
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 11 June 2018
Sec. Clinical Diabetes
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00323
Background: Cytochrome P450 family 17 subfamily A member 1 (CYP17A1) gene encodes a key enzyme in the synthesis and metabolism of steroid hormones and has been associated with various factors, such as hypertension, insulin resistance, and polycystic ovary syndrome. However, whether the gene was associated with type 2 diabetes mellitus (T2DM) has not been reported yet. Therefore, we sought to investigate whether CYP17A1 was associated with T2DM and related traits among Han Chinese.
Methods: Three tagging single nucleotide polymorphisms (rs1004467, rs17115149, and rs12413409), in the CYP17A1 gene region were selected and genotyped in a case–control study that included 440 diabetes and 1,320 control subjects. Effects of genetic loci were studied using univariate unconditional logistic regression and multivariate logistic regression analysis adjusted for age, sex, family history, body mass index, smoking, and drinking. Bioinformatics analysis was also conducted using the GEO DataSets and PROMO database to gain hints of possible mechanism.
Results: Rs17115149 and rs12413409 polymorphisms were significantly associated with the risk of T2DM, even after adjusting for age, sex, family history, body mass index, smoking, and drinking. In stratified analyses, rs1004467 and rs12413409 showed significant association with T2DM in the older age group (≥65 years) and, in the case of rs12413409, the risk of T2DM was significant in men but not in women. Rs17115149 had significant association with T2DM in the hypertension subgroup, and rs12413409 in the non-hypertension subgroup. Moreover, rs12413409 showed significant association with plasma glucose levels in the recessive model (P = 0.020) among subjects not taking hypoglycemic measures. Bioinformatics analysis revealed significantly higher CYP17A1 gene expression in T2DM patients compared to healthy controls. Finally, the mutant T allele of the rs17115149 polymorphism allowed binding to the RBP-Jkappa transcription factor.
Conclusion: This is the first report to identify that variants rs1004467, rs17115149, and rs12413409 of CYP17A1, are related to plasma glucose levels and T2DM among Han Chinese. Our results suggest that CYP17A1 might constitute a risk gene for progression to T2DM.
Type 2 diabetes (T2DM) is a complex multifactorial disorder caused by various susceptibility genes and a variety of environmental determinants, and is one of the main challenges of modern health (1). There is convincing evidence that genetic factors contribute strongly to an individual’s risk of developing T2DM (2). Several large-scale association studies have reported numerous common, rare, and functional variants of T2DM (3, 4). To date, more than 100 susceptibility loci have been identified to influence the risk for T2DM, and recent studies have argued that many additional risk loci remain to be determined (5).
The cytochrome P450 family 17 subfamily A member 1 (CYP17A1) gene, located on chromosome 10q24.3, consists of eight exons and seven introns and is expressed mainly in the adrenal glands and gonads (6, 7). In humans, CYP17A1 encodes the P450c17 protein, a key enzyme in the steroidogenic pathway. It can catalyze two distinct types of substrate oxidation (8, 9): 17alpha-hydroxylation of steroids and the 17,20-lyase reaction, which are essential for corticoid biosynthesis and sex steroid precursors generation, respectively (10). Some studies show that deficient expression of P450c17 can impair androgen, estrogen, and cortisol hormone synthesis, while producing excessive amounts of mineralocorticoid, and may cause hypertension, hypokalemia, pseudohermaphroditism, and delayed sexual maturation (11, 12).
Genetic association studies have revealed that the CYP17A1 gene plays an important role in various pathological conditions, such as visceral and subcutaneous fat accumulation (13), coronary artery disease (CAD) (14), hypertension (12), prostate cancer (15), insulin resistance, and polycystic ovary syndrome (16), which are often related to T2DM. Moreover, considering that corticoids are also associated with glycometabolism, CYP17A1 is likely to be involved in T2DM. However, no studies have investigated the relationship between CYP17A1 and T2DM in the Han Chinese population. Therefore, here, we aimed to assess the association between CYP17A1 polymorphisms and T2DM among Han Chinese.
An age- (±5 years) and sex-frequency matched case–control study was conducted, which included 440 T2DM patients and 1,420 non-diabetic controls. Patients and controls were recruited from three hospitals in Chongqing city between October 2013 and July 2015. The three hospitals were the Second Affiliated Hospital of Chongqing Medical University, the Chongqing Zhongshan Hospital, and the Chongqing Hospital of Traditional Chinese Medicine. All type 2 diabetic patients included in the study had to meet the 1999 WHO criteria for diabetes (17): a fasting glucose level ≥7.0 mmol/L or a 2-h glucose level ≥11.1 mmol/L, treatment with insulin and/or oral hypoglycemic agents following a diagnosis of T2DM, and having been diagnosed after the age of 35 years. The non-diabetic controls resided in the same communities as the cases, and inclusion criteria were as follows: (1) >50 years of age, (2) a fasting glucose level <6.1 mmol/L or 2-h glucose level <7.8 mmol/L and no family history of T2D, (3) no past history of diabetes, and (4) no severe liver disease and/or kidney disease. All participants underwent a questionnaire-based interview aimed at collecting their family history, medical history, as well as information pertaining to medication, home environment, and lifestyle factors.
Subjects who had smoked >100 cigarettes or had drunk <3 times per week for >1 year in their lifetime were defined as smokers and drinkers, respectively. Notably, during the interview, some subjects whose systolic and diastolic blood pressure measured slightly >140 and >90 mmHg, respectively, but had never been diagnosed with hypertension, were classified as the uncertain hypertension group. This study was approved by the ethics committee of Chongqing Medical University and was performed in accordance with the guidelines of the Helsinki World Medical Association Declaration. After a full explanation of the study, all participants approved the study and written informed consents were obtained.
Blood samples were collected from all participants after overnight fasting (at least 12 h). Then, extensive anthropometric and biochemical traits related to glucose metabolism were measured using standard laboratory procedures in the clinical laboratories of the respective hospitals. Parameters included blood pressure, fasting blood glucose (FBG), total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglyceride.
Three tagging SNPs, rs1004467, rs17115149, and rs12413409, were selected using the pairwise tagging method in Haploview 4.0, based on R2 < 0.8 and minor allele frequency >0.05 across the CYP17A1 gene region using 1,000 Chinese Han population genome data sets. The selected SNPs were the most frequently analyzed SNPs at this locus in the Chinese population.
Human genomic DNA samples were extracted from peripheral blood (QIAamp DNA blood kit; QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. A NanoDrop 2000 spectrophotometer (Thermo Scientific, DE, USA) was used to measure the concentration. The eluted and qualified DNA samples were stored at −80°C prior to further use.
Genotyping was performed using a MassARRAY time-of-flight mass spectrometer (Sequenom, San Diego, CA, USA), as described in our previous study (18). Success rates for rs1004467, rs17115149, and rs12413409 were 98.8, 100.0, and 98.8%, respectively.
We conducted a bioinformatics analysis to further explore the possible mechanisms of the CYP17A1 gene and rs17115149 variant. We used the GEO DataSets (https://www.ncbi.nlm.nih.gov/gds/) to explore CYP17A1 gene expression in type 2 diabetic patients compared with non-diabetics, and the PROMO database (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) to inspect whether the rs17115149 polymorphism included any transcription factor binding sites (TFBS). If so, a variant in this allele could cause gain/loss of binding to TFBS in humans.
For baseline characteristics, continuous variables were reported as mean ± SD, and categorical variables were reported as frequencies in percentages. Normal distribution of data was analyzed using the Kolmogorov–Smirnov normality test. Student’s t-test was used to compare the data with a normal distribution, and data with unequal variance and/or without a normal distribution were assessed using the Mann–Whitney rank sum test. The chi-square test and Hardy–Weinberg equilibrium (HWE) analyses were used for categorical variables. Univariate unconditional logistic regression analysis was performed to compare the case and control groups by computing the odds ratios and their 95% confidence intervals (CIs). Adjusted logistic regression analysis was conducted after adjusting for age, sex, family history, body mass index, smoking, and drinking. Each model was composed of allele A versus allele B, with A being the major allele and B the minor allele. This generated the following models: dominant (AB + BB versus AA), recessive (BB versus AB + AA), codominant (BB versus AA and AB versus AA), overdominant (AA + BB versus AB), and addictive (AA versus AB versus BB). A haplotype analysis of rs1004467, rs17115149, and rs12413409 SNPs was performed using Phase 2.0 software (19). The association between FBG and variant genotypes were measured using the Mann–Whitney rank sum test. We used the Haploview 4.0 program to analyze pairwise linkage disequilibrium (LD) based on data extracted from 1,000 genomes. A P-value <0.05 was defined as significant. Statistical analyses were performed with SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA).
Table 1 lists the baseline characteristics of study participants. Among the 1,860 participants, 440 were type 2 diabetic patients and 1,420 were non-diabetic controls. The mean ages of the case and control groups were 70.04 and 66.32 years, respectively. No significant difference was observed in sex distribution between diabetic and control groups (P = 0.660). Diabetic cases had higher FBG and systolic blood pressure levels and higher rates of hypertension, CAD, and hyperlipidemia compared to non-diabetic controls.
The characteristics of the three selected SNPs are shown in Table S1 in Supplementary Material, and no apparent deviations in genotype distributions were observed based on HWE analysis for all SNPs in the control group (P > 0.05). The LD pattern of the three SNPs is shown in Figure S1 in Supplementary Material among the 1,000 genome of Chinese Han population, the diabetic group and control group. Rs1004467 and rs12413409 SNPs displayed moderate LD, whereas rs1004467 and rs17115149 exhibited no LD, even though their distance was relatively close.
As presented in Table 2, among the selected three SNPs, univariate analysis and adjusted logistic regression analysis both indicated that rs17115149 and rs12413409 were significantly associated with T2DM. The risk genotype of rs17115149 was the GT variant (OR = 1.373; 95% CI, 1.020–1.849); moreover, the T allele appeared to be a risk allele when compared to the C allele (OR = 1.345; 95% CI, 1.029–1.760). After adjusting for multiple risk factors, rs17115149 was associated with T2DM in the dominant, overdominant, and addictive models. As for rs13413409, the AA genotype was found to pose a greater risk than the GG genotype (OR = 1.682; 95% CI, 1.122–2.522), and the association between rs12413409 and T2DM remained significant in the recessive model in multivariate analyses.
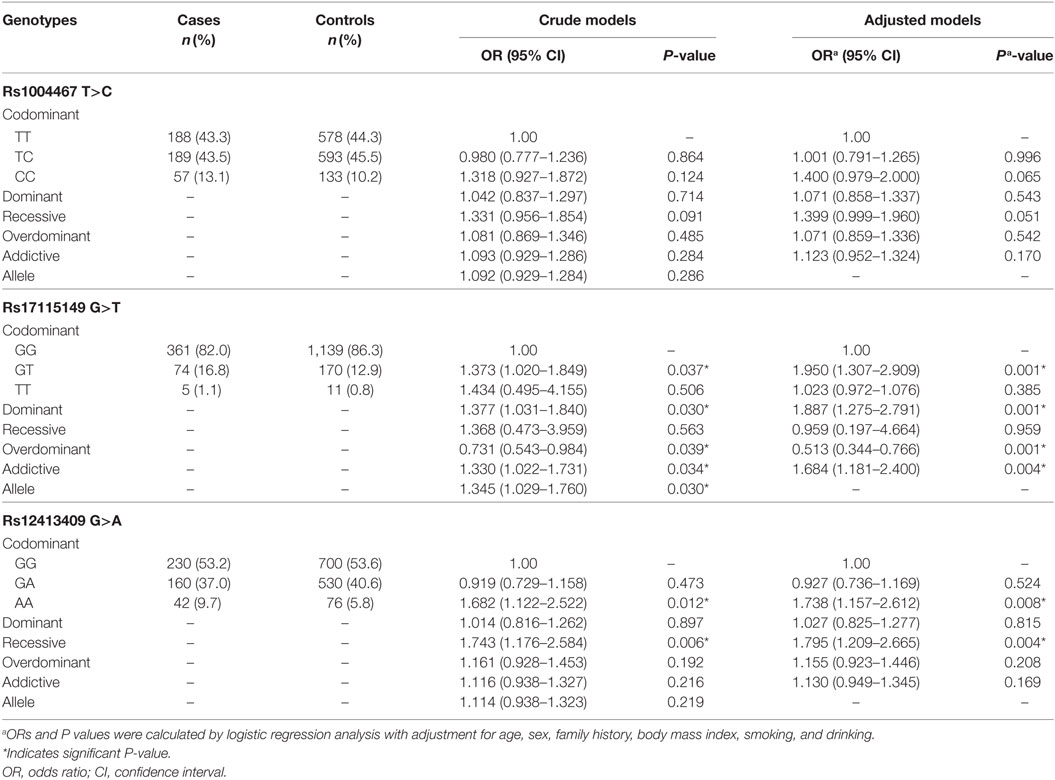
Table 2. Association of three genotyped single nucleotide polymorphisms with type 2 diabetes mellitus in the Han Chinese population.
We then performed stratified analyses to explore the relationship between SNPs and conventional T2DM risk factors, including age, gender, and hypertension.
As shown in Table S2 in Supplementary Material, stratified analyses based on age and gender revealed that SNPs rs1004467 and rs12413409 were both significantly associated with T2DM in the older age group (≥65 years), whereas no significant associations were found in the other group (<65 years). Moreover, when the analysis was performed separately in men and women, significant associations of rs12413409 (codominant model and recessive model) and T2DM were observed in men, but not in women.
In the stratified analyses of hypertension, we found rs17115149 to be nominally associated with T2DM in the hypertension group, whereas rs12413409 was associated with T2DM in the non-hypertension group (Table 3).
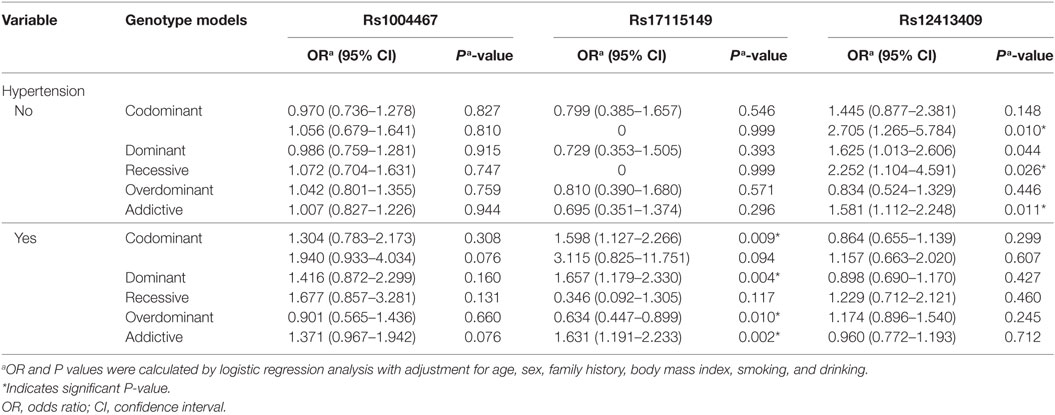
Table 3. Stratified analysis of rs1004467, rs17115149, and rs12413409 polymorphisms on type 2 diabetes mellitus in the hypertension group.
To determine whether the three SNPs in the CYP17A1 gene cluster accounted for any other associations with T2DM when tested together, a haplotype analysis for rs1004467, rs17115149, and rs12413409 in the T2DM and control groups was performed.
A total of eight haplotypes were found in both type 2 diabetic and control groups. The haplotypes with <1% frequency were excluded from further analysis. Finally, four haplotypes were compared between type 2 diabetic and control groups. As indicated in Table 4, compared with the haplotype TGG carriers, two other haplotype (TTG and CGG) carriers had significantly higher risk of diabetes (P < 0.05), with TTG displaying the strongest association with T2DM (OR = 1.70; 95% CI, 1.27–2.28; P = 3.09 × 10−4). However, no difference was found between CGA and TGG haplotypes in type 2 diabetic and control groups.
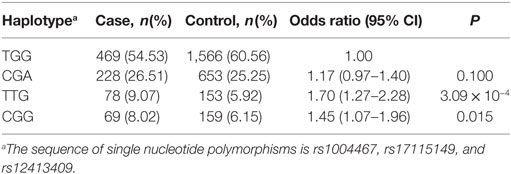
Table 4. Associations between three-site haplotypes in the cytochrome P450 family 17 subfamily A member 1 gene region and type 2 diabetes mellitus risk in Han Chinese participants.
Among non-oral hypoglycemic agent and/or insulin takers, who included controls and T2DM patients not taking hypoglycemic measures, individuals with AA homozygous genotypes at rs12413409 exhibited significantly higher FBG levels than those with GA and GG carriers (P = 0.020). Furthermore, we found that the carriers of genotype AA had higher FBG levels compared to GA and GG genotype carriers in males (P = 0.020) but not in females (P = 0.339) (Figure 1).
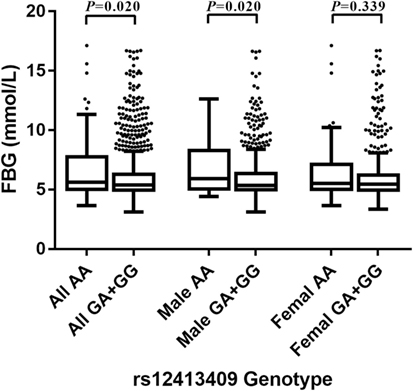
Figure 1. Box-whisker plot of fasting blood glucose (FBG) levels in the non-oral hypoglycemic agents and/or insulin takers study subgroup (N = 1,459), stratified by rs12413409 genotype and gender. The plot shows the median within the interquartile range box, with whiskers extending to the 5th and 95th percentiles; data points beyond the whiskers are displayed as dots. Groups were compared by the Mann–Whitney nonparametric test.
Notably, rs1004467 and rs17115149 showed insignificant difference in FBG levels in different genotype carriers in all models.
To determine CYP17A1 gene expression in T2DM patients compared with healthy controls, we analyzed the high-throughput microarray gene expression database of GDS3782 data from the GEO DataSets and found that the CYP17A1 gene expression was significantly higher in pancreatic beta-cells in T2DM patients compared to healthy controls (Figure 2A).
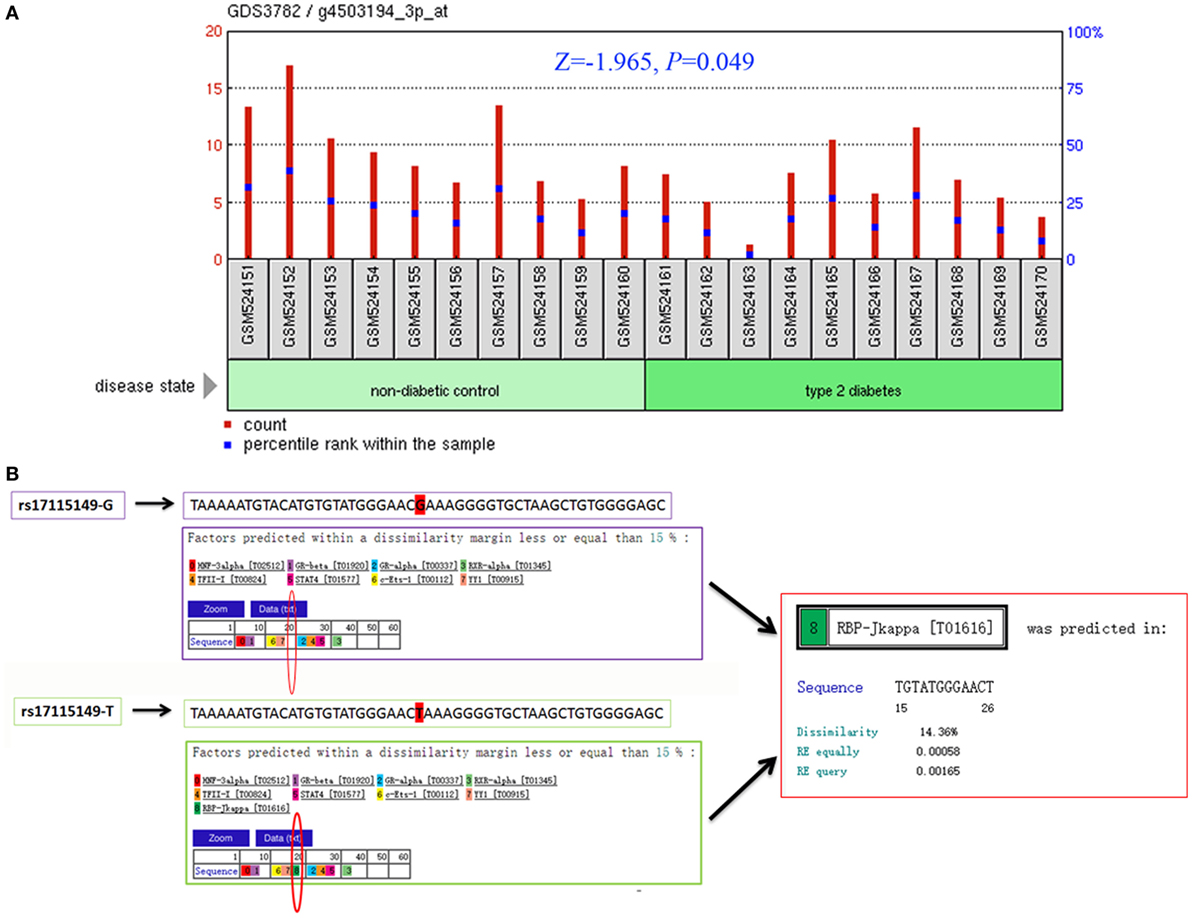
Figure 2. Bioinformatics analysis of the cytochrome P450 family 17 subfamily A member 1 (CYP17A1) gene and rs17115149 polymorphism. Analysis of CYP17A1 gene expression in type 2 diabetes mellitus patients compared with the healthy control group was performed using the high-throughput microarray gene expression database of GDS3782 data and Mann–Whitney U nonparametric test (A). Bioinformatics analysis using data from the PROMO transcription factor binding site database showed that the mutant T allele at the rs17115149 polymorphism allowed binding to the RBP-Jkappa transcription factor (B).
Considering that rs17115149 is located in the 5′ untranslated region of CYP17A1, which often harbors TFBS, the PROMO database was used to determine potential transcription factor binding to this SNP. Bioinformatics analysis showed that the mutant T allele at the rs17115149 polymorphism allowed binding to the RBP-Jkappa transcription factor (Figure 2B).
In the present study, we explored the potential relationship between CYP17A1 genetic polymorphisms and T2DM susceptibility. We report for the first time that SNPs rs17115149 and rs12413409 in the CYP17A1 gene cluster were strongly associated with T2DM risk in the Chinese Han population. Moreover, we observed that SNP rs17115149 was mainly responsible for the increased risk of T2DM among the hypertension group. Additionally, patients carrying rs12413409 AA genotypes had a higher FBG level and risk of T2DM among males. Consequently, our results indicate that CYP17A1 may be a candidate gene for T2DM susceptibility, and rs17115149 and rs12413409 polymorphisms may play an important role in the progression of T2DM.
Cytochrome P450 family 17 subfamily A member 1 encodes the P450c17 protein and plays a key part in the synthesis and metabolism of steroid hormones (10). Studies have shown that mutations in certain CYP17A1 sites could reduce the expression of P450c17, which may result in impaired androgen, estrogen, and cortisol hormone synthesis, while producing excessive mineralocorticoids, which may cause hypertension, hypokalemia, pseudohermaphroditism, and delayed sexual maturation (11, 12). Furthermore, several genetic association studies suggest that CYP17A1 plays an important role in different pathological conditions, such as visceral and subcutaneous fat accumulation (13), CAD (14), hypertension (12), prostate cancer (15), insulin resistance, and polycystic ovary syndrome (16). There is a strong epidemiologic, clinical, and phenotypic overlap between these conditions and T2DM. Furthermore, a large number of studies have shown that steroids, such as androgen (20), estrogen (21), cortisol (22), and mineralocorticoid (23) play important roles in the pathogenesis of diabetes. This evidence has raised the question of whether CYP17A1 contributed to the development of T2DM. So far, no obvious association between CYP17A1 gene polymorphisms and T2DM incidence has been observed. Wu et al. showed that serum P450c17 expression was lower in type 2 diabetic rats than in the normal control group (24). Ueshiba et al. showed that patients with T2DM had low 17,20-lyase and high 17α-hydroxylase activities (25). In the present study, we conducted bioinformatics analysis by analyzing the high-throughput microarray gene expression database of GDS3782 data, and found that CYP17A1 gene expression was higher in T2DM patients compared with the healthy control group. These findings reveal that CYP17A1 may contribute to the progression of T2DM. To further study the relationship between CYP17A1 genetic polymorphisms and T2DM, we choose three common genetic variants, rs1004467, rs17115149, and rs12413409 of CYP17A1, to explore their effects on risk of T2DM and related traits in the Han Chinese population.
Rs1004467, which is in the intron region of CYP17A1, has been associated with cardiovascular diseases. Specifically, two case–control studies have reported rs1004467 to be significantly associated with CAD in Chinese populations (14, 26). Furthermore, another case–control study found that rs1004467 in CYP17A1 was associated with arterial stiffness in 326 prediabetic and 743 diabetic subjects (27). In our study, we found that rs1004467 was associated with T2DM in the older age group (≥65 years). Our finding suggests that this variant might represent a genetic locus that plays a role in the development and progression of T2DM. Owing to the absence of additional studies on the rs1004467 polymorphism and T2DM, a large sample size association study and meta-analysis on rs1004467 with T2DM should be performed on Han Chinese in the future.
Rs17115149, a functional regulatory SNP, is located at −600 bp before the transcription site within the CpG islands of the CYP17A1 promoter. It has been associated with CYP17A1 RNA expression, and may represent a genetic risk factor for male infertility and testosterone levels (28). Furthermore, it is significantly associated with histologic aggressiveness and may be linked to development of prostate cancer (15). However, an association between rs17115149 and T2DM has never been reported, and its function remains unknown. In this study, we demonstrated that the polymorphism was associated with T2DM and could augment the risk of T2DM in the hypertension group, which suggests its importance in glucose metabolism. Using bioinformatics data from the PROMO transcription factor binding site database, we found that the mutant T allele at the rs17115149 locus allowed binding to the RBP-Jkappa transcription factor. RBP-Jkappa is a transcription-inhibiting factor of many target genes (29, 30) and has also been associated with the occurrence and development of diabetes mellitus (31, 32). Moreover, by analyzing the high-throughput microarray gene expression database of GDS3782 data, CYP17A1 gene expression was found to be higher in T2DM patients than in the healthy control group. Therefore, we hypothesize that the rs17115149 locus, located at the 5′ untranslated region of CYP17A1, may be associated with binding of RBP-Jkappa and, consequently, affect the expression of p450c17 and onset of T2DM. Rs17115149 may influence protein expression also via alternative splicing of mRNA.
The rs12413409 SNP is located in the CYP17A1-CNNM2-NT5C2 gene region on chromosome 10q24.32 and was associated with myocardial infarction (MI) in a Japanese population (33). It has also been associated with waist/hip ratio, heart rate, and MI in a Chinese population (34). Moreover, the association between this SNP and CAD has been confirmed within a Southern Han Chinese study (35). However, no research regarding the association between the SNP and T2DM has been reported so far. In the present study, significant association was found between rs12413409 and T2DM in the Han Chinese population, even after adjusting for age, sex, family history, body mass index, smoking, and drinking. Among non-oral hypoglycemic agent and/or insulin takers, rs12413409 polymorphism was significantly associated with plasma FBG levels, particularly in males. Further research is required to determine whether the association between CYP17A1 and T2DM is mediated through its effect on glucose metabolism.
Although we performed a rigorous case–control study to reveal a link and possible mechanisms between CYP17A1 polymorphisms and T2DM using meticulous multilevel statistics and bioinformatics analysis, there are also several limitations to our study. One of them is the lack of a functional and mechanistic investigation of rs17115149 and rs12413409. Therefore, future functional studies are warranted. In addition, during control group selection, we excluded individuals with a T2DM family history. Even though we adjusted the family history in the association analysis, such exclusion might induce a spurious association as it excludes genetically susceptible controls. Finally, as the association between CYP17A1 polymorphisms and T2DM has not been studied, the result of this study should be confirmed in a larger sample in the future.
In summary, the associations between CYP17A1 polymorphisms and T2DM and FBG levels described in this study have not been reported previously. To the best of our knowledge, this is the first report linking CYP17A1, which shows high affinity for steroid hormone metabolism and has been widely associated with cardiovascular disease, to glucose metabolism and the progression of T2DM.
This study reveals that CYP17A1 rs17115149 and rs12413409 polymorphisms are associated with T2DM in the Han Chinese population. Furthermore, rs17115149 is associated with T2DM in the hypertension subgroup, and rs12413409 is associated with FBG levels. These observations suggest that CYP17A1 polymorphisms could be involved in glucose metabolism and increased risk of T2DM. The findings should be verified in further studies with larger and independent populations.
This study was carried out in accordance with the recommendations of Medical Informed Consent Reference Guide with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committee of Chongqing Medical University.
Y-MN, S-SW, and LW conceived and designed the study, carried out the SNP genotyping and the statistical analysis of the genotype data, and drafted the manuscript. CZ and PW collected the data and contributed to the statistical analyses. S-SW and LZ participated in drafting the manuscript. H-XZ and LW revised the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors particularly thank all volunteers for assisting in collecting the data and samples and participating in the present study in the Second Affiliated Hospital of Chongqing Medical University, Chongqing Zhongshan Hospital, and Chongqing Hospital of Traditional Chinese Medicine.
This work was supported by the grants from the National Natural Science Foundation of China (81202274 and 81372998) and the Funding Project of Taihe Hospital (2018JJXM048).
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fendo.2018.00323/full#supplementary-material.
Figure S1. Linkage disequilibrium (LD) patterns between three genotyped single nucleotide polymorphism (SNPs). LD patterns between genotyped SNPs had shown among the 1,000 genome of Chinese Han population (A), the diabetic group (B), and control group (C).
1. Gaulton KJ. Mechanisms of type 2 diabetes risk loci. Curr Diab Rep (2017) 17:72. doi:10.1007/s11892-017-0908-x
2. Willemsen G, Ward KJ, Bell CG, Christensen K, Bowden J, Dalgård C, et al. The concordance and heritability of type 2 diabetes in 34,166 twin pairs from international twin registers: the discordant twin (DISCOTWIN) consortium. Twin Res Hum Genet (2015) 18:762–71. doi:10.1017/thg.2015.83
3. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet (2012) 44:981–90. doi:10.1038/ng.2383
4. Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, Ferreira T, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet (2014) 46:234–44. doi:10.1038/ng.2897
5. Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature (2016) 536:41–7. doi:10.1038/nature18642
6. Matteson KJ, Picado-Leonard J, Chung BC, Mohandas TK, Miller WL. Assignment of the gene for adrenal P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase) to human chromosome 10. J Clin Endocrinol Metab (1986) 63:789–91. doi:10.1210/jcem-63-3-789
7. Picado-Leonard J, Miller WL. Cloning and sequence of the human gene for P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA (1987) 6:439–48. doi:10.1089/dna.1987.6.439
8. Wang YH, Tee MK, Miller WL. Human cytochrome p450c17: single step purification and phosphorylation of serine 258 by protein kinase a. Endocrinology (2010) 151:1677–84. doi:10.1210/en.2009-1247
9. Tee MK, Miller WL. Phosphorylation of human cytochrome P450c17 by p38α selectively increases 17,20 lyase activity and androgen biosynthesis. J Biol Chem (2013) 288:23903–13. doi:10.1074/jbc.M113.460048
10. Gregory MC, Denisov IG, Grinkova YV, Khatri Y, Sligar SG. Kinetic solvent isotope effect in human P450 CYP17A1-mediated androgen formation: evidence for a reactive peroxoanion intermediate. J Am Chem Soc (2013) 135:16245–7. doi:10.1021/ja4086403
11. Soveid M, Rais-Jalali GA. Seventeen alpha-hydroxylase deficiency associated with absent gonads and myelolipoma: a case report and review of literature. Iran J Med Sci (2016) 41:543–7.
12. Auchus RJ. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J Steroid Biochem Mol Biol (2017) 165:71–8. doi:10.1016/j.jsbmb.2016.02.002
13. Hotta K, Kitamoto A, Kitamoto T, Mizusawa S, Teranishi H, Matsuo T, et al. Genetic variations in the CYP17A1 and NT5C2 genes are associated with a reduction in visceral and subcutaneous fat areas in Japanese women. J Hum Genet (2012) 57:46–51. doi:10.1038/jhg.2011.127
14. Dai CF, Xie X, Yang YN, Li XM, Zheng YY, Fu ZY, et al. Relationship between CYP17A1 genetic polymorphism and coronary artery disease in a Chinese Han population. Lipids Health Dis (2015) 14:16. doi:10.1186/s12944-015-0007-4
15. Han JH, Lee YS, Kim HJ, Lee SY, Myung SC. Association between cytochrome CYP17A1, CYP3A4, and CYP3A43 polymorphisms and prostate cancer risk and aggressiveness in a Korean study population. Asian J Androl (2015) 17:285–91. doi:10.4103/1008-682X.133320
16. Baptiste CG, Battista MC, Trottier A, Baillargeon JP. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol (2010) 122:42–52. doi:10.1016/j.jsbmb.2009.12.010
17. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med (1998) 15:539–53. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0CO;2-S
18. Fan J, Huang X, Chen J, Cai Y, Xiong L, Mu L, et al. Host genetic variants in HLA loci influence risk for hepatitis B virus infection in children. Hepat Mon (2016) 16:e37786. doi:10.5812/hepatmon.37786
19. Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet (2003) 73:1162–9. doi:10.1086/379378
20. Taylor SR, Meadowcraft LM, Williamson B. Prevalence, pathophysiology, and management of androgen deficiency in men with metabolic syndrome, type 2 diabetes mellitus, or both. Pharmacotherapy (2015) 35:780–92. doi:10.1002/phar.1623
21. Weigt C, Hertrampf T, Flenker U, Hülsemann F, Kurnaz P, Fritzemeier KH, et al. Effects of estradiol, estrogen receptor subtype-selective agonists and genistein on glucose metabolism in leptin resistant female Zucker diabetic fatty (ZDF) rats. J Steroid Biochem Mol Biol (2015) 154:12–22. doi:10.1016/j.jsbmb.2015.06.002
22. Yuen KC, Chong LE, Riddle MC. Influence of glucocorticoids and growth hormone on insulin sensitivity in humans. Diabet Med (2013) 30:651–63. doi:10.1111/dme.12184
23. Colussi G, Catena C, Lapenna R, Nadalini E, Chiuch A, Sechi LA. Insulin resistance and hyperinsulinemia are related to plasma aldosterone levels in hypertensive patients. Diabetes Care (2007) 30:2349–54. doi:10.2337/dc07-0525
24. Wu XY, Wang WY, Wang RR, Xie L, Fang ZX, Chen GR. [Ginkgo biloba extract enhances testosterone synthesis of Leydig cells in type 2 diabetic rats]. Zhonghua Nan Ke Xue (2008) 14:371–6.
25. Ueshiba H, Shimizu Y, Hiroi N, Yakushiji F, Shimojo M, Tsuboi K, et al. Decreased steroidogenic enzyme 17,20-lyase and increased 17-hydroxylase activities in type 2 diabetes mellitus. Eur J Endocrinol (2002) 146:375–80. doi:10.1530/eje.0.1460375
26. Dai CF, Xie X, Ma YT, Yang YN, Li XM, Fu ZY, et al. Haplotype analyses of CYP17A1 genetic polymorphisms and coronary artery disease in a Uygur population. J Renin Angiotensin Aldosterone Syst (2015) 16:389–98. doi:10.1177/1470320314565840
27. Yang SJ, Lee ST, Kim WJ, Park SE, Park SW, Kim JW, et al. Genetic variation in CYP17A1 is associated with arterial stiffness in diabetic subjects. Exp Diabetes Res (2012) 2012:827172. doi:10.1155/2012/827172
28. Park JH, Lee J, Kim CH, Lee S. The polymorphism (-600 C>A) of CpG methylation site at the promoter region of CYP17A1 and its association of male infertility and testosterone levels. Gene (2014) 534:107–12. doi:10.1016/j.gene.2013.09.088
29. Oswald F, Winkler M, Cao Y, Astrahantseff K, Bourteele S, Knöchel W, et al. RBP-Jkappa/SHARP recruits CtIP/CtBP corepressors to silence Notch target genes. Mol Cell Biol (2005) 25:10379–90. doi:10.1128/MCB.25.23.10379-10390.2005
30. Wang H, Zang C, Liu XS, Aster JC. The role of Notch receptors in transcriptional regulation. J Cell Physiol (2015) 230:982–8. doi:10.1002/jcp.24872
31. Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, Kitajewski J, et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med (2011) 17:961–7. doi:10.1038/nm.2378
32. Cras-Méneur C, Conlon M, Zhang Y, Pasca DMM, Bernal-Mizrachi E. Early pancreatic islet fate and maturation is controlled through RBP-Jκ. Sci Rep (2016) 6:26874. doi:10.1038/srep26874
33. Matsuoka R, Abe S, Tokoro F, Arai M, Noda T, Watanabe S, et al. Association of six genetic variants with myocardial infarction. Int J Mol Med (2015) 35:1451–9. doi:10.3892/ijmm.2015.2115
34. Wang Y, Wang L, Liu X, Zhang Y, Yu L, Zhang F, et al. Genetic variants associated with myocardial infarction and the risk factors in Chinese population. PLoS One (2014) 9:e86332. doi:10.1371/journal.pone.0086332
Keywords: cytochrome P450 family 17 subfamily A member 1, polymorphism, type 2 diabetes, susceptibility, steroid hormone
Citation: Wang L, Niu Y-M, Wu S-S, Zhang C, Zhou L, Zuo H-X and Wang P (2018) A Study on the Association Between Polymorphisms in the Cytochrome P450 Family 17 Subfamily A Member 1 Gene Region and Type 2 Diabetes Mellitus in Han Chinese. Front. Endocrinol. 9:323. doi: 10.3389/fendo.2018.00323
Received: 13 October 2017; Accepted: 28 May 2018;
Published: 11 June 2018
Edited by:
Mohamed Abu-Farha, Dasman Diabetes Institute, KuwaitReviewed by:
Dubravka Jurišić Eržen, University of Rijeka, CroatiaCopyright: © 2018 Wang, Niu, Wu, Zhang, Zhou, Zuo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Ming Niu, bjRvbmVvbmVAMTI2LmNvbQ==;
Shi-Shi Wu, c3Vuc2hpbmUuYXNodG9uQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.