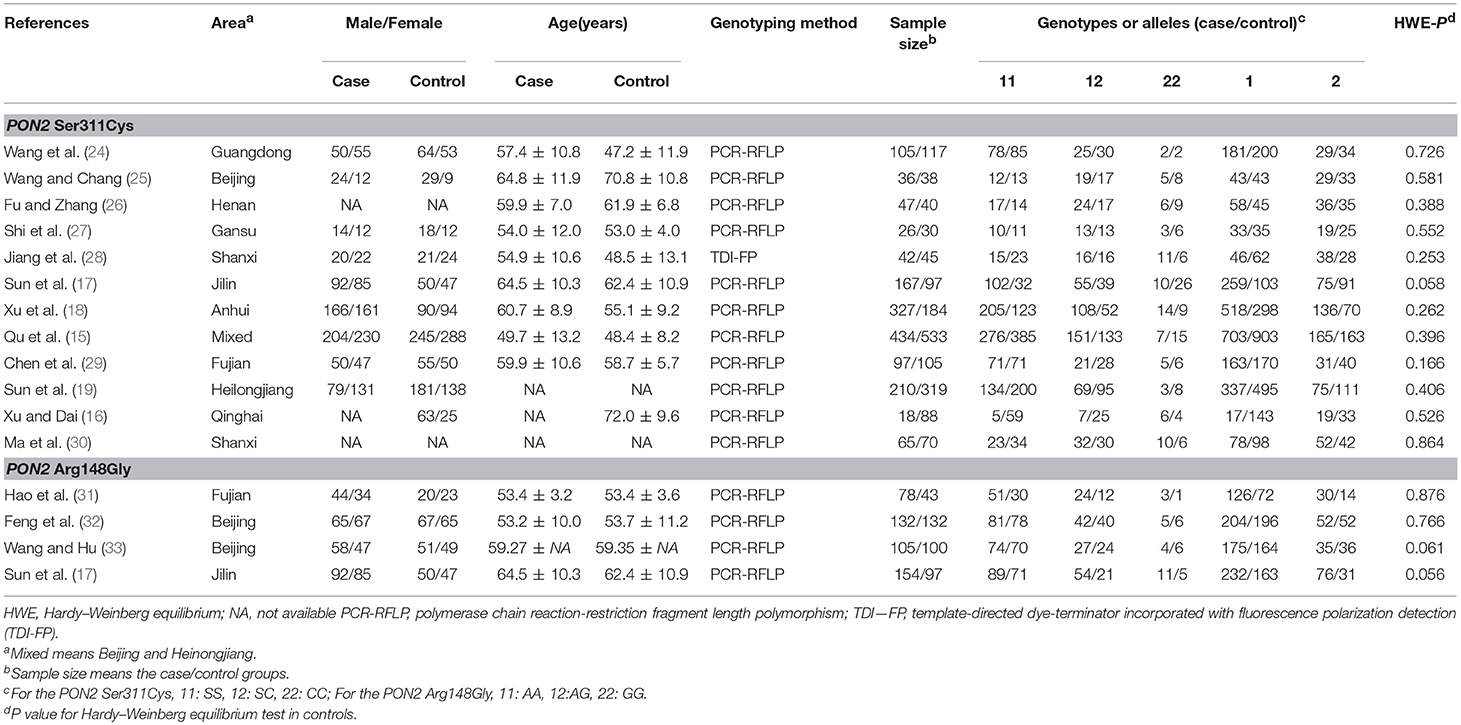
Table 1. Characteristics of included studies of the association of PON2 Ser311Cys and Arg148Gly genetic polymorphisms with type 2 diabetes mellitus.
Pooled Analyses
The main results of this meta-analysis for the association between PON2 Ser311Cys polymorphism and risk of developing T2DM are shown in Table 2. There was no significant association between the PON2 Ser311Cys polymorphism and T2DM risk under all genetic models: allelic (OR = 1.06, 95% CI = 0.77–1.45; P = 0.721), heterozygous (OR = 1.13, 95% CI = 0.87–1.45; P = 0.362), dominant (OR = 1.10, 95% CI = 0.80–1.51; P = 0.562), recessive (OR = 0.87, 95% CI = 0.48–1.58; P = 0.648), homozygous (OR = 0.94, 95% CI = 0.47–1.89; P = 0.865) (Figure 2, Table 2).
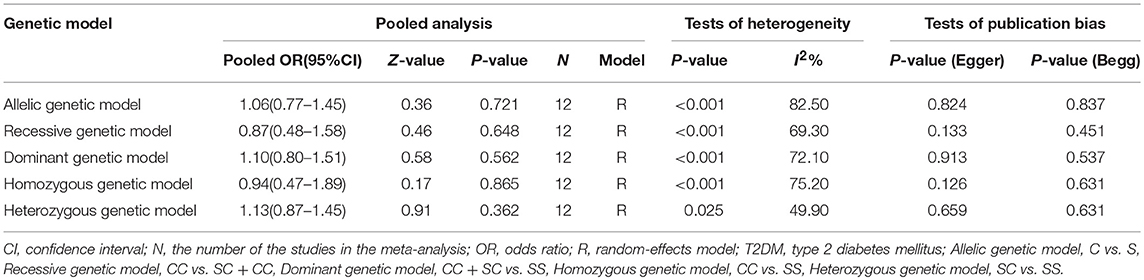
Table 2. Summary of meta-analysis of association between PON2 Ser311Cys genetic polymorphism and risk of type 2 diabetes mellitus in the Chinese population.

Figure 2. Forest plot of the meta-analysis for association between PON2 Ser311Cys polymorphism and type 2 diabetes risk under the allelic (A), homozygous (B), recessive (C), heterozygous (D), and dominant (E) genetic model.
The main results of this meta-analysis in the association between PON2 Arg148Gly polymorphism and risk of developing T2DM are listed in Table 3. No significant association between the PON2 Arg148Gly polymorphism and T2DM risk was also found in all genetic models: allelic (OR = 1.17, 95% CI = 0.91–1.50; P = 0.218), heterozygous (OR = 1.28, 95% CI = 0.94–1.74; P = 0.117), dominant (OR = 1.25, 95% CI = 0.93–1.67; P = 0.142), recessive (OR = 0.99, 95% CI = 0.52–1.88; P = 0.973), homozygous (OR = 1.08, 95% CI = 0.57–2.07; P = 0.808) (Figure 3, Table 3).
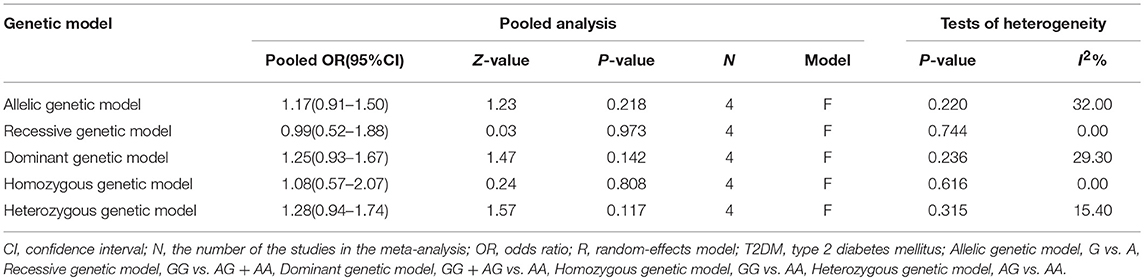
Table 3. Summary of meta-analysis of association between PON2 Arg148Gly genetic polymorphism and risk of type 2 diabetes mellitus in the Chinese population.
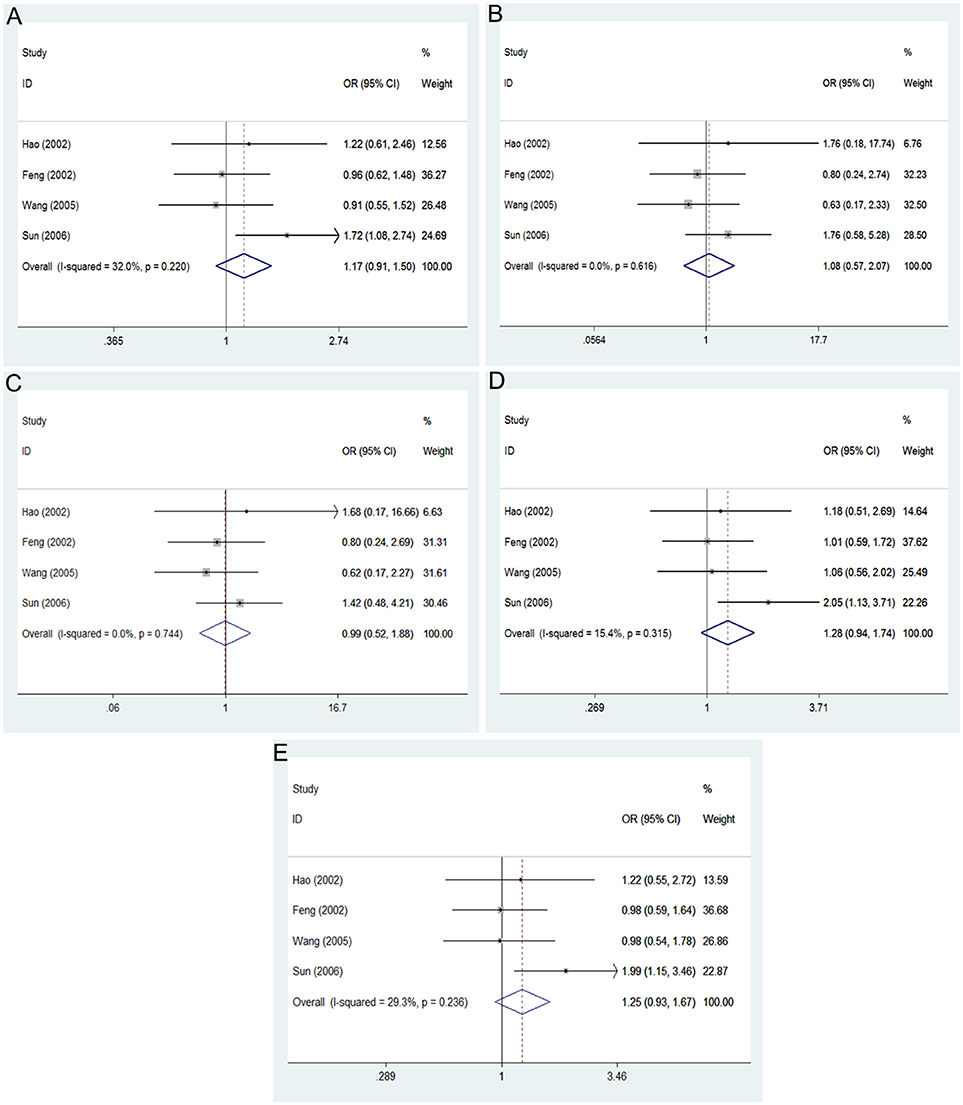
Figure 3. Forest plot of the meta-analysis for association between PON2 Ala148Gly polymorphism and type 2 diabetes risk under the allelic (A), homozygous (B), recessive (C), heterozygous (D), and dominant (E) genetic model.
Sources of Heterogeneity
The results of the heterogeneity analysis in PON2 Ser311Cys polymorphism are summarized in Table 2. There was significant between-study heterogeneity under all genetic models (allelic: I2 = 82.50%, Pheterogeneity < 0.001; recessive: I2 = 69.30%, Pheterogeneity < 0.001; dominant: I2 = 72.10%, Pheterogeneity < 0.001; homozygous: I2 = 75.20%, Pheterogeneity < 0.001; heterozygous: I2 = 49.90%, Pheterogeneity = 0.025). By contrast, as is shown in Table 3, there was no heterogeneity in the meta-analysis in PON2 Arg148Gly polymorphism under all five genetic models (allelic: I2 = 32.00%, Pheterogeneity = 0.220; recessive: I2 = 0%, Pheterogeneity = 0.744; dominant: I2 = 29.30%, Pheterogeneity = 0.236; homozygous: I2 = 0%, Pheterogeneity = 0.616; heterozygous: I2 = 15.40%, Pheterogeneity = 0.315).
Galbraith plot was performed to detect whether there were outliers which could be the potential sources of between-study heterogeneity in the meta-analysis of PON2 Ser311Cys polymorphism. The analysis showed that the studies conducted by Sun et al. (17) and Xu and Dai (16) were the outliers under the allelic (Figure 4A), homozygous (Figure 4B) and recessive (Figure 4C) genetic models. For the heterozygous (Figure 4D) and dominant (Figure 4E) genetic models, Galbraith plot analysis indicated that Sun YD and Qu YC's study were the outliers. In addition, Xu HN's study may also contribute to the significant heterogeneity under a dominant genetic model.
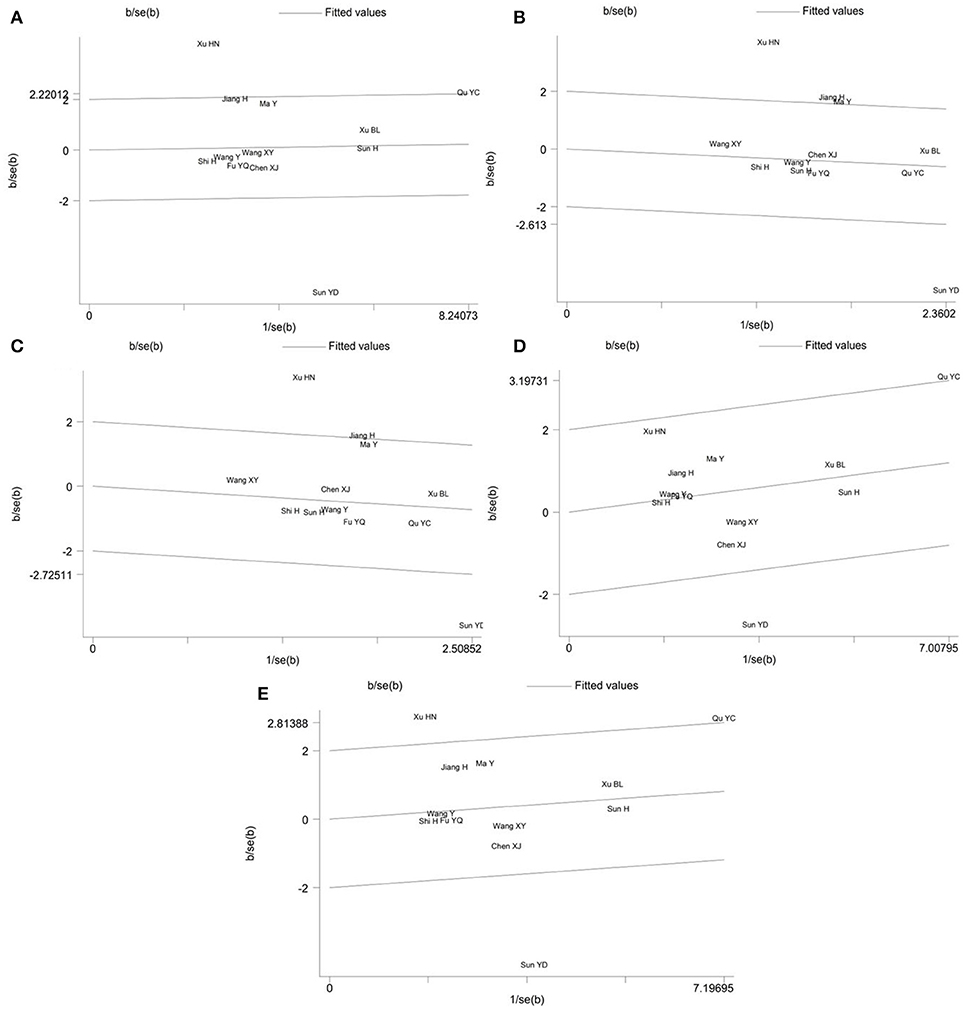
Figure 4. Galbraith plot of the meta-analysis for association between PON2 Ser311Cys polymorphism and type 2 diabetes risk under the allelic (A), homozygous (B), recessive (C), heterozygous (D), and dominant (E) genetic model.
After exclusion of these outliers studies from the meta-analysis, the recalculated summary ORs were still insignificant but the between-study heterogeneity significantly decreased in all genetic models: allelic (I2 = 14%, Pheterogeneity = 0.314), homozygous (I2 = 0%, Pheterogeneity = 0.475), recessive (I2 = 0%, Pheterogeneity = 0.512), heterozygous (I2 = 0%, Pheterogeneity = 0.706) and dominant (I2 = 0%, Pheterogeneity = 0.721) (Table 4).
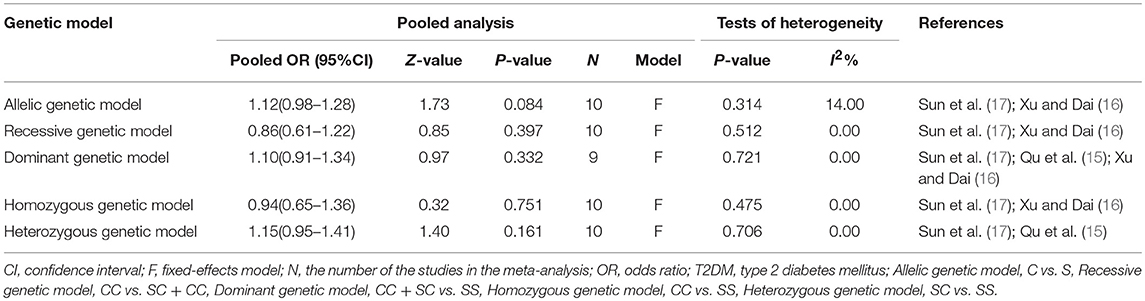
Table 4. Summary of meta-analysis of association between PON2 Ser311Cys genetic polymorphism and risk of type 2 diabetes mellitus in the Chinese population after omitting the outliers.
Publication Bias Analyses
The publication bias in the meta-analysis of PON2 Ser311Cys polymorphism was assessed by Begg's funnel plot (Figure 5, Table 2) and Egger's test (Table 2). The Begg's funnel plot appeared symmetric in all genetic models, with P = 0.824 for allelic genetic model (Figure 5A); P = 0.126 for homozygous genetic model (Figure 5B); P = 0.133 for recessive genetic model (Figure 5C); P = 0.659 for heterozygous genetic model (Figure 5D); P = 0.913 for dominant genetic model (Figure 5E), suggesting no evidence of publication bias.
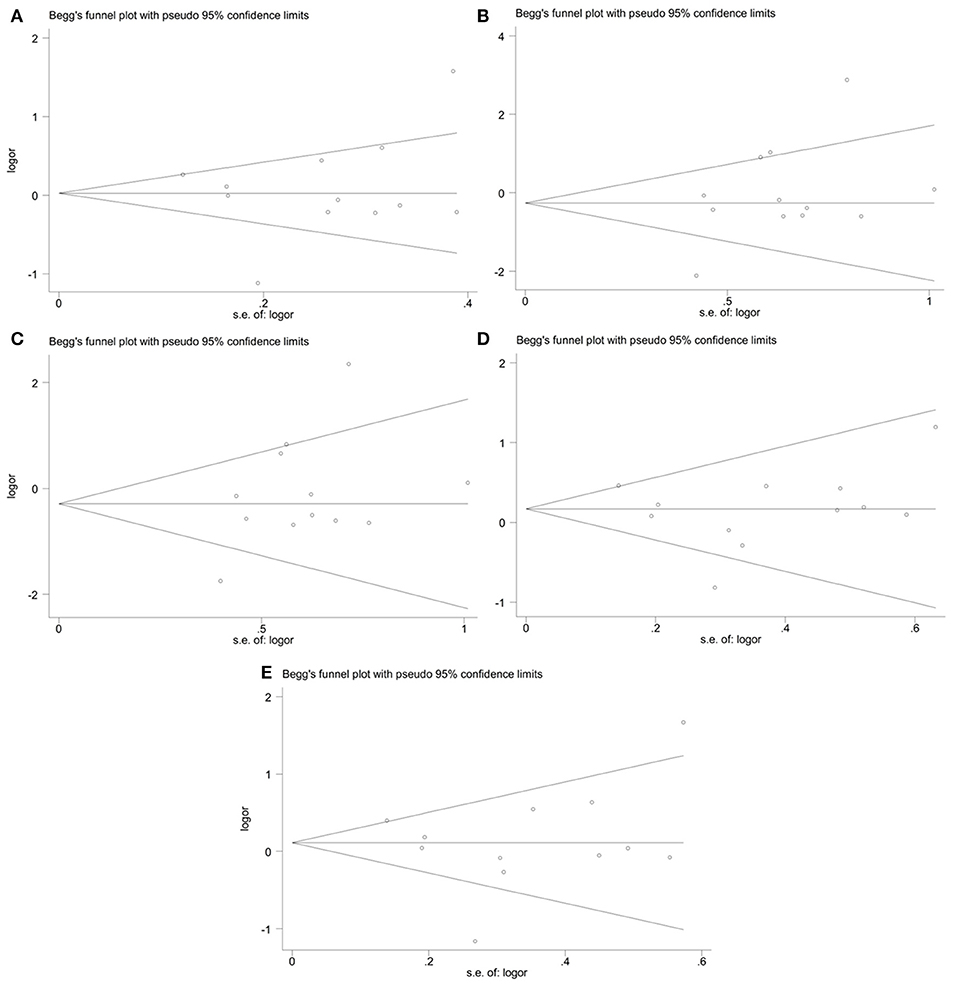
Figure 5. Begg's funnel plot of the meta-analysis for association between PON2 Ser311Cys polymorphism and type 2 diabetes risk under the allelic (A), homozygous (B), recessive (C), heterozygous (D), and dominant (E) genetic model.
Moreover, no evidence of publication bias was also detected by Egger's test (P = 0.837 for allelic genetic model; P = 0.451 for recessive genetic model; P = 0.537 for dominant genetic model; P = 0.631 for homozygous genetic model; P = 0.631 for heterozygous genetic model).
Discussion
T2DM is a silent progressive polygenic disease and associated with a number of genetic factors. The role of PON2 gene in the glycemic control and risk of developing T2DM may be attributed to the widespread tissue expression of PON2, especially the expression in the pancreas. In addition, the expression in the cardiac and skeletal muscle suggests that PON2 could play important roles in the peripheral utilization of glucose (34). Consequently, a large number of researchers have focused on the associations of the PON2 Ser311Cys and Ala148Gly variations with T2DM risk in the Chinese population. However, the genetic association between the two PON2 SNPs and the risk of developing 2DM was uncertain owing to conflicting results generated by various independent case-control studies.
To our knowledge, this is the first comprehensive meta-analysis to assess the genetic association between PON2 Ser311Cys and Ala148Gly polymorphisms and T2DM risk in the Chinese population. Our findings demonstrated that PON2 Ser311Cys and Ala148Gly genetic polymorphisms were not significantly associated with the risk of developing of T2DM in the Chinese population. The pooled OR and 95% CI were examined with five genetic models including the allelic, homozygous, heterozygous, recessive and dominant, and consistently no significant effects of PON2 Ser311Cys and Ala148Gly genotypes on T2DM risk were found.
Interestingly, both PON2 Ser311Cys and Ala148Gly polymorphisms were found not to be associated with the susceptibility of T2DM. The two polymorphisms, Ser311Cys and Ala148Gly, were located at the exon nine and exon five of the PON2 gene, respectively. By checking the two SNPs (Ser311Cys/rs6954345/rs7493 and Arg148Gly/rs11545942/rs12026) information available for Chinese population in the 1000 genomics database (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/), we found that the minor allele frequency of PON2 Ser311Cys and Ala148Gly were the same each other: 0.20 in Southern Han Chinese and 0.18 in Beijing Han Chinese. In addition, Dasgupta et al. reported that PON2 Ser311Cys and Ala148Gly polymorphisms were in strong linkage disequilibrium (LD) with each other (r2 = 0.81) (35). Therefore, strong LD between PON2 Ser311Cys and Ala148Gly polymorphisms may explain the consistent findings existed in the two PON2 genetic polymorphisms.
In the meta-analysis of PON2 Ser311Cys polymorphism, there was substantial between-study heterogeneity under all five genetic models. Therefore, the Galbraith plot was employed to identify the outliers that could be the possible sources of between-study heterogeneity. The Galbraith plot analyses indicated that the studies conducted by Sun et al. (17), Xu and Dai (16), and Qu et al. (15) were the outliers and could largely account for the significant heterogeneity when all eligible studies were pooled into our meta-analysis. Xu HN's study had the largest OR while Sun YD's study had the smallest OR among the included studies. After omitting these outlier studies, the I2 values immediately decreased to 14% in an allelic genetic model and 0% in other four genetic models, and the P-values of the Q-test in all genetic models were >0.1. Moreover, the pooled ORs remained statistically insignificant in all genetic models, which demonstrated that our meta-analysis results were stable and reliable.
One previous meta-analysis assessed the association of PON2 Ser311Cys and Ala148Gly gene polymorphisms with diabetic nephropathy and retinopathy in Caucasian populations (36). However, only three studies included PON2 Ser311Cys and two studies included Ala148Gly in the meta-analysis. The results showed these two PON2 genetic polymorphisms were not associated with diabetic nephropathy and retinopathy in Caucasians. In addition, numerous meta-analyses have been conducted to determine the association of PON2 Ser311Cys and Ala148Gly gene polymorphisms with the risk of developing other diseases, such as coronary heart disease (37–39), ischemic stroke (40, 41) and Alzheimer Disease (42). This current meta-analysis only focused on the association of PON2 Ser311Cys and Ala148Gly gene polymorphisms and the risk of developing T2DM.
Our study has some limitations. First, some of the included studies were based on small sample size, which may have resulted in a decreased power to detect a significant difference in the distribution of genotypes or alleles between cases and controls. Second, the pooled OR is based on the crude OR in the original studies. Because we could not obtain enough raw data from individual studies, the pooled data were not adjusted by potential confounding factors such as gender, age, smoking status, body mass index and waist-hip ratio. Third, T2DM is a polygenic hereditary disorder. The current study only focused on the role of PON2 genetic polymorphisms in the susceptibility to T2DM. Other susceptibility genes such as PON1 and PON3 gene may interact with PON2 gene by gene-to-gene effect for T2DM (34, 43). Fourth, various environmental factors may be involved in the T2DM risk, the effect of gene-to-environment interactions should be taken into account. For example, synergistic effects between the PON2 Ala148Gly polymorphism and obesity were found in the risk of T2DM (32). Finally, the current meta-analysis was based on data from candidate gene association studies because the genome-wide association studies (GWAS) data was not collected. Nevertheless, there is no evidence of publication bias assessed by Begg's funnel plot and Egger's test in the meta-analysis.
In conclusion, our meta-analysis confirmed that PON2 Ser311Cys and Ala148Gly gene polymorphisms did not have a significant association with the risk of developing T2DM in the Chinese population. A well-designed study, with consideration of gene-to-gene and gene-to-environment interactions, should be conducted in the future.
Author Contributions
HR and J-QL conceived and designed the study. J-QL, HR, S-LT, and M-ZL performed the search. HR, S-LT, and J-QL analyzed the data. J-QL, HR, and M-ZL contributed reagents, material, analysis tools. HR, S-LT, J-QL, and HB wrote and review the manuscript. HR and S-LT revised the manuscript. HR and J-QL reference collection, data management, statistical analyses, paper writing, and study design.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81703623), and the Scientific Foundation of Hunan (No. 2018JJ3719).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
In addition, we would like to thank Dr. Andrew J. Cave at University of Alberta for support with English language expression.
References
1. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA (2013) 310:948–59. doi: 10.1001/jama.2013.168118
2. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. (2010) 362:1090–101. doi: 10.1056/NEJMoa0908292
3. Liu L, Lou Q, Guo X, Yuan L, Shen L, Sun Z, et al. Management status and its predictive factors in patients with type 2 diabetes in China: a nationwide multicenter study: a nationwide multicenter study. Diabetes Metab Res Rev. (2015) 31:811–6. doi: 10.1002/dmrr.2757
4. Guo XH, Yuan L, Lou QQ, Shen L, Sun ZL, Zhao F, et al. A nationwide survey of diabetes education, self-management and glycemic control in patients with type 2 diabetes in China. Chin Med J. (2012) 125:4175–80. doi: 10.3760/cma.j.issn.0366-6999.2012.23.003
5. Bianco A, Chiefari E, Nobile CG, Foti D, Pavia M, Brunetti A. The Association between HMGA1 rs146052672 Variant and Type 2 Diabetes: a transethnic meta-analysis. PLoS ONE (2015) 10:e0136077. doi: 10.1371/journal.pone.0136077
6. Li YY, Wang H, Yang XX, Geng HY, Gong G, Kim HJ, et al. Small ubiquitin-like modifier 4 (SUMO4) gene M55V polymorphism and type 2 diabetes mellitus: a meta-analysis including 6,823 subjects. Front Endocrinol. (2017) 8:303. doi: 10.3389/fendo.2017.00303
7. Li S, Xiao J, Ji L, Weng J, Jia W, Lu J, et al. BMI and waist circumference are associated with impaired glucose metabolism and type 2 diabetes in normal weight Chinese adults. J Diabetes Complic. (2014) 28:470–6. doi: 10.1016/j.jdiacomp.2014.03.015
8. Pullinger CR, Goldfine ID, Tanyolaç S, Movsesyan I, Faynboym M, Durlach V, et al. Evidence that an HMGA1 gene variant associates with type 2 diabetes, body mass index, and high-density lipoprotein cholesterol in a Hispanic-American population. Metab Syndr Relat Disord. (2014) 12:25–30. doi: 10.1089/met.2013.0086
10. Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, et al. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. (2001) 276:44444–9. doi: 10.1074/jbc.M105660200
11. Calle R, McCarthy MI, Banerjee P, Zeggini E, Cull CA, Thorne KI, et al. Paraoxonase 2 (PON2) polymorphisms and development of renal dysfunction in type 2 diabetes: UKPDS 76. Diabetologia (2006) 49:2892–9. doi: 10.1007/s00125-006-0436-8
12. Witte I, Foerstermann U, Devarajan A, Reddy ST, Horke S. Protectors or traitors: the roles of PON2 and PON3 in atherosclerosis and cancer. J Lipids (2012) 2012:342806. doi: 10.1155/2012/342806
13. Gluba A, Pietrucha T, Banach M, Piotrowski G, Rysz J. The role of polymorphisms within paraoxonases (192 Gln/Arg in PON1 and 311Ser/Cys in PON2) in the modulation of cardiovascular risk: a pilot study. Angiology (2010) 61:157–65. doi: 10.1177/0003319709351258
14. Erlich PM, Lunetta KL, Cupples LA, Huyck M, Green RC, Baldwin CT, et al. Polymorphisms in the PON gene cluster are associated with Alzheimer disease. Hum Mol Genet. (2006) 15:77–85. doi: 10.1093/hmg/ddi428
15. Qu Y, Yang Z, Jin F, Sun L, Zhang C, Ji L, et al. The Ser311Cys variation in the paraoxonase 2 gene increases the risk of type 2 diabetes in northern Chinese. J Genet. (2008) 87:165–9. doi: 10.1007/s12041-008-0025-3
16. Xu HN, Dai QX. Relationship between PON2 Cys311Ser gene polymorphism and elderly diabetic complications in plateau. World Latest Med Inform. (2014) 14:11–3. doi: 10.1089/gtmb.2010.0207
17. Sun YD, Sun SC, Zuo J, Lin YL, Kan Y, Shao H, et al. Polymorphism of paraoxonase in diabetic nephropathies. J Jilin Univ Med Edn. (2005) 31:598–601. doi: 10.1007/s001250051566
18. Xu BL, Xu JX, Yang MG, Liu SQ, Wang CJ, Yao SH, et al. Association of paraoxonase gene 2 polymorphism with type 2 diabetes mellitus. Acta Univ Med Anhui (2007) 42:326–7. doi: 10.1016/j.gene.2013.05.013
19. Sun H, Li YP, Yang Y, Wang XY, Yan HH, Miao CQ, et al. PON2 Gene 9 Ser311C → G polymorphism in Chinese families with type 2 diabetes. Prog Modern Biomed. (2012) 12:210–4. doi: 10.1111/j.1464-5491.2007.02328.x
20. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629–34.
23. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50:1088–101.
24. Wang XY, Xue YM, Wen SJ, Zhang NL, Ji Z, Pan SY. The association of paraoxonase 2 gene C311S variant with ischemic stroke in Chinese type 2 diabetes mellitus patients. Chin J Med Genet. (2003) 20:215–9. doi: 10.3760/j.issn:1003-9406.2003.03.011
25. Wang Y, Chang ZW. The association of paraoxonase 2 gene 311Cys/Ser polymorphism and type 2 diabetes mellitus complicated by coronary heart disease. Chin J Pathophysiol. (2003) 19:211–4. doi: 10.3321/j.issn:1000-4718.2003.02.016
26. Fu YQ, Zhang SH. Relationships between paraoxonas family and diabetic vascular complications in type 2 diabetes mellitus. Zheng Zhou Univ. (2004) 241:1489–96. doi: 10.1177/1535370216641786
27. Shi H, Ning YY, Zhu XZ, Ren JG, Chen AR. Relationship between paraoxonase 2 C311S polymorphism and diabetic nephropathy. Clin Focus (2004) 19:623–5. doi: 10.1002/cbf.1519
28. Jiang H, Jiao K, Zhang J. Association between the onset of type 2 diabetes mellitus and diabetic nephropathy and the polymorphism of paraoxonase 2 S311C gene. Chin J Clin Rehabil. (2005) 9:126–8. doi: 10.3321/j.issn:1673-8225.2005.19.050
29. Chen XJ, Pan SZ, Zeng J. Relationship between PON1,PON2 polymorphism and type 2 diabetic nephropathy. Med J Chin People's Armed Pol Forces (2011) 22:153–6. doi: 10.1210/jc.2003-031252
30. Ma Y, Yang XX, Wang F. The study of gene PON2 and AGT polymorphism in type 2 diabetic nephropathy. China Continuing Med Educ. (2017) 9:219–21. doi: 10.1007/s001250051586
31. Hao YL, Lin LX, Chen G. Relationship between paraoxonase 2 A148G polymorphism and diabetic nephropathy. Chin J Nephrol. (2002) 18:422–4. doi: 10.1016/j.ancard.2015.02.006
32. Feng Y, Li QF, Qian RL, Hong TP, Guan BX, Chen CZ, et al. An association study of paraoxonase gene G/A148 polymorphism with type 2 diabtets mellitus and blood lipids level in Chinese population. Chin J Diabetes (2002) 10:195–8. doi: 10.1371/journal.pone.0154369
33. Wang SL, Hu Z. Study of the Susceptibility Genes of Type 2 Diabetic Nephropathy in Nothern Chinese. Shandong: A Dissertation of Shandong University (2005):22–42.
34. Hegele RA, Connelly PW, Scherer SW, Hanley AJ, Harris SB, Tsui LC, et al. Paraoxonase-2 gene (PON2) G148 variant associated with elevated fasting plasma glucose in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. (1997) 82:3373–7. doi: 10.1210/jcem.82.10.4289
35. Dasgupta S, Demirci FY, Dressen AS, Kao AH, Rhew EY, Ramsey-Goldman R, et al. Association analysis of PON2 genetic variants with serum paraoxonase activity and systemic lupus erythematosus. BMC Med Genet. (2011) 12:7. doi: 10.1186/1471-2350-12-7
36. Wang J, Yang MM, Rong SS, Ng TK, Li YB, Liu XM. Association of paraoxonase gene polymorphisms with diabetic nephropathy and retinopathy. Mol Med Rep. (2013) 8:1845–51. doi: 10.3892/mmr.2013.1710
37. Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet (2004) 363:689–95. doi: 10.1016/s0140-6736(04)15642-0
38. Wang M, Lang X, Zou L, Huang S, Xu Z. Four genetic polymorphisms of paraoxonase gene and risk of coronary heart disease: a meta-analysis based on 88 case-control studies. Atherosclerosis (2011) 214:377–85. doi: 10.1016/j.atherosclerosis.2010.11.028
39. Chen ML, Zhao H, Liao N, Xie ZF. Association between paraoxonase 2 Ser311Cys polymorphism and coronary heart disease risk: a meta-analysis. Med Sci Monit. (2016) 22:3196–201. doi: 10.12659/MSM.896601
40. Rodriguez-Esparragon F, Lopez-Fernandez JC, Buset-Rios N, Garcia-Bello MA, Hernandez-Velazquez E, Cappiello L, et al. Paraoxonase 1 and 2 gene variants and the ischemic stroke risk in Gran Canaria population: an association study and meta-analysis. Int J Neurosci. (2017) 127:191–8. doi: 10.3109/00207454.2016.1165675
41. Li BH, Zhang LL, Yin YW, Pi Y, Yang QW, Gao CY, et al. Association between paraoxonase 2 Ser311Cys polymorphism and ischemic stroke risk: a meta-analysis involving 5,008 subjects. Mol Biol Rep. (2012) 39:5623–30. doi: 10.1007/s11033-011-1367-0
42. Nie Y, Luo D, Yang M, Wang Y, Xiong L, Gao L, et al. A meta-analysis on the relationship of the PON genes and Alzheimer disease. J Geriatr Psychiatry Neurol. (2017) 30:303–10. doi: 10.1177/0891988717731825
Keywords: PON2, gene polymorphism, Ser311Cys, Ala148Gly, susceptibility, type 2 diabetes
Citation: Ren H, Tan S-L, Liu M-Z, Banh HL and Luo J-Q (2018) Association of PON2 Gene Polymorphisms (Ser311Cys and Ala148Gly) With the Risk of Developing Type 2 Diabetes Mellitus in the Chinese Population. Front. Endocrinol. 9:495. doi: 10.3389/fendo.2018.00495
Received: 21 March 2018; Accepted: 07 August 2018;
Published: 27 August 2018.
Edited by:
Antonio Brunetti, Università degli Studi Magna Græcia di Catanzaro, ItalyReviewed by:
Guoqiang Gu, Vanderbilt University, United StatesZoi Michailidou, University of Edinburgh, United Kingdom
Copyright © 2018 Ren, Tan, Liu, Banh and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Quan Luo, bHVvamlhbnF1YW54eUBjc3UuZWR1LmNu
†These authors have contributed equally to this work
 Huan Ren
Huan Ren Sheng-Lan Tan
Sheng-Lan Tan Mou-Ze Liu1,2,3
Mou-Ze Liu1,2,3 Hoan L. Banh
Hoan L. Banh Jian-Quan Luo
Jian-Quan Luo