- 1Laboratory of Human Genetics, Institute of Genetics and Cytology of the National Academy of Sciences of Belarus, Minsk, Belarus
- 2Faculty of Medicine, Vilnius University, Vilnius, Lithuania
- 3Department of Cardiology and Internal Diseases, Belarusian State Medical University, Minsk, Belarus
- 4Department of Cardiology and Rheumatology, Belarusian Medical Academy of Postgraduate Education, Minsk, Belarus
- 5Minsk City Center for Osteoporosis and Bone-Muscular Diseases Prevention, Minsk City Clinical Hospital, Minsk, Belarus
Vitamin D receptor (VDR) is one of the main mediators of vitamin D biological activity. VDR dysfunction might substantially contribute to development of postmenopausal osteoporosis (PMO). Numerous studies have revealed the effects of several VDR gene variants on osteoporosis risk, although significant variation in different ethnicities have been suggested. The main purpose of this work was to assess the frequency of distribution of VDR genetic variants with established effect and evaluate their haplotype association with the risk of PMO in a cohort of Belarusian and Lithuanian women. Case group included women with PMO (n = 149), the control group comprised women with normal bone mineral density (BMD) and without previous fragility fractures (n = 172). Both groups were matched for age, height, sex, and BMI—no statistically significant differences observed. VDR gene polymorphic variants (ApaI rs7975232, BsmI rs1544410, TaqI rs731236, and Cdx2 rs11568820) were determined using polymerase chain reaction and restriction fragment length polymorphism. The lumbar spine (L1-L4) and femoral neck BMD was measured using dual-energy X-ray absorptiometry. Association between each VDR variant and PMO risk was assessed using multiple logistic regression. The genotyping revealed statistically significant difference in the rs7975232 genotype frequencies between the patients and the controls (homozygous C/C genotype was overrepresented in patients, p = 0.008). Patients with osteoporosis were also three times more likely to carry the rs1544410 G/G genotype, when compared to controls. We found that rs7975232, rs1544410, and rs731236 variants were in a strong direct linkage disequilibrium (p < 0.0001), suggesting that risk alleles of these markers are preferably inherited jointly. For the bearers of C-G-C haplotype (consisting of rs7975232, rs1544410, and rs731236 unfavorable alleles), the risk of PMO was significantly higher (OR = 4.7, 95% CI 2.8–8.1, p < 0.0001) compared to controls. This haplotype was significantly over-represented in PMO group compared to all other haplotypes. Our findings highlight the importance of identified haplotypes of VDR gene variants. Complex screening of these genetic markers can be used to implement personalized clinical approach for prevention, treatment, and rehabilitation programs.
Introduction
Vitamin D and its active metabolites are important components of the immune and hormonal systems that not only control phosphorus and calcium homeostasis but also play important role in providing numerous biological effects, involved in the regulation of processes of cell differentiation and proliferation in many target organs and tissues. So-called classical effects of 1.25(OH)2D and other vitamin D metabolites are participated in the processes of mineralization of bone tissue, maintaining calcium homeostasis and, finally, direct effect on bone remodeling mediated through the vitamin D receptor (VDR), encoded by VDR gene. Research published during the past two decades has established that pleiotropic effects of vitamin D and their genetic revelations are associated with a wide variety of diseases (1). This study is focused on association of VDR gene variants with susceptibility to postmenopausal osteoporosis (PMO).
Osteoporosis is characterized by reduced bone mineral density (BMD) and increased bone fragility. Homeostasis of bone tissue during lifetime is mainly maintained by balanced processes of bone resorption and formation, resulting from the combined action of multiple genes and environmental factors. Identification of gene variants, responsible for low BMD, will help to reveal individuals with increased risk of osteoporosis and suggest a personalized clinical approach to prevent or at least to delay the development of this pathology. The prevalence of osteoporosis is different in various ethnicities (2). Evaluation of genetic predisposition to osteoporosis is especially important, because this disease is asymptomatic; the first clinical manifestation in the majority of cases is low energy fractures, and the number of older people, having elevated risk of fragility fractures, is increasing. Postmenopausal women lose important bone protectors (estrogens), and their bone resorption rate increases dramatically (3).
The human VDR gene is located on the short arm of chromosome 12. It consists of 9 exons and encodes a 427 amino acid protein (4). In the VDR gene, several polymorphic sequence variations have been reported, which can occur in coding or noncoding parts of the gene and lead to changes in the protein sequence or affect the degree of gene expression (5). These include single nucleotide polymorphisms (SNP) that can be identified with the appropriate restriction endonuclease enzymes, such as ApaI (rs7975232), BsmI (rs1544410), FokI (rs2228570), TaqI (rs731236), and Cdx2 (rs11568820).
VDR ApaI (rs7975232) gene polymorphism is located in the 3′-regulatory region of VDR gene (in intron 8). There is no functional effect of this variant described, although some authors suggested its effect on mRNA stability (5). It was found that BsmI (rs1544410) variant is significantly associated with the increased risk of developing PMO (6) and antiresorbtive treatment responses (7). VDR TaqI (rs731236) gene polymorphism is located in exon 9 and it has been proved to affect mRNA stability, influencing biological function of vitamin D (8). These three SNPs are located at the 3′-terminus of VDR gene and frequently reported to be in linkage disequilibrium (LD). The FokI (rs2228570) polymorphism is located in the second exon of the VDR gene, plays an important role in post transcriptional processes and causes the production of two different VDR protein variants: long and short variants (5). Compared with the long VDR form, the short form has greater transcriptional activation capability. In meta-analysis, VDR FokI polymorphism was significantly associated with higher risk of developing PMO in Asian, but not in Caucasian populations (6). Cdx2 variant is located in the 5′-promoter region of the VDR gene. The VDR Cdx2 G-variant reduces transcriptional activity of the gene to 70% of the A allele (9).
In recent years, multiple studies have been performed to investigate correlation between VDR gene variants and osteoporosis risk, suggesting the presence of ethnic differences in the genetic association with osteoporosis. But there is still no clear evidence about their effects in performed recent meta-analyses (6, 10–12). Therefore, to improve the significance of associations, it is necessary to perform evaluation of combinations of genetic variants with established effects on independent cohort.
The aim of this study was to compare the associations of selected polymorphic variants within VDR gene with the risk of osteoporosis in Belarusian and Lithuanian postmenopausal women.
Materials and Methods
Subjects and Clinical Assessment
Based on a case–control design, 149 patients with postmenopausal osteoporosis (PMO group) and 172 asymptomatic controls (CON group) participated in the study. One hundred twenty-one Belarusian patients and 127 controls were recruited from Minsk City Center for osteoporosis and bone-muscular diseases prevention (Minsk, Belarus), while 28 Lithuanian patients and 45 controls were recruited from National Osteoporosis Center (Vilnius, Lithuania). All subjects signed written informed consent after being fully informed about the nature of the study according to Helsinki Declaration of 1975, as revised in 2000. Local Research Ethics Committee at the Belarusian Medical Academy of Postgraduate Education and Lithuanian Regional Biomedical Research Ethics Committee approved the study protocol. The exclusion criteria for both groups were chronic diseases, oncology, hypercalcemia, and use of glucocorticosteroids. The women with the clinical diagnosis of osteoporosis (the BMD T-score of −2.5 or lower at the femoral neck or the lumbar spine) and at least 2 years postmenopausal were defined as the patients with PMO. The control group comprised postmenopausal women with BMD T-score of >−2.5 and without previous fragility fractures. The data of the medical history and the fracture history were obtained by a clinical expert.
BMD Measurement
Bone mineral density was measured at the lumbar spine and both proximal femurs using dual-energy X-ray absorptiometry (Prodigy, GE Lunar, Madisson, WI, USA). The lowest value from right or left femur and lumbar spine L1–L4 BMD was taken and used in further comparative analysis.
Genotyping
For genetic analyses, venous blood samples were taken from the cubital vein using the Vacutainer system with EDTA (Beckton-Dickinson, Franklin Lakes, NJ, USA). DNA was isolated from bloodspots dried on special NucleoSafe cards (Macherey-Nagel, Germany) using the standard proteinase K digestion, phenol–chloroform extraction, and ethanol precipitation. The DNA solution was extracted with a phenol–chloroform–isoamyl alcohol mixture to remove protein contaminants and then was precipitated with 100% ethanol. The DNA was pelleted after the precipitation step, washed with 70% ethanol to remove salts and small organic molecules, and resuspended in a buffer at a concentration suitable for further investigation (20–120 ng/µL). The quality and purity of DNA samples were checked using Qubit 2 Fluorimeter (Thermo Fisher Scientific, USA).
Selected polymorphic variants (ApaI rs7975232, BsmI rs1544410, TaqI rs731236, and Cdx2 rs11568820) in VDR gene were determined using the polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) analysis as described earlier (13). Briefly, the PCR reaction system consisted of 10-µL 10 × PCR buffer (1 × buffer = 10 mM Tris–HCl, pH 8.3; 50 mM KCl; 1.25 mM MgCl2), 1.0 µL of 10 × dNTPs (0.2 mM), 1.0 µL of each primer, 0.5 µL of polymerase, 3.5 µL of mQ water, and 10 ng of genomic DNA. The PCR was performed with an initial denaturation at 95°C for 15 min, followed by 28 cycles of denaturation at 99°C for 1 s, annealing at 60°C for 10 s, and extension at 72°C for 10 s. The PCR amplification was carried out in an automated thermal cycler (C1000, Bio-Rad, USA). The final extension was performed at 72°C for 1 min. The PCR products were size-separated by electrophoresis on the 10% polyacrylamide gel at 125 V for 1 h. The 100-bp DNA ladder (Thermo Fisher Scientific, Lithuania) was used to determine the fragments size.
Statistical Analysis
Kolmogorov–Smirnov test was used to assess the normality of data distribution. Normally distributed data are presented as mean and compared using Student’s t-test. The data which are not normally distributed are presented as median (25, 75% interquartile range) and compared using Mann–Whitney U-test.
Based on the determined frequencies of genotypes and by using the Pearson chi-square (χ2) test, the Hardy–Weinberg equilibrium was assessed. Crude odds ratios (OR) were reported with 95% confidence intervals (95% CI) and calculated in comparison to reference (wild-type) genotype. Logistic regression models were used to assess difference between the characteristics of PMO and CON groups for categorical data and for comparison of allele, genotype, and haplotype frequencies between these groups. The statistical analysis was performed using the freely available programming language R (http://r-project.org) with package “SNPassoc” (version 1.9-2). LD between the genetic variants was determined using “haplo.stats” R-package. The differences between the groups were considered statistically significant at p < 0.05.
Results
Participant Characteristics
Each subject was supplied with questionnaire, containing personal information and variables, necessary for the study. The participants within the PMO and CON groups were matched for age, height, sex, and BMI—no statistically significant differences were found (Table 1). Both groups included Belarusian and Lithuanian individuals—postmenopausal women of the same age. The comparison of Belarusian and Lithuanian groups has not revealed any significant differences in alleles and genotypes distribution. The CON group had significantly higher spine and femur neck BMD level compared to PMO group (p < 0.01).
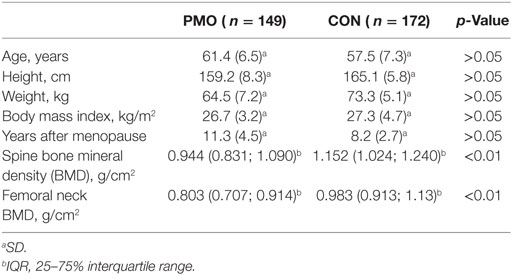
Table 1. Clinical characteristics of analyzed patients with postmenopausal osteoporosis (PMO) and control (CON) groups.
Genotype and Allele Frequencies Distribution of Single VDR Gene Variants
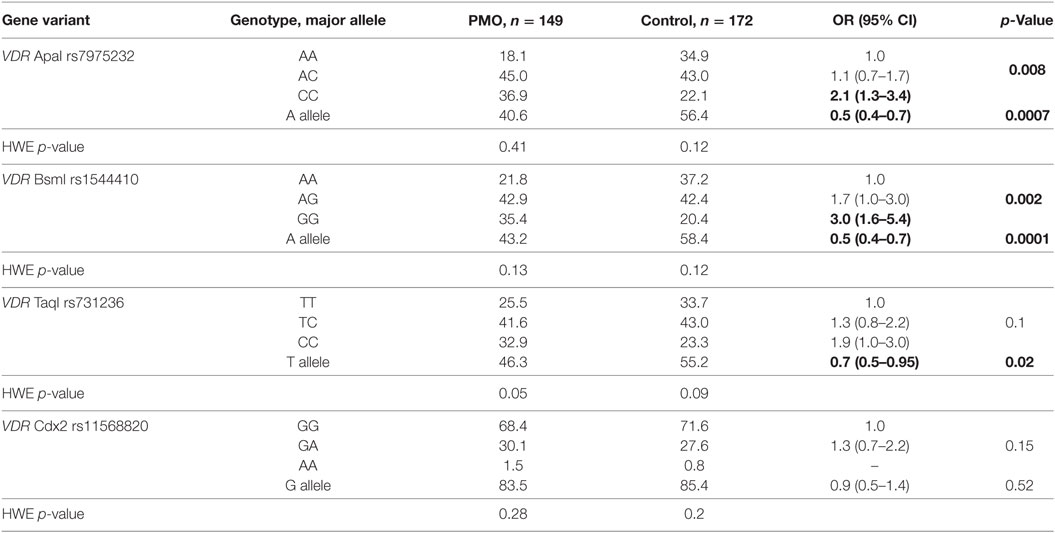
Four VDR gene restriction fragment length polymorphisms (ApaI, BsmI, TaqI, and Cdx2) were selected following the literature review for consideration in Caucasian population (5, 6, 14, 15). Search of risk alleles and assessment of their combined action in present study was performed on the independent cohort. The genotype and allele frequencies of the analyzed VDR gene single SNPs are presented in Table 2. The distribution of all four analyzed gene polymorphisms in controls and in patients with postmenopausal osteoporosis was in correspondence with the one expected from the Hardy–Weinberg equilibrium (p > 0.05 in all cases).
There was a statistically significant difference in genotype and allele distribution for VDR ApaI, BsmI, and TaqI gene variants revealed between PMO and CON groups (Table 2). The CC genotype of VDR ApaI was significantly over-represented in PMO (36.9%) group when compared to the CON (22.1%) group (OR = 2.1, 95% CI 1.3–3.4, p = 0.008). The CON group individuals were more likely to carry VDR ApaI A allele (56.4%), compared to the PMO group (40.6%, OR = 0.5, 95% CI 0.4–0.7, p = 0.0007). The differences in the genotype (p = 0.002) and allele (p = 0.0001) frequency distributions of the VDR BsmI gene variant between the PMO and CON groups were also significant. More specifically, the risk of PMO was significantly higher for the bearers of GG-genotype compared to reference wild-type AA-genotype (OR = 3.0, 95% CI 1.6–5.4, p = 0.002).
There was, however, no statistically significant difference in VDR Taq genotype distribution (p = 0.1), though the T allele was over-represented in the asymptomatic CON (55.2%) group compared to the PMO (46.3%) group (OR = 0.7, 95% CI 0.5–0.95) and the C-allele was over-represented in the PMO (53.7%) group compared to the CON (44.8%) group (OR = 1.4, 95% CI 1.1–2.0, p = 0.02 in both cases). No significant differences in the VDR Cdx2 genotype distribution (p = 0.15) and allele frequencies (p = 0.52) between the PMO and CON groups were observed.
LD Analysis of VDR Gene
The results of LD analysis are presented in the Figure 1. LD plot was constructed using combined genotype data from PMO and CON groups. The three SNPs, ApaI (A/C), BsmI (A/G), and TaqI (T/C) of VDR gene are in a very strong LD (the measure D’ is very close to 1, p = 0.01). The positive coefficient of correlation r suggests that major alleles of VDR ApaI, BsmI, and TaqI gene variants are likely to be inherited together, as well as minor alleles.
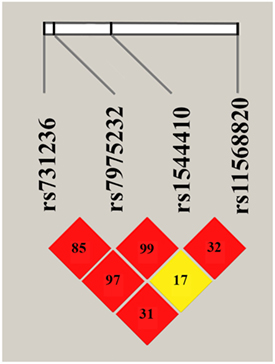
Figure 1. Linkage disequilibrium plot for VDR ApaI, BsmI, TaqI, and Cdx2 variants. The LD plot was build using combined genotype data from postmenopausal osteoporosis and control groups (constructed with “haplo.stats” package for R). LD is displayed as pairwise D’ values multiplied by 100 and given for each single nucleotide polymorphism combination within each cell. Red cells correspond to p < 0.0001, yellow—p > 0.1.
No significant LD was found between VDR Cdx2 and other three analyzed gene variants. Thus, two haplotypes A-A-T and C-G-C are inferred with high probability from revealed VDR polymorphisms with high D’ measure, allowing further complex analysis of allelic combinations.
Haplotype Analysis
The haplotype analysis was performed for three VDR gene polymorphisms: ApaI, BsmI, and TaqI (Figure 2). Haplotypes were constructed from all possible allelic combinations of three VDR polymorphisms and compared between the PMO and CON groups.
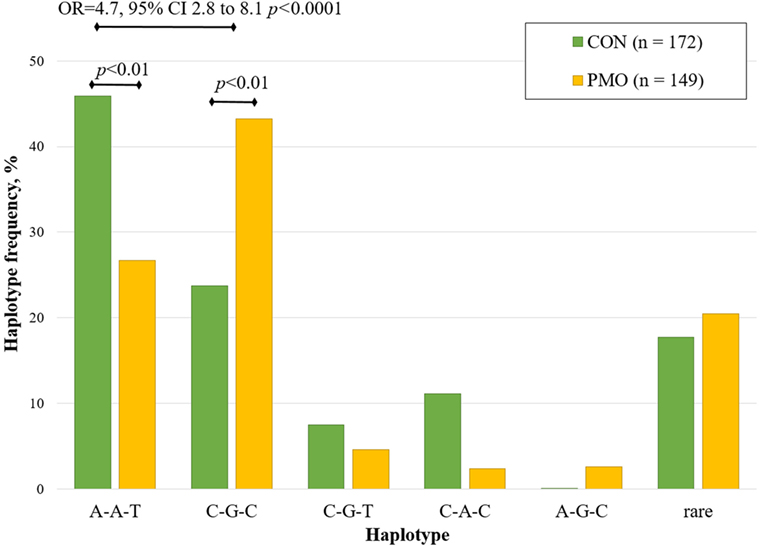
Figure 2. Estimated haplotype frequency distribution of VDR ApaI, BsmI, and TaqI variants in the postmenopausal osteoporosis (PMO) and control (CON) groups.
Five haplotypes (A-A-T, C-G-C, C-A-C, A-G-C, and C-G-T) of the possible eight combinations were inferred at a frequency of greater than 5%. The A-A-T haplotype was the most frequent haplotype (total frequency 36.8%), constructed from three wild-type allele variants. This haplotype was significantly over-represented in CON group (46.0%) compared to the PMO group (26.7%, p < 0.01). The total frequency of C-G-C haplotype was 32.4%, it was significantly under-represented in CON group (23.7%) compared to PMO group (43.2%, p < 0.01). For the bearers of C-G-C haplotype, the risk of PMO was significantly higher compared to the reference (wild-type) haplotype (OR = 4.7, 95% CI 2.8–8.1, p < 0.0001). None significant association was found for other revealed haplotypes.
Discussion
This case–control study was performed to investigate the role of VDR gene in PMO in combined cohorts of Caucasian (Belarusian and Lithuanian) women. Because PMO is a multifactorial disorder, genetic variants within the VDR gene, which is an important regulator of bone remodeling, may be a contributing risk factor. For the best of our knowledge, this is the first study demonstrating an association of four VDR variants with PMO risk in cohort of Belarusian and Lithuanian patients.
VDR gene variants were selected from key publications with established associations with PMO (5, 6, 14, 15) and analyzed in independent population for assessing their combined action. A total of four polymorphic variants of VDR gene were selected and evaluated for this study. These variants are established as PMO risk factors, so we are mainly interested to know if their effect is similar to other populations or may be different and what will be their combined action.
The cases and controls involved in the analysis were well defined with similar inclusion criteria. The descriptive analysis of our random selection of subjects showed that PMO and CON groups were matched for age, height, weight, sex, and BMI. Spine and femoral BMD levels were significantly different between PMO and CON groups. The observed genotype frequencies in CON group did not deviate from Hardy–Weinberg equilibrium (p > 0.05 in all cases), while in PMO group it deviated (VDR Taq, p = 0.05). We also revealed significant differences in VDR polymorphic alleles (ApaI, BsmI, and TaqI) and genotypes (ApaI and BsmI) frequency distribution between PMO and CON groups.
Obtained genotype and allele frequencies in CON group (Table 2) for VDR ApaI were close to those of Caucasian subjects reported in HapMap (16), for VDR BsmI—in British (17), Spanish (18), and Slovenian (19) populations, VDR TaqI—in Czech population (20), VDR Cdx2—in Slovenian population (19). The contrast with the data reported in other Caucasian studies (6) is likely related to both ethnic differences and differences in inclusion criteria.
The main finding of single VDR gene variants association analysis is that the presence of VDR ApaI CC and BsmI GG homozygous genotypes is significantly associated with increased PMO risk, being over-represented in PMO group. By comparison of VDR TaqI allele frequencies in CON and PMO groups, we found that allele C also represented a risk factor (OR = 1.4, p = 0.02). This data are in accordance with the data reported in meta-analysis (6), where ApaI and BsmI were the most frequent markers, associated with PMO risk. Currently it is accepted that VDR BsmI polymorphism is related to BMD level, but its effect is relatively small and strongly influenced by external factors like diet (1). Unlike VDR BsmI, ApaI, and TaqI polymorphisms affect mRNA stability, which results in change of biological functions of vitamin D (6, 21).
The absence of significant association for VDR Cdx2 may be explained by very low number of individuals with AA-genotypes (less than five individuals in each subgroup).
As shown in Figure 1, three of four investigated variants were in strong LD. This finding is in agreement with previous studies that have found strong linkage between ApaI, BsmI, and TaqI variants of VDR gene located within the chromosomal region 12q12. The greatest degree of LD was found between ApaI and BsmI, followed by BsmI and TaqI, and then ApaI and TaqI. The same trend was reported in the meta-study for Caucasian populations (22), as well as for British population (23), opposite trend observed in Italian population (24). Most likely, the strong LD coefficient may be explained by the location of all three markers in the ninth exon of VDR gene, as well as by the adaptive advantage of a particular allelic combination. The absence of LD between Cdx2 (located in the promoter region of VDR gene) and other three variants is in good accordance with the literature (23).
Positive correlation between VDR ApaI, BsmI, and TaqI variants suggests that their major alleles are associated together, making them likely to be inherited jointly. Using logistic regression, we have analyzed the global distribution of all revealed haplotypes in PMO and CON groups. Inferred haplotypes of ApaI, BsmI, and TaqI variants were significantly associated with PMO risk (global haplotype association p < 0.0001).
The most frequent haplotype was wild-type A-A-T (36.8%) followed by C-G-C (32.4%). These haplotypes were more common than other haplotypes in CON and PMO groups. Very close haplotype frequency distribution was reported for Caucasian (22), Dutch (25), and Italian (24) women. By excluding too rare haplotypes, most common were compared with reference haplotype A-A-T. The data show that for the bearers of non-favorable haplotype C-G-C, the risk of PMO is significantly higher. No protective variants were revealed in this study.
It is necessary to mention that, in contrast to ApaI and BsmI, VDR TaqI variant is constituted by the silent replacement of thymine (T) by cytosine (C) and does not change the amino acid sequence of VDR protein (5). This evidence partly contradicts with the result of this study, where significant association of PMO risk with single VDR TaqI allele variants or VDR ApaI-BsmI-TaqI haplotypes was found. These three SNPs are located at the 3′ end of the VDR gene and are at very high degree of LD. Therefore, with high probability the risk allele of TaqI variant is inherited together with risk alleles of ApaI and BsmI variants, and vice versa. For this reason, the effect of VDR TaqI variant can be explained by strong LD, when they are found in specific haplotypes, and due to the effects of ApaI and BsmI polymorphisms on PMO risk. Another possible explanation of VDR TaqI significant association with PMO risk are epigenetic (methylation) processes (26).
Considering the contrasting results in different studies of the genetic predisposition to osteoporosis, it seems that the best interpretations are possible if a complex of genetic variants and haplotypes is evaluated. In addition, it is very important to analyze the association of established risk variants on independent cohort, which will help to escape risk score summarization paradox. The present study is the first such research, performed on independent cohorts for evaluation of combined effect of different genetic variants with known associations, as well as to assess if the overall pattern of association is similar to other populations. Moreover, basic studies will help to reveal a deeper knowledge of the molecular mechanisms of bone disorders regulated by vitamin D through its receptors.
The limitations of current study include a low number of Lithuanian participants, which is not sufficient for comparison of VDR variants genotype distribution between Lithuanian and Belarusian populations. Also, the sample size was not enough big to perform the adjustments for additional covariates (such as BMI, height, weight, vitamin D level, fractures incidence). Further, well-designed studies with larger sample sizes of both ethnic populations will clarify data, obtained in present studies.
Thus, the most important result of this study is that for the women-bearers of C-G-C haplotype, there is a 4.7-fold risk of developing PMO. Our study suggests that variants of VDR gene are associated with the risk of PMO, although different polymorphisms might have different influences. The overall pattern of known VDR markers of PMO risk in combined Belarusian and Lithuanian population is close to other Caucasian populations. Further work will also include analysis of interaction between VDR variants and other genetic markers, playing important roles in genetic predisposition to osteoporosis.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
PM collected the data, performed statistical analysis, and drafted the research paper. ER, IM, MT, and VA supervised and guided the design and analysis of the research project. AR and VS collected patient sample and performed clinical survey of Belarusian patients. MT collected patient sample and performed clinical survey of Lithuanian patients. KK performed DNA extraction and genotyping. All authors were involved in drafting and revising the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors would like to thank all participants of the study.
Funding
This study was performed within joint Belarusian-Lithuanian cooperation program in science and technology and supported by State Committee on Science and Technology of the Republic of Belarus (No. B11LIT-017, B17LITG-008) and Research Council of Lithuania (No. TAP-21/2011, TAP-LB-17-005).
References
1. Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta (2006) 371(1–2):1–12. doi:10.1016/j.cca.2006.02.016
2. Leslie WD. Clinical review: ethnic differences in bone mass – clinical implications. J Clin Endocrinol Metab (2012) 97(12):4329–40. doi:10.1210/jc.2012-2863
3. Baill IC, Castiglioni A. Health maintenance in postmenopausal women. Am Fam Physician (2017) 95(9):561–70.
4. Miyamoto K, Kesterson RA, Yamamoto H, Taketani Y, Nishiwaki E, Tatsumi S, et al. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol (1997) 11(8):1165–79. doi:10.1210/mend.11.8.9951
5. Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene (2004) 338(2):143–56. doi:10.1016/j.gene.2004.05.014
6. Zhang L, Yin X, Wang J, Xu D, Wang Y, Yang J, et al. Associations between VDR gene polymorphisms and osteoporosis risk and bone mineral density in postmenopausal women: a systematic review and meta-analysis. Sci Rep (2018) 8(1):981. doi:10.1038/s41598-017-18670-7
7. Creatsa M, Pliatsika P, Kaparos G, Antoniou A, Armeni E, Tsakonas E, et al. The effect of vitamin D receptor BsmI genotype on the response to osteoporosis treatment in postmenopausal women: a pilot study. J Obstet Gynaecol Res (2011) 37(10):1415–22. doi:10.1111/j.1447-0756.2011.01557.x
8. Fang Y, van Meurs JB, d’Alesio A, Jhamai M, Zhao H, Rivadeneira F, et al. Promoter and 3’-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the Rotterdam study. Am J Hum Genet (2005) 77(5):807–23. doi:10.1086/497438
9. Arai H, Miyamoto KI, Yoshida M, Yamamoto H, Taketani Y, Morita K, et al. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J Bone Miner Res (2001) 16(7):1256–64. doi:10.1359/jbmr.2001.16.7.1256
10. Zintzaras E, Rodopoulou P, Koukoulis GN. BsmI, TaqI, ApaI and FokI polymorphisms in the vitamin D receptor (VDR) gene and the risk of osteoporosis: a meta-analysis. Dis Markers (2006) 22(5–6):317–26. doi:10.1155/2006/921694
11. Jia F, Sun RF, Li QH, Wang DX, Zhao F, Li JM, et al. Vitamin D receptor BsmI polymorphism and osteoporosis risk: a meta-analysis from 26 studies. Genet Test Mol Biomarkers (2013) 17(1):30–4. doi:10.1089/gtmb.2012.0267
12. Qin G, Dong Z, Zeng P, Liu M, Liao X. Association of vitamin D receptor BsmI gene polymorphism with risk of osteoporosis: a meta-analysis of 41 studies. Mol Biol Rep (2013) 40(1):497–506. doi:10.1007/s11033-012-2086-x
13. Marozik P, Mosse I, Alekna V, Rudenko E, Tamulaitiene M, Ramanau H, et al. Association between polymorphisms of VDR, COL1A1, and LCT genes and bone mineral density in Belarusian women with severe postmenopausal osteoporosis. Medicina (Kaunas) (2013) 49(4):177–84. doi:10.3390/medicina49040028.
14. Ralston SH. Genetics of osteoporosis. Ann N Y Acad Sci (2010) 1192:181–9. doi:10.1111/j.1749-6632.2009.05317.x
15. Ji GR, Yao M, Sun CY, Li ZH, Han Z. BsmI, TaqI, ApaI and FokI polymorphisms in the vitamin D receptor (VDR) gene and risk of fracture in Caucasians: a meta-analysis. Bone (2010) 47(3):681–6. doi:10.1016/j.bone.2010.06.024
16. The International HapMap Consortium. A haplotype map of the human genome. Nature (2005) 437(7063):1299–320. doi:10.1038/nature04226
17. Houston LA, Grant SF, Reid DM, Ralston SH. Vitamin D receptor polymorphism, bone mineral density, and osteoporotic vertebral fracture: studies in a UK population. Bone (1996) 18(3):249–52. doi:10.1016/8756-3282(95)00483-1
18. Fontova Garrofe R, Gutierrez Fornes C, Broch Montane M, Aguilar Crespillo C, Pujol del Pozo A, Vendrell Ortega J, et al. [Polymorphism of the gene for vitamin D receptor, bone mass, and bone turnover in women with postmenopausal osteoporosis]. Rev Clin Esp (2000) 200(4):198–202. doi:10.1016/S0014-2565(00)70605-9
19. Mencej-Bedrac S, Prezelj J, Kocjan T, Teskac K, Ostanek B, Smelcer M, et al. The combinations of polymorphisms in vitamin D receptor, osteoprotegerin and tumour necrosis factor superfamily member 11 genes are associated with bone mineral density. J Mol Endocrinol (2009) 42(3):239–47. doi:10.1677/JME-08-0108
20. Zajickova K, Zofkova I, Bahbouh R, Krepelova A. Vitamin D receptor gene polymorphisms, bone mineral density and bone turnover: FokI genotype is related to postmenopausal bone mass. Physiol Res (2002) 51(5):501–9.
21. Sassi R, Sahli H, Souissi C, Sellami S, Ben Ammar El Gaaied A. Polymorphisms in VDR gene in Tunisian postmenopausal women are associated with osteopenia phenotype. Climacteric (2015) 18(4):624–30. doi:10.3109/13697137.2015.1007123
22. Thakkinstian A, D’Este C, Attia J. Haplotype analysis of VDR gene polymorphisms: a meta-analysis. Osteoporos Int (2004) 15(9):729–34. doi:10.1007/s00198-004-1601-x
23. Chen L, Davey Smith G, Evans DM, Cox A, Lawlor DA, Donovan J, et al. Genetic variants in the vitamin d receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer Epidemiol Biomarkers Prev (2009) 18(11):2874–81. doi:10.1158/1055-9965.EPI-09-0544
24. Colombini A, Brayda-Bruno M, Lombardi G, Croiset SJ, Ceriani C, Buligan C, et al. BsmI, ApaI and TaqI polymorphisms in the vitamin D receptor gene (VDR) and association with lumbar spine pathologies: an Italian case-control study. PLoS One (2016) 11(5):e0155004. doi:10.1371/journal.pone.0155004
25. Uitterlinden AG, Weel AE, Burger H, Fang Y, van Duijn CM, Hofman A, et al. Interaction between the vitamin D receptor gene and collagen type I alpha 1 gene in susceptibility for fracture. J Bone Miner Res (2001) 16(2):379–85. doi:10.1359/jbmr.2001.16.2.379
Keywords: vitamin D receptor, genetic variants, polymorphism, haplotype, postmenopausal osteoporosis
Citation: Marozik P, Tamulaitiene M, Rudenka E, Alekna V, Mosse I, Rudenka A, Samokhovec V and Kobets K (2018) Association of Vitamin D Receptor Gene Variation With Osteoporosis Risk in Belarusian and Lithuanian Postmenopausal Women. Front. Endocrinol. 9:305. doi: 10.3389/fendo.2018.00305
Received: 02 April 2018; Accepted: 23 May 2018;
Published: 05 June 2018
Edited by:
Pawel Pludowski, Children’s Memorial Health Institute, PolandReviewed by:
Ihor Shymanskyi, Palladin Institute of Biochemistry (NAS Ukraine), UkraineJacek Lukaszkiewicz, Medical University of Warsaw, Poland
Copyright: © 2018 Marozik, Tamulaitiene, Rudenka, Alekna, Mosse, Rudenka, Samokhovec and Kobets. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavel M. Marozik, cC5tYXJvemlrQGlnYy5ieQ==
 Pavel M. Marozik
Pavel M. Marozik Marija Tamulaitiene
Marija Tamulaitiene Ema Rudenka
Ema Rudenka Vidmantas Alekna
Vidmantas Alekna Irma Mosse
Irma Mosse Alena Rudenka
Alena Rudenka Volha Samokhovec
Volha Samokhovec Katsiaryna Kobets
Katsiaryna Kobets