- 1Memphis VA Hospital, Memphis, TN, United States
- 2University of Memphis, Memphis, TN, United States
- 3All India Institute of Medical Sciences (AIIMS), Rishikesh, India
- 4University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 5Biostatistics, Life University, Marietta, GA, United States
- 6Baylor College of Medicine, Houston, TX, United States
- 7Texas Heart Institute, Houston, TX, United States
- 8University of Kansas Medical Center, Kansas City, KS, United States
- 9Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 10Texas Cardiac Arrhythmia Institute at St. David's Medical Center, Austin, TX, United States
- 11University of Tennessee Health Science Center, Memphis, TN, United States
- 12New York University School of Medicine, New York, NY, United States
- 13Mayo Clinic College of Medicine, Rochester, MN, United States
Background: For patients with atrial fibrillation who are at high risk for bleeding or who cannot tolerate oral anticoagulation, left atrial appendage (LAA) closure represents an alternative therapy for reducing risk for thromboembolic events.
Objectives: To compare the efficacy and safety of the Amplatzer and WatchmanTM LAA closure devices.
Methods: A meta-analysis was performed of studies comparing the safety and efficacy outcomes of the two devices. The Newcastle-Ottawa Scale was used to appraise study quality.
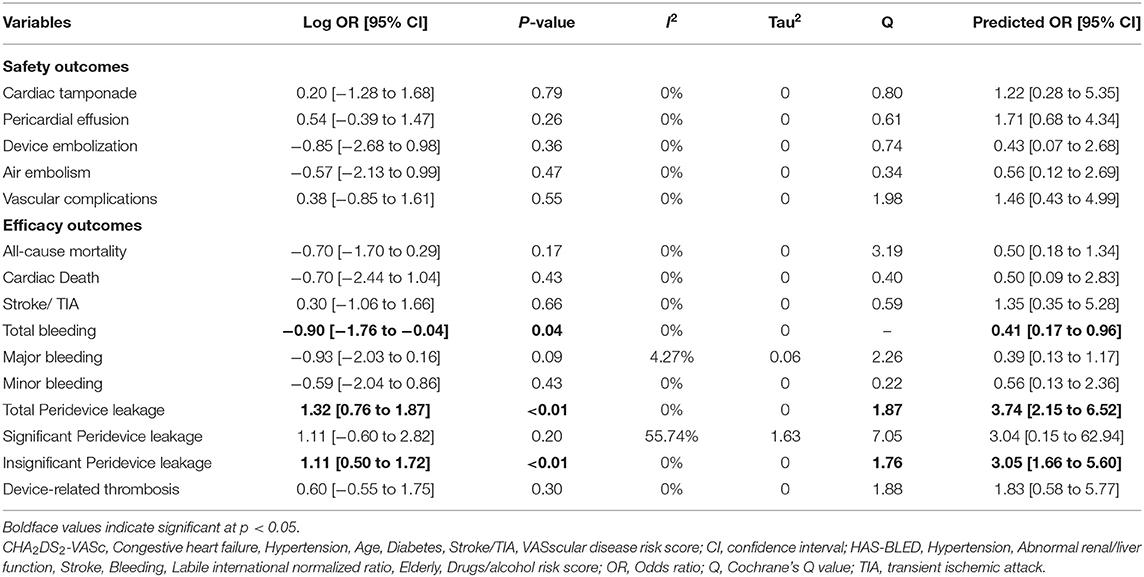
Results: Six studies encompassing 614 patients were included in the meta-analysis. Overall event rates were low for both devices. No significant differences between the devices were found in safety outcomes (i.e., pericardial effusion, cardiac tamponade, device embolization, air embolism, and vascular complications) or in the rates of all-cause mortality, cardiac death, stroke/transient ischemic attack, or device-related thrombosis. The total bleeding rate was significantly lower in the WatchmanTM group (Log OR = −0.90; 95% CI = −1.76 to −0.04; p = 0.04), yet no significant differences was found when the bleeding rate was categorized into major and minor bleeding. Total peridevice leakage rate and insignificant peridevice leakage rate were significantly higher in the WatchmanTM group (Log OR = 1.32; 95% CI = 0.76 to 1.87; p < 0.01 and Log OR = 1.11; 95% CI = 0.50 to 1.72; p < 0.01, respectively). However, significant peridevice leakages were similar in both the devices.
Conclusions: The LAA closure devices had low complication rates and low event rates. Efficacy and safety were similar between the systems, except for a higher percentage of insignificant peridevice leakages in the WatchmanTM group. A randomized controlled trial comparing both devices is underway, which may provide more insight on the safety and efficacy outcomes comparison of the devices.
Introduction
Left atrial appendage (LAA) closure is an alternative means of reducing the risk for stroke in patients with atrial fibrillation (AF) who cannot tolerate long-term oral anticoagulation (1–4), as the vast majority of thrombus formation in patients with AF occurs in the LAA (5, 6). Several LAA closure systems have been developed, including the WatchmanTM Left Atrial Appendage Closure Device (Boston Scientific, Marlborough, Massachusetts) and the AmplatzerTM Cardiac Plug and Amulet devices (St. Jude Medical-Abbott, St. Paul, Minnesota) (7). Of these, the WatchmanTM is the most studied device and the only device approved by the U.S. Food and Drug Administration (FDA) for LAA closure. FDA approval was based on data from two multicenter randomized controlled trials comparing the WatchmanTM and warfarin: the PROTECT AF (WatchmanTM Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation) trial (8) and the PREVAIL (Prospective Randomized Evaluation of the WatchmanTM LAA Closure Device in Patients With Atrial Fibrillation vs. Long-Term Warfarin Therapy) trial (9). Notably, in the PREVAIL trial, non-inferiority was established for ischemic stroke prevention but not for overall efficacy (a composite of stroke, systemic emboli, and cardiovascular or unexplained death) (9). A 2-year follow-up study of data from the prospective, multicenter, multinational EWOLUTION (Evaluating Real-Life Clinical Outcomes in Atrial Fibrillation Patients Receiving the WatchmanTM Left Atrial Appendage Closure Technology) registry showed that patients with AF who received a WatchmanTM device had consistently low rates of stroke and non-procedural bleeding (10). Despite their limited use in the United States, the Amplatzer LAA closure devices are popular in the rest of the world. Many observational studies showing favorable safety and efficacy outcomes have been published (11–20). Several cohort studies have directly compared the safety and efficacy of the Amplatzer cardiac plug or amulet vs. the WatchmanTM device (21–26). However, these studies were limited in sample size and therefore we conducted a meta-analysis of published data that directly compared the Amplatzer and WatchmanTM devices, focusing primarily on efficacy and safety outcomes (27).
Methods
Search Strategy
A systematic review of existing literature was performed to search for literature prior to July 2019. Two physician-reviewers (DK and SS) queried PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases for published literature, using the search terms WatchmanTM, Amplatzer Cardiac Plug, Amplatzer Amulet, left atrial appendage occlusion, left atrial appendage closure, and combinations of these keywords. Additional literature was sought by searching for references of eligible articles. Any discrepancies were resolved by a third reviewer (IBR).
Study Selection
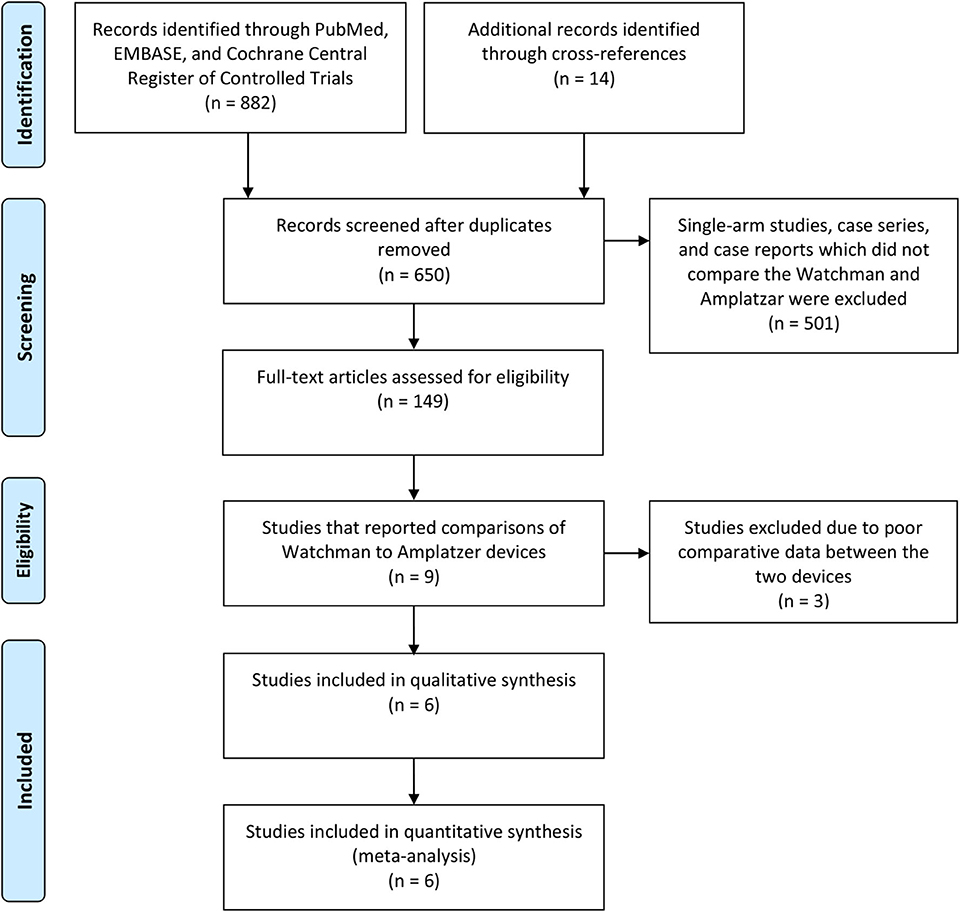
For the meta-analysis, we selected studies that directly compared the Amplatzer and WatchmanTM devices, provided periprocedural and at least 90 days long-term efficacy data (similar follow up duration for both the devices), and had a sample size of at least 10 (Figure 1). Studies that involved both the Amplatzer and WatchmanTM devices but did not report comparative outcomes data for each device were excluded (28–30). Single-arm studies, case reports, case series, and cohort studies that had fewer than 10 patients or that did not present adequate safety/efficacy outcomes data also were excluded.
The Newcastle-Ottawa Scale was used to assess whether the studies included in the meta-analysis were of good or poor quality (31). Good quality is indicated by 3–4 points in the selection domain, 1–2 points in the comparability domain, and 2–3 points in the outcome domain (for an overall rating of 6–9 points) (Supplementary Table 1).
Data Extraction
Data on baseline characteristics and safety and efficacy outcomes for each device were extracted from each of the selected studies and entered into a Microsoft Excel spreadsheet by authors DK and SS. Baseline characteristics include the total number of participants and implantation success rate for individual studies of age, sex, previous stroke, and CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes, stroke/TIA, and VAScular disease) score (for determining stroke risk and prophylaxis). Also included were HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding, labile international normalized ratio, elderly, and drugs/alcohol) score (for determining risk for major bleeding associated with oral anticoagulation), mean follow-up periods, follow up duration for post-procedural trans-esophageal echocardiography (TEE), and size for assessment of significant peridevice leakages. Safety outcomes included periprocedural complications such as cardiac tamponade, pericardial effusion, device embolization, air embolism, and vascular complications. Long-term efficacy outcomes included all deaths, cardiac death, stroke or TIA, bleeding, peridevice leakage, and device-related thrombosis. The study outcome definitions from the individual studies were used for the above outcomes.
Data Analysis
To compare the safety and efficacy outcomes of the two devices, a hypergeometric-normal model to approximate the exact likelihood was used, as the number of events in each study is small relative to the group and included many zero events (32). To negate the small study effect, Log OR with 95% confidence intervals (CIs) was calculated, and that was back-transformed to predict exponential OR along with 95% confidence intervals (CIs) using R software (33).
Results
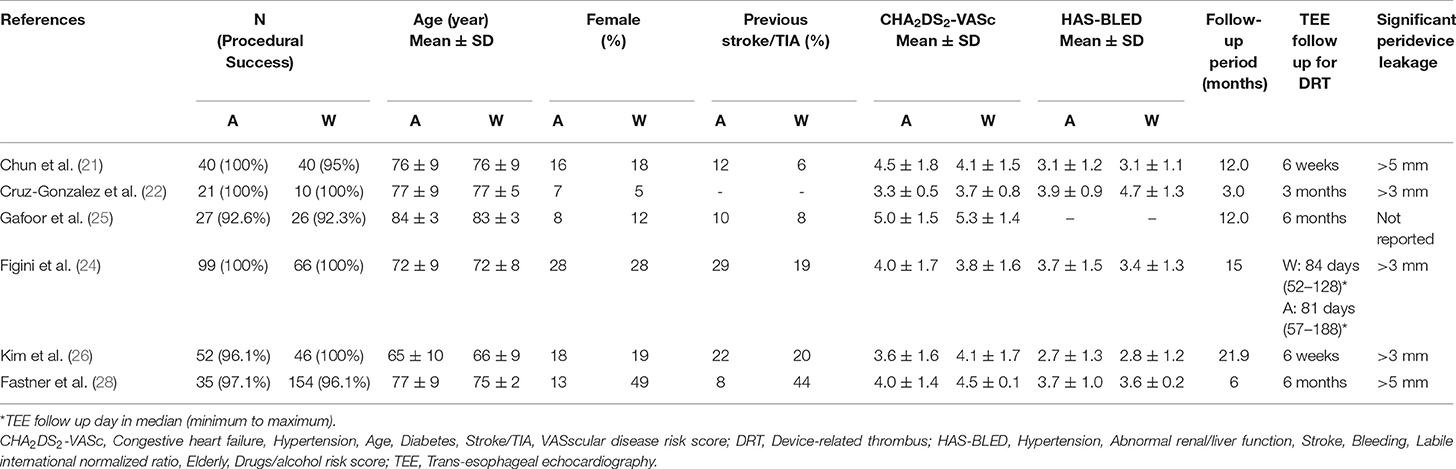
Six studies with a total of 342 patients in the WatchmanTM group and 274 (Table 1) patients in the Amplatzer group were included in the meta-analysis (Figure 1) (21–26). All of the studies scored 7 points on the Newcastle-Ottawa Scale (Supplementary Table 1), indicating good quality. Descriptive comparison of baseline characteristics from the six studies used in the meta-analysis is shown in Table 1. Follow-up durations for post-procedural trans-esophageal echocardiography (TEE) were different across the studies and have been summarized in Table 1. The definitions of significant peridevice leakages were also variable across the studies and have been summarized in Table 1. The implantation success rate was high for both the Amplatzer (98.2%) and the WatchmanTM (96.8%) devices. The mean procedural time was 55.1 ± 6.2 (minutes ± standard deviation) for WatchmanTM and 59.3 ± 11 for Amplatzer, while the mean fluoroscopy time was 9.9 ± 3.8 for the WatchmanTM and 12 ± 4.8 for the Amplatzer. The I2 statistic for heterogeneity was 0% for most outcomes, with the highest statistic being 55.74%, signifying consistency of results. A statistical comparison of baseline characteristics, procedural outcomes, and safety and efficacy outcomes is shown in Table 2. In most studies, the background anticoagulants regimens included single antiplatelet therapies for patients for whom oral anticoagulants were contraindicated. Post-operatively, most of these patients were discharged on dual antiplatelet treatment.
Safety Outcomes
Overall, periprocedural complication rates were low in both groups (Table 2). The only vascular complications were local groin hematoma and pseudoaneurysm formation. The meta-analysis found no significant differences in the safety outcome measures, which included cardiac tamponade, pericardial effusion, device embolization, air embolism, and vascular complications.
Efficacy Outcomes
The mean follow-up period to assess the outcomes ranged from 3 to 22 months. The overall complication event rates were low in both groups (Table 2). No significant differences were observed in all-cause mortality, cardiac death, and stroke or TIA occurrences. The total bleeding rate was significantly lower in the WatchmanTM group (Log OR = −0.90; 95% CI = −1.76 to −0.04; p = 0.04). However, no significant differences were observed when major bleeding and minor bleeding outcomes were compared.
Device-related thrombosis and peridevice leakage data were taken from transesophageal echocardiography results obtained at the first follow-up visit after the LAA closure procedure (Table 1). However, because definition of peridevice leakage varied across the studies, comparisons were made for significant, insignificant, and total peridevice leakage rates. The rates of total peridevice leakage were significantly higher in the WatchmanTM group (Log OR = 1.32; 95% CI = 0.76 to 1.87; p < 0.01). Significant peridevice leakage rate was similar among the WatchmanTM and the Amplatzer group, whereas insignificant peridevice leakage rate was significantly higher in the WatchmanTM group (Log OR = 1.11; 95% CI = 0.50 to 1.72; p < 0.01). Device-related thrombosis rate did not differ significantly between the WatchmanTM and Amplatzer groups.
Discussion
In this study, we performed a systematic review and meta-analysis to compare the two most popular LAA closure devices worldwide: the WatchmanTM Left Atrial Appendage Closure Device and the Amplatzer Cardiac Plug and Amulet devices. Of these devices, only the WatchmanTM has been approved in the United States for stroke prophylaxis in patients with AF. FDA approval was granted in response to two randomized controlled trials (PROTECT AF and PREVAIL) that demonstrated the Watchman'sTM non-inferiority to anticoagulant in preventing strokes (8, 9, 34). However, although ample efficacy and safety data on the Amplatzer devices are available from European and Asian reports (11–20), the devices have not been broadly adopted in the United States.
This spurred the FDA to recommend a head-to-head trial comparing WatchmanTM and Amplatzer devices in patients undergoing LAA closure (34). To this end, a randomized clinical trial of the WatchmanTM device and the Amplatzer Amulet is now underway in Switzerland. The primary endpoints of the SWISS-APERO trial (NCT03399851) are composites of many of the outcome measures evaluated in our study, including a safety composite (procedure-related complications, all-cause death, and major bleeding through 12 months), an efficacy composite (ischemic stroke and systemic embolism through 18 months), and a mechanism-of-action endpoint (device closure at 45 days, as evaluated by transesophageal echocardiography). The trial is expected to begin providing results by no later than 2021.
Safety Outcomes
In terms of real-world data, many of the WatchmanTM safety outcomes were derived from the PROTECT AF (8, 35) and PREVAIL (9) trials and their subsequent registries, whereas Amplatzer data came from smaller cohort studies. Reddy et al. (34) observed that, over time, increased user experience with the WatchmanTM resulted in a trend toward fewer periprocedural complications. Our meta-analysis showed that, overall, both devices had relatively low complication rates. We found no differences in the rates of periprocedural complications with regard to pericardial effusion, cardiac tamponade, major bleeding, vascular complications, device emboli, or air embolization; nonetheless, the strength and generalizability of these results is tempered by the small sample size and low number of adverse events.
Efficacy Outcomes
The meta-analysis showed no significant difference between the two devices with respect to overall mortality, cardiac deaths, and stroke or TIA occurrences. Even though the total bleeding rate was higher in the Amplatzer group, major and minor bleeding rates were not significantly different for the devices. Low chi2 value in the presence of a tau2 value of 0 signifies the consistency of results and is unlikely to be tampered with by the variable follow-up period. It should be noted that post-operative antithrombotic therapy varied for the devices and for each study. Manufacturer recommendations regarding post-procedural antithrombotic protocols are different for the Amplatzer vs. the WatchmanTM. After Amplatzer implantation, patients are usually started on dual antiplatelet therapy and then transitioned to aspirin monotherapy; conversely, after WatchmanTM implantation, patients typically receive oral anticoagulation for 45 days before transitioning to dual antiplatelet therapy and eventually aspirin monotherapy. This may be one of the possible explanations for the significant difference in total bleeding incidences.
Significant peridevice leakage was defined differently in the six studies included in the analyses. Total peridevice leakages were significantly more frequent in the Amplatzer group. However, significant peridevice leakages were similar among both the devices which matches the finding of the EWOLUTION registry, where the rate of significant leaks in WatchmanTM devices was <1% (10). Low chi2 value in the presence of tau2, value of 0, signifies the consistency of results that are unlikely to be tampered with by the variable follow-up period. Device-related thrombus (DRT) events were similar for both the devices despite different follow-up durations across the studies. Not all patients who followed up underwent TEE (21–26). However, in a large meta-analysis combining 66 studies, there was no difference in DRT rates between the Amplatzer and WatchmanTM devices (3.6 vs. 3.1%, p = 0.24) (36).
The device design might also contribute to the discrepancy in peridevice leakage rates between the two closure systems. The Amplatzer devices comprise a lobe and a disk, connected by a central waist. The self-expanding lobe engages the LAA, while the disk covers the orifice. The WatchmanTM is a parachute-shaped device consisting of a self-expanding frame with a permeable polyester fabric cover that accommodates the LAA. Several studies have examined mechanisms of peridevice leakage, which represents suboptimal engagement of the device with the LAA (37, 38). Post-operative imaging studies have shown that WatchmanTM leaks stem primarily from gaps between the device and the LAA ostium, whereas Amplatzer leaks typically involve off-axis alignment of the lobe portion with respect to the LAA neck (37). However, Wolfrum et al. (38) found no correlation between periprocedural complications and the positioning of the Amplatzer device.
Notably, a study of the anatomical impact of the devices in canine models showed more complete neo-endocardialization of the WatchmanTM device surface vs. the Amplatzer after 28 days. This was postulated to result from the Amplatzer disk's extension outside of the LAA orifice, which may affect surrounding structures and delay healing (39). These anatomical differences do not appear to significantly affect either device's overall efficacy in stroke prevention, and subsequent studies of the effect of incomplete LAA closure and peridevice leakage found no significant increases in long-term adverse events (40).
Limitations
The number of prospective studies included in the meta-analysis were limited. Of the six studies, two were prospective non-randomized studies, four were retrospective studies, and no randomized controlled trials were available. Although funnel plots were generated to appraise efficacy and safety outcomes, because our meta-analysis included fewer than 10 studies, the data were probably too scant to detect true asymmetry from chance, and thus no definitive conclusions could be drawn.
Conclusions
These devices have different implant techniques. The Amplatzer Device includes a lobe with a covering disk (connected by a waist) and the WatchmanTM device includes a self-expanding frame covered by a fabric. Post-implantation anti-coagulation regimens are also different for the devices. The WatchmanTM typically has a longer duration of both anti-coagulants and dual anti-platelet therapy post-implantation in comparison to the Amplatzer. However, the results of this study reveal an overall low and similar complication rate for both devices. The total bleeding rates were higher in the Amplatzer group (but not major or minor bleeding), and other efficacy endpoints were similar in both groups, except for a higher percentage of insignificant peridevice leakages in the WatchmanTM group. Our observations were limited by the small number of available studies. An FDA-mandated randomized controlled trial directly comparing these two popular devices is currently underway, and its results may provide more comparative information about the safety and efficacy of the devices.
Perspectives
Competency in Medical Knowledge 1: Patients with atrial fibrillation (AF) are at a relatively higher risk of developing transient ischemic attack (TIA) or stroke. However, antithrombotic therapy may not be a feasible option for such patients if they are at a higher risk of bleeding or cannot tolerate the therapy.
Competency in Medical Knowledge 1: Contemporary left atrial appendage (LAA) closure devices (The WatchmanTM and the Amplatzer) provide an alternative option to mechanically occlude the LAA, thereby eliminating the need for antithrombotic therapy in patients with higher bleeding rates or who cannot tolerate antithrombotic therapy.
Competency in Patient Care: Given the high implantation success frequency and low frequency of adverse outcomes, LAA closure is a promising avenue for the specific cohort of patients to minimize the risk of TIA or strokes. However, regular long-term follow-ups are recommended for both devices to ensure their functionality and to record the safety and efficacy outcomes.
Competency in Interpersonal and Communication Skills: Even though the newer anticoagulants have proved superior in providing effective anticoagulation and lower internal bleeding rates as compared to warfarin, indolent bleeding is still a critical issue for certain populations. Since the devices work mechanically and have a low risk of adverse outcomes, alternative possible treatment options should be discussed with eligible candidates.
Translational Outlook 1: Even though only the WatchmanTM is approved in the United States, the Amplatzer is approved in other countries and has been successful in demonstrating low rates of adverse events in other countries. However, further research and long-term comparison of safety and efficacy outcomes of both devices is required due to their structural dissimilarities.
Translational Outlook 2: Additional prospective clinical trials are needed to evaluate the long-term outcomes of both devices.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
IB conceptualized and reviewed the analyses. DK and SS performed the literature review, analyses, and drafted the manuscript. SC, XJ, WL, and BJ also contributed to drafting the manuscript and critical review of the text. Critical review of the manuscript and inputs regarding the analyses, results, and interpretation of the results were received from NM, MR, DL, SK, AN, AA, JJ, SB, SA, and MS. All authors contributed to the article and approved the submitted version.
Conflict of Interest
SK receives research grants from, and is a consultant for, Boston Scientific, Abbott Vascular, Gore Medical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Jeanie F. Woodruff, BS, ELS, contributed to the editing of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.00089/full#supplementary-material
Abbreviations
AF, atrial fibrillation; CI, confidence interval; FDA, US Food and Drug Administration; LAA, left atrial appendage; OR, odds ratio; TIA, transient ischemic attack.
References
1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. (2001) 285:2370–5. doi: 10.1001/jama.285.18.2370
2. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
3. Verheugt FW. Novel oral anticoagulants to prevent stroke in atrial fibrillation. Nat Rev Cardiol. (2010) 7:149–54. doi: 10.1038/nrcardio.2009.235
4. Sievert H, Lesh MD, Trepels T, Omran H, Bartorelli A, Della Bella P, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation. (2002) 105:1887–9. doi: 10.1161/01.CIR.0000015698.54752.6D
5. Camm AJ, Colombo A, Corbucci G, Padeletti L. Left atrial appendage closure: a new technique for clinical practice. Heart Rhythm. (2014) 11:514–21. doi: 10.1016/j.hrthm.2013.11.030
6. Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. (1995) 25:452–9. doi: 10.1016/0735-1097(94)00396-8
7. Romero J, Perez IE, Krumerman A, Garcia MJ, Lucariello RJ. Left atrial appendage closure devices. Clin Med Insights Cardiol. (2014) 8:45–52. doi: 10.4137/CMC.S14043
8. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. (2009) 374:534–42. doi: 10.1016/S0140-6736(09)61343-X
9. Holmes DR Jr., Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. (2014) 64:1–12. doi: 10.1016/j.jacc.2014.04.029
10. Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, et al. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the Watchman left atrial appendage closure technology. Circ Arrhythm Electrophysiol. (2019) 12:e006841. doi: 10.1161/CIRCEP.118.006841
11. Guérios EE, Schmid M, Gloekler S, Khattab AA, Wenaweser PM, Windecker S, et al. Left atrial appendage closure with the Amplatzer Cardiac Plug in patients with atrial fibrillation. Arq Bras Cardiol. (2012) 98:528–36. doi: 10.1590/S0066-782X2012005000044
12. Kebernik J, Jose J, Abdel-Wahab M, Stöcker B, Geist V, Richardt G. Safety and efficacy of left atrial appendage closure with the Amplatzer Cardiac Plug in very high stroke and bleeding risk patients with non-valvular atrial fibrillation. Cardiol Ther. (2015) 4:167–77. doi: 10.1007/s40119-015-0053-z
13. Kefer J, Tzikas A, Freixa X, Shakir S, Gafoor S, Nielsen-Kudsk JE, et al. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Int J Cardiol. (2016) 207:335–40. doi: 10.1016/j.ijcard.2016.01.003
14. Lam YY, Yip GW, Yu CM, Chan WWM, Cheng BCW, Yan BP, et al. Left atrial appendage closure with Amplatzer cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv. (2012) 79:794–800. doi: 10.1002/ccd.23136
15. López-Minguez JR, Eldoayen-Gragera J, González-Fernández R, Fernández-Vegas C, Fuentes-Cañameroet ME, Millán-Nuñez V, et al. Immediate and one-year results in 35 consecutive patients after closure of left atrial appendage with the Amplatzer cardiac plug. Rev Esp Cardiol. (2013) 66:90–7. doi: 10.1016/j.rec.2012.04.017
16. Nielsen-Kudsk JE, Johnsen SP, Wester P, Damgaard D, Airaksinen J, Lund J, et al. Left atrial appendage occlusion versus standard medical care in patients with atrial fibrillation and intracerebral haemorrhage: a propensity score-matched follow-up study. Eurointervention. (2017) 13:371–8. doi: 10.4244/EIJ-D-17-00201
17. Nietlispach F, Gloekler S, Krause R, Shakir S, Schmid M, Khattab AA, et al. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv. (2013) 82:283–9. doi: 10.1002/ccd.24872
18. Park JW, Bethencourt A, Sievert H, Santoro G, Meier B, Walsh K, et al. Left atrial appendage closure with Amplatzer Cardiac Plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. (2011) 77:700–6. doi: 10.1002/ccd.22764
19. Saw J, Tzikas A, Shakir S, Gafoor S, Omran H, Nielsen-Kudsk JE, et al. Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the Amplatzer Cardiac Plug. JACC Cardiovasc Interv. (2017) 10:391–9. doi: 10.1016/j.jcin.2016.11.029
20. Tzikas A, Freixa X, Llull L, Gafoor S, Shakir S, Omran H, et al. Patients with intracranial bleeding and atrial fibrillation treated with left atrial appendage occlusion: results from the Amplatzer Cardiac Plug registry. Int J Cardiol. (2017) 236:232–6. doi: 10.1016/j.ijcard.2017.02.042
21. Chun KR, Bordignon S, Urban V, Perrotta L, Dugo D, Fürnkranz A, et al. Left atrial appendage closure followed by 6 weeks of antithrombotic therapy: a prospective single-center experience. Heart Rhythm. (2013) 10:1792–9. doi: 10.1016/j.hrthm.2013.08.025
22. Cruz-Gonzalez I, Perez-Rivera A, Lopez-Jimenez R, Rodriguez-Collado J, Martín-Moreiras J, Cascon M, et al. Significance of the learning curve in left atrial appendage occlusion with two different devices. Catheter Cardiovasc Interv. (2014) 83:642–6. doi: 10.1002/ccd.25230
23. Fastner C, Hoffmann L, Aboukoura M, Behnes M, Lang S, Martin Borggrefe M, et al. Real-world experience comparing two common left atrial appendage closure devices. BMC Cardiovasc Disord. (2018) 18:171. doi: 10.1186/s12872-018-0899-9
24. Figini F, Mazzone P, Regazzoli D, Rega F, Budts W. Left atrial appendage closure: a single center experience and comparison of two contemporary devices. Catheter Cardiovasc Interv. (2017) 89:763–72. doi: 10.1002/ccd.26678
25. Gafoor S, Franke J, Bertog S, Boehm P, Heuer L, Gonzaga M, et al. Left atrial appendage occlusion in octogenarians: short-term and 1-year follow-up. Catheter Cardiovasc Interv. (2014) 83:805–10. doi: 10.1002/ccd.25297
26. Kim JS, Lee H, Suh Y, Pak HN, Hong GR, Shim CY, et al. Left atrial appendage occlusion in non-valvular atrial fibrillation in a Korean multi-center registry. Circ J. (2016) 80:1123–30. doi: 10.1253/circj.CJ-15-1134
27. Basu Ray I, Jia X, Liu J. Meta-analysis of two popular left atrial appendage closure systems for stroke prevention in atrial fibrillation. Paper Presented at: The Heart Rhythm Society's 38th Annual Scientific Sessions, May 10–13. Chicago, IL (2017).
28. Fastner C, Behnes M, Sartorius B, Wenke A, El-Battrawy I, Ansari U, et al. Procedural success and intra-hospital outcome related to left atrial appendage morphology in patients that receive an interventional left atrial appendage closure. Clin Cardiol. (2017) 40:566–74. doi: 10.1002/clc.22699
29. Merella P, Lorenzoni G, Marziliano N, Berne P, Viola G, Pischedda P, et al. Nonvalvular atrial fibrillation in high-hemorrhagic-risk patients: state of the art of percutaneous left atrial appendage occlusion. J Cardiovasc Med. (2019) 20:1–9. doi: 10.2459/JCM.0000000000000735
30. Pracon R, Bangalore S, Dzielinska Z, Konka M, Kepka C, Kruk M, et al. Device thrombosis after percutaneous left atrial appendage occlusion is related to patient and procedural characteristics but not to duration of postimplantation dual antiplatelet therapy. Circ Cardiovasc Interv. (2018) 11:e005997. doi: 10.1161/CIRCINTERVENTIONS.117.005997
31. Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. (2017) 5:80–4. doi: 10.13105/wjma.v5.i4.80
32. Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. (2010) 29:3046–67. doi: 10.1002/sim.4040
33. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2014). Available online at: http://www.R-project.org/
34. Reddy VY, Gibson DN, Kar S, O'Neill W, Doshi SK, Horton RP, et al. Post-approval U.S. Experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. (2017) 69:253–61. doi: 10.1016/j.jacc.2016.10.010
35. Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. (2011) 123:417–24. doi: 10.1161/CIRCULATIONAHA.110.976449
36. Alkhouli M, Busu T, Shah K, Osman M, Alqahtani F, Raybuck B. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC Clin Electrophysiol. (2018) 4:1629–37. doi: 10.1016/j.jacep.2018.09.007
37. Saw J, Fahmy P, DeJong P, Lempereur M, Spencer R, Tsang M, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging. (2015) 16:1198–206. doi: 10.1093/ehjci/jev067
38. Wolfrum M, Attinger-Toller A, Shakir S, Gloekler S, Seifert B, Moschovitis A, et al. Percutaneous left atrial appendage occlusion: effect of device positioning on outcome. Catheter Cardiovasc Interv. (2016) 88:656–64. doi: 10.1002/ccd.26646
39. Kar S, Hou D, Jones R, Werner D, Swanson L, Tischler B, et al. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc Interv. (2014) 7:801–9. doi: 10.1016/j.jcin.2014.03.003
40. Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. (2012) 59:923–9. doi: 10.1016/j.jacc.2011.11.028
Keywords: atrial fibrillation, stroke, left atrial appendage closure, Amplatzer Cardiac Plug, Amplatzer Amulet, WatchmanTM device
Citation: Basu Ray I, Khanra D, Shah S, Char S, Jia X, Lam W, Mathuria N, Razavi M, Jain B, Lakkireddy D, Kar S, Natale A, Adeboye A, Jefferies JL, Bangalore S, Asirvatham S and Saeed M (2020) Meta-Analysis Comparing WatchmanTM and Amplatzer Devices for Stroke Prevention in Atrial Fibrillation. Front. Cardiovasc. Med. 7:89. doi: 10.3389/fcvm.2020.00089
Received: 15 January 2020; Accepted: 27 April 2020;
Published: 22 June 2020.
Edited by:
Shimon Rosenheck, Meir Medical Center, IsraelReviewed by:
Jacqueline Joza, McGill University Health Centre, CanadaOsmar Antonio Centurion, Universidad Nacional de Asunción, Paraguay
Copyright © 2020 Basu Ray, Khanra, Shah, Char, Jia, Lam, Mathuria, Razavi, Jain, Lakkireddy, Kar, Natale, Adeboye, Jefferies, Bangalore, Asirvatham and Saeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Indranill Basu Ray, ibasuray@yahoo.com
†These authors have contributed equally to this work
 Indranill Basu Ray
Indranill Basu Ray Dibbendhu Khanra
Dibbendhu Khanra Sumit Shah4
Sumit Shah4 Wilson Lam
Wilson Lam Adedayo Adeboye
Adedayo Adeboye