- 1University of Arizona, Tucson, AZ, United States
- 2Lovelace Respiratroy Research Institute, Albuquerque, NM, United States
- 3Regina Montis Regalis Hospital, Mondovì, Italy
- 4University Hospital of Padua, Padua, Italy
- 5San Martino Hospital Istituto di Ricovero e Cura a Carattere Scientifico, Genoa, Italy
- 6Agostino Gemelli University Polyclinic, Catholic University of the Sacred Heart, Rome, Italy
- 7Sapienza University of Rome, Rome, Italy
- 8University of Ferrara, Ferrara, Italy
- 9University of Pavia, Pavia, Italy
- 10Azienda Socio Sanitaria Territoriale Bergamo EST, Seriate, Italy
- 11Ospedale Papa Giovanni XXIII, Bergamo, Italy
- 12Azienda Unità Sanitaria Locale-Istituto di Ricovero e Cura a Carattere Scientifico di Reggio Emilia, Reggio Emilia, Italy
- 13Marche Polytechnic University, Ancona, Italy
- 14Department of Medical Sciences, San Giuseppe Hospital MultiMedica IRCCS, Milan, Italy
- 15Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 16San Luca Hospital, Vallo della Lucania, Italy
- 17Department of Infectious Diseases, Colli Hospital, Naples, Italy
- 18L. Pierantoni GB Morganis Hospital, Forlì, Italy
- 19Mauro Scarlato Hospital, Azienda Sanitaria Locale Salerno, Scafati, Italy
- 20ASL Lanciano Vasto Chieti, Chieti, Italy
- 21University Hospital Major of Charity of Novara, Novara, Italy
- 22Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
- 23University of Milan, Milan, Italy
Background: Italy has one of the world's oldest populations, and suffered one the highest death tolls from Coronavirus disease 2019 (COVID-19) worldwide. Older people with cardiovascular diseases (CVDs), and in particular hypertension, are at higher risk of hospitalization and death for COVID-19. Whether hypertension medications may increase the risk for death in older COVID 19 inpatients at the highest risk for the disease is currently unknown.
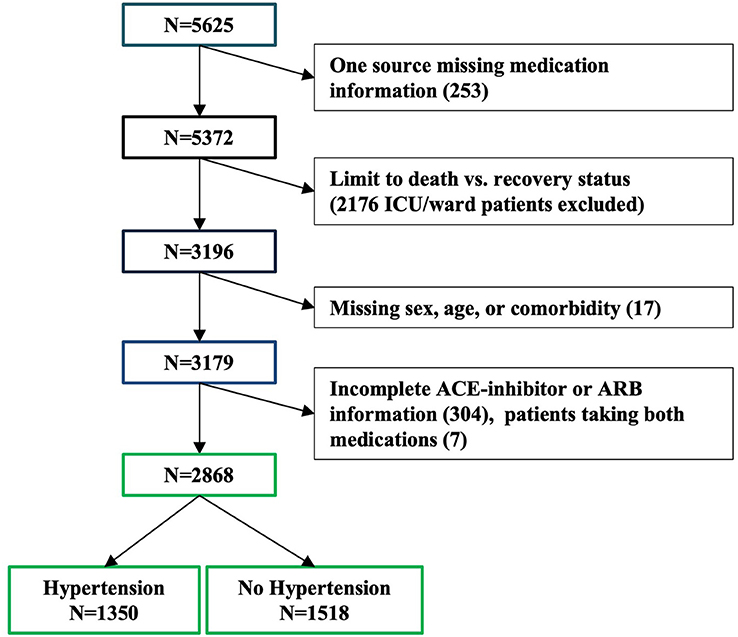
Methods: Data from 5,625 COVID-19 inpatients were manually extracted from medical charts from 61 hospitals across Italy. From the initial 5,625 patients, 3,179 were included in the study as they were either discharged or deceased at the time of the data analysis. Primary outcome was inpatient death or recovery. Mixed effects logistic regression models were adjusted for sex, age, and number of comorbidities, with a random effect for site.
Results: A large proportion of participating inpatients were ≥65 years old (58%), male (68%), non-smokers (93%) with comorbidities (66%). Each additional comorbidity increased the risk of death by 35% [adjOR = 1.35 (1.2, 1.5) p < 0.001]. Use of ACE inhibitors, ARBs, beta-blockers or Ca-antagonists was not associated with significantly increased risk of death. There was a marginal negative association between ARB use and death, and a marginal positive association between diuretic use and death.
Conclusions: This Italian nationwide observational study of COVID-19 inpatients, the majority of which ≥65 years old, indicates that there is a linear direct relationship between the number of comorbidities and the risk of death. Among CVDs, hypertension and pre-existing cardiomyopathy were significantly associated with risk of death. The use of hypertension medications reported to be safe in younger cohorts, do not contribute significantly to increased COVID-19 related deaths in an older population that suffered one of the highest death tolls worldwide.
Introduction
Italy, after Japan, tops the list of the world's oldest countries, with over 22% of its population aged 65 or older (1). Italy has been one of the hardest hit countries during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. As of September 1 2020 over 35,500 persons had died due to Coronavirus disease 2019 (COVID-19), especially in the northern regions of the country, with 84.5% of deaths occurring in patients age 70 or older (Istituto Superiore di Sanitá, ISS, https://www.epicentro.iss.it/coronavirus/), and a crude case fatality rate in the region Lombardy of 18.3% (2). Previous study showed that comorbid conditions play a relevant role in increasing the risk of death in patients with COVID-19 (3–9). In particular, hypertension and underlying cardiovascular diseases (CVDs) have been strongly associated with death in COVID-19 inpatients (7, 10, 11), and case fatality rates tend to be high in older people and hypertensive individuals (12). Indeed, the prevalence of CVDs in COVID-19 patients across studies ranges from 8 to 42% (13). Hypertension, heart failure (HF) and ischemic heart disease are often treated with renin-angiotensin-aldosterone system (RAAS) blockers such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). The use of ACE inhibitors/ARBs in patients with COVID-19 or at risk of infection with the virus is currently a subject of intense debate (14, 15), due to the evidence that SARS-CoV-2 uses the ACE2 receptor for entry into target cells (16). ACE2 and its related axis are an endogenous counter-regulatory system, with effects opposite to those of the ACE axis (17, 18). Nonetheless, ACE inhibitor/ARBs have also been associated with a reduction of mortality and re-hospitalization in patients with cardiovascular diseases due to their anti-thrombotic and anti-inflammatory effects, and protective effects against endothelial dysfunction (13). Whether the use of ACE inhibitors and ARB affects the mortality of COVID-19 patients has been debated. The only and largest survey published so far assessing the association between comorbidities, use of ACE inhibitors/ARBs, and COVID-19 death included 4,480 patients from Denmark (12). The authors found no evidence that either ACE inhibitors or ARB increased the risk for death among persons hospitalized for COVID-19. However, the COVID-19-related death burden in Denmark has been tremendously lower than Italy (628 vs. 35,595, respectively, as of September 1 2020) which poses questions on the heterogeneity of the Italian and Danish populations and the ways COVID-19 hit and was handled by the two countries. A second study from China also excluded subjects aged ≥75 years (19). To date, there is no study available of the relation between chronic use of ACE inhibitors and ARB, considered separately, and mortality in hospitalized COVID-19 patients that includes sufficient patients in the older age group, and that accounts for concomitant cardiovascular therapies or comorbid conditions. Moreover, due to higher fatality of COVID-19 infection in patients affected by CVDs, there is an unmet need to understand the link between cardiovascular therapies, CVD and COVID-19 severity and mortality.
In this study, we describe baseline characteristics and factors associated with death among 3,179 patients hospitalized for COVID-19, who were either discharged or died, and who were residents of 19 out of Italy's 21 regions, including the main islands. In assessing potential risks for death, we specifically assessed the comorbidities and the role of pre-hospitalization ACE inhibitors and ARBs and other commonly used CVD medications (such as beta-blockers, calcium channel blockers and diuretics). By age distribution, our sample is representative of older people, that are most severely affected by COVID-19 since the beginning of the pandemic (5, 6).
Methods
Patient Inclusion
Data for 5,625 patients hospitalized for COVID-19 and with a positive nasopharyngeal swab for SARS-CoV-2 virus were manually extracted from medical charts from 61 hospitals across Italy. Patients were included in this analysis if they had been either discharged or had died at the time of ascertainment (n = 3,179, Figure 1, 56 sites). The status for each patient was reported at the time of data collection by the local investigators and represents an assessment of the patient's condition between March 25 and April 22, 2020. All the patients' information was obtained by manual review of the medical charts by the attending physician or nurse during their shifts. Each participating center was provided, upon enrollment, with a database to fill with patients' demographic, social, and clinical information and detailed instructions about the data collection. Smoking history was manually extracted from the chart for each patient. Information about smoking was not available for 316 patients. The collection and analysis of data in the registry have been deemed exempt from ethics review.
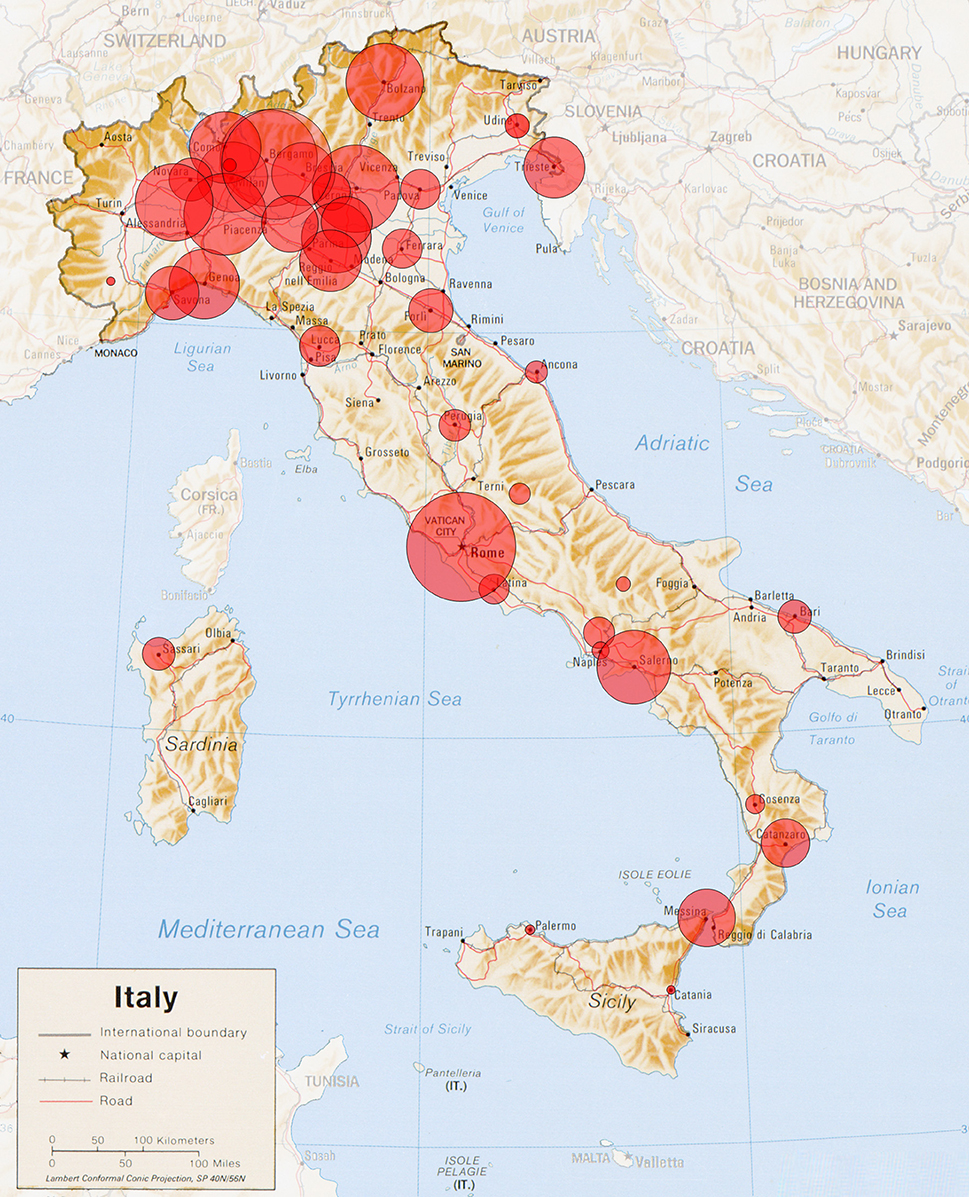
Figure 1. Italian Cartographic representation of the study subjects: Cartographic representation of the patients in this study cohort, with the area of each red circle proportional to the combined number of patients from each compact metropolitan area.
Comorbidities
Investigators manually extracted information about preexisting comorbidities known or suspected to be associated with COVID-19 mortality from the chart of each patient that was still hospitalized in their hospital or discharged within 30 days from the collection of the data. Information was available for atrial fibrillation, blood cancer, organ cancer, coronary artery disease, cardiomyopathy, chronic heart failure, chronic obstructive pulmonary disease (COPD), chronic renal failure, diabetes, hypertension, obesity, and stroke. We used a count of the reported number of comorbidities for each patient to assess their combined effect on mortality. Patients missing comorbidity information were excluded from these analyses (n = 17, Figure 2).
Cardiovascular Medications
For this study, we specifically targeted extraction of detailed information from the patient's chart regarding use of ACE inhibitors and ARB at the time of admission. We also extracted information about other medications usually prescribed for hypertension (beta-blockers, diuretics, and Ca-antagonists).
Statistics
A generalized linear mixed model, mixed-effects logistic regression, was used to assess the relations of sex, age, comorbidity count and hypertension medication use to death relative to recovery (STATA 16, StataCorp, College Station, TX, USA). The primary outcome was inpatient mortality. Since data were clustered by hospital site, site was included in the models as a random effect to account for potential within site correlation of patient characteristics. The number of patients contributed by each hospital site varied, ranging from 2 to 242 patients (Supplementary Figure 1). A dummy category for those patients missing smoking information was included in the model for Figure 3.
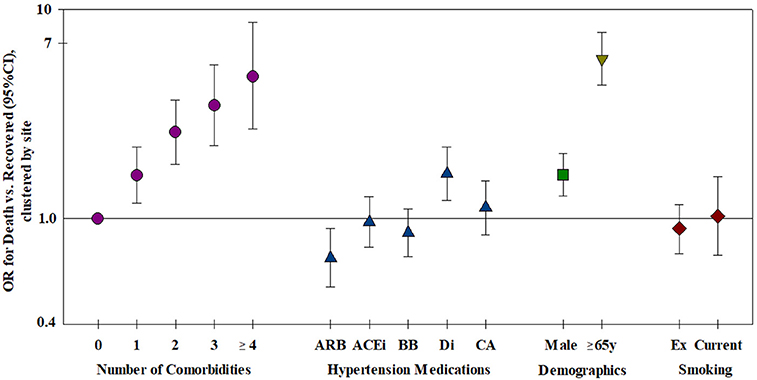
Figure 3. Risk factors for mortality—all risk factors were included in the model, clustered by site (n = 2,868). ARB, Angiotensin receptor blocker; ACEi, Angiotensin converting enzyme inhibitor; BB, Beta-blocker; Di, Diuretic; CA, Ca-antagonist.
Results
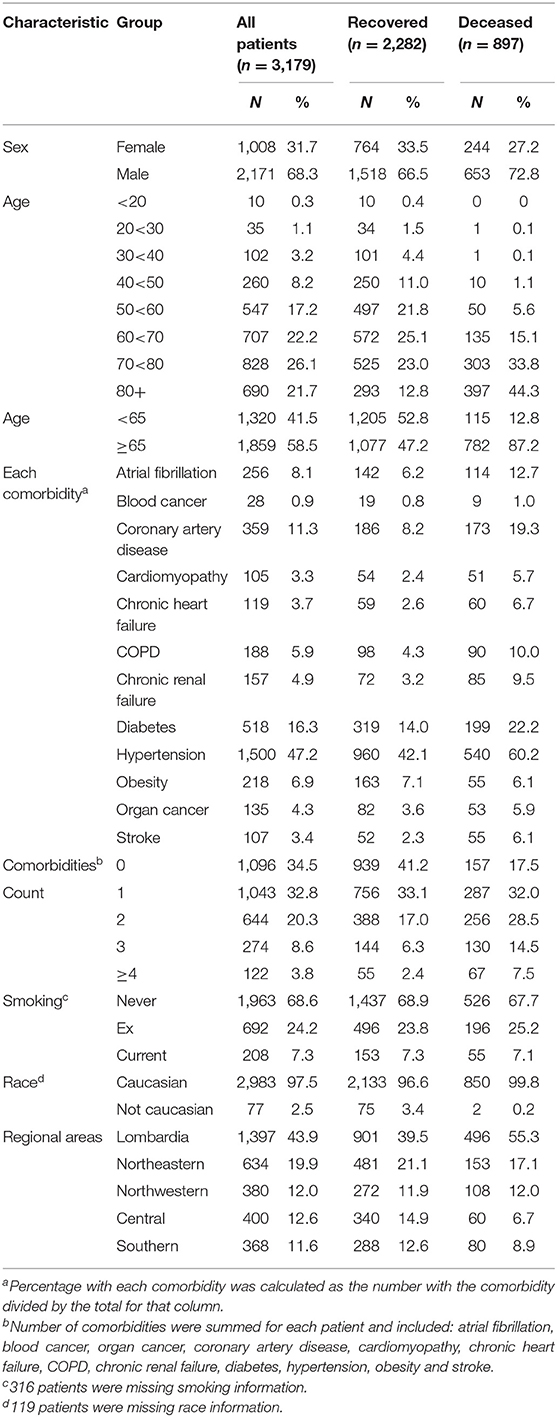
There were 3,179 patients with complete data for sex, age, status, and comorbidities (Table 1); 2,282 (71.8%) had been discharged from the hospital and 897 (28.2%) had died. The median age was 69.0 years, with an interquartile range of 57 to 78 years (Supplementary Figure 2).
Risk Factors for Death
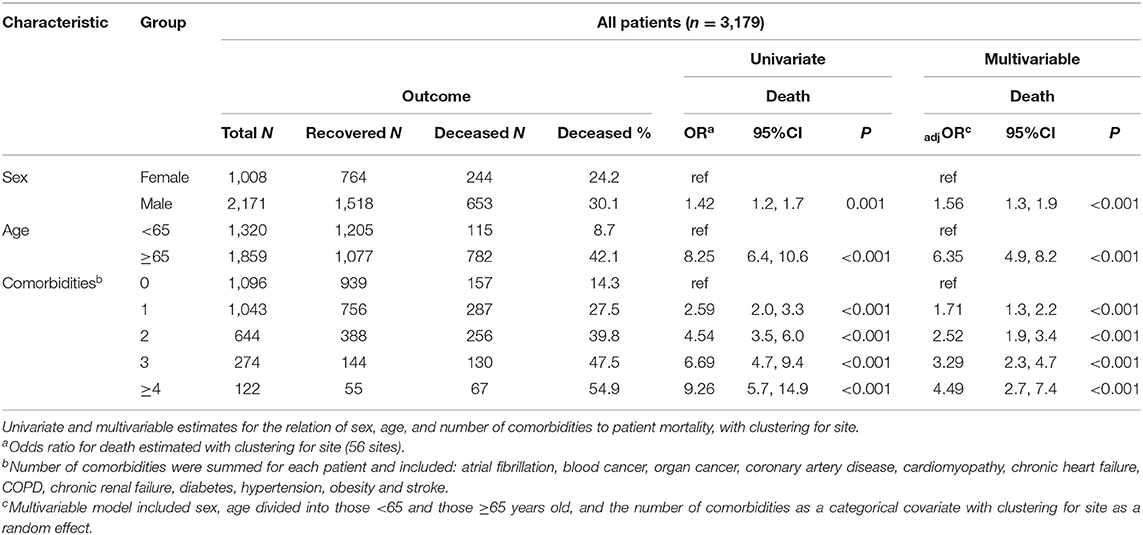
The relation of age and death was non-linear; very few patients under the age of 50 died (Table 1). Males were more likely to die than females and patients aged ≥65 years were over six times more likely to die compared to younger patients (Table 2). Current smoking was unrelated to death in either univariate or multivariable analyses (Figure 3). Hypertension (47.2%) was the most frequent comorbidity, followed by diabetes (16.3%), coronary artery disease (11.3%), atrial fibrillation (8.1%), and obesity (6.9%) (Table 1). In a model including all comorbidities and adjusted for sex, age and site, the comorbidities cardiomyopathy, COPD, chronic renal failure, hypertension, obesity, organ cancer, and stroke were all independent risk factors for death (Table 3). Interestingly, while only 5.9% of COVID-19 inpatients had COPD, the latter was highly and significantly associated with increased risk of death (Table 3). In addition to evaluating the relation of the individual comorbidities to death, the count of comorbidities reported for each patient was strongly associated with risk of death (Table 2), with the odds for death increasing by 35% for each additional comorbidity (evaluated as an ordinal count; adjOR =1.35 [1.2, 1.5] p < 0.001), after adjusting for sex, age, and site.
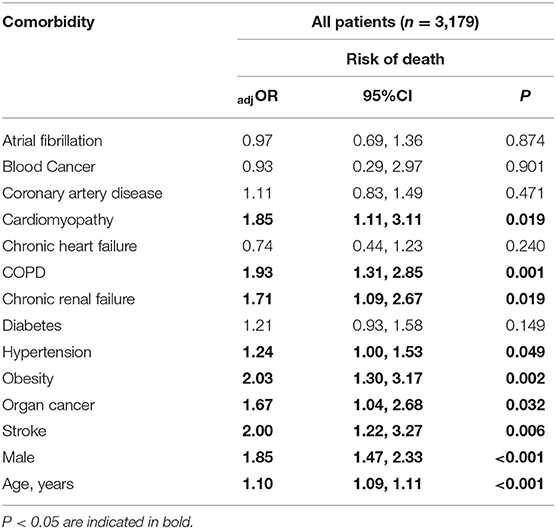
Table 3. Multivariable model for the risk of death associated with each comorbidity after adjustment for sex and age, clustering for site as a random effect.
Analysis of Variability Across Geographic Regions and Hospitals
There were differences in the number of comorbidities across the hospitals and regional areas that provided data (Supplementary Table 1). Patients from the Central Italy were reported to have the fewest number of comorbidities and those from the Northeastern region to have the most. Therefore, in order to assess whether the geographic/hospital variation in diagnostic labeling of comorbidities could have influenced the meaning of the associations made between comorbidities and mortality, in addition to the random effect adjustment for hospital site, we added regional area to the multivariable model shown in Table 2. There was no appreciable change in the relation of any risk factor to death, including comorbidity count, after this additional adjustment (data not shown).
Risk of Death by Hypertension Medication Use
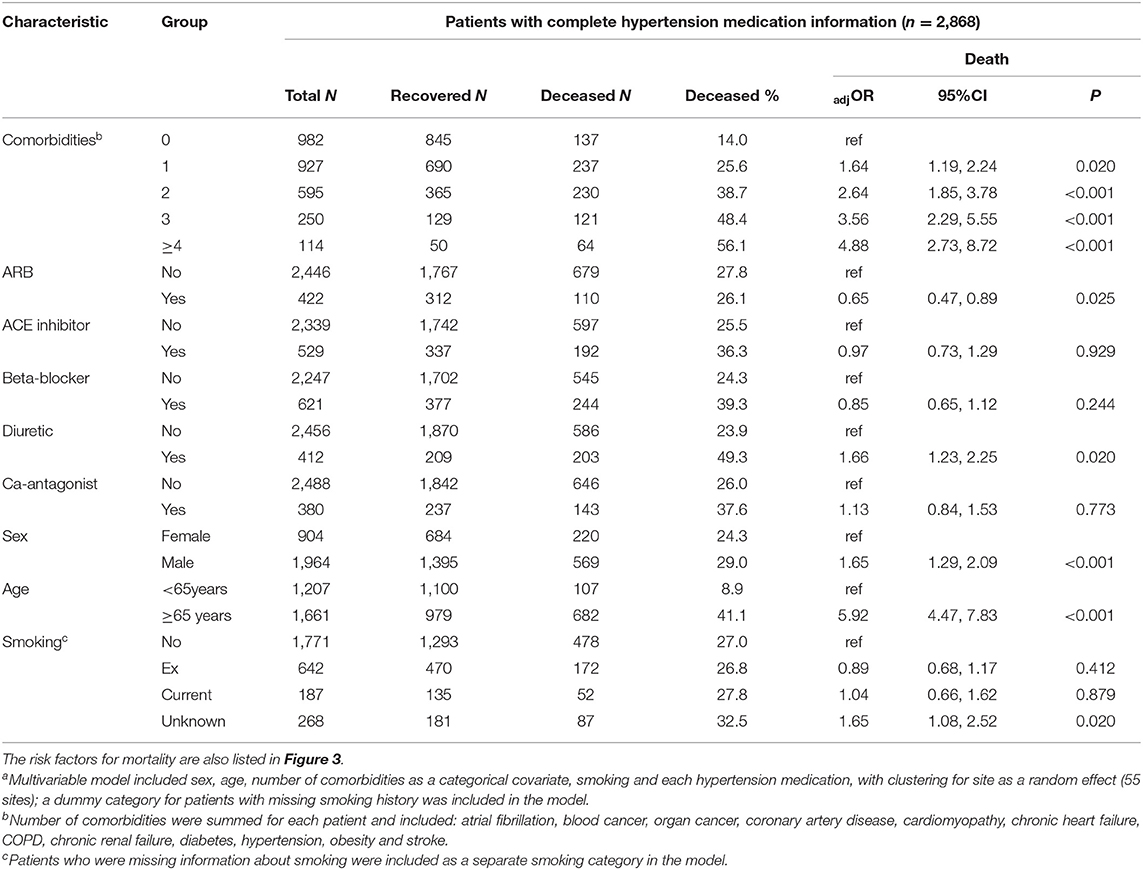
Of the 3,179 patients, 2,868 had complete information for hypertension medication use. Patients with no comorbidities were less likely to use ARBs and ACE inhibitors but there was no trend for increased use of ACE inhibitors or ARBs among patients with one or more comorbidities (Supplementary Table 2). The use of diuretics, beta-blockers and Ca-antagonists increased significantly with the number of comorbidities reported for each patient. Comorbidities were strongly associated with age but not with sex (Supplementary Table 2).
Most of the 951 patients taking either ACE inhibitors or ARB at admission had hypertension, 87.9 and 90.3%, respectively, and beta-blocker use was reported for 29.9%, diuretic use for 24.3% and Ca-antagonist use for 22.8%. After adjustment for age, sex, number of comorbidities, smoking and site, we found no increased risk of death associated with the use of ACE inhibitors, ARBs, beta-blockers or Ca-antagonists (Figure 3 and Table 4). There was a marginal negative association between ARB use and a marginal positive association between diuretic use and death (Figure 3, p = 0.025 and p = 0.020, respectively).
Discussion
This is the first and largest Italian countrywide study to date of COVID-19 inpatients, the majority of which was aged over 65, who either died or were discharged from hospital in 19 out of the 21 Italian regions, including the major islands (Figure 1). We found, in line with previous publications (3–9), that pre-existing comorbidities are major risk factors for death in COVID-19 patients. We report that the number of comorbidities is linearly and strongly associated with the risk of COVID 19-related death. However, after adjusting for comorbidities, age, and sex, we report that the lack of an association between risk of inpatient death due to COVID-19 and use of CVD medications reported in younger patients (3–9), extends to the older population at highest risk for COVID-19. Diuretics were associated with a marginal increased risk of death, and ARBs with a marginal decreased risk of death in this sample. Each comorbidity increased mortality risk independently from age and gender. Among CVD, cardiomyopathies and hypertension were related to a poor outcome. These findings are in line with Inciardi et al. (20) who showed that, in the Northern Italian population, COVID-19 patients with pre-existing CVD had an increased rate of death compared to patients without CVD. The underlying systemic inflammation in patients with CVD (21) might contribute to the increase immune responses and inflammatory cascade known to lead to a worse prognosis in COVID-19 patients (22).
Prior therapy with ACE inhibitors/ARBs was not related to worse prognosis in this cohort. The use of ACE inhibitors and ARB in patients with COVID-19 has been called into question by some (14), due to the evidence that SARS-CoV-2 uses the ACE2 receptor for entry into target cells (16). On the other hand, it has been recently shown that treatment with ACE inhibitors and ARBs does not increase ACE2 plasma levels in patients with heart failure (23). A recent study by Fosbol et al. (12) showed no association between risk of mortality in the Danish population and ACE inhibitor/ARB use. However, the COVID-19-related death burden in Denmark has been ~57-fold lower than Italy (https://coronavirus.jhu.edu/data/mortality), which calls for further studies investigating the characteristics of the Italian COVID-19 population. Additionally, Fosbol et al. did not compare the use of ACE inhibitors to the use of ARB, and the data were computed from national electronic health record review. Here we report that a similar conclusion is applicable to the Italian population. As the north of Italy, and especially the region Lombardia, suffered from one of the highest COVID-19 mortality rates worldwide (2), our data (carefully collected by manual review of medical charts from Italian 56 hospital distributed throughout the peninsula) are particularly important in order to understand the characteristics of the Italian COVID-19 patients with regional specificity. Also, in our study, a higher number of inpatients taking either ACEi or ARB than the Danish cohort was enrolled, thus allowing a stronger statistical power to rule out the individual effects of ACE inhibitors and ARB treatments. In another study, Reynolds et al. reported that previous treatment with ACE inhibitors or ARBs was not associated with a higher risk of testing positive for COVID-19 (10). Similarly, Mancia et al. recently showed that the use of ARBs and ACE inhibitors was more frequent among patients from one Italian region (Lombardy) who were infected with SARS-CoV-2 than among a large population of controls who were matched for age, sex, and place of residence (9). However, in the same study, neither ACE inhibitors nor ARBs showed an independent association with COVID-19 in patients with mild-to-moderate disease or in those with severe disease. Neither of these two latter studies had mortality as primary outcome, but rather the likelihood of the subjects of testing positive for COVID-19, or experiencing severe manifestations of COVID-19.
In a study of 5,700 patients hospitalized with COVID-19 in the New York City area, the mortality rates for patients with hypertension not taking an ACE inhibitors or ARBs at admission, taking an ACE inhibitor, or taking an ARB were comparable (8). Moreover, among 1,128 hospitalized COVID-19 patients with hypertension from Hubei, China, the inpatient use of ACE inhibitors/ARB was reported to be associated with lower risk of all-cause mortality compared with ACE inhibitors/ARB non-users (19). However, in the first study (8) the results were unadjusted for known confounders, including age, sex, race, ethnicity, and comorbidities. In the second study (19), the sample-size included only 188 patients who received ACE inhibitors/ARB, and thus it did not have the power to test the effects of ACE inhibitors and ARBs separately.
This is the first study assessing the safety of CVD medications, and in particular ACE inhibitors and ARB studied separately, in a nation-wide representative sample of COVID-19 inpatients, mostly aged >65. We provide strong evidence suggesting that, regardless of a person's risks for COVID19, the five drugs most frequently used for the treatment of CVDs in outpatient settings are not associated with increased inpatient mortality due to COVID-19.
Among CVD therapies, diuretics were associated with a marginally significant increased risk of mortality in our final models. However, the proportion of patients who were using diuretics at the time of admission increased markedly with the number of comorbidities in each patient. Although we adjusted for number of comorbidities, it is likely that residual confounding may be present. Similarly, use of ARBs was associated with a small decrease in death rates after adjustment for confounders, but the effect was marginally significant and may have been influenced by unaccounted confounding.
Our study has several strengths: (1) it is the first Italian country-wide study describing the characteristics of a highly heterogeneous cohort of COVID-19 inpatients both in terms of severity of the clinical manifestations, and also in terms of geographical distribution. We believe that the analyses performed in this study are clinically informative given that the north of Italy suffered from one of the highest mortalities for COVID-19 worldwide; and (2) the availability of a sample representative of the older people (>65 years of age) at highest risk for morbidity and mortality due to COVID-19. Limitations include: (1) the observational nature of the study, and the fact that data on comorbidities were collected by manual review of the medical charts with no objective assessment; this methodological limitation did not allow the disentanglement of the independent associations of antihypertensive medications with mortality (i.e., adequate control for disease related-, hypertension-, and other medication use- related variables); (2) lack of other outcome measures apart from death or hospital discharge, and of information about the cause of death of the patients (such as severe respiratory distress, acute kidney injury, myocardial infarction, pulmonary, and systemic thromboemobilsm); (3) the absence of information about specific treatments received during hospitalization and in-hospital ACE inhibitors/ARBs continuation or discontinuation; (4) the lack of a severity score for COVID-19 patients during hospitalization; and (5) the potential bias introduced by excluding patients that were still hospitalized at the time of data collection. However, we felt that including the latter could have introduced an even bigger bias, because these individuals could have had associations between the variables analyzed and the outcome that could be different from patients that were included because they had an outcome.
In summary, the results of our nationwide Italian study of a population of COVID19 inpatients, that suffered from one of the highest mortality rates worldwide, confirms that the number of comorbidities appears to be independently associated with increased COVID-19-related death. Among cardiovascular comorbidities both hypertension and pre-existing cardiomyopathy were associated with COVID-19 risk of death. Nonetheless, we provide reassuring evidence that use of commonly-used CVD medications is not associated with increased risk of death due to COVID-19, regardless of the person's risk for the disease due to age, sex, or comorbid conditions. Our findings show that there is no need to interrupt treatments for CVD in COVID-19 patients; in particular, the treatment with ACE inhibitors/ARBs should be continued in order to reduce potential cardiovascular derangement in COVID-19 patients. Further studies are needed in order to shed light onto the relationship between CVD therapies, underlying CVD, and prognosis in COVID-19 patients.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Arizona IRB waiver #2003521629. The ethics committee approvals/waivers were also obtained from each of the participating hospitals. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
FP, GR, EB, MB, MCa, BC, MCo, AC, FD'A, ED'E, GF, SGa, SGu, SH, MK, LM, AP, RP, PP, VP, MP, CT, and RT collected the data. FP, DAS, SGu, JCW, and FDiM analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the FAMRI and the Asthma and Airway Disease Research Center, University of Arizona research funds.
Conflict of Interest
MB has served on advisory boards and has received travel fundings, honoraria for speaking from Angelini, Astra Zeneca, bayer, Cubist, Pfizer, Menarini. MCo has received personal fees from Chiesi, AstraZeneca, Boehringer-Ingelheim, Alk-Abello, GSK, Novartis, Zambon, and scientific grants from Chiesi and University of Ferrara, Italy. FDiM has received personal fees from Chiesi, AstraZeneca, Boehringer-Ingelheim, GSK, Novartis, Zambon, Guidotti/Malesci, Menarini, Mundipharma, TEVA, Almiral, Levante Pharma, Sanophi, and scientific grants from AstraZeneca, Boehringer-Ingelheim, GSK, Novartis. AP has received board membership and consultancy fees, payment for lectures, grants for research, travel expenses reimbursements from GSK, AZ, Boehringer Ingelheim, Chiesi Farmaceutici, TEVA < Mundipharma, Zambon, Novartis, Menarini, Sanofi,Roche, Edmondpharma, Fondazione Maugeri, Fondazione Chiesi. JW has received investigator initiated research funding from Vertex pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the investigators of the ItaliCO study group for collecting the patients' data included in this manuscript.
ITALICO: Italian National Study on Risk Factors Associated With COVID-19
List of Contributors
F. Polverino MD PhD1, D. Stern MS1, M. Polverino MD2, F. D'Amico MD3, E. D'Elia, MD PhD4, A. Agarossi MD5, S. Agati MD6, E. Agosteo MD7, F. Ando' MD8, M. Andreoni MD9, IF. Angelillo DDS MPH10, G. Arcoleo MD11, C. Arena MD12, P. Baiamonte MD11, E. Balestro MD13, L. Ball MD, PhD14, P. Banfi MD15, G. Bartoletti MD16, R. Bartolotta RN17, M. Bassetti MD PhD14, D. Battaglini MD14, M. Bellan MD PhD18, I. Benzoni MD PhD19, R. Bertolini MD20, M. Bevilacqua MD21, M. Bezzi22 MD, A. Bianco MD23, A. Bisbano MD24,F. Bobbio MD18, G. Bocchialini MD22, F. Bonetti MD20, F. Boni MD25, M. Bonifazi MD26, G. Borgonovo MD25, S. Borre' MD27, M. Bosio MD28, G. Brachini MD29, I. Brunetti MD14, L. Calagna3, F. Calò10, M. Candelli MD PhD30, A. Capuozzo MD2, T. Carr MD1, A. Castellani MD22, F. Catalano MD PhD11, G. Catania MD31, E. Catena MD5, M. Cattaneo32, 33, A. Cattelan MD13, V. Ceruti MD21, F. Chiumiento MD34, G. Cicchitto MD2, B. Cirillo MD29, M. Confalonieri MD35, P. Confalonieri MD35, M. Contoli MD PhD36, N. Coppola MD PhD10, A. Corsico MD28, R. Cosentina MD3, R. Costantino MD37, C. Crimi MD PhD38, A. Currà MD39, M. D'Abbraccio MD40, A. Dalbeni MD21, F. Daleffe MD22, R. Davide MD41, M. Del Donno MD42, F. Di Marco, MD PhD43, F. Di Pastena MD44, F. Di Perna MD45, Z. Di Rosa MD46, A. Di Sabatino MD28, O. Elesbani MD40, D. Elia MD32, V. Esposito MD PhD47, L. Fabiani MD48, G. Falco MD25, G. Falo MD35, C. Fanelli MD49, A. Fantin MD50, F. Ferrigno MD51, G. Fiorentino MD46, F. Franceschi MD PhD30, M. Fronza MD52, G. Gardini Gardenghi MD40, S. Gasparini MD26, D.R. Giacobbe MD14, C. Giannotti MD5, G. Giannotti MD19, A. Gidari MD53, F. Giovanardi MD PhD25, P. Gnerre MD31, F. Gonnelli MD26, M. Graziano MD54, S. Greco MD47, A. Grosso PhD28, S. Guarino MD32, S. Guerra MD PhD1, S. Harari MD32, A. Iannarelli MD55, P. Imitazione MD39, F. Inglese MD56, V. Iodice MD46, A. Izzo MD43, C. La Greca MD16, M. Kraft MD1, A. Lax MD15, F. Legittimo MD20, A. Leo MD57, S. Leone MD53, V. Lepidini MD9, M. Leto RN36, F. Licata MD23, F. Locati MD3, L. Lorini MD4, B. Lucchetti MD20, I. Maida MD48, M. Macera MD10, E. Manzillo MD46, A. March MD49, D. Mascheroni MD58, A. Mastroianni MD17, I. Mauro MD2, M. Mazzitelli MD23, E. Mazzuca MD11, L. Mennella MD16, C. Micheletto MD12, A. Mingoli MD29, P. Minuz MD21, M. Moioli MD14, L. Monti MD58, R. Morgagni, MD PhD9, L. Mucci MD59, M. Muselli MD47, S. Negri MD6, C.G.A. Nobile MD60, S. Oldani MD61, C. Olivieri MD27, A. Papi MD35, G. Parati MD62, L. Parodi MD31, R. Parrella MD PhD46, E. Pastorelli MD27, V. Patruno MD49, F. Pellegrino MD7, P. Pelosi MD FERS14, M.F. Pengo, MD PhD62, D. Pepe MD23, A. Perotti MD5, R. Petrino MD27, M. Petrucci MD30, R.M. Piane MD16, G. Pignataro MD PhD30, M. Pino MD23, M. Pirisi MD18, V. Poletti MD61, F. Porru MD63, F. Pugliese MD29, R. Punzi MD46, D.A. Ramaroli MD12, C. Robba MD PhD14, R. Rostagno MD27, G. Ruocco MD64, U. Sabatini MD28, P.P. Sainaghi MD PhD18, F. Salton MD34, C. Salzano MD65, A. Sanduzzi MD39, S. Sanduzzi Zamparelli MD10, V. Sangiovanni MD46, D. Santopuoli MD45, P. Sapienza MD29, L. Sarmati MD9, E. Schiaroli MD66, F. Scienza MD27, M. Senni MD4, L. Serchisu PhD51, S. Sgherzi MD57, D. Soddu MD18, D. Soranna MD61, C. Sorino MD PhD6, S. Spadaro MD35, E. Stirpe MD49, C. Tana MD58, S. Tardivo MD12, S. Tartaglia MD27, E. Teopompi MD20, R. Terribile MD27, M. Tomchaney1, E. Torelli MD30, C. Torlasco MD61, C. Torti MD23, E. Tupputi MD56, C. Ugolinelli MD5, A. Vatrella MD68, A.G. Versace MD8, M. Villani MD68, L. Vincenzo MD40, C.A. Volta MD35, N. Voraphani MD1, J.C. Woods PhD69, E. Zekaj MD70, R. Zoppellari MD35, F.D. Martinez MD1
1Asthma and Airway Disease Research Center, University of Arizona
2Ospedale Scarlato, Scafati
3ASST Bergamo Est, Seriate
4ASST Papa Giovanni XXIII, Bergamo
5Ospedale Sacco, Milano
6ASST Lariana, Ospedale Sant'Anna di Como
7Clinica San Carlo- Paderno Dugnano, Milano
8Policlinico di Messina, Messina
9Policlinico Universitario Tor Vergata, Roma
10Università Vanvitelli, Napoli
11A.O.O.R. Villa Sofia Cervello, Palermo
12Università di Verona, Verona
13Azienda Ospedaliera Universitaria di Padova, Padova
14Policlinico San Martino and Universita' di Genova, Genova
15IRCCS Fondazione Don Carlo Gnocchi, Milano
16Azienda USL Toscana Nord Ovest, Ospedale di Lucca
17zienda Ospedaliera di Cosenza, Cosenza
18AOU Maggiore della Carità and Università del Piemonte Orientale UPO, Novara, Italy
19Ospedale di Cremona, Cremona
20AUSL-IRCCS RE Ospedale di Guastalla
21Policlinico GB Rossi, Verona
22Spedali Civili di Brescia, Brescia
23Università degli studi Magna Graecia, Catanzaro
24Ospedale San Giovanni di Dio, Crotone
25AUSL-IRCCS, Reggio Emilia
26Polytechnic University of Marche Region, Azienda Ospedali Riuniti, Ancona
27Ospedale S Andrea, Vercelli
28IRCCS Policlinico San Matteo Foundation, Pavia
29Universita La Sapienza, Roma
30Fondazione Universitaria Policlinico Gemelli - IRCCS. Università Cattolica del Sacro Cuore di Roma, Roma
31Ospedale San Paolo, Savona
32Department of Medical Sciences, San Giuseppe Hospital MultiMedica IRCCS, Milan, Italy
33Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
34Ospedale di Eboli, Eboli
35Ospedale di Trieste, Trieste
36Universita' di Ferrara, Ferrara
37Azienda Ospedaliera Pugliese Ciaccio, Catanzaro
38Azienda Ospedaliera Universitaria Policlinico-Vittorio Emanuele, Catania
39Ospedale Giulio Jazzolino, Vibo Valentia
40Ospedale Monaldi and Federico II University, Napoli
41ASST del Garda, Ospedale di Desenzano del Garda
42Ospedale Rummo, Benevento
43ASST Papa Giovanni XXIII, Bergamo, and University of Milan
44Ospedale Dono Svizzero, Formia
45Ospedale di Caserta, Caserta
46Ospedale Cardarelli, Campobasso
47Ospedale Cotugno, Napoli
48Universita' de L'Aquila
49Ospedale di Sassari, Sassari
50Ospedale Universitario di Bolzano
51ASL Salerno, Scafati
52Ospedale di Bolzano, Bolzano
53Ospedale di Perugia, Perugia
54Azienda Sanitaria Locale and San Giuseppe Moscati Hospital, Avellino
55Ospedale Santa Maria Goretti, Latina
56ASST Mantova, Mantova
57ASL BT. Pneumologia Andria
58IC Villa Aprica, Gruppo San Donato, Como
59ASL Lanciano Vasto Chieti, Chieti, Italy
60Università della Calabria, Cosenza
61Ospedale Morgagni, Forli'
62IRCCS Istituto Auxologico Italiano, Ospedale San Luca, Milano
63Erasmus MC, Rotterdam
64Ospedale Regina Montis Regalis, Mondovì, Cuneo
65Hospital “Buon Consiglio-Fatebenefratelli,” Napoli
66Ospedale di Perugia, Perugia
67Universita' di Salerno
68Ospedale di Crema, Crema
69Cincinnati Children's Hospital and University of Cincinnati
70IRCCS Istituto Ortopedico Galeazzi Milano
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.585866/full#supplementary-material
References
1. International Institute for Applied Systems Analysis (IIASA). Aging Demographic Data Sheet: Laxenburg, AI: International Institute for Applied Systems Analysis (IIASA) (2018).
2. Odone A, Delmonte D, Scognamiglio T, Signorelli C. COVID-19 deaths in Lombardy, Italy: data in context. Lancet Public Health. (2020) 5:e310. doi: 10.1016/S2468-2667(20)30099-2
3. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
4. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. (2020) 55:2000547 doi: 10.1183/13993003.01227-2020
5. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
6. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
7. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
8. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
9. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N Engl J Med. (2020) 382:2431–40. doi: 10.1056/NEJMoa2006923
10. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of covid-19. N Engl J Med. (2020) 382:2441–8. doi: 10.1056/NEJMoa2008975
11. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–260. doi: 10.1038/s41569-020-0360-5
12. Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. (2020) 324:168–77. doi: 10.1001/jama.2020.11301
13. Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, et al. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. (2020) 22:957–66. doi: 10.1002/ejhf.1871
14. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. (2020) 8:e21. doi: 10.1016/S2213-2600(20)30116-8
15. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N Engl J Med. (2020) 382:1653–9. doi: 10.1056/NEJMsr2005760
16. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80e278. doi: 10.1016/j.cell.2020.02.052
17. Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. (2012) 487:477–81. doi: 10.1038/nature11228
18. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. (2016) 118:1313–26. doi: 10.1161/CIRCRESAHA.116.307708
19. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. (2020) 126:1671–81. doi: 10.1161/CIRCRESAHA.120.317242
20. Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. (2020) 41:1821–9. doi: 10.1093/eurheartj/ehaa388
21. Paquissi FC. The role of inflammation in cardiovascular diseases: the predictive value of neutrophil-lymphocyte ratio as a marker in peripheral arterial disease. Ther Clin Risk Manag. (2016) 12:851–60. doi: 10.2147/TCRM.S107635
22. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–14. doi: 10.1016/S0140-6736(20)30628-0
23. Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J. (2020) 41:1810–7. doi: 10.1093/eurheartj/ehaa373
Keywords: COVID-19, comorbidities, ACE inhibitors, mortality, cohort study
Citation: Polverino F, Stern DA, Ruocco G, Balestro E, Bassetti M, Candelli M, Cirillo B, Contoli M, Corsico A, D'Amico F, D'Elia E, Falco G, Gasparini S, Guerra S, Harari S, Kraft M, Mennella L, Papi A, Parrella R, Pelosi P, Poletti V, Polverino M, Tana C, Terribile R, Woods JC, Di Marco F, Martinez FD and the ItaliCO study group (2020) Comorbidities, Cardiovascular Therapies, and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO). Front. Cardiovasc. Med. 7:585866. doi: 10.3389/fcvm.2020.585866
Received: 22 July 2020; Accepted: 19 August 2020;
Published: 09 October 2020.
Edited by:
Shuyang Zhang, Peking Union Medical College Hospital, ChinaReviewed by:
Bastiaan Geelhoed, University Medical Center Groningen, NetherlandsChristoph Sinning, University Heart and Vascular Center Hamburg (UHZ), Germany
Copyright © 2020 Polverino, Stern, Ruocco, Balestro, Bassetti, Candelli, Cirillo, Contoli, Corsico, D'Amico, D'Elia, Falco, Gasparini, Guerra, Harari, Kraft, Mennella, Papi, Parrella, Pelosi, Poletti, Polverino, Tana, Terribile, Woods, Di Marco, Martinez and the ItaliCO study group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Polverino, ZnBvbHZlcmlub0Bjb3BkbmV0Lm9yZw==
†These authors have contributed equally to this work
 Francesca Polverino
Francesca Polverino Debra A. Stern1
Debra A. Stern1 Gaetano Ruocco
Gaetano Ruocco Matteo Bassetti
Matteo Bassetti Marcello Candelli
Marcello Candelli Marco Contoli
Marco Contoli Filippo D'Amico
Filippo D'Amico Luigi Mennella
Luigi Mennella Paolo Pelosi
Paolo Pelosi Venerino Poletti
Venerino Poletti Claudio Tana
Claudio Tana Fabiano Di Marco
Fabiano Di Marco